Abstract
[Fe–S] clusters are essential cofactors in all domains of life. They play many biological roles due to their unique abilities for electron transfer and conformational control. Yet, producing and analyzing Fe–S proteins can be difficult and even misleading if not done anaerobically. Due to unique redox properties of [Fe–S] clusters and their oxygen sensitivity, they pose multiple challenges and can lose enzymatic activity or cause their component proteins to be structurally disordered due to [Fe–S] cluster oxidation and loss in air. Here we highlight tested protocols and strategies enabling efficient and stable [Fe–S] protein production, purification, crystallization, X-ray diffraction data collection, and structure determination. From multiple high-resolution anaerobic crystal structures, we furthermore analyze exemplary data defining [Fe–S] clusters, substrate entry, and product exit for the functional oxidation states of type II molybdo-bis(-molybdopterin guanine dinucleotide) (Mo-bisMGD) enzymes. Notably, these enzymes perform electron shuttling between quinone pools and specific substrates to catalyze respiratory metabolism. The identified structure–activity relationships for this enzyme class have broad implications germane to perchlorate environments on Earth and Mars extending to an alternative mechanism underlying metabolic origins for the evolution of the oxygen atmosphere. Integrated structural analyses of type II Mo-bisMGD enzymes unveil novel distinctive shared molecular mechanisms for dynamic control of substrate entry and product release gated by hydrophobic residues. Collective findings support a prototypic model for type II Mo-bisMGD enzymes including insights for a fundamental molecular mechanistic understanding of selectivity and regulation by a con-formationally gated channel with general implications for [Fe–S] cluster respiratory enzymes.
1. INTRODUCTION
Life evolved in an anaerobic world, which can frequently be overlooked from the focus of most funded research on aerobic eukaryotes. Due to this anaerobic origin, our experimental approaches must consider fundamental enzymatic mechanisms and biochemical pathways that were developed and integrated into metabolism in the absence of selective pressure to avoid oxygen reactivity (Imlay, 2008). The [Fe–S] cluster is prone to oxygen sensitivity due to its redox properties enabling conversion from Fe2+ to Fe3+, and the fact that its redox potential can range from −700 to +450mV (Bak & Elliott, 2014; Imlay, 2006). As the redox potential of molecular oxygen ranges from +300 to +800mV (Segel, 1976), the aerobic purification of [Fe–S] proteins can make [Fe–S] clusters prone to oxidation resulting in the loss of [Fe–S] clusters and consequently protein dysfunction and even domain misfolding, as seen for XPD (Fan et al., 2008). Such oxidative damage has biological and practical relevance as oxidation causing mis-metalation can occur in cells, especially under conditions of oxidative stress (Cotruvo & Stubbe, 2012; Imlay, 2014). In aerobic cells, reactive oxygen species are scavenged by oxygen into superoxide that can accelerate DNA and other damage by destroying [Fe–S] clusters with a rate constant of 106–107M−1s−1 and elevating free-iron levels (Imlay, 2008; Keyer & Imlay, 1996). As a result, superoxide dismutase metalloenzymes, which convert superoxide radicals to molecular oxygen and hydrogen peroxide that is further reduced to oxygen by peroxidases and catalases, are critical to the stability of [Fe–S] cluster enzymes in many cells including many anaerobes that may generate oxygen during metabolism (Imlay, 2008; Perry, Shin, Getzoff, & Tainer, 2010). The fundamental sensitivity of [Fe–S] clusters to superoxide and nitric oxide, which is tightly controlled by its synthesis from arginine in eukaryotes (Crane et al., 1998) and some microbes (Adak et al., 2002), drove the development of multiple protective superoxide dismutases with several different folds and metal ion active sites (Perry et al., 2010). In human cells, DNA sequence regions without stable double-helical conformations are especially vulnerable to iron-mediated damage, and translocation breakpoints in patient tumor samples are associated with these regions (Bacolla, Tainer, Vasquez, & Cooper, 2016). Strikingly, in higher eukaryotes both growth defects and DNA damage caused by superoxide develop primarily from its toxic damage to iron–sulfur clusters (ISCs) (Keyer & Imlay, 1996).
Whenever doing experiments with [Fe–S] cluster proteins, it is therefore crucial to appreciate dioxygen toxicity to [Fe–S] clusters, despite the fact that they are truly ancient cofactors essential for all domains of life (Johnson, Dean, Smith, & Johnson, 2005; Lill, 2009). Cubane [4Fe–4S] and rhombic [2Fe–2S] clusters are those found most frequently in proteins (Beinert, 1997; Johnson et al., 2005) although more complex [Fe–S] clusters such as H-cluster, P-cluster, and FeMo cofactor also exist (Broderick et al., 2014; Burgess & Lowe, 1996; Hoffman, Lukoyanov, Yang, Dean, & Seefeldt, 2014). They play essential roles for protein function and are even required for DNA replication, transcription, and repair critical for genome integrity and stability (Fuss, Tsai, Ishida, & Tainer, 2015) where their function may include detection of non-B-DNA areas, which are generally associated with increased breaks and translocations linked with cancer (Bacolla et al., 2016). In prokaryotes, the formation, regulation, and diversity of [Fe–S] clusters have been most studied for Escherichia coli and Azotobacter vinelandii (Frazzon & Dean, 2003; Roche et al., 2013). In eukaryotes, maturation of both cytosolic and nuclear Fe–S proteins requires the cooperation of the mitochondrial systems derived from microbial ancestors (Lill & Muhlenhoff, 2006; Paul & Lill, 2015). In fact, [Fe–S] cluster proteins are key to mitochondrial respiration including the assembly, stability, and function of respiratory complexes I, II, and III, which underscores the fundamental roles of Fe–S clusters in biological respiration (Melber et al., 2016). The maturation and insertion of [Fe–S] clusters into apo-target proteins require mitochondrial ISC assembly machinery that contains scaffold protein IscU, cysteine desulfurase (Nfs1), accessory protein Isd11, activator/iron donor Frataxin, and Ferredoxin (Agar et al., 2000; Colin et al., 2013; Cory et al., 2017; Johnson et al., 2005; Lill, 2009; Pandey et al., 2013; Raulfs, O’Carroll, Santos Dos, Unciuleac, & Dean, 2008; Schmucker et al., 2011; Tsai & Barondeau, 2010; Webert et al., 2014; Yoon & Cowan, 2003; Zheng, Cash, Flint, & Dean, 1998). [Fe–S] clusters are assembled in mitochondrial and cytosolic machineries (Braymer & Lill, 2017; Bridwell-Rabb, Fox, Tsai, Winn, & Barondeau, 2014; Lill et al., 2006), and clusters are transferred to apo-target proteins aided by chaperones HscA/HscB upon maturation (Bonomi, Iametti, Morleo, Ta, & Vickery, 2011; Chandramouli & Johnson, 2006; Fox, Chakrabarti, McCormick, Lindahl, & Barondeau, 2015; Fox, Das, Chakrabarti, Lindahl, & Barondeau, 2015; Uzarska, Dutkiewicz, Freibert, Lill, & Muhlenhoff, 2013; Vranish et al., 2015). Defects in Fe–S cluster assembly and maturation lead to multiple human diseases and protein dysfunctions (Rouault, 2015; Stehling, Wilbrecht, & Lill, 2014) that encompass many processes including metabolism, DNA maintenance, Fe–S cluster assembly machineries, respiratory chain metabolism, ribosome function, and tRNA modification (Andreini, Banci, & Rosato, 2016). The roles of [Fe–S] clusters include key functions in gene expression activation, substrate binding, electron transfer, sulfur donation, and structural stabilization when ligated to protein cysteine residues (Johnson et al., 2005). Of these many functions, a remarkable and exemplary function in [Fe–S] proteins is electron shuttling, which was proposed as the rate-limiting step, between multi-[Fe–S] clusters functioning in respiratory metabolism (de Vries, Dorner, Strampraad, & Friedrich, 2015; Martin & Matyushov, 2017; Rikken, Kroon, & van Ginkel, 1996).
For such respiratory electron transfer, an underappreciated and critical [Fe–S] function involves the respiration of perchlorate (ClO4−) and chlorate (ClO3−) [collectively, (per)chlorate] that occurs in dissimilatory (per)chlorate-reducing bacteria (Youngblut, Wang, Barnum,& Coates, 2016). Perchlorate is a water-soluble chemical that can be reduced to chlorate and subsequently reduced to chlorite (ClO2−) by (per)chlorate reductase (PcrAB). The product chlorite, a harmful molecule, is then dismutated to chloride and oxygen by chlorite dismutase (Cld) (Lee, Streit, Zdilla, Abu-Omar, & DuBois, 2008) in a manner somewhat reminiscent of the superoxide dismutase reaction (Perry et al., 2010). The oxygen is further used by cytochrome cbb3 oxidase for quinone oxidation (Bak & Elliott, 2014; Buschmann et al., 2010; Coates & Achenbach, 2004; Hosler, Ferguson-Miller, & Mills, 2006; Imlay, 2006). A remarkable feature of (per)chlorate respiration is electron shuttling (2e− at a time) from the quinone pool to heme to five [Fe–S] clusters, and finally to the Mo-bisMGD cofactor for (per)chlorate reduction (Youngblut, Tsai, et al., 2016; Youngblut, Wang, et al., 2016). The structure–activity relationships for these enzymes are relevant to removal of perchlorate (ClO4−) and nitrate (NO3−) from drinking water (Matos, Velizarov, Crespo, & Reis, 2006), to environmental controls of perchlorate and nitrate co-occurrence in arid and semiarid environments (Jackson, Böhlke, et al., 2015), to the control of H2S production from industrial oil recovery and mining (Carlson et al., 2015), and even to the generation of water and oxygen from perchlorate on Mars (Carlson et al., 2015; Ojha et al., 2015). Given its emblematic and understudied features, we focus on PcrAB to examine relationships of protein structure to the regulation of [Fe–S] cluster function.
Importantly, examination of PcrAB enzyme structures discovered distinct conformational states in reduced and oxidized [Fe–S] cluster forms that inform a detailed mechanism for (per)chlorate reduction. These structures showcase the fundamental importance of [Fe–S] cluster enzymes for cellular energy and their dramatic effects on altering protein functional conformations. The identified aromatic gate residues guard substrate entry and regulate product release to control the electron flow for (per)chlorate reduction (Youngblut, Tsai, et al., 2016). Yet, we find that in light of comparative structural analyses, the seemingly unique aromatic gate opening and closing for PcrAB may in fact serve as a prototypic model for Mo-bisMGD- and [Fe–S] cluster-containing respiratory proteins, which can be usefully grouped into the DMSO reductase family (Schindelin, Kisker, Hilton, Rajagopalan, & Rees, 1996; Youngblut, Tsai, et al., 2016). To furthermore robustly enable research on [Fe–S] cluster-containing proteins, we herein share our tactics, protocols, and strategies for [Fe–S] protein production, purification, crystallization, data collection, and X-ray structure determination and use PcrAB as a prototypic system for structure analysis.
2. RECOMBINANT [Fe–S] PROTEIN PRODUCTION AND PURIFICATION IN GENERAL: WHAT IS NEEDED AND WHAT NEEDS TO BE CONSIDERED?
Aerobic oxidative damage for eukaryotic DNA polymerases α, δ, and ε provides a sobering warning regarding metalloenzyme studies. These were only found to contain [4Fe–4S] clusters in 2011 (Netz et al., 2011) about 30 years after DNA polymerase α was isolated (Johnson, Snyder, Chang, & Davis, 1985). This unrecognized existence of [Fe–S] clusters in eukaryotic DNA polymerases, which delayed progress for decades, resulted from their aerobic protein production and purification, which caused oxidation damage and loss of the clusters resulting in dysfunctional polymerases. This is a general problem for native and recombinant proteins, as the metalloprotein remains largely undefined (Yannone, Hartung, Menon, Adams, & Tainer, 2012) even for microbes (Cvetkovic et al., 2010). Here we share general protocols and strategies that have proven successful for working with and analyzing proteins containing [Fe–S] clusters in ways that can protect them from oxidation damage and thus allow detailed structure determinations and analyses, as seen for multiple DNA repair proteins (Banda, Nuñez, Burnside, Bradshaw, & David, 2017), for sulfite reductase (Crane, Siegel, & Getzoff, 1995), and for the XPD helicase that acts in transcription and repair (Fan et al., 2008).
2.1 [Fe–S] Protein Production
Recombinant [Fe–S] protein overexpression in E. coli is often a first choice for large-scale protein production at low cost, although baculovirus methods continue to improve especially for multiprotein complexes such as TFIIH (Gradia, Ishida, Tsai, Jeans, & Tainer, 2017), and an exciting novel system has been developed in Pyrococcus furiosus that should be considered as well (McTernan et al., 2014). Importantly, E. coli contains the ISC biosynthetic system (iscS, iscU, iscA, hscB, hscA, and fdx) for general housekeeping Fe–S cluster assembly (Johnson et al., 2005; Takahashi & Nakamura, 1999; Zheng et al., 1998) and the sulfur-utilization factor (SUF) system (sufA, sufB, sufC, sufD, sufS, and sufE) for Fe–S cluster assembly when iron or sulfur sources are scarce (Barras, Loiseau, & Py, 2005; Outten, Djaman, & Storz, 2004). Thus, functional [Fe–S] clusters can be incorporated into E. coli that recombinantly expressed [Fe–S] proteins. However, in some cases, overexpressed [Fe–S] proteins lack [Fe–S] clusters or have reduced levels of clusters. In such cases, the [Fe–S] cluster maturation can be facilitated by coexpressing the isc operon in E. coli strains (Nakamura, Saeki, & Takahashi, 1999) or by adding cysteine and iron supplement (Jaganaman, Pinto, Tarasev, & Ballou, 2007; Kuchenreuther et al., 2010) before induction or by expressing the bacteria anaerobically (Kuchenreuther et al., 2010). For example, in the case of perchlorate reductase (PcrAB) from Azospira suillum PS, overexpression of PcrAB in E. coli resulted the loss of enzymatic activity due to the lack of Mo-bisMGD cofactor incorporation, which required expression of the PcrAB in the A. suillum PS native host (Youngblut, Tsai, et al., 2016; Youngblut, Wang, et al., 2016).
Five tips for [Fe–S] protein production
Subcloning the gene of interest into the vector of choice. pET vector is one of the most common vectors used for protein expression in bacteria. Many [Fe–S] proteins were expressed using His-tag as their affinity tag of choice. However, the potential risk in employing His-tag constructs for [Fe–S] proteins is that the metal chelate affinity purification process may chelate iron and thereby lower the occupancy of [Fe–S] cluster bound to the target protein (Daniels et al., 2000). Furthermore, the nonspecific binding of untagged protein may increase the complexity and time of purification process (Bornhorst & Falke, 2000). Alternatively, GST (glutathione S-transferase) tag and MBP (maltose-binding protein) tags may have more specific binding that can increase the purity of [Fe–S] protein, decrease the time of purification process, and increase the solubility of the [Fe–S] protein (Lebendiker & Danieli, 2017; Schafer, Seip, Maertens, Block, & Kubicek, 2015). Moreover, addition of 1–10mM reducing agents like dithiothreitol (DTT) or β-mercaptoethanol (BME) or tris(2-carboxyethyl)phosphine (TCEP) may help to stabilize [Fe–S] protein and increase binding affinity of GST fusion (Schafer et al., 2015). Glutathione used in GST elution buffer can provide additional oxidation resistance protection for [Fe–S] protein. If the binding affinity of GST-tagged protein is low, incubation of GST-fusion protein with the GST resin for 1h or slow application rate (0.5mL/min) or addition of stabilization agents (500mM NaCl and 10% glycerol) may help to increase the binding affinity.
Choosing host strains. BL21(DE3) strains are often a good candidate for bacterial host strain choice. For human [Fe–S] protein expression, Rosetta 2(DE3) host strain (Novagen) is a BL21 derivative designed to increase eukaryotic protein expression. Alternatively, yeast strains or other native strains can help the expression and solubility of [Fe–S] proteins (Pierik, Netz, & Lill, 2009; Youngblut, Tsai, et al., 2016). For difficult eukaryotic [Fe–S] protein or protein complex, insect cells Sf9 or Hi5 are the commonly used for protein expression and the Hi-5 insect cell is designed to enhance the protein expression level (Kidd & Emery, 1993). QB3 Macrolab from University of California, Berkeley developed baculovirus expression vectors with biobrick polypromotor, called MacroBac (Gradia et al., 2017), that is suitable for difficult eukaryotic [Fe–S] protein or multiprotein complex expression. The concept of MacroBac system was adapted from MultiBac system (Berger, Fitzgerald, & Richmond, 2004; Fitzgerald et al., 2006) along with the ligation-independent cloning technique (Aslanidis & de Jong, 1990) to provide efficient and robust cloning system for multiprotein complex expression. Other baculovirus expression system such as biGBac system (Weissmann et al., 2016) adapted from the Gibson assembly cloning concept (Gibson et al., 2008, 2009) was also developed.
Choosing the growth medium. Terrific broth (TB) was tested to produce at least 25% more [Fe–S] protein than minimal media (M9) and Luria-Broth (LB) for bacteria growth due to its higher buffering capacity when induced at high OD (OD600 >1) (Jaganaman et al., 2007).
Adding the supplement. When [Fe–S] protein expression level or [Fe–S] cluster occupancy is low, addition of 1mM cysteine and 1mM ferric ammonium citrate or ferric citrate during induction may help to boost the protein expression (Jaganaman et al., 2007).
Selecting the temperature for protein expression. We routinely screen temperature for protein expression in bacteria from 16°C to 37°C. We normally pick three to four temperatures to test, e.g., 16°C, 22°C, 28°C, and 37°C. For eukaryotic protein expression, we generally see better solubility and folding (i.e., less protein aggregation) at lower temperature (16°C or 22°C).
2.2 [Fe–S] Protein Purification: Use Anaerobic Hood and Check for Full Fe–S Occupancy
A dark brown color for concentrated sample is usually characteristic of [Fe–S] clusters ligated to cysteine residues. If an [Fe–S] protein solution turns a light yellow color, it is typically a warning sign of [Fe–S] cluster oxidation or loss. Although some [Fe–S] proteins were purified aerobically from many laboratories with some success due to the lack of an anaerobic chamber, the purification of [Fe–S] proteins anaerobically could be critical to successfully maintain the [Fe–S] cluster and enzyme activity, especially for more oxygen-labile [Fe–S] proteins like FNR (Yan & Kiley, 2009) and XPD (Fan et al., 2008). Here we share our tips to maintain full [Fe–S] cluster occupancy during purification.
The ideal way to purify an [Fe–S] protein is to purify it anaerobically in a well-designed anaerobic chamber with low oxygen level (<0.1ppm) based upon a reliable oxygen monitor. We have found that the vinyl anaerobic chamber from Coy Labs can provide a suitable anaerobic environment for reliable oxygen-sensitive protein preparation (Fig. 1A). The anaerobically chamber is typically equipped with an infuser (Coy Labs), which can constantly maintain H2 level (usually set at 3%) to ensure that the active palladium catalyst converts oxygen to water. Cells can be homogenized inside the glovebox by using a sonicator, and protein can be purified by using an AKTA FPLC system that is connected to the anaerobic chamber, as shown for our laboratory setup (Fig. 1A).
All buffer should be purged with Argon or N2 (≥99.999% purity with ≤1ppm O2) inert gas to minimize the oxygen level by using a Schlenk line manifold system with or even without an anaerobic chamber. Moreover, we suggest the use of the reducing agent TCEP, which has more resistance to oxidation in air (Burns, Butler, Moran, & Whitesides, 1991) compared to DTT (E= −0.33V at pH 7) (Cleland, 1964), BME (E=−0.28V at pH 7), and glutathione (E=−0.28V at pH 7) (Mickey & Howard, 1995). It is a good idea to include reducing agents in the buffer to scavenge oxygen and keep cysteine ligands reduced unless the [Fe–S] protein is sensitive to reducing agents, such as the disulfide bond-dependent protein pyruvate–ferredoxin oxidoreductase (Vita, Hatchikian, Nouailler, Dolla, & Pieulle, 2008).
The iron content of a purified [Fe–S] protein can be usefully measured by colorimetric assay (Cowart, Singleton, & Hind, 1993; Fish, 1988) or inductively coupled plasma mass spectrometry (ICP-MS) (Fox, Chakrabarti, et al., 2015; Yan & Kiley, 2009) to calculate the occupancy of [Fe–S] clusters.
In the case of low [Fe–S] occupancy, the [Fe–S] cluster may be reconstituted using chemical or enzymatic methods in vitro as noted here.
Fig. 1.
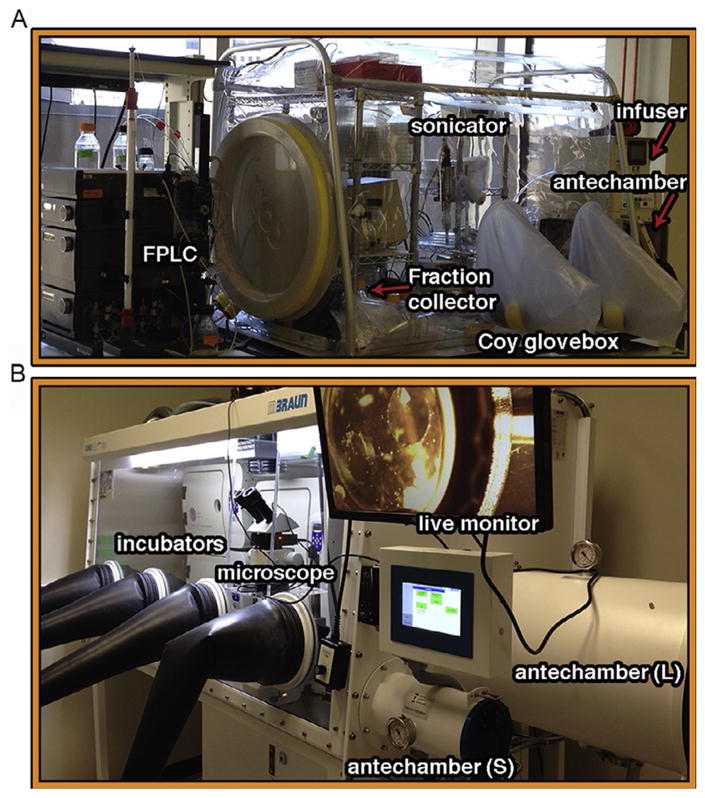
Glovebox setup for anaerobic purification and crystallization. (A) Cells can be homogenized inside the glovebox by using a sonicator. The supernatant is loaded onto FPLC columns through a superloop. Fractions are all collected inside the glovebox. An infuser from the CoyLab is used to automatically maintain the 3%H2/97%N2 level. (B) Exemplary setup for crystallizations and screening in the glovebox (<0.1ppm O2). Three incubators are included to maintain three different temperatures. Crystals, which are visualized and harvested under the microscope, can be immediately transferred out of the glovebox through the small antechamber for flash cooling.
For the chemical method, ferrous ammonium sulfate Fe(NH4)2 (SO4)2 (>100 M excess) and DTT (>100 M excess) are added to the apoprotein, followed by adding sodium sulfide Na2S (>100 M excess) dropwise into the mixture with stirring to prevent the immediate generation of insoluble Fe–S colloids. After the mixture is stirred for an additional hour, the excess iron and sulfide materials can be removed on a desalting column (Coghlan & Vickery, 1991; Malkin & Rabinowitz, 1966; Venkateswara Rao & Holm, 2004).
Alternatively, the enzymatic method is cleaner and more efficient. Basically, excess Fe2+ or Fe3+ (>10M excess), L-cysteine (>10M excess), and DTT (>100M excess) are added to the apoprotein. The assembly reaction is initiated by adding catalytic amount of cysteine desulfurase (IscS). IscS generates persulfide from L-cysteine, followed by transferring to apoprotein for [Fe–S] cluster assembly process (Agar et al., 2000; Fox, Chakrabarti, et al., 2015; Fox, Das, et al., 2015; Kiley & Beinert, 2003). Excess materials can then be removed on a desalting column.
3. [Fe–S] PROTEIN CRYSTALLIZATION, DATA COLLECTION, AND STRUCTURE DETERMINATION
3.1 Considerations and Strategies for Anaerobic Crystallization
High-resolution X-ray structures provide unmatched detail that often uniquely enables insights into the biological activity and function of a given [Fe–S] cluster protein. However, the loss of [Fe–S] cluster due to oxidation may result in structure disorder that impacts the protein function. For example, [4Fe–4S] cluster acts in the structure ordering role in [Fe–S] cluster domain of XPD helicase. The loss of [4Fe–4S] cluster reveals a disordered structure that significantly decreases XPD catalytic activity and DNA-binding affinity (Fan et al., 2008). Therefore, anaerobic crystallization of an [Fe–S] protein is a key step to obtain the intact protein structure, especially for oxygen-labile [Fe–S] proteins such as XPD, IRP1, and PcrAB (Dupuy et al., 2005; Fan et al., 2008; Youngblut, Tsai, et al., 2016). The order or full occupancy of [Fe–S] cluster in the structure depends on how sensitive of [Fe–S] cluster is to oxygen. And the level of oxygen sensitivity can depend upon shifts in the [Fe–S] redox potential, such as occurring from changes in the electrostatic environment of the cluster upon DNA binding, as seen for XPD, MutY, and endonuclease III (Boon, Livingston, Chmiel, David, & Barton, 2003; Gorodetsky, Boal, & Barton, 2006; Sontz, Mui, Fuss, Tainer, & Barton, 2012). When the purification and crystallization are performed in air, there is always a question whether [Fe–S] protein remains stable and functional. E.g., perchlorate reductase PcrAB lost its brown color and activity (i.e., loss of [Fe–S] clusters) after overnight incubation at 4°C in air. For oxygen-sensitive [Fe–S] proteins, such as PcrAB, the crystallization buffer must be purged with N2 or argon, and crystallization trials must be set up in the anaerobic chamber (Fig. 1B).
Tips for setting up anaerobic crystallization are outlined below based upon our experience.
Without an anaerobic chamber, the crystallization buffer should minimally be purged with high purity N2 or argon to prolong the half-life of [Fe–S] clusters.
It is usually a good idea to include 0.5–2.0mM TCEP and/or 1–10mM DTT and/or 5–20mM BME in your protein buffer to help keep cysteines reduced and slow down the oxidation of any [Fe–S] clusters.
With an anaerobic chamber, the crystallization screening solution in a 96-deep-well block and any necessary crystallization plates, pipette tips, and reagents should be brought into the anaerobic chamber at least 3 days in advance. This is important to allow the chamber catalyst to remove most of the dioxygen from the plastic or solution before setting up crystallization trials. If the screening solution cannot be preequilibrated in the glovebox a few days in advance, you can equilibrate the screening solution (50–100μL) by exposing it to high purity N2 in the glovebox for 30min to exchange the oxygen after being pipetted into the crystallization plate.
For setting up sparse-matrix crystallization screening trials, a multi-channel pipette is usually efficient, which usually takes about 10min per plate, and 0.5–17mu;L protein per drop for good accuracy on pipetting. For some less oxygen-sensitive [Fe–S] proteins, crystallization screening trials can be setup with pipetting robot located outside the anaerobic chamber (note: it is preferable if the robot can locate inside the chamber), followed by immediately bringing the crystallization plate into the anaerobic chamber for incubation. The robot offers the advantage of less time (about 1–2min per plate) and lower amounts of protein (0.1–0.27mu;L per drop, 10–207mu;L protein per plate) if the protein sample is limited. For this method, crystallization screens should be preequilibrated in the glovebox using tip #3 above. After the plates have been set up by the robot, the plates should be sealed tightly with the optical transparent sealing film and clamped with large paper clips to prevent the evaporation during evacuation cycle using the vacuum pump (Fig. 2A).
For growing crystals in the reduced state, a glovebox is optimal. E.g., PcrAB crystals were grown in an oxidized state where Mo atom is in +6 oxidation state (Fig. 2B). To reduce the oxidation state, a reducing agent like sodium dithionite (Na2S2O4, E =−0.66V at pH 7) or zinc powder (E=−0.76V at pH 7) combined with the electron carrier methyl viologen (E=−0.446V) can be used to reduce [Fe–S] clusters (Mayhew, 1978; Najmudin et al., 2008; Youngblut, Tsai, et al., 2016). To prepare reduced methyl viologen solution (10mM), sodium dithionite (100mM) or excess Zn powder (100mg) is added into 1mL of methyl viologen solution in tube. The solution should turn deep-blue color (absorption 600nm, ε600 =8.25mM−1cm−1) when the methyl viologen is reduced (Yu & Wolin, 1969); it turns colorless when it is oxidized. The excess Zn powder can be removed by centrifugation, and the supernatant containing reduced methyl viologen is then added into the protein solution or crystals for reduction (Najmudin et al., 2008; Youngblut, Tsai, et al., 2016). Reduced methyl viologen can be added to the purification or assay buffer to scavenge oxygen and maintain the anaerobic condition by monitoring the blue color change.
A microscope can be brought into the anaerobic chamber or mounted on the panel of the anaerobic chamber for efficient screening of crystallization trays (Fig. 1B). Automatic crystallization plate tracking and imaging system provides periodically recorded images that can be stored and analyzed from each crystallization drop to monitor the crystal growth over time (Mayo et al., 2005).
Fig. 2.
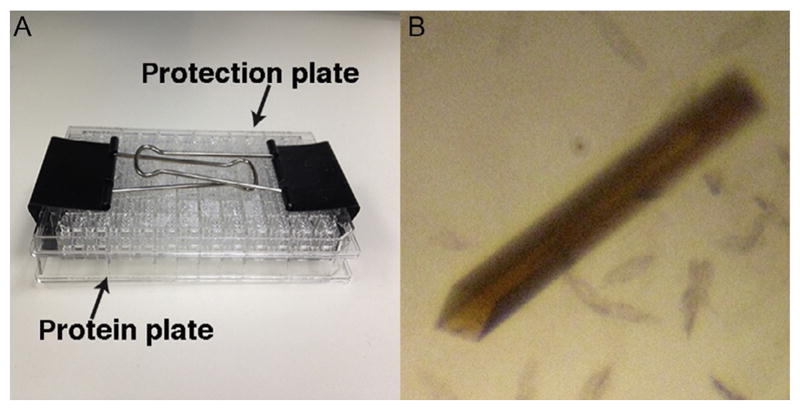
Anaerobic crystallization setup and transfer. (A) Simple but effective setup to bring a setup crystallization plate into the glovebox for less oxygen-sensitive [Fe–S] proteins using pipetting robot located outside the glovebox. The protection plate was placed on top of a protein crystallization plate and clamped down with paper clips to prevent evaporation from the drops and reservoir during the vacuum pump evacuation cycle. (B) Oxygen-sensitive PcrAB crystal grown in the glovebox at −20°C. The brown crystal color shows the presence of intact [Fe–S] clusters.
3.2 Protocols for Anaerobic Crystal Mounting
Mounting crystals with those thick gloves attached to the anaerobic chamber are quite challenging. Often crystallographers replace the standard gloves with more flexible ones that can be clamped off when not in use. They also may bring in liquid nitrogen into the glovebox to freeze the protein crystals. Normally, the liquid nitrogen in the dewar is placed in the antechamber. The antechamber is then evacuated to about halfway of the vacuum pressure gauge reads, followed by refilling. By repeating this process for ~10–15 times, we found it challenging to bring liquid nitrogen into the glovebox without contaminating the glovebox with some dioxygen. An alternative is to employ the room temperature mounting kit from MiTeGen (MicroRT capillary system), which is usually used to test diffraction without cryoprotectant or to do controllable crystal dehydration experiments. We found the MiTeGen kit to be a suitable tool to mount crystals anaerobically. Drawbacks are that only one crystal is mounted and taken out at a time from the glovebox, and it may not work well if you are doing time-resolved crystallography due to the longer transfer time.
Protocol for mounting crystals anaerobically with a room temperature mounting kit
To harvest the crystals from 96-well crystallization plates is usually harder anaerobically. You need to cut off the tape and mount your crystal with thick gloves or with replaced more sensitive gloves that may allow oxygen into the chamber. Alternatively, if you can optimize the crystal conditions for a screw-cap crystallization plate (Qiagen EasyXtal 15-Well Tool). This will then be much easier to work with when mounting the crystals.
Before mounting the crystals, pipette 40μl of artificial cryoprotectant solution into the MicroRT capillary with a gel-loading pipette tip to maintain the crystal hydration.
Pipette 10μL of cryoprotectant solution on the siliconized glass cover slide (Hampton Research). Transfer single crystals from the drop into cryoprotectant solution (only if your crystals need added cryoprotectant).
Mount the crystal with a loop that has been inserted into an 18-mm MiTeGen goniometer base (MiTeGen B3S) with a magnetic wand. Slide the capillary tubing past your crystal and onto the goniometer base with the MicroRT aligner. The cryoprotectant solution inside the MicroRT capillary should prevent the dehydration of the mounted crystal for a few hours, which allows the time to transfer the mounted crystal out of the glovebox.
Fasten the magnetic wand on the aligner with a laboratory labeling tape to secure the position and transfer the crystal out of the anaerobic chamber.
Carefully remove the capillary tubing without touching the crystal, and flash cool it in liquid nitrogen. This procedure was used for anaerobic [Fe–S] protein crystals that have yielded a 1.86Å resolution structure with low mosaic spread (0.2 degrees) (Youngblut, Tsai, et al., 2016).
3.3 Data Collection Strategy for [Fe–S] Proteins Employing Fe Phasing With Minimum Exposure and Maximum Redundancy
A key benefit of [Fe–S] proteins for structure determination is that you may be able to use the Fe anomalous signal to solve the phase problem (Bowman, Bridwell-Rabb, & Drennan, 2016), provided you are careful to avoid loss of the damage-sensitive anomalous signal. The Fe anomalous absorption peak is at ~7112eV (1.7433Å ), which is accessible at most of synchrotron beamlines, such as SIBYLS at the Advanced Light Source (Classen et al., 2013).
Protein crystals with heavy atoms are often more sensitive to radiation damage. In the case of PcrAB, each PcrAB heterodimer contains 20 Fe atoms and 1 Mo atom. When the protein crystal suffers radiation damage, diffraction spots at a high-resolution shell start to fade away quickly, but even more importantly, the anomalous signal is lost before the diffraction peak disappears. You can compare images to see if the diffraction spots in the higher resolution shells are fading. Image quality including radiation damage should be carefully assessed by checking the Rsym to see if the number increases dramatically. Additionally, X-ray absorption spectroscopy (XAS) can be measured to assess the dose-dependent photoreduction of crystals (Corbett et al., 2007). We recommend that diffraction data are collected using minimal exposure and with high redundancy ~20-fold. Notably, collecting the data repeatedly at the same spot in the crystal for over 360 degrees will increase redundancy, but may not improve data quality. Ideally, multiple datasets from different spots in the same single crystal can be merged to increase redundancy and improve data quality. For an optimal anomalous data collection strategy, collecting 30 degrees wedges for peak wavelength with a reverse beam for total 360 degrees is followed by remote and inflection wavelength data collection. This strategy ensures at least a complete SAD dataset at peak wavelength in case radiation damage happens more quickly than completion of multiple wavelength datasets. Alternatively, you can use helical (Flot et al., 2010) or vector (Hilgart et al., 2011) data collection strategies. These minimize radiation damage by collecting images helically around your crystals or by collecting from two endpoints of your crystals. These strategies work if your crystal is long enough to obtain a complete dataset by moving along the crystal length, and the beamline is capable of these data collection strategies. Overall, the key to collect good diffraction data from [Fe–S] protein crystals is to minimize the radiation damage by collecting data quickly (with the shortest practical exposures) and with high redundancy.
3.4 Structure Determination: Proper Refinement to Maintain Cluster Geometry and Occupancy
Improper structure determination and refinement may distort the geometry of [Fe–S] clusters. Proper refinement is important as active sites may show actual functional distortions in the [Fe–S] clusters: you will wish to distinguish possible small functional distortions from poor refinement practices. Experimental phases can be obtained from an anomalous dataset by using heavy-atom phasing and refinement with Phenix.autosol (Terwilliger et al., 2009), SHARP (Bricogne, Vonrhein, Flensburg, Schiltz, & Paciorek, 2003), or SHELX (Sheldrick, 2008). Alternatively, molecular replacement (MR) using homology models (generally >30% sequence identity) by PHASER (McCoy et al., 2007) or other MR programs in CCP4 suite (Winn et al., 2011) may be a good method to develop structure solutions. For difficult structure solution, especially for a low-resolution dataset or poor homology models (<30% sequence identity), MR combined with structure-modeling software Rosetta (Das & Baker, 2008) may help to generate a better solution (DiMaio et al., 2011). Once an electron density map is generated, proper refinement with correct cluster geometry needs to be carefully done.
Restraints of geometry for the [Fe–S] cluster should be included in programs like refmac (Murshudov, Vagin, & Dodson, 1997) or Phenix.refine (Afonine et al., 2012), where typically the ideal Fe–S bond distance is set as 2.30Å with sigma 0.05 for bond distance and no restraint is set for bond angle. Besides the geometry of [Fe–S] clusters, occupancy and B-factor of [Fe–S] clusters should be refined as well. Changing the occupancy of each [Fe–S] cluster to below 1 (e.g., 0.95) will allow the Phenix.refine to refine the occupancy automatically based on your experimental data or you can specifically refine the occupancy of a particular cluster by selecting its atoms in the Phenix.refine parameter file. Observation of electron density maps to check positive and negative difference electron density peaks around the [Fe–S] clusters allows checks of Fourier truncation effects and the Fe atom position, occupancy, and temperature factor. More refinement cycles with refinement of occupancy and anisotropic atomic displacement parameters of the metal ions may help to resolve these parameters, but do not resolve Fourier truncation ripples. Furthermore, the electron density map of [Fe–S] cluster should show good to strong peaks that indicate little to no radiation damage was produced (Fig. 3). The proper refinement of [Fe–S] cluster structures may reveal the extend to which their distortion impacts enzymatic activity and biological function (Bowman et al., 2016). Such distortion can be further measured by Mossbauer spectroscopy (Pandelia, Lanz, Booker, & Krebs, 2015) and XAS (Einsle, Andrade, Dobbek, Meyer, & Rees, 2007; Ha et al., 2017) to evaluate the coordination geometry, solvation, and redox states of [Fe–S] clusters. These data can then be tested by computational modeling to investigate the electronic structures of the [Fe–S] clusters (Fee et al., 2003; Sandala, Hopmann, Ghosh, & Noodleman, 2011; Shomura, Yoon, Nishihara, & Higuchi, 2011).
Fig. 3.
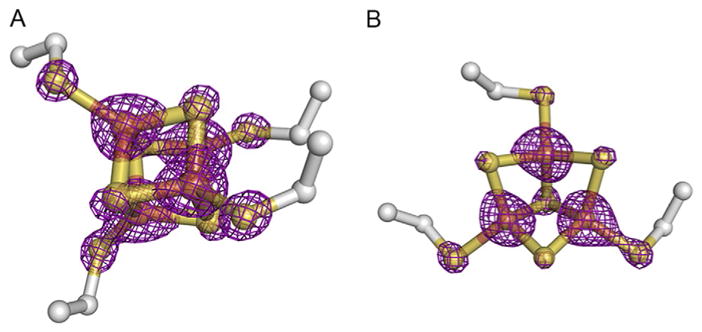
Electron density maps of (A) [4Fe–4S] (FS1) and (B) [3Fe–4S] (FS4) cluster of PcrAB (PDB: 4ydd). The 2Fo–Fc CCP4 map was generated using Phenix.fft with a grid resolution factor of 0.167. The graphic figure was contoured at 7.0 sigma and prepared on PyMOL. Comparatively, the electron density of the [4Fe–4S] cluster shown here suggests that it may suffer less radiation damage than the [3Fe–4S] cluster, reflecting the direct solvent exposure of the [3Fe–4S] cluster.
4. STRUCTURAL ANALYSES: WHAT TO LOOK FOR IN Fe–S CLUSTER STRUCTURES: CASE STUDIES IN TYPE II MO-BISMGD ENZYMES
Structural analysis can be critical to provide insight into protein function and mechanism. For example, DNA binding to EndoIII and MutY glycosylases shifts the redox potential of their [4Fe–4S] clusters into physiological range to form a redox-active state (Boal et al., 2005; Boon et al., 2003). This shift results in part by the position of the DNA polyanion near the [Fe–S] cluster with accompanying structural changes. These changes include significantly altering Fe–S cluster loop (FCL) between the first two ligating cysteines that reside along the DNA-bound interface, as were revealed by the crystal structures of EndoIII and MutY (Chepanoske, Golinelli, Williams, & David, 2000; Fromme & Verdine, 2003; Guan et al., 1998; Kuo et al., 1992; Thayer, Ahern, Xing, Cunningham, & Tainer, 1995). These electrostatic and protein structural changes create a large increase in the covalency of the Fe–S bond upon DNA binding, which can be measured by sulfur K-edge XAS, despite the similarity of their [Fe–S] cluster structures with and without DNA (Ha et al., 2017). At the same time, some [Fe–S] protein structure domain folds depend upon their [Fe–S] clusters, such as Sulfolobus acidocaldarius XPD (Fan et al., 2008), human primase (Baranovskiy et al., 2015; Vaithiyalingam, Warren, Eichman, & Chazin, 2010), Bacillus subtilis AddAB (Saikrishnan et al., 2012), human exo-nuclease 5 (Sparks et al., 2012), and Mus musculus DNA2 (Zhou, Pourmal, & Pavletich, 2015). Loss of such [Fe–S] clusters may cause domain disorders that decrease DNA-binding affinity and lead to functional defects. Indeed, metal site loss or defects may in general cause decreased fold stability and even human diseases, as seen, for example, for human superoxide dismutase (Pratt et al., 2014).
For [Fe–S] cluster-containing respiratory protein complexes, we herein compare three enzymes (perchlorate reductase PcrAB, nitrate reductase NarGHI, and ethylbenzene dehydrogenase, EbdABC). These enzymes are key members of the type II DMSO reductase family, which commonly contains the signature bis (molybdopterin guanine dinucleotide)Mo cofactor (Mo-bisMGD) and an Asp-Mo ligand (Iobbi-Nivol & Leimkuhler, 2013; Jormakka, Richardson, Byrne, & Iwata, 2004; McDevitt, Hugenholtz, Hanson, & McEwan, 2002; Moura, Brondino, Trincão, & Romão, 2004; Schindelin et al., 1996; Schwarz, Mendel, & Ribbe, 2009).
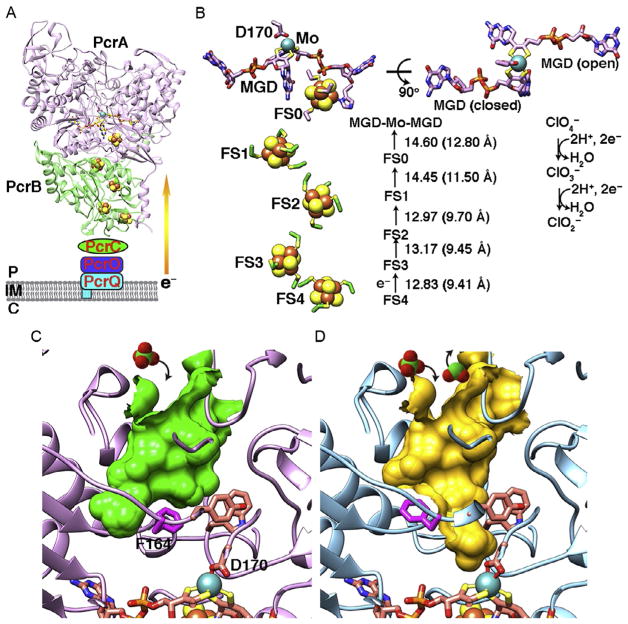
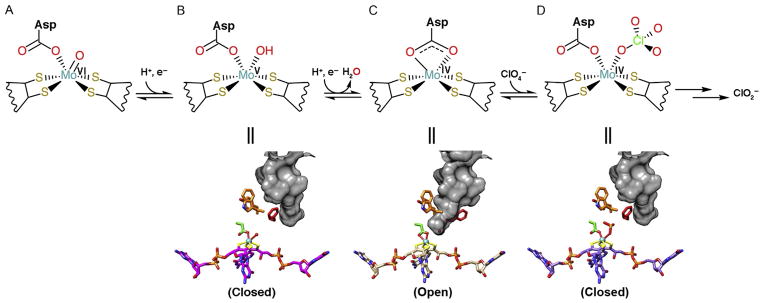
Interestingly, these three protein complexes furthermore have as a hallmark five [Fe–S] clusters that are responsible for electron shuttling to Mo atom or to heme cofactor (Figs. 4, 6, and 7). PcrAB is an anaerobic respiratory protein found in dissimilatory (per)chlorate-reducing bacteria that use (per)chlorate as terminal electron acceptors by converting perchlorate to chlorate and then to chlorite (Bender et al., 2005; Coates & Achenbach, 2004; Youngblut, Wang, et al., 2016). PcrAB from A. suillum PS is a peri-plasmic heterodimeric protein complex that contains a Mo-bisMGD and [4Fe–4S] cluster (FS0) in PcrA, plus three [4Fe–4S] clusters (FS1–3) and one [3Fe–4S] cluster (FS4) in PcrB, respectively (Fig. 4A). The reduction of perchlorate to chlorite requires two separate 2e− transfer reactions that may be transferred from heme-containing proteins PcrQ, PcrO, and PcrC to PcrB and PcrA (Fig. 4A) (Youngblut, Tsai, et al., 2016; Youngblut, Wang, et al., 2016). It remains elusive if the PcrAB forms a complex with heme proteins PcrC, PcrO, and PcrQ to thereby resemble the hetero-trimeric cytosolic nitrate reductase cNarGHI (Bertero et al., 2003). The distances between the five [Fe–S] clusters and the Mo atom can range from 9.41 to 12.8Å (Fig. 4B), which are similar to the electron transfer distance in respiratory complex I (Hayashi & Stuchebrukhov, 2010) and fall within the proposed limit of 14Å for an electron transfer distance (Page, Moser, Chen, & Dutton, 1999). In the oxidized form of PcrA, the substrate tunnel to the Mo-bisMGD active site is closed by the gate residue F164 (Fig. 4C). Furthermore, here we identified the surface topography of these proteins through the CASTp server (http://sts.bioe.uic.edu/castp/index.php), which also shows volume and area of the protein pockets and cavities (Liang, Edelsbrunner, Fu, Sudhakar, & Subramaniam, 1998a, 1998b; Liang, Edelsbrunner, & Woodward, 1998). We also identified gate residues by carefully examining the residues lining the tunnel to the active site. During catalysis, ligand D170 shifts from a mondentate to the bidentate coordination state when the Mo atom is reduced, and the substrate tunnel is opened via the shifting of F164 to different rotamers (Figs. 4C–D and 5). The F164 gate residue controls substrate entry and product release to enhance the catalytic efficiency when the (per)chlorate concentration is limited in the bacterial environment (Youngblut, Tsai, et al., 2016; Youngblut, Wang, et al., 2016). Indeed, this explains why PcrAB has a relatively high perchlo- rate affinity (Km =6 μM) and substrate inhibition over 200 μM compared to nitrate reductase NarGHI, which has a lower perchlorate affinity (Km =1.1mM). In fact, mutation of F164A in A. suillum PcrA resulted in a loss of its ability to grow by (per)chlorate respiration (Youngblut, Tsai, et al., 2016).
Fig. 4.
PcrAB structure from Azospira suillum PS containing a Mo-bisMGD cofactor plus five [Fe–S] clusters (PDB: 4ydd and 5ch7). (A) Periplasmic PcrAB is proposed to receive electrons from membrane-bound heme protein PcrQ and two heme-binding proteins PcrO and PcrC via quinone oxidation. The electrons are then transferred to downstream [Fe–S] clusters and Mo-bisMGD for (per)chlorate catalysis. Labels show P (periplasm), IM (inner membrane), and C (cytoplasm). (B) The layout of the active site Mo-bisMGD, conserved Asp170 ligand, and [Fe–S] clusters. MGD exhibits tricyclic pterin (closed) and bicyclic dihydropterin (open) forms in both oxidized and reduced Mo states. The distances between each cofactor are shown. The edge-to-edge cluster distance is calculated from the closest two atoms of two neighboring cofactors; center-to-center distance is calculated from the average of the longest+shortest distance between two cofactors. (C) The substrate tunnel (green surface) is unveiled in oxidized PcrA (PDB: 4ydd), while F164 is in the closed conformation. (D) PcrA is in the reduced state (PDB: 5ch7); D170 was shifted from monodentate to the bidentate coordination state. F164 rotates to a different conformation and opens the substrate tunnel (yellow surface) into the active site for (per)chlorate catalysis. Substrate perchlorate and product chlorite are shown in space-filling models.
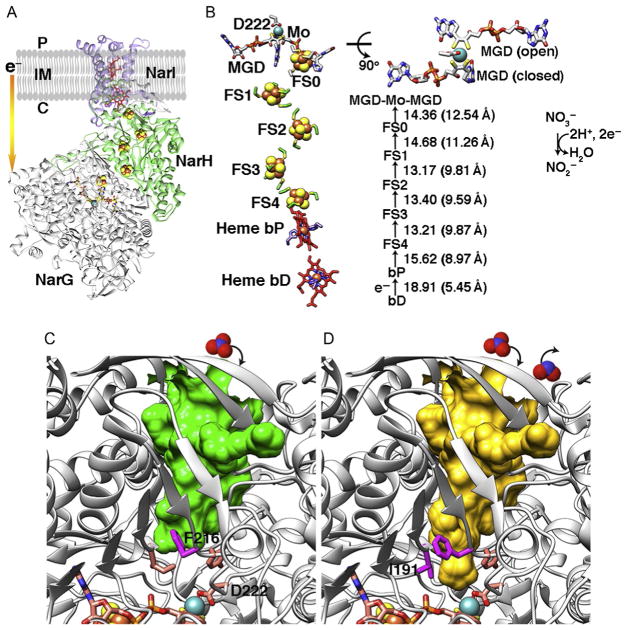
Fig. 6.
Cytosolic NarGHI structure from E. coli (PDB: 1q16) and comparative implications from PcrAB structures. (A) Overall structure of cNarGHI. The membrane-bound b-type heme protein NarI transfers electrons from the quinone pool to the cytosolic-facing nitrate reductase NarGH via the [Fe–S] clusters. (B) The layout and cofactor distances in cNarGHI are shown. MGD cofactor shows open and closed forms resembling those found in PcrA. (C) cNarG is in the closed state. Based upon our data and analyses, residue F216 is predicted to control the substrate tunnel access. (D) I191 may also be required to change conformation along with F216 to open full access to the active site. Substrate nitrate and product nitrite are shown in space-filling models (top).
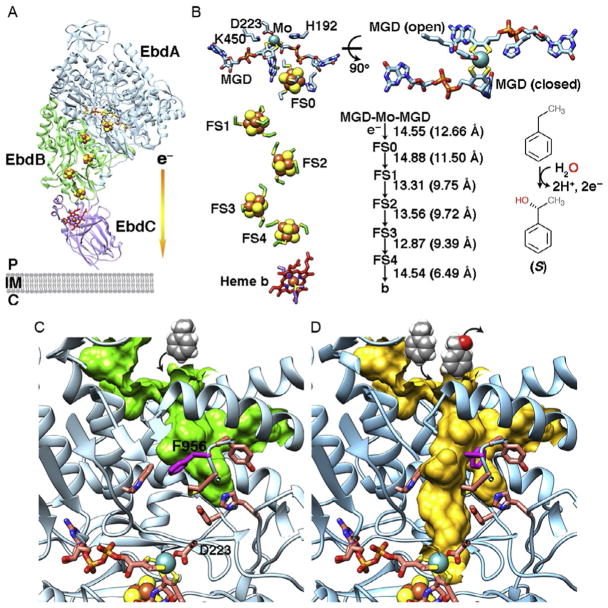
Fig. 7.
EdbABC structure from Aromatoleum aromaticum (PDB: 2ivf). (A) The overall structure of EdbABC. EbdABC is a periplasmic heterotrimer. The terminal electron acceptor is still unknown. (B) The layout and distances of cofactors and active site. Residues D223, K450, and H192 that act in catalysis are shown. MGD cofactors show the bicyclic dihydropterin open state and tricyclic pterin closed forms in the crystal structure. The electrons are transferred from Mo-bisMGD to the [Fe–S] clusters to heme b cofactor by converting ethylbenzene to (S)-1-phenylethanol. (C) Residue F956 is predicted to gate the substrate tunnel to active site. (D) The switch of F956 to a different conformation allows substrate access to the active site for catalysis. Substrate ethylbenzene and product (S)-1-phenylethanol are shown in space-filling models.
Fig. 5.
Dissecting the mechanism of (per)chlorate catalysis based on PcrAB structures. PcrAB structures reveal different redox states corresponding to each catalytic step (A–D labels). The gate residue F164 (red sticks) controls the substrate/product access (shown in gray surface) to the Mo-bisMGD active site. In step (D) the substrate analog SeO32− bound to Mo atom was visible in the structure. Notably, the Asp side chain (green sticks with red oxygen atoms) shifts coordination states from monodentate to bidentate and back to monodentate in order to maintain a six-coordination geometry with the Mo atom.
Comparative examination of [Fe–S] protein structures aids the identification of functional vs circumstantial elements. We therefore examined the [Fe–S] clusters, Mo-bisMGD cofactor, and substrate tunnels in cytosolic nitrate reductase cNarGHI (Bertero et al., 2003) and periplasmic ethylben-zene dehydrogenase EbdABC (Kloer, Hagel, Heider, & Schulz, 2006) (Figs. 6 and 7). For cNarGHI, the layout of the [Fe–S] clusters and Mo-bisMGD active site in cNarGH resembles PcrAB (Fig. 6A and B). In fact, nitrate reductase can also reduce perchlorate and chlorate at a slower rate than nitrate (Marangon et al., 2012; Youngblut, Tsai, et al., 2016). The reduction of nitrate requires 2e− from quinone pool oxidation that generates a 2H+ proton-motive force from cytoplasm to periplasm and delivers 2e− to cNarI heme protein, to cNarH, and then to cNarG (Bertero et al., 2003). It was proposed that formate dehydrogenase transfers 2e− to the quinone pool and forms a redox loop to cytosolic nitrate reductase (Bertero et al., 2003; Jormakka, Tornroth, Byrne, & Iwata, 2002). Using comparative analyses and the above methodology, we identify here the gate residue F216 in E.colicNarG.ShiftoftheF216rotamerconformationissuitabletoenableaccess of nitrate substrate to the Mo-bisMGD active site for catalysis (Fig. 6C and D).
The crystal structure of ethylbenzene dehydrogenase EbdABC from Aromatoleum aromaticum forms a heterotrimeric complex that is located in periplasm (Johnson, Pelletier, & Spormann, 2001; Kloer et al., 2006) (Fig. 7). EbdABC catalyzes oxygen-independent oxidation on hydrocarbon by hydroxylating ethylbenzene to (S)-1-phenylethanol. This generates 2H+ and 2e− that are transferred to unknown terminal electron acceptors (Szaleniec et al., 2007). It was proposed that EbdABC generates a redox loop by transferring electrons to the quinone pool and then to cytosolic nitrate reductase for nitrate respiration (Kloer et al., 2006). Using CASTp surface topography search, we predict here that the substrate tunnel is gated by residue F956 (Fig. 7C). A different rotamer conformation of F956 is suitable to open access of the substrate tunnel from outside the active site (Fig. 7D). This observation supports the feasibility of identifying the gate residue for type II Mo-bisMGD enzymes based on comparative structural analyses that include the [Fe–S] sites and protein surface topography.
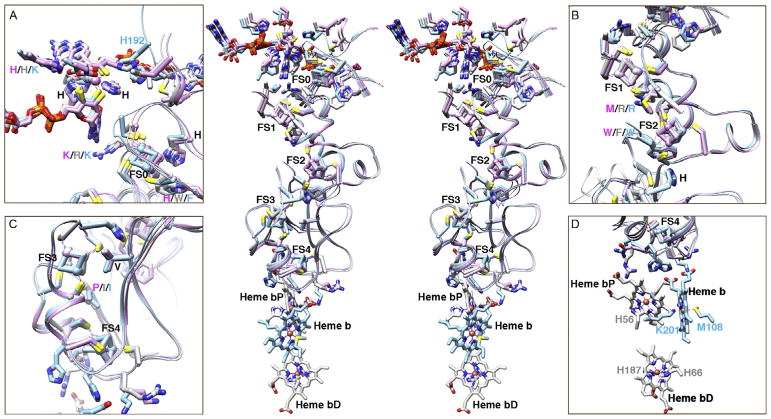
It remains difficult to predict the existence and function of [Fe–S] clusters based on the protein sequence, as there is no conserved protein motif or domain for all [Fe–S] clusters (Fuss et al., 2015; Lill, 2009). In fact, the prediction of all metal-binding sites remains challenging (Cvetkovic et al., 2010; Yannone et al., 2012). Therefore, structures become a critical tool to identify, interpret, and investigate the existence and function of [Fe–S] clusters. Here, we demonstrate the power of comparative metalloenzyme structural analyses. We superimpose the structures of type II Mo-bisMGD enzymes PcrAB, cNarGHI, and EbdABC to assess the function from ligands and [Fe–S] cluster environments (Fig. 8). A common feature of [Fe–S] clusters consists of conserved cysteine ligands. In type II Mo-bisMGD enzymes, FS0 contains specific H/CxxxCxxxC(x)nC sequence motif except EbdA (Coelho & Romão, 2015; Jormakka et al., 2004) (Figs. 4 and 6–8). The non-cysteine ligands in the [Fe–S] protein are seen in many proteins, and their unique features tune the redox potential of the clusters for diverse protein functions (Bak & Elliott, 2014). For example, the His to Cys mutation of FS0 in cNarG shows that enzyme activity is compromised due to a decrease in the redox potential by at least 500mV (Magalon et al., 1998; Rothery et al., 2010). The neighboring Arg/Lys residue between FS0 and Mo-bisMGD also plays a critical role for mediating the electron transfer and enzyme activity (Rothery et al., 2010) (Fig. 8A). Interestingly, the loop containing H192 in the EbdA structure shifts closer to the Mo-bisMGD cofactor for catalysis, while the loops in PcrA and cNarG stay away (Fig. 8A).
Fig. 8.
Unified insights for a fundamental mechanistic understanding of selectivity and regulation come from comparative superimposition of structures of PcrAB (PDB: 4ydd, purple), cNarGHI (PDB: 1q16, light gray), and EdbABC (PDB: 2ivf, sky blue). Stereo view (center panel) of the superimposed structures (center) reveals striking structural conservation. Close-up views of the Mo-bisMGD and [Fe–S] clusters are shown in boxes A–D. Superimposition of the structures was based on the PcrB as the reference structure and used Chimera software. RMSD between 228 pruned atom pairs out of 326 pairs is 0.779Å for cNarH; RMSD between 281 pruned atom pairs out of 324 pairs is 0.786Å for EbdB.
The residues around [Fe–S] clusters in the respiratory protein shown here are mostly hydrophobic with positively charged His or Lys or Arg residues between the clusters (Fig. 8). Interestingly, many His/Arg/Lys residues are positioned between [3Fe–4S] cluster and heme b cofactor that may facilitate the electron transfer (Kloer et al., 2006; Rothery, Blasco, & Weiner, 2001), although the roles of those residues remain to be tested (Fig. 8D). Therefore, we show here how attentive examination of the [Fe–S] cluster environment including geometry, ligands, fold, surrounding residues, and surface topography may prove critical to understanding the function of [Fe–S] clusters in proteins.
5. SUMMARY AND PROSPECTS FOR ADVANCES
The great importance of [Fe–S] cluster-containing enzymes for cellular energy and for controlling protein conformations (Beinert, 1997) makes the ability to prepare and study these proteins with intact [Fe–S] clusters central to biochemistry, bioinorganic chemistry, and cell biology. As [Fe–S] clusters predate the origin of an oxygen atmosphere, they became critical in cell biology before dioxygen sensitivity became a possible evolutionary selection. Yet, humans and other eukaryotes have surprisingly not only retained [Fe–S] clusters but furthermore made them a requirement for DNA replication, transcription, and repair as well as for genome integrity (Fuss et al., 2015).
These eukaryotic [Fe–S] clusters are formed in the mitochondria and are critical for mitochondrial metabolism (Lill et al., 2012; Rouault, 2015), showing that eukaryotes captured and kept the ability to make and use these clusters from microbes. Indeed, disruption of [Fe–S] cluster assembly biosynthesis results in human diseases, as typified by Friedreich ataxia (Martelli & Puccio, 2014; Rotig et al., 1997; Stemmler, Lesuisse, Pain, & Dancis, 2010; Tsai, Bridwell-Rabb, & Barondeau, 2011). Furthermore, the proper formation of [Fe–S] cluster assembly complexes in mitochondria is connected via an allosteric regulation between respiratory chain complexes and apoptosis-inducing factor that links mitochondrial redox state to cell growth vs apoptosis (Brosey et al., 2016). These observations underscore the unique and powerful features of [Fe–S] clusters and their electron transfer abilities that have evidently provided strong evolutionary pressure to not only keep and furthermore to expand [Fe–S] cluster uses for cell biology, despite associated oxygen sensitivity.
Ironically, PcrAB [Fe–S] clusters function to create oxygen, which can damage the PcrAB [Fe–S] cluster, by reducing perchlorate to chlorite such that chlorite dismutase transforms the chlorite into chloride and oxygen. Notably, oxygen biogenesis initiated by PcrAB thus provides an intriguing early direct metabolic link between aerobic and anaerobic metabolisms (Coates & Achenbach, 2004; Coates et al., 1999; Rikken et al., 1996). As perchlorate respiration likely developed before oxygen production by photosynthesis (and oxygen respiration), PcrAB reveals a chemistry suitable for the initial origin of an oxygen atmosphere and for providing provocative insights into when and how life may have adapted to oxidizing conditions (Nerenberg, 2013).
Besides possibly contributing to the evolution of the oxygen atmosphere, the widespread occurrence of perchlorate as a pollutant, in arid regions on Earth, and on Mars (Jackson, Davila, et al., 2015; Ming et al., 2014) highlights the need to understand the basic geochemical cycling of perchlorate as well as the related chemistries of other Mo-bisMGD enzymes. Furthermore, the use of microbes and their PcrAB enzymes provides an effective means for environmental removal of perchlorate (Xu, Song, Min, Steinberg, & Logan, 2003). On Mars, the activity of PcrAB makes the ClO4− ion with its central chlorine atom surrounded by four tetrahedral oxygen atoms, a possible rich oxygen source. Bio-beads or other stabilized constructs with purified PcrAB plus chlorite dismutase could transform perchlorate on Mars to form O2 for astronauts and fuel as the measured amounts of perchlorate in Martian regolith appear sufficient to make this process feasible (Davila, Willson, Coates, & McKay, 2013).
Here exemplary structural analyses of multiple structures of the five [Fe–S] clusters and molybdenum cofactor enzyme perchlorate reductase uncovered new biology and aided the accurate definition of the mechanism for multi-[Fe–S] cluster electron transfer in this and related systems. In considering these and other metalloenzyme results, it is important to recall the need for rigorous refinement of [Fe–S] clusters and for considering the coupled challenges from accurately modeling bound solvent and flexible regions. All existing crystal structures contain errors in refined models for flexible regions and bound solvent that greatly exceed the errors in the crystallographic data, so modeling and refining these aspects merit careful attention (Holton, Classen, Frankel, & Tainer, 2014). Going forward, the combination of X-ray crystallography with other methods such as X-ray scattering in solution may be key to defining the protein structure with flexibility and bound solvent (Rambo & Tainer, 2013a, 2013b). X-ray scattering from [Fe–S] cluster sites, as well as other metal sites such as gold, may allow direct examination of metal site distances (Grishaev, Anthis, & Clore, 2012; Hura et al., 2013). In part, bound waters and ligands, two of the parameters analyzed here for [Fe–S] cluster proteins, depend upon protein surface topology (Kuhn et al., 1992) that is influenced by side-chain flexibility (Schnecke, Swanson, Getzoff, Tainer, & Kuhn, 1998). Importantly, the high-resolution structures and combined mutational analyses, as obtained with the anaerobic approaches described here, are sufficient to discover an informed mechanistic model. Such detailed structures are furthermore key to the design of metal sites in proteins including antibodies (Roberts et al., 1990; Tainer, Roberts, & Getzoff, 1991), which has recently become a rapidly advancing area of science and engineering, as seen for protein cages (Lai et al., 2016) and repeat protein folds (Brunette et al., 2015). Indeed as part of this report on methods including data interpretation and analysis, we were able to delineate novel comparative structural analyses of key Mo-bisMGD- and [Fe–S] cluster-containing proteins that disclose specific gate residues regulating substrate entry and product release mechanisms to inform biology and possible design.
With the above points in mind, we maintain that PcrAB structures and mutations serve as an archetype for Mo-bisMGD- and [Fe–S] cluster-containing respiratory proteins. In particular, our described methods and findings provide a prototypic model for substrate entry and product release that can be tested and applied to other Mo-bisMGD multi-[Fe–S] cluster enzymes. By presenting analyses of key examples including cNarGHI and EbdABC to identify proposed gating residues (Figs. 6 and 7), these ideas can now be functionally tested by other researchers. Importantly, we show how to implement PcrAB analyses in order to bring out a unified testable model for other Mo-bisMGD enzymes. Of course, all such analyses may require the successful anaerobic [Fe–S] protein production, purification, crystallization, data collection, and structure determination methods noted here. By noting tested strategies and protocols for the anaerobic handling of [Fe–S] proteins, we hope that these procedures and ideas will benefit the rigorous study of anaerobic [Fe–S] protein and Mo-bisMGD-containing enzymes and help other researchers advance our knowledge of these biologically and environmentally critical systems.
Acknowledgments
We acknowledge that the work and ideas reported here build upon the many pioneering researchers in the [Fe–S] field, especially Roland Lill, Michael Johnson, Helmut Beinert, Richard Holm, Eckard Munck, Patricia Kiley, Tracey Rouault, James Imlay, F. Wayne Outten, David Stout, Lou Noodleman, Barbara Burgess, Joan Broderick, John Peters, Brian Hoffman, Fraser Armstrong, Dennis Winge, Brian Crane, Harry Gray, Elizabeth Getzoff, Michael Adams, David Barondeau, Timothy Stemmler, Cathy Drennan, JoAnne Stubbe, and Doug Rees. These investigators taught us many interesting things about metalloproteins and how to study them properly. Unfortunately this expertise is disappearing, so we hope to have recorded some useful points we developed from others as well as from our own efforts. This work was supported in part by the Robert A. Welch Distinguished Chair in Chemistry, startup funds from the Cancer Prevention and Research Institute of Texas, and the University of Texas STARs program. Integrated X-ray structural efforts at the Advanced Light Source SIBYLS beamline are supported in part by the United States Department of Energy program Integrated Diffraction Analysis Technologies (IDAT).
References
- Adak S, Bilwes AM, Panda K, Hosfield D, Aulak KS, McDonald JF, et al. Cloning, expression, and characterization of a nitric oxide synthase protein from Deinococcus radiodurans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):107–112. doi: 10.1073/pnas.012470099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica. Section D, Biological Crystallography. 2012;68(Pt. 4):352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: Sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39(27):7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- Andreini C, Banci L, Rosato A. Exploiting bacterial operons to illuminate human iron-sulfur proteins. Journal of Proteome Research. 2016;15(4):1308–1322. doi: 10.1021/acs.jproteome.6b00045. [DOI] [PubMed] [Google Scholar]
- Aslanidis C, de Jong PJ. Ligation-independent cloning of PCR products (LIC-PCR) Nucleic Acids Research. 1990;18(20):6069–6074. doi: 10.1093/nar/18.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacolla A, Tainer JA, Vasquez KM, Cooper DN. Translocation and deletion breakpoints in cancer genomes are associated with potential non-B DNA-forming sequences. Nucleic Acids Research. 2016;44(12):5673–5688. doi: 10.1093/nar/gkw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak DW, Elliott SJ. Alternative FeS cluster ligands: Tuning redox potentials and chemistry. Current Opinion in Chemical Biology. 2014;19:50–58. doi: 10.1016/j.cbpa.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Banda DM, Nuñez NN, Burnside MA, Bradshaw KM, David SS. Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals and medicine. Free Radical Biology & Medicine. 2017;107:202–215. doi: 10.1016/j.freeradbiomed.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranovskiy AG, Zhang Y, Suwa Y, Babayeva ND, Gu J, Pavlov YI, et al. Crystal structure of the human primase. The Journal of Biological Chemistry. 2015;290(9):5635–5646. doi: 10.1074/jbc.M114.624742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barras F, Loiseau L, Py B. How Escherichia coli and Saccharomyces cerevisiae build Fe/S proteins. Advances in Microbial Physiology. 2005;50:41–101. doi: 10.1016/S0065-2911(05)50002-X. [DOI] [PubMed] [Google Scholar]
- Beinert H. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science. 1997;277(5326):653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- Bender KS, Shang C, Chakraborty R, Belchik SM, Coates JD, Achenbach LA. Identification, characterization, and classification of genes encoding perchlorate reductase. Journal of Bacteriology. 2005;187(15):5090–5096. doi: 10.1128/JB.187.15.5090-5096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nature Biotechnology. 2004;22(12):1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- Bertero MG, Rothery RA, Palak M, Hou C, Lim D, Blasco F, et al. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nature Structural Biology. 2003;10(9):681–687. doi: 10.1038/nsb969. [DOI] [PubMed] [Google Scholar]
- Boal AK, Yavin E, Lukianova OA, O’Shea VL, David SS, Barton JK. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry. 2005;44(23):8397–8407. doi: 10.1021/bi047494n. [DOI] [PubMed] [Google Scholar]
- Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE. Facilitated transfer of IscU-[2Fe2S] clusters by chaperone-mediated ligand exchange. Biochemistry. 2011;50(44):9641–9650. doi: 10.1021/bi201123z. [DOI] [PubMed] [Google Scholar]
- Boon EM, Livingston AL, Chmiel NH, David SS, Barton JK. DNA-mediated charge transport for DNA repair. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12543–12547. doi: 10.1073/pnas.2035257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhorst JA, Falke JJ. Purification of proteins using polyhistidine affinity tags. Methods in Enzymology. 2000;326:245–254. doi: 10.1016/s0076-6879(00)26058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SEJ, Bridwell-Rabb J, Drennan CL. Metalloprotein crystallography: More than a structure. Accounts of Chemical Research. 2016;49(4):695–702. doi: 10.1021/acs.accounts.5b00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braymer JJ, Lill R. Iron-sulfur cluster biogenesis and trafficking in mitochondria. The Journal of Biological Chemistry. 2017;292(31):12754–12763. doi: 10.1074/jbc.R117.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricogne G, Vonrhein C, Flensburg C, Schiltz M, Paciorek W. Generation, representation and flow of phase information in structure determination: Recent developments in and around SHARP 2.0. Acta Crystallographica. Section D, Biological Crystallography. 2003;59(Pt. 11):2023–2030. doi: 10.1107/s0907444903017694. [DOI] [PubMed] [Google Scholar]
- Bridwell-Rabb J, Fox NG, Tsai CL, Winn AM, Barondeau DP. Human frataxin activates Fe–S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry. 2014;53(30):4904–4913. doi: 10.1021/bi500532e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JB, Byer AS, Duschene KS, Duffus BR, Betz JN, Shepard EM, et al. H-cluster assembly during maturation of the [FeFe]-hydrogenase. Journal of Biological Inorganic Chemistry. 2014;19(6):747–757. doi: 10.1007/s00775-014-1168-8. [DOI] [PubMed] [Google Scholar]
- Brosey CA, Ho C, Long WZ, Singh S, Burnett K, Hura GL, et al. Defining NADH-driven allostery regulating apoptosis-inducing factor. Structure (London, England: 1993) 2016;24(12):2067–2079. doi: 10.1016/j.str.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette TJ, Parmeggiani F, Huang PS, Bhabha G, Ekiert DC, Tsutakawa SE, et al. Exploring the repeat protein universe through computational protein design. Nature. 2015;528(7583):580–584. doi: 10.1038/nature16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess BK, Lowe DJ. Mechanism of molybdenum nitrogenase. Chemical Reviews. 1996;96(7):2983–3012. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- Burns JA, Butler JC, Moran J, Whitesides GM. Selective reduction of disulfides by tris(2-carboxyethyl)phosphine. Journal of Organic Chemistry. 1991;56(8):2648–2650. [Google Scholar]
- Buschmann S, Warkentin E, Xie H, Langer JD, Ermler U, Michel H. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science. 2010;329(5989):327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- Carlson HK, Kuehl JV, Hazra AB, Justice NB, Stoeva MK, Sczesnak A, et al. Mechanisms of direct inhibition of the respiratory sulfate-reduction pathway by (per)chlorate and nitrate. The ISME Journal. 2015;9(6):1295–1305. doi: 10.1038/ismej.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli K, Johnson MK. HscA and HscB stimulate [2Fe-2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry. 2006;45(37):11087–11095. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepanoske CL, Golinelli MP, Williams SD, David SS. Positively charged residues within the iron-sulfur cluster loop of E. coli MutY participate in damage recognition and removal. Archives of Biochemistry and Biophysics. 2000;380(1):11–19. doi: 10.1006/abbi.2000.1890. [DOI] [PubMed] [Google Scholar]
- Classen S, Hura GL, Holton JM, Rambo RP, Rodic I, McGuire PJ, et al. Implementation and performance of SIBYLS: A dual endstation small-angle X-ray scattering and macromolecular crystallography beamline at the advanced light source. Journal of Applied Crystallography. 2013;46(Pt. 1):1–13. doi: 10.1107/S0021889812048698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland WW. Dithiothreitol, a new protective reagent for SH groups. Biochemistry. 1964;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Coates JD, Achenbach LA. Microbial perchlorate reduction: Rocket-fuelled metabolism. Nature Reviews Microbiology. 2004;2(7):569–580. doi: 10.1046/j.1462-2920.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- Coates JD, Michaelidou U, Bruce RA, O’Connor SM, Crespi JN, Achenbach LA. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Applied and Environmental Microbiology. 1999;65(12):5234–5241. doi: 10.1128/aem.65.12.5234-5241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C, Romão MJ. Structural and mechanistic insights on nitrate reductases. Protein Science: A Publication of the Protein Society. 2015;24(12):1901–1911. doi: 10.1002/pro.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan VM, Vickery LE. Site-specific mutations in human ferredoxin that affect binding to ferredoxin reductase and cytochrome P450scc. The Journal of Biological Chemistry. 1991;266(28):18606–18612. [PubMed] [Google Scholar]
- Colin F, Martelli A, Clémancey M, Latour JM, Gambarelli S, Zeppieri L, et al. Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. Journal of the American Chemical Society. 2013;135(2):733–740. doi: 10.1021/ja308736e. [DOI] [PubMed] [Google Scholar]
- Corbett MC, Latimer MJ, Poulos TL, Sevrioukova IF, Hodgson KO, Hedman B. Photoreduction of the active site of the metalloprotein putidaredoxin by synchrotron radiation. Acta Crystallographica. Section D, Biological Crystallography. 2007;63(Pt. 9):951–960. doi: 10.1107/S0907444907035160. [DOI] [PubMed] [Google Scholar]
- Cory SA, Van Vranken JG, Brignole EJ, Patra S, Winge DR, Drennan CL, et al. Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(27):E5325–E5334. doi: 10.1073/pnas.1702849114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotruvo JA, Stubbe J. Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: The class I ribonucleotide reductases as a case study. Metallomics: Integrated Biometal Science. 2012;4(10):1020–1036. doi: 10.1039/c2mt20142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart RE, Singleton FL, Hind JS. A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Analytical Biochemistry. 1993;211(1):151–155. doi: 10.1006/abio.1993.1246. [DOI] [PubMed] [Google Scholar]
- Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, et al. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279(5359):2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- Crane BR, Siegel LM, Getzoff ED. Sulfite reductase structure at 1.6 A: Evolution and catalysis for reduction of inorganic anions. Science. 1995;270(5233):59–67. doi: 10.1126/science.270.5233.59. [DOI] [PubMed] [Google Scholar]
- Cvetkovic A, Menon AL, Thorgersen MP, Scott JW, Poole FL, Jenney FE, et al. Microbial metalloproteomes are largely uncharacterized. Nature. 2010;466(7307):779–782. doi: 10.1038/nature09265. [DOI] [PubMed] [Google Scholar]
- Daniels DS, Mol CD, Arvai AS, Kanugula S, Pegg AE, Tainer JA. Active and alkylated human AGT structures: A novel zinc site, inhibitor and extrahelical base binding. The EMBO Journal. 2000;19(7):1719–1730. doi: 10.1093/emboj/19.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Baker D. Macromolecular modeling with rosetta. Annual Review of Biochemistry. 2008;77:363–382. doi: 10.1146/annurev.biochem.77.062906.171838. [DOI] [PubMed] [Google Scholar]
- Davila AF, Willson D, Coates JD, McKay CP. Perchlorate on Mars: A chemical hazard and a resource for humans. International Journal of Astrobiology. 2013;12(4):321–325. doi: 10.1017/S1473550413000189. [DOI] [Google Scholar]
- de Vries S, Dorner K, Strampraad MJF, Friedrich T. Electron tunneling rates in respiratory complex I are tuned for efficient energy conversion. Angewandte Chemie (International Ed in English) 2015;54(9):2844–2848. doi: 10.1002/anie.201410967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio F, Terwilliger TC, Read RJ, Wlodawer A, Oberdorfer G, Wagner U, et al. Improved molecular replacement by density- and energy-guided protein structure optimization. Nature. 2011;473(7348):540–543. doi: 10.1038/nature09964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy J, Darnault C, Brazzolotto X, Kuhn LC, Moulis JM, Volbeda A, et al. Crystallization and preliminary X-ray diffraction data for the aconitase form of human iron-regulatory protein 1. Acta Crystallographica. Section F, Structural Biology and Crystallization Communications. 2005;61(Pt. 5):482–485. doi: 10.1107/S1744309105010444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsle O, Andrade SLA, Dobbek H, Meyer J, Rees DC. Assignment of individual metal redox states in a metalloprotein by crystallographic refinement at multiple X-ray wavelengths. Journal of the American Chemical Society. 2007;129(8):2210–2211. doi: 10.1021/ja067562o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Fuss JO, Cheng QJ, Arvai AS, Hammel M, Roberts VA, et al. XPD helicase structures and activities: Insights into the cancer and aging phenotypes from XPD mutations. Cell. 2008;133(5):789–800. doi: 10.1016/j.cell.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee JA, Castagnetto JM, Case DA, Noodleman L, Stout CD, Torres RA. The circumsphere as a tool to assess distortion in [4Fe-4S] atom clusters. Journal of Biological Inorganic Chemistry. 2003;8(5):519–526. doi: 10.1007/s00775-003-0445-8. [DOI] [PubMed] [Google Scholar]
- Fish WW. Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods in Enzymology. 1988;158:357–364. doi: 10.1016/0076-6879(88)58067-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DJ, Berger P, Schaffitzel C, Yamada K, Richmond TJ, Berger I. Protein complex expression by using multigene baculoviral vectors. Nature Methods. 2006;3(12):1021–1032. doi: 10.1038/nmeth983. [DOI] [PubMed] [Google Scholar]
- Flot D, Mairs T, Giraud T, Guijarro M, Lesourd M, Rey V, et al. The ID23-2 structural biology microfocus beamline at the ESRF. Journal of Synchrotron Radiation. 2010;17(1):107–118. doi: 10.1107/S0909049509041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NG, Chakrabarti M, McCormick SP, Lindahl PA, Barondeau DP. The human iron-sulfur assembly complex catalyzes the synthesis of [2Fe-2S] clusters on ISCU2 that can be transferred to acceptor molecules. Biochemistry. 2015;54(25):3871–3879. doi: 10.1021/bi5014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NG, Das D, Chakrabarti M, Lindahl PA, Barondeau DP. Frataxin accelerates [2Fe-2S] cluster formation on the human Fe-S assembly complex. Biochemistry. 2015;54(25):3880–3889. doi: 10.1021/bi5014497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazzon J, Dean DR. Formation of iron-sulfur clusters in bacteria: An emerging field in bioinorganic chemistry. Current Opinion in Chemical Biology. 2003;7(2):166–173. doi: 10.1016/s1367-5931(03)00021-8. [DOI] [PubMed] [Google Scholar]
- Fromme JC, Verdine GL. Structure of a trapped endonuclease III-DNA covalent intermediate. The EMBO Journal. 2003;22(13):3461–3471. doi: 10.1093/emboj/cdg311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss JO, Tsai CL, Ishida JP, Tainer JA. Emerging critical roles of Fe-S clusters in DNA replication and repair. Biochimica et Biophysica Acta. 2015;1853(6):1253–1271. doi: 10.1016/j.bbamcr.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, et al. Complete chemical synthesis, assembly, and cloning of a Myco-plasma genitalium genome. Science. 2008;319(5867):1215–1220. doi: 10.1126/science.1151721. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Gorodetsky AA, Boal AK, Barton JK. Direct electrochemistry of endonu-clease III in the presence and absence of DNA. Journal of the American Chemical Society. 2006;128(37):12082–12083. doi: 10.1021/ja064784d. [DOI] [PubMed] [Google Scholar]
- Gradia SD, Ishida JP, Tsai MS, Jeans C, Tainer JA. Chapter one—MacroBac: New technologies for robust and efficient large-scale production of recombinant multiprotein complexes. Methods in Enzymology. 2017;592:1–26. doi: 10.1016/bs.mie.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishaev A, Anthis NJ, Clore GM. Contrast-matched small-angle X-ray scattering from a heavy-atom-labeled protein in structure determination: Application to a lead-substituted calmodulin-peptide complex. Journal of the American Chemical Society. 2012;134(36):14686–14689. doi: 10.1021/ja306359z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Manuel RC, Arvai AS, Parikh SS, Mol CD, Miller SL, et al. MutY catalytic core, mutant and bound adenine structures define specificity for DNA repair enzyme superfamily. Nature Structural Biology. 1998;5(12):1058–1064. doi: 10.1038/4168. [DOI] [PubMed] [Google Scholar]
- Ha Y, Arnold AR, Nuñez NN, Bartels PL, Zhou A, David SS, et al. Sulfur K-edge XAS studies of the effect of DNA binding on the [Fe4S4] site in EndoIII and MutY. Journal of the American Chemical Society. 2017;139(33):11434–11442. doi: 10.1021/jacs.7b03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Stuchebrukhov AA. Electron tunneling in respiratory complex I. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19157–19162. doi: 10.1073/pnas.1009181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgart MC, Sanishvili R, Ogata CM, Becker M, Venugopalan N, Stepanov S, et al. Automated sample-scanning methods for radiation damage mitigation and diffraction-based centering of macromolecular crystals. Journal of Synchrotron Radiation. 2011;18(Pt. 5):717–722. doi: 10.1107/S0909049511029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chemical Reviews. 2014;114(8):4041–4062. doi: 10.1021/cr400641x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton JM, Classen S, Frankel KA, Tainer JA. The R-factor gap in macromolecular crystallography: An untapped potential for insights on accurate structures. The FEBS Journal. 2014;281(18):4046–4060. doi: 10.1111/febs.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler JP, Ferguson-Miller S, Mills DA. Energy transduction: Proton transfer through the respiratory complexes. Annual Review of Biochemistry. 2006;75:165–187. doi: 10.1146/annurev.biochem.75.062003.101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hura GL, Tsai CL, Claridge SA, Mendillo ML, Smith JM, Williams GJ, et al. DNA conformations in mismatch repair probed in solution by X-ray scattering from gold nanocrystals. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(43):17308–17313. doi: 10.1073/pnas.1308595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. Iron-sulphur clusters and the problem with oxygen. Molecular Microbiology. 2006;59(4):1073–1082. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annual Review of Biochemistry. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. The mismetallation of enzymes during oxidative stress. The Journal of Biological Chemistry. 2014;289(41):28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iobbi-Nivol C, Leimkuhler S. Molybdenum enzymes, their maturation and molybdenum cofactor biosynthesis in Escherichia coli. Biochimica et Biophysica Acta. 2013;1827(8–9):1086–1101. doi: 10.1016/j.bbabio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Jackson WA, Bohlke JK, Andraski BJ, Fahlquist L, Bexfield L, Eckardt FD, et al. Global patterns and environmental controls of perchlorate and nitrate co-occurrence in arid and semi-arid environments. Geochimica et Cosmochimica Acta. 2015;164:502–522. doi: 10.1016/j.gca.2015.05.016. [DOI] [Google Scholar]
- Jackson WA, Davila AF, Sears DWG, Coates JD, McKay CP, Brundrett M, et al. Widespread occurrence of (per)chlorate in the solar system. Earth and Planetary Science Letters. 2015;430:470–476. doi: 10.1016/j.epsl.2015.09.003. [DOI] [Google Scholar]
- Jaganaman S, Pinto A, Tarasev M, Ballou DP. High levels of expression of the iron-sulfur proteins phthalate dioxygenase and phthalate dioxygenase reductase in Escherichia coli. Protein Expression and Purification. 2007;52(2):273–279. doi: 10.1016/j.pep.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annual Review of Biochemistry. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Pelletier DA, Spormann AM. Isolation and characterization of anaerobic ethylbenzene dehydrogenase, a novel Mo-Fe-S enzyme. Journal of Bacteriology. 2001;183(15):4536–4542. doi: 10.1128/JB.183.15.4536-4542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM, Snyder M, Chang L, Davis RW. Isolation of the gene encoding yeast DNA polymerase I. Cell. 1985;43:369–377. doi: 10.1016/0092-8674(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Jormakka M, Richardson D, Byrne B, Iwata S. Architecture of NarGH reveals a structural classification of Mo-bisMGD enzymes. Structure (London, England: 1993) 2004;12(1):95–104. doi: 10.1016/j.str.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Jormakka M, Tornroth S, Byrne B, Iwata S. Molecular basis of proton motive force generation: Structure of formate dehydrogenase-N. Science. 2002;295(5561):1863–1868. doi: 10.1126/science.1068186. [DOI] [PubMed] [Google Scholar]
- Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd IM, Emery VC. The use of baculoviruses as expression vectors. Applied Biochemistry and Biotechnology. 1993;42(2–3):137–159. doi: 10.1007/BF02788049. [DOI] [PubMed] [Google Scholar]
- Kiley PJ, Beinert H. The role of Fe–S proteins in sensing and regulation in bacteria. Current Opinion in Microbiology. 2003;6:181–185. doi: 10.1016/s1369-5274(03)00039-0. [DOI] [PubMed] [Google Scholar]
- Kloer DP, Hagel C, Heider J, Schulz GE. Crystal structure of ethylbenzene dehydrogenase from Aromatoleum aromaticum. Structure (London, England: 1993) 2006;14(9):1377–1388. doi: 10.1016/j.str.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kuchenreuther JM, Grady-Smith CS, Bingham AS, George SJ, Cramer SP, Swartz JR. High-yield expression of heterologous [FeFe] hydrogenases in Escherichia coli. PLoS One. 2010;5(11):e15491. doi: 10.1371/journal.pone.0015491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn LA, Siani MA, Pique ME, Fisher CL, Getzoff ED, Tainer JA. The interdependence of protein surface topography and bound water molecules revealed by surface accessibility and fractal density measures. Journal of Molecular Biology. 1992;228(1):13–22. doi: 10.1016/0022-2836(92)90487-5. [DOI] [PubMed] [Google Scholar]
- Kuo CF, McRee DE, Fisher CL, O’Handley SF, Cunningham RP, Tainer JA. Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science. 1992;258(5081):434–440. doi: 10.1126/science.1411536. [DOI] [PubMed] [Google Scholar]
- Lai YT, Hura GL, Dyer KN, Tang HYH, Tainer JA, Yeates TO. Designing and defining dynamic protein cage nanoassemblies in solution. Science Advances. 2016;2(12):e1501855. doi: 10.1126/sciadv.1501855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebendiker M, Danieli T. Purification of proteins fused to maltose-binding protein. Methods in Molecular Biology (Clifton, NJ) 2017;1485:257–273. doi: 10.1007/978-1-4939-6412-3_13. [DOI] [PubMed] [Google Scholar]
- Lee AQ, Streit BR, Zdilla MJ, Abu-Omar MM, DuBois JL. Mechanism of and exquisite selectivity for O-O bond formation by the heme-dependent chlorite dismutase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(41):15654–15659. doi: 10.1073/pnas.0804279105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Edelsbrunner H, Fu P, Sudhakar PV, Subramaniam S. Analytical shape computation of macromolecules: I. Molecular area and volume through alpha shape. Proteins. 1998a;33(1):1–17. [PubMed] [Google Scholar]
- Liang J, Edelsbrunner H, Fu P, Sudhakar PV, Subramaniam S. Analytical shape computation of macromolecules: II. Inaccessible cavities in proteins. Proteins. 1998b;33(1):18–29. [PubMed] [Google Scholar]
- Liang J, Edelsbrunner H, Woodward C. Anatomy of protein pockets and cavities: Measurement of binding site geometry and implications for ligand design. Protein Science. 1998;7(9):1884–1897. doi: 10.1002/pro.5560070905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460(7257):831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- Lill R, Dutkiewicz R, Elsasser HP, Hausmann A, Netz DJA, Pierik AJ, et al. Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes. Biochimica et Biophysica Acta. 2006;1763(7):652–667. doi: 10.1016/j.bbamcr.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, et al. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochimica et Biophysica Acta. 2012;1823(9):1491–1508. doi: 10.1016/j.bbamcr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: Components and mechanisms. Annual Review of Cell and Developmental Biology. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- Magalon A, Asso M, Guigliarelli B, Rothery RA, Bertrand P, Giordano G, et al. Molybdenum cofactor properties and [Fe-S] cluster coordination in Escherichia coli nitrate reductase A: Investigation by site-directed mutagenesis of the conserved his-50 residue in the NarG subunit. Biochemistry. 1998;37(20):7363–7370. doi: 10.1021/bi972858f. [DOI] [PubMed] [Google Scholar]
- Malkin R, Rabinowitz JC. The reconstitution of clostridial ferredoxin. Biochemical and Biophysical Research Communications. 1966;23(6):822–827. doi: 10.1016/0006-291x(66)90561-4. [DOI] [PubMed] [Google Scholar]
- Marangon J, Paes de Sousa PM, Moura I, Brondino CD, Moura JJG, González PJ. Substrate-dependent modulation of the enzymatic catalytic activity: Reduction of nitrate, chlorate and perchlorate by respiratory nitrate reductase from Marinobacter hydrocarbonoclasticus 617. Biochimica et Biophysica Acta. 2012;1817(7):1072–1082. doi: 10.1016/j.bbabio.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Martelli A, Puccio H. Dysregulation of cellular iron metabolism in Friedreich ataxia: From primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Frontiers in Pharmacology. 2014;5:130. doi: 10.3389/fphar.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DR, Matyushov DV. Electron-transfer chain in respiratory complex I. Scientific Reports. 2017;7(1):5495. doi: 10.1038/s41598-017-05779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos CT, Velizarov S, Crespo JG, Reis MAM. Simultaneous removal of perchlorate and nitrate from drinking water using the ion exchange membrane bioreactor concept. Water Research. 2006;40(2):231–240. doi: 10.1016/j.watres.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Mayhew SG. The redox potential of dithionite and SO-2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. European Journal of Biochemistry. 1978;85(2):535–547. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
- Mayo CJ, Diprose JM, Walter TS, Berry IM, Wilson J, Owens RJ, et al. Benefits of automated crystallization plate tracking, imaging, and analysis. Structure (London, England: 1993) 2005;13(2):175–182. doi: 10.1016/j.str.2004.12.010. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. Journal of Applied Crystallography. 2007;40(Pt. 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt CA, Hugenholtz P, Hanson GR, McEwan AG. Molecular analysis of dimethyl sulphide dehydrogenase from Rhodovulum sulfidophilum: Its place in the dimethyl sulphoxide reductase family of microbial molybdopterin-containing enzymes. Molecular Microbiology. 2002;44(6):1575–1587. doi: 10.1046/j.1365-2958.2002.02978.x. [DOI] [PubMed] [Google Scholar]
- McTernan PM, Chandrayan SK, Wu CH, Vaccaro BJ, Lancaster WA, Yang Q, et al. Intact functional fourteen-subunit respiratory membrane-bound [NiFe]-hydrogenase complex of the hyperthermophilic archaeon Pyrococcus furiosus. The Journal of Biological Chemistry. 2014;289(28):19364–19372. doi: 10.1074/jbc.M114.567255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melber A, Na U, Vashisht A, Weiler BD, Lill R, Wohlschlegel JA, et al. Role of Nfu1 and Bol3 in iron-sulfur cluster transfer to mitochondrial clients. eLife. 2016;5:e15991. doi: 10.7554/eLife.15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey B, Howard J. Rigidity of microtubules is increased by stabilizing agents. The Journal of Cell Biology. 1995;130(4):909–917. doi: 10.1083/jcb.130.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming DW, Archer PD, Glavin DP, Eigenbrode JL, Franz HB, Sutter B, et al. Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale crater, Mars. Science. 2014;343(6169):1245267. doi: 10.1126/science.1245267. [DOI] [PubMed] [Google Scholar]
- Moura JJG, Brondino CD, Trincão J, Romão MJ. Mo and W bis-MGD enzymes: Nitrate reductases and formate dehydrogenases. Journal of Biological Inorganic Chemistry. 2004;9(7):791–799. doi: 10.1007/s00775-004-0573-9. [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallographica. Section D, Biological Crystallography. 1997;53(Pt. 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Najmudin S, González PJ, Trincão J, Coelho C, Mukhopadhyay A, Cerqueira NMFSA, et al. Periplasmic nitrate reductase revisited: A sulfur atom completes the sixth coordination of the catalytic molybdenum. Journal of Biological Inorganic Chemistry. 2008;13(5):737–753. doi: 10.1007/s00775-008-0359-6. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Saeki K, Takahashi Y. Hyperproduction of recombinant ferre-doxins in Escherichia coli by coexpression of the ORF1-ORF2-iscS-iscU-iscA-hscB-hs cA-fdx-ORF3 gene cluster. Journal of Biochemistry. 1999;126(1):10–18. doi: 10.1093/oxfordjournals.jbchem.a022409. [DOI] [PubMed] [Google Scholar]
- Nerenberg R. Breathing perchlorate. Science. 2013;340(6128):38–39. doi: 10.1126/science.1236336. [DOI] [PubMed] [Google Scholar]
- Netz DJA, Stith CM, Stumpfig M, Kopf G, Vogel D, Genau HM, et al. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nature Chemical Biology. 2011;8(1):125–132. doi: 10.1038/nchembio.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha L, Wilhelm MB, Murchie SL, McEwen AS, Wray JJ, Hanley J, et al. Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nature Geo-science. 2015;8(1):829–832. doi: 10.1038/ngeo2546. [DOI] [Google Scholar]
- Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Molecular Microbiology. 2004;52(3):861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- Page CC, Moser CC, Chen X, Dutton PL. Natural engineering principles of electron tunnelling in biological oxidation-reduction. Nature. 1999;402(6757):47–52. doi: 10.1038/46972. [DOI] [PubMed] [Google Scholar]
- Pandelia ME, Lanz ND, Booker SJ, Krebs C. Mossbauer spectroscopy of Fe/S proteins. Biochimica et Biophysica Acta. 2015;1853(6):1395–1405. doi: 10.1016/j.bbamcr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Pandey A, Gordon DM, Pain J, Stemmler TL, Dancis A, Pain D. Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly. The Journal of Biological Chemistry. 2013;288(52):36773–36786. doi: 10.1074/jbc.M113.525857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul VD, Lill R. Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochimica et Biophysica Acta. 2015;1853(6):1528–1539. doi: 10.1016/j.bbamcr.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Perry JJP, Shin DS, Getzoff ED, Tainer JA. The structural biochemistry of the superoxide dismutases. Biochimica et Biophysica Acta. 2010;1804(2):245–262. doi: 10.1016/j.bbapap.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik AJ, Netz DJA, Lill R. Analysis of iron-sulfur protein maturation in eukaryotes. Nature Protocols. 2009;4(5):753–766. doi: 10.1038/nprot.2009.39. [DOI] [PubMed] [Google Scholar]
- Pratt AJ, Shin DS, Merz GE, Rambo RP, Lancaster WA, Dyer KN, et al. Aggregation propensities of superoxide dismutase G93 hotspot mutants mirror ALS clinical phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(43):E4568–4576. doi: 10.1073/pnas.1308531111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambo RP, Tainer JA. Accurate assessment of mass, models and resolution by small-angle scattering. Nature. 2013a;496(7446):477–481. doi: 10.1038/nature12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambo RP, Tainer JA. Super-resolution in solution X-ray scattering and its applications to structural systems biology. Annual Review of Biophysics. 2013b;42:415–441. doi: 10.1146/annurev-biophys-083012-130301. [DOI] [PubMed] [Google Scholar]
- Raulfs EC, O’Carroll IP, Santos Dos PC, Unciuleac MC, Dean DR. In vivo iron-sulfur cluster formation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(25):8591–8596. doi: 10.1073/pnas.0803173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikken GB, Kroon AGM, van Ginkel CG. Transformation of (per)chlorate into chloride by a newly isolated bacterium: Reduction and dismutation. Applied Microbiology and Biotechnology. 1996;45(3):420–426. doi: 10.1007/s002530050707. [DOI] [Google Scholar]
- Roberts VA, Iverson BL, Iverson SA, Benkovic SJ, Lerner RA, Getzoff ED, et al. Antibody remodeling: A general solution to the design of a metal-coordination site in an antibody binding pocket. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(17):6654–6658. doi: 10.1073/pnas.87.17.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche B, Aussel L, Ezraty B, Mandin P, Py B, Barras F. Iron/sulfur proteins biogenesis in prokaryotes: Formation, regulation and diversity. Biochimica et Biophysica Acta. 2013;1827(3):455–469. doi: 10.1016/j.bbabio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Rothery RA, Bertero MG, Spreter T, Bouromand N, Strynadka NCJ, Weiner JH. Protein crystallography reveals a role for the FS0 cluster of Escherichia coli nitrate reductase A (NarGHI) in enzyme maturation. The Journal of Biological Chemistry. 2010;285(12):8801–8807. doi: 10.1074/jbc.M109.066027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothery RA, Blasco F, Weiner JH. Electron transfer from heme bL to the [3Fe-4S] cluster of Escherichia coli nitrate reductase A (NarGHI) Biochemistry. 2001;40(17):5260–5268. doi: 10.1021/bi002393k. [DOI] [PubMed] [Google Scholar]
- Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nature Genetics. 1997;17(2):215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- Rouault TA. Mammalian iron-sulphur proteins: Novel insights into biogenesis and function. Nature Reviews Molecular Cell Biology. 2015;16(1):45–55. doi: 10.1038/nrm3909. [DOI] [PubMed] [Google Scholar]
- Saikrishnan K, Yeeles JT, Neville SG, Krajewski WW, Dillingham MS, Wigley DB. Insights into Chi recognition from the structure of an AddAB-type helicase-nuclease complex. The EMBO Journal. 2012;31(6):1568–1578. doi: 10.1038/emboj.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandala GM, Hopmann KH, Ghosh A, Noodleman L. Calibration of DFT functionals for the prediction of Fe Mossbauer spectral parameters in iron-nitrosyl and iron-sulfur complexes: Accurate geometries prove essential. Journal of Chemical Theory and Computation. 2011;7(10):3232–3247. doi: 10.1021/ct200187d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer F, Seip N, Maertens B, Block H, Kubicek J. Purification of GST-tagged proteins. Methods in Enzymology. 2015;559:127–139. doi: 10.1016/bs.mie.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Schindelin H, Kisker C, Hilton J, Rajagopalan KV, Rees DC. Crystal structure of DMSO reductase: Redox-linked changes in molybdopterin coordination. Science. 1996;272(5268):1615–1621. doi: 10.1126/science.272.5268.1615. [DOI] [PubMed] [Google Scholar]
- Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donzé M, Reutenauer L, et al. Mammalian frataxin: An essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS One. 2011;6(1):e16199. doi: 10.1371/journal.pone.0016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnecke V, Swanson CA, Getzoff ED, Tainer JA, Kuhn LA. Screening a peptidyl database for potential ligands to proteins with side-chain flexibility. Proteins. 1998;33(1):74–87. [PubMed] [Google Scholar]
- Schwarz G, Mendel RR, Ribbe MW. Molybdenum cofactors, enzymes and pathways. Nature. 2009;460(7257):839–847. doi: 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- Segel IH. Biochemical calculations: How to solve mathematical problems in general biochemistry. 2. New York: John Wiley & Sons; 1976. Retrieved from http://www.wiley.com/WileyCDA/WileyTitle/productCd-0471774219.html. [Google Scholar]
- Sheldrick GM. A short history of SHELX. Acta Crystallographica. Section A: Foundations of Crystallography. 2008;64(Pt. 1):112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Shomura Y, Yoon KS, Nishihara H, Higuchi Y. Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase. Nature. 2011;479(7372):253–256. doi: 10.1038/nature10504. [DOI] [PubMed] [Google Scholar]
- Sontz PA, Mui TP, Fuss JO, Tainer JA, Barton JK. DNA charge transport as a first step in coordinating the detection of lesions by repair proteins. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):1856–1861. doi: 10.1073/pnas.1120063109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JL, Kumar R, Singh M, Wold MS, Pandita TK, Burgers PM. Human exonuclease 5 is a novel sliding exonuclease required for genome stability. The Journal of Biological Chemistry. 2012;287(51):42773–42783. doi: 10.1074/jbc.M112.422444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling O, Wilbrecht C, Lill R. Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie. 2014;100:61–77. doi: 10.1016/j.biochi.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Stemmler TL, Lesuisse E, Pain D, Dancis A. Frataxin and mitochondrial FeS cluster biogenesis. The Journal of Biological Chemistry. 2010;285(35):26737–26743. doi: 10.1074/jbc.R110.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaleniec M, Hagel C, Menke M, Nowak P, Witko M, Heider J. Kinetics and mechanism of oxygen-independent hydrocarbon hydroxylation by ethylbenzene dehydrogenase. Biochemistry. 2007;46(25):7637–7646. doi: 10.1021/bi700633c. [DOI] [PubMed] [Google Scholar]
- Tainer JA, Roberts VA, Getzoff ED. Metal-binding sites in proteins. Current Opinion in Biotechnology. 1991;2(4):582–591. doi: 10.1016/0958-1669(91)90084-i. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. Journal of Biochemistry. 1999;126(5):917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- Terwilliger TC, Adams PD, Read RJ, McCoy AJ, Moriarty NW, Grosse-Kunstleve RW, et al. Decision-making in structure solution using Bayesian estimates of map quality: The PHENIX AutoSol wizard. Acta Crystallographica. Section D, Biological Crystallography. 2009;65(Pt. 6):582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer MM, Ahern H, Xing D, Cunningham RP, Tainer JA. Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure. The EMBO Journal. 1995;14(16):4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CL, Barondeau DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49(43):9132–9139. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Bridwell-Rabb J, Barondeau DP. Friedreich’s ataxia variants I154F and W155R diminish frataxin-based activation of the iron-sulfur cluster assembly complex. Biochemistry. 2011;50(29):6478–6487. doi: 10.1021/bi200666h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzarska MA, Dutkiewicz R, Freibert SA, Lill R, Muhlenhoff U. The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Molecular Biology of the Cell. 2013;24(12):1830–1841. doi: 10.1091/mbc.E12-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaithiyalingam S, Warren EM, Eichman BF, Chazin WJ. Insightsintoeukaryotic DNA priming from the structure and functional interactions of the 4Fe-4S cluster domain of human DNA primase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(31):13684–13689. doi: 10.1073/pnas.1002009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswara Rao P, Holm RH. Synthetic analogues of the active sites of iron-sulfur proteins. Chemical Reviews. 2004;104(2):527–559. doi: 10.1021/cr020615. [DOI] [PubMed] [Google Scholar]
- Vita N, Hatchikian EC, Nouailler M, Dolla A, Pieulle L. Disulfide bond-dependent mechanism of protection against oxidative stress in pyruvate-ferredoxin oxi-doreductase of anaerobic Desulfovibrio bacteria. Biochemistry. 2008;47(3):957–964. doi: 10.1021/bi7014713. [DOI] [PubMed] [Google Scholar]
- Vranish JN, Russell WK, Yu LE, Cox RM, Russell DH, Barondeau DP. Fluorescent probes for tracking the transfer of iron-sulfur cluster and other metal cofactors in biosynthetic reaction pathways. Journal of the American Chemical Society. 2015;137(1):390–398. doi: 10.1021/ja510998s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webert H, Freibert SA, Gallo A, Heidenreich T, Linne U, Amlacher S, et al. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nature Communications. 2014;5:5013. doi: 10.1038/ncomms6013. [DOI] [PubMed] [Google Scholar]
- Weissmann F, Petzold G, VanderLinden R, Huis In ’t Veld PJ, Brown NG, Lampert F, et al. biGBac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2564–9. doi: 10.1073/pnas.1604935113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallographica. Section D, Biological Crystallography. 2011;67(Pt. 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Song Y, Min B, Steinberg L, Logan BE. Microbial degradation of perchlorate: Principles and applications. Environmental Engineering Science. 2003;20(5):405–422. doi: 10.1089/109287503768335904. [DOI] [Google Scholar]
- Yan A, Kiley PJ. Techniques to isolate O2-sensitive proteins: [4Fe-4S]-FNR as an example. Methods in Enzymology. 2009;463:787–805. doi: 10.1016/S0076-6879(09)63042-1. [DOI] [PubMed] [Google Scholar]
- Yannone SM, Hartung S, Menon AL, Adams MWW, Tainer JA. Metals in biology: Defining metalloproteomes. Current Opinion in Biotechnology. 2012;23(1):89–95. doi: 10.1016/j.copbio.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Cowan JA. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. Journal of the American Chemical Society. 2003;125(20):6078–6084. doi: 10.1021/ja027967i. [DOI] [PubMed] [Google Scholar]
- Youngblut MD, Tsai CL, Clark IC, Carlson HK, Maglaqui AP, Gau-Pan PS, et al. Perchlorate reductase is distinguished by active site aromatic gate residues. The Journal of Biological Chemistry. 2016;291(17):9190–9202. doi: 10.1074/jbc.M116.714618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblut MD, Wang O, Barnum TP, Coates JD. (Per)chlorate in biology on earth and beyond. Annual Review of Microbiology. 2016;70:435–457. doi: 10.1146/annurev-micro-102215-095406. [DOI] [PubMed] [Google Scholar]
- Yu L, Wolin MJ. Hydrogenase measurement with photochemically reduced methyl viologen. Journal of Bacteriology. 1969;98(1):51–55. doi: 10.1128/jb.98.1.51-55.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters: Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. The Journal of Biological Chemistry. 1998;273(21):13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- Zhou C, Pourmal S, Pavletich NP. Dna2 nuclease-helicase structure, mechanism and regulation by rpa. eLife. 2015;4:e09832. doi: 10.7554/eLife.09832. [DOI] [PMC free article] [PubMed] [Google Scholar]