Abstract
The purpose of this study was to evaluate the anti-inflammatory and anti-genotoxic activity of branched-chain amino acids (BCAAs) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. BCAAs inhibited LPS-induced NO production, with 100 mM leucine having the most pronounced effect, suppressing NO production by 81.15%. Valine and isoleucine also reduced NO production by 29.65 and 42.95%, respectively. Furthermore, BCAAs suppressed the inducible nitric oxide synthase mRNA expression. Additionally, BCAAs decreased the mRNA expression of interleukin-6 and cyclooxygenase-2 which are proinflammatory mediators. Anti-genotoxic activities of BCAAs were assessed using the alkaline comet assay and valine, isoleucine, and leucine significantly (p < 0.05) decreased tail length of DNA (damaged portion) to 254.8 ± 7.5, 235.6 ± 5.6, and 271.5 ± 19.9 μm compared than positive control H2O2 (434.3 ± 51.3 μm). These results suggest that BCAAs can be used in the pharmaceutical or functional food industries as anti-inflammatory agents or anti-cancer agents.
Keywords: Branched-chain amino acid, Anti-inflammatory activity, Anti-genotoxic activity, RAW 264.7 macrophage
Introduction
Macrophages are well known that involved in the inflammatory responses. Activated macrophages by stimulation with lipopolysaccharide (LPS), which is the major component of Gram-negative bacteria cell wall, produce a large amount of proinflammatory cytokines and mediators, and inducible enzymes [1]. The activation of macrophages is an important part of initiating immune defence mechanisms, since the inflammatory mediators produced by macrophages enhance immune response [2].
Inflammation is a biological response that is triggered by several stimuli and conditions, such as infection and tissue injury [3]. However, chronic inflammation is closely associated with cancer, cardiovascular diseases, sepsis, and rheumatoid arthritis [4]. These chronic inflammatory diseases are characterized by overproduction of proinflammatory cytokines which are interleukin-6 (IL-6), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and inducible enzymes which are inducible nitric oxide (iNOS) and cyclooxygenase-2 (COX-2), which, in turn, produce nitric oxide (NO) and prostaglandin E2 (PGE2), respectively [5, 6]. Therefore, substances suppressing these cytokines and enzymes can be used as anti-inflammatory agents.
Reactive species (RS), which include free radicals derived from nitrogen or oxygen, are well known that be involved in many human diseases. These RS can attack DNA, lipids and proteins, thereby leading to cellular death [7]. DNA damage can lead to the modifications of purine and pyrimidine bases and 2-deoxyribose, and can break the single and double strand of DNA [8]. RS are considered to play important roles in neuronal death in vascular and chronic inflammatory diseases, and cancer [9]. The break of balance between the production and elimination of free radicals can induce the aforementioned diseases. RS which damages DNA and modulates certain cellular pathways such as the phosphorylation of p53 at the serine and threonine residues are involved in the formation of cancer [10].
Branched-chain amino acids (BCAAs: valine, isoleucine, and leucine) are the most hydrophobic amino acids and essential amino acids and share common structural features of their aliphatic side chain [11]. BCAAs play an important role in formation of the globular protein structure. BCAAs usually account for 20–25% of dietary proteins and, particularly, account for ~33% of the essential amino acids in muscle proteins [12, 13]. BCAAs have been used as nutritional supplements to improve mental and physical health [14]. Furthermore, BCAAs have been reported to affect gene expression [15], protein metabolism [16], and apoptosis [17].
In this study, the anti-inflammatory activity of BCAAs was evaluated by measuring the production of NO, the levels of expression of iNOS and proinflammatory mediators (IL-6 and COX-2) in RAW 264.7 macrophages. Furthermore, we evaluated the antigenotoxic activity of BCAAs in RAW 264.7 macrophages.
Materials and methods
Materials
BCAAs were obtained from Cremar, Co. (Seongnam, South Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin were purchased from Hyclone Laboratories, Inc. (Logan, UT, USA). MTT, lipopolysaccharides, sulfanilamide, and N-(1-naphthyl) ethylenediamine dihydrochloride were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). All other chemicals and organic solvents used in this study were of analytical grade and the highest purity.
Cell lines and culture conditions
RAW 264.7 macrophages were purchased from Korean Cell Line Bank (Seoul, Korea). The RAW 264.7 macrophages were cultured in DMEM supplemented with 10% heat inactivated FBS, and 1% penicillin–streptomycin at 37 °C in a 5% CO2 incubator (MCO-18AIC, Sanyo, Osaka, Japan). The culture medium was replaced every 3–4 days.
Measurement of cell viability and nitrite
MTT assay was used for measuring cell viability as described by Moon et al. [18]. For measuring nitrite production, RAW 264.7 macrophages were incubated in a 96-well plate (1 × 105 cells/well) for 2 h, following which they were incubated further for 24 h with or without 1 μg/mL of LPS to stimulate NO production. Four concentrations (12.5, 25, 50, and 100 mM) of BCAAs were used. NO reacts with oxygen to produce nitrite under aerobic condition, the quantity of NO was determined using Griess reagent [19]. One hundred μL of griess reagent was mixed with 100 μL of medium for 15 min to measuring nitrite. The absorbance at 540 nm was measured using a microplate reader. The NO concentration was calculated by comparison with the absorbance at 540 nm of standard nitrite solution in culture medium.
RNA isolation and cDNA synthesis
RAW 264.7 macrophages were incubated in a 24-well plate (6 × 105 cells/well) for 24 h, followed by another 24 h incubation period with or without 100 mM of BCAAs. The macrophages were then incubated with 1 μg/mL of LPS for 24 h (1st condition); Cells were cultured in the presence of 100 mM of BCAAs and LPS at the same time for 24 h (2nd condition). Trizol methods were used for isolating total RNA from LPS-stimulated RAW 264.7 macrophages. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (PCR) was performed to observe the influence of BCAAs on inflammation-associated gene expression. The template used was cDNA synthesized from RNA by using reverse transcriptase. The target cDNA was amplified using real-time DNA thermal cycler (iQTM5 multicolour, BIO-RAD, Hercules, CA, USA). PCR amplification was carried out under the following reaction conditions: 95 °C for 15 min (initiation) followed by 55 cycles of 95 °C for 10 s (denaturation), 55 °C for 30 s (annealing), with a final extension at 72 °C for 20 s. Amplification results were analysed by delta–delta Ct method, and the purity of PCR products was assessed by analysing the melting curve from PCR reaction. β-Actin was used as the housekeeping gene. PCR primers used for quantitative PCR in this study were synthesized according to the following oligonucleotide sequences. iNOS: forward 5′-CTTGGTGAGGGGACTGGACT-3′, and reverse 5′-GGGGTTTTCTCCACGTTGTT-3′; and IL-6: forward 5′-CCTTCCTACCCCAATTTCCA-3′, and reverse 5′-CGCACTAGGTTTGCCGAGTA-3′; and COX-2: forward 5′-TGGGAAGCTTTCTCCAACCT-3′, and reverse 5′-GTGAAGTGCTGGGCAAAGAA-3′; and β-actin: forward 5′-GATTACTGCTCTGGCTCCTA-3′, and reverse 5′-ATCGTACTCCTGCTTGCT-3′.
Measurement of anti-genotoxic activity
To determine the anti-genotoxic activity of BCAAs, we used the comet assay [18]. The incubated RAW 264.7 macrophages (2 × 105 cells) were washed with 1.0 mL of PBS and used in comet assays. BCAAs were dissolved in distilled water and macrophages incubated with each BCAA at 400 μM at 37 °C for 30 min in the dark. To stimulate oxidation, macrophages were treated with 200 μM H2O2 and incubated on ice for 5 min, then washed with PBS. Samples that were not exposed to H2O2 were used as negative controls. After H2O2 treatment, samples were mixed with 75 μL of 0.7% low-melting agarose and distributed over slides pre-coated with 1% normal melting agarose (NMA). Slides were then immersed in a cold alkali lysis buffer (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1.0% sodium laurylsarcosine, 1.0% Triton X-100, and 10% DMSO) for 1 h at 4 °C in the dark. Slides were placed in an electrophoresis tank containing a basic buffer (300 mM NaOH, 10 mM Na2EDTA, pH 13.0) for 20 min and subjected to electrophoresis (25 V, 300 ± 3 mA) for 20 min at 4 °C. Slides were washed three times in a neutral buffer (0.4 M Tris, pH 7.5) at 4 °C and then immersed in ethanol for 5 min and dried. Slides were stained using a 20 μg/mL ethidium bromide solution. Slides were examined using Komet version 5.0 (Kinetic imaging, Liverpool, UK) and fluorescence microscopy (LEICA DMLB, Wetzlar, Germany), to measure the fluorescent intensity in tails. We quantified tail intensity using 50 cells from two replicate slides.
Statistical analysis
All results are presented as the mean ± standard deviation (SD) of three replicates. Statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test, with a p value less than 0.05 considered statistically significant.
Results and discussion
Effect of BCAAs on the production of NO in RAW 264.7 cells
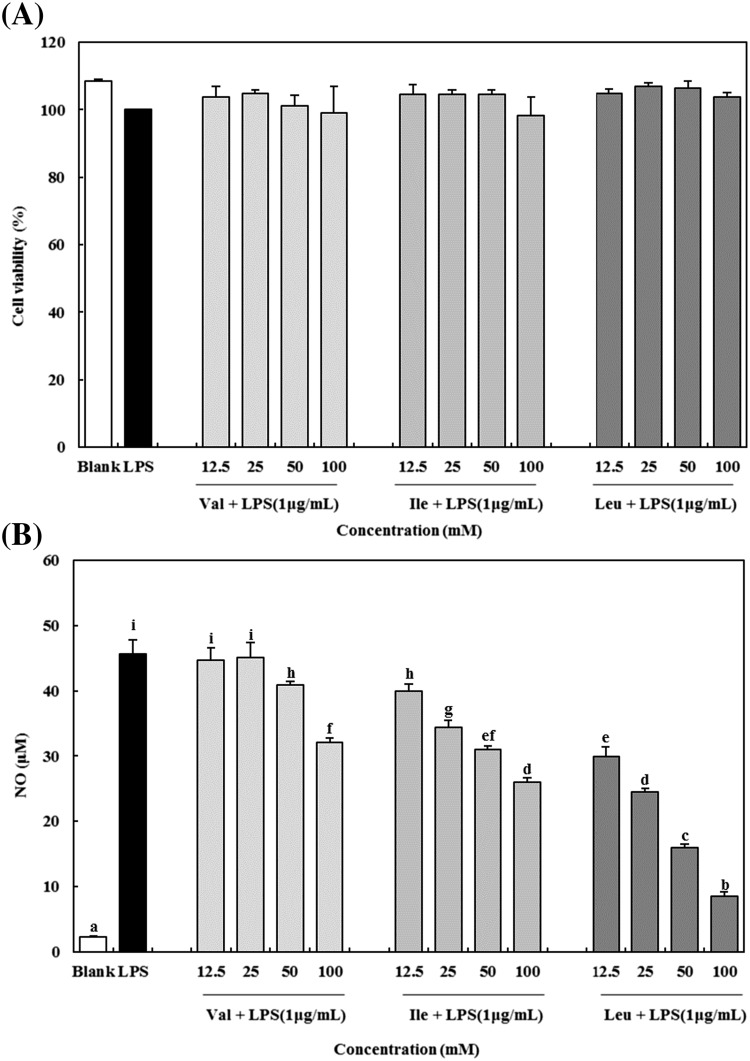
LPS, a well-known endotoxin, are a component of cell wall in Gram-negative bacteria. LPS have been known to induce the production proinflammatory cytokines and mediators such as IL-6, IL-1β, TNF-α, and NO in RAW 264.7 macrophages [20, 21]. Particularly, overproduction of NO can be harmful and may cause various inflammatory diseases [22]. Therefore, substances which have inhibitory effect on NO production can be used as a potential anti-inflammatory agent. In this study, the cytotoxicity of BCAAs on RAW 264.7 macrophages was determined by using MTT assay and the concentrations of BCAAs used in this study (12.5–100 mM) did not affect cell viability [Fig. 1(A)].
Fig. 1.
Effect of BCAAs on cell viability (A) and NO production (B) in LPS-induced RAW 264.7 macrophages. Val, valine; Ile, isoleucine; Lue, leucine. The data are expressed as the mean ± standard deviation (SD) of three separate experiments. a–i Different letters among samples indicate significant differences by Duncan’s multiple range test (p < 0.05)
To investigate the inhibitory effect on NO production in RAW 264.7 macrophages, the quantity of nitrite in the culture media was determined by using Griess reagent. As shown in Fig. 1(B), addition of LPS significantly increased the production of NO in the media by approximately 22-fold (from 2.29 ± 0.22 to 45.64 ± 2.20 μM). After adding BCAAs, the levels of NO decreased in a dose-dependent manner. Among the BCAAs, leucine appeared to have the greatest inhibitory effect on NO production. At 12.5, 25, 50, and 100 mM concentration of leucine, the levels of NO decreased to 29.97 ± 1.4, 24.47 ± 0.52, 15.97 ± 0.50, and 8.60 ± 0.64 μM, respectively. The rate of inhibition at leucine concentration of 100 mM was 81.15 ± 1.39%. Meanwhile, valine and isoleucine showed a weak inhibitory effect on NO production compared to leucine, but at a concentration of 100 mM, these showed inhibitory effects of as much as 29.65 ± 1.54 and 42.95 ± 1.28%, respectively.
Nitric oxide (NO) is synthesized by a family of nitric oxide synthases, which are neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS), and plays a dual role as either a beneficial or a harmful molecule in the inflammatory process [23]. Among these nitric oxide synthases, iNOS produces a large amount of NO under pathological conditions. The other synthases are constitutively expressed at low levels [24]. The effect on expression of iNOS messenger RNA (mRNA) was examined to elucidate the mechanism of the inhibitory effect on NO production by BCAAs in RAW 264.7 macrophage. RAW 264.7 macrophages were cultured in the presence of 100 mM of BCAAs and LPS at the same time for 24 h. As shown in Fig. 2, it appeared that the iNOS mRNA expression was significantly (p < 0.05) up-regulated by LPS stimulation to 3.79 times compared to the blank (without LPS stimulation), however, BCAAs suppressed the expression of iNOS mRNA. In this study, valine, isoleucine, and leucine reduced iNOS expression by 47.31, 23.66, and 89.61%, respectively.
Fig. 2.
Effect of BCAAs on iNOS mRNA expression in LPS-stimulated RAW 264.7 macrophages. Val, valine; Ile, isoleucine; Lue, leucine. The data are expressed as the mean ± standard deviation (SD) of three separate experiments. a–d Different letters among samples indicate significant differences by Duncan’s multiple range test (p < 0.05)
Yang et al. [25] reported that excessive production of NO has been suggested to lead to hypotension, vascular hyporeactivity and death. It indicated that the overproduction of NO plays an important role in septic shock. Additionally, van der Woude et al. [26] reported that the over-expression of iNOS also leads to DNA damage, mutation, increased cell proliferation, and oxidative stres. Therefore, the modulation of iNOS-mediated NO release is one of the contributing factors during the inflammatory process [23]. In our previous study, we showed that BCAAs have scavenging effect on NO in SNP (sodium nitroprusside) system [27]. The present study also shows that BCAAs have inhibitory activity on NO production by suppressing the iNOS mRNA expression.
Effect of BCAAs on the expression mRNA of inflammatory mediators
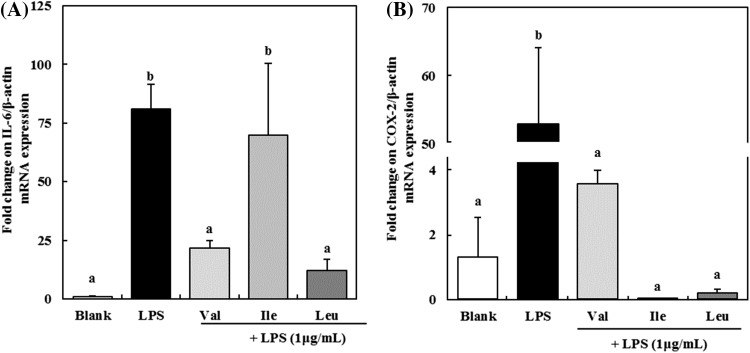
RAW 264.7 macrophages were incubated with or without 100 mM of BCAAs for 24 h to investigate the effect of BCAAs on the transcription levels of proinflammatory mediators such as IL-6 and COX-2. After then, cells were stimulated with 1 μg/mL LPS for another 24 h. The mRNA levels of proinflammatory mediators were increased by LPS stimulation (Fig. 3). IL-6 levels were significantly (p < 0.05) increased in cells incubated with LPS for 24 h compared to that in cells without LPS stimulation. However, the expression of IL-6 mRNA was suppressed by 100 mM of valine, isoleucine, and leucine to 73.32, 13.79, and 85.04%, respectively [Fig. 3(A)]. In this study, leucine showed the strongest inhibition effect on expression of IL-6 mRNA. Dinarello et al. [28] reported that IL-6 is multifunctional cytokine, which is upregulated by bacterial LPS, and plays an important role in the pathogenesis of inflammatory diseases. High serum IL-6 levels are observed frequently in many pathological conditions such as inflammation, autoimmune diseases, and sepsis. In case of sepsis, IL-6 remains elevated for a long period of time and the levels correlate with sepsis severity and mortality [29]. These results suggest that BCAAs can be used as anti-inflammatory agents via regulating IL-6 expression.
Fig. 3.
Effect of BCAAs on expression of IL-6 (A) and COX-2 (B) mRNA in LPS-induced RAW 264.7 macrophages. Val, valine; Ile, isoleucine; Lue, leucine. The data are expressed as the mean ± standard deviation (SD) of three separate experiments. a,b Different letters among samples indicate significant differences by Duncan’s multiple range test (p < 0.05)
The COX-2 mRNA expression also was decreased by treatment with BCAAs [Fig. 3(B)]. After stimulation with LPS on RAW 264.7 macrophages, the COX-2 mRNA expression was increased by as much as 52.96 times compared to the blank. Valine suppressed the COX-2 mRNA expression by 93.22%. Moreover, in case of isoleucine and leucine, it was suppressed by more than 99%. Smith et al. [30] reported that COX-2 is an inducible enzyme that causes the conversion of arachidonic acid to prostaglandin H2, which is a precursor of an important biological mediator PGE2. PGE2 is involved in the pathogenesis of various inflammatory diseases, and invasion and growth of tumor [31]. Our study showed that BCAAs have the ability to suppress the COX-2 mRNA expression.
Effect of BCAAs on the protection of DNA damages in macrophages induced by H2O2
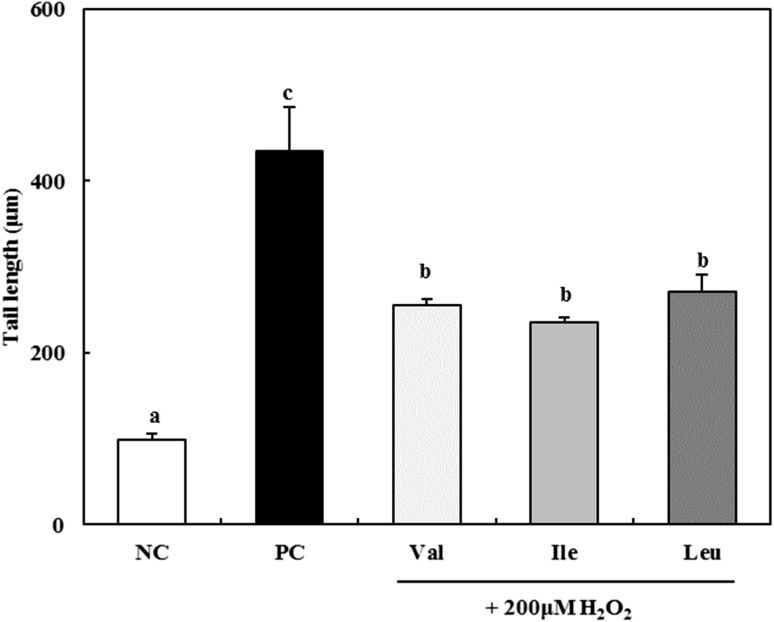
The alkaline comet assay is a relatively simple, rapid, low-cost, and quantitative technique for analyzing DNA damage in eukaryotic cells [32]. The protection activity of DNA damages of BCAAs was assessed in RAW 264.7 macrophages (Figs. 4, 5). The tail length (damaged portion of DNA) of negative control was 98.8 ± 6.8 μm, while that of positive control was 434.3 ± 51.3 μm. It was greater fourfold than negative control (Fig. 4). When BCAAs were treated at the concentration 400 μM, tail length decreased to 254.8 ± 7.5 μm for valine, 235.6 ± 5.6 μm for isoleucine, and 271.5 ± 19.9 μm for leucine. However, there was no significant difference (p < 0.05) between each of BCAAs. Comet images of DNA treated with BCAAs were shown in Fig. 5. Negative control showed clear circle images, however, the tail of DNA was increased by treatment of 200 μM H2O2. After treated with BCAAs, the tail of DNA was decreased compared with positive control.
Fig. 4.
Antigenotoxic effect of 400 μM BCAAs on 200 μM H2O2 induced DNA damage in RAW 264.7 macrophages. Val, valine; Ile, isoleucine; Lue, leucine. NC negative control; PC: 200 μM H2O2 treated positive control. The data are expressed as the mean ± standard deviation (SD) of three separate experiments. a–c Different letters among samples indicate significant differences by Duncan’s multiple range test (p < 0.05)
Fig. 5.
Comet images of RAW 264.7 macrophages treated with BCAAs. A Negative control; B 200 μM H2O2 treated positive control; C Valine 400 μM + 200 μM H2O2; D Isoleucine 400 μM + 200 μM H2O2; E Leucine 400 μM + 200 μM H2O2
Damage to cellular DNA can threaten genome stability, which can subsequently result in carcinogenesis or tissue aging [33]. DNA damage can also lead to mutations, and under certain circumstances can activate the nuclear transcription factor p53. Culmsee et al. [34] reported that transcription factor p53 is known to regulate major cellular functions which are transcription, DNA synthesis, DNA repair, cell cycle regulation, and cell death. BCAAs have the protection effect of H2O2-induced DNA damage in macrophages (Fig. 4).
Boyacioglu et al. [35] reported that methionine, one of the nine essential amino acids, increased the percentage of tail DNA contents in comet assays. However, when vitamin C was added as an antioxidant in rats where chronic hyperhomocysteinemia was induced, the proportion of tail DNA contents was decreased. Other researchers have reported that antioxidants protect DNA from genotoxic agents and are able to eliminate reactive oxygen species [36].
In conclusion, in this study we found that BCAAs significantly reduce the levels of proinflammatory cytokines and mediators in RAW 264.7 macrophages stimulated by LPS. Furthermore, BCAAs can protect the damages induced by H2O2 in macrophages. From these results, it can be surmised that BCAAs can be utilized in the pharmaceutical or functional food industries as anti-inflammatory agents or anti-cancer agents.
Acknowledgements
This research was supported by the High Value-added Food Technology Development Program (313021-3) of the Ministry of Agriculture, Food and Rural Affairs (Korea).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Kim J, Kim JS, Park E. Cytotoxic and anti-inflammatory effects of onion peel extract on lipopolysaccharide stimulated human colon carcinoma cells. Food Chem. Toxicol. 2013;62:199–204. doi: 10.1016/j.fct.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Ahn CB, Cho YS, Je JY. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015;168:151–156. doi: 10.1016/j.foodchem.2014.05.112. [DOI] [PubMed] [Google Scholar]
- 3.Jeong JB, Shin YK, Lee SH. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem. Toxicol. 2013;55:229–233. doi: 10.1016/j.fct.2012.12.062. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Zhang W, Zhen Q, Gao R, Du T, Xiao X, Wang Z, Ge Q, Hu J, Ye P, Zhu Q, Li Q. Impaired adipogenesis in adipose tissue associated with hepatic lipid deposition induced by chronic inflammation in mice with chew diet. Life Sci. 2015;137:7–13. doi: 10.1016/j.lfs.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol. Rev. 2005;208:228–251. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 7.Cini M, Fariello RG, Bianchetti A, Moretti A. Studies on lipid peroxidation in the rat brain. Neurochem. Res. 1994;19:283–288. doi: 10.1007/BF00971576. [DOI] [PubMed] [Google Scholar]
- 8.El-Khamisy SF, Caldecott KW. TDP1-dependent DNA single-strand break repair and neurodegeneration. Mutagenesis. 2006;21:219–224. doi: 10.1093/mutage/gel024. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernstrom JD. Branched-chain amino acids and brain function. J. Nutr. 2005;135:1539S–1546S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 12.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J. Nutr. 2006;136:207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites. 2013;3:931–945. doi: 10.3390/metabo3040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleeson MC. Interrelationship between physical activity and branched-chain amino acids. J. Nutr. 2005;135:1591S–1595S. doi: 10.1093/jn/135.6.1591S. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa K, Okabayashi T, Shima Y, Iiyama T, Takezaki Y, Munekage M, Namikawa T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Branched-chain amino acid-enriched nutrients stimulate antioxidant DNA repair in a rat model of liver injury induced by carbon tetrachloride. Mol. Biol. Rep. 2012;39:10803–10810. doi: 10.1007/s11033-012-1974-4. [DOI] [PubMed] [Google Scholar]
- 16.Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, Kubota T, Nagano Y, Matsuo K, Endo I, Sekido H, Shimada H. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480–486. doi: 10.1016/j.nut.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y, Kido Y. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr. Res. 2012;32:522–529. doi: 10.1016/j.nutres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Moon SH, Lee JH, Lee M, Park E, Ahn DU, Paik HD. Cytotoxic and antigenotoxic activities of phosvitin from egg yolk. Poult. Sci. 2004;93:2103–2107. doi: 10.3382/ps.2013-03784. [DOI] [PubMed] [Google Scholar]
- 19.Marcocci L, Magurie JJ, Droy-Lefaiz MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGB 761. Biochem. Biophys. Res. Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 20.Guastadisegni C, Nicolini A, Balduzzi M, Ajmone-Cat MA, Minghetti L. Modulation of PGE2 and TNF-alpha by nitric oxide in resting and LPS-activated RAW 264.7 cells. Cytokine. 2002;19:175–180. doi: 10.1006/cyto.2002.1955. [DOI] [PubMed] [Google Scholar]
- 21.Kim HH, Park GH, Park KS, Lee JY, An BJ. Anti-oxidant and anti-inflammation activity of fractions from Aster glehni Fr. Schm. J. Microbiol. Biotechnol. 2010;38:434–441. [Google Scholar]
- 22.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim ND, Kim EM, Kang KW. Cho Mk, Choi SY, Kim SG. Ginsenoside Rg3 inhibits phenylephrine-induced vascular contraction through induction of nitric oxide synthase. Brit. J. Pharmacol. 2003;140:661–670. doi: 10.1038/sj.bjp.0705490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar AP, Ryan C, Cordy V, Reynolds WF. Inducible nitric oxide synthase expression is inhibited by myeloperoxidase. Nitric Oxide. 2005;13:42–53. doi: 10.1016/j.niox.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Comtois AS, Fang L, Hartman NG, Blaise G. Nitric oxide-derived nitrate anion contributes to endotoxic shock and multiple organ injury/dysfunction. Crit. Care Med. 2002;30:650–657. doi: 10.1097/00003246-200203000-00026. [DOI] [PubMed] [Google Scholar]
- 26.van der Woude CJ, Kleibeuker JH, Jansen PL, Moshage H. Chronic inflammation, apoptosis and (pre-)malignant lesions in the gastro-intestinal tract. Apoptosis. 2004;9:123–130. doi: 10.1023/B:APPT.0000018794.26438.22. [DOI] [PubMed] [Google Scholar]
- 27.Jin HJ, Lee JH, Kim DH, Kim KT, Lee GH, Choi SJ, Chang PS, Paik HD. Antioxidative and nitric oxide scavenging activity of branched-chain amino acids. Food Sci. Biotechnol. 2015;24:1555–1558. doi: 10.1007/s10068-015-0200-2. [DOI] [Google Scholar]
- 28.Dinarello CA. Cytokines as endogenous pyrogens. J. Infect. Dis. 1999;179:S294–S304. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- 29.Nasraway SA. The problems and challenges of immunotherapy in sepsis. Chest. 2003;123:451S–459S. doi: 10.1378/chest.123.5_suppl.451S. [DOI] [PubMed] [Google Scholar]
- 30.Smith WL, Garavito RM, Dewitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 31.Claria J. Cyclooxygenass-2 biology. Curr. Pharm. Design. 2003;9:2177–2190. doi: 10.2174/1381612033454054. [DOI] [PubMed] [Google Scholar]
- 32.Liao W, McNutt MA, Zhu WG. The comet assay: a sensitive method for detecting DNA damage in individual cells. Methods. 2009;48:46–53. doi: 10.1016/j.ymeth.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi M, Niida H, Murakami H, Shimada M. DNA damage responses in skin biology-implications in tumor prevention and aging acceleration. J. Dermatol. Sci. 2009;56:76–81. doi: 10.1016/j.jdermsci.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 35.Boyacioglu M, Sekkin S, Kum C, Korkmaz D, Kiral F, Yalinkilinc HS, Ak MO, Akar F. The protective effects of vitamin C on the DNA damage, antioxidant defenses and aorta histopathology in chronic hyperhomocysteinemia induced rats. Exp. Toxicol. Pathol. 2014;66:407–413. doi: 10.1016/j.etp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Aydin S, Tokac M, Taner G, Arikok AT, Dundar HZ, Ozkardes AB, Taslipinar MY, Kilic M, Basaran AA, Basaran N. Antioxidant and antigenotoxic effects of lycopene in obstructive jaundice. J. Surg. Res. 2013;182:285–295. doi: 10.1016/j.jss.2012.10.031. [DOI] [PubMed] [Google Scholar]