Abstract
Wine fermentations using rice media containing either Monascus koji or rice nuruk were performed and fermentative characteristics based on the koji type were investigated. Cultivations were performed in a 20 °C room in a 20 L bottle with the rice media that included Monascus rice koji at both 20 and 30%, or rice nuruk at 20%. After 22 days of cultivation, the ethanol yield reached 14.2–14.6% (v/v) for M. koji and 16.5% (v/v) for rice nuruk. This lower yield with use of M. koji was thought to be due to rapid cell concentration decreases in the later stage. Total amounts of organic acids and volatile compounds in fermentations using M. koji were 166–172 and 1779–1874 mg/L, respectively, being 8.7–12.9% and 46.3–54.1% higher than with use of rice nuruk. With M. koji, a high quality rice wine was produced with high levels of volatile compounds and monacolin K.
Keywords: Rice wine, Monascus koji, Rice nuruk, Ethanol fermentation, Monacolin K
Introduction
Different types of traditional rice wines have been developed in Korea. Currently, there are more than 100 different rice wine brands in Korea that constitute a market share of half a billion dollars [1, 2]. Recently, the demand for alcohol beverages with health benefits has increased [2].
Nuruk has been used in Korea as an enzyme source for traditional alcohol fermentation. It is made by natural cultivation of wheat grains with different fungi and bacteria present in air. Hydrolytic enzymes, such as amylase, protease, and lipase are produced in nuruk [3–6]. During alcohol fermentation and/or aging, starch, proteins, and lipids can be degraded into small molecules by enzymes. As a result, the quality of alcohol beverages is greatly affected by the characteristic properties of nuruk added to fermentation media. Types and amounts of flavor and aroma compounds formed in alcohol fermentation broths depend considerably on nuruk [7]. Traditional nuruk has advantages of bringing out a variety of flavors and aromas in alcohol beverages, but it has also some disadvantages, such as unpleasant odors and flavors [5]. Lactic acid bacteria, fungi, and yeasts have been isolated from traditional nuruk and they are often used for production of commercial nuruk products [8]. Nuruk products using Aspergillus kawachii or Rhizopus japonicus have recently appeared. Alcohol beverages using nuruk contain considerable amounts of proteins, fibers, and carbohydrates, and small amounts of vitamins, organic acids, and physiologically active compounds [9]. In spite of these merits, problems, such as formation of unpleasant flavors and odors, and/or fusel oils remain to be solved.
Monascus koji is called red yeast rice and has been traditionally used in the oriental countries of China, Japan, and Korea for production of red grain wines and red soybean products [10, 11]. M. koji usually made in solid culture after inoculating cooked rice with Monascus strains contains the hydrolytic enzymes amylase, protease, and lipase [12]. In addition, red, orange, and yellow pigments are also produced in Monascus culture that can be used as coloring agents in jam, tomato ketchup, and gochujang [13]. M. koji had been used as a traditional oriental medicine. There are reports [14–16] that Monascus strains produce the secondary metabolites monacolin K, a statin that inhibits cholesterol synthesis, and γ-amino butyric acid (GABA), that induce different physiological effects. The physiologically active compounds saponin and isoflavones are known to exist in M. koji [17], which is currently registered with the Korea Food and Drug Administration (KFDA) as a functional food material for prevention of adult diseases [18].
In this study, M. koji and rice nuruk were made by cultivation with Monascus and Aspergillus strains, respectively. Then, alcohol fermentations using a rice medium containing M. koji or rice nuruk were performed. The different fermentative characteristics affecting wine quality attributes of cell growth, ethanol yield, and organic acid and volatile compound contents were compared between koji types.
Materials and methods
Materials
Monascus sp. KCTC 6121 was obtained from the Korean Culture Type Center (KCTC) of KRIBB (Daejeon, South Korea), and Aspergillus kawachi and Saccharomyces cerevisiae S-2 were supplied by the Japanese Brewing Council (Brewing Society of Japan, Tokyo, Japan).
Commercial white rice was purchased from Jangseong Nonghyup (Jangseong, South Korea). Potato Dextrose Agar (PDA) was obtained from Becton–Dickinson (Sparks, USA). Standard compounds used for analysis of organic acids and volatile compounds were bought from Sigma-Aldrich (St. Louis, USA). Other chemicals, including soluble starch, casein, glucose, ethanol, monacolin K, citrinin, 3, 5-dinitrosalicylic acid (DNS), trifluoro acetic acid, phosphoric acid, and acetonitrile, were also purchased from Sigma–Aldrich (St. Louis, USA). Soft water was prepared using the ion exchange treatment plant of Bohae Brewing Co. (Jangseong, South Korea).
Procedures for production of M. koji and rice nuruk
Seed cultures of Monascus sp. KCTC 6121 and Aspergillus kawachi were prepared by cultivation for 7 days on PDA in an incubator at 25 °C. Five kilograms of white rice were washed with tap water, then immersed in 20 °C water for 2 h. After the water was filtered, wet rice was cooked in a steamer for 30 min. Cooked rice was then put on plates and inoculated with either of the seed cultures at a ratio of a Petri dish per 1 kg. After 88 h of cultivation in an incubator at 35 °C, preparation of M. koji and rice nuruk was completed.
Ethanol fermentation process for production of rice wines
A yeast solution was made by adding dried yeast (Saccharomyces cerevisiae S-2), sucrose, and soft water (1:0.5:10) (w/w) to a flask. After 30 min of incubation at 35 °C, the solution was used as an inoculum for ethanol fermentation. Cooked rice was prepared in the method specified in the previous section.
Fermentations were performed in cultivations using each of two different kojis at 20 or 30% (w/v) for M. koji and 20% (w/v) for rice nuruk. The first cultivation was started by adding 0.48 g of a yeast solution to a 20 L bottle with a cotton plug that contained 1.2 kg of M. koji or rice nuruk, and 1.8 L water. Bottles were then incubated for 2 days in a 20 °C room. During the 2nd stage, 2.4 kg of cooked rice was added to the bottles, followed by incubation for 2 days in a 20 °C room. During the 3rd stage, 2.4 kg of cooked rice was again added to the bottles, followed by incubation in a 20 °C room. The total culture time was 22 days.
For 30% (w/v) M. koji (1.8 kg), 0.72 g of yeast was used as an inoculum. Cooked rice was added (1.8 kg during the 2nd stage and 2.4 kg during the 3rd stage). Other conditions were the same as for use of 20% (w/v) M. koji and 20% (w/v) rice nuruk.
Measurement of acidity
Acidity was measured according to the analytical method of the Korean Food Standards Codex of KFDA [19]. Titration was done with 0.1 N NaOH. The acidity was calculated as: acidity = a × f, where a is the spent amount (mL) of 0.1 N NaOH and f is a factor of 0.1 N NaOH.
Measurement of cell number, ethanol and sugar contents, and color intensity
The cell concentration was expressed as total cell number. After culture samples were diluted with distilled water, the number of cells was counted with a Haemacytometer (Marienfeld- Superior) (Lauda-Konigshofen, Germany). The total cell number was calculated by multiplication using the dilution rate.
The ethanol amount was determined by the alcohol analysis method of the Korean Technical Service Institute [20]. After culture samples were filtered through a 100 mesh, the solution was distilled until the distillate reached 80–90% (v/v) of the original solution. The ethanol amount in the distillate (%; v/v) was measured with a density meter from Anton Paar Inc. (DMA 5000) (Graz, Austria).
The amount of reducing sugars was measured by the 3, 5-dinitrosalicylic acid (DNS) method [21]. A 30 mL capped tube containing 1 mL of sample and 3 mL of a 1% DNS solution was boiled for 5 min, then cooled, followed by addition of 21 mL of distilled water to the reacted solution. The absorbance was measured at 550 nm with a spectrophotometer (Cary 60 UV–VIS/Agilent Technology; Santa Clara, USA). The sugar amount was expressed in glucose equivalents. The intensity of red color was determined by measuring the absorbance at 515 nm with a spectrophotometer (Cary 60 UV–VIS/Agilent Technology; Santa Clara, USA).
Determination of amylase, protease, and lipase activities
Activities of glucoamylase, protease, and lipase were measured based on the Korean Food Additives Code of KFDA [22].
Determination of organic acids, volatile compounds, and others
Amounts of organic acids were measured at 436 nm with an HPLC (Waters 2695; Aminex HPX-87H exclusion column (300 × 7.8 mm); and a Waters 2487 UV/Visible detector). The flow rate of 4 mm H2SO4 as a solvent was 0.6 mL per min.
Amounts of volatile compounds were measured with a gas chromatograph (Agilent 7890A GC; HP-INNOWAX column (30 m × 0.25 mm; 0.5 μm); Flame Ionization Detector (H2: 30 mL/min; air: 300 mL/min)). One microliter of solution was injected with an Agilent 7697A Headspace sampler in split mode (30:1) at 200 °C. The column temperature was held for 3 min at 40 °C, then, increased to 100 °C at the rate of 7 °C per min, and again increased to 200 °C at 8 °C per min and held for 10 min. Nitrogen gas was used as a mobile phase at 1 mL per min. The detection temperature was 250 °C.
The amount of monacolin K was measured at 237 nm with an HPLC (Waters 2695; Waters C18 column (10 μm, 3.9 × 300 mm) and a Waters 2487 UV/Visible detector). The solvent was 70% acetonitrile in 0.2% phosphoric acid with a flow rate of 1.0 mL per min.
The amount of citrinin was measured at 502 nm with an HPLC (Waters 2695; Waters C18 column (10 μm, 3.9 × 300 mm) and a Waters 2487 UV/Visible detector). The solvent was composed of 0.1% trifluoro acetic acid and acetonitrile (65:35) with a flow rate of 1.0 mL per min.
Statistical analysis
Data were expressed as mean ± SEM and analyzed using the SPSS software package (IBM SPSS Statistics, Version 23). Differences between means were assessed using Scheffe’s multiple-range and Dunnet T3 tests. Statistical significance was defined as p < 0.05.
Results and discussion
Cell growth and ethanol production in fermentations using M. koji or rice nuruk
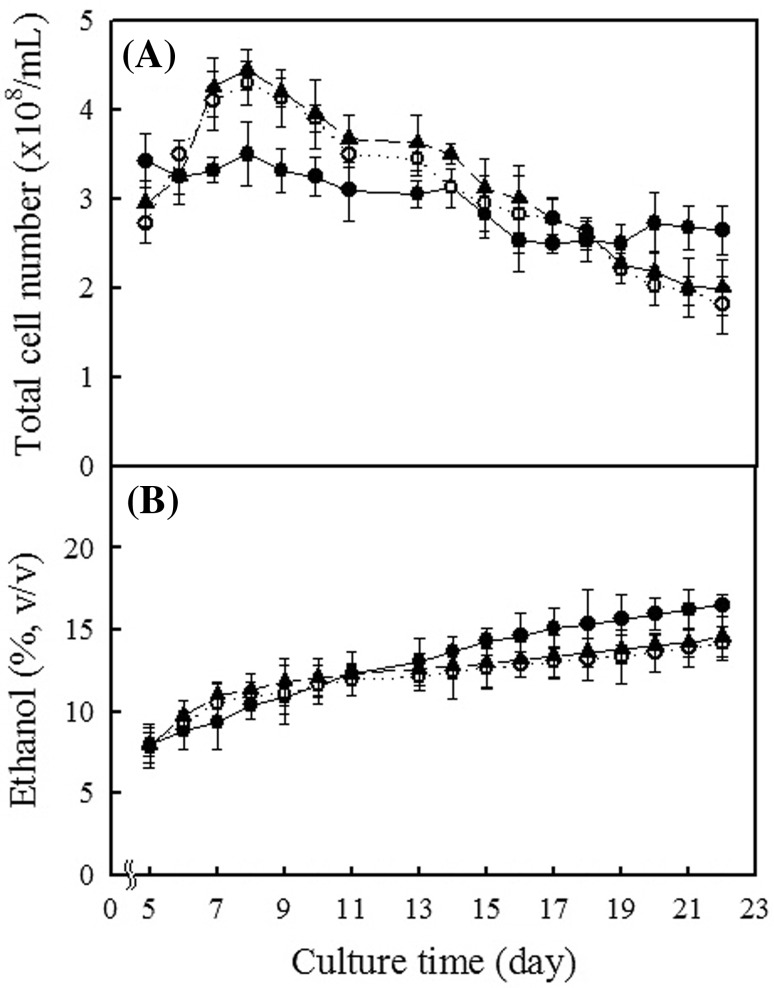
Rice wines were produced by alcohol fermentation using 20% rice nuruk, 20% M. koji, and 30% M. koji and fermentative characteristics were compared. The number of yeast cells in fermentations using either rice nuruk or M. koji reached a maximum value on the 8th day of cultivation (Fig. 1A). Fermentations using both 20 and 30% M. koji showed a similar cell concentration pattern and maximum cell numbers were both larger than with use of 20% rice nuruk. In later stages, the total cell number with 20% rice nuruk was greater than with use of M. koji. The ethanol concentration in rice nuruk and M. koji fermentations reached approximately 8% (v/v) on the 5th day of cultivation and gradually increased with an increasing cultivation time until the 22nd day (Fig. 1B). The final ethanol yield with use of 20% rice nuruk was 16.5% (v/v), and 14.2–14.6% (v/v) for the two M. koji fermentations.
Fig. 1.
Total cell number (A) and ethanol content (B) during fermentation cultivation using rice nuruk and Monascus koji. (filled circle): 20% rice nuruk; (circle): 20% Monascus koji; (filled triangle): 30% Monascus koji
The ethanol yield was dependent on the yeast cell number during fermentation (Fig. 1). During the first eleven days of cultivation, both ethanol concentrations and cell numbers with M. koji were maintained at higher levels than with use of rice nuruk. However, at later stages, the situation was reversed. The ethanol concentration with rice nuruk was maintained at a higher level than with use of M. koji.
Variations in acidity and sugar content during cultivation
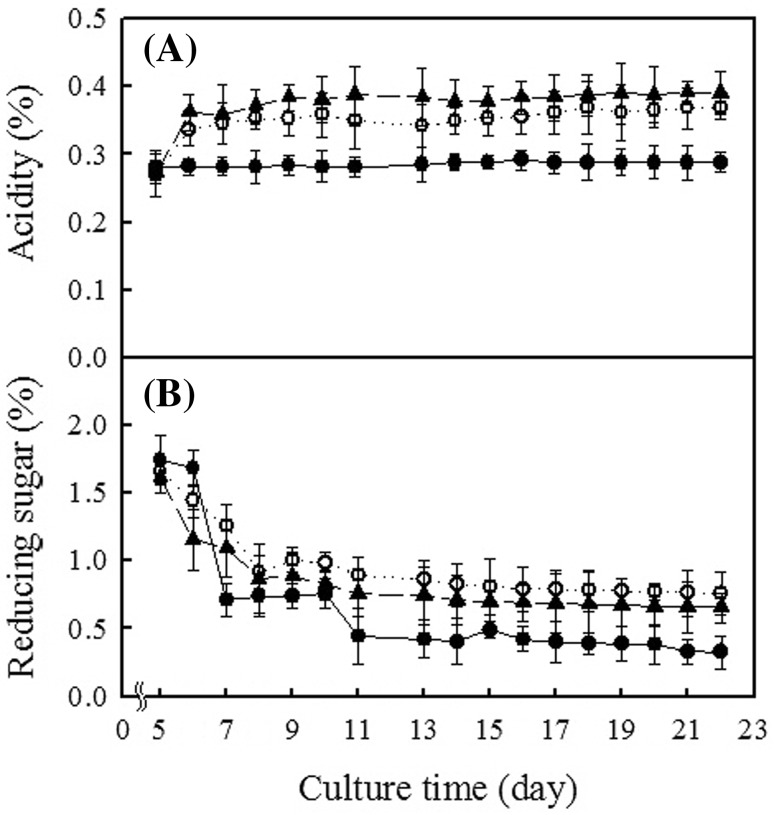
Acidity values in fermentations using rice nuruk or M. koji were both 0.28 on the 5th day of cultivation (Fig. 2A). As the cultivation time increased from 5 to 22 days, the acidity value with use of M. koji gradually increased to 0.37–0.39, but with use of rice nuruk the value was maintained at approximately 0.28, suggesting that organic acids with use of M. koji were produced in larger amounts than with use of rice nuruk.
Fig. 2.
Acidity (A) and reducing sugar content (B) during fermentation cultivation using rice nuruk and Monascus koji. The reducing sugar content (%, w/v) was expressed as glucose equivalents. (filled circle): 20% rice nuruk; (circle): 20% Monascus koji; (filled triangle): 30% Monascus koji
Reducing sugar contents with use of either rice nuruk or M. koji were greatly decreased during the first 7–8 days, and subsequently gradually decreased until the 22nd day (Fig. 2B). During fermentation, the sugar content with use of rice nuruk was maintained at a lower level than with use of both M. koji types, indicating that more sugar was consumed for ethanol production with rice nuruk.
Changes in activities of hydrolases in the fermentation broth with cultivation time
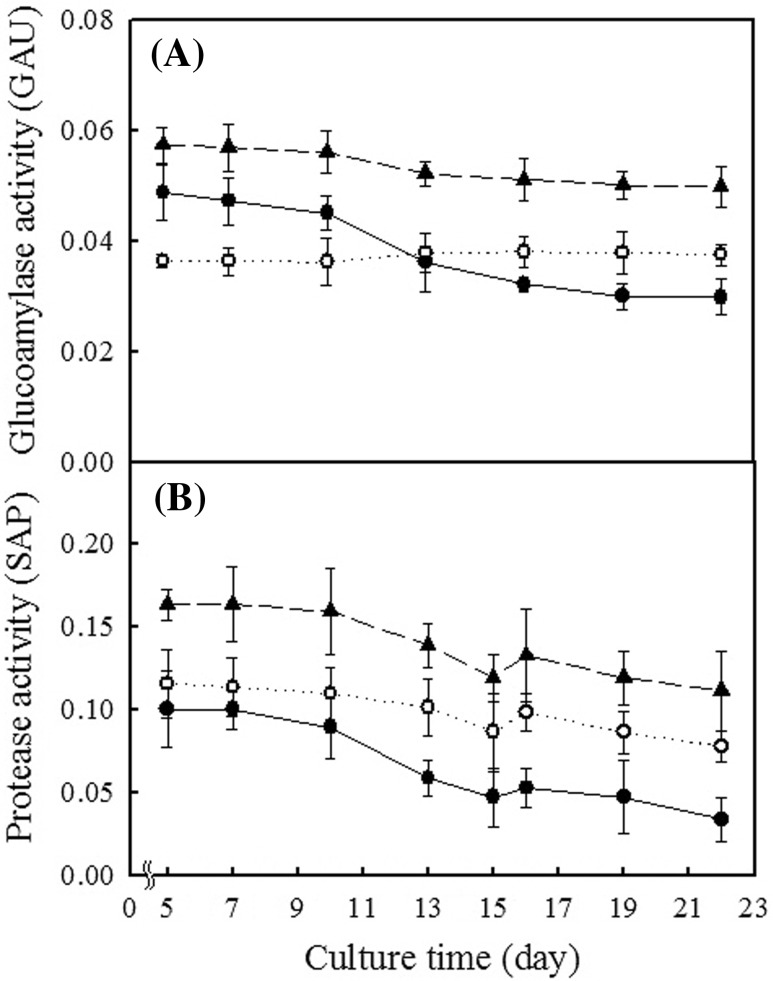
The glucoamylase activity in the fermentation using rice nuruk gradually decreased from 0.048 GAU on the 5th day to 0.030 GAU on the 22nd day (Fig. 3A). However, with M. koji, the enzyme activity was 0.037–0.050 GAU throughout the cultivation time. The protease activity with M. koji was maintained at higher levels than with use of rice nuruk throughout the cultivation time (Fig. 3B). No lipase activity was detected with use of either M. koji or rice nuruk.
Fig. 3.
Glucoamylase activity (A) and protease activity (B) during fermentation cultivation using rice nuruk and Monascus koji. (filled circle): 20% rice nuruk; (circle): 20% Monascus koji; (filled triangle): 30% Monascus koji
There was little difference in the glucoamylase activity between rice nuruk and M. koji throughout the cultivation time. The glucoamylase activity level affects conversion of starch to reducing sugar. Therefore, there was no difficulty in sugar conversion with use of M. koji. However, the protease activity with M. koji was maintained at a higher level than with rice nuruk throughout the cultivation time. Thus, greater amounts of flavor and aroma compounds, which are partially derived from hydrolysis of proteins [23], were produced with M. koji than with rice nuruk.
Varying patterns in organic acid content based on the koji type
Amounts of organic acids in fermentations using either M. koji or rice nuruk were measured (Table 1). At the end of cultivation (after 22 days), total amounts of organic acids with M. koji (20 and 30%) were higher than with rice nuruk by 8.7 and 12.9%, respectively. The amount of citric acid with rice nuruk was responsible for 20.0% of the total, whereas citric acid was not found with use of M. koji. The proportion of lactic acid to total amount of organic acids was 30.3% with rice nuruk and 39.6–43.1% with M. koji. The amounts of lactic acid with use of M. koji were 1.4–1.6 times greater than the amount with rice nuruk. Besides, malic acid and succinic acid also existed as major components in all fermentations using M. koji or rice nuruk.
Table 1.
Organic acids in alcohol fermentation broths at the end of cultivation
| Compounds | Content (mg/L) | ||
|---|---|---|---|
| 20% Rice nuruk | 20% Monascus koji | 30% Monascus koji | |
| Citric acid | 22.62 ± 2.00 | – | – |
| Tartaric acid | – | – | – |
| Malic acid | 33.87 ± 1.46a | 25.20 ± 1.56b | 31.42 ± 1.47a |
| Succinic acid | 40.95 ± 0.69b | 55.27 ± 0.81a | 53.02 ± 1.34a |
| Lactic acid | 46.39 ± 2.03c | 65.81 ± 2.27b | 74.51 ± 1.59a |
| Acetic acid | 9.17 ± 0.35c | 20.05 ± 0.73a | 13.74 ± 1.77b |
| Total | 152.99 ± 0.91b | 166.34 ± 3.94a | 172.69 ± 2.38a |
– not detected
Data (n = 3) were expressed as mean ± SEM and analyzed using the SPSS software package (IBM SPSS Statistics, Version 23). Differences between means were assessed using Scheffe’s multiple-range and Dunnet T3 tests. Values with different superscript letters are significantly different at p < 0.05
Total amounts of citric acid, malic acid, and succinic acid were similar, regardless of using M. koji or rice nuruk. Differences in the total amount of organic acids with M. koji or rice nuruk probably related to differences in lactic acid contents.
Varying volatile compound contents based on the koji type
At the end of fermentation, total amounts of volatile compounds using M. koji were 46.3–54.1% higher than for use of rice nuruk (Table 2).The amounts of each compound with rice nuruk were highest in the order of isoamyl alcohol, isobutanol, n-propanol, ethyl acetate, acetaldehyde, and isoamyl acetate. Isoamyl alcohol was responsible for 64.2% (781 mg/L) of the total. In addition, methyl alcohol, n-butyl alcohol, acetone, methyl acetate, and ethyl caproate existed in small amounts. On the other hand, with M. koji, amounts of each compound were highest in the order of isoamyl alcohol, isobutanol, n-propanol, acetaldehyde, ethyl acetate, and isoamyl acetate. Isoamyl alcohol was responsible for 63.6–63.9% (1136–1191 mg/L) of the total. Methyl alcohol, n-butyl alcohol, ethyl caproate, ethyl caprylate, and isoamyl acetate were detected in small amounts.
Table 2.
Volatile compounds in alcohol fermentation broths at the end of cultivation
| Compounds | Content (mg/L) | ||
|---|---|---|---|
| 20% Rice nuruk | 20% Monascus koji | 30% Monascus koji | |
| Acetaldehyde | 40.41 ± 1.59c | 79.37 ± 1.84b | 107.74 ± 0.92a |
| Acetone | 4.32 ± 0.23 | – | – |
| Methyl acetate | 1.38 ± 0.07 | – | – |
| Ethyl acetate | 66.09 ± 0.70a | 62.61 ± 0.65b | 63.59 ± 0.91ab |
| Methyl alcohol | 8.65 ± 0.19b | 8.71 ± 0.10b | 10.11 ± 0.62a |
| n-Propanol | 110.90 ± 0.87c | 199.69 ± 0.85b | 169.88 ± 1.20a |
| Isobutanol | 185.07 ± 1.07c | 272.56 ± 1.21b | 311.44 ± 2.74a |
| Isoamyl acetate | 9.72 ± 0.48a | 3.02 ± 0.04c | 4.52 ± 0.26b |
| n-Butanol | 7.21 ± 0.23b | 13.52 ± 0.36a | 12.03 ± 0.66a |
| Isoamyl alcohol | 781.42 ± 1.11c | 1136.36 ± 3.48b | 1191.93 ± 2.68a |
| Ethyl caproate | 1.62 ± 0.18a | 1.90 ± 0.11a | 1.54 ± 0.07a |
| Ethyl caprylate | – | 1.97 ± 0.02a | 1.85 ± 0.09a |
| Total | 1216.79 ± 1.01c | 1779.71 ± 6.62b | 1874.63 ± 7.88a |
– not detected
Data (n = 3) were expressed as mean ± SEM and analyzed using the SPSS software package (IBM SPSS Statistics, Version 23). Differences between means were assessed using Scheffe’s multiple-range and Dunnet T3 tests. Values with different superscript letters are significantly different at p < 0.05
The total amount of volatile compounds with use of M. koji was much higher than with use of rice nuruk. This difference was due to higher amounts of isoamyl alcohol, isobutanol, and n-propanol with M. koji than with rice nuruk. In particular, acetone and methyl acetate were produced only with rice nuruk, whereas ethyl caprylate was present with M. koji.
Occurrence of monacolin K and citrinin in fermentations using M. koji
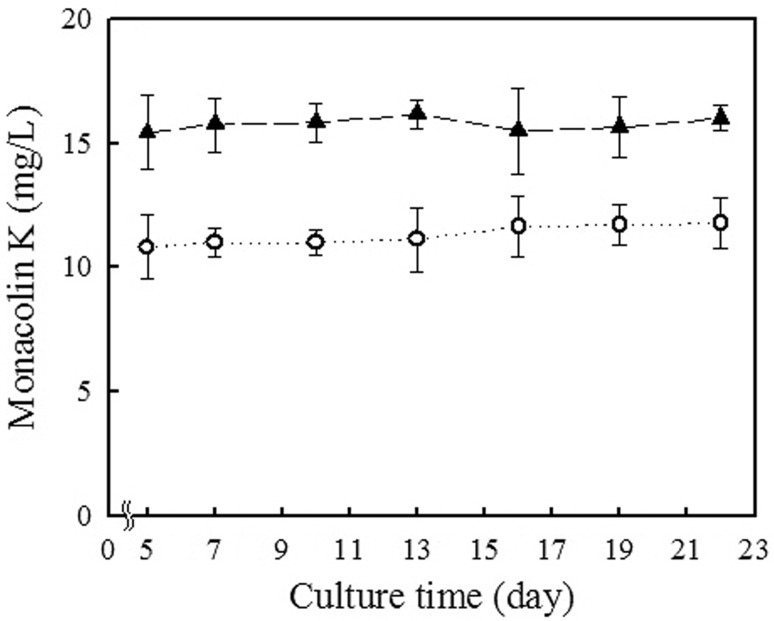
Monacolin K, which is a statin [14], was produced at 12 and 16 mg/L in fermentation broths using 20% koji and 30% koji, respectively (Fig. 4), but was not detected using rice nuruk. Citrinin, a mycotoxin, which is produced by some strains of Monascus species [24], was not found with use of either M. koji or rice nuruk.
Fig. 4.
Monacolin K content during fermentation cultivation using Monascus koji. (circle): 20% Monascus koji; (filled triangle): 30% Monascus koji
The ethanol yield of fermentations was apparently related to the number of yeast cells rather than to the activity level of glucoamylase for conversion of starch to reducing sugars because the higher ethanol yield was in agreement with a higher cell number with use of rice nuruk, despite a lower glucoamylase activity. Sugar conversion was considered to be sufficient, regardless of use of either rice nuruk or M. koji, considering that large amounts of reducing sugars still remained at the end of fermentation.
Organic acids found in fermentation broths were considered to be derived from glycolysis or/and TCA metabolism [25]. Larger amounts of organic acids were produced using M. koji than for using rice nuruk, indicating that with use of M. koji, sugars would have been actively converted to other metabolites, but not to ethanol. Volatile compounds probably originate from reducing sugars and/or amino acids in fermentation broths [26]. The protease activity level with use of M. koji was higher than for use of rice nuruk, indicating that more amino acids with M. koji can be made via enzymatic hydrolysis of proteins. As expected, much higher amounts of volatile compounds were obtained using M. koji than with use of rice nuruk. Organic acids and volatile compounds, which are the flavor and aroma components of ethanol fermentation, are responsible for the quality of alcohol beverages [26]. They were produced in larger amounts using M. koji than with use of rice nuruk, suggesting that a better quality of alcohol beverage product can be made using M. koji.
Monacolin K, which was produced in alcohol fermentations using M. koji, is known to lower the cholesterol plasma level [14, 27, 28]. It is also known as the commercial statin drug lovastatin. There are reports [14, 15, 29] that monacolin K is present in metabolites of Monascus ruber. Monascus-cultivated rice is known as red yeast rice and can be used as a food supplement that is comparable in effect with statin drugs [30]. In the United States, use of red yeast rice in patients with statin intolerance is medically recommended [31]. On the other hand, citrinin, which is a hepatotoxic and nephrotoxic mycotoxin, is produced by some Monascus species [27, 32]. According to the Ministry of Health and Welfare of Japan, the amount of citrinin in Monascus pigments used as food additives should be less than 200 ppb [27]. Fortunately, since citrinin was not detected in any fermentation broths herein, there is no problem with use of M. koji as a food supplement. In this respect, alcohol beverages made using M. koji are expected to have advantages of both good flavor and an active biological effect.
In conclusion, if rice nuruk is substituted for M. koji in ethanol fermentation, formation of organic acids and volatile compounds in fermentation broths will be greatly enhanced. As a result, a higher quality of alcohol beverage can be prepared using M. koji and alcohol beverages can function as a health-care product due to the presence of monacolin K.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Jang JH. History of Korean traditional rice wine. Korean J Dietary Cult. 1989;4:271–274. [Google Scholar]
- 2.Yeo SH, Jeong YJ. Current trends and development a plan in the Korean makeolli industry. Food Sci Ind. 2010;43:55–64. [Google Scholar]
- 3.So MH. Aptitudes for takju brewing of wheat flour-nuruks made with different mold species. Korean J Food Nutr. 1995;8:6–12. [Google Scholar]
- 4.Huh CK, Kim SM, Kim YD. Comparison for enzymic activity of nuruk and quality properties of yakju by different fungi. Korean J Food Preserv. 2014;24:573–580. doi: 10.11002/kjfp.2014.21.4.573. [DOI] [Google Scholar]
- 5.Baek SY, Kim JY, Choi JH, Choi JS, Choi HS, Jeong ST, Yeo SH. Assessment of the quality characteristics of mixed-grain nuruk made with different fungal strains. J East Asian Soc Dietary Life. 2012;22:103–108. [Google Scholar]
- 6.Park KH, Liu Z, Park C-S, Ni L. Microbiota associated with the starter cultures and brewing process of traditional Hong Qu glutinous rice wine. Food Sci Biotechnol. 2016;25:649–658. doi: 10.1007/s10068-016-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han EH, Lee TS, Noh BS, Lee DS. Volatile flavor components in mash of takju prepared by using different nuruk. Korean J Food Sci Technol. 1997;29:759–766. [Google Scholar]
- 8.Jo GY, Lee CW. Isolation and identification of the fungi from nuruk. J Korean Soc Food Sci Nutr. 1997;26:759–766. [Google Scholar]
- 9.Song JC, Park HJ. Takju brewing using the uncooked germinated brown rice at second stage mash. J Korean Soc Food Sci Nutr. 2003;32:847–854. doi: 10.3746/jkfn.2003.32.6.847. [DOI] [Google Scholar]
- 10.Kim EY, Rhyu MR. The chemical properties of doenjang prepared by Monascus koji. Korean J Food Sci Technol. 2000;32:1114–1121. [Google Scholar]
- 11.Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, Zhang D, Cooper R, Chang M. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem. 2000;48:5220–5225. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 12.Kohama Y, Matsumoto S, Mimura T, Tanabe N, Inada A, Nakanishi T. Isolation and identification of hypertensive principles in red-mold rice. Chem Pharm Bull. 1987;35:2484–2489. doi: 10.1248/cpb.35.2484. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Lim SI. Quality characteristics of muffin added red yeast rice flour. Korean J Food Sci Technol. 2007;39:272–275. [Google Scholar]
- 14.Endo A, Monacolin K. A new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. 1979;32:852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- 15.Park JY, Han SI, Seo WD, Ra JE, Sim EY, Nam MH. Study on Monascus strains and characteristic for manufacturing red yeast rice with high production of monacolin K. Korean J Crop Sci. 2014;59:167–173. doi: 10.7740/kjcs.2014.59.2.167. [DOI] [Google Scholar]
- 16.Jin YJ, Pyo YH. Effect of Monascus fermentation on the content of bioactive compounds in white and black soybeans. Korean J Food Sci Technol. 2015;47:409–412. doi: 10.9721/KJFST.2015.47.3.409. [DOI] [Google Scholar]
- 17.Choi MJ, Yu DS. Effects of red-yeast-rice supplementation on bone mineral density and bone mineral content in ovariectomized rats. Korean J Nutr. 2004;37:423–430. [Google Scholar]
- 18.KFDA. Health functional food office book, 2–52: Monascus koji, Sejong, South Korea, pp.124–125 (2016).
- 19.KFDA. Korean food standards codex, 27: Alcohol beverage, Sejong, South Korea, pp. 162 (2016).
- 20.National Tax Service . Alcohol Beverage Analysis Code, 6–4: Alcohol content. South Korea: Sejong; 2013. pp. 39–41. [Google Scholar]
- 21.The Korea Society of Food Science and Nutrition . HandBook of Experiments in Food Science and Nutrition. Seoul: Hyoil Publishing Co.; 2000. pp. 151–152. [Google Scholar]
- 22.KFDA. Korean food additives code, 9: Glucoamylase, 20: Lipase, 87: Protease, Sejong, South Korea, pp. 892–1074 (2016).
- 23.Huang Y, Wu Q, Xu Y. Isolation and identification of a black Aspergillus strain and the effect of its novel protease on the aroma of Moutai-flavoured liquor. J Inst Brew. 2014;120:268–276. doi: 10.1002/jib.135. [DOI] [Google Scholar]
- 24.Blanc PJ, Laussac JP, Lebars J, Lebars P, Loret MO, Pareilleux A, Prome D, Prome JC, Santerre AL, Goma G. Characterization of monascidin-a from Monascus as citrinin. Int J Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- 25.Whiting GC. Organic acid metabolism of yeasts during fermentation of alcoholic beverages-a review. J Inst Brew. 1976;82:84–91. doi: 10.1002/j.2050-0416.1976.tb03731.x. [DOI] [Google Scholar]
- 26.Chung H, Yoon MK, Han J, Kim YS. Evaluation of volatile organic compounds in alcoholic beverages consumed in Korea. J Korean Soc Appl Biol Chem. 2015;58:423–432. doi: 10.1007/s13765-015-0059-1. [DOI] [Google Scholar]
- 27.Lin Y-L, Wang T-H, Lee M-H, Su N-W. Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Appl Microbiol Biotechnol. 2008;77:965–973. doi: 10.1007/s00253-007-1256-6. [DOI] [PubMed] [Google Scholar]
- 28.Endo A. Monacolin-K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme a reductase. J Antibiot. 1980;33:334–336. doi: 10.7164/antibiotics.33.334. [DOI] [PubMed] [Google Scholar]
- 29.Jia X-Q, Mo E-K, Sun B-S, Gu L-J, Fang Z-M, Sung C-K. Solid-state fermentation for production of monacolin K on soybean by Monascus ruber GM011. Food Sci Biotechnol. 2006;15:814–816. [Google Scholar]
- 30.Heber D, Yip I, Ashley JM, Elashoff DA, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice supplements. Am J Clin Nutr. 1999;69:231–236. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- 31.Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;16:830–839. doi: 10.7326/0003-4819-150-12-200906160-00006. [DOI] [PubMed] [Google Scholar]
- 32.Patakova P. Monascus secondary metabolites: production and biological activity. J Ind Microbiol Biotechnol. 2013;40:169–181. doi: 10.1007/s10295-012-1216-8. [DOI] [PubMed] [Google Scholar]