Abstract
Background
Dizziness, a common chief complaint, has an extensive differential diagnosis that includes both benign and serious conditions. Emergency physicians must distinguish the majority of patients with self-limiting conditions from those with serious illnesses that require acute treatment.
Objective of the Review
This article presents a new approach to diagnosis of the acutely dizzy patient that emphasizes different aspects of the history to guide a focused physical examination with the goal of differentiating benign peripheral vestibular conditions from dangerous posterior circulation strokes in the emergency department.
Discussion
Currently, misdiagnoses are frequent and diagnostic testing costs are high. This relates in part to use of an outdated, prevalent, diagnostic paradigm. The traditional approach, which relies on dizziness symptom quality or type (i.e., vertigo, presyncope, or disequilibrium) to guide inquiry, does not distinguish benign from dangerous causes, and is inconsistent with current best evidence. A new approach divides patients into three key categories using timing and triggers, guiding a differential diagnosis and targeted bedside examination protocol: 1) acute vestibular syndrome, where bedside physical examination differentiates vestibular neuritis from stroke; 2) spontaneous episodic vestibular syndrome, where associated symptoms help differentiate vestibular migraine from transient ischemic attack; and 3) triggered episodic vestibular syndrome, where the Dix-Hallpike and supine roll test help differentiate benign paroxysmal positional vertigo from posterior fossa structural lesions.
Conclusions
The timing and triggers diagnostic approach for the acutely dizzy patient derives from current best evidence and offers the potential to reduce misdiagnosis while simultaneously decreases diagnostic test overuse, unnecessary hospitalization, and incorrect treatments.
Keywords: dizziness, vertigo, diagnosis, misdiagnosis, BPPV, vestibular neuritis, nystagmus, posterior circulation stroke
INTRODUCTION
Approximately 3.5% of emergency department (ED) visits are for dizziness (1,2). Using the fewest possible resources, physicians must distinguish between the large majority of dizzy patients with self-limiting or easily treatable conditions and the minority with life- or brain-threatening conditions. Compared to those without dizziness, dizzy patients undergo more testing, more imaging, have longer ED lengths of stay, and are more likely to be admitted (1). In 2013, total health care–related costs for patients with dizziness in the United States was estimated to exceed ≥10 billion (3,4). Additional “costs” included adverse events, such as patient anxiety, injuries from falls, and preventable major strokes following misdiagnosed minor cerebrovascular events (5).
The existing diagnostic paradigm for dizziness, based on symptom quality or type of dizziness (i.e., asking the question “what do you mean ‘dizzy’?”), is taught across specialties; however, newer research has questioned its scientific basis (6). Taking a history from a dizzy patient should be no different than taking a history in other patients. The timing, triggers, and evolution over time; associated symptoms; and context (and not the descriptor used) best inform the differential diagnosis (7). Bedside examination can frequently establish a specific diagnosis (8). A confident diagnosis of a peripheral vestibular problem obviates the need for specialty consultation, expensive imaging, and hospitalization. When the evaluation suggests a central problem, especially stroke, steps can be taken to prevent harm by early initiation of secondary stroke prevention for milder presentations or thrombolysis or surgical interventions for more malignant presentations (9). We propose a new diagnostic algorithm to guide one’s approach to the acutely dizzy patient (see Figure 1). In this article, we use the general term dizziness to encompass various words patients use to describe disturbed balance or spatial orientation, such as lightheaded, spinning, rocking, vertigo, off balance, and others.
Figure 1.
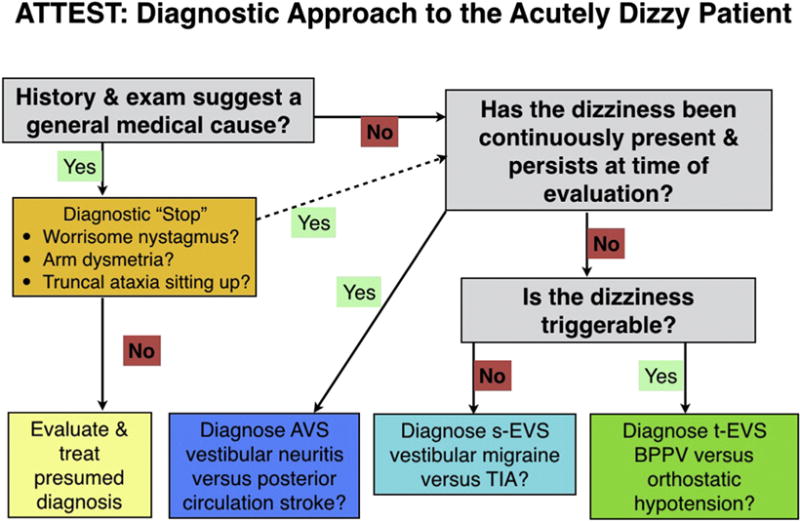
Diagnostic approach to the acutely dizzy patient. ATTEST = A, associated symptoms; TT, timing and triggers; ES, examination signs; and T, additional testing as needed. The first step is to take a history focused on associated symptoms, timing and triggers of the dizziness, and the overall context. Many patients’ histories will suggest a general medical cause (various toxic, metabolic, infectious, or cardiovascular causes). In this group of patients, we recommend a very brief diagnostic “stop” in order to reduce misdiagnosis. As part of this stop, first make sure there are no suspicious neurovestibular signs (nystagmus, limb ataxia, or gait/truncal ataxia). If a general medical cause still seems likely, evaluate and treat for the presumed diagnosis or diagnoses. For patients with a positive stop or whose history does not suggest a general medical cause, ask questions aimed at timing and triggers to place the patient into one of three categories. For patients in the acute vestibular syndrome (AVS) and triggered, episodic vestibular syndrome (t-EVS), physical examination (see text) will often allow a specific diagnosis to be made. For patients with the spontaneous episodic vestibular syndrome (s-EVS), use history to try to distinguish vestibular migraine from transient ischemic attack (TIA) or other causes (see text) since, by definition, these patients will no longer have symptoms and their dizziness cannot be triggered at the bedside. BPPV = benign paroxysmal positional vertigo.
DISCUSSION
Differential Diagnosis
Numerous conditions cause acute dizziness. A study from a national database (National Hospital Ambulatory Medical Care Survey), over a 13-year period, of 9472 patients with dizziness reported general medical (including non-stroke cardiovascular) diagnoses (~50%), otovestibular diagnoses (~33%), and neurologic (including stroke) diagnoses (~11%) (1). In this study, 22% of patients received a symptom-only dizziness (not otherwise specified) diagnosis. Although assigning a symptom-only diagnosis is common in emergency medicine practice, this was much more common in dizzy patients than in non-dizzy controls (22.1% vs. 8.4%; odds ratio 3.1) (1). Prospectively defined “dangerous” diagnoses (a mix of medical and neurologic conditions for which a poor outcome was likely without treatment) were found in 15% of patients and were more common in older patients (21% dangerous diagnoses in patients ≥50 years vs. 9.35 in patients < 50). The most common serious diagnoses were fluid and electrolyte disturbances (5.6%), cerebrovascular diseases (4.0%), cardiac dysrhythmias (3.2%), acute coronary syndromes (1.7%), anemia (1.6%), and hypoglycemia (1.4%) (1).
In a prospective single-institution study, 23 of 413 (6%) adult ED patients with dizziness had a central nervous system (CNS) diagnosis (2). Another 3-year study of 907 ED patients with dizziness, vertigo, or imbalance as a primary symptom reported that 1 in 5 were admitted (68% to an intensive care unit) (10). Most patients had benign conditions, such as peripheral vestibular problems (32%), orthostatic hypotension (13%), and migraine (4%). No specific diagnosis was made in 22% of cases. Serious neurologic disease was found in 49 (5%) patients, of which 37 were cerebrovascular. Only 2 patients with serious neurologic disease presented with isolated dizziness, although patients were not systematically imaged, so some may have been missed.
Thus, the overall incidence of important CNS disease in adult ED patients with acute dizziness is approximately 5%, most of which is stroke. Risk factors for CNS causes of dizziness include increasing age, history of vascular disease or previous stroke, the complaint of “instability,” abnormal gait, and focal neurologic findings (1,10–14).
Origin and Flaws of the Traditional Approach
The symptom-quality paradigm comes from a methodologically flawed study published in 1972 (Appendix 1) that was embraced by the medical establishment (15,16). This approach is based on first asking a dizzy patient, “What do you mean by, ‘dizzy’?” and then using the response to generate a differential diagnosis (e.g., vestibular problems if “vertigo,” cardiovascular disorders if “presyncope” or “near-syncope,” neurologic issues for “disequilibrium” and psychiatric, or metabolic causes if “other”). For this approach to work, two facts must be true. Patients should be able to consistently choose one (and only one) dizziness type and each symptom type should be tightly linked with a given differential diagnosis. Both are demonstrably false (6,16,17).
In a 2007 study, ED patients with dizziness were asked a series of questions aimed at determining the reliability and consistency of eliciting “symptom quality” and timing and triggers of their dizziness (18). When the main question was re-asked an average of 6 min later, half of the patients changed their primary dizziness type (19). More than 60% of the patients endorsed more than one dizziness type. This finding alone severely undercuts the logic of a diagnostic process based on dizziness type. Beyond imprecision and inconsistency, the differential diagnosis is not tightly linked with the use of a given word. Use of the term vertigo was not associated with stroke in a large series of ED patients with dizziness (20). Patients with a cardiovascular cause of dizziness describe “vertigo” in almost 40% of cases, more than the fraction that described presyncope (21). Patients with benign paroxysmal positional vertigo (BPPV) often describe non-vertiginous dizziness, especially in elderly patients (22). Despite these newer data, most physicians still use a symptom-quality approach with dizzy patients without considering the timing and triggers (6,23).
By way of analogy to another common medical complaint, patients with “sharp” chest pain are more likely to have pulmonary embolism and those with “dull”’ chest pain are more likely to have acute coronary syndromes, but one does not use the quality of the pain in such a binary way (17). Timing and triggers are more important in creating and rank-ordering a differential diagnosis. Intermittent exertional chest pain that resolves with rest suggests angina, whereas constant chest pain lasting hours is more likely to be myocardial infarction. Chest pain regularly triggered by swallowing suggests an esophageal problem. The history for virtually all chief complaints exploits the concept of timing and triggers. Patients are far more consistent in their responses to timing and triggers than they are for dizziness type (19). Context and associated symptoms are also important in diagnosis; for example, a smoker with chest pain, fever, and purulent sputum is conceptually different from a cancer patient with chest pain, leg swelling, and dyspnea.
DIAGNOSTIC PITFALLS
In a retrospective German study of 475 consecutive ED dizzy patients seen by neurologists (who routinely performed a detailed ocular motor examination using Frenzel lenses), the neurologists diagnosed benign conditions in 73% of cases and serious conditions (mostly cerebrovascular and inflammatory CNS disease) in 27% of cases (24). A neurologist masked to the initial ED visit changed the diagnosis at follow-up in 44% of those revisiting within 30 days. Benign vestibular diagnoses were deemed wrong in 58% (n = 21 of 36), including 17% (n = 6 of 36) with missed ischemic stroke or transient ischemic attack (TIA).
The most common reason for misdiagnosis was an evolution of the clinical course over time, which factored in 70% of misdiagnoses (24). This is part and parcel of emergency medicine; initially ambiguous (or nonexistent) symptoms or signs evolve over time. There has never been (and likely never will be) a head-to-head comparison of emergency physicians vs. neurologists diagnosing patients with dizziness at the same phase of care, but this German study shows that diagnosing dizziness is complicated, even for those with specialized training and focus.
Posterior circulation strokes mimic peripheral causes of dizziness (25–27). In one study from an ear, nose, and throat clinic, almost 3% of patients referred for vertigo had a missed cerebellar stroke (26). A missed stroke diagnosis is important because the underlying vascular pathology goes untreated (leaving the patient vulnerable to another stroke that might be prevented with secondary prophylaxis) and because patients can develop posterior fossa edema, which can be fatal (28–30). Lost opportunity for thrombolysis is another negative consequence of missing a posterior circulation stroke; however, many cerebrovascular dizzy patients have minor deficits and present late, and may not be thrombolysis candidates (31–33).
Younger age and vertebral dissection as the cause for acute dizziness were found to be risk factors for missed cerebellar stroke (34,35). Posterior circulation location is a risk factor for stroke misdiagnosis in general (31,36–38). To put these data into context, only a very small proportion (0.18–0.7%) of ED patients diagnosed with a benign or peripheral vestibular diagnosis return to the ED within 30 days and are hospitalized with a cerebrovascular diagnosis (38–41). However, because dizziness is so common, this very small percentage probably translates into tens of thousands of missed strokes and likely thousands of patients harmed each year (42).
Knowledge gaps regarding eye-movement findings also contribute to misdiagnosis (6). In a study of 1091 dizzy patients in U.S. EDs, physicians used templates to document the presence or absence of nystagmus in 887 (80%). Nystagmus was said to be present in 185 (21%) (43). Of these 185 patients, sufficient information regarding the nystagmus to be diagnostically useful was recorded in only 10 (5.4%). Of patients given a peripheral vestibular diagnosis, the nystagmus description conflicted with the final diagnosis in 81%. Misdiagnosis of common peripheral vestibular problems, such as BPPV and vestibular neuritis, leads to ineffective treatments and resource overutilization (42).
Misdiagnosis of patients with dizziness results from five common pitfalls: over-reliance on a symptom-quality approach to diagnosis, underuse of a timing and triggers approach, lack of familiarity with key physical examination findings, overweighting traditional factors such as age and vascular risk factors to screen patients, and over-reliance on computed tomography (CT) (6). Not considering stroke in young patients is a particularly important contributing factor (30,35).
GOALS OF CARE
Primary goals of care in ED patients with dizziness not due to medical causes are to differentiate benign peripheral vestibular conditions from posterior circulation strokes or other dangerous causes (rather than to make a definitive diagnosis) and to manage symptoms appropriately before discharge in those who do not have serious diseases. It is usually the case that a definitive, final diagnosis is not the goal of ED care. However, with dizziness, the most certain way to “rule out” dangerous causes is to “rule in” one of two common, benign inner ear conditions (BPPV or vestibular neuritis) by bedside examination. Furthermore, doing so leads to the correct choice of symptomatic treatment (repositioning maneuvers for BPPV or vestibular suppressant medications for vestibular neuritis).
A NEW DIAGNOSTIC PARADIGM
A new diagnostic paradigm is based on the timing, triggers, associated symptoms, and context of the dizzy symptoms. Although the use of this new paradigm has not yet been proven to reduce misdiagnosis in prospective clinical trials, it is supported by a very strong evidence base in the specialty literature; it is also noteworthy that the traditional paradigm is not evidence-based and likely predisposes to misdiagnosis (16,44). We believe that this new method allows one to confidently make an accurate diagnosis more frequently than the traditional paradigm. Although other acronyms have been used, here we use the mnemonic: ATTEST (associated symptoms, timing and triggers, bedside examination signs, and additional testing as needed) (7).
In the traditional paradigm, a patient endorsing vertigo would undergo an evaluation for peripheral vestibular vs. central causes of dizziness. This has led to confusion, such as using CT imaging for diagnosis and meclizine for treatment of vertigo, no matter what the cause (45). Physicians tend to generalize their management of patients with peripheral vertigo, whereas the 2 most common causes (BPPV and vestibular neuritis) should be managed very differently (42). We present three distinct timing and triggers categories that are important for emergency physicians to recognize (Table 1 and Figure 1).
Table 1.
| Syndrome | Description | Common Benign Causes | Common Serious Causes |
|---|---|---|---|
| AVS | Acute, continuous dizziness lasting days, accompanied by nausea, vomiting, nystagmus, head motion intolerance, and gait unsteadiness | Vestibular neuritis Labyrinthitis | Posterior circulation ischemic stroke |
| s-EVS | Episodic dizziness that occurs spontaneously, is not triggered,‡ and usually last minutes to hours | Vestibular migraine Menière’s disease | TIA |
| t-EVS | Episodic dizziness brought on by a specific, obligate trigger (typically a change in head position or standing up), and usually lasting <1 min | BPPV | CPPV Orthostatic hypotension due to serious medical illness |
AVS = acute vestibular syndrome; BPPV = benign paroxysmal positional vertigo; CPPV = central paroxysmal positional vertigo; s-EVS = spontaneous, episodic vestibular syndrome; TIA = transient ischemic attack; t-EVS = triggered, episodic vestibular syndrome.
Note that the use of the word vestibular here connotes vestibular symptoms (e.g., dizziness or vertigo or imbalance or lightheadedness), rather than underlying vestibular diseases (e.g., benign paroxysmal positional vertigo, vestibular neuritis).
This table lists the more common diseases causing these presenting syndromes and is not intended to be exhaustive.
Dizziness is “triggered” (not dizzy at baseline, dizziness develops with movement), as in positional vertigo due to BPPV. This must be distinguished from dizziness that is “exacerbated” (dizzy at baseline, worse with movement); such exacerbations are common in AVS, whether peripheral (neuritis) or central (stroke).
Acute Vestibular Syndrome
Spontaneous acute vestibular syndrome (AVS) is defined as the acute onset of persistent dizziness associated with nausea or vomiting, gait instability, nystagmus, and head-motion intolerance lasting days to weeks (7,17,46). Patients are usually symptomatic at presentation and focused physical examination is often diagnostic. The most common cause is vestibular neuritis (dizziness only) or labyrinthitis (dizziness plus hearing loss or tinnitus) (46). The most frequent dangerous cause is posterior circulation ischemic stroke, generally in the cerebellum or brainstem (46). A small minority are due to multiple sclerosis (47,48). Rare causes of an isolated AVS include cerebellar hemorrhage; thiamine deficiency; and various autoimmune, infectious, or other metabolic conditions (47,49,50).
A key concept is understanding the distinction between symptoms that are exacerbated (dizzy at baseline, worse with movement) vs. triggered (not dizzy at baseline, dizziness develops with movement). Patients with an AVS typically experience worse dizziness with head movement (exacerbation), such as when performing the Dix-Hallpike maneuver, but this is not a sign of BPPV. Confusion on this point contributes to difficulty differentiating BPPV from vestibular neuritis or stroke (6,7,23). Occasionally, BPPV patients may endorse mild, persistent symptoms of malaise or unsteadiness between triggered, brief bouts of vertigo; this may be due to repeated symptoms triggered by small, inadvertent head movements or anticipatory anxiety about moving, and is more common among older patients. This can usually be teased out by careful history-taking. When such patients lack obvious features of vestibular neuritis or stroke, the Dix-Hallpike and supine roll tests can be performed to assess for an atypical, AVS-like presentation of BPPV (51).
Vestibular neuritis (the most common cause of an AVS) is a benign, self-limited, presumed viral or post-viral inflammatory condition affecting the vestibular nerve (similar to Bell’s palsy affecting the facial nerve). The diagnosis is therefore clinical and requires excluding other causes. Most cases are idiopathic, possibly linked to herpes simplex infections (52). Ramsay Hunt Syndrome due to herpes zoster presents with AVS, usually in conjunction with hearing loss, facial palsy, and a vesicular eruption in the ear or palate (53). While not recommended in the diagnoses of vestibular neuritis, routine magnetic resonance imaging (MRI) is usually normal in both cases (54).
Posterior fossa strokes can mimic vestibular neuritis or labyrinthitis (55). The prevalence of cerebrovascular disease in patients presenting to the ED with dizziness is 3–5%, but among those with the AVS, it is estimated at ~25% (7,46). Almost all (96%) of these strokes are ischemic (46,49). Sensitivity of CT for acute posterior fossa ischemic stroke may be as low as 7–16% in the first 24 h (56,57). Therefore, CT cannot rule out ischemic stroke in AVS, a factor contributing to misdiagnosis (6,23,30). Importantly, even MRI with diffusion-weighted imaging (DWI) misses 10–20% of strokes presenting with an AVS during the first 24–48 h after onset, more for small strokes (57–59). Delayed MRI (3–7 days post symptom onset) may be required to confirm the presence of a new infarct (46,58,59).
Fortunately, the physical examination can make the distinction between vestibular neuritis and posterior circulation stroke with greater sensitivity than early MRI (58–60). These studies were conducted by neurootologists performing a targeted three-component ocular motor examination—the head impulse test (HIT), gaze testing for nystagmus, and alternate cover test for skew-deviation (HINTS [head impulse, nystagmus, test of skew]). Trained general neurologists may achieve similar accuracy (61). Preliminary evidence suggests that “specially trained” emergency physicians using Frenzel lenses can also successfully use components of this approach (nystagmus testing and HIT) (62,63). Our own anecdotal experience also suggests that emergency physicians can learn to perform and interpret this examination without any special lenses or equipment. However, because emergency physicians use of this approach has not been fully validated, we suggest performing two additional components for the basic evaluation of the AVS patient—a targeted neurologic examination and gait testing.
For several reasons, we perform these tests in the following sequence (Table 2): 1) gaze testing; 2) alternate cover test; 3) HIT; 4) targeted neurologic examination, focusing on cranial nerves (including hearing), cerebellar testing, and long-tract signs; and 5) gait testing.
Table 2.
Use of the Physical Examination to Diagnose Patients With Acute Vestibular Syndrome
| Exam Component | Peripheral (All Must Be Present to Diagnose Vestibular Neuritis) | Central (Any One of These Findings Suggests Posterior Fossa Stroke) |
|---|---|---|
| Nystagmus (straight-ahead gaze and rightward and leftward gaze) | Dominantly horizontal, direction-fixed, beating away from the affected side* | Dominantly vertical or torsional or dominantly horizontal, direction-changing on left/right gaze† |
| Test of Skew (alternate cover test) | Normal vertical eye alignment and no corrective vertical movement (i.e., no skew deviation) | Skew deviation (small vertical correction on uncovering the eye)‡ |
| Head Impulse Test | Unilaterally abnormal with head moving towards the affected side (presence of a corrective re-fixation saccade towards the normal side)§ | Usually bilaterally normal (no corrective saccade) |
| Targeted neurologic examination (see text) | No cranial nerve, brainstem, or cerebellar signs | Presence of limb ataxia, dysarthria, diplopia, ptosis, anisocoria, facial sensory loss (pain/temperature), unilateral decreased hearing |
| Gait and truncal ataxia | Able to walk unassisted and to sit up in stretcher without holding on or leaning against bed or rails | Unable to walk unassisted or sit up in stretcher without holding on or leaning against bed or rails |
Inferior branch vestibular neuritis will present with downbeat-torsional nystagmus, but this is a rare disorder. From the emergency medicine perspective, vertical nystagmus in a patient with an acute vestibular syndrome patient should be considered to be central (a stroke).
More than half of posterior fossa strokes will have direction-fixed horizontal nystagmus that, alone, cannot be distinguished from that typically seen with vestibular neuritis.
More than half of posterior fossa strokes will have no skew deviation, so, on this criterion alone, cannot be distinguished from vestibular neuritis.
Strokes in the anterior inferior cerebellar artery territory may produce a unilaterally abnormal head impulse test that mimics vestibular neuritis, but hearing loss is usually present as a clue. If a patient has bilaterally abnormal Head Impulse Test, this is also suspicious for a central lesion if nystagmus is present (as may be seen in Wernicke’s syndrome).
The major reason for gaze testing first is that nystagmus is part of the definition of AVS, and the diagnostic meaning of HIT differs dramatically in dizzy patients without nystagmus (64). Gaze testing is also the least intrusive part of the examination and represents an important branch point in decision-making based on the presence or absence of nystagmus and its details. Thus, nystagmus helps to anchor and inform the rest of the process. All or nearly all patients with an AVS due to a peripheral vestibular cause will have nystagmus if carefully examined within the first days, so its absence makes the diagnosis of vestibular neuritis unlikely (65). Nystagmus may, however, be absent if vestibular suppressant medications (e.g., benzodiazepines) are applied before examination, so it is preferable to search for nystagmus before appropriate medications to manage symptoms are administered (45).
Nystagmus is usually visible with the naked eye, but normal visual fixation can completely suppress mild nystagmus in patients with vestibular neuritis (66). Sub-specialists often use Frenzel lenses to block visual fixation and magnify the view, making detection easier. One simple technique that emergency physicians can use to block visual fixation without the need for any special equipment is to simply place a piece of white paper close to the patient’s eyes, and then instruct them to “look through the paper” and examine for nystagmus from the side. This is only required if there is no nystagmus during the basic examination. If nystagmus is truly absent, an acute vestibular neuritis is very unlikely and the HIT can yield false information (64).
Despite known pitfalls, bedside examination for nystagmus is quite simple and, with practice, becomes easy to interpret. The details of the nystagmus are diagnostically important (45). Have the patient look straight ahead (“neutral” or “primary” gaze) and observe for eye movements. By convention, the direction of nystagmus is named by the direction of the fast component. Patients whose eyes drift leftward and snap back horizontally to the right have right-beating horizontal nystagmus. After observing for nystagmus in primary gaze, look for “gaze-evoked” nystagmus by having the patient look to the right and then to the left, each for several seconds. Again, observe for the presence of nystagmus and the direction of its fast-beating component. Many normal individuals have a few beats of physiologic horizontal nystagmus on extreme lateral gaze that is very low amplitude, extinguishes quickly, beats in the direction of gaze and is symmetric to the two sides. This finding does not “count” as pathologic nystagmus. Table 2 shows the nystagmus findings for patients with the AVS. Two patterns suggest stroke: 1) dominantly vertical or torsional nystagmus in any gaze position; 2) dominantly horizontal nystagmus that changes direction in different gaze positions (e.g., bilateral, gaze-evoked nystagmus). Note, however, that the most common pattern seen in stroke patients presenting AVS is direction-fixed horizontal nystagmus (i.e., the same as that seen in acute vestibular neuritis), which is why further testing is often needed (46).
Skew deviation is a vertical misalignment of the eyes due to imbalance in gravity-sensing vestibular pathways (67). Skew deviation is elicited using the “alternate cover” test. With the patient looking directly at the examiner’s nose, the physician covers one eye, then the other, and continues alternating back and forth, roughly every 1–2 s. If skew deviation is present, each time the covered eye is uncovered, a slight vertical correction occurs (one side corrects upward and the other corrects downward); if no vertical movement occurs, there is no skew (horizontal movements do not count). The amplitude of correction is small (1–2 mm) and appears at the moment the eye is uncovered; therefore, it is key for the examiner to focus on one eye (either one) for several cycles, rather than following the uncovered eye. A normal response is no vertical correction, and an abnormal response suggests a stroke in an AVS presentation.
The next component is the HIT, a test of the vestibulo-ocular reflex (VOR) described in 1988 (68). Standing in front of the patient, the examiner holds the patient’s head by each side, instructs the patient to focus on the examiner’s nose and to keep their head and neck loose. The examiner gently displaces the patient’s head about 10–20 degrees from the midline to one side; from there, a flick of the wrists brings the head back toward the center position rapidly (>120 degrees/s) where it stops “dead” at the midline, while the examiner observes the eyes carefully. The normal response (normal vestibular function) is that the patient’s gaze remains locked on the examiner’s nose. The presence of a corrective saccade (the eyes move with the head, then snap back in a fast corrective movement to again look at the examiner’s nose, as instructed) is a “positive” test (abnormal VOR), which generally indicates a peripheral process, usually vestibular neuritis. The HIT is performed to one side then the other, and the absence of a corrective saccade on both sides suggests a stroke in AVS. It may seem counterintuitive that a normal finding predicts a dangerous disease. This is why the HIT is only useful in patients with AVS (with nystagmus). A HIT done in a patient without nystagmus (e.g., a dizzy patient with urosepsis or dehydration), will be normal (i.e., worrisome for stroke) and, therefore, misleading.
Because the circuit of the VOR does not loop through the cerebellum, cerebellar stroke patients typically have a negative (normal) HIT (27,69). Occasional patients with posterior circulation stroke will have a falsely “positive” (abnormal) HIT, usually from an infarct involving the region where the vestibular nerve enters the brainstem or a stroke of the inner ear itself (labyrinthine stroke) (67). When abnormal HITs occur in stroke, hearing is often also affected because blood supply to both structures is generally from the anterior inferior cerebellar artery (AICA), which also supplies the cochlea (70). Adding a bedside test of hearing (“HINTS plus”) helps to diagnose these patients (60). This last point is important because traditional teaching is that coincident hearing loss and dizziness is always peripheral (in the labyrinth). However, combined audio-vestibular loss is often a sign of stroke (71–73). The relative frequency of labyrinthitis vs. AICA stroke is unknown (74). Because so-called “viral labyrinthitis” (i.e., AVS with unilateral hearing loss) is uncommon, combined audiovestibular loss should be treated with extreme caution in the ED (7,60,75).
Patients with the AVS who have worrisome nystagmus, skew deviation, or a bilaterally normal HIT have a presumed stroke and should be admitted. If all three tests are reassuring (direction-fixed, predominantly horizontal nystagmus beating opposite a unilaterally abnormal HIT, and without skew deviation), perform a targeted neurologic examination to search for anisocoria, facial weakness or sensory asymmetry, dysarthria/dysphonia, or limb ataxia (8). Lateral medullary stroke (Wallenberg’s syndrome) merits special attention. In addition to acute dizziness, patients may complain of dysarthria, dysphagia, or hoarseness due to lower cranial neuropathy. They may have Horner’s syndrome with subtle ptosis and anisocoria only evident in dim light (the normal larger pupil fully dilates, accentuating the difference in pupil size) (76). The physical finding of hemi-facial decreased pain and temperature sensation may be missed if one only tests light touch.
Finally, if these four examination components (nystagmus, skew deviation, HIT, and targeted neurologic examination) are benign, test the gait. Ideally, have the patient walk unassisted, but for severely nauseated patients too symptomatic to walk, test for truncal ataxia by asking the patient to sit upright in the stretcher with arms crossed. Patients who cannot walk or sit up unassisted are unsafe for discharge and are more likely to have a stroke (or other CNS pathology) rather than vestibular neuritis (27,44,77). Although American emergency physicians are uncomfortable using HINTS testing and instead overuse CT, one study reported that specially trained emergency physicians using these bedside examination elements decreased both CT use and hospitalization (62,63,78).
The key takeaway is that bedside examination trumps brain imaging in the early diagnosis of patients with the AVS. Instructional videos are available at http://novel.utah.edu/Newman-Toker/collection.php.
Spontaneous Episodic Vestibular Syndrome
The spontaneous episodic vestibular syndrome (s-EVS) is marked by recurrent, spontaneous episodes of dizziness that range in duration from seconds to days, the majority lasting minutes to hours. If patients are still symptomatic at presentation (e.g., 3 h in), use this approach to AVS (79). However, most are asymptomatic at the time of clinical assessment and, by definition, the dizziness cannot be triggered at the bedside, so the evaluation usually relies entirely on the history. Perfusion-based imaging may help diagnose some cerebrovascular causes when examination and history are inconclusive (79).
Spells sometimes occur up to several times a day, but are usually less frequent and can be separated by months or even years, depending on the cause. The most common benign cause is vestibular migraine (80–82). The most common dangerous cause is posterior circulation TIA (83,84). Menière’s disease also presents with the s-EVS, but is less common (81). Other causes include reflex (e.g., vasovagal) syncope, and panic attacks (85,86). Diagnosis may be obvious but classical features may be absent (87–89). Uncommon dangerous causes of s-EVS are cardiovascular (cardiac dysrhythmia, unstable angina pectoris, pulmonary embolus), endocrine (hypoglycemia, neuro-humoral neoplasms), or toxic (intermittent carbon monoxide exposure).
Vestibular migraine presentations are quite variable. Attack duration ranges from seconds to days (81). Nystagmus, if present, can be peripheral, central, or mixed type (90). Headache, often absent during the attack, may begin before, during, or after the dizziness and may differ from the patient’s “typical” migraine headaches (81,90). Nausea, vomiting, photophobia, phonophobia, and visual auras may accompany vestibular migraine, but these features are often absent. Hearing loss or tinnitus sometimes occurs, mimicking Meniere’s disease (91,92). Given the variable presentations, the diagnosis of vestibular migraine is made based on a combination of clinical findings, with the most important benign feature being a history of repeated recurrences over years without permanent sequelae (8,81).
Menière’s disease is a relatively uncommon cause of dizziness in the ED. Patients classically present with episodic vertigo accompanied by unilateral tinnitus and aural fullness, often with reversible sensorineural hearing loss (92). Episodes typically last minutes to hours. Only 1 in 4 patients initially present with the complete symptom triad, and non-vertiginous dizziness is common (93,94).
Reflex (or neurocardiogenic) syncope includes vasovagal syncope, carotid sinus hypersensitivity, and situational syncope (e.g., micturition, defecation, cough) (95). Presyncope without loss of consciousness outnumber spells with full syncope (85). Dizziness is the most common presyncopal symptom and it may be of any type, including vertigo (21). Diagnosis is based on clinical history, excluding dangerous mimics (especially dysrhythmia), and can be confirmed by formal head-up tilt table testing (96).
Episodic dizziness from panic attacks (with or without hyperventilation) begins rapidly, peaks within 10 min and, by definition, is accompanied by at least three other symptoms (97). Although a situational precipitant (e.g., claustrophobia) may be present, spells often occur without obvious reasons and classical symptoms are absent in 30% of cases (87). Ictal panic attacks from temporal lobe epilepsy generally last only seconds, and altered mental status is frequent (86). Hypoglycemia, cardiac dysrhythmias, pheochromocytoma, and basilar TIA can also mimic panic attacks by producing a combination of neurologic and autonomic features.
The principal dangerous diagnosis presenting as s-EVS is TIA (83). Traditionally, isolated vertigo was not considered a TIA symptom, but epidemiologic evidence now suggests that isolated attacks of spontaneous dizziness are the most common vertebrobasilar TIAs (84,98). In a study of 86 patients with “acute transient vestibular syndrome” 23 (27%) were diagnosed with stroke or TIA (79). The authors defined “transient” as duration < 24 h and excluded BPPV and orthostatic hypotension (which are triggered); therefore, most (if not all) of these patients had s-EVS. Among 23 patients examined while still symptomatic (15 vestibular neuritis, 3 stroke, 5 undetermined), the HINTS plus approach was 100% sensitive and 75% specific for stroke; it could not be applied in the remaining 63 patients examined after their symptoms had resolved. Although TIAs can last seconds to hours, the highest risk subgroup in this study was those with symptoms lasting minutes (79,99). Focal neurologic symptoms and head or neck pain were associated with stroke and TIA. Of the 27 patients diagnosed with a cerebrovascular cause, DWI imaging was only 58% sensitive, presumably because these patients with transient symptoms had either smaller lesions or ischemia without infarction. The authors found that perfusion-weighted MRI was able to nearly double the proportion of patients in whom they could make a definite diagnosis. However, despite the intensive investigations these patients underwent, the authors could not determine a cause in 56% (79).
TIA causing dizziness or vertigo is easily missed; in a population-based study of transient symptoms preceding vertebrobasilar stroke, 9 of 10 who sought medical attention were initially missed (84). Dizziness is the most common symptom in basilar artery occlusion occurring without other neurologic symptoms in 20% (100,101). Dizziness is the most common presenting symptom of vertebral artery dissection, which affects younger patients, mimics migraine, and is easily misdiagnosed (30,102). Because 5% of TIA patients suffer a stroke within 48 h, prompt diagnosis is critical (103). Patients with posterior circulation TIA may have a higher stroke risk than those with anterior circulation TIA (104,105). Prompt treatment lowers stroke risk after TIA by roughly 80% (28,29). Patients with new symptoms in the past 12 months, even if repetitive, should be considered “at risk” for cerebrovascular causes; extra caution should be taken in those with ABCD2 risk scores ≥ 3 or with sudden, severe, or sustained craniocervical pain, as the latter may represent arterial dissection (14,84,99,102).
Cardiac dysrhythmias should also be considered in patients with s-EVS, particularly when true syncope occurs (106). Although some clinical features may increase or decrease the odds of a cardiac cause, additional testing (e.g., cardiac loop recording) is often required to confirm the final diagnosis (95,96).
Triggered Episodic Vestibular Syndrome
Patients with triggered episodic vestibular syndrome (t-EVS) have brief episodes of dizziness lasting seconds to minutes, depending on the underlying etiology. There is an “obligate” trigger; a specific trigger consistently causes dizziness. Common triggers are changes in head position or body posture. Patients with nausea and vomiting may overestimate episode duration. Again, clinicians must distinguish exacerbating features (worsens pre-existing baseline dizziness) from triggers (provokes new dizziness not present at baseline). The most common etiologies of t-EVS are BPPV and orthostatic hypotension. Dangerous causes include central (neurologic) mimics of BPPV and serious causes of orthostatic hypotension. By definition, physicians should be able to reproduce the dizziness at the bedside.
BPPV, the most common vestibular cause of dizziness, results from mobile crystalline debris in one or more semicircular canals (“canaliths”) of the vestibular labyrinth. Classical symptoms are repetitive brief, triggered episodes of rotational vertigo lasting less than a minute, though non-vertiginous dizziness is frequent (22,107,108). The diagnosis is confirmed by reproducing symptoms using canal-specific positional testing maneuvers and identifying a canal-specific nystagmus (Table 3) (108–110). We recommend starting with the Dix-Hallpike maneuver, which tests the posterior canal (most commonly involved) (111). A detailed recent review of these examination maneuvers includes instructive video clips (45). Should the Dix-Hallpike maneuver not reproduce the symptoms, the supine head roll test (where one starts with the patient supine and turns, turning the head to 90 degrees right and left) can be attempted to diagnose horizontal canal BPPV. Once the correct canal is identified, bedside treatment with canal repositioning maneuvers can follow (108). Although BPPV is common, emergency physicians often do not use guideline-supported Dix-Hallpike (diagnostic) or Epley (therapeutic) maneuvers (78,112,113).
Table 3.
Use of the Physical Examination to Diagnose Patients With Triggered Episodic Vestibular Syndrome
| Positional Diagnostic Test in t-EVS | BPPV (Posterior Canal) | BPPV (Horizontal Canal) | Central |
|---|---|---|---|
| Dix-Hallpike test | Upbeat-torsional* 5-30 s (crescendo-decrescendo intensity pattern) | None† | Variable direction (usually pure downbeat or horizontal; almost never upbeat or torsional) Variable duration (often persists >90 s if position is held; rarely varies significantly in intensity) |
| Supine roll test | None† | Pure horizontal‡ 30–90 s (crescendo-decrescendo intensity pattern) | Variable direction (usually pure downbeat or horizontal; rarely upbeat or torsional) Variable duration (often persists >90 s if position is held; rarely varies significantly in intensity) |
BPPV = benign paroxysmal positional vertigo; t-EVS = triggered, episodic vestibular syndrome.
The nystagmus of posterior canal BPPV will have a prominent torsional component, and the 12 o’clock pole of the eye will beat toward the down-facing (tested) ear. Upon arising from the down (Dix-Hallpike) position, the nystagmus will reverse direction because the canaliths are now moving in the opposite direction. This reversal is not the same as gaze-evoked, direction-changing nystagmus seen in patients with acute vestibular syndrome, and does not imply central disease (see text describing HINTS).
Although the Dix-Hallpike test is fairly specific to posterior canal BPPV and the supine roll test is fairly specific to horizontal canal BPPV, the maneuvers may sometimes stimulate the other canal. If so, the nystagmus direction will depend on the affected canal, not on the type of maneuver eliciting the nystagmus. The nystagmus may be considerably weaker and less obvious than if one were using the “correct” canal-specific maneuver.
The nystagmus of horizontal canal BPPV may beat toward the down ear or away from it. It may spontaneously reverse after an initial decay. When the other side is tested, the nystagmus will usually beat in the opposite direction (e.g., if right-beating initially with right ear down, then it will usually be left-beating initially with left ear down). These reversals are not the same as gaze-evoked, direction-changing nystagmus seen in patients with acute vestibular syndrome, and does not imply central disease (see text describing HINTS).
Central mimics of BPPV (central paroxysmal positional vertigo [CPPV]) caused by posterior fossa neoplasm, infarction, hemorrhage, and demyelination are rare. Distinguishing factors between BPPV from CPPV are summarized in Table 4 (114).
Table 4.
Characteristics of Patients With Triggered, Episodic Vestibular Syndrome that Suggest a Central Mimic (Central Paroxysmal Positional Vertigo) rather than Typical Benign Paroxysmal Positional Vertigo
| Presence of symptoms or signs that are not seen in BPPV |
| Headache |
| Diplopia |
| Abnormal cranial nerve or cerebellar function |
| Atypical nystagmus characteristics or symptoms during positional tests |
| Downbeating nystagmus* |
| Nystagmus that starts instantaneously, persists for >90 s, or lacks a crescendo-decrescendo pattern of intensity |
| Prominent nystagmus with mild or no associated dizziness or vertigo |
| Poor response to therapeutic maneuvers |
| Repetitive vomiting during positional maneuvers |
| Unable to cure patient with canal-specific canalith repositioning maneuver† |
| Frequent recurrent symptoms |
BPPV = benign paroxysmal positional vertigo.
Downbeating nystagmus can be seen with anterior canal BPPV. However, because BPPV of this canal is relatively rare and because downbeating nystagmus is most often the result of central structural lesions, it is safer for emergency physicians to consider this finding always worrisome prompting imaging or specialty consultation or referral.
Modified Epley maneuver or equivalent for posterior canal BPPV. Supine roll test or equivalent for horizontal canal BPPV.
Orthostatic hypotension accounts for 24% of acute syncopal presentations (115). The classical symptom is lightheadedness or presyncope on arising, but vertigo is common and underappreciated (21,23). Orthostatic hypotension is a sustained decline in blood pressure of at least 20 mm Hg systolic or 10 mm Hg diastolic within 3 min of standing (116). Optimal cutoffs may need to be adjusted based on baseline blood pressure (117). Emergency physicians are familiar with the common causes of orthostatic hypotension. Dangerous but uncommon causes include myocardial infarction, occult sepsis, adrenal insufficiency, and diabetic ketoacidosis (118).
Because BPPV produces dizziness on arising in 58%, it can mimic the postural lightheadedness of orthostatic hypotension, and often goes undiagnosed in the elderly (22,119,120). Also, orthostatic hypotension may be incidental and misleading, especially in older patients taking antihypertensive medications (121). Positional triggers, such as rolling over in bed or reclining, are common in BPPV, but should not occur with orthostatic hypotension, helping to distinguish between these two entities. Orthostatic dizziness and orthostatic hypotension are not always related (122,123). Orthostatic dizziness without systemic orthostatic hypotension has been reported with hemodynamic TIA (due low flow across a vascular stenosis) and in patients with intracranial hypotension (124,125).
CONCLUSIONS
Dizziness, vertigo, and unsteadiness are common complaints caused by numerous diseases that span organ systems. Diagnosis can be difficult. Misconceptions, resource overutilization, and misdiagnosis are common. The current “symptom quality” paradigm was created 45 years ago and is not evidence-based; a newer paradigm, based on timing and triggers, is more consistent with best evidence. History and physical examination are more accurate than imaging, and more likely to result in a specific diagnosis than the traditional paradigm.
ARTICLE SUMMARY.
1. Why is this topic important?
Dizziness is an extremely common symptom that is associated with numerous conditions. Distinguishing serious from benign causes using the fewest possible resources is key for emergency physicians. The ability to make this distinction could reduce the current rate of misdiagnosis for dangerous etiologies, including posterior circulation stroke, while simultaneously improving the care for patients with benign causes.
2. What does this review attempt to show?
This review shows that the traditional approach to diagnosing the dizzy patient based on the “type” of dizziness is inconsistent with scientific evidence. By contrast, an approach based on “timing and triggers” of the dizziness coheres well with best evidence. Using this new approach, physicians can more accurately identify patients with posterior circulation stroke and more promptly diagnose and treat the more common peripheral vestibular causes.
3. What are the key findings?
After first using history to place the patient into a “timing and triggers” category, physicians can apply a diagnostic strategy that highly leverages the physical examination, utilizing specific bedside tests depending on which category the patient fits within. This approach helps to diagnose and treat common peripheral causes of dizziness and to confidently identify posterior circulation stroke. Computed tomography is a poor test for ischemic stroke, and even magnetic resonance imaging is not sufficiently sensitive for detecting stroke in the first 48 h after onset of dizziness symptoms.
4. How is patient care impacted?
Patients with peripheral vestibular causes of dizziness can be managed according to best evidence and without the need for specialty consultation or advanced imaging, while those patients with posterior circulation stroke can be correctly identified and treated appropriately.
Acknowledgments
Dr. Newman-Toker’s effort was supported by a grant from the National Institutes of Health (NIDCD U01 DC013778). The funding agency was not involved in design of the study, the collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
APPENDIX 1. SHORTCOMINGS OF THE 1972 SUBSPECIALTY REFERRAL CLINIC STUDY THAT LED TO ADOPTION AND PERSISTENCE OF THE “SYMPTOM QUALITY” APPROACH (15,16)
Methodological weaknesses in study design and analysis
Tautological hypothesis for primacy of symptom quality
Methods placed patients into one of four categories of dizziness type by design
Related “appropriate” follow-up questions or special diagnostic tests applied selectively based on dizziness type assigned (work-up bias)
Dizziness type used to help determine final diagnoses (e.g., “The diagnosis of a peripheral vestibular disorder was, typically, applied to a patient who complained of unmistakable rotational vertigo.”) (incorporation bias)
Weak standards for assignment of diagnoses
Diagnostic criteria not defined in detail (“A discussion of the hierarchical ordering of criteria for each diagnosis is beyond the scope of this paper.”)
Resident physicians (not senior consultants) performed most examinations
Single individual assigned all final diagnoses; no independent verification
Individual assigning diagnoses not masked to dizziness type or other clinical features, nor to study hypothesis/objectives (risking diagnostic-review bias)
No long-term follow-up of patients to verify accuracy of diagnoses
Small, biased sample and lack of statistical rigor
Tertiary-care subspecialty referral clinic population limited to those fluent in English and available to return for four half days of laboratory testing
Small sample (n = 125 over 2 years) with large fraction excluded in analysis or not diagnosed (18%, n = 23 of 125; 12 inadequate data, 9 uncertain diagnoses, 2 inappropriate referrals)
Very small subgroups for nonperipheral vestibular etiologies (e.g., psychiatric, n = 9; stroke, n = 5; non-stroke neurologic, n = 4; cardiovascular, n = 4, other, n = 6).
No quantitative analysis of type-etiology association and exceptions ignored (e.g., anemia classed as cardiovascular but symptoms not “syncope-like”; psychosis classed as psychiatric but symptoms “variable and numerous”; 2 patients with hyperventilation syndrome complained of “positional vertigo”; multisensory dizziness [mostly peripheral neuropathy combined with minor visual or vestibular loss] complained of “light-head-edness”).
Unavoidable issues related to era in which study was performed
Lack of modern imaging
When the study was conducted, neither CT nor MRI were available
Era-specific lack of disease understanding
Vestibular migraine (a common cause of s-EVS) had not yet been described
Posterior circulation TIA/stroke presenting isolated dizziness not recognized
Footnotes
Reprints are not available from the authors.
Both Dr. Edlow and Dr. Newman-Toker review medical-legal cases for both plaintiff and defense firms in cases involving neurologic conditions, including dizziness and stroke. Dr. Newman-Toker has conducted funded research related to stroke misdiagnosis and has been loaned research equipment by two commercial companies (GN Otometrics and Interacoustics).
Author contributions: JAE wrote the first draft and the diagnostic algorithm. All authors reviewed and edited multiple revisions. JAE takes responsibility for the paper as a whole.
References
- 1.Newman-Toker DE, Hsieh YH, Camargo CA, Jr, Pelletier AJ, Butchy GT, Edlow JA. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765–775. doi: 10.4065/83.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung CS, Mak PS, Manley KV, et al. Predictors of important neurological causes of dizziness among patients presenting to the emergency department. Emerg Med J. 2010;27:517–521. doi: 10.1136/emj.2009.078014. [DOI] [PubMed] [Google Scholar]
- 3.Saber Tehrani AS, Coughlan D, Hsieh YH, et al. Rising annual costs of dizziness presentations to U.S. emergency departments. Acad Emerg Med. 2013;20:689–696. doi: 10.1111/acem.12168. [DOI] [PubMed] [Google Scholar]
- 4.Newman-Toker DE. Missed stroke in acute vertigo a time for action, not debate. Ann Neurol. 2016;79:27–31. doi: 10.1002/ana.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman-Toker DE, McDonald KM, Meltzer DO. How much diagnostic safety can we afford, and how should we decide? A health economics perspective. BMJ Qual Saf. 2013;22(Suppl 2):ii11–20. doi: 10.1136/bmjqs-2012-001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerber KA, Newman-Toker DE. Misdiagnosing dizzy patients: common pitfalls in clinical practice. Neurol Clin. 2015;33:565–75. doi: 10.1016/j.ncl.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577–99. doi: 10.1016/j.ncl.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman-Toker DE. Symptoms and signs of neuro-otologic disorders. Continuum (Minneap Minn) 2012;18:1016–40. doi: 10.1212/01.CON.0000421618.33654.8a. [DOI] [PubMed] [Google Scholar]
- 9.Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–64. doi: 10.1016/S1474-4422(08)70216-3. [DOI] [PubMed] [Google Scholar]
- 10.Navi BB, Kamel H, Shah MP, et al. Rate and predictors of serious neurologic causes of dizziness in the emergency department. Mayo Clin Proc. 2012;87:1080–8. doi: 10.1016/j.mayocp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chase M, Joyce NR, Carney E, et al. ED patients with vertigo: can we identify clinical factors associated with acute stroke? Am J Emerg Med. 2012;30:587–91. doi: 10.1016/j.ajem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Moubayed SP, Saliba I. Vertebrobasilar insufficiency presenting as isolated positional vertigo or dizziness: a double-blind retrospective cohort study. Laryngoscope. 2009;119:2071–6. doi: 10.1002/lary.20597. [DOI] [PubMed] [Google Scholar]
- 13.Kerber KA, Meurer WJ, Brown DL, et al. Stroke risk stratification in acute dizziness presentations: A prospective imaging-based study. Neurology. 2015;85:1869–78. doi: 10.1212/WNL.0000000000002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navi BB, Kamel H, Shah MP, et al. Application of the ABCD2 score to identify cerebrovascular causes of dizziness in the emergency department. Stroke. 2012;43:1484–9. doi: 10.1161/STROKEAHA.111.646414. [DOI] [PubMed] [Google Scholar]
- 15.Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323–34. doi: 10.1212/wnl.22.4.323. [DOI] [PubMed] [Google Scholar]
- 16.Newman-Toker D. Diagnosing Dizziness in the Emergency Department: Why “what do you mean by ’dizzy’?” Should Not be the First Question You Ask [PhD] Baltimore: Johns Hopkins School of Medicine; 2007. [Google Scholar]
- 17.Edlow J. Diagnosing dizziness: we are teaching the wrong paradigm! Acad Emerg Med. 2013;20:1064–6. doi: 10.1111/acem.12234. [DOI] [PubMed] [Google Scholar]
- 18.Newman-Toker DE. Charted records of dizzy patients suggest emergency physicians emphasize symptom quality in diagnostic assessment. Ann Emerg Med. 2007;50:204–5. doi: 10.1016/j.annemergmed.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Newman-Toker DE, Cannon LM, Stofferahn ME, Rothman RE, Hsieh YH, Zee DS. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. 2007;82:1329–40. doi: 10.4065/82.11.1329. [DOI] [PubMed] [Google Scholar]
- 20.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37:2484–7. doi: 10.1161/01.STR.0000240329.48263.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman-Toker DE, Dy FJ, Stanton VA, Zee DS, Calkins H, Robinson KA. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087–94. doi: 10.1007/s11606-008-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson J, Johnson I, Bamiou DE, Newton JL. Benign paroxysmal positional vertigo: clinical characteristics of dizzy patients referred to a Falls and Syncope Unit. QJM. 2005;98:357–64. doi: 10.1093/qjmed/hci057. [DOI] [PubMed] [Google Scholar]
- 23.Stanton VA, Hsieh YH, Camargo CA, Jr, et al. Overreliance on symptom quality in diagnosing dizziness: results of a multicenter survey of emergency physicians. Mayo Clin Proc. 2007;82:1319–28. doi: 10.4065/82.11.1319. [DOI] [PubMed] [Google Scholar]
- 24.Royl G, Ploner CJ, Leithner C. Dizziness in the emergency room: diagnoses and misdiagnoses. Eur Neurol. 2011;66:256–63. doi: 10.1159/000331046. [DOI] [PubMed] [Google Scholar]
- 25.Braun EM, Tomozik PV, Ropposch T, Nemetz U, Lackner A, Walch C. Misdiagnosis of acute peripheral vestibulopathy in central nervous ischemic infarction. Otol Neurotol. 2011;32:1518–21. doi: 10.1097/MAO.0b013e318238ff9a. [DOI] [PubMed] [Google Scholar]
- 26.Casani AP, Dallan I, Cerchiai N, Lenzi R, Cosottini M, Sellari-Franceschini S. Cerebellar infarctions mimicking acute peripheral vertigo: how to avoid misdiagnosis? Otolaryngol Head Neck Surg. 2013;32:1518–21. doi: 10.1177/0194599812472614. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Sohn SI, Cho WY, et al. Cerebellar infarction presenting with isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67:1178–83. doi: 10.1212/01.wnl.0000238500.02302.b4. [DOI] [PubMed] [Google Scholar]
- 28.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–60. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 29.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–42. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 30.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med. 2007;14:63–8. doi: 10.1197/j.aem.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 31.Kuruvilla A, Bhattacharya P, Rajamani K, Chaturvedi S. Factors associated with misdiagnosis of acute stroke in young adults. J Stroke Cerebrovasc Dis. 2011;20:523–7. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Martin-Schild S, Albright KC, Tanksley J, et al. Zero on the NIHSS does not equal the absence of stroke. Ann Emerg Med. 2011;57:42–5. doi: 10.1016/j.annemergmed.2010.06.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandelzweig L, Goldbourt U, Boyko V, Tanne D. Perceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute stroke. Stroke. 2006;37:1248–53. doi: 10.1161/01.STR.0000217200.61167.39. [DOI] [PubMed] [Google Scholar]
- 34.Masuda Y, Tei H, Shimizu S, Uchiyama S. Factors associated with the misdiagnosis of cerebellar infarction. J Stroke Cerebrovasc Dis. 2013;22:1125–30. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Newman-Toker DE, Moy E, Valente E, Coffey R, Hines AL. Misssed diagnosis of stroke in the ED: a cross-sectional analysis of a large population based sample. Diagnosis (Berl) 2014;2:29–40. doi: 10.1515/dx-2013-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda S, Inatomi Y, Yonehara T, et al. Discrimination of acute ischemic stroke from nonischemic vertigo in patients presenting with only imbalance. J Stroke Cerebrovasc Dis. 2014;23:888–95. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima MH, Hirano T, Uchino M. Patients with acute stroke admitted on the second visit. J Stroke Cerebrovasc Dis. 2008;17:382–7. doi: 10.1016/j.jstrokecerebrovasdis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Lee CC, Ho HC, Su YC, et al. Increased risk of vascular events in emergency room patients discharged home with diagnosis of dizziness or vertigo: a 3-year follow-up study. PLoS One. 2012;7:e35923. doi: 10.1371/journal.pone.0035923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atzema CL, Grewal K, Lu H, Kapral MK, Kulkarni G, Austin PC. Outcomes among patients discharged from the emergency department with a diagnosis of peripheral vertigo. Ann Neurol. 2016;79:32–41. doi: 10.1002/ana.24521. [DOI] [PubMed] [Google Scholar]
- 40.Kerber KA, Zahuranec DB, Brown DL, et al. Stroke risk after nonstroke emergency department dizziness presentations: a population-based cohort study. Ann Neurol. 2014;75:899–907. doi: 10.1002/ana.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim AS, Fullerton HJ, Johnston SC. Risk of vascular events in emergency department patients discharged home with diagnosis of dizziness or vertigo. Ann Emerg Med. 2011;57:34–41. doi: 10.1016/j.annemergmed.2010.06.559. [DOI] [PubMed] [Google Scholar]
- 42.Newman-Toker DE, Camargo CA, Jr, Hsieh YH, Pelletier AJ, Edlow JA. Disconnect between charted vestibular diagnoses and emergency department management decisions: a cross-sectional analysis from a nationally representative sample. Acad Emerg Med. 2009;16:970–7. doi: 10.1111/j.1553-2712.2009.00523.x. [DOI] [PubMed] [Google Scholar]
- 43.Kerber KA, Morgenstern LB, Meurer WJ, et al. Nystagmus assessments documented by emergency physicians in acute dizziness presentations: a target for decision support? Acad Emerg Med. 2011;18:619–26. doi: 10.1111/j.1553-2712.2011.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarnutzer AA, Lee SH, Robinson KA, Wang Z, Edlow JA, Newman-Toker DE. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: a meta-analysis. Neurology. 2017;88:1468–77. doi: 10.1212/WNL.0000000000003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edlow JA, Newman-Toker D. Using the physical examination to diagose patients with acute dizziness and vertigo. J Emerg Med. 2016;50:617–8. doi: 10.1016/j.jemermed.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. 2011;183:E571–92. doi: 10.1503/cmaj.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edlow JA, Newman-Toker DE. Medical and nonstroke neurologic causes of acute, continuous vestibular symptoms. Neurol Clin. 2015;33:699–716. doi: 10.1016/j.ncl.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pula JH, Newman-Toker DE, Kattah JC. Multiple sclerosis as a cause of the acute vestibular syndrome. J Neurol. 2013;260:1649–54. doi: 10.1007/s00415-013-6850-1. [DOI] [PubMed] [Google Scholar]
- 49.Kerber KA, Burke JF, Brown DL, et al. Does intracerebral haemorrhage mimic benign dizziness presentations? A population based study. Emerg Med J. 2012;29:43–6. doi: 10.1136/emj.2010.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kattah JC, Dhanani SS, Pula JH, Mantokoudis G, Saber Tehrani AS, Newman-Toker D. Vestibular signs of thiamine deficiency during the early phase of suspected Wernicke encephalopathy. Neurol Clin Pract. 2013;3:260–468. doi: 10.1212/01.CPJ.0000435749.32868.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cutfield NJ, Seemungal BM, Millington H, Bronstein AM. Diagnosis of acute vertigo in the emergency department. Emerg Med J. 2011;28:538–9. doi: 10.1136/emj.2010.105874. [DOI] [PubMed] [Google Scholar]
- 52.Arbusow V, Theil D, Strupp M, Mascolo A, Brandt T. HSV-1 not only in human vestibular ganglia but also in the vestibular labyrinth. Audiol Neurootol. 2001;6:259–62. doi: 10.1159/000046131. [DOI] [PubMed] [Google Scholar]
- 53.Lu YC, Young YH. Vertigo from herpes zoster oticus: superior or inferior vestibular nerve origin? Laryngoscope. 2003;113:307–11. doi: 10.1097/00005537-200302000-00020. [DOI] [PubMed] [Google Scholar]
- 54.Strupp M, Jager L, Muller-Lisse U, Arbusow V, Reiser M, Brandt T. High resolution Gd-DTPA MR imaging of the inner ear in 60 patients with idiopathic vestibular neuritis: no evidence for contrast enhancement of the labyrinth or vestibular nerve. J Vestib Res. 1998;8:427–33. [PubMed] [Google Scholar]
- 55.Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027–32. doi: 10.1056/NEJMcp021154. [DOI] [PubMed] [Google Scholar]
- 56.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi JH, Kim HW, Choi KD, et al. Isolated vestibular syndrome in posterior circulation stroke: frequency and involved structures. Neurol Clin Pract. 2014;4:410–8. doi: 10.1212/CPJ.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–10. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saber Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and non-lacunar mechanisms. Neurology. 2014;83:169–73. doi: 10.1212/WNL.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986–96. doi: 10.1111/acem.12223. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Lee W, Chambers BR, Dewey HM. Diagnostic accuracy of acute vestibular syndrome at the bedside in a stroke unit. J Neurol. 2011;258:855–61. doi: 10.1007/s00415-010-5853-4. [DOI] [PubMed] [Google Scholar]
- 62.Vanni S, Nazerian P, Casati C, et al. Can emergency physicians accurately and reliably assess acute vertigo in the emergency department? Emerg Med Australas. 2015;27:126–31. doi: 10.1111/1742-6723.12372. [DOI] [PubMed] [Google Scholar]
- 63.Vanni SP, Pecci R, Casati C, et al. Standing, a four-step bedside algorithm for differential diagnosis of acute vertigo in the emergency department. ACTA Otorhinolaryngol Ital. 2014;34:419–26. [PMC free article] [PubMed] [Google Scholar]
- 64.Newman-Toker DE, Kattah JC. In reply. Acad Emerg Med. 2014;21:348–9. doi: 10.1111/acem.12338. [DOI] [PubMed] [Google Scholar]
- 65.Taylor RL, McGarvie LA, Reid N, Young AS, Halmagyi GM, Welgampola MS. Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology. 2016;87:1704–12. doi: 10.1212/WNL.0000000000003223. [DOI] [PubMed] [Google Scholar]
- 66.Newman-Toker DE, Sharma P, Chowdhury M, Clemons TM, Zee DS, Della Santina CC. Penlight-cover test: a new bedside method to unmask nystagmus. J Neurol Neurosurg Psychiatry. 2009;80:900–3. doi: 10.1136/jnnp.2009.174128. [DOI] [PubMed] [Google Scholar]
- 67.Newman-Toker DE, Curthoys IS, Halmagyi GM. Diagnosing stroke in acute vertigo: the HINTS family of eye movement tests and the future of the “Eye ECG”. Semin Neurol. 2015;35:506–21. doi: 10.1055/s-0035-1564298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 69.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70:2378–85. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- 70.Hausler R, Levine RA. Auditory dysfunction in stroke. Acta Otolaryngol. 2000;120:689–703. doi: 10.1080/000164800750000207. [DOI] [PubMed] [Google Scholar]
- 71.Lee H. Neuro-otological aspects of cerebellar stroke syndrome. J Clin Neurol. 2009;5:65–73. doi: 10.3988/jcn.2009.5.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee H, Kim JS, Chung EJ, et al. Infarction in the territory of anterior inferior cerebellar artery: spectrum of audiovestibular loss. Stroke. 2009;40:3745–51. doi: 10.1161/STROKEAHA.109.564682. [DOI] [PubMed] [Google Scholar]
- 73.Lee SH, Kim JS. Acute diagnosis and management of stroke presenting dizziness or vertigo. Neurol Clin. 2015;33:687–98. xi. doi: 10.1016/j.ncl.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Newman-Toker DE, Reich SG. Wrong-way nystagmus in the AICA syndrome [letter to the editor] Laryngoscope. 2008;118:378–9. doi: 10.1097/MLG.0b013e318157f77f. [DOI] [PubMed] [Google Scholar]
- 75.Pogson JM, Taylor RL, Young AS, et al. Vertigo with sudden hearing loss: audio-vestibular characteristics. J Neurol. 2016;263:2086–96. doi: 10.1007/s00415-016-8214-0. [DOI] [PubMed] [Google Scholar]
- 76.Kim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003;126:1864–72. doi: 10.1093/brain/awg169. [DOI] [PubMed] [Google Scholar]
- 77.Carmona S, Martinez C, Zalazar G, et al. The diagnostic accuracy of truncal ataxia and HINTS as cardinal signs for acute vestibular syndrome. Front Neurol. 2016;7:125. doi: 10.3389/fneur.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kene MV, Ballard DW, Vinson DR, Rauchwerger AS, Iskin HR, Kim AS. Emergency physician attitudes, preferences, and risk tolerance for stroke as a potential cause of dizziness symptoms. West J Emerg Med. 2015;16:768–76. doi: 10.5811/westjem.2015.7.26158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi JH, Park MG, Choi SY, et al. Acute transient vestibular syndrome: prevalence of stroke and efficacy of bedside evaluation. Stroke. 2017;48:556–62. doi: 10.1161/STROKEAHA.116.015507. [DOI] [PubMed] [Google Scholar]
- 80.Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67:1028–33. doi: 10.1212/01.wnl.0000237539.09942.06. [DOI] [PubMed] [Google Scholar]
- 81.Seemungal B, Kaski D, Lopez-Escamez JA. Early diagnosis and management of acute vertigo from vestibular migraine and meniere’s disease. Neurol Clin. 2015;33:619–628. ix. doi: 10.1016/j.ncl.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Strupp M, Versino M, Brandt T. Vestibular migraine. Handb Clin Neurol. 2010;97:755–771. doi: 10.1016/S0072-9752(10)97062-0. [DOI] [PubMed] [Google Scholar]
- 83.Blum CA, Kasner SE. Transient ischemic attacks presenting with dizziness or vertigo. Neurol Clin. 2015;33:629–642. ix. doi: 10.1016/j.ncl.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Paul NL, Simoni M, Rothwell PM, Oxford Vascular S Transient isolated brainstem symptoms preceding posterior circulation stroke: a population-based study. Lancet Neurol. 2013;12:65–71. doi: 10.1016/S1474-4422(12)70299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romme JJ, van Dijk N, Boer KR, et al. Influence of age and gender on the occurrence and presentation of reflex syncope. Clin Auton Res. 2008;18:127–133. doi: 10.1007/s10286-008-0465-0. [DOI] [PubMed] [Google Scholar]
- 86.Kanner AM. Ictal panic and interictal panic attacks: diagnostic and therapeutic principles. Neurol Clin. 2011;29:163–175. ix. doi: 10.1016/j.ncl.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Chen J, Tsuchiya M, Kawakami N, Furukawa TA. Non-fearful vs. fearful panic attacks: a general population study from the National Comorbidity Survey. J Affect Disord. 2009;112:273–278. doi: 10.1016/j.jad.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246:883–892. doi: 10.1007/s004150050478. [DOI] [PubMed] [Google Scholar]
- 89.Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet. 2001;357:348–353. doi: 10.1016/S0140-6736(00)03642-4. [DOI] [PubMed] [Google Scholar]
- 90.Lempert T, Neuhauser H, Daroff RB. Vertigo as a symptom of migraine. Ann N Y Acad Sci. 2009;1164:242–251. doi: 10.1111/j.1749-6632.2009.03852.x. [DOI] [PubMed] [Google Scholar]
- 91.Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107(Pt 4):1123–1142. doi: 10.1093/brain/107.4.1123. [DOI] [PubMed] [Google Scholar]
- 92.Sajjadi H, Paparella MM. Meniere’s disease. Lancet. 2008;372:406–414. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 93.Mancini F, Catalani M, Carru M, Monti B. History of Meniere’s disease and its clinical presentation. Otolaryngol Clin North Am. 2002;35:565–580. doi: 10.1016/s0030-6665(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 94.Faag C, Bergenius J, Forsberg C, Langius-Eklof A. Symptoms experienced by patients with peripheral vestibular disorders: evaluation of the Vertigo Symptom Scale for clinical application. Clin Otolaryngol. 2007;32:440–446. doi: 10.1111/j.1749-4486.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 95.van Dijk JG, Thijs RD, Benditt DG, Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol. 2009;5:438–448. doi: 10.1038/nrneurol.2009.99. [DOI] [PubMed] [Google Scholar]
- 96.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Katon WJ. Clinical practice. Panic disorder. N Engl J Med. 2006;354:2360–2367. doi: 10.1056/NEJMcp052466. [DOI] [PubMed] [Google Scholar]
- 98.Hoshino T, Nagao T, Mizuno S, Shimizu S, Uchiyama S. Transient neurological attack before vertebrobasilar stroke. J Neurol Sci. 2013;325:39–42. doi: 10.1016/j.jns.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Grad A, Baloh RW. Vertigo of vascular origin. Clinical and electrony stagmographic features in 84 cases. Arch Neurol. 1989;46:281–284. doi: 10.1001/archneur.1989.00520390047014. [DOI] [PubMed] [Google Scholar]
- 100.von Campe G, Regli F, Bogousslavsky J. Heralding manifestations of basilar artery occlusion with lethal or severe stroke. J Neurol Neurosurg Psychiatry. 2003;74:1621–1626. doi: 10.1136/jnnp.74.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fisher CM. Vertigo in cerebrovascular disease. Arch Otolaryngol. 1967;85:529–534. doi: 10.1001/archotol.1967.00760040531010. [DOI] [PubMed] [Google Scholar]
- 102.Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18:245–254. doi: 10.1097/NRL.0b013e31826754e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shah KH, Kleckner K, Edlow JA. Short-term prognosis of stroke among patients diagnosed in the emergency department with a transient ischemic attack. Ann Emerg Med. 2008;51:316–323. doi: 10.1016/j.annemergmed.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 104.Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126:1940–1954. doi: 10.1093/brain/awg197. [DOI] [PubMed] [Google Scholar]
- 105.Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke. 2009;40:2732–2737. doi: 10.1161/STROKEAHA.109.553859. [DOI] [PubMed] [Google Scholar]
- 106.Newman-Toker DE, Camargo CA., Jr ’Cardiogenic vertigo’—true vertigo as the presenting manifestation of primary cardiac disease. Nat Clin Pract Neurol. 2006;2:167–172. doi: 10.1038/ncpneuro0125. quiz 73. [DOI] [PubMed] [Google Scholar]
- 107.Baloh RW, Honrubia V, Jacobson K. Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. 1987;37:371–378. doi: 10.1212/wnl.37.3.371. [DOI] [PubMed] [Google Scholar]
- 108.Fife TD, von Brevern M. Benign Paroxysmal positional vertigo in the acute care setting. Neurol Clin. 2015;33:601–617. viii–ix. doi: 10.1016/j.ncl.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(Suppl):S47–81. doi: 10.1016/j.otohns.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 110.Fife TD, Iverson DJ, Lempert T, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:2067–2074. doi: 10.1212/01.wnl.0000313378.77444.ac. [DOI] [PubMed] [Google Scholar]
- 111.Welgampola MS, Bradshaw AP, Lechner C, Halmagyi GM. Bedside assessment of acute dizziness and vertigo. Neurol Clin. 2015;33:551–564. vii. doi: 10.1016/j.ncl.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 112.Bashir K, Qotb MA, Alkahky S, Fathi AM, Mohamed MA, Cameron PA. Are emergency physicians and paramedics providing canalith repositioning manoeuvre for benign paroxysmal positional vertigo? Emerg Med Australasia. 2015;27:179–180. doi: 10.1111/1742-6723.12362. [DOI] [PubMed] [Google Scholar]
- 113.Kerber KA, Burke JF, Skolarus LE, et al. Use of BPPV processes in emergency department dizziness presentations: a population-based study. Otolaryngol Head Neck Surg. 2013;148:425–430. doi: 10.1177/0194599812471633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soto-Varela A, Rossi-Izquierdo M, Sanchez-Sellero I, Santos-Perez S. Revised criteria for suspicion of non-benign positional vertigo. QJM. 2013;106:317–321. doi: 10.1093/qjmed/hct006. [DOI] [PubMed] [Google Scholar]
- 115.Sarasin FP, Louis-Simonet M, Carballo D, Slama S, Junod AF, Unger PF. Prevalence of orthostatic hypotension among patients presenting with syncope in the ED. Am J Emerg Med. 2002;20:497–501. doi: 10.1053/ajem.2002.34964. [DOI] [PubMed] [Google Scholar]
- 116.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 117.Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27:976–982. doi: 10.1097/hjh.0b013e3283279860. [DOI] [PubMed] [Google Scholar]
- 118.Gilbert VE. Immediate orthostatic hypotension: diagnostic value in acutely ill patients. South Med J. 1993;86:1028–1032. [PubMed] [Google Scholar]
- 119.von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg. 2000;122:630–634. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- 121.Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;30:173–178. doi: 10.1111/j.1365-2710.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 122.Radtke A, Lempert T, von Brevern M, Feldmann M, Lezius F, Neuhauser H. Prevalence and complications of orthostatic dizziness in the general population. Clin Auton Res. 2011;21:161–168. doi: 10.1007/s10286-010-0114-2. [DOI] [PubMed] [Google Scholar]
- 123.Wu JS, Yang YC, Lu FH, Wu CH, Chang CJ. Population-based study on the prevalence and correlates of orthostatic hypoten-sion/hypertension and orthostatic dizziness. Hypertens Res. 2008;31:897–904. doi: 10.1291/hypres.31.897. [DOI] [PubMed] [Google Scholar]
- 124.Stark RJ, Wodak J. Primary orthostatic cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1983;46:883–891. doi: 10.1136/jnnp.46.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blank SC, Shakir RA, Bindoff LA, Bradey N. Spontaneous intracranial hypotension: clinical and magnetic resonance imaging characteristics. Clin Neurol Neurosurg. 1997;99:199–204. doi: 10.1016/s0303-8467(97)00015-2. [DOI] [PubMed] [Google Scholar]


