Abstract
Neural pathways regulate immunity and inflammation via the inflammatory reflex and specific molecular targets can be modulated by stimulating neurons. Neuroimmunomodulation by nonpharmacological methods is emerging as a novel therapeutic strategy for inflammatory diseases, including kidney diseases and hypertension. Electrical stimulation of vagus neurons or treatment with pulsed ultrasound activates the cholinergic anti-inflammatory pathway (CAP) and protects mice from acute kidney injury (AKI). Direct innervation of the kidney, by afferent and efferent neurons, might have a role in modulating and responding to inflammation in various diseases, either locally or by providing feedback to regions of the central nervous system that are important in the inflammatory reflex pathway. Increased sympathetic drive to the kidney has a role in the pathogenesis of hypertension, and selective modulation of neuroimmune interactions in the kidney could potentially be more effective for lowering blood pressure and treating inflammatory kidney diseases than renal denervation. Use of optogenetic tools for selective stimulation of specific neurons has enabled the identification of neural circuits in the brain that modulate kidney function via activation of the CAP. In this Review we discuss evidence for a role of neural circuits in the control of renal inflammation as well as the therapeutic potential of targeting these circuits in the settings of AKI, kidney fibrosis and hypertension.
The prevalence of kidney disease is increasing worldwide1,2. In the USA, estimates suggest that more than 20 million people have kidney disease, of whom nearly 700,000 have reached end-stage renal disease (ESRD) requiring dialysis or transplantation2. The incidence of acute kidney injury (AKI) is also increasing3,4. AKI is associated with high mortality, morbidity, risk of chronic kidney disease (CKD) and ESRD5. As a consequence of gaps in our understanding of the pathophysiology of AKI, limited clinical and pathological data, adverse effects of pharmacological agents6,7, and a lack of data in good animal models8, effective treatment of AKI in well-designed clinical trials remains elusive. No approved pharmacological agents exist for the prevention and treatment of AKI, thus novel therapies and innovative approaches are needed.
Neuroimmunomodulation is an important area of therapeutics that is based on the bidirectional control by the brain of the physiological functions of various organ systems, including the cardiovascular, digestive, respiratory, and immune systems. Advances in the elucidation of neural circuits that control immunity and inflammation9–12 offer new approaches to therapies for kidney disease13. An NIH initiative on peripheral nerve control of organ function (Stimulating Peripheral Activity to Relieve Conditions) aims to catalyse the development of next-generation therapies based on neuromodulatory control of organ function14.
Accumulating evidence exists of the value in using electrodes to modulate nerve signalling as a new nonpharmacologic method for the control of hypertension, heart failure, obesity, epilepsy, inflammation, diabetes mellitus, bronchoconstriction (forming the basis of anticholinergic treatment of chronic obstructive pulmonary disease), migraine and other diseases15–18. In particular, vagus nerve stimulation (VNS) attenuates inflammation, and preliminary early clinical trials show efficacy of VNS for the treatment of rheumatoid arthritis19 and Crohn disease20. To date, however, none of the published clinical studies of VNS include kidney disease.
Collaborative, multidisciplinary approaches have the potential to shed new light on the pathogenesis of AKI and other kidney diseases, as well as on kidney-related diseases such as hypertension, and could lead to innovative therapeutic interventions. In this Review, we high-light the molecular and cellular basis for neural control of immunity as a paradigm to guide novel approaches for the treatment of kidney disease.
Regulation of immunity by neural reflexes
The nervous and immune systems have long been studied independently. Over the last few decades, however, considerable advances have led to the understanding that these two systems are inextricably linked to maintain normal homeostasis as well as to respond to stress and pathophysiological disorders21.
In the 19th century, the germ theory advanced by Louis Pasteur22, and later by Robert Koch23, demonstrated that pathogens cause disease. Subsequently, Claude Bernard described the milieu or environment necessary to maintain equilibrium and health24, and Lewis Thomas elegantly described the importance of host sensing of bacteria and the host response to bacterial products, such as lipopolysaccharide (LPS)25. During sepsis, immediate activation of both pro-inflammatory and anti-inflammatory immune responses occurs and the balance of these pathways is important for favourable outcomes26. A hyper-inflammatory pathway in the absence of the counterbalancing anti-inflammatory pathway might lead to overwhelming inflammation and death. Thus, the immediate early anti-inflammatory response seems to be critical to maintain balance.
A well-known mechanism of the neuroimmune control of inflammation is dependent upon the hypothalamic–pituitary–adrenal axis27. During stress, the pituitary gland produces adrenocorticotropic hormone and the adrenal glands are stimulated to release hormones that affect almost all types of immune cells. These immune cells perform immunosuppressive and anti-inflammatory functions through various genomic and non-genomic mechanisms27.
A seminal study by Linda Watkins provided early evidence that the nervous system also directly initiates the response to inflammation28. She demonstrated that IL-1β-induced hyperthermia could be blocked by subdiaphragmatic vagotomy, indicating that the afferent limb of the vagus nerve is necessary to induce the febrile response to intraperitoneal injection of IL-1β. Sensory neurons in close proximity to immune cells in the periphery respond to inflammatory products and send signals to the central nervous system (CNS)29–31. Afferent sensory nerve fibres also detect bacterial products (pathogen-associated molecular patterns), pro-inflammatory cytokines, immunoglobulins and ATP32.
The sensory afferent vagus nerve expresses IL-1β receptors33, tumour necrosis factor (TNF) receptors34, Fc receptors35, Toll-like receptor 4 and P2X purinergic receptors36,37. Upon activation, this nerve sends signals to and activates neurons of the brainstem nucleus tractus solitarius30,38,39, which is one of its primary terminal fields40. This activation culminates in activation of efferent vagus nerve signals that suppress monocyte and/or macrophage production of inflammatory cytokines such as TNF and IL-69, and attenuate inflammation in the liver, heart41, pancreas42, gastrointestinal tract43–45 and kidney13. This vagus nerve circuit is referred to as the inflammatory reflex. VNS has been exploited to modulate the immune system46 and is being studied as a nonpharmacological tool for neuroimmunomodulation in disorders including myocardial infarction, colitis, pancreatitis, ischaemia–reperfusion injury, sepsis and arthritis47.
The cholinergic anti-inflammatory pathway
Once bacteria or bacterial products penetrate the initial barriers and gain access to the blood, the next line of defence is the reticuloendothelial system — tissues including those of the spleen, liver, lung, and peritoneum48 that contain phagocytic myeloid cells (presumably macrophages) and are the terminal target of the inflammatory reflex. The spleen is a major component of the reticuloendothelial system and an important source of TNF production as evidenced by the finding that VNS-induced inhibition of TNF production is attenuated in splenectomized animals49.
The efferent arm of the inflammatory reflex, which mediates inhibition of systemic inflammation by VNS, is termed the cholinergic-anti-inflammatory pathway (CAP)9. This pathway also requires the spleen49,50. Vagus efferent neurons are cholinergic and upon activation release acetylcholine from their nerve terminals. Although the spleen contains acetylcholine, vagus nerve fibres, which originate in the brainstem in the dorsal motor nucleus of the vagus, do not innervate the spleen51,52. Noradrenergic nerve fibres in the spleen, which originate in the coeliac ganglion and produce noradrenaline as their primary neurotransmitter, terminate in the white pulp around splenic T cells50,51. Despite the absence of cholinergic innervation, splenic acetylcholine levels increase following inflammatory reflex stimulation53.
Some immune cells, such as T cells, dendritic cells and macrophages, can synthesize and secrete neurotransmitters and express neurotransmitter receptors that permit control of the immune response to infection by the CNS and peripheral nervous system54. Rosas-Ballina et al. found that CD4+ T cells provide a link between VNS-mediated activation of the inflammatory reflex, sympathetic innervation of the spleen and increased acetylcholine levels53. In response to noradrenaline, CD4+ T cells release acetylcholine53, likely via β-adrenergic receptor stimulation55. In mice, VNS produced a rapid increase in acetylcholine levels in the spleen that peaked within 20 min53.
To identify the cells that produce acetylcholine in the spleen, Rosas-Ballina et al. used mice that express enhanced green fluorescent protein (eGFP) under the control of transcriptional regulatory elements for choline acetlytransferase (ChAT), which catalyses the biosynthesis of acetylcholine53. Using flow cytometry analysis, they showed that CD4+ T cells that express ChAT (ChAT-eGFP + T cells) can be defined phenotypically as CD44highCD62Llow memory T cells. In dual labelling studies, neuronal synapses expressing synaptophysin were localized adjacent to ChAT-eGFP + T cells within the white pulp of the spleen, providing an anatomical basis for splenic nerve fibres interacting with acetylcholine-producing T cells. Acetylcholine released from these T cells is thought to bind to macrophages in the spleen53.
Macrophages express α7 nicotinic acetylcholine receptors (α7nAChR), and VNS or nicotine, which is an α7nAChR agonist, suppress LPS-induced increases in serum TNF levels and splenic TNF content, or macrophage TNF release, respectively56. Moreover, VNS failed to inhibit LPS-induced increases in serum TNF in α7nAChR-deficient mice56. Thus acetylcholine-synthesizing splenic CD4 + T cells link vagus nerve signals with the anti-inflammatory role of the spleen53 (FIG. 1). The cellular mechanism underlying α7nAChR-induced suppression of TNF production involves inhibition of STAT3 phosphorylation and sequestration of NF-κB21,57,58. Although many of the functional components of the CAP have been defined, ongoing studies of these mechanisms suggest that the principles of reflex control of immunity can be exploited in other tissues32.
Figure 1 |. The inflammatory reflex.
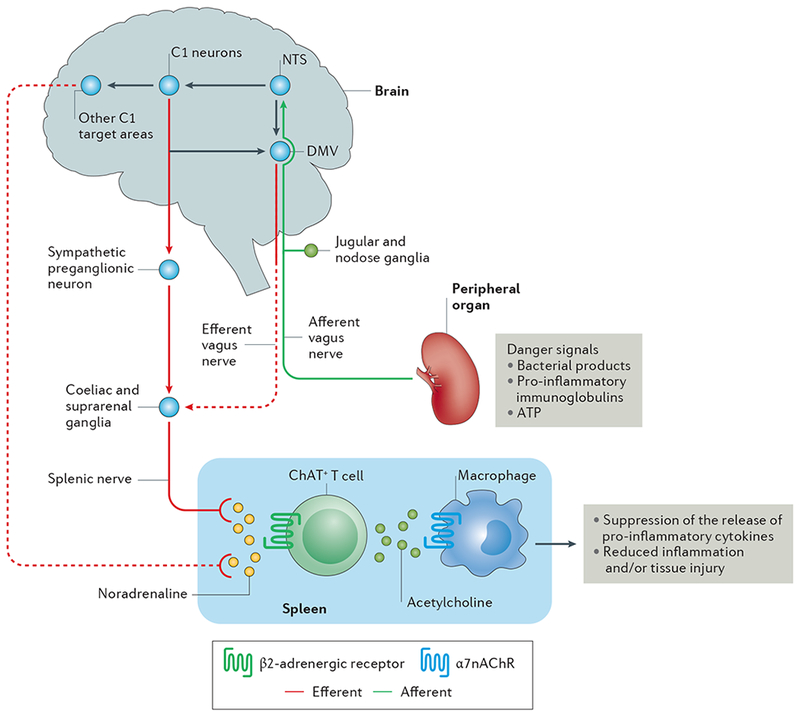
Afferent vagus nerve fibres transmit danger signals from peripheral organs to the nucleus tractus solitarius (NTS). This signalling leads to activation of the efferent vagus nerve arising in the dorsal motor nucleus of the vagus (DMV), and of the splenic nerve, leading to noradrenaline release in the spleen. This noradrenaline release activates choline acetyltransferase positive (ChAT+) T cells, which express β2-Activated ChAT + T cells release acetylcholine, which binds to α7 nicotinic acetylcholine receptors (α7nAChRs) on macrophages, leading to suppression of the release of pro-inflammatory cytokines and reduced inflammation and/or tissue injury. In addition to activation of this cholinergic anti-inflammatory pathway (CAP) by this vagal preganglionic efferent pathway, direct stimulation of brainstem C1 neurons (or indirect stimulation by a variety of physiological stressors) elicits activation of the CAP via a sympathetic efferent pathway76 that might involve C1 projections to sympathetic preganglionic neurons (which innervate sympathetic ganglia such as the coeliac and suprarenal ganglia) or other C1 projections in the brain that stimulate a sympathetic pathway. Dashed lines represent unconfirmed pathways, and solid lines represent confirmed pathways.
The inflammatory reflex pathway in AKI
Despite the critical role of inflammation in the pathogenesis of AKI, few studies have addressed the role of the vagus nerve or the inflammatory reflex pathway in this setting. In rodents, pretreatment with nicotine or the nicotine analogue GTS-21 attenuated renal ischaemia–reperfusion injury (IRI)59 or septic AKI60, suggesting that nicotinic acetylcholine receptors might mediate an anti-inflammatory response after kidney injury. Preclinical studies of VNS and pulsed ultrasound application have also provided evidence of a role of the CAP in protection from AKI.
Vagus nerve stimulation
In mice, electrical stimulation of vagus afferent or efferent nerves 24 h before IRI markedly attenuated kidney injury and caused a decrease in plasma TNF levels13. This protective effect could be abolished by splenectomy 7 days before VNS. The protective effect of VNS was also lost in mice deficient in α7nAChR, and conditioned α7nAChR splenocytes from VNS-treated donor mice transferred protection when injected into naive recipients subjected to IRI. These findings demonstrate that α7nAChR positive splenocytes are required for VNS-induced attenuation of AKI and systemic inflammation.
AKI has an important role in determining outcomes in transplantation. In particular, brain death of the donor results in the release of pro-inflammatory mediators and an increase in adhesion molecules that leads to inflammation and pretransplantation injury to peripheral organs, including the kidney61. The inflammatory milieu in the deceased donor could, therefore, contribute to shortened graft survival in the recipient61.
Hoeger and co-workers showed that in rats, VNS attenuated the pro-inflammatory response to brain death (that is, an increase in serum TNF levels and in the expression of pro-inflammatory genes in the small intestine, liver, kidney and heart)61. Moreover, VNS of brain-dead donor rats before kidney transplantation resulted in improved recipient and organ survival61,62. At 16 weeks post-transplantation, serum creatinine levels were significantly lower in recipients of kidneys from donors that had undergone VNS than in recipients of kidneys from donors that had not undergone VNS62. Moreover, histological analysis showed that kidneys from rats that had undergone VNS had reduced vasculopathy and tubulopathy compared with controls. These findings suggest that VNS of deceased kidney donors before transplantation could potentially lead to improved outcomes in the recipient, but this hypothesis has not yet been evaluated clinically. This concept also raises questions of whether VNS in transplant recipients might also be beneficial, and whether performing VNS in both donors and recipients might further increase the efficacy of the intervention.
VNS therapy using a device that is surgically implanted to stimulate the vagus nerve was approved for the treatment of medically refractory epilepsy in Europe in 1994 and in the USA by the FDA in 1997 (REF. 63). In addition, the FDA approved VNS therapy for treatment-resistant depression in 2005 (REF. 63). As of August 2014, over 100,000 VNS devices had been implanted in more than 75,000 patients worldwide64. Clinical trials of VNS for the treatment of a wide variety of disorders, such as heart failure, hypertension, inflammation and diabetes, are on-going.
Two non-invasive external devices have now been developed to stimulate the vagus nerve through the skin63,64. Transcutaneous VNS can be accomplished using a dedicated intra-auricular electrode (similar to an earphone) that stimulates the auricular branch of the vagus nerve64. In addition, a transcutaneous device has been developed that can deliver a proprietary, low-voltage electrical signal to the cervical vagus nerve64. In 2016, transcutaneous cervical VNS was reported to protect against cerebral ischaemic injury in rats65. In addition, a pilot trial showed that VNS using a gammaCore® transcutaneous device could downregulate inflammatory cytokine release in healthy individuals66. These findings suggest that a non-invasive vagus nerve stimu lator could be safely applied in clinical practice and should be evaluated as a potential preventive therapy for ischaemic AKI and other inflammatory conditions.
Ultrasound stimulation
Similar to VNS, delivery of pulsed ultrasound to the spleen using a clinical ultrasound machine could prevent AKI in mice67,68. A single ultrasound application 1–2 days before IRI suppressed systemic inflammation and attenuated AKI. The efficacy waned in a time-dependent manner when ultrasound was applied up to 7 days before IRI68. The mechanism of this persistent activation state is unknown, but the effect can be thought of as analogous to that of a vaccine that protects against tissue injury resulting from AKI or sepsis, as well as against the systemic dysregulated immune response in these conditions. A protective effect of ultrasound in AKI has also been shown in a model of sepsis induced by caecal ligation and puncture69.
Several findings suggest that ultrasound-induced protection of the kidney from IRI occurs through activation of the CAP67,68. First, the spleen is required for this protective effect as evidenced by its abrogation in mice that were splenectomized 7 days before ultrasound treatment68. Second, chemical sympathectomy of the spleen using direct splenic injections of 6-hydroxydopamine (a neurotoxin that destroys cate cholaminergic neurons70) 14 days before ultrasound abolished the protective effect69, indicating a requirement for innervation of the spleen. Third, the protective effect of ultrasound was absent in Rag1−/− mice, which lack T cells and B cells, but could be reconstituted by adoptive transfer of CD4+ T cells68. Fourth, a series of experiments demonstrated that α7nAChR-positive splenocytes were necessary for the protective effect of ultrasound69. This protective effect was observed in chimeric mice that expressed α7nAChR in bone-marrow-derived cells, but not in those that lacked α7nAChR expression in these cells. Moreover, splenocytes that were isolated from ultrasound-treated wild-type donor mice protected wild-type recipients from IRI, whereas splenocytes from ultrasound-treated α7nAChR−/− donor mice did not confer such protection69. Finally, the protective effect of ultrasound was absent in mice that were treated with α-bungarotoxin, which blocks α7nAChRs, and was mimicked in mice treated with an α7nAChR agonist68. Taken together, these results strongly suggest that similar to VNS, ultrasound-mediated protection from IRI is due to activation of the CAP, and that stimu lation of the CAP using ultrasound is a promising nonpharmacological and non-invasive strategy for prevention of AKI.
CNS regulation of the inflammatory reflex
In addition to activating the inflammatory reflex pathway by signals in the periphery (involving neurons of the peripheral nervous system), evidence from early studies suggests that the brain can control the inflammatory reflex. For example, in rats, intracerebroventricular injection of the anti-inflammatory agent CNI-1493 (a tetravalent guanylhydrazone) suppressed carrageenan-induced paw oedema and inflammation71. These effects of CNI-1493 were abrogated by atropine, a muscarinic acetylcholine receptor (mAChR) antagonist, or by bilateral cervical vagotomy. These findings are consistent with activation of the inflammatory reflex pathway from sites in the CNS and demonstrate the requirement for an intact vagus nerve in mediating the central effects of CNI-1493. Similarly intracerebroventricular injection of a mAChR agonist blocked endotoxin-induced systemic inflammation in rats72. These studies demonstrate an important ability of the CNS to control the inflammatory reflex pathway; however, they do not specifically identify regions that regulate this pathway.
Role of C1 neurons
C1 neurons reside in the medullary reticular formation and are glutamatergic, catecholaminergic and peptidergic73–75. These neurons have several properties that make them good candidates for a potential role as part of an integrative centre for the neuroimmune reflex in the CNS. Stress responses mobilize the autonomic nervous system, especially the sympathetic division73–75, and C1 neurons are important for central regulation of autonomic function (particularly cardiovascular function) and also seem to be sensors for various autonomic physical stressors. They project to sympathetic preganglionic neurons in the spinal cord and innervate a variety of brain regions, including the dorsal motor nucleus of the vagus and the paraventricular nucleus of the hypothala mus. Furthermore, subsets of C1 cells are activated by circulating IL-1 and LPS73, suggesting that they could regulate the immune system by controlling the corticotropin-releasing factor/adrenocorticotropic hormone/corticosterone cascade or the autonomic nervous system.
To investigate whether C1 neurons control the inflammatory reflex pathway to attenuate inflammation and AKI, Abe et al. used optogenetic technology76 (described below). Using transgenic mice that selectively expressed channel rhodopsin-2 in C1 neurons, they showed that optogenetic stimulation of these neurons protected the kidneys from IRI. This C1 neuron-activated protection was dependent on the spleen, β2-adrenergic receptors and α7nAChRs, suggesting that it was mediated by the CAP. Additional experiments suggested that C1 neuron-mediated CAP activation occurred through activation of a sympathetic pathway76. These findings suggest that the CNS controls the inflammatory reflex in part through C1 neurons (FIG. 1).
Neural circuits in the kidney
In addition to a role of inflammatory reflexes, including the CAP, in modulating the response to AKI13,68,69,76, direct innervation of the kidney might be important in regulating immunity and inflammation. Anatomical and physiological evidence suggest that neural circuits, including afferent and efferent innervation of the kidney, provide pathways for regulation of kidney function and disease states by the peripheral nervous system, and for feedback within the kidney and likely to the CNS77,78 (FIG. 2).
Figure 2 |. Neural circuitry of efferent and afferent innervation in the kidney.
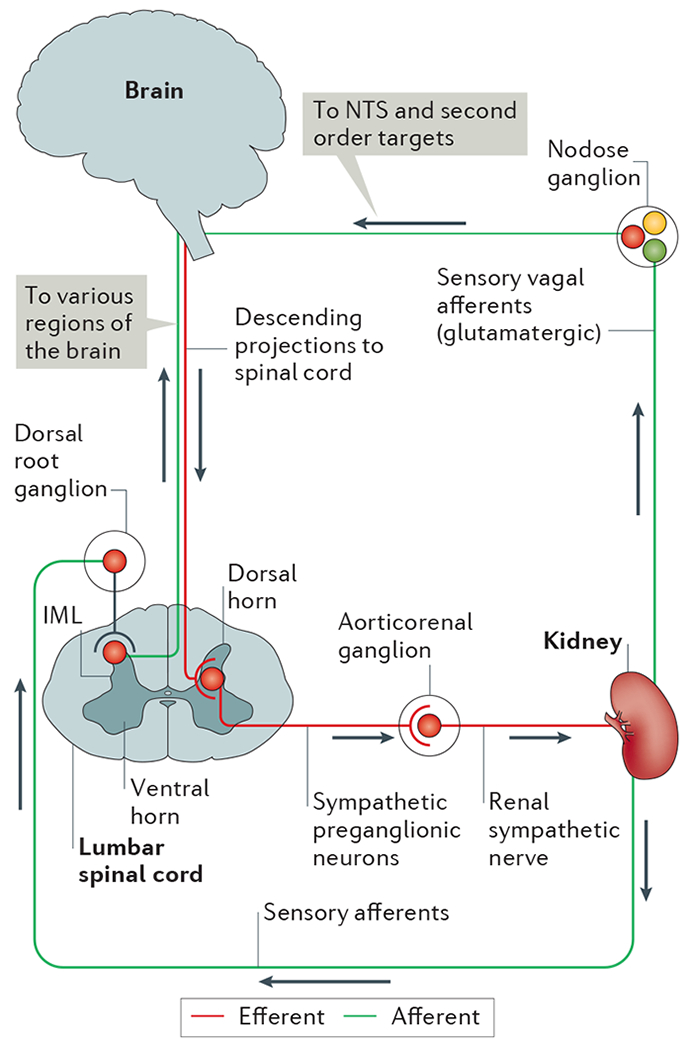
In the efferent pathway, descending projections from the brain (for example, from brainstem and hypothalamic regions) synapse on sympathetic preganglionic neurons in the intermediolateral cell column (IML) of the thoracic and lumbar spinal cord. Sympathetic preganglionic neurons in the lumbar spinal cord project through the ventral horn to the aorticorenal ganglion and activate the renal sympathetic nerve, which innervates the kidney and releases noradrenaline, neuropeptide Y and ATP. Sensory afferents in the kidney, which release substance P and calcitonin gene-related peptide, have their cells bodies in the dorsal root ganglia and synapse centrally on interneurons in the dorsal horn of the spinal cord that transmit information to various regions of the brain. Although the kidney does not seem to have vagal efferent input, some evidence suggests that vagal afferent neurons arising in the nodose ganglion innervate the kidney, thus providing a pathway for integration with the nucleus tractus solitarius (NTS) and subsequent second order neuronal targets in the brain.
Efferent sympathetic renal neurons arborize in a widely distributed pattern in the kidney, with noradrenergic terminals contacting the vasculature and tubules, particularly along the proximal tubules79. Increased renal sympathetic nerve activity (RSNA), and hence increased noradrenaline release, decrease renal blood flow and increase sodium retention and renin release80,81.
Terminal fields of afferent renal sensory neurons, whose cell bodies reside in the dorsal root ganglion82, have a much sparser distribution than sympathetic efferents. Sensory nerve fibres in the kidney contain substance P83,84 and calcitonin gene-related protein (CGRP)84 and are mechanosensitive and chemosensitive85–87. Afferent renal sensory neurons project to the dorsal horn of the spinal cord88, where they synapse with neurons projecting to regions of the brainstem and hypothalamus, and perhaps directly to the brainstem89, thus providing pathways for feedback to the CNS. At present, whether sensory information from the kidney can activate the inflammatory reflex is not known. However these sensory afferents have been shown to respond to various stimuli, such as bradykinin, substance P, capsaicin, and prostaglandins90,91. Sensory afferents, including vagus afferents92, are glutamatergic, which enables them to be discriminated from cholinergic vagus efferents, as has been elegantly shown in the lung93. Within the kidney, interactive relationships exist between sympathetic renal efferent and sensory renal afferent nerves; these reno-renal reflexes have been studied in various disease states, such as hypertension and diabetes81,90,94. RSNA results in the release of noradrenaline, which increases renal afferent nerve activity and provides feedback to inhibit RSNA.
No evidence exists of parasympathetic innervation of the kidney (that is, vagus efferents). By contrast, evidence for vagus afferents in the kidney is controversial and requires additional investigation. Retrograde tract tracing studies in rats revealed that the nodose ganglion (the inferior ganglion of the vagus nerve) contains cell bodies of neurons that project to the kidney95,96. These results suggest the presence of vagus afferents in the kidney; however, further studies are needed to determine their function. Moreover, these vagus afferents target the nucleus of the solitary tract in the brain, an area that is important for regulation of autonomic function in the periphery97. Electrophysiological data showing that neurons in the hypothalamus are excited after stimulation of renal sensory afferents through a pathway that may involve areas of the medulla, suggest that renal sensory afferent information might also be integrated in the hypothalamus98.
Role of renal nerves in kidney fibrosis
Experimental evidence suggests a role of renal nerves in the development of renal fibrosis. In animal models, renal denervation prevented the development of renal fibrosis after unilateral ureteral obstruction99 or IRI100. Fibrotic and inflammatory responses in these denervated kidneys were, however, observed following local infusion of noradrenaline (which is found in sympathetic efferents) or CGRP (which is found in sensory afferents). These fibrotic responses to noradrenaline and CGRP were blocked by antagonists of their respective receptors, suggesting that neural signalling contributes to the development of renal inflammation and fibrosis. The denervation method used in these studies does not discriminate between sympathetic efferent and sensory afferent nerves; however, the roles of noradrenaline and CGRP, respectively, suggest involvement of both pathways in kidney fibrosis. Future studies with selective denervation of sensory afferents101,102 are needed to dissect the individual roles of these neural circuits.
Although the findings described above suggest that renal nerves contribute to fibrosis, other endogenous factors leading to an abnormal microenvironment also contribute to the progression of kidney disease following an acute insult103–105. Such factors include, but are not limited to, capillary rarefaction and hypoxia106,107, altered fatty acid oxidation108, inflammatory cells109–111, cell cycle arrest112, mitochondrial function113, partial epithelial-to-mesenchymal transition114,115 and the endothelium116. The contribution of these factors explains why renal allografts undergo fibrosis despite the renal denervation that occurs during transplantation117.
Neural-immune mechanisms in hypertension
Regulation of blood pressure involves a complex intertwining of mechanisms involving the sympathetic nervous system (effects on heart rate and vascular tone), the CNS (brainstem mechanisms regulating cardiovascular function), hormones, environmental factors, and the kidney, particularly in terms of renal sodium handling, renin secretion, and the renal vasculature81,118–121. Perturbations in these regulatory mechanisms lead to hypertension. Increased sympathetic drive to the kidney in hypertension forms the basis for the experimental and clinical use of renal denervation for blood-pressure lowering, but the contribution from renal sensory afferents in the renorenal reflex is not well understood122.
Role of the immune system
Considerable evidence exists for a role of the immune system in blood pressure control in animal models123, and some studies suggest that the immune system might also be important in human hypertension124 (TABLE 1). Passive transfer studies in rodents have demonstrated that immunosuppression can attenuate hypertension125–127. In rats with CKD, elimination of T cells owing to thymectomy or splenectomy prevented the development of hypertension, whereas transfer of lymph node cells from rats with renal infarction induced hyper tension126. Similarly, in spontaneously hypertensive rats (SHR), depletion of lymphocytes with anti-thymocyte serum or chronic cyclophosphamide administration attenuated hypertension128,129. Moreover, Rag-1−/− mice, which are deficient in T cells and B cells, show a blunted increase in blood pressure in response to angiotensin II or deoxycorticosterone acetate salt130. This effect could be reversed by adoptive transfer of T cells from wild-type mice.
Table 1 |. Therapeutic use of neuromodulation in kidney disease and HTN.
| Setting | Treatment | Key findings | Refs |
|---|---|---|---|
| Acute kidney injury (CAP) | |||
| Preclinical | Ultrasound | Ultrasound treatment of mice prior to IRI protected kidney structure and function consistent with activation of the CAP | 69 |
| Vagus nerve stimulation | Stimulation of vagal afferents or efferents in mice prior to IRI protected kidney structure and function consistent with activation of the CAP | 13 | |
| Optogenetic stimulation of C1 neurons | Optogenetic stimulation of C1 neurons located in the medulla oblongata attenuated IRI by activating the CAP predominantly through a sympathetic pathway | 76 | |
| Transplantation (CAP) | |||
| Preclinical | Vagus nerve stimulation | VNS activation of the CAP in brain-dead donor rats attenuated the production of inflammatory cytokines in the donors and chronic allograft nephropathy in the recipients | 61,62 |
| Renal fibrosis (SNS) | |||
| Preclinical | Renal denervation | Renal nerve stimulation after ureteral obstruction stimulates fibrosis, whereas renal denervation prevents inflammation and fibrosis | 99 |
| Renal denervation at the time of or 1 day after IRI preserved histology, attenuated pro-inflammatory and profibrotic responses and apoptosis, and prevented G2/M cell cycle arrest in the kidney | 100 | ||
| Hypertension (SNS) | |||
| Preclinical | Renal denervation | • In angiotensin II-induced HTN, renal sympathetic nerves contribute to dendritic cell activation, subsequent T cell infiltration and end-organ damage in the kidney • Bilateral renal denervation reduced inflammation and associated renal fibrosis, albuminuria, and nephrinuria |
146 |
| In CKD, rhizotomy from T9 to L1 (a procedure that interrupts the afferent nerve input to the hypothalamus) blocks HTN, indicating that the afferent pathway contributes to HTN | 156 | ||
| Clinical | Bilateral nephrectomy | Bilateral nephrectomy corrected HTN, sympathetic nerve activity and calf vascular resistance, indicating that the kidney was the source of the afferent signal causing HTN | 157 |
| Renal denervation | In a proof-of-principle trial in patients with resistant HTN, renal sympathetic denervation led to a decrease in blood pressure without serious adverse events | 152 | |
| The SYMPLICITY HTN-2 trial in patients with treatment-resistant HTN showed that renal denervation resulted in sustained lowering of blood pressure at 3 years compared with medical therapy alone | 153 | ||
| The SYMPLICITY HTN-3 in patients with resistant HTN showed that renal denervation did not result in a significant reduction in systolic blood pressure at 6 months compared with a sham procedure | 158 | ||
| Cardiorenal syndrome (SNS) | |||
| Preclinical | Renal denervation | SNS had a detrimental effect on renal blood flow and renal vascular resistance in rabbits with heart failure; this effect was prevented by renal denervation | 167 |
| CKD and resistant hypertension (SNS) | |||
| Clinical | Renal denervation | In patients with resistant hypertension and stage 3–4 CKD, bilateral renal denervation was safe and effective in lowering blood pressure | 168 |
| Resistant hypertension and albuminuria (SNS) | |||
| Clinical | Renal denervation | In patients with resistant HTN, renal denervation reduced blood pressure, renal resistive index, and the incidence of albuminuria without adversely affecting glomerular filtration rate | 169 |
CAP, cholinergic anti-inflammatory pathway; CKD, chronic kidney disease; HTN, hypertension; IRI, ischaemia-reperfusion injury; SNS, sympathetic nervous system.
Cytokines have also been implicated in the pathogenesis of hypertension. T helper type 17 cells produce IL-17, which has a role in immune-mediated diseases such as psoriasis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and asthma131. In Il17−/− mice, angiotensin II infusion induced an initial increase in blood pressure, but this response was not sustained compared to controls (C57BL/6 mice)132. TNF and IL-6 have also been shown to contribute to hypertension in animal models132–137.
Both TNF and IL-6 were independent risk factors for high blood pressure in a population-based study that included 196 healthy individuals138. In addition, a small study that included eight patients with hypertension and either psoriasis or rheumatoid arthritis, showed that treatment with the immunosuppressant myco phenolate mofetil was associated with a significant reduction in blood pressure and urinary excretion of TNF139. Together, these studies provide evidence for a role of the immune system in the pathogenesis of hypertension.
Neuroimmunomodulatory pathways
Interactions between neural circuits and the immune system in hypertension have also been identified. Although increased sympathetic nerve activity seems to have both activating and suppressive effects on the immune system (dependent in part on the effects on immune cells in different locations), direct evidence indicates that increased sympathetic outflow and the resulting increase in noradrenaline levels activates T cells, promotes renal inflammation and contributes to hypertension140. These neural-immune mechanisms are mediated at least in part by T cells in the kidney, which have roles in immune-mediated damage141–143 and salt-sensitive hypertension144,145. In a seminal study by Burne, Rabb and co-workers, T cell-deficient nu/nu mice were shown to be protected from IRI, an effect that was reversed upon reconstitution of these mice with wild-type T cells141. Additional studies have demonstrated that natural killer T cells contribute to early innate-immune-mediated IRI142.
Renal sympathetic nerves drive T cell infiltration, kidney damage and hypertension140,146. In turn, hypertension causes accumulation of oxidized products (γ-ketoaldehydes) in dendritic cells, which promotes dendritic cell cytokine production and activates T cells146. Both sympathetic efferents and renal sensory afferents have a role in mediating hypertension. Selective renal denervation studies showed that renal sympathetic nerves are primarily responsible for renal inflammation, whereas afferent nerves mediate salt-induced hypertension101. Furthermore, two models of experimental hypertension showed that increased sympathetic splenic nerve activity and T cell recruitment to the kidney were mediated by hypertension-induced activation of a cholinergic–sympathetic immuno modulatory mechanism with marked similarities to the CAP147. Interruption of this pathway by selective splenic denervation prevented T cell activation, egress from the spleen, and kidney infiltration, and protected mice from hypertension147.
These new concepts are provocative but the mechanisms need to be more clearly defined to resolve potential inconsistences140. A role for neural-immune mechanisms in the kidney also needs to be confirmed in human hypertension. Perhaps more intriguing, and not yet understood, is the apparent contradiction between the anti-inflammatory role stimulated by increased sympathetic drive to the spleen (the noradrenaline-dependent CAP) and the renal pro-inflammatory response driven by increased sympathetic drive to the kidney. Future investigations are needed to understand mechanisms in the kidney, including the role that other neuromodulators released from afferent and efferent nerve terminals (for example, ATP, nitric oxide and neuro peptides) have in this pro-inflammatory, hypertensive pathway in the kidney as well as feedback mechanisms from sensory afferent pathways to the brain that could drive compensatory mechanisms, perhaps by activating the CAP.
Implications for therapy
Despite the importance of the sympathetic nervous system in the pathophysiology of hypertension, targeting this pathway has been underutilized. From a clinical standpoint, immunosuppressive drugs that target lympho cytes or specific immunotherapy directed at T cells have been investigated for treatment of hypertension in animals128,129 and humans139. In addition to the obvious limitations of immunosuppression, this approach is complicated by the observation that patients with hypertension show increased susceptibility to secondary infections. Case and Zimmerman suggested that this susceptibility might be related to an immuno suppressive state in lymphoid organs, separate from kidney and vasculature T cell activation, that is driven by the suppressive effects of increased sympathetic drive148. Directly targeting the neuroimmune axis in the kidney or the CAP could provide a more selective approach to the treatment of hypertension than broad immunosuppression.
As sympathetic outflow is enhanced in essential hypertension, radiofrequency ablation has been utilized to denervate the kidney149–151. In a proof-of-principal study, sympathetic renal denervation was performed in 45 patients with resistant hypertension, but the effect on renal noradrenaline spillover (a measure of sympathetic denervation) was incomplete with a mean decrease of only 47.5%152. Despite incomplete denervation, blood-pressure control was substantial with a mean decrease in office blood pressure of 27/17 mmHg (relative to baseline measurement at enrolment) at 12 months after denervation. Follow-up studies demonstrated sustained lowering of blood pressure at 3 years153.
Notably, the renal denervation procedure ablates both afferent and efferent nerves. Renal afferent nerves project to the hypothalamus and can stimulate sympathetic outflow81,151,154–156. In patients with kidney failure on haemodialysis, bilateral nephrectomy corrected hypertension and increased muscle sympathetic nerve activity and calf muscle vascular resistance, demonstrating that the diseased kidneys were the source of the afferent signal157.
Despite these early, encouraging findings, the SYMPLICITY HTN-3 prospective randomized control trial in 535 patients with resistant hypertension failed to demonstrate a significant blood-pressure lowering effect of radiofrequency renal denervation in comparison to a sham procedure158. This result precipitated the termination of other planned studies of renal denervation for the treatment of resistant hypertension. SYMPLICITY HTN-3, while carefully designed to obviate the problems associated with prior studies (such as lack of a sham control group), was limited, however, by problems associated with patient selection (differences in the pathogenesis of obesity-induced hypertension and possible variations in response owing to age and ethnicity) and compliance (lack of confirmation of adherence to antihypertensive medication) as well as by ineffective ablation of renal nerves owing to inexperienced operators. Second-generation multi-electrode devices for renal denervation are currently being tested with the aim of improving efficacy and safety, in part by delivering multiple lesions simultaneously to enable shorter procedure times with a reduced risk of clotting. Further defining the neural circuitry and the underlying molecular mechanisms is important to enable the development of selective strategies for the therapeutic use of renal denervation in hypertension.
Tools to investigate neural pathways
Combining molecular and genetic approaches in a variety of ways, particularly with the introduction of optogenetic techniques, has given neuroscientists, nephro logists and immunologists a powerful toolset with fine resolution for selectively probing specific subsets of phenotypically distinct neurons.
Optogenetic technology enables the use of light for stimulation of specific neurons in transgenic mice. Light-sensitive ion channels (microbial opsins) can be introduced into the neurons of interest using two different techniques. Either Cre-dependent viral vectors harbouring the opsin downstream of a floxed-stop or floxed-inverse cassette are injected into specific brain regions of mice that express Cre recombinase under the control of a specific promoter of interest, or Cretransgenic mice are crossed with mice harbouring the floxed-stop or floxed-inverse cassette. Spatially targeted application of light of a specific wavelength can then be used to open the light-sensitive channels (either membrane cation channels such as channel rhodopsin-2 or chloride channels such as halorhodopsin), enabling selective activation or silencing, respectively, of the opsin-expressing neurons159,160.
To date, such investigations have mostly focused on the CNS, but these techniques are now being extended to the regulation of organ function by the peripheral nervous system. For example, vagus sensory neurons were found to be divided into several phenotypic subgroups based on specific markers, and optogenetic stimulation of each subgroup modulated the function of the lung, heart, and gastrointestinal tract in different ways93,161. This elegant technique could be exploited to finely examine the specific function of phenotypically different neural circuits in the kidney and to more clearly define specific mechanisms underlying the role of the inflammatory reflex in kidney function in injury and disease.
Analogous to optogenetics, Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) uses a chemogenetics approach to selectively activate neural circuits that express mutated mAChRs. These mAChRs (which are introduced using adenoviral or Cre-LoxP techniques) respond to an inactive ligand, clozapine-N-oxide (CNO)162, or its metabolite clozapine, rather than acetylcholine163. Treatment with low-dose CNO, therefore, induces selective activation of neural circuits expressing mAChRs.
Imaging of neural pathways can be enhanced using a transgenic multicolour labelling strategy known as Brainbow164. This method uses the Cre-LoxP recombination strategy to label neurons with multiple distinct colours, thus defining specific neural pathways. Use of these approaches in combination with other techniques such as tract tracing will enable precise understanding of neural pathways that mediate physiological functions and disease pathways.
Conclusions
Neuroimmunomodulation of organ function is attracting a great deal of interest as a novel means of treating disease. A growing understanding of the roles of neural circuits, such as the immune reflex pathway and particularly the CAP, as well as of sympathetic efferent and sensory afferent innervation, in kidney function, responses to acute and chronic kidney injury, and hypertension, is providing new therapeutic approaches, particularly through nonpharmacological, and in some cases non-invasive, techniques.
Stimulating the vagus nerve modulates peripheral nerve activity and suppresses inflammation, and clinical trials examining the effects of VNS in inflammatory disorders, such as rheumatoid arthritis165 and inflammatory bowel diseases are ongoing166. Experimental evidence suggests that VNS and therapeutic ultrasound protect the kidney from acute ischaemic injury by stimulating the CAP13,67–69. Furthermore, data suggest that neural pathways mediate inflammation and hyper tension124 as well as kidney fibrosis99,100, and that stimulation of C1 neurons in the brainstem activates the CAP76. C1 neurons respond to a variety of stimuli, including danger signals, and could be a central component of the immune reflex circuit.
The use of optogenetics, DREADDs and Brainbow labelling in combination with tract tracing, immunofluorescent labelling and electrophysiological techniques is enabling greater understanding of neural circuits that regulate kidney function. These circuits could potentially be manipulated to modulate immune responses in kidney disease. Further elucidation of neuroimmune mechanisms in AKI, kidney disease and hypertension might lead to the identification of neural circuits that could be exploited for therapy.
Key points.
Neural circuits that control immunity and inflammation provide novel targets for the treatment of kidney disease and hypertension
Activation of the cholinergic anti-inflammatory pathway (CAP) blocks splenic-dependent systemic inflammation and acute kidney injury (AKI); non-invasive, nonpharmacological approaches to activate the CAP include ultrasound application and vagus nerve stimulation
Neural circuits that directly regulate kidney function or carry sensory feedback from the kidney to the central nervous system could provide additional mechanisms for bidirectional neuroimmunomodulation of kidney disease
Interactions between neural circuits and the immune system have important roles in the pathogenesis of hypertension and renal fibrosis
Further defining neuroimmunomodulatory pathways in hypertension could enable the development of selective neuronal stimulatory or inhibitory strategies for lowering blood pressure that could potentially be more effective than renal denervation
Optogenetic tools provide unprecedented opportunities to dissect the neural pathways that control immunity and inflammation and enable the identification of novel approaches to therapy for kidney diseases
Acknowledgements
The authors’ research summarized in this review was supported by the NIH under awards 1R01DK105133, R01 DK062324, U18EB021787 and 1S10RR026799-01.
Glossary
- Vagus nerve
The vagus or 10th cranial nerve is a pair of nerve bundles (right and left) that contains axons of both efferent and afferent neurons. The efferent neurons provide cholinergic input to almost all organs in the periphery and some skeletal muscles. Their cell bodies are located in the medulla oblongata. The cell bodies of afferent neurons are located in the nodose ganglia. These neurons carry the majority of sensory information from visceral organs to the CNS.
- Subdiaphragmatic vagotomy
Denervation by surgical transection of both trunks of the vagus nerve either below or caudal to the diaphragm.
- Nucleus tractus solitarius
A group of neuronal cell bodies in the brainstem that form an integrative centre for sensory information from the vagus, glossopharyngeal and facial nerves. The nucleus tractus solitarius projects to a wide variety of brain regions, including the hypothalamus, thalamus, and medulla, and participates in circuits that regulate autonomic function.
- Coeliac ganglion
A cluster of nerve cells in the abdomen that forms part of the prevertebral sympathetic chain and provides sympathetic innervation to most of the digestive tract. The coeliac ganglion is regulated by the preganglionic neurons in the intermediolateral cell column of the spinal cord.
- Bilateral cervical vagotomy
Bilateral cervical vagotomy is a surgical transection of the vagus nerve at the level of the neck on both sides. This procedure prevents the flow of information through the nerve in both directions (afferent and efferent) between its origin in the CNS and its targets in the periphery.
- Retrograde tract tracing
A technique for identifying neuronal pathways that exploits constitutive axonal transport to trace the movement of specialized proteins, markers or viruses (which can be visualized by various means) from their point of exogenous introduction in a target field of interest (synaptic terminals) to the cell bodies of those axons (retrograde transport). By contrast, anterograde tracing traces the transport of markers from the cell body region in the direction of the synapse.
Footnotes
Competing interests statement
M.D.O. and D.L.R. own equity in Adenosine Therapeutics, LLC. K.J.T. is a consultant for SetPoint Medical, Inc.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Okusa MD, Chertow GM & Portilla D The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin. J. Am. Soc. Nephrol 4, 520–522 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojo A Addressing the global burden of chronic kidney disease through clinical and translational research. Trans. Am. Clin. Climatol Assoc 125, 229–243; discussion 243–246 (2014). [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu RK, McCulloch CE, Dudley RA, Lo LJ & Hsu CY Temporal changes in incidence of dialysis-requiring AKI. J. Am. Soc. Nephrol 24, 37–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molitoris BA, Okusa MD, Palevsky PM, Kimmel PL & Star RA Designing clinical trials in acute kidney injury. Clin. J. Am. Soc. Nephrol 7, 842–843 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Okusa MD et al. Design of clinical trials in acute kidney injury: a report from an NIDDK workshop — prevention trials. Clin. J. Am. Soc. Nephrol 7, 851–855 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV et al. AKI: a path forward. Clin. J. Am. Soc. Nephrol 8, 1606–1608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo SK, Rosner MH & Okusa MD Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin. J. Am. Soc. Nephrol 2, 256–365 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Perrin S Preclinical research: make mouse studies work. Nature 507, 423–425 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Borovikova LV et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). Study demonstrating an important and previously unrecognized anti-inflammatory role of the parasympathetic nervous system.
- 10.Tracey KJ The inflammatory reflex. Nature 420, 853–859 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Chiu IM, von Hehn CA & Woolf CJ Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci 15, 1063–1067 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG & Yoshida Y Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci 19, 784–787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Invest 126, 1939–1952 (2016). Study demonstrating that VNS activates the CAP, resulting in activation of anti-inflammatory effects via α7 nicotinic acetylcholine receptor–expressing splenic macrophages.
- 14.National Institutes of Health. Stimulating peripheral activity to relieve conditions. National Institutes of Health; https://commonfund.nih.gov/SPARC (2017). [Google Scholar]
- 15.Ogbonnaya S & Kaliaperumal C Vagal nerve stimulator: evolving trends. J. Nat. Sci. Biol. Med 4, 8–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Undem BJ & Kollarik M The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc. Am. Thorac Soc 2, 355–360; discussion 371–372 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chunchai T et al. Vagus nerve stimulation exerts the neuroprotective effects in obese-insulin resistant rats, leading to the improvement of cognitive function. Sci. Rep 6, 26866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Ferrari GM et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J 32, 847–855 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Koopman FA et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 113, 8284–8289 (2016). Pilot study demonstrating that VNS inhibited TNF production and improved disease severity in patients with rheumatoid arthritis.
- 20.Bonaz B et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 28, 948–953 (2016). Pilot study demonstratating the potential efficacy and safety of chronic VNS in patients with Crohn disease.
- 21.Andersson U & Tracey KJ Neural reflexes in inflammation and immunity. J. Exp. Med 209, 1057–1068 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith KA Louis Pasteur, the father of immunology? Front. Immunol 3, 68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blevins SM & Bronze MS Robert Koch and the ‘golden age’ of bacteriology. Int. J. Infect. Dis 14, e744–e751 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Bernard C Leçons sur les phénomènes de la vie, communs aux animaux et aux végétaux [French]. Paris 307, (1878). [Google Scholar]
- 25.Thomas L Germs. N. Engl. J. Med 287, 553–555 (1972). [DOI] [PubMed] [Google Scholar]
- 26.Hotchkiss R, Monneret G & Payen D Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol 13, 862–874 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellavance MA & Rivest S The HPA — immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol 5, 136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watkins LR et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett 183, 27–31 (1995). Seminal discovery that an intact vagus nerve is required for the development of fever in rodents following intra-abdominal administration of IL-1β. This finding demonstrates an essential early role for the sensory nervous system in a fundamental mechanism of host defence.
- 29.Niijima A The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst 61, 287–291 (1996).8988487 [Google Scholar]
- 30.Goehler LE et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci 85, 49–59 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Ordovas-Montanes J et al. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 36, 578–604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov VA & Tracey KJ Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci 20, 156–166 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Ek M, Kurosawa M, Lundeberg T & Ericsson A Activation of vagal afferents after intravenous injection of interleukin-1β: role of endogenous prostaglandins. J. Neurosci 18, 9471–9479 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermann GE & Rogers RC TNFα: a trigger of autonomic dysfunction. Neuroscientist 14, 53–67 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Kleij H et al. Evidence for neuronal expression of functional Fc (ε and γ) receptors. J. Allergy Clin. Immunol 125, 757–760 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page AJ, O’Donnell TA & Blackshaw LA P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J. Physiol 523, 403–411; part 2 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouns I, Adriaensen D, Burnstock G & Timmermans JP Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X3 receptors. Am. J. Respir. Cell Mol. Biol 23, 52–61 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Mascarucci P, Perego C, Terrazzino S & De Simoni M G. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1β. Neuroscience 86, 1285–1290 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Ericsson A, Kovacs KJ & Sawchenko PE A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci 14, 897–913 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalia M & Sullivan JM Brainstem projections of sensory and motor components of the vagus nerve in the rat. J. Comp. Neurol 211, 248–265 (1982). [DOI] [PubMed] [Google Scholar]
- 41.Bernik TR et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med 195, 781–788 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Westerloo DJ et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Ghia JE, Blennerhassett P & Collins SM Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol 293, G560–G567 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF & Collins SM The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130 (2006). [DOI] [PubMed] [Google Scholar]
- 45.de Jonge WJ et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol 6, 844–851 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Andersson U & Tracey KJ Reflex principles of immunological homeostasis. Annu. Rev. Immunol 30, 313–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tracey KJ Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest 117, 289–296 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mebius RE & Kraal G Structure and function of the spleen. Nat. Rev. Immunol 5, 606–616 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Huston JM et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med 203, 1623–1628 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosas-Ballina M et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reilly FD, McCuskey PA, Miller ML, McCuskey RS & Meineke HA Innervation of the periarteriolar lymphatic sheath of the spleen. Tissue Cell 11, 121–126 (1979). [DOI] [PubMed] [Google Scholar]
- 52.Bellinger DL, Lorton D, Hamill RW, Felten SY & Felten DL Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun 7, 191–204 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Rosas-Ballina M et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011). Landmark study demonstrating that an acetylcholine-producing, memory T cell population is integral to the inflammatory reflex following VNS in mice.
- 54.Franco R, Pacheco R, Lluis C, Ahern GP & O’Connell PJ The emergence of neurotransmitters as immune modulators. Trends Immunol. 28, 400–407 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Vida G et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 25, 4476–4485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Pena G et al. Unphosphorylated STAT3 modulates alpha7 nicotinic receptor signaling and cytokine production in sepsis. Eur. J. Immunol 40, 2580–2589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metz CN & Tracey KJ It takes nerve to dampen inflammation. Nat. Immunol 6, 756–757 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Yeboah MM et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 74, 62–69 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatterjee PK et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS ONE 7, e35361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoeger S et al. Modulation of brain dead induced inflammation by vagus nerve stimulation. Am. J. Transplant 10, 477–489 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Hoeger S et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol. Dial. Transplant 29, 544–549 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Howland RH Vagus nerve stimulation. Curr. Behav. Neurosci. Rep 1, 64–73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Menachem E, Revesz D, Simon BJ & Silberstein S Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur. J. Neurol 22, 1260–1268 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ay I, Nasser R, Simon B & Ay H Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stimul. 9, 166–173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lerman I et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation 19, 283–290 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Gigliotti JC & Okusa MD The spleen: the forgotten organ in acute kidney injury of critical illness. Nephron Clin. Pract 127, 153–157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gigliotti JC et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol 24, 1451–1460 (2013). First study demonstrating that ultrasound treatment of mice prior to ischaemia–reperfusion injury protects kidney structure and function consistent with activation of the CAP.
- 69.Gigliotti JC et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J. Am. Soc. Nephrol 26, 2407–2481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brimijoin S & Molinoff PB Effects of 6-hydroxydopamine on the activity of tyrosine hydroxylase and dopamine-β-hydroxylase in sympathetic ganglia of the rat. J. Pharmacol. Exp. Ther 178, 417–424 (1971). [PubMed] [Google Scholar]
- 71.Borovikova LV et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci 85, 141–147 (2000). [DOI] [PubMed] [Google Scholar]
- 72.Pavlov VA et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl Acad. Sci. USA 103, 5219–5223 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li HY, Ericsson A & Sawchenko PE Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc. Natl Acad. Sci. USA 93, 2359–2364 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC & Loewy AD Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270, 644–646 (1995). [DOI] [PubMed] [Google Scholar]
- 75.Guyenet PG et al. C1 neurons: the body’s EMTs. Am. J. Physiol. Regul. Integr. Comp. Physiol 305, R187–R204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abe C et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci 20, 700–707 (2017). First demonstration that optogenetic stimulation of C1 neurons located in the medulla oblongata protects kidneys from ischaemia–reperfusion injury.
- 77.Nishi EE, Bergamaschi CT & Campos RR The crosstalk between the kidney and the central nervous system: the role of renal nerves in blood pressure regulation. Exp. Physiol 100, 479–484 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Abdulla MH & Johns EJ The innervation of the kidney in renal injury and inflammation: a cause and consequence of deranged cardiovascular control. Acta Physiol. (Oxf.) 220, 404–416 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Barajas L, Liu L & Powers K Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can. J. Physiol. Pharmacol 70, 735–749 (1992). [DOI] [PubMed] [Google Scholar]
- 80.Burnstock G & Loesch A Sympathetic innervation of the kidney in health and disease: emphasis on the role of purinergic cotransmission. Auton. Neurosci 204, 4–16 (2017). [DOI] [PubMed] [Google Scholar]
- 81.DiBona GF & Kopp UC Neural control of renal function. Physiol. Rev 77, 75–197 (1997). Comprehensive review of the role of the neural system in controlling renal function.
- 82.Ferguson M, Ryan GB & Bell C Localization of sympathetic and sensory neurons innervating the rat kidney. J. Auton. Nerv. Syst 16, 279–288 (1986). [DOI] [PubMed] [Google Scholar]
- 83.Ferguson M & Bell C Substance P-immunoreactive nerves in the rat kidney. Neurosci. Lett 60, 183–188 (1985). [DOI] [PubMed] [Google Scholar]
- 84.Kopp UC, Cicha MZ, Smith LA & Hokfelt T Nitric oxide modulates renal sensory nerve fibers by mechanisms related to substance P receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol 281, R279–R290 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Kopp UC, Olson LA & DiBona GF Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am. J. Physiol 246, F67–F77 (1984). [DOI] [PubMed] [Google Scholar]
- 86.Kopp UC, Smith LA & DiBona GF Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am. J. Physiol 249, F507–F517 (1985). [DOI] [PubMed] [Google Scholar]
- 87.Johns EJ & Kopp UC in Seldin and Giebisch: The Kidney: Physiology and Pathophysiology (eds Alpern RJ, Moe OW & Caplan M) 451–486 (Academic, 2013). [Google Scholar]
- 88.Ciriello J & Calaresu FR Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J. Auton. Nerv. Syst 8, 273–285 (1983). [DOI] [PubMed] [Google Scholar]
- 89.Wyss JM & Donovan MK A direct projection from the kidney to the brainstem. Brain Res. 298, 130–134 (1984). [DOI] [PubMed] [Google Scholar]
- 90.Kopp UC Role of renal sensory nerves in physiological and pathophysiological conditions. Am. J. Physiol. Regul. Integr. Comp. Physiol 308, R79–R95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stella A & Zanchetti A Functional role of renal afferents. Physiol. Rev 71, 659–682 (1991). [DOI] [PubMed] [Google Scholar]
- 92.Hermes SM, Colbert JF & Aicher SA Differential content of vesicular glutamate transporters in subsets of vagal afferents projecting to the nucleus tractus solitarii in the rat. J. Comp. Neurol 522, 642–653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang RB, Strochlic DE, Williams EK, Umans BD & Liberles SD Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ditting T et al. Tonic postganglionic sympathetic inhibition induced by afferent renal nerves? Hypertension 59, 467–476 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Norvell JE & Anderson JM Assessment of possible parasympathetic innervation of the kidney. J. Auton. Nerv. Syst 8, 291–294 (1983). [DOI] [PubMed] [Google Scholar]
- 96.Gattone VH 2nd, Marfurt CF & Dallie S Extrinsic innervation of the rat kidney: a retrograde tracing study. Am. J. Physiol 250, F189–F196 (1986). [DOI] [PubMed] [Google Scholar]
- 97.Weiss ML & Chowdhury SI The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 812, 227–241 (1998). [DOI] [PubMed] [Google Scholar]
- 98.Zheng H & Patel KP Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton. Neurosci 204, 57–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J & Padanilam BJ Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J. Am. Soc. Nephrol 24, 229–242 (2013). Study showing that renal denervation prevents fibrogenesis and the inflammatory cascade following ureteral obstruction.
- 100.Kim J & Padanilam BJ Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 87, 350–358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banek CT et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68, 1415–1423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foss JD, Wainford RD, Engeland WC, Fink GD & Osborn JW A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am. J. Physiol. Regul. Integr. Comp. Physiol 308, R112–R122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chawla LS, Eggers PW, Star RA & Kimmel PL Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med 371, 58–66 (2014). A summmary of the bidirectional relationship between AKI and CKD.
- 104.Basile DP et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol 27, 687–697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venkatachalam MA, Weinberg JM, Kriz W & Bidani AK Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol 26, 1765–1776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Basile DP, Donohoe D, Roethe K & Osborn JL Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 281, F887–F899 (2001). [DOI] [PubMed] [Google Scholar]
- 107.Mimura I & Nangaku M The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol 6, 667–678 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Kang HM et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med 21, 37–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee S et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol 22, 317–326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao Q, Harris DC & Wang Y Macrophages in kidney injury, inflammation, and fibrosis. Physiology 30, 183–194 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Jang HR & Rabb H Immune cells in experimental acute kidney injury. Nat. Rev. Nephrol 11, 88–101 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Yang L, Besschetnova TY, Brooks CR, Shah JV & Bonventre JV Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med 16, 535–543 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szeto HH et al. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J. Am. Soc. Nephrol 28, 1437–1449 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lovisa S et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med 21, 998–1009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grande MT et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med 21, 989–997 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Perry HM et al. Endothelial sphingosine 1phosphate receptor1 mediates protection and recovery from acute kidney injury. J. Am. Soc. Nephrol 27, 3383–3393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Venner JM, Famulski KS, Reeve J, Chang J & Halloran PF Relationships among injury, fibrosis, and time in human kidney transplants. JCI Insight 1, e85323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dampney RA Central neural control of the cardiovascular system: current perspectives. Adv. Physiol. Educ 40, 283–296 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Guyenet PG The sympathetic control of blood pressure. Nat. Rev. Neurosci 7, 335–346 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Joyner MJ, Charkoudian N & Wallin BG Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56, 10–16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coffman TM The inextricable role of the kidney in hypertension. J. Clin. Invest 124, 2341–2347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DiBona GF & Esler M Translational medicine: the antihypertensive effect of renal denervation. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R245–R253 (2010). [DOI] [PubMed] [Google Scholar]
- 123.Wenzel U et al. Immune mechanisms in arterial hypertension. J. Am. Soc. Nephrol 27, 677–686 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harrison DG The immune system in hypertension. Trans. Am. Clin. Climatol Assoc 125, 130–138; discussion 138–140 (2014). [PMC free article] [PubMed] [Google Scholar]
- 125.Renaudin C, Bataillard A & Sassard J Partial transfer of genetic hypertension by lymphoid cells in Lyon rats. J. Hypertens 13, 1589–1592 (1995). [PubMed] [Google Scholar]
- 126.Okuda T & Grollman A Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med 25, 257–264 (1967). [PubMed] [Google Scholar]
- 127.White FN & Grollman A Autoimmune factors associated with infarction of the kidney. Nephron 1, 93–102 (1964). [DOI] [PubMed] [Google Scholar]
- 128.Dzielak DJ Immune mechanisms in experimental and essential hypertension. Am. J. Physiol 260, R459–R467 (1991). [DOI] [PubMed] [Google Scholar]
- 129.Bendich A, Belisle EH & Strausser HR Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem. Biophys. Res. Commun 99, 600–607 (1981). [DOI] [PubMed] [Google Scholar]
- 130.Guzik TJ et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med 204, 2449–2460 (2007). Study demonstrating a role of T cells in the genesis of hypertension. This finding supports a role of inflammation in the pathogenesis of hypertension.
- 131.Tesmer LA, Lundy SK, Sarkar S & Fox DA Th17 cells in human disease. Immunol. Rev 223, 87–113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Madhur MS et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM & Didion SP IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler. Thromb. Vasc. Biol 27, 2576–2581 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Lee DL et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. Heart Circ. Physiol 290, H935–H940 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Brands MW et al. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56, 879–884 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Venegas-Pont M et al. Tumor necrosis factor-α antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56, 643–649 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tran LT, MacLeod KM & McNeill JH Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol. Cell Biochem 330, 219–228 (2009). [DOI] [PubMed] [Google Scholar]
- 138.Bautista LE, Vera LM, Arenas IA & Gamarra G Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J. Hum. Hypertens 19, 149–154 (2005). [DOI] [PubMed] [Google Scholar]
- 139.Herrera J, Ferrebuz A, MacGregor EG & Rodriguez-Iturbe B Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol 17, S218–S225 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Touyz RM The neuroimmune axis in the kidney: role in hypertension. Circ. Res 117, 487–489 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Burne MJ et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Invest 108, 1283–1290 (2001). The seminal observation that CD4+ T cells are important mediators of ischaemic acute renal failure, which has led to therapeutic approaches to block T cells in the pathogenesis of AKI.
- 142.Li L et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J. Immunol 178, 5899–5911 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Day Y-J et al. Renal ischemia-reperfusion and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and inteferon-γ J. Immunol 176, 3108–3114 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Zhang J & Crowley SD Role of T lymphocytes in hypertension. Curr. Opin. Pharmacol 21, 14–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez-Iturbe B, Pons H & Johnson RJ Role of the immune system in hypertension. Physiol. Rev 97, 1127–1164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao L et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ. Res 117, 547–557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carnevale D et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun 7, 13035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Case AJ & Zimmerman MC Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J. Physiol 594, 527–536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rumantir MS et al. Neural mechanisms in human obesity-related hypertension. J. Hypertens 17, 1125–1133 (1999). [DOI] [PubMed] [Google Scholar]
- 150.Esler M et al. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol. Rev 70, 963–985 (1990). [DOI] [PubMed] [Google Scholar]
- 151.Esler M et al. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11, 3–20 (1988). [DOI] [PubMed] [Google Scholar]
- 152.Krum H et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009). This proof-of-principle trial in patients with resistant hypertension demonstrated that renal denervation decreased blood pressure without serious adverse events.
- 153.Esler MD et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur. Heart J 35, 1752–1759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Katholi RE Renal nerves and hypertension: an update. Fed. Proc 44, 2846–2850 (1985). [PubMed] [Google Scholar]
- 155.Katholi RE Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am. J. Physiol 245, F1–F14 (1983). [DOI] [PubMed] [Google Scholar]
- 156.Campese VM & Kogosov E Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 25, 878–882 (1995). [DOI] [PubMed] [Google Scholar]
- 157.Converse RL Jr. et al. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med 327, 1912–1918 (1992). [DOI] [PubMed] [Google Scholar]
- 158.Bhatt DL et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med 370, 1393–1401 (2014). The prospective, single-blind, randomized, sham-controlled SYMPLICITY HTN-3 trial showed that radiofrequency renal denervation did not significantly reduce systolic blood pressure in patients with resistant hypertension at 6 months.
- 159.Deisseroth K Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci 18, 1213–1225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deisseroth K Optogenetics. Nat. Methods 8, 26–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Williams EK et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roth BL DREADDs for neuroscientists. Neuron 89, 683–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gomez JL et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cai D, Cohen KB, Luo T, Lichtman JW & Sanes JR Improved tools for the Brainbow toolbox. Nat. Methods 10, 540–547 (2013). [PubMed] [Google Scholar]
- 165.Koopman FA, Schuurman PR, Vervoordeldonk MJ & Tak PP Vagus nerve stimulation: a new bioelectronics approach to treat rheumatoid arthritis? Best Pract. Res. Clin. Rheumatol 28, 625–635 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Matteoli G & Boeckxstaens GE The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Clayton SC, Haack KK & Zucker IH Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am. J. Physiol. Renal Physiol. 300, F31–F39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hering D et al. Renal denervation in moderate to severe CKD. J. Am. Soc. Nephrol 23, 1250–1257 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mahfoud F et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60, 419–424 (2012). [DOI] [PubMed] [Google Scholar]


