Abstract
Callus was induced from mangosteen (Garcinia mangostana L.) young purple–red leaves on Murashige and Skoog basal medium with various combinations of plant growth regulators. Murashige and Skoog medium with 4.44 µM 6-benzylaminopurine and 4.52 µM 2,4-dichlorophenoxyacetic acid was the best for friable callus induction. This friable callus was used for the initiation of cell suspension culture. The effects of different combinations of 6-benzylaminopurine and 2,4-dichlorophenoxyacetic acid, carbon sources and inoculum sizes were tested. It was found that combination of 2.22 µM 6-benzylaminopurine + 2.26 µM 2,4-dichlorophenoxyacetic acid, glucose (30 g/l) and 1.5 g/50 ml inoculum size was the best for cell growth. Callus and cell suspension cultures were then treated either with 100 µM methyl jasmonate as an elicitor for 5 days, or 0.5 g/l casein hydrolysate as an organic supplement for 7 days. Metabolites were then extracted and profiled using liquid chromatography-time of flight mass spectrometry. Multivariate discriminant analyses revealed significant metabolite differences (P ≤ 0.05) for callus and suspension cells treated either with methyl jasmonate or casein hydrolysate. Based on MS/MS data, methyl jasmonate stimulated the production of an alkaloid (thalsimine) and fatty acid (phosphatidyl ethanolamine) in suspension cells while in callus, an alkaloid (thiacremonone) and glucosinolate (7-methylthioheptanaldoxime) was produced. Meanwhile casein hydrolysate stimulated the production of alkaloids such as 3ß,6ß-dihydroxynortropane and cis-hinokiresinol and triterpenoids such as schidigerasaponin and talinumoside in suspension cells. This study provides evidence on the potential of secondary metabolite production from in vitro culture of mangosteen.
Keywords: Callus induction, Cell suspension culture, Methyl jasmonate, Casein hydrolysate, Liquid chromatography–time of flight–mass spectrometry
Introduction
Mangosteen (Garcinia mangostana L.) belongs to the Clusiaceae family. The pericarp of the fruit is particularly known to contain secondary metabolites such as xanthone (Shan and Zhang 2010), anthocyanins (Zarena and Udaya Sankar 2012), proanthocyanidins (Fu et al. 2007) and phenolic acids (Zadernowski et al. 2009). These metabolites contribute to the anti-inflammatory, anti-bacterial, anti-tumor and anti-oxidative properties reported (Pedraza-Chaverri et al. 2008). Hence, there is a high demand for mangosteen fruit not only due to its delicious taste but also due to its medicinal and pharmaceutical values. Mangosteen, however, takes approximately 8–10 years to mature and it produces fruits once or twice a year (Te-Chato and Lim 1999), causing limitation in getting the secondary metabolites throughout the year. For these reasons, in vitro culture would undoubtedly provide an alternative approach for the production of these metabolites from mangosteen.
Plant cell, tissue and organ culture technology has been shown to be a successful alternative to the whole plant system for producing commercially important natural products (Smetanska 2008), from callus and cell suspension culture such as terpenoid (Misra et al. 2014), triterpene (Han et al. 2014), flavonoid (Wang et al. 2015) and phenylpropanoid (Kamalipourazad et al. 2016). Callus induction for plantlet production from young leaves of mangosteen was previously reported by Te-Chato and Lim (2000) and Maadon et al. (2016). However, there is no report on the establishment of cell suspension culture for production of secondary metabolites for mangosteen.
The application of elicitors and organic supplements to the cells has been shown to enhance the production of secondary metabolites. Methyl jasmonate (MeJA) is one of the widely used elicitors for stimulating secondary metabolite production in plant cell culture. MeJA has been reported to induce the accumulation of terpenoids in sweet basil (Ocimum basilicum) (Misra et al. 2014), flavonoids in St. John’s wort (Hypericum perforatum) (Wang et al. 2015) and alkaloids in Indian pennywort (Centella asiatica) (Rao and Usha 2015). In contrast, casein hydrolysate (CH) being an organic supplement to culture medium could enhance the yield of desired secondary metabolites such as phenolic compounds and anthocyanin in grapevine (Cetin and Baydar 2014) and sennosides in senna (Cassia angustifolia) (Chetri et al. 2016). Production of metabolites of mangosteen through in vitro culture using an elicitor or an organic supplement, however, has not been researched. Therefore, the establishment of in vitro protocols of mangosteen for secondary metabolite production would be of great potential for commercial and research applications. Hence, the aim of this work was to establish callus and cell suspension cultures of mangosteen and to investigate the effects of MeJA and CH on the production of secondary metabolites from both types of cultures.
Materials and methods
Preparation of plant material and establishment of callus culture
Young purple–red leaves of mangosteen (Garcinia mangostana L.) from 6-month-old seedlings grown in the greenhouse, Plant Biotechnology Laboratory, Universiti Kebangsaan Malaysia were used. The leaves (3–5 cm) were rinsed under tap water for 2 min followed by surface-sterilization for 2 min in 80% alcohol (v/v) solution. This was followed by 5 min of shaking in 20% Clorox bleach (contained 5% sodium hypochloride and 1% sodium hydroxide) solution with few drops of Tween 20 (Sigma-Aldrich, Missouri, USA). The leaves were subsequently rinsed with sterile-distilled water. The leaves were cut transversely into four or five segments (1.0 cm × 1.0 cm) based on the leaf size and cultured on Murashige and Skoog (1962) (MS) medium with 4.44 µM 6-benzylaminopurine (BAP) in combination with 4.52 µM 2,4-dichlorophenoxyacetic acid (2,4-D), 4.14 µM picloram (4-Amino-3,5,6-trichloropicolinic acid) or 4.52 µM dicamba (2-methoxy-3,6-dichlorobenzoic acid), and 4.65 µM kinetin (N-(2-Furylmethyl)-3H-purin-6-amine) in combination with 4.52 µM 2,4-D, 4.14 µM picloram or 4.52 µM dicamba with 88 µM sucrose and 3.0 g/L of gelrite (Duchefa Biochemie, Haarlem, The Netherlands) for callus induction. Chemicals for MS medium, plant growth regulators and sucrose were purchased from Sigma-Aldrich, Missouri, USA. The pH was adjusted to 5.7–5.8 with 0.1 N sodium hydroxide (NaOH) and 0.1 N hydrochloric acid (HCl) before autoclaving at 121 °C for 20 min. Each six treatment combinations consisted of three replicates with four leaves in individual Petri dish for each replicate. All cultures were incubated at 23 ± 2 °C in dark condition. The cultures were observed weekly based on the morphological structures (compact, friable, and spongy) formed. Subcultures were carried out every 4 weeks and final data was taken at 120 days of culture.
Histological analysis
Histological analysis was performed to confirm the morphological features of the friable callus. Histological technique using resin developed by ORSTOM-CIRAD team (Mari et al. 1995) was used. The two types of friable callus obtained were fixed in gluteraldehyde–fomaldehyde–caffein (Sigma-Aldrich, USA) solution (consist of 0.2 M phosphate buffer, 10% parafomaldehyde, 25% gluteraldehyde, 1% caffeine and distilled water) for 1–3 days at room temperature and dehydrated with the ethanol series (v/v) (30, 50, 70, 80, 90, 95, 100%). This was followed by soaking in 100% butanol for 3 days for better penetration of the resin. Samples were then placed at 4 °C for 3 days in Technovit® 7100 resin (Kulzer, Wehrheim, Germany) followed by inclusion with the same resin. An automated rotary microtome (Leica Biosystems, Nussloch, Germany) was used to cut the samples to 3–5 µm sections. The sections were then double-stained using the periodic acid/Schiff reaction and naphthol blue black (Sigma-Aldrich, Missouri, USA), then viewed under a light microscope (Carl ZE, Carl Zeiss, Jena, Germany). This double staining allows penetration of the stains to the cell wall, enabling clear observation of the cell arrangement in the sections. Three replicates were done for each type of callus.
Establishment of cell suspension culture
Friable callus (callus that falls apart easily), obtained from medium with 4.44 µM BAP + 4.52 µM 2,4-D were used for cell suspension establishment. The friable callus with 1.0 g fresh weight was transferred into 50 ml MS liquid medium, in a 100 ml size conical flask. Cultures were maintained on an orbital shaker (Protech, Malaysia) at 110 rpm and incubated under a 16 h photoperiod (22.26 µmol m−2 s−1), at the temperature of 23 ± 2 °C. Three experiments were carried out to investigate the effects of different combinations of BAP and 2,4-D, types of carbon source and inoculum sizes on cell growth. The effects of BAP and 2,4-D were tested using four different combinations (2.22 µM BAP + 2.26 µM 2,4-D; 2.22 µM BAP + 4.52 µM 2,4-D; 4.44 µM BAP + 2.26 µM 2,4-D; 4.44 µM BAP + 4.52 µM 2,4-D). BAP and 2,4-D were used because these combinations of plant growth regulators produced high percentage of friable callus in the callus induction experiment. The effect of carbon sources was tested using sucrose, glucose, galactose and maltose at 30 g/l each (all carbon sources except sucrose were purchased from Duchefa Biochemie, Haarlem, The Netherlands). The effect of inoculum sizes was tested at 0.5, 1.0 and 1.5 g. Each experiment consisted of three replicates with five flasks per replicate. The medium was replaced with a fresh one every 5 days. All media contained 2.83 µM ascorbic and 2.60 µM citric acid (both from Sigma-Aldrich, Missouri, USA), and concentration used based on Sundram et al. (2012) to overcome browning of the cultures. The growth of cells was recorded using Settled Cell Volume (SCV) method (Mustafa et al. 2011).
Elicitation of friable callus and cell suspension cultures with MeJA and CH
A batch of friable callus was cultured on MS solid medium supplemented with 100 µM methyl jasmonate (MeJA) (Sigma-Aldrich, USA) as an elicitor. Another batch of friable callus was cultured on MS solid medium containing 0.5 g/l of casein hydrolysates (CH) (Duchefa Biochemie, Haarlem, The Netherlands). For the cell suspension culture, 2.0 ml of Settled Cell Volume (SCV) with spent medium of the suspension stock were transferred into each conical flask, containing 50 ml of fresh medium (based on previous experiments with 30 g/l glucose and 2.22 µM BAP + 2.26 µM 2,4-D) and maintained under same conditions for 15 days where the cells were at exponential phase (Wang et al. 2015; Farag et al. 2016). After which 100 µM MeJA and 0.5 g/l of CH were then added separately to the respective cultures. Cultures consisted of three replicates with five flask per replicate and were kept at 23 ± 2 °C, with a 16 h photoperiod and maintained on an orbital shaker at 110 rpm. Friable callus and suspension cells treated with MeJA were harvested 5 days after elicitation (James et al. 2013; Wang et al. 2015) while friable callus and suspension cells treated with CH were harvested seven days after treatment. The control treatments were cultures not treated with MeJA or CH.
Extraction and chemical analysis of callus and suspension cell extracts
Callus was ground with liquid nitrogen into powdered form while suspension cells were sieved using Whatman filter paper grade 1 (GE Healthcare Life Sciences, Singapore) before grinding them. Both non-treated callus and suspension cells were used as controls. All samples in powdered form were kept in − 80 °C before metabolite extraction on the same day. Extraction of metabolites was done using methanol:chloroform:water (3:1:1) (Cadahia et al. 2015). Chloroform (200 µL) was used to extract 50 mg (in powdered form) of samples and the extracts were sonicated at room temperature for 15 min. Then, 600 µl of methanol and 200 µl of water were added followed by sonication for 20 min at room temperature. The extracts were then centrifuged at 10,000×g for 10 min and 200 µl of the supernatant were transferred into vials and diluted with 795 µl of methanol and spiked with 5 µL of vanillin (Sigma-Aldrich, Missouri, USA) as internal standard with a total of 1.0 ml of extract. Sample extracts were analyzed using the liquid chromatography-time of flight-mass spectrometry (LC-TOF-MS) platform. Separation was performed on a Reversed-Phase C-18 column (Acclaim™ Polar Advantage II, 3 mm × 150 mm, 3 µm particle size) (Thermo Scientific, Waltham, Massachusetts, USA) on an UltiMate 3000 UHPLC system (Dionex, Idstein, Germany). Gradient elution was performed at 0.4 mL/min and 40 °C using H2O + 0.1% Formic Acid (A) and 100% ACN (B) with 22 min total run time. The injection volume of sample was 1.0 µl with three biological replicates and three technical replicates. The gradient started at 5% B (0–3 min); 80% B (3–10 min); 80% B (10–15 min) and 5% B (15–22 min). Eluted compounds were detected using a high-resolution mass spectrometry MicroTOF QIII (Bruker Daltonics, Bremen, Germany) with ESI-positive ionization mode using the following settings: capillary voltage: 4500 V; nebulizer pressure: 1.2 bar; drying gas: 8 l/min at 200 °C and the mass range was at 50–1000 m/z.
Statistical and multivariate analyses
Statistical Analysis Software (SAS, Institute Inc., Cary, NC, USA) was used for the analyses of all data obtained from tissue culture experiments. One-way analysis of variance (ANOVA) was used followed by comparison of means using Duncan’s Multiple Range Test (DMRT).
Data from generated LC-TOF-MS files were extracted using Profile Analysis 2.0 (Bruker Daltonic, Germany). Principal component analysis (PCA) was performed using SIMCA-P + 12.0 software (Umetrics, Umea, Sweden) to assess the separation in metabolite composition in response to MeJA and CH treatment. Heatmaps were generated using MetaboAnalyst 3.0 (Xia Lab, McGill, Quebec, Canada) to visualize the metabolite intensities detected and based on ANOVA at P ≤ 0.05. Metabolite identification was done via METLIN (La Jolla, California, USA) database, retention time relative to internal standards, mass spectra and comparison to both the reference literature and phytochemical dictionary of database.
Results
Friable callus induction
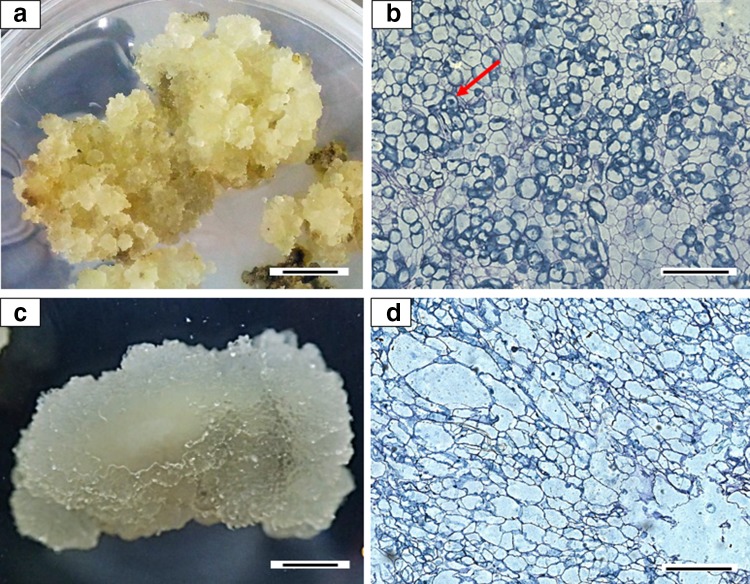
Callus was produced from cut ends of young leaves of mangosteen after three weeks of culture. Friable callus was successfully established on 4.44 µM BAP + 4.52 µM 2,4-D (65.00%) and 4.44 µM BAP + 4.14 µM picloram (84.15%) as shown in Table 1. Combination of BAP + dicamba, kinetin + 2,4-D and kinetin + picloram produced compact and spongy/dusty callus while combinations of kinetin + dicamba only produced spongy/dusty callus. Both friable callus on 4.44 µM BAP + 4.52 µM 2,4-D and 4.44 µM BAP + 4.14 µM picloram were further analyzed histologically to observe the structure or type of cells for each callus. Friable callus on 4.44 µM BAP + 4.52 µM 2,4-D (Fig. 1a) has isodiametric cells with clearly seen nuclei (Fig. 1b) compared to the friable watery callus on 4.44 µM BAP + 4.14 µM picloram (Fig. 1c) which has non-isodiametric elongated cells with large vacuoles (Fig. 1d). Therefore, callus on MS medium supplemented with 4.44 µM BAP + 4.52 µM 2,4-D was used as a starting material for cell suspension culture.
Table 1.
The effect of different combinations of plant growth regulators on mangosteen callus induction
| Cytokinin | Auxin | Friable callus | Compact callus | Spongy/dusty callus | Browning/died |
|---|---|---|---|---|---|
| 4.44 µM BAP | 4.52 µM 2,4-D | 65.00 b | 22.22ab | 0.00b | 7.41b |
| 4.14 µM Picloram | 84.15 a | 41.67ab | 0.00b | 5.55b | |
| 4.52 µM Dicamba | 0.00c | 33.34ab | 44.44a | 8.33b | |
| 4.65 µM Kinetin | 4.52 µM 2,4-D | 0.00c | 38.89ab | 63.89a | 19.44ab |
| 4.14 µM Picloram | 0.00c | 16.67ab | 47.22a | 27.77a | |
| 4.52 µM Dicamba | 0.00c | 0.00b | 66.67a | 5.55b |
a,b,cMean value is from three replications with four explants each. Mean value that does not have the same letters within each column differ significantly at P ≤ 0.05 using Duncan’s Multiple Range Test.
Value in bold shows the significant difference number producing friable callus
Fig. 1.
Callus developed (morphologically and histologically) from segmented mangosteen young leaves after 120 days of culture on MS medium. a Friable callus on medium supplemented with 4.44 µM BAP and 4.52 µM 2,4-D, showing b the isodiametric cells with dense nucleus (red arrow). c Friable and watery callus on medium supplemented with 4.44 µM BAP and 4.14 µM picloram, showing d cells with large vacuoles. Bars for a and c represents 1 cm; b, d represents 400 µm
Establishment of cell suspension culture
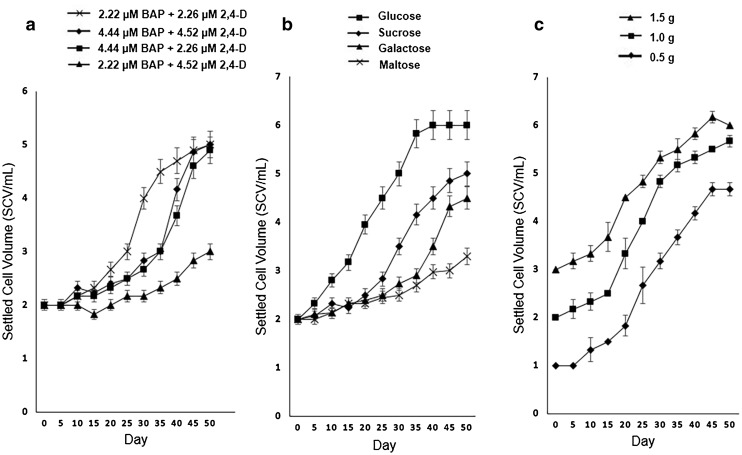
Cell suspension culture of mangosteen was established in MS liquid medium with the different treatments tested. Figure 2a shows that 2.22 µM BAP + 2.26 µM 2,4-D gave the fastest rate of growth where the exponential growth of cells were recorded on day 25 and reached stationary phase subsequent to day 50. All treatments reached stationary phase with 5.0 mL of yield except for treatments in 2.22 µM BAP + 4.52 µM 2,4-D which produced 3.0 mL of yield. As for carbon sources, the best carbon source for suspension cell growth with significantly different final yield was glucose with 6.0 ml of SCV followed by sucrose (5.0 ml), galactose (4.5 ml) and maltose (3.3 ml) (Fig. 2b). Initial inoculum size also influenced the final yield of cells. Figure 2c shows the result on the effect of different inoculum sizes. The growth pattern for all inoculum sizes was similar but the yield differed based on the inoculum size. Inoculum size of 1.5 g yielded higher cell volume throughout the culture period. However, on day 45 of culture with 1.5 g of inoculum size, the cells started to decrease.
Fig. 2.
Growth curves of cell suspension cultures of mangosteen, affected by different treatments: a combination of BAP and 2,4-D; b different carbon sources and c inoculum sizes
Metabolite profile of callus
Following chemical analysis of extracts by LC-TOF-MS and the m/z value and retention times of mass-spectrometry spectral data observed in positive ionization mode are presented in Table 2.
Table 2.
Metabolites identified in mangosteen callus extract using LC-TOF-MS
| m/z value (Retention time) | Ion adduct | Putative metabolites | Class | |
|---|---|---|---|---|
| 1 | 114.126 (15.63 min) | – | Unknown 1 | Others |
| 2 | 116.071 (1.82 min) | M + H | Proline | Amino acid |
| 3 | 125.107 (17.31 min) | M + Na | N-Methylputrescine | Amino acid |
| 4 | 128.110 (17.33 min) | M + H-H2O | Heptanoic acid | Carboxylic acid |
| 5 | 128.112 (9.41 min) | – | Unknown 2 | Others |
| 6 | 130.050 (1.71 min) | M + H | Pyroglutamic acid | Amino acid |
| 7 | 130.050 (2.92 min) | M + ACN + H | Pyruvate | Carboxylic acid |
| 8 | 130.158 (16.50 min) | M + H | Octylamine | Others |
| 9 | 132.101 (2.50 min) | M + H | Leucine | Amino acid |
| 10 | 141.140 (16.22 min) | M + ACN + H | 2-Methylpiperidine | Others |
| 11 | 143.996 (16.98 min) | – | Unknown 3 | Others |
| 12 | 147.076 (1.70 min) | M + H | Glutamine | Amino acid |
| 13 | 153.137 (17.35 min) | M + H | 1,8-Diazabicyclo[5.4.0]undec-7-ene | Others |
| 14 | 156.173 (16.47 min) | M + H | Isopentylideneisopentylamine | Others |
| 15 | 161.026 (0.28 min) | M + H | Thiacremonone | Alkaloid |
| 16 | 162.005 (16.98 min) | – | Unknown 4 | Others |
| 17 | 198.096 (1.84 min) | M + Na | 7-Methylthioheptanaldoxime | Others |
| 18 | 203.051 (1.85 min) | M + Na | beta-D-Hamamelopyranose | Carbohydrate |
| 19 | 235.117 (1.65 min) | M + H-H2O | p-(3,4-Dihydro-6-methoxy-2-naphthyl)phenol | Phenolic acid |
| 20 | 239.149 (7.25 min) | M + H | Agroclavine | Alkaloid |
| 21 | 242.923 (0.27 min) | M + Na | 2-Iodophenol | Phenolic acid |
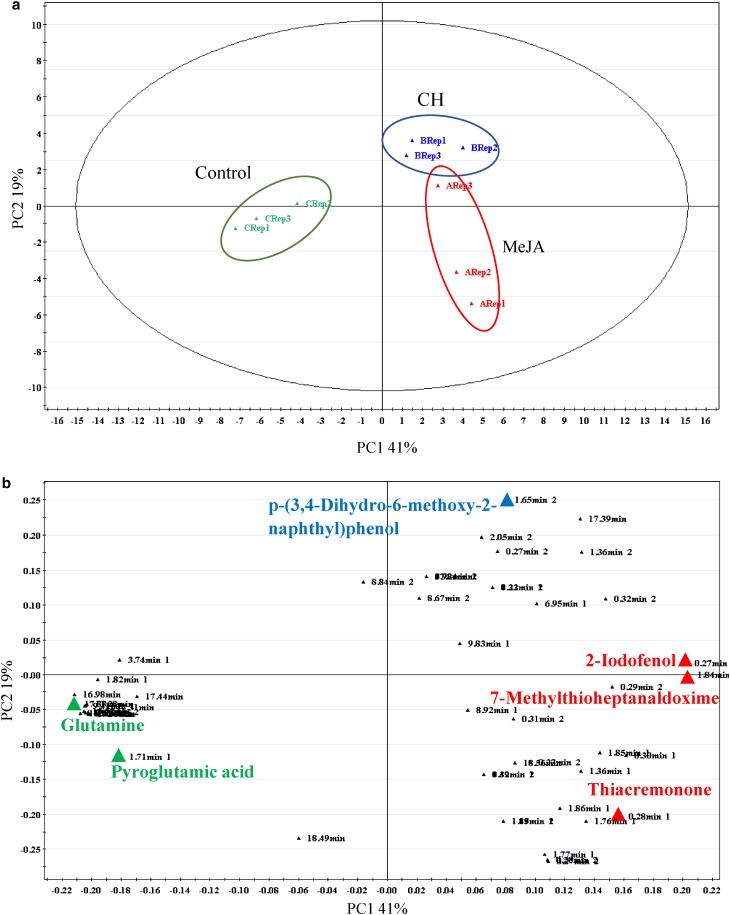
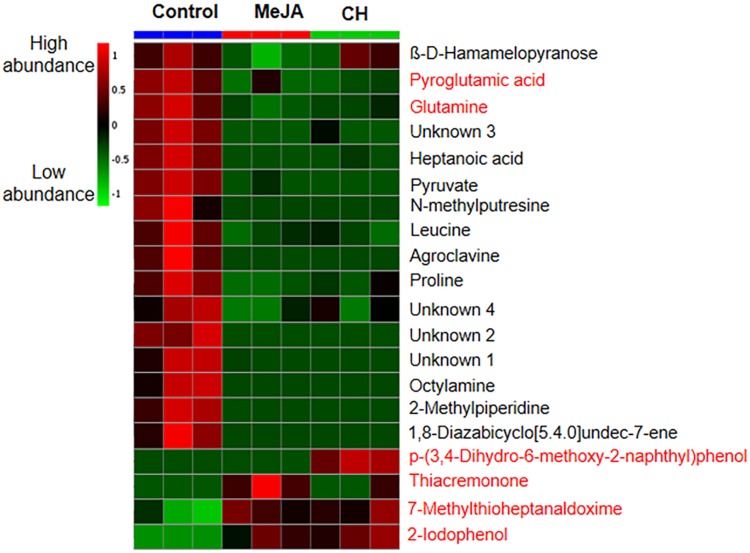
A score contribution plot constructed using the first two principal components (PC1 and PC2, explained the total of 60% variance) (Fig. 3a), and showed that samples were clustered based on metabolites detected in the treatments. The PCA models provided an overall and qualitative visual representation of similarity/dissimilarity between and within the samples (on x- and y-axes respectively). Metabolites from control samples are grouped together and significantly separated from the samples treated with MeJA and CH (Fig. 3a). As revealed in Fig. 3b, amino acid (glutamine and pyroglutamic acid) were highly significant in this plot. Loading plot results also showed that thiacremonone (alkaloid) levels were found in the MeJA-treated samples. Meanwhile, p-(3,4-dihydro-6-methoxy-2-naphthyl) phenol (phenolic acid) was found at greater levels in the CH-treated samples. Higher levels of a glucosinolate, 7-methylthioheptanaldoxime and 2-iodophenol (phenolic acid) were found in both MeJA and CH treatment. Metabolite intensities in the callus are demonstrated by the heatmap (Fig. 4), from which the number of metabolites detected was high in control samples compared to samples treated with MeJA and CH.
Fig. 3.
Principal component analyses of mangosteen untreated callus, callus treated with 100 µM MeJA and callus treated with 500 mg/L CH. a Score plot shows the metabolome clusters located at distinct positions of PC1 (41%) and PC2 (19%); b Loading plot for PC1 and PC2 with contributing mass peaks and their assignments, with each metabolite denoted by its mass/retention time pair
Fig. 4.
Heatmap showing relative abundance of metabolites from callus treated with MeJA and CH. Data means from three biological replicates. Colour scale is relative to the abundance of each compound
Metabolite profile of suspension cells
Cell suspension culture was also treated with MeJA treatment for five days and CH treatment for seven days prior to LC-TOF-MS analysis. Chemical constituents of cell suspension extracts treated with MeJA and CH were analyzed via LC-TOF-MS and the m/z value and retention times MS spectral data observed in positive ionization mode are presented in Table 3.
Table 3.
Metabolites identified in mangosteen suspension cell extract using LC-TOF-MS
| m/z value (Retention time) | Ion adduct | Putative metabolites | Class | |
|---|---|---|---|---|
| 1 | 113.023 (11.85 min) | M + H | Mesaconate | Carboxylic acid |
| 2 | 120.075 (3.84 min) | – | Unknown 5 | Others |
| 3 | 132.098 (2.61 min) | M + H | Isoleucine | Amino acid |
| 4 | 147.074 (1.80 min) | M + H | Glutamine | Amino acid |
| 5 | 166.081 (3.86 min) | M + Na | 3ß,6ß-Dihydroxynortropane | Alkaloid |
| 6 | 175.114 (1.44 min) | M + H | Hexanethioic acid S-propyl ester | Fatty acid |
| 7 | 219.129 (9.06 min) | M + H | L-Lysopine | Amino acid |
| 8 | 223.090 (11.59 min) | – | Unknown 6 | Others |
| 9 | 228.045 (7.55 min) | M + H-H2O | 1-Isothiosianato-6-(metylsulphynil)hexane | Sulfoxide |
| 10 | 235.111 (1.74 min) | M + H-H2O | cis-Hinokiresinol | Alkaloid |
| 11 | 247.021 (9.06 min) | M + Na | 2,3-Dihydroxy-2,4-cyclopentadien-1-one | Ketone |
| 12 | 251.154 (1.31 min) | M + H | Isopentyl beta-D-glucoside | Glycoside |
| 13 | 254.942 (18.67 min) | M + H | Tricamba | Others |
| 14 | 256.076 (2.06 min) | M + H | Nicotinate D-ribonucleoside | Glycosylamines |
| 15 | 275.069 (3.19 min) | M + Na | 7-Methoxyisoflavone | Flavonoid |
| 16 | 332.125 (1.73 min) | M + Na | Koenigine | Alkaloid |
| 17 | 343.114 (1.99 min) | M + Na | Conchosine B | Terpenoid |
| 18 | 526.249 (2.20 min) | M + H | 6-Acetoxyangolensic acid methyl ester | Ester |
| 19 | 530.284 (8.21 min) | M + Na | Ergosine | Alkaloid |
| 20 | 637.295 (15.57 min) | M + Na | Thalsimine | Alkaloid |
| 21 | 652.382 (7.61 min) | – | Unknown 7 | Others |
| 22 | 674.330 (7.35 min) | M + Na | Scutianine F | Alkaloid |
| 23 | 689.414 (7.86 min) | M + H | Glycerophosphate | Lipid |
| 24 | 751.488 (11.85 min) | M + H | Phosphoglycerol | Lipid |
| 25 | 758.386 (8.17 min) | – | Unknown 8 | Others |
| 26 | 758.557 (15.51 min) | – | Unknown 9 | Others |
| 27 | 760.569 (15.40 min) | – | Unknown 10 | Others |
| 28 | 782.550 (15.87 min) | M + H-H2O | Phosphatidyl- ethanolamine | Fatty acid |
| 29 | 784.570 (14.14 min) | M + H | Phosphoglycerol | Lipid |
| 30 | 788.390 (7.26 min) | – | Unknown 11 | Others |
| 31 | 821.421 (9.16 min) | M + H-H2O | Talinumoside I | Triterpene |
| 32 | 889.456 (8.57 min) | M + H | Schidigerasaponin F1 | Triterpene |
| 33 | 907.439 (7.11 min) | M + Na | Schidigerasaponin B1 | Triterpene |
| 34 | 962.474 (8.56 min) | – | Unknown 12 | Others |
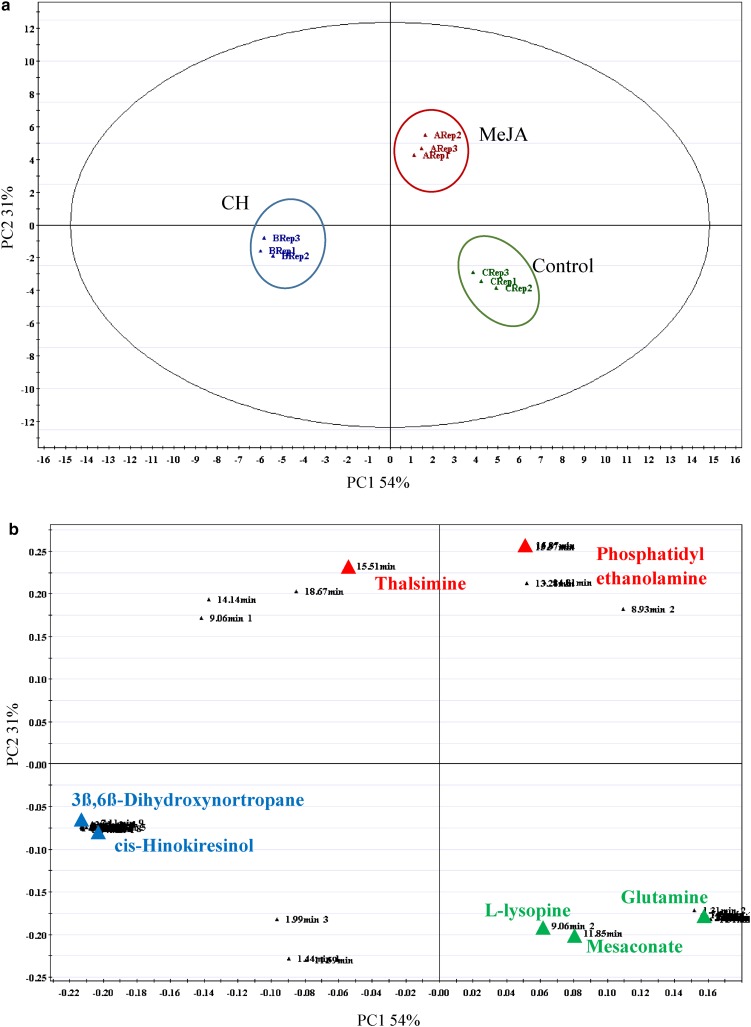
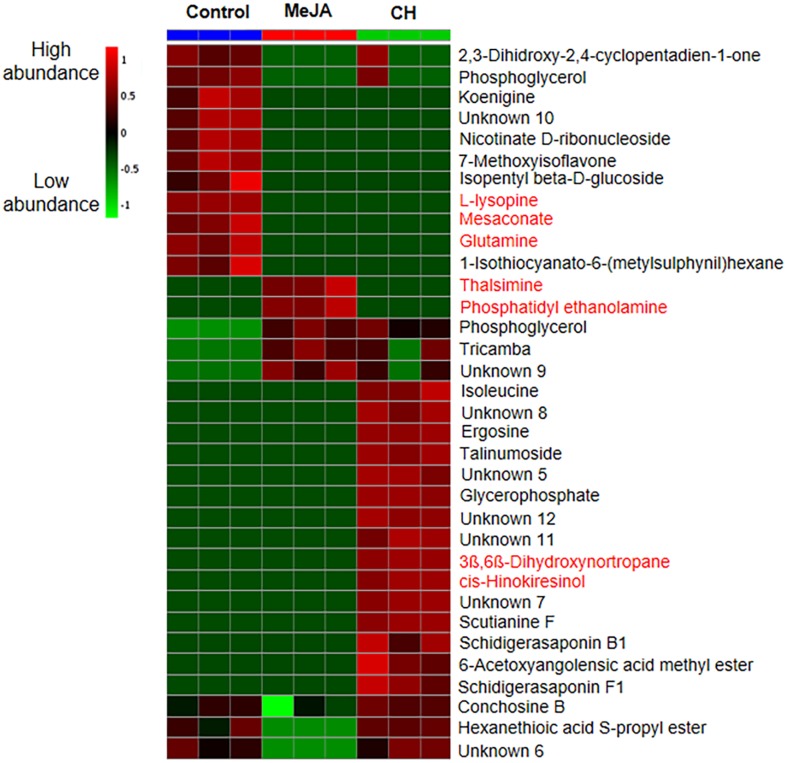
The multivariate data analysis performed on MS data revealed a significant metabolite separation between control, MeJA and CH samples (Fig. 5a) with 85% variance on the PCA score plot. As revealed in Fig. 5b, glutamine, L-lysopine and mesaconic acid were found to be significantly high in the control samples. Treatment of MeJA in cell suspension culture triggered the production of thalsimine (alkaloid) and phosphatidyl ethanolamine (fatty acid). Meanwhile, 3ß,6ß-dihydroxynortropane and cis-hinokiresinol levels, both from class of alkaloids were higher in the CH-treated samples. The metabolite intensities detected in suspension cells are demonstrated by the heatmap (Fig. 6) from which the number of metabolites detected was high in CH treatments compared to MeJA and control treatments.
Fig. 5.
Principal component analyses of mangosteen between untreated suspension cells, suspension cells treated with 100 µM MeJA and suspension cells treated with 500 mg/L CH. a Score plot shows the metabolome clusters located at distinct positions of PC1 (54%) and PC2 (31%). b Loading plot for PC1 and PC2 with contributing mass peaks and their assignments, with each metabolite denoted by its mass/retention time pair
Fig. 6.
Heatmap showing relative abundance of metabolites from suspension cells treated with MeJA and CH. Data means from three biological replicates. Colour scale is relative to the abundance of each compound
Discussion
Induction of callus and establishment of cell suspension culture
Callus induction of mangosteen was carried out previously by Te-chato et al. (1995), Rohani et al. (2012) and Maadon et al. (2016), where different types of callus such as embryogenic, compact, friable, nodular and globular were reported. In this study, different plant growth regulators were tested for friable callus formation, as this type of callus is essential for the establishment of a cell suspension culture where in a liquid medium with agitation, the cells disperse to form single cell suspension throughout the liquid medium (Mustafa et al. 2011). Friable callus induction was highly induced on MS media with BAP + 2,4-D and BAP + picloram. However, the callus induced on medium with BAP + picloram was watery (Fig. 1c) and had non-isodiametric cells with large vacuoles (Fig. 1d) compared to the callus induced on BAP + 2,4-D (Fig. 1a) where the cells were isodiametric with clearly seen nuclei (Fig. 1b). Hence, it was shown that the combination of cytokinin and auxin produced high yield of friable callus and this was also reported in plants such as Eurasian tree (Elaeagnus angustifolia) (Zeng et al. 2009) and ‘Melong kecil’ (Hymenocallis littoralis) (Sundarasekar et al. 2012) when combinations of BAP and 2,4-D were used. In the present study, 65% of the explants produced friable callus when cultured on MS medium supplemented with 4.44 µM BAP + 4.52 µM 2,4-D compared to 26% obtained by Maadon et al. (2016) on the same medium. Friable callus on medium 4.44 µM BAP + 4.52 µM 2,4-D was selected for cell suspension culture studies. Watery friable callus produced in this study was not used for cell suspension culture because the elongated cells with large vacuoles were reported to be of low viability (Padua et al. 2014).
In the present study, three factors were tested: combinations of BAP and 2,4-D, carbon source and inoculum size. Combinations of different concentration of plant growth regulator play important roles in cell division and enlargement (Ikeuchi et al. 2013) to increase the number of suspension cells. In this study, 2.22 µM BAP + 2.26 µM 2,4-D was found to give the fastest growth of cells compared to the other concentrations. Higher rates of cell growth were found when the cells were cultured in a liquid medium containing high or same concentrations of BAP to 2,4-D whereas much lower rates of cell division occurred in medium containing lower concentration (2.22 µM) of BAP than 2,4-D (4.56 µM). The best combination of BAP with 2,4-D, where the concentration of 2,4-D was the same or lower than BAP concentration were reported in other woody plant species for cell suspension culture, such as piper (Piper solmsianum) (Balbuena et al. 2009) and seagrass (Halodule pinifolia) (Subhashini et al. 2014).
Carbon acts as an energy source and can also be signaling molecules that affect growth, development and metabolism of cultured cells (Murthy and Praveen 2013). The present study showed that glucose was the best carbon source followed by sucrose, galactose and maltose. Cell suspension culture of Indian madder (Rubia cardifolia) biomass yield produced was high in glucose compared to sucrose-containing medium (Suzuki et al. 1984). Glucose is more efficiently utilized form of sugars and had preferential uptake by rudgea (Rudgea jasminoides) cell suspension culture where an increase in the biomass production was observed (Kretzschmar et al. 2007). Krishnan et al. (2014) reported that cells treated with glucose showed rapid cell growth which resulted in higher biomass of a type of liverworts (Marchantia linearis) suspension cultures. Glucose also enhanced the growth of mangosteen cells by giving about 17% (1 mL of cells volume) more cells compared to sucrose. Early exponential phase on day 5 was observed when glucose was used, compared to the other carbon sources (day 25) as shown in Fig. 2b.
The present results showed that inoculum size influenced the biomass growth of cell suspension culture. Inoculum size had a positive correlation on the growth of mangosteen suspension cells, with larger inoculum size led to high biomass growth or higher SCV, at 3–4 SCV/mL within 15 days of culture. However, with 1.5 g of inoculum, there was a decrease in growth observed after day 45. Similar growth pattern was observed for cell suspension culture of rose periwinkle (Catharanthus roseus) (Mustafa et al. 2011) and they suggested that the nutrient and carbon sources may have depleted sooner with larger inoculum size. In mangosteen suspension culture, 1.5 g of inoculum in 50 mL medium resulted in optimum level of biomass. Teixeira et al. (1995) also found that 1.5 g was the best inoculum size in 50 mL medium for oil palm cell suspension culture. Meanwhile, Jin et al. (2017) found that 2.0 g was the best inoculum size in 50 mL of culture medium for cell suspension culture of chrysanthemum (Dendrathema indicum). Hence, the inoculum size has to be tested for each species studied as it may vary according to species and culture conditions used.
Metabolite profiles of callus and suspension cells
Plant cell cultures are able to accumulate secondary metabolites in low quantities but the accumulation could be further enhanced by elicitation (Gandi et al. 2012). Secondary metabolites are produced intracellularly in plant cells (Chattopadhyay et al. 2002). In the present study, only intracellular metabolites were studied, as intracellular metabolite concentration is usually much higher than the extracellular one (Villas-Boas 2007). Indeed, in this study, there were several primary and secondary metabolites found even in non-treated cultures, which were not treated with either MeJA or CH. Identified metabolites belonged to various compound classes (Tables 2, 3) including amino acids, phenolic acids, alkaloids, flavonoids, fatty acids and triterpenes. MeJA was introduced as a stress to the cells for the release of secondary metabolites due to a self-defence response (Giri and Zaheer 2016), while CH was introduced as an organic supplement for the cells which interestingly in the present study, produced 16 secondary metabolites more compared to cells treated with MeJA.
An elicitor such as MeJA acts in the cell plasma membrane where the elicitor-binding site induced reactions as defense response (Ramirez-Estrada et al. 2016), and in doing so the cells produce secondary metabolites. In a related research, it was noted that MeJA increased the production of anthraquinones and phenolic acids in Fallopia multiflora (Polygonum multiflorum) cell suspension culture (Thiruvengadam et al. 2016). Similar mechanism probably occurred in the callus and cell culture of mangosteen where MeJA stimulated the production of secondary metabolites. MeJA was also found to be effective in increasing the production of alkaloid yield of rose periwinkle (Catharanthus roseus) (Tonk et al. 2017) and fatty acids in coral tree (Erythrina lysistemon) (Farag et al. 2016).
Adding organic supplements is another approach to increase the yield of secondary metabolites in plant cell culture systems, as shown in antitoothache plant (Spilanthes acmella) suspension cells (Abyari et al. 2016). In the present study, CH was added to stimulate secondary metabolite production in callus and suspension cells. CH is produced by enzymatic or acid hydrolysis of casein, digested with hydrochloric acid and consisting of a mixture of amino acids and peptides (Abyari et al. 2016). CH is usually added to media as it acts as a good organic substrate and is suitable for micropropagation and secondary metabolite production (Kayser and Quax 2008).
CH stimulated the production of terpenoids, alkaloids and fatty acids in callus and cell suspension culture of mangosteen. Phenolic acid p-(3,4-dihydro-6-methoxy-2-naphthyl) phenol found in callus is a metabolite from a group of plant phenolics. CH was reported to increase the production of phenolics in callus culture of Ephedra alata also known as perennial tough shrub (Hegazi and El-Lamey 2012). The 3ß,6ß-dihydroxynortropane which belongs to the alkaloid group was found in suspension cells in this study. The production of alkaloid was reported to be high in suspension cells of coniferous tree (Taxus spp.) (Wu et al. 2001) and wild rue (Peganum harmala L.) (Ebrahimi and Zarinpanjeh 2015) when treated with CH. The production of secondary metabolites in suspension cells treated with CH was higher than those treated with MeJA due to its sterols or amino acid content which might have stimulated the secondary metabolite production as suggested by Abyari et al. (2016).
In the present study, three types of triterpenes (a class of lipids from isoprene moieties) were detected in suspension cells treated with CH, namely schidigerasaponin B1, schidigerasaponin F1 and talinumoside 1. Previous study also found that, the addition of CH could stimulate production of terpenes such as saponin in ginseng (Panax ginseng) (Wu et al. 2005) and artemisin in sweet wormword (Artemisia annua) (Woerdenbag et al. 1993).
In general, the number of secondary metabolite production in callus treated with MeJA or CH was low (21 significant metabolites) compared to that of suspension cells, which produced 34 significant metabolites. This might be due to the condition of cells where the callus was not agitated on the agar medium compared to suspension cells in the liquid medium where agitation was involved. Agitation promotes better growth by enhancing the transfer of nutrients or stresses from the liquid to the cells and adequate mixing occurs for the production of certain or specific metabolites (Murthy et al. 2014). Since the suspension cells were continuously grown in culture, even in the non-treated cell cultures, dynamic metabolite profiles were exhibited as shown in the present study where not only the primary metabolites were produced but also secondary metabolites. Alkaloids such as koenigine and flavonoids such as 7-methoxyisoflavone were detected in the non-treated suspension cells.
The results obtained in this study showed that it was possible to induce friable callus and suspension cell culture from mangosteen young leaves. MeJA and CH were shown to trigger differential changes in the metabolome of mangosteen callus and suspension cells, leading to the biosynthesis of a broad range of metabolites, some of which are of significant potential. This research was the first attempt on metabolite profiling of mangosteen callus and suspension cells based on the application of an elicitor (MeJA) and an organic supplement (CH). The groups of identified compounds could serve as a basis in the search of novel or important bioactive compounds. This work demonstrates the possibility of producing desired metabolites from in vitro cultures for pharmaceutical and nutraceutical industries.
Acknowledgements
This research was funded by Research University Grant under the Economic Transformation Programme (UKM-ETP-2013-015) from Universiti Kebangsaan Malaysia. We also acknowledge Centre for Research and Instrumentation Management (CRIM), UKM for the Research Instrumentation Development Grants, PIP-CRIM 2010 and PIP-CRIM 2012.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Abyari M, Nasr N, Soorni J, Sadhu D. Enhanced accumulation of scopoletin in cell suspension culture of Spilanthes acmella Murr. using precursor feeding. Braz Arch Biol Technol. 2016;59:1–7. doi: 10.1590/1678-4324-2016150533. [DOI] [Google Scholar]
- Balbuena TS, Santa-Catarina C, Silveira V, Kato MJ, Floh EIS. In vitro morphogenesis and cell suspension culture establishment in Piper solmsianum C. DC. (Piperaceae) Acta Bot Bras. 2009;23:274–281. doi: 10.1590/S0102-33062009000100029. [DOI] [Google Scholar]
- Cadahia E, Fernandez de Simon B, Aranda I, Sanz M, Sanchez-Gomez D, Pinto E. Non-targeted metabolomic profile of Fagus sylvatica L. leaves using liquid chromatography with mass spectrometry and gas chromatography with mass spectrometry. Phytochem Anal. 2015;26:171–182. doi: 10.1002/pca.2549. [DOI] [PubMed] [Google Scholar]
- Cetin ES, Baydar NG. Elicitor application to cell suspension culture for production of phenolic compounds in grapevine. J Agric Sci. 2014;22:42–53. [Google Scholar]
- Chattopadhyay S, Farkya S, Srivastava AK, Bisaria VS. Bioprocess consideration for production of secondary metabolites by plant cell suspension cultures. Biotechnol Bioprocess Eng. 2002;7:138–149. doi: 10.1007/BF02932911. [DOI] [Google Scholar]
- Chetri SK, Kapoor H, Agrawal V. Marked enhancement of sennoside bioactive compounds through precursor feeding in Cassia angustifolia Vahl and cloning of isochorismate synthase gene involved in its biosynthesis. Plant Cell Tiss Organ Cult. 2016;124:431–446. doi: 10.1007/s11240-015-0905-1. [DOI] [Google Scholar]
- Ebrahimi MA, Zarinpanjeh N. Bio-elicitation of β-carboline alkaloids in cell suspension culture of Peganum harmala L. J Med Plants Res. 2015;14:43–57. [Google Scholar]
- Farag MA, Mekky H, El-Masry S. Metabolomics driven analysis of Erythrina lysistemon cell suspension culture in response to methyl jasmonate elicitation. J Adv Res. 2016;7:681–689. doi: 10.1016/j.jare.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Loo AEK, Chia FPP, Huang D. Oligomeric proanthocyanidins from mangosteen pericarps. J Agric Food Chem. 2007;55:7689–7694. doi: 10.1021/jf071166n. [DOI] [PubMed] [Google Scholar]
- Gandi S, Rao K, Chodisetti B, Giri A. Elicitation of andrographolide in the suspension cultures of Andrographis paniculata. Appl Biochem Biotechnol. 2012;168:1729–1738. doi: 10.1007/s12010-012-9892-4. [DOI] [PubMed] [Google Scholar]
- Giri CC, Zaheer M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tiss Organ Cult. 2016;126:1–18. doi: 10.1007/s11240-016-0985-6. [DOI] [Google Scholar]
- Han JY, Wang HY, Choi YE. Production of dammarenediol-II triterpene in a cell suspension culture of transgenic tobacco. Plant Cell Rep. 2014;33:225–233. doi: 10.1007/s00299-013-1523-1. [DOI] [PubMed] [Google Scholar]
- Hegazi GAE-M, El-Lamey TM. In vitro production of some phenolic compounds from Ephedra alata Decne. J Appl Environ Biol Sci. 2012;1:158–163. [Google Scholar]
- Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25:3159–3173. doi: 10.1105/tpc.113.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JT, Tugizimana F, Steenkamp PA, Dubery IA. Metabolomic analysis of methyl jasmonate-induced triterpenoid production in the medicinal herb Centella asiatica (L.) Urban. Molecules. 2013;18:4267–4281. doi: 10.3390/molecules18044267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yang Y, Gao W, Gong M, Wang J, Anderson NO, He M. Establishment of callus induction and cell suspension cultures of Dendrathema indicum var. aromaticum a scented chrysanthemum. J Pl Sci. 2017;6:38–44. [Google Scholar]
- Kamalipourazad M, Sharifi M, Maivan HZ, Behmanesh M, Chashmi NA. Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. Plant Physiol Biochem. 2016;107:374–384. doi: 10.1016/j.plaphy.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Kayser O, Quax WJ. Medicinal plant biotechnology: from basic research to industrial applications. Weinheim: Wiley-VCH Verlag GmbH; 2008. [Google Scholar]
- Kretzschmar FS, Oliveira CJF, Jr, Braga MR. Differential sugar uptake by cell suspension cultures of Rudgea jasminoides, a tropical woody Rubiaceae. In Vitro Cell Dev Biol Plant. 2007;43:71–78. doi: 10.1007/s11627-006-9001-x. [DOI] [Google Scholar]
- Krishnan R, Anil Kumar VS, Murugan K. Establishment of cell suspension culture in Marchantia linearis Lehm & Lindenb. for the optimum production of flavonoids. 3 Biotech. 2014;4:49–56. doi: 10.1007/s13205-013-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maadon SN, Rohani ER, Ismail I, Baharum SN, Normah MN. Somatic embryogenesis and metabolic differences between embryogenic and non-embryogenic structures in mangosteen. Plant Cell Tiss Organ Cult. 2016;127:443–459. doi: 10.1007/s11240-016-1068-4. [DOI] [Google Scholar]
- Mari S, Engelmann F, Chabrillange N, Huet C, Michaux-Ferriere Histo-cytological study of apices of coffee (Coffea recemosa and C. sessiliflora) in vitro plantlets during their cryopreservation using the encapsulation-dehydration technique. Cryo Letters. 1995;16:289–298. [Google Scholar]
- Misra RC, Maiti P, Chanotiya CS, Shanker K, Ghosh S. Methyl jasmonate-elicited transcriptional responses and pentacyclic triterpene biosynthesis in sweet basil. Plant Physiol. 2014;164:1028–1044. doi: 10.1104/pp.113.232884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murthy HN, Praveen N. Carbon sources and medium pH affects the growth of Withania somnifera (L.) Dunal. adventitious roots and withanolide A production. Nat Prod Res. 2013;27:185–189. doi: 10.1080/14786419.2012.660691. [DOI] [PubMed] [Google Scholar]
- Murthy HN, Lee EJ, Paek KY. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Organ Cult. 2014;118:1–16. doi: 10.1007/s11240-014-0467-7. [DOI] [Google Scholar]
- Mustafa NR, de Winter W, van Iren F, Verpoorte R. Initiation, growth and cryopreservation of plant cell suspension cultures. Nat Protoc. 2011;6:715–742. doi: 10.1038/nprot.2010.144. [DOI] [PubMed] [Google Scholar]
- Padua MS, Paiva LV, Silva LCD, Alves E, Castro AHF. Morphological characteristics and cell viability of coffee plants calli. Cienc Rural. 2014;44:660–665. doi: 10.1590/S0103-84782014000400014. [DOI] [Google Scholar]
- Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusido RM, Palazon J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182. doi: 10.3390/molecules21020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Usha K. Production of secondary metabolites from callus cultures of Centella asiatica (L.) Urban. Ann Phytomed. 2015;4:74–78. [Google Scholar]
- Rohani ER, Ismanizan I, Noor NM. Somatic embryogenesis of mangosteen. Plant Cell Tiss Organ Cult. 2012;110:251–259. doi: 10.1007/s11240-012-0147-4. [DOI] [Google Scholar]
- Shan Y, Zhang W. Preparative separation of major xanthones from mangosteen pericarp using high-performance centrifugal partition chromatography. J Sep Sci. 2010;33:1274–1278. doi: 10.1002/jssc.200900774. [DOI] [PubMed] [Google Scholar]
- Smetanska I. Production of secondary metabolites using plant cell cultures. In: Stahl U, Donalies UE, Nevoigt E, editors. Food biotechnology. Advances in biochemical engineering/biotechnology. Berlin: Springer, Verlag; 2008. pp. 187–228. [DOI] [PubMed] [Google Scholar]
- Subhashini P, Raja S, Thangaradjou T. Establishment of cell suspension culture protocol for a seagrass (Halodule pinifolia): growth kinetics and histomorphological characterization. Aquat Bot. 2014;117:33–40. doi: 10.1016/j.aquabot.2014.04.005. [DOI] [Google Scholar]
- Sundarasekar J, Anthony JJJ, Murugaiyah V, Subramaniam S. Preliminary responses of 2,4-D and BAP on callus initiation of an important medicinal-ornamental Hymenocallis littoralis plants. J Med Plants Res. 2012;6:2088–2093. [Google Scholar]
- Sundram TCM, Annuar MSM, Khalid N. Optimization of culture condition for callus induction from shoot buds for establishment of rapid growing cell suspension cultures of Mango ginger (Curcuma mangga) Aust J Crop Sci. 2012;6:1139–1146. [Google Scholar]
- Suzuki S, Matsumoto T, Mikami Y. Effects of nutritional factors on the formation of anthraquinones by Rubia cordifolia plant cells in suspension cultures. Agric Biol Chem. 1984;48:603–610. [Google Scholar]
- Te-Chato S, Lim M. Plant regeneration of mangosteen via nodular callus formation. Plant Cell Tiss Organ Cult. 1999;59:89–93. doi: 10.1023/A:1006482513727. [DOI] [Google Scholar]
- Te-Chato S, Lim M. Improvement of mangosteen micropropagation through meristematic nodular callus formation from in vitro-derived leaf explants. Sci Hort. 2000;86:291–298. doi: 10.1016/S0304-4238(00)00154-0. [DOI] [Google Scholar]
- Te-chato S, Lim M, Suranilpong P. Embryogenic callus induction in mangosteen (Garcinia mangostana L.) Songklanakarin J Sci Technol. 1995;1:115–120. [Google Scholar]
- Teixeira JB, Söndahl MR, Nakamura T, Kirby EG. Establishment of oil palm cell suspensions and plant regeneration. Plant Cell Tiss Organ Cult. 1995;40:105–111. doi: 10.1007/BF00037662. [DOI] [Google Scholar]
- Thiruvengadam M, Rekha K, Rajakumar G, Lee T-j, Kim S-h, Chung I-m (2016) Enhanced production of anthraquinones and phenolic compounds and biological activities in the cell suspension cultures of Polygonum multiflorum. Int J Mol Sci 17:1912 [DOI] [PMC free article] [PubMed]
- Tonk D, Mujib A, Ali M, Zafar N. Elicitors enhance alkaloid yield in Catharanthus roseus. In: Naeem M, Aftab T, Khan M, editors. Catharanthus roseus. Cham: Springer; 2017. pp. 101–119. [Google Scholar]
- Villas-Boas SG. Sampling and sample preparation. In: Villas-Boas SG, Roessner U, Hansen MAE, Smedsgaard J, Nielsen J, editors. Metabolome analysis: an introduction. Hoboken: Wiley; 2007. pp. 39–82. [Google Scholar]
- Wang J, Qian J, Yao L, Lu Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour Bioprocess. 2015;2:5. doi: 10.1186/s40643-014-0033-5. [DOI] [Google Scholar]
- Woerdenbag H, Luers J, van Uden W, Pras N, Malingre T, Alfermann A. Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tiss Organ Cult. 1993;32:247–257. doi: 10.1007/BF00029850. [DOI] [Google Scholar]
- Wu J, Wang C, Mei X. Stimulation of taxol production and excretion in Taxus spp. cell cultures by rare earth chemical lanthanum. J Biotechol. 2001;85:67–73. doi: 10.1016/S0168-1656(00)00383-7. [DOI] [PubMed] [Google Scholar]
- Wu JY, Wong K, Ho KP, Zhou LG. Enhancement of saponin production in Panax ginseng cell culture by osmotic stress and nutrient feeding. Enzyme Microb Technol. 2005;36:133–138. doi: 10.1016/j.enzmictec.2004.07.010. [DOI] [Google Scholar]
- Zadernowski R, Czaplicki S, Naczk M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana) Food Chem. 2009;112:685–689. doi: 10.1016/j.foodchem.2008.06.030. [DOI] [Google Scholar]
- Zarena AS, Udaya Sankar K. Isolation and identification of pelargonidin 3-glucoside in mangosteen pericarp. Food Chem. 2012;130:665–670. doi: 10.1016/j.foodchem.2011.07.106. [DOI] [Google Scholar]
- Zeng F-S, Wang W-W, Zhan Y-G, Xin Y. Establishment of the callus and cell suspension culture of Elaeagnus angustifolia for the production of condensed tannins. Afr J Biotechnol. 2009;8:5005–5010. [Google Scholar]