Abstract
The effects of aronia extract (AE) supplementation on the lifespan and age-associated oxidative stress was investigated in the fruit fly Drosophila melanogaster. Supplementation with 2.5 mg/mL of AE (AE 2.5) extended the mean lifespan in D. melanogaster by 18%. AE supplementation significantly improved locomotor activity at both 10 and 40 days. In 40-days-old flies, reactive oxygen species production was significantly lower and accumulation of the lipid oxidation product malondialdehyde had markedly decreased by 49.3% in the AE 2.5 group. The extended longevity and improved locomotion in the AE 2.5 group were probably because of increases in the level of antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase) and expression of stress resistance genes (Hsp68, l(2)efl, and Jafrac1). Present study provides the evidence that dietary supplementation with AE extended lifespan and ameliorated age-related oxidative damages in D. melanogaster.
Keywords: Aronia, Drosophila melanogaster, Aging, Lifespan, Oxidative stress
Introduction
Aging is a complex chronological process that is characterized by senescence, which is an indication of a decline in biological functions [1]. An imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense system in the body leads to oxidative damage, and oxidative stress is known to be a major cause of aging [2].
The fruit fly Drosophila melanogaster is a suitable model system for studying aging or age-related diseases because of its short lifespan, large number of offspring, and availability at a low cost. In addition, some mechanisms that are responsible for age-related functional decline, including oxidative damage, and disease-causing genes are highly conserved in flies and humans [3, 4]. Polyphenols are widely distributed in fruits and vegetables and they comprise some of the major dietary antioxidants. It has been reported that the consumption of dietary polyphenols improves the antioxidant capacity of the body and provides protection against degenerative diseases and aging by activating endogenous antioxidant defense systems and regulating cellular signaling pathways [5]. Berries are rich source of dietary antioxidants and have attracted considerable attention because they contain significant amounts of anthocyanins. Wu et al. [6] reported that Aronia melanocarpa (choke berry) has higher anthocyanins content and antioxidant capacity compared with other berries such as Ribes grossularia (gooseberry) and Sambucus nigra (elderberry). Aronia also has the highest proanthocyanidins (condensed oligomeric flavan-3-ols) content (1800 ± 370 mg/100 g fresh weight) among 99 selected common food products of plant origin [7].
The previous studies have shown that anthocyanins from various sources including cranberry [8], blueberry [9], and black rice [10] are effective in the extending the mean life span and alleviating of oxidative stresses in a fly model system. Oligomeric proanthocyanidins also exhibit diverse bioactivities including antioxidant, anti-inflammatory and cardio protective activities [11].
Up to present, various bioactivities of aronia extract (AE) effect have been reported including hepato protective, lipid lowering, and antihypertensive effects [12] but its effects on age-related oxidative stress and possibly on lifespan extension remain unclear. In the present study, we systemically investigated the effects of AE on ROS production, activity of endogenous antioxidant enzymes, lifespan, and expression level of related genes using Drosophila melanogaster model system.
Materials and methods
Materials
Folin–Ciocalteau reagent, 4-(dimethyl) amino cinnamaldehyde, bromophenol blue, sodium azide, 3,5-dichloro-2-hydroxybenzenesulfonic acid, 4-aminoantipyrine, 1, 1, 3, 3-tetramethoxy propane, thiobarbituric acid (TBA), butylated hydroxytoluene, bicinchoninic acid solution, copper (II) sulfate solution, bovine serum albumin, 2′,7′-dichlorofluorescein diacetate (DCF-DA), 2, 2′-azobis(2-amidinopropane) dihydrochloride (AAPH), and superoxide dismutase (SOD) were purchased from Sigma Chemical Co. (St. Louis, USA). Propionic acid and p-hydroxybenzonic acid methyl ester were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Hydrogen peroxide was purchased from Yakuri Pure Chemicals Co., Ltd. (Kyoto, Japan). Peroxidase was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Drosophila rearing and diets
The Canton S line of D. melanogaster was obtained from the Bloomingston Drosophila Stock Center at Indiana University. The flies were raised at 24 ± 1 °C under 55% relative humidity with a 12 h/12 h light/dark cycle. The basal fly diet (1000 mL) was prepared by dissolving 90.6 g sucrose, 68 g yeast, 42.8 g corn meal, 6.5 g agar, 4.5 mL propionic acid, and 10 mL p-hydroxybenzonic acid methyl ester solution (20%, w/v). Control group was maintained with the basal diet, whereas experiment group was fed with an AE-supplemented (1 and 2.5 mg/mL) basal diet.
Preparation of AE
Aronia melanocarpa was purchased from Happy-farmer (Seongnam, Korea) and carefully washed before extraction. Aronia was extracted with 70% acetone (v/v) in a shaking incubator at room temperature for 24 h. Dried AE was obtained after solvent removal followed by lyophilization.
Chemical analysis of AE
Total phenolic content
The total phenolic content of AE was determined by the method of Xu et al. [13] and expressed as milligrams of gallic acid equivalents (GAE) per gram AE.
Total flavonoids content
The total flavonoid content of AE was determined using the method reported by Kim et al. [14] and expressed as milligrams of catechin equivalents (CAE) per gram AE.
Total anthocyanins content
Total anthocyanin content in AE was determined using the pH differential method [15]. In brief, absorbance of sample was measured at 520 and 700 nm in buffers at pH 1 and 4.5 using a spectrophotometer (Amhersham Pharmacia Biotech, Little Chalfont, UK) and the total anthocyanin content was calculated based on following formula:
Cyanidin-3-glucoside (C3G) with a molar extinction coefficient of 29,600 L/cm mol and molecular weight of 449.2 g/mol was used as standard and the result was expressed as milligrams C3G equivalents/per gram AE.
Total proanthocyanidins content
Total proanthocyanidin content in AE was determined by vanillin method [16] using catechin as a standard and the result was expressed as milligrams proanthocyanidin per gram AE.
Oxygen radical absorbance capacity (ORAC)
The ORAC value of AE was determined by the method of Kurilich et al. [17] with a slight modification. In brief, sample (20 μL) and fluorescein (120 μL at a final concentration of 70 nM) were mixed in 75 mM phosphate buffer (pH 7.4) and pre-incubated for 15 min at 37 °C in a microplate reader (Biotek Instruments Inc., Winooski, VT, USA). AAPH solution (60 uL, 40 mM) was added to the assay mixture and the fluorescence intensity was recorded at an excitation wavelength of 485 nm and an emission wavelength of 520 nm for 80 min with 1 min interval. The results were calculated as the area under curve and expressed as Trolox equivalents (TE) per gram AE.
In vivo bioassays
Lifespan assay
Male flies (1–3-day old, 200 flies/group) were randomly allocated to groups, and the lifespan of each fly was individually assessed by recording the age of spontaneous death. The dead flies were counted every 2–3 days, and the remaining live flies were placed in new vials and fed with fresh diet twice each week. The entire life span assay was replicated.
Gustatory assay
Male flies (1–3 days old) were reared in vials containing standard diet with or without AE for 10 days (100 flies/group). In the gustatory assay, flies were transferred into new vials with diet containing bromophenol blue (0.05%, w/v). After feeding for 24 h, the flies were transferred into Eppendorf tubes and washed with PBS, and then homogenized with 0.5 mL of distilled water. The supernatant was collected and absorbance of sample was measured at 595 nm using a microplate reader.
SOD activity assay
Flies (50 flies/group) were homogenized in ice-cold 0.1 M Tris–HCl buffer (500 μL, pH 7.8) containing 0.5% Triton X-100 using a pestle and mortar. The supernatant was obtained after centrifugation at 12,000×g for 10 min at 4 °C. SOD assay kit (Dojindo, Japan) was used to measure the SOD activity.
Catalase (CAT) activity assay
Flies (50 flies/group) were homogenized as described for the SOD assay. Hydrogen peroxide solution (25 μL, 400 mM) was mixed with the supernatant from the fly homogenate (10 μL) and allowed to react for 1 min. Sodium azide (900 μL, 15 mM) was then added to stop reaction, and the reaction mixture (10 μL) was mixed with 270 μL of color reagent (2 mM 3,5-dichloro-2-hydroxybenzenesulfonic acid and 0.25 mM 4-aminoantipyrine) and 20 μL of fresh peroxidase (25 unit/mL). After 15 min, the absorbance of each sample was measured at 520 nm.
Glutathione peroxidase (GPx) activity
Flies (50 flies/group) were homogenized in 500 μL of Tris–HCl buffer (50 mM, pH 7.5) containing 5 mM ethylenediaminetetraacetic acid and 1 mM dithiothreitol and were centrifuged at 10,000×g for 15 min at 4 °C. The supernatants were transferred into new tubes and the GPx activity was determined using an assay kit (Cayman Chemicals, Ann Arbor, MI, USA) according to the manufacturer’s protocol.
Lipid hydroperoxides (LPO)
Lipid peroxidation was measured using the TBA reaction method [18]. Flies (50 flies/group) were homogenized in 0.1 M sodium phosphate buffer (pH 7.4) and 5% butylated hydroxytoluene and centrifuged at 4500×g for 10 min at 4 °C. The supernatant (50 μL) was reacted with 150 μL acetic acid (pH 3.5, 20%), 150 μL of TBA (0.2%, w/v), 20 μL of 8% sodium lauryl sulfate. The mixture was heated in a water bath at 95 °C for 1 h and 300 μL of 1-butanol was added to the reaction mixture. The absorbance of each sample was analyzed at 532 nm and calculated as malondialdehyde (MDA) equivalents using 1,1,3,3- tetramethoxypropane as a standard.
Reactive oxygen species (ROS)
The ROS levels were determined in the head and body regions using the DCF-DA method [19]. Fluorescence was measured using a microplate reader with excitation of 485 nm and emission of 640 nm. The relative ROS levels were calculated based on the ratio between control and AE treatments.
Climbing ability
A climbing ability test was performed to evaluate changes in the locomotor function with age. Flies (20 flies/group) were gently tapped the bottom of the vial (2.3 × 9.5 cm) and allowed to climb up to the top of the vial for 20 s. The number of flies that could climb up over 7 cm from the bottom to the top was counted at the end of each experiment. The assay was performed three times on days 10 and 40.
mRNA expression levels
Total RNA was extracted from flies (20 flies/group) using a Quiazol (Quiazen Sciences, Maryland, MD, USA). The cDNA was constructed from RNA samples (A260/A280 > 1.6) using a high capacity RNA-to-cDNA kit (Applied Biosystems, Foster city, CA, USA). Quantitative real-time PCR (qRT-PCR) was performed using a StepOne Plus real time PCR system (Applied Biosystems) with gene specific TaqMan® probes and primers. For DNA amplification, the initial denaturation cycle was performed at 90 °C for 20 min, followed by 40 amplification cycles at 95 °C for 1 min and 60 °C for 20 min. Quantitative analyses of the PCR products was carried out using the comparative cycle threshold method described in the StepOne Plus manual and the results were normalized against those for ribosomal protein L32 (RpL32, NM_001144655.3), a housekeeping gene. The target genes used in the analysis were as follows:
SOD1 (NM_057387.5), SOD2 (NM_001299574.1), CAT (NM_080483.3), MTH (NM_001259606.2), GstD1 (NM_001038953.2), Hsp68 (NM_079750.4), l(2)efl (NM_001274227.1), bsk (NM_001169459.1), and Jafrac1 (NM_001298273.1).
Statistical analysis
All assays were performed in triplicate unless indicated otherwise. Data were expressed as the mean values ± standard error (SE), and statistical analyses were performed using SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Kaplan–Meier test was used to detect significant differences in the lifespan curves and an independent t test was performed at p < 0.05.
Results and discussion
Antioxidant compounds and antioxidant activity of AE
The major antioxidant components in AE are shown in Table 1. Total phenol content of AE (105 mg GAE/g) was similar to that of an ethanol extract (110 mg GAE/g) of previous report [20]. Aronia melanocarpa is well-known as a rich source of anthocyanins and proanthocyanidins. However, anthocyanin and proanthocyanidin contents of AE vary greatly according to different studies [20, 21]. This is probably due to differences in the cultivars, habitat, maturation stages and extraction methods employed [22]. The antioxidant activity determined by ORAC assay was 50 TE μmol/g extract. A strong positive relationship has been demonstrated between antioxidant activities and total proantocyanidin and anthocyanin content of AE [23].
Table 1.
Antioxidant components of aronia acetone extract
| Antioxidant compounds or activity | Aronia extract |
|---|---|
| Total phenolics (mg GAE/g extract) | 105 ± 1 |
| Total flavonoids (mg CATE/g extract) | 87.3 ± 2 |
| Total anthocyanins (mg C3G/g extract) | 13.2 ± 1 |
| Total proanthocyanidins (mg CATE/g extract) | 52.5 ± 0.4 |
| ORAC (TE μmol/g extract) | 573 ± 18 |
GAE Gallic acid equivalent, CATE Catechin equivalent, C3G Cyanidin-3glucoside, TE Trolox equivalent
Effect of AE supplementation on lifespan and locomotor activity
The primary hypothesis tested in the present study was whether AE supplementation could extend the lifespan by reducing oxidative stress. The effect of AE supplementation (1 and 2.5 mg/mL) on the lifespan of flies was determined. As shown in Fig. 1(A), both AE 1 and AE 2.5 supplementation increased maximum lifespan by 9% compared with control group. However, the median and mean lifespan were only increased significantly from 51 to 60 days with AE 2.5. Therefore, AE 2.5 was selected for use in the subsequent experiments. Gustatory assay based on the intensity of the dye (bromophenol blue) consumed by flies during feeding for 24 h indicated that there was no significant difference in food intake between groups (Fig. 1B). The mean lifespan extension obtained using AE 2.5 was 18% and it was greater than observed in previous supplementation studies, e.g., in comparison with the control diet, supplementation with 20 mg/mL cranberry anthocyanin extract or 5 mg/mL blueberry extract prolonged the mean lifespan by 10% [7, 8]. A direct comparison is not possible, but AE supplementation obtained good performance even at a low dosage. The greater lifespan extension obtained with AE compared with that using anthocyanin rich cranberry or blueberry extracts is possibly related to the combined effect anthocyanins and proanthocyanins. Thus the relative contributions of the anthocyanins and proanthocyanidins in AE should be clarified further in the future.
Fig. 1.
Lifespan curve (A) and gustatory behavior (B) of flies fed control (CON) diet or two experimental diets supplemented with 1 (AE 1) and 2.5 mg/mL aronia acetone extract (AE 2.5). The mean, median, and maximum lifespan (average of the last surviving 5% of flies) are presented. Data are expressed as the mean ± SE. The stomach color intensity was used as an index of gustatory behavior
It has been demonstrated that degradation of the locomotor activity by flies is closely associated with aging [24]. A climbing ability assay was performed on days 10 and 40 days as an indicator of locomotive activity. The climbing ability was decreased significantly with age and it was significantly higher in the AE 2.5 supplementation group on both days 10 and 40 (Fig. 2). This finding suggests that AE supplementation ameliorates the aging associated impairment of locomotor activity.
Fig. 2.
The effect of AE 2.5 supplementation on the climbing ability of Drosophila on days 10 and 40. Data are expressed as the mean ± SE, where * and **indicate significant differences within the same age at *p < 0.05 and at **p < 0.01, respectively
The strong inverse relationship between aging and locomotor ability has been validated in various species including flies. In particular, vertical climbing has been suggested as a good indicator of physical fitness [25]. According to the results of climbing ability test, we found that AE supplementation was helpful in maintaining locomotor activity on day 40 day (Fig. 2). This suggested that dietary AE supplementation not only improves longevity and contributes to improving the health. In accordance with our results curcumin supplementation (250 μM) significantly improved spontaneous locomotion and climbing ability in 5-week-old flies [26].
Effects of AE supplementation on antioxidant enzyme activities and oxidative stress
The effect of AE 2.5 supplementation on the antioxidant enzyme activities was evaluated on days 10 and 40. As aging progressed, the levels of all the antioxidant enzymes significantly decreased in all groups. The activity of SOD, the first enzyme to remove superoxide anion was significantly higher in the AE 2.5 group than that in the control group on days 10 and 40 (Fig. 3A) but CAT activity was only significantly greater in the AE 2.5 group on day 40 (Fig. 3B). The greatest difference between control and AE 2.5 supplementation groups was found in GPx activity on day 40, which increased by 54% (Fig. 3C). These results suggest that AE 2.5 supplementation was effective in the removal of free radicals from the body and reducing oxidative stress.
Fig. 3.
Effects of AE 2.5 supplementation in Drosophila on the activities of (A) superoxide dismutase (SOD), (B) catalase (CAT), and (C) glutathione peroxidase (GPx) on days 10 and 40. Data are expressed as the mean ± SE, where * and **indicate significant differences within the same age at *p < 0.05 and at **p < 0.01, respectively
A plausible mechanism responsible for the anti-aging effect of AE supplementation is reduced oxidative stress. The activities of all the antioxidant enzymes tested, i.e., SOD, CAT, and GPX were significantly higher in the AE 2.5 supplementation group compared with those in the control group. GPx is an endogenous selenium containing enzyme that converts hydrogen peroxide into water, but its role in flies is not fully understood [27].
AE supplementation decreased the relative ROS and total body LPO levels on day comparison with the basal diet (Fig. 4A, B). In particular, the accumulation of MDA was 49.3% lower in the AE 2.5 group, which was consistent with the analysis of the antioxidant enzyme activities. A previous study showed that the administration of AE (3 × 100 mg/day) for 2 months has an antioxidant effect on patients with metabolic according to significant differences observed in the GPx, SOD, and lipid oxidation products levels [28].
Fig. 4.
Effects of AE 2.5 supplementation in Drosophila on the (A) relative reactive oxygen species (ROS) production and (B) lipid hydroperoxide (MDA) levels on day 40. Data are expressed as the mean ± SE, where **indicates a significant difference at p < 0.01
Effect of AE supplementation on age related gene expression
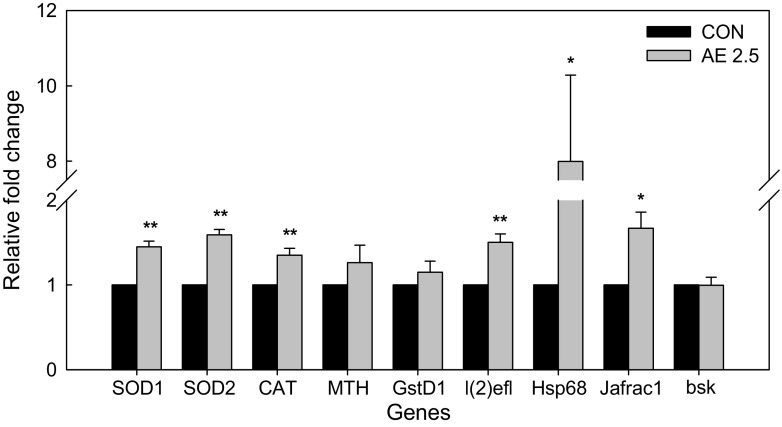
Polyphenolic compounds not only directly remove ROS but also induce the expression of endogenous antioxidant enzymes [29]. The changes in gene expression associated with oxidative stress and longevity were assessed by qRT-PCR. As shown in Fig. 5, the gene expression levels of antioxidant enzymes, including SOD1, SOD2, and CAT were significantly increased by AE 2.5 supplementation. The upregulation of antioxidant gene expression agreed with the greater resistance to oxidative stress. Li et al. [30] reported that green tea catechins extended the lifespan of flies by upregulating the activities of SOD and CAT. The over-expression of SOD or CAT alone marginally increased the lifespan in flies but the overexpression of both SOD and CAT was much more effective in extending the lifespan [31]. This result suggests that balance between SOD and CAT is important for decreasing oxidative stress.
Fig. 5.
Effects of AE 2.5 supplementation in Drosophila on the relative expression levels of antioxidant and longevity genes on day 40. SOD1 copper-zinc containing superoxide dismutase, SOD2 manganese containing superoxide dismutase, CAT catalase, MTH Methuselah, GstD1 glutathione S transferase D1, Hsp68 heat shock protein 68, l(2)efl lethal (2) essential for life, bsk basket, Jafrac1 thioredoxin peroxidase 1. The gene expression levels were normalized against that of RpL32, a house keeping gene. Data are expressed as the mean ± SE, where * and ** indicate significant difference at *p < 0.05 and at **p < 0.01, respectively
In terms of longevity genes, the expression levels of Hsp68, l(2)efl, and Jafrac1 were significantly increased but there was no differences in the expression levels of MTH, GstD1 and bsk. The increased expression of stress resistance genes such as Hsp68 and l(2)efl probably favored lifespan extension. It has been reported that MTH encodes a G protein-coupled receptor and is one of the major contributors to lifespan extension in MTH mutant flies [32]. However, Peng et al. [8] failed to find differences in MTH gene expression levels, although a significant lifespan extending effect was observed after theaflavin supplementation. In the present study, there was no significant difference in MTH gene expression levels between control and AE 2.5 groups. Hsp is a molecular chaperone that combats thermal and oxidative stress and it is involved in lifespan and stress resistance during aging [33]. The induction of l(2)efl also improves the resistance to oxidative stress and protects against age-related neuron damages [34] The AE supplementation significantly upregulated these two genes belonging to the heat shock protein family. Jafrac1 is a peroxiredoxin family member that detoxifies peroxide and possibly increases the lifespan in flies [35]. Taken together, AE-induced longevity extension in flies may occur by modulating the Jun-N-terminal kinase signaling pathway because Hsp68, l(2)efl, and Jafrac1 are central target genes of this pathway.
AE is a natural antioxidant mixture containing various polyphenols and flavonoids including anthocyanins and proanthocyanidin. Liu [36] reported that combination of phytochemicals can provide greater health benefit than single antioxidant compound by additive and synergistic effect. AE supplementation can extend the lifespan and has anti-aging effects in Drosophila melanogaster. The extended longevity and improved locomotion in files supplemented with AE 2.5 were probably due to increases in the level of antioxidant enzymes (SOD, CAT, and GPx) and the expressions of stress resistance genes (Hsp68, l(2)efl, and Jafrac1).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Iliadi KG, Knight D, Boulianne GL. Healthy aging–insights from Drosophila. Front. Psychol. 10.3389/fphys.2012.00106 (2012) [DOI] [PMC free article] [PubMed]
- 2.Wickens AP. Ageing and the free radical theory. Resp. Physiol. 2001;128:379–391. doi: 10.1016/S0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 3.Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 6.Wu X, Gu L, Prior RL, McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Li YM, Lei L, Liu Y, Wang X, Ma KY, Chen ZY. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Exp. Gerontol. 2015;69:189–195. doi: 10.1016/j.exger.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Peng C, Zuo Y, Kwan KM, Liang Y, Ma KY, Chan HYE, Huang Y, Yu H, Chen ZY. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp. Gerontol. 2012;47:170–178. doi: 10.1016/j.exger.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Y, Peng C, Liang Y, Ma KY, Yu H, Chan HYE, Chen ZY. Black rice extract extends the lifespan of fruit flies. Food Funct. 2012;3:1271–1279. doi: 10.1039/c2fo30135k. [DOI] [PubMed] [Google Scholar]
- 10.Hellstorm JK, Torronen AR, Mattila PH. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem. 2009;57:7899–7906. doi: 10.1021/jf901434d. [DOI] [PubMed] [Google Scholar]
- 11.Fine AM. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Alter. Med. Rev. 2000;5:144–151. [PubMed] [Google Scholar]
- 12.Denev PN, Kratchanov CG, Ciz M, Lojek A, Kratchanova MG. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: in vitro and in vivo evidences and possible mechanisms of action: a review. Comp. Rev. Food Sci. Food Safety. 2012;11:471–488. doi: 10.1111/j.1541-4337.2012.00198.x. [DOI] [Google Scholar]
- 13.Xu BJ, Yuan SH, Chang SKC. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007;72:S167–177. doi: 10.1111/j.1750-3841.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi: 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- 15.Lee J, Rennaker C, Wrolstad RE. Correlation of two anthocyanin quantification methods: hPLC and spectrophotometric methods. Food Chem. 2008;110:782–786. doi: 10.1016/j.foodchem.2008.03.010. [DOI] [Google Scholar]
- 16.Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998;46:4267–4274. doi: 10.1021/jf980366j. [DOI] [Google Scholar]
- 17.Smith P, Krohn RI, Hermanson G, Mallia A, Gartner F, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Jung JW, Ahn YJ, Kwon HW. Neuroprotective properties of phytochemicals against paraquat-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Pestic. Biochem. Physiol. 2012;104:118–125. doi: 10.1016/j.pestbp.2012.07.006. [DOI] [Google Scholar]
- 20.Hwang SJ, Yoon WB, Lee OH, Cha SJ, Kim JD. Radical-scavenging-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in Korea. Food Chem. 2014;146:71–77. doi: 10.1016/j.foodchem.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Bijak M, Borowski M, Borowiecka M, Podsedek A, Golanski J, Nowak P. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia. 2011;82:811–817. doi: 10.1016/j.fitote.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Kulling SE, Rawel HM. Chokeberry (Aronia melanocarpa) –A review on the characteristic components and potential health effects. Planta Med. 2008;74:1625–1634. doi: 10.1055/s-0028-1088306. [DOI] [PubMed] [Google Scholar]
- 23.Rugina D, Sconta Z, Leopold L, Pintea A, Bunea A, Socaciu C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on Hela human cervical tumor cells. J. Med. Food. 2012;15:700–706. doi: 10.1089/jmf.2011.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon AF, Liang DF, Krantz DE. Differential decline in behavioral performance of Drosophila melanogaster with age. Mech. Ageing Dev. 2006;127:647–651. doi: 10.1016/j.mad.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Jones MA, Grotewiel M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011;46:320–325. doi: 10.1016/j.exger.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, Seong KM, Yu K, Min KJ, Jafari M. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13:561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 27.Helfand SL, Rogina B. Molecular genetics of aging in the fly: is that the end of the beginning? BioEssays. 2003;25:3063–3073. doi: 10.1002/bies.10225. [DOI] [PubMed] [Google Scholar]
- 28.Broncel M, Kozirog M, Duchnowicz P, Koter-Michalak M, Sikora J, Chojnowska-Jezierska J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2009;16:CR28–34. [PubMed] [Google Scholar]
- 29.Peng C, Chan HYE, Huang Y, Yu H, Chen ZY. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J. Agric. Food Chem. 2011;59:2097–2106. doi: 10.1021/jf1046267. [DOI] [PubMed] [Google Scholar]
- 30.Li YM. HYE Chan, Huang Y, Yu H, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol. Nutr. Food Res. 2007;51:546–554. doi: 10.1002/mnfr.200600238. [DOI] [PubMed] [Google Scholar]
- 31.Orr W, Sohal RS. Extension of lifespan by the overexpression of superoxide-dismutase and catalase. Science. 1994;263:1128. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 32.Paaby AB, Schmidt PS. Functional significance of allelic variation at methuselah, an aging gene in Drosophila. PLoS One. 2008;3:e1987. doi: 10.1371/journal.pone.0001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 34.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]