Abstract
Background
Endoscopic experience is known to correlate with outcomes of endoscopic mucosal resection (EMR), particularly complete resection of the polyp tissue. Whether specialist endoscopists can protect against incomplete polypectomy in the setting of known risk factors for incomplete resection (IR) is unknown.
Aims
We aimed to characterize how specialist endoscopists may help to mitigate the risk of IR of large sessile polyps.
Methods
This is a retrospective cohort study of patients who underwent EMR at the University of Michigan from January 1, 2006, to November 15, 2015. The primary outcome was endoscopist-reported polyp tissue remaining at the end of the initial EMR attempt. Specialist endoscopists were defined as endoscopists who receive tertiary referrals for difficult colonoscopy cases and completed at least 20 EMR colonic polyp resections over the study period.
Results
A total of 257 patients with 269 polyps were included in the study. IR occurred in 40 (16%) cases. IR was associated with polyp size ≥ 40 mm [adjusted odds ratio (aOR) 3.31, 95% confidence interval (CI) 1.38–7.93], flat/laterally spreading polyps (aOR 2.61, 95% CI 1.24–5.48), and difficulty lifting the polyp (aOR 11.0, 95% CI 2.66–45.3). A specialist endoscopist performing the initial EMR was protective against IR, even in the setting of risk factors for IR (aOR 0.13, 95% CI 0.04–0.41).
Conclusions
IR is associated with polyp size ≥ 40 mm, flat and/or laterally spreading polyps, and difficulty lifting the polyp. A specialist endoscopist initiating the EMR was protective of IR.
Keywords: Endoscopic mucosal resection, Polypectomy, Colon
Introduction
Endoscopic mucosal resection (EMR) has become the preferred method for the removal of sessile or laterally spreading lesions ≥10 mm given the lower rate of complications and mortality as compared to surgical management [1–6]. EMR of large colonic polyps is also roughly three times less expensive than surgical management, and this effect is amplified among older patients with multiple comorbidities [5, 7]. Thus, surgery should be reserved for large polyps that have been deemed endoscopically unresectable [3, 8–10].
Incomplete resection (IR) of large sessile or laterally spreading polyps occurs in up to 10% of colonic EMR procedures [11, 12]. The British Society guidelines for the management of large non-pedunculated colorectal polyps have indicated that an increased risk of IR is associated with polyps ≥ 40 mm, location of the polyp, prior failed attempt at resection, and a non-lifting sign [11]. Prior studies have also shown that the experience of the endoscopists performing the EMR affects the rate of incomplete resections [12, 13]. Clinically, incomplete resection of large colonic polyps has been implicated in the development of interval colon cancers, failure of additional EMR attempts due to tethering and fibrosis, and the eventual need for surgical resection [14–16].
To define the most cost-effective care in patients with large colonic polyps, it will become critical to identify factors that maximize successful outcomes of EMR and minimize multiple futile procedures. Our hypothesis is that endoscopists specializing in colonic EMR protect against IR of large colonic polyps as compared to non-specialized endoscopists. To our knowledge, this is the first study in the USA that characterizes how endoscopists with specialized experience in EMR may help to mitigate the risk of IR of large sessile or laterally spreading lesions, especially in the setting of known risk factors for IR.
Methods
Patient Population and Definitions
Adult colonoscopies with sessile or flat polyps ≥ 10 mm resected using an inject and lift technique performed at a University of Michigan (UM) Medical Procedures Unit (MPU), located in either the main hospital or an off-campus ambulatory unit, were identified from an endoscopy database (Provation MD, Provation Medical, Minneapolis, MN) from January 1, 2006, to November 15, 2015. Search terms used to identify patients from the database included “colonoscopy,” “single polyp,” “polyp(s), multiple,” “multiple polyps,” and “size in mm.” The endoscopy reports were manually reviewed, and patients were excluded if they had inflammatory bowel disease (IBD), familial polyposis syndrome, had no follow-up colonoscopy after their initial EMR procedure, had a polyp ≥ 10 mm that was not removed through an inject and lift technique, or had a pedunculated polyp ≥ 10 mm.
Specialist endoscopists (SE) were defined as endoscopists who receive tertiary referrals for difficult colonoscopy cases, completed at least 20 large (≥10 mm) EMR colonic polyp resections over the study period, and are at least 5 years out from fellowship training. IR was defined as endoscopist-reported incomplete polypectomy at the time of the initial EMR, and complete resection (CR) was defined as endoscopist-reported complete polypectomy at the time of the initial EMR.
Study Variables and Primary Outcomes
Data were manually abstracted for demographic data (age, gender, race); date and location (main hospital vs. off-site) of colonoscopy; estimated size, description of polyp shape (sessile or flat/laterally spreading polyp), and location of polyp; and provider (SE or non-SE). Therapeutic aspects of the initial EMR recorded included any reported difficulty in lifting the polyp; choice of resection tool including cold snare, hot snare, or both; use of cold forceps; use of other modalities such as APC or hemoclips; type of resection (piecemeal vs. en bloc); and reported success (IR or CR). Adverse events, such as immediate bleeding, delayed post-polypectomy bleed, perforation, or hospitalization, were reviewed. Final histology of the polyps was recorded and included adenocarcinoma, adenoma, sessile serrated adenoma/sessile serrated polyp (SSA/SSP), hyperplastic, or benign mucosal polyp with or without dysplasia. Follow-up, including subsequent colonoscopies and surgeries, was tracked. Study data were collected and managed using REDCap electronic data capture tools, and the study was approved by the Institutional Review Board at The University of Michigan [17].
The primary outcome was IR defined as endoscopist-reported residual polyp tissue remaining at the end of the initial EMR. Secondary outcomes included clinical implications of IR based on SE versus non-SE providers at UM.
Statistical Analysis
Student’s t tests, Fisher exact tests, or Chi-square tests were used as appropriate for univariable analysis. All factors with a p value of ≤0.1 were included in our multivariable analysis. Stepwise backward logistic regression was used to develop a model to predict IR. A two-sided p value of <0.05 was considered statistically significant. Adjusted probabilities were calculated based on our final multivariable model. Stata version 14 was used to perform all analyses (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Database Query
The initial query of the endoscopy database using the search terms “colonoscopy,” “single polyp,” “polyp(s), multiple,” “multiple polyps,” and “size in mm” identified 3196 colonoscopies with at least one polyp from January 2006 to November 2015. After limiting the findings to polyps ≥ 10 mm, 696 patients were identified. Furthermore, after manual review of the endoscopy report excluded patients with IBD, familial polyposis syndrome, those patients who had no follow-up colonoscopy or did not have an inject and lift technique described in the report, and those patients who did not have a sessile or flat polyp; a total of 257 patients, with 269 polyps, were included in our study. 67% of the EMRs were performed in the last 4 years.
EMR Procedural Characteristics and Adverse Events
Of the 257 patients included in our study, 57% of patients were male, 88% were Caucasian, and the mean age at the time of the first EMR was 62 ± 11 years (Table 1). Patient follow-up interval was a mean of 22 ± 25 months during which patients underwent 2.7 ± 1.0 colonoscopies (Table 1). All resection attempts were performed with submucosal injections with saline, with or without epinephrine or a blue dye as chosen by the attending endoscopist. 67% of polyps were located on the right side of the colon, and the mean size of the polyps removed was 28 ± 11 mm (Table 1). Polyps ≥ 40 mm made up 47 (18%) of EMR cases (Table 1). A total of 183 (71%) of polyps were adenomas, 42 (16%) were SSA/SSP, and 11 (4%) of polyps were adenocarcinoma (Table 1). The remainder of the polyps found were either benign mucosal polyps or hyperplastic polyps. Further procedural characteristics are listed in Table 1.
Table 1.
Clinical and endoscopic characteristics of 257 EMR patients
| Baselines characteristic (n = 257) | n (% or ±SD) |
|---|---|
| Male gender | 147 (57%) |
| Caucasian | 226 (88%) |
| Age at first EMR | 61.8 ± 11.1 |
| Months of follow-up | 21.9 ± 25.2 |
| Mean number of colonoscopies | 2.7 ± 1.01 |
| Mean polyp size | 28.1 ± 11.1 |
| Polyps ≥ 40 mm | 47 (18%) |
| Right-sided colonic polyps | 171 (67%) |
| Difficulty lifting | 18 (7%) |
| Off-site UM endoscopy unit | 16 (6%) |
| Polyp shape | |
| Sessile | 168 (65%) |
| Non-sessile polyp | 89 (35%) |
| Pathology of EMR-removed polyp | |
| Adenoma | 183 (71%) |
| SSA/SSPa | 42 (16%) |
| Adenocarcinoma | 11 (4%) |
| Dysplasia | |
| Low grade | 2 (0.8%) |
| High grade | 6 (2%) |
| Cytological dysplasia | 1 (0.4%) |
| Procedural characteristics | |
| Piecemeal removal | 192 (75%) |
| Argon plasma coagulation use | 50 (19%) |
| Snare type | |
| Hot snare | 178 (69% |
| Cold snare | 29 (11%) |
| Hot and cold snare | 47 (18%) |
| Adverse events | n (% or ±SD) |
| Perforation | 3 (1%) |
| Hospitalizations | 1 (0.4%) |
| Immediate bleeding | 8 (3%) |
| Post-polypectomy bleeding | 9 (4%) |
SSA/SSP sessile serrated adenoma/sessile serrated polyp
Adverse events were uncommon in our cohort of patients. Three (1.2%) patients had a perforation in the right colon during the initial EMR which was treated endoscopically, and one patient (0.4%) required hospitalization in the intensive care unit for delayed perforation which required a right hemicolectomy (Table 1). Eight patients (3.1%) had immediate bleeding, and nine patients (3.5%) had delayed post-polypectomy bleeding which required endoscopic interventions (Table 1). Of the nine patients with delayed bleeding, six (66.7%) had hemoclips deployed for defect closure.
Incomplete Resection
At the initial EMR attempt, IR occurred in 40 of 257 (16%) cases. Patient age and gender, number of right colon lesions, and polyp pathology were similar among the IR and CR group (Table 2). Specialist endoscopists accounted for 20% of the 40 IR cases (Table 3). Polyps removed at the initial EMR were slightly larger in the IR group when compared to polyps removed in the CR group (33 ± 15 vs. 27 ± 10 mm, p = 0.002). There was a higher percentage of polyps ≥ 40 mm in the IR cohort than in the CR cohort (30% vs. 16%, p = 0.04) (Table 2). Furthermore, polyps in the IR cohort were more likely to undergo piecemeal resection (93 vs. 71%, p = 0.002) and more often described as difficult to lift (15 vs. 6%, p = 0.03) than polyps in the CR cohort (Table 2). In addition, flat and/or laterally spreading polyps were more likely to be incompletely resected compared to sessile polyps (Table 2). Last, patients with IR were more likely to be referred to surgery than patients with CR (30 vs. 9%, p < 0.001) (Table 2).
Table 2.
Characteristics of patients with incomplete polypectomy at the time of the first EMR
| Incomplete (n = 40) | Complete (n = 217) | p value | |
|---|---|---|---|
| Male gender | 26 (65%) | 121 (56%) | 0.28 |
| Mean age at time of EMR | 63.0 ± 10.7 | 61.6 ± 11.1 | 0.45 |
| Mean number of colonoscopy | 3.1 (range 2–6) | 2.6 (range 2–7) | 0.01 |
| Mean polyp size | 33.1 mm (range 15–80 mm) | 27.2 (range 10–60 mm) | 0.002 |
| Polyps ≥ 40 mm | 12 (30%) | 28 (16%) | 0.04 |
| Mean months of follow-up | 24 ± 26.0 | 22 ± 25.1 | 0.66 |
| Right colon lesions | 24 (60%) | 147 (68%) | 0.34 |
| Piecemeal removal | 37 (93%) | 155 (71%) | 0.002 |
| Difficulty lifting | 6 (15%) | 12 (6%) | 0.03 |
| Surgery referral | 12 (30%) | 19 (9%) | <0.001 |
| Pathology of EMR-removed polyp | |||
| Adenoma | 31 (78%) | 152 (70%) | 0.34 |
| SSA/SSP | 5 (13%) | 37 (17%) | 0.33 |
| High-grade dysplasia | 3 (8%) | 3 (1.5%) | 0.05 |
| Adenocarcinoma | 1 (2.5%) | 10 (5%) | 0.47 |
| Polyp shape | 0.003 | ||
| Sessile | 18 (45%) | 150 (69%) | |
| Flat and/or laterally spreading | 22 (55%) | 67 (31%) | |
Table 3.
Characteristics of patients by endoscopist
| Specialist (n = 107) | Non-specialist (n = 150) | p value | |
|---|---|---|---|
| Male gender | 60 (56%) | 87 (58%) | 0.76 |
| Mean age at time of EMR | 63 ± 10.6 | 61 ± 11.4 | 0.36 |
| Mean number of colonoscopy | 2.5 ± 0.87 | 2.8 ± 1.1 | 0.005 |
| Mean polyp size | 31 ± 12.0 | 26 ± 10.0 | 0.002 |
| Polyps ≥ 40 mm | 28 (26%) | 19 (13%) | 0.006 |
| Mean months of follow-up | 13.7 ± 18.2 | 27.7 ± 27.8 | <0.001 |
| Right colon lesions | 69 (64%) | 102 (68%) | 0.56 |
| Piecemeal removal | 96 (90%) | 96 (60%) | <0.001 |
| Difficulty lifting | 16 (15%) | 2 (1.3%) | <0.001 |
| Incomplete resection | 8 (7%) | 32 (21%) | 0.003 |
| Surgery referral | 8 (7%) | 23 (15%) | 0.06 |
| Pathology of EMR-removed polyp | |||
| Adenoma | 77 (72%) | 106 (71%) | 0.82 |
| SSA/SSP | 18 (17%) | 24 (16%) | 0.86 |
| Adenocarcinoma | 4 (4%) | 7 (5%) | 0.72 |
| Polyp shape | 0.003 | ||
| Sessile | 81 (76%) | 87 (58%) | |
| Flat and/or laterally spreading | 26 (24%) | 63 (42%) | |
Role of Specialist Endoscopists
Four endoscopists fulfilled our criteria of specialist endoscopist at the University of Michigan. These specialists receive tertiary referrals for difficult colonoscopy cases, performed at least 20 EMR procedures over the study period, and are at least 5 years out from fellowship training. Furthermore, they have significant experience in the removal of large complex colonic polyps.
Specialist endoscopists completed 107 (42%) of the initial EMR procedures, and non-specialist endoscopists completed 150 (58%) of the initial EMR procedures (Table 3). There was no difference in patient gender, age at the time of EMR, number of right-sided colon lesions, or polyp pathology in cases with specialist endoscopists versus non-specialist endoscopists (Table 3).
Specialist endoscopists were less likely to have IR of polyps than non-specialist endoscopists (7% (8/107) vs. 21% (32/150), p = 0.003) (Table 3). The polyps resected by specialist endoscopists were generally larger than those removed by non-specialist endoscopists (31 ± 12.0 vs. 26 ± 10.0 mm, p = 0.002) and more often removed piecemeal than en bloc (90 vs. 60%, p < 0.001) (Table 3). The polyps resected by specialist endoscopists were also more likely to be ≥40 mm (28 mm versus 19 mm, p = 0.006), were more often described as difficult to lift than those by non-specialist endoscopists (15 vs. 1.3%, p < 0.001) (Table 3).
Outcomes of IR
In total, 28/40 (70%) patients with IR were managed non-surgically. Among those 28, 14 patients achieved CR. These patients required a median of 2.1 colonoscopies (interquartile range 2–4) to achieve CR, in contrast to a median of 1 colonoscopy for patients with CR at the initial EMR (p < 0.001). In the 14 patients who did not achieve CR, 8 are actively undergoing further EMR and adjunctive ablative interventions, 5 were lost to follow-up, and one person passed away from causes unrelated to their EMR procedure.
Twelve patients were referred to surgery after a mean of 2 colonoscopies. The indications for surgery included location (proximity to a diverticulum, n = 1), inadequate lifting (n = 3), difficult access or positioning (n = 1), endoscopist-reported high risk of continued incomplete resection (n = 2), high-grade dysplasia or adenocarcinoma (n = 2), interval development of adenocarcinoma during a subsequent EMR (n = 1), and progressive or residual disease (n = 2).
Multivariable Model
Based on our multivariable logistic regression, the probability of IR was associated with polyp size ≥ 40 mm (adjusted odds ratio (aOR) 3.31, 95% CI 1.38–7.93), a flat and/or laterally spreading polyp (aOR 2.61, CI 1.24–5.48), and difficulty lifting (aOR 11.0, 95% confidence interval (CI) 2.66–45.3) (Table 4). A specialist endoscopist performing the initial EMR was protective of IR (aOR 0.13, 95% CI 0.04–0.42) (Table 4). Piecemeal resection was not included in our initial model due to collinearity with specialist endoscopists.
Table 4.
Univariable and multivariable analysis regression models for incomplete polypectomy
| Incomplete EMR OR (95% CI) | ||
|---|---|---|
|
| ||
| Univariable | Multivariable | |
| Female gender | 0.68 (0.34–1.37) | – |
| Mean age at initial EMR | 1.01 (0.981–1.04) | – |
| Difficulty lifting | 3.01 (1.06–8.57) | 11.0 (2.66–45.3) |
| Adenoma | 1.47 (0.66–3.27) | – |
| SSA/SSP | 0.70 (0.26–1.89) | – |
| Polyp ≥ 40 mm | 2.23 (1.04–4.80) | 3.31 (1.38–7.93) |
| Specialist endoscopists | 0.30 (0.13–0.68) | 0.13 (0.04–0.42) |
| Polyp shape (n = 253) | ||
| Sessile | Ref | Ref |
| Flat and/or laterally spreading | 2.74 (1.38–5.44) | 2.61 (1.24–5.48) |
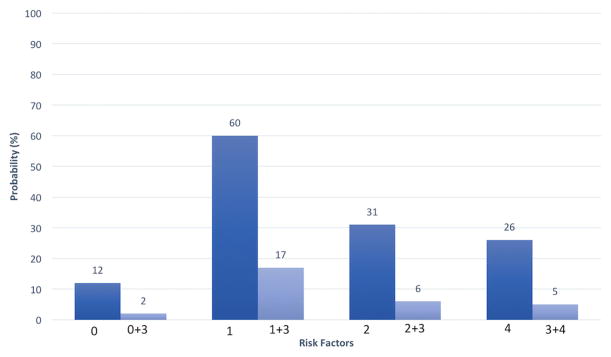
Adjusted probabilities of IR were calculated from our multivariable regression model. The probability of IR for a polyp without any risk factors (i.e., easy to lift, <40 mm, and sessile shaped) was 12% if performed by a non-specialist endoscopist and 2% if performed by a specialist endoscopist (Fig. 1).
Fig. 1.
Adjusted probability of incomplete polypectomy for individual risk factors. 0 no risk factor present, 1 difficulty lifting, 2 polyp size ≥ 40 mm, 3 specialist endoscopists, 4 flat and/or laterally spreading polyp, based on our multivariable model
When evaluating individual negative risk factors, the risk of polyp IR when performed by a non-specialist endoscopist was 31% for polyps ≥ 40 mm, 26% for flat/laterally spreading polyps, and 60% for polyps difficult to lift (Fig. 1). These rates dropped to 6, 5, and 17%, respectively, when performed by a specialist endoscopist (Fig. 1).
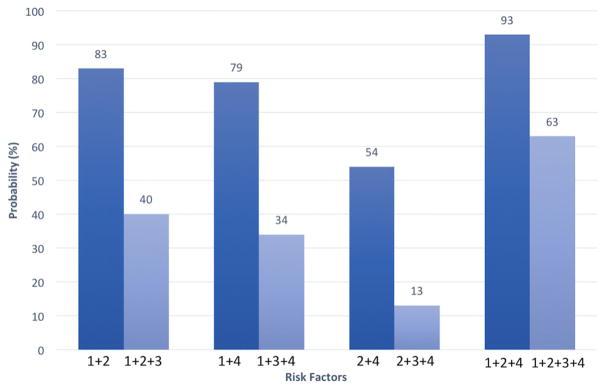
In our model, the risk of IR in the setting of combined risk factors was also lower if a specialist endoscopist initiated the procedure versus a non-specialist endoscopist (Fig. 2). When two negative risk factors are present, the probability of IR ranges from 54 to 83% (see Fig. 2), but drops at least 40% to 13–40% when performed by a specialist endoscopist. When all three negative risk factors are present (a flat/laterally spreading polyp that is ≥40 mm and difficult to lift), the probability of IR is 93%, but drops to 63% when performed by a specialist endoscopist (Fig. 2).
Fig. 2.
Adjusted probability of incomplete polypectomy based on combined risk factors. 1 difficulty lifting, 2 polyp size ≥ 40 mm, 3 specialist endoscopists, 4 flat and/or laterally spreading polyp, based on our multivariable model
Discussion
Our retrospective cohort study has found that incomplete resection of large sessile and/or flat polyps occurs in 16% of EMR cases and we validated known risk factors for incomplete resection, including difficulty lifting and polyp size ≥ 40 mm [12]. Importantly, the results from our study confirm that specialist endoscopists are more likely to be successful at complete EMR of large colonic polyps despite the presence of either a single or multiple risk factors for IR. To our knowledge, our study is the first US study to evaluate whether specialist endoscopists reduce the risk of IR for large colonic polyps ≥ 10 mm.
Incomplete resection of colonic polyps has important implications. First, residual polyp tissue is a known risk factor for malignant transformation. Studies have found that 19–27% of interval cancers occur in the same portion of the colon as the site of prior polypectomy [15, 18, 19]. Since some patients are lost to follow-up, as demonstrated in our study, it is important to maximize the probability of CR at the index colonoscopy. In our cohort of IR patients with follow-up, one patient progressed to adenocarcinoma on a subsequent colonoscopy over a 9-month time period and we speculate that a complete resection likely would have prevented malignant transformation and/or provided earlier detection of adenocarcinoma.
Some might argue that IR is less important if one can assure CR after adequate follow-up. If achieved, CR may require multiple endoscopic procedures, which has important implications on healthcare utilization and costs (e.g., lost work time for patients). In our study, 35% of our IR cases achieved CR but they required 2–4 colonoscopies, as compared to 1 colonoscopy, to achieve CR. Moreover, achieving CR is not always guaranteed. 30% of our cohort required surgery due to cancer, progressive disease, or suboptimal endoscopic positioning. The number of therapeutic colonoscopies before surgical resection of large colonic polyps becomes more cost-effective has not yet been identified.
It is important to note that the overall rate of IR in our study (16.7%) was slightly higher than previously published studies, which ranged from 7 to 10% [12, 20]. Prior studies that have evaluated risk factors for incomplete resection of large colonic polyps have included only specialized endoscopists at their center, while our study included general and specialist endoscopists, which may explain the higher rate of IR [13, 21]. Another reason may be our study’s higher percentage of polyps ≥ 40 mm, which is a known risk factor for IR. Our cohort had over 18% of polyps that were ≥40 mm, whereas a previous study reported having about 13% of polyps ≥ 40 mm [12].
We believe that the question of who should perform large colon EMRs is an important one, especially as EMRs of large polyps become widely accepted as the preferred management strategy. For example, at our institution, most of our EMRs were performed in the last 4 years, reflecting our institution’s changing practice and referral patterns. Previous studies have found that successful resection rates and adverse events vary widely by the experience of the endoscopist [13, 19]. A 2002 study that evaluated endoscopic resection of large sessile colonic polyps by specialist and non-specialist endoscopists found that the estimated end cost of management by specialists was less than half of that by non-specialist endoscopists, which the authors attributed to avoiding surgical resection and/or performing a complete resection at the initial EMR session [13]. Our data support that the highest probability of complete resection occurs when a specialist endoscopist performs the procedure, even in the presence of known risk factors for incomplete resections. For these reasons, we believe that general endoscopists should consider referral to specialist endoscopists for removal of large colonic polyps.
Given the strength of the existing data, including from our own study, it will be important in the future to clarify what training and endoscopic traits define a specialist endoscopist. We chose to define specialist endoscopists as providers who receive tertiary referrals for difficult colonoscopies, performed at least 20 EMR procedures over the study period, and are at least 5 years from fellowship with significant experience in EMR of large complex colonic polyps. The four specialists in our study are all interventional endoscopists who perform ERCP, EUS, and esophageal EMR, which may or may not be applicable in other centers. We speculate that our specialist endoscopists had lower incomplete resection rates because they are more comfortable with large resections and the use of advanced techniques for the removal of these polyps, including underwater EMR, grasp/snare technique for defiant polyps, and dynamic injection techniques [3]. They also have less time constraints on their endoscopy schedule due to longer blocks of time per case and have access to anesthesia support during their procedures, which has been shown to positively impact the success of advanced endoscopic procedures [3, 22]. There is no formal recommendation in the ASGE core curriculum as to which providers should perform these EMR, but we believe that formally defining specialist endoscopists could potentially have healthcare policy implications and merits further study [2, 12, 13].
There are some important limitations to our study. First, the overall number of EMR cases over the 10-year period is lower than might be expected. This is in part because the majority of our EMR cases (67%) were from the last 4 years of the study period (2012–2015), which reflects a growing referral and practice pattern at our institution. Furthermore, we believe that we may have missed cases due to “free texting” (rather than using pre-populated variables) in the endoscopy report, which would result in polyps not being captured in the search function of our endoscopy reporting system. Moreover, given the retrospective nature of our study, there was no standardized protocol in place for the characterization of polyps, especially size, shape, difficulty lifting, amount of polyp tissue left behind, degree of lumen involvement, the choice of resection techniques, or the timing of scheduled follow-up. Lastly, given that a quarter of patients with IR are still undergoing colonoscopy for primary polyp removal, their outcomes and the mean number of procedures required for IR patients to achieve CR remain unclear.
In conclusion, our study has shown that specialist endoscopists are less likely to have incomplete resection of polyps despite the presence of risk factors for incomplete resection, specifically polyp size and difficulty lifting. We believe our findings support that specialist endoscopists should perform large colonic EMR to maximize the chances of complete resection and decrease cost due to eventual surgical resection and/or progression to cancer. Future research is needed to further define specialist endoscopists and to quantify the effects of incomplete resection in the cost-effective care of patients with large colon polyps.
Acknowledgments
Funding provided by the NIH T32 training grant. REDCap was used to collect and manage study data and is supported by CTSA: UL1TR000433.
Footnotes
Compliance with ethical standards
Conflict of interest No disclosures or conflicts of interest to report.
References
- 1.Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2012;10:969–979. doi: 10.1016/j.cgh.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 2.ASGE Technology Committee. Hwang JH, Konda V, et al. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215–226. doi: 10.1016/j.gie.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Klein A, Bourke MJ. Advanced polypectomy and resection techniques. Gastrointest Endosc Clin N Am. 2015;25:303–333. doi: 10.1016/j.giec.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Saunders BP, Tsiamoulos ZP. Endoscopic mucosal resection and endoscopic submucosal dissection of large colonic polyps. Nat Rev Gastroenterol Hepatol. 2016;13:486–496. doi: 10.1038/nrgastro.2016.96. [DOI] [PubMed] [Google Scholar]
- 5.Keswani RN, Law R, Ciolino JD, et al. Adverse events after surgery for nonmalignant colon polyps are common and associated with increased length of stay and costs. Gastrointest Endosc. 2016;84:296e1–303.e1. doi: 10.1016/j.gie.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Ahlenstiel G, Hourigan LF, Brown G, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80:668–676. doi: 10.1016/j.gie.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Jayanna M, Burgess NG, Singh R, et al. Cost analysis of endoscopic mucosal resection vs surgery for large laterally spreading colorectal lesions. Clin Gastroenterol Hepatol. 2016;14:271e1–278.e2. doi: 10.1016/j.cgh.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. YMGE. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 9.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) YMGE. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Metz AJ, Bourke MJ, Moss A, et al. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy. 2011;43:506–511. doi: 10.1055/s-0030-1256346. [DOI] [PubMed] [Google Scholar]
- 11.Rutter MD, Chattree A, Barbour JA, et al. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut. 2015;64:1847–1873. doi: 10.1136/gutjnl-2015-309576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. YGAST. 2011;140:1909–1918. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 13.Brooker JC, Saunders BP, Shah SG, et al. Endoscopic resection of large sessile colonic polyps by specialist and non-specialist endoscopists. Br J Surg. 2002;89:1020–1024. doi: 10.1046/j.1365-2168.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- 14.Leung K, Pinsky P, Laiyemo AO, et al. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc. 2010;71:111–117. doi: 10.1016/j.gie.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar WD, Sawhney MS, Nelson DB, et al. Colorectal cancers found after a complete colonoscopy. YJCGH. 2006;4:1259–1264. doi: 10.1016/j.cgh.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and work-flow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74e1–80.e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 20.Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 21.Pellisé M, Burgess NG, Tutticci N, et al. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut. 2016 doi: 10.1136/gutjnl-2015-310249. [DOI] [PubMed] [Google Scholar]
- 22.Buxbaum J, Roth N, Motamedi N, et al. Anesthetist-directed sedation favors success of advanced endoscopic procedures. Am J Gastroenterol. 2017;112:290–296. doi: 10.1038/ajg.2016.285. [DOI] [PubMed] [Google Scholar]