Abstract
Background
Cognitive deficit associated with cancer and its treatment is called cancer-related cognitive impairment (CRCI). Increases in cancer survival have made understanding the basis of CRCI more important. CRCI neuroimaging studies have traditionally used dedicated research brain MRIs in breast cancer survivors after chemotherapy with small sample sizes; little is known about other non-central nervous system (CNS) cancers after chemotherapy as well as those not exposed to chemotherapy. However, there may be a wealth of unused data from clinically-indicated MRIs that could be used to study CRCI.
Objective
Evaluate brain cortical structural differences in those with various non-CNS cancers using clinically-indicated MRIs.
Design
Cross-sectional
Patients
Adult non-CNS cancer and non-cancer control (C) patients who underwent clinically-indicated MRIs.
Methods
Brain cortical surface area and thickness were measured using 3D T1-weighted images. An age-adjusted linear regression model was used and the Benjamini and Hochberg false discovery rate (FDR) corrected for multiple comparisons. Group comparisons were: cancer cases with chemotherapy (Ch+), cancer cases without chemotherapy (Ch−) and subgroup of lung cancer cases with and without chemotherapy vs C.
Results
Sixty-four subjects were analyzed: 22 Ch+, 23 Ch− and 19 C patients. Subgroup analysis of 16 lung cancer (LCa) patients was also performed. Statistically significant decreases in either cortical surface area or thickness were found in multiple regions of interest (ROIs) primarily within the frontal and temporal lobes for all comparisons. Effect sizes were variable with the greatest seen in the left middle temporal surface area ROI (Cohen’s d −0.690) in the Ch− vs C group comparison.
Limitations
Several limitations were apparent including a small sample size that precluded adjustment for other covariates.
Conclusions
Our preliminary results suggest that, in addition to breast cancer, other types of non-CNS cancers treated with chemotherapy may result in brain structural abnormalities. Similar findings also appear to occur in those not exposed to chemotherapy. These results also suggest that there is potentially a wealth of untapped clinical MRIs that could be used for future CRCI studies.
Keywords: Cancer-related cognitive impairment (CRCI), chemobrain, chemotherapy, psychological/behavioral oncology, complications and late effects of therapy, cognition, structural MRI
1. INTRODUCTION
Cancer is a major public health problem and the second leading cause of death in the United States [1]. Fortunately, advances in non-central nervous system (CNS) cancer diagnosis and therapy have resulted in greater numbers of cancer survivors [2–4]. Given these statistics, increasing attention has been focused on the morbidity associated with cancer survival, including the cognitive deficits secondary to cancer and its treatment. This has been referred to as cancer-related cognitive impairment (CRCI) [2] and “chemobrain,” CRCI thought to be due to chemotherapy, may affect up to 78% of cancer survivors [5] and can adversely affect survivors’ quality-of-life. Cognitive deficits associated with chemobrain usually manifest as loss of episodic and working memory, slower processing speed and executive dysfunction. The degree of dysfunction is often mild to modest and it can be chronic in up to 35% of patients [6, 7].
The underlying biological mechanisms and neural substrates affected in patients with CRCI have recently become a focus of intense study. Several theories about how chemotherapeutic agents cause cognitive dysfunction include, but are not limited to, injury to neural progenitor cells and postmitotic oligodendrocytes [8, 9], oxidative injury [10], reduced neurogenesis and white matter integrity [8, 11]. With regard to how cancer itself could result in CRCI, several mechanisms have been proposed including inflammation and inadequate DNA repair mechanisms [2].
Neuroimaging is a powerful method to study the neuroanatomical alterations associated with CRCI. So far, most of what is known comes from MRI studies of breast cancer survivors after chemotherapy [4, 12] generally using a cross-sectional design with small sample sizes. Structural T1-weighted, diffusion tensor imaging (DTI) and functional MRI (fMRI) studies suggest that there are significant anatomical and functional differences between breast cancer survivors and healthy controls [13–25]; comparatively little work has focused on breast cancer survivors who have not been exposed to chemotherapy. Furthermore, little is known about cancers outside of the breast, but recent publications using structural and functional MRI (fMRI) focusing on other types of cancers [26–29] also suggest that significant brain differences exist between these cancer patients and controls.
Additionally, CRCI MRI studies have generally used research-dedicated brain scans. Like many other biomedical fields, it is becoming more and more critical to show reproducible effects across large, well-powered cohorts. Interestingly, there may be a large body of untapped brain MRI data performed for clinical purposes which could help to address many unanswered questions regarding CRCI. We hypothesize that there are statistically significant differences in cortical structures among patients with various non-CNS cancers treated with and without chemotherapy compared to non-cancer controls. The primary objective of this cross-sectional pilot study was to test this hypothesis in a cohort of patients with different types of non-CNS cancers. Rather than using research-dedicated scans, we used already- existing clinical MRI scans that were intended to exclude brain metastatic disease.
2. METHODOLOGY
2.1 Study Design and Setting
This was a HIPAA-compliant, single-institution, IRB-approved, retrospective cross-sectional pilot study performed at the Keck Hospital of the University of Southern California (USC). A waiver of informed consent was obtained.
2.2 Participants
Our inclusion criteria included adults ≥ 18 years of age with various non-CNS cancers (cases) and those without cancer (controls) who underwent brain MRI that included volumetric non-contrast-enhanced T1-weighted imaging from January 2016 to June 2017. Subjects were found using the Montage neuroimaging database. Cases were scanned to exclude brain metastases and controls to evaluate a variety of clinical indications including headache, dizziness or vertigo, among others. Exclusion criteria included patients with histories of intrathecal or intraventricular chemotherapy, cranial radiation, metastatic brain tumors and documented neuropsychiatric disorders including histories of migraine, depression, anxiety and other neuropsychiatric conditions.
2.3 Variables and Image Processing
For each subject, structural T1-weighted magnetic resonance imaging (MRI) brain scans were performed on one of three 3T MRI scanners. Images were analyzed using the open-source, fully automated and validated segmentation software FreeSurfer version 5.3 [30]. Image acquisition parameters are given in Table 1. The segmentations of 141 regions were extracted, including the lateral ventricles and total ICV. Average values of bilateral regions were also calculated. All segmentations were visually inspected for accuracy by (FR, 2 years of experience) following a thorough and standardized quality control protocol designed by the ENIGMA Consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols/). Each image segmentation was also independently verified by a neuroimaging expert (MSS, 7 years of experience) at each site by overlaying the segmentation label of each structure on the T1-weighted brain scan.
Table 1.
Image acquisition parameters.
| Manufacturer | Field Strength | Model | Head Coil | T1w-Sequence | TR (ms) | TE (ms) | FOV (mm) | Matrix | Slice thickness (mm) | Slice Orientation |
|---|---|---|---|---|---|---|---|---|---|---|
| GE | 3T | Signa HDxt | 8 channel phased array | 3D FSPGR | 6.64 | 2.86 | 220×298 | 224×256 | 1 (no gap) | coronal |
| Toshiba | 3T | Titan | 32 channel phased array | 3D FFE | 5.3 | 2.5 | 210×284 | 240×240 | 1 (no gap) | coronal |
| GE | 3T | Signa HDxt | 8 channel phased array | 3D FSPGR | 8.42 | 3.16 | 220×298 | 224×256 | 1 (no gap) | coronal |
2.4 Data Sources
All clinical characteristics were obtained through a search of the electronic medical record.
2.5 Study Size
As this was a pilot study, formal sample size calculations were not performed.
2.6 Statistical Methods
Data for all regions of interest (ROIs) were corrected by the intracranial volume (ICV) by using the percent of ICV. Histograms and the D’Agostino’s K-squared test were used to determine the distribution of the data. For data not normally distributed, a Wilcoxon score transformation was used so that the comparison was conducted in a non-parametric fashion. We utilized an age-adjusted linear regression model and the residuals after age adjustment were used for the group comparisons. Independent t-tests were used for group comparisons using data with a normal distribution. Wilcoxon Rank Sum tests were used when the data distribution was not normal. The Benjamini and Hochberg false discovery rate (FDR) was used for multiple comparisons correction to control the false positive rate at 5%. Effect sizes (Cohen’s d) were also determined. SAS 9.4 was used for the statistical analysis. The following group comparisons were made: cancer cases treated with chemotherapy (Ch+) vs control (C); cancer cases without chemotherapy (Ch−) vs C; subgroup analysis of lung cancer cases with and without chemotherapy (LCa) was also compared vs C.
3. RESULTS
3.1 Participants
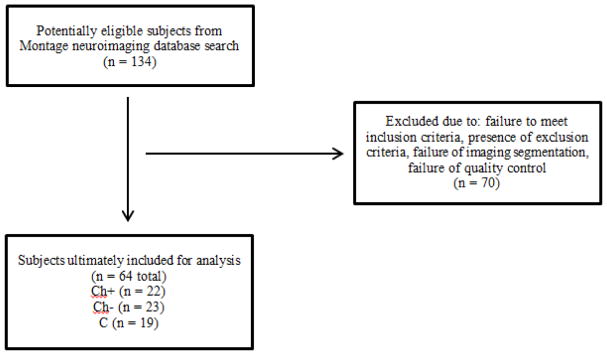
A total of 64 subjects were ultimately included in our analysis. There were 45 case and 19 C patients. Of the cancer cases, 22 were scanned after chemotherapy (Ch+) while 23 were scanned without chemotherapy (Ch−). Please refer to the flow diagram (Figure 1) for further details. The most common cancer in our cohort was lung cancer (n = 16). Six cancer cases had developed 2 different types of cancers. Clinical characteristics of our entire cohort are listed in Table 2. Table 3 depicts the different chemotherapy drugs that were used in our Ch+ cohort.
Figure 1.
Flow diagram documenting subjects who were included in the analysis of this study
Table 2.
Clinical characteristics of cancer cases and controls.
| Ch+ (n = 22) | Ch− (n = 23) | C (n = 19) | |
|---|---|---|---|
|
| |||
| Age (years, mean) | 60.7 | 58.7 | 50.6 |
|
| |||
| Sex | |||
| Male | 14 | 11 | 8 |
| Female | 8 | 12 | 11 |
|
| |||
| Cancer type | 1 Bladder | 3 Breast | NA |
| 1 Bladder and prostate | 4 Kidney | ||
| 4 Breast | 11 Lung | ||
| 1 Breast and Ewing’s sarcoma | 1 Lymphoma | ||
| 1 Colon and prostate | 2 Melanoma | ||
| 1 Esophageal | 2 Testicular | ||
| 1 Gastric | |||
| 3 Lung | |||
| 1 Lung and rectal | |||
| 1 Lung and prostate | |||
| 1 Lymphoma | |||
| 1 Lymphoma and rectal | |||
| 1 Neuroendocrine | |||
| 1 Prostate | |||
| 2 Rectal | |||
| 1 Testicular | |||
|
| |||
| Treatment other than chemotherapy | NA | ||
| Hormonal | 3 | 4 | |
| Biological | 9 | 4 | |
| Radiation | 9 | 4 | |
| Surgery | 16 | 13 | |
|
| |||
| Smoking | |||
| Never | 13 | 14 | 0 |
| Ever | 7 | 6 | 0 |
| Current | 2 | 3 | 2 |
|
| |||
| Cerebrovascular risk factors (any combination of hypertension, diabetes mellitus, dyslipidemia) | 11 | 10 | 7 |
|
| |||
| Non-cancer control clinical indications for MRI | NA | NA | 6 headache |
| 2 dizziness | |||
| 1 ataxia | |||
| 1 facial nerve hemangioma | |||
| 1 hand tremor | |||
| 1 hearing loss | |||
| 1 optic neuropathy | |||
| 1 optic disc papilledema | |||
| 1 leg weakness | |||
| 1 pulsatile tinnitus | |||
| 2 vertigo | |||
| 1 Osler-Weber-Rendu syndrome | |||
Table 3.
Chemotherapy regimens used in the Ch+ group.
| Ch+ (n = 22) | Chemotherapy Regimen* | |
|---|---|---|
|
| ||
| Cancer type | 1 Bladder | Cisplatin, gemcitabine |
| 1 Bladder and prostate | Cisplatin | |
| 4 Breast | Cyclophosphamide, doxorubicin; doxorubicin, cyclophosphamide, paclitaxel, capecitabine; unknown**; paclitaxel, vinorelbine | |
| 1 Breast and Ewing’s sarcoma | Irinotecan, vincristine, doxorubicin, cyclophosphamide, paclitaxel | |
| 1 Colon and prostate | Leucovorin, fluorouracil, oxaliplatin | |
| 1 Esophageal | Leucovorin, fluorouracil, oxaliplatin | |
| 1 Gastric | Docetaxel, cisplatin, leucovorin, fluorouracil, oxaliplatin | |
| 3 Lung | Pemetrexed; pemetrexed, carboplatin; cisplatin, docetaxel, carboplatin, Nab-paclitaxel | |
| 1 Lung and rectal | Fluorouracil | |
| 1 Lung and prostate | Carboplatin, paclitaxel, cisplatin | |
| 1 Lymphoma | Cyclophosphamide, doxorubicin, vincristine, prednisolone | |
| 1 Lymphoma and rectal | Etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin | |
| 1 Neuroendocrine | Cisplatin, etoposide | |
| 1 Prostate | Docetaxel | |
| 2 Rectal | Folinic acid, fluorouracil, oxaliplatin; Folinic acid, fluorouracil, ininotecan, capecitabine, trifluridine-tipiracil hydrochloride | |
| 1 Testicular | Bleomycin, etoposide, cisplatin | |
Semicolons separate each subject’s chemotherapy regimen.
One breast cancer patient’s chemotherapy regimen could not be found in the medical records.
3.2 Main Results
Our analysis detected statistically significant decrease in either cortical surface area or cortical thickness in ROIs of primarily the frontal and temporal lobes for the comparisons between Ch+ vs. C, Ch− vs. C, and LCa vs. C. An overview of these ROIs is listed in Table 4. Effect sizes were variable with the greatest seen in the left middle temporal surface area ROI (Cohen’s d −0.690, 95% confidence interval (−1.329, −0.051)) in the Ch− vs C group comparison.
Table 4.
Group comparisons with listing of ROIs which demonstrated a statistically significant decrease in either cortical surface area or cortical thickness compared to non-cancer controls. Adjusted p values* are also noted.
| Group Comparison | Region of Interest | p value* |
|---|---|---|
| Ch+ (N = 22) vs C (N = 19) | Average banks of the superior temporal sulcus thickness | 0.007 |
| Average superior temporal thickness | 0.012 | |
| Left superior frontal surface area | 0.013 | |
| Left lateral orbitofrontal surface area | 0.037 | |
| Average fusiform surf area | 0.041 | |
| Ch− (N = 23) vs C (N = 19) | Average superior temporal thickness | 0.030 |
| Left middle temporal surface area | 0.028 | |
| Average fusiform surface area | 0.025 | |
| Average middle temporal surface area | 0.018 | |
| Average lateral occipital surface area | 0.023 | |
| Left isthmus cingulate surface area | 0.021 | |
| Right isthmus cingulate surface area | 0.021 | |
| Right banks superior temporal sulcus surface area | 0.028 | |
| Left inferior temporal surface area | 0.027 | |
| Left fusiform surface area | 0.026 | |
| Average banks superior temporal sulcus surface area | 0.025 | |
| Left lateral occipital surface area | 0.024 | |
| Right inferior parietal surface area | 0.029 | |
| Right parahippocampal surface area | 0.044 | |
| Right middle temporal surface area | 0.042 | |
| Left medial orbitofrontal surface area | 0.041 | |
| Right fusiform surface area | 0.041 | |
| Average inferior temporal surface area | 0.039 | |
| Right lateral occipital surface area | 0.039 | |
| Average parahippocampal surface area | 0.045 | |
| Left surface area | 0.050 | |
| LCa (N = 16) vs C (N = 19) | Average banks superior temporal sulcus thickness | 0.007 |
| Left lateral orbitofrontal surface area | 0.024 | |
| Left medial orbitofrontal surface area | 0.047 | |
| Average superior temporal surface area | 0.042 | |
| Right inferior parietal surface area | 0.047 |
4. DISCUSSION
Cancer survivors make up an ever-increasing proportion of the public. There were an estimated 15.5 million cancer survivors in the US as of January 2016 and this is projected to increase to 26.1 million survivors by 2040 [31]. Given these statistics, it is critical to better understand the biological mechanisms and neuroanatomical substrates that underlie CRCI. In contrast to prior CRCI-related neuroimaging studies which have generally used dedicated research imaging scans, we used clinical brain MRI data collected to assess for possible CNS metastases of multiple types of primary non-CNS cancers.
Both the neuroimaging [4, 12] and neuropsychological literature [2] generally support the notion of the existence of structural/functional neuroimaging abnormalities and cognitive dysfunction due to cancer and chemotherapy. However, much of this evidence should still be considered preliminary due to the lack of data available outside of breast cancer, preponderance of cross-sectional designs with small sample sizes and a paucity of well-powered prospective, longitudinal cohort studies [2].
Our results have shown that, compared to non-cancer controls, those with a various non-CNS cancers have statistically significant decreases in either cortical surface area or cortical thickness, primarily affecting the frontal and temporal lobes. This difference was present in both Ch+ and Ch− groups. Furthermore, subgroup analysis of the LCa group showed similar differences. These findings are consistent with the prior work where the majority of structural T1-weighted CRCI neuroimaging studies after chemotherapy have been performed using breast cancer patients. Overall, these studies have found reductions of GM and WM throughout the brain [13–19], and when the findings have been focal, involvement of the frontotemporal regions is common [12]. Some of these structural abnormalities [16, 19, 32, 33] have also been associated with cognitive deficits. This appears to reflect the notion that cancer and chemotherapy are more prone to injure the frontotemporal regions that are critical to cognitive functions like episodic memory and executive function [12] Other structural MRI studies from DTI [20–22] as well as fMRI studies [23–25] have also provided complementary evidence documenting abnormal WM integrity and brain activation patterns due to breast cancer and chemotherapy.
The findings from our Ch− cohort are also in agreement with the few neuroimaging studies that have studied cancer patients not exposed to chemotherapy. Structural [15, 19, 34] and functional MRI [23, 24, 35] studies have found that abnormalities may already exist in the absence of chemotherapy for breast cancer patients. More recent neuroimaging studies of other types of cancer including lung [26] and testicular cancer [27] also suggest that structural abnormalities exist without chemotherapy. This is further supported by neuropsychological data in breast [36, 37], testicular [27] and lung cancer [26] patients not treated with chemotherapy.
Little is known whether neuroimaging changes are also present in individuals with other types of non-CNS cancers outside of the breast. Our overall findings that also included subgroup analysis of our LCa cohort are in line with a recent cross sectional MRI study of lung cancer [26]. In this study, cognitive dysfunction and diffuse WM abnormalities from DTI were present prior to chemotherapy. In addition, cognitive dysfunction, GM reduction in the left anterior cingulate cortex and the bilateral parahippocampal gyri and insula, as well as focally decrease WM integrity in bilateral inferior longitudinal fasciculi and left cingulum were found in chemotherapy-exposed subjects compared to healthy controls. A few other recent publications of structural MRI of testicular cancer [27] as well as fMRI studies of prostate [28] and various cancers [29], including breast, colorectal, Hodgkin’s lymphoma, leukemia and myeloma, similarly support the presence of structural/functional neuroimaging and cognitive abnormalities in these malignancies following treatment.
Several limitations should be kept in mind when interpreting the findings of this study. First, the cross-sectional design makes it difficult to establish whether structural brain alterations developed after cancer and chemotherapy. Second, we used a sample of convenience given the retrospective nature of the study. In addition, the cancer cases in this cohort were compared to what was readily available on the same MRI scanners, a non-cancer control group, rather than a healthy control group. Third, there was a heterogeneous mixture of non-CNS cancer types and treatment regimens. Fourth, the relatively small sample size precluded adjustment for other covariates that could impact the results. Fifth, both the cancer cases and non-cancer controls were scanned for clinical indications, and could differ from cancer survivors in general as well as healthy controls. Lastly, our method of surface-based image processing with FreeSurfer differed from most other structural MRI studies of CRCI which have largely relied on voxel-based morphometry (VBM) [4, 12].
5. CONCLUSIONS
While considering these limitations, these preliminary results support the notion that non-CNS cancers other than breast cancer treated with chemotherapy may be associated with brain structural abnormalities. Similar findings also appear to exist in these cancer patients even in the absence of chemotherapy. These findings should be validated in larger studies, ideally with a prospective, longitudinal cohort design focusing on a wide variety of cancers. Furthermore, while CRCI brain MRI studies have generally used research-dedicated scans, our results suggest that there is a wealth of untapped neuroimaging data obtained for clinical indications that could form the basis of future CRCI neuroimaging studies.
Acknowledgments
Funding: MSS was partially supported by SC CTSI (NIH/NCRR/NCATS) Grant KL2TR000131, NIH Loan Repayment Program Award 1 L30 CA209248-01 and the Wright Foundation Pilot Grant; †MSS, VG, FR, NJ and PMT were also partially supported by NIH Big Data to Knowledge (BD2K) Initiative under U54EB020403 awarded to PMT.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–138. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain. Oncology (Williston Park) 2014;28(9):797–804. [PubMed] [Google Scholar]
- 4.Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(8):1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 6.Boykoff N, Moieni M, Subramanian SK. ‘Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3(4):223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaya E, Keskin L, Aydogdu I, Kuku I, Bayraktar N, Erkut MA. Oxidant/antioxidant parameters and their relationship with chemotherapy in Hodgkin’s lymphoma. J Int Med Res. 2005;33(6):687–692. doi: 10.1177/147323000503300611. [DOI] [PubMed] [Google Scholar]
- 11.Seigers R, Schagen SB, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013;7(4):453–459. doi: 10.1007/s11682-013-9250-3. [DOI] [PubMed] [Google Scholar]
- 12.McDonald BC, Saykin AJ. Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav. 2013;7(4):374–387. doi: 10.1007/s11682-013-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saykin AJ, Wishart HA. Mild cognitive impairment: conceptual issues and structural and functional brain correlates. Semin Clin Neuropsychiatry. 2003;8(1):12–30. doi: 10.1053/scnp.2003.50002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 15.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergouignan L, Lefranc JP, Chupin M, Morel N, Spano JP, Fossati P. Breast cancer affects both the hippocampus volume and the episodic autobiographical memory retrieval. PLoS One. 2011;6(10):e25349. doi: 10.1371/journal.pone.0025349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, Vrooman H, Niessen WJ, Breteler MM, Schagen SB. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132(3):1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 19.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):S117–125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer. 2008;8(1):88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 21.Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Smeets A, Christiaens MR, Leemans A, Van Hecke W, Vandenberghe J, Vandenbulcke M, Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 23.Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, Berman MG, Hayes DF, Noll DC, Peltier S, Welsh RC. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32(3):324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 24.Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an fMRI study. Front Hum Neurosci. 2011;5:122. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simo M, Root JC, Vaquero L, Ripolles P, Jove J, Ahles T, Navarro A, Cardenal F, Bruna J, Rodriguez-Fornells A. Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol. 2015;10(1):38–45. doi: 10.1097/JTO.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amidi A, Agerbaek M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, Demontis D, Borglum AD, Harboll A, Zachariae R. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11(3):769–783. doi: 10.1007/s11682-016-9552-3. [DOI] [PubMed] [Google Scholar]
- 28.Chao HH, Uchio E, Zhang S, Hu S, Bednarski SR, Luo X, Rose M, Concato J, Li CS. Effects of androgen deprivation on brain function in prostate cancer patients - a prospective observational cohort analysis. BMC Cancer. 2012;12:371. doi: 10.1186/1471-2407-12-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Apple AC, Schroeder MP, Ryals AJ, Voss JL, Gitelman D, Sweet JJ, Butt ZA, Cella D, Wagner LI. Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors postchemotherapy compared with healthy controls. Cancer. 2016;122(2):258–268. doi: 10.1002/cncr.29737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 31.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, Klaunig JE, Champion VL, Unverzagt FW, Saykin AJ. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl):S109–116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherling C, Collins B, MacKenzie J, Lepage C, Bielajew C, Smith A. Structural brain differences in breast cancer patients compared to match controls prior to chemotherapy. International Journal of Biology. 2012;4:3–25. [Google Scholar]
- 35.Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A. Prechemotherapy differences in response inhibition in breast cancer patients compared to controls: a functional magnetic resonance imaging study. J Clin Exp Neuropsychol. 2012;34(5):543–560. doi: 10.1080/13803395.2012.666227. [DOI] [PubMed] [Google Scholar]
- 36.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110(1):143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange M, Giffard B, Noal S, Rigal O, Kurtz JE, Heutte N, Levy C, Allouache D, Rieux C, Le Fel J, Daireaux A, Clarisse B, Veyret C, Barthelemy P, Longato N, Eustache F, Joly F. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer. 2014;50(13):2181–2189. doi: 10.1016/j.ejca.2014.05.026. [DOI] [PubMed] [Google Scholar]