Abstract
Owing to their toxicity, phthalate plasticizers are currently being replaced with terephthalates in many consumer products. Nevertheless, data on human exposure to and toxicity of terephthalates are still scarce. In this study, we developed a robust analytical method for the measurement of six terephthalate metabolites (TPhMs) in human urine through their successful separation from phthalate metabolites (PhMs). Target analytes were identified, using commercially available standards, and quantified with isotopically labeled internal standards (IS). The limit of quantification (LOQ) of TPhMs were in the range of 0.12 to 0.4 ng/mL, with the exception of 2.8 ng/mL for terephthalic acid (TPA) and 3.75 ng/mL for mono-(2-ethylhexyl) terephthalate (mEHTP), which were found in procedural blanks at notable levels. The method developed in this study showed excellent accuracy (recoveries: 86–117%) and precision (RSD: 0.6–12.2%) for TPhMs. The method was successfully applied for the analysis of 30 human urine samples collected from individuals with no known history of occupational exposure. The detection frequencies (df %) of TPhMs in urine ranged between 26.6 and 100%. This is one of the first studies that report a method for the analysis of emerging class of environmental chemicals in human specimens.
Keywords: Terephthalate metabolites, Plasticizers, Phthalate replacements, Human biomonitoring, HPLC-MS/MS, Method validation
1. Introduction
Terephthalates are esters (alkyl/cycloalkyl/aryl) of benzene-1,4-dicarboxylic acid (terephthalic acid) that are widely used in polyester plastics. Terephthalates are structural isomers of phthalates, the most widely used plasticizers in the global market [1, 2]. Due to their structural and chemical similarities, terephthalates have been widely used in many consumer products similar to those of phthalates. Among terephthalates, polyethylene terephthalate (PET) is the most widely used compound, especially in the manufacture of plastic bottles/containers for packaging beverages, water, and food [3]. Dimethyl terephthalate (DMTP) is a major methanolysis product of PET [4, 5] and a major starting material for the synthesis of polyesters [6] and the most commonly used terephthalate derivative. Global PET production was approximately 46 million tons in 2014, with a forecast of 81 million tons in 2020 [7]. Di-2-ethylhexyl terephthalate (DEHTP) is a high-volume production chemical with a global production volume estimated at 50,000 tons in 2007 [8]. DEHTP is used in medical devices, vinyl flooring, bottle caps, toys, electric connectors, automobile flooring, and glues, among other products [8, 9]. Several regulatory bodies, such as the U.S. Food and Drug Administration (FDA), Australian National Industrial Chemicals Notification and Assessment Scheme (NICNA), and European Food Safety Authority (EFSA), have recommended the need for less-toxic alternatives of phthalates in consumer products [10]. Terephthalates are thought to be safer alternatives, and, therefore, the production and usage of these plasticizers are increasing. Terephthalates undergo metabolic and excretion pathways similar to those of phthalates in the human body [11].
The major metabolic pathway of DEHTP [1, 12] is like that of its isomeric phthalate, di-2-ethylhexylphthalate (DEHP) [11, 13] (Fig. S1). Similarly, other terephthalates are metabolized to their respective monoesters and excreted in urine. Although several studies have reported phthalate exposures in populations worldwide, little is known about terephthalate exposures in humans. Development of an analytical method for the measurement of terephthalate metabolites (TPhMs) is required for an accurate determination of human exposure to these chemicals. Although analytical methods have been reported for the analysis of phthalate metabolites (PhMs) in urine, the lack of authentic analytical standards hindered the development of methods for TPhMs analysis. Furthermore, there exists analytical challenges in the accurate determination of TPhMs, which include background contamination, co-elution, and possible similar mass fragment transitions with PhMs. A few recent HPLC-MS/MS methods developed for the analysis of TPhMs in human urine focused only on DEHTP metabolites [1, 8, 12]. In the present study, we developed and validated an analytical method for the separation and simultaneous analysis of six TPhMs and seven PhMs in human urine.
2. Materials and Methods
2.1 Chemicals/standards, solvents, and urine samples
The six target TPhMs were mono-(2-ethylhexyl) terephthalate (mEHTP), mono-benzyl terephthalate (mBzTP), mono-methyl terephthalate (mMTP), mono-ethyl terephthalate (mETP), mono-tert-butyl terephthalate (mTBTP), and terephthalic acid (TPA). The analytical standards, mBzTP, mMTP, mTBTP, and TPA, were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada); mETP, from Matrix Scientific (Columbia, SC, USA); and mEHTP, from Cambridge Isotope Laboratories (Andover, MA, USA). The corresponding isomeric standards of phthalate metabolites, mEHP, mBzP, mMP, mBP, mIBP, mEP, and PA, were purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Isotopically labeled terephthalate standards were not commercially available yet (except for 13C6-mEHTP), and, therefore, we used labeled PhMs as internal standards (ISs), except for TPA, for which 2D4-TPA (Sigma-Aldrich, St. Louis, MO, USA) was used. 13C2-mMP, 13C2-mBP, 13C2-mEP, 13C2-mBzP, 13C4-mEHP, and 13C6-mEHTP were purchased from Cambridge Isotope Laboratories (Andover, MA, USA) and 2D4-mIBP was from C/D/N Isotopes, Inc. (Pointe Claire, QC, Canada).
Formic acid and β-glucuronidase (≥100,000 units/mL β-glucuronidase; ≤7,500 units/mL sulfatase) from Helix pomatia (Type HP-2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol, ethyl acetate (ACS grade), acetic acid, acetonitrile, and other solvents used in the analysis were purchased from Mallinckrodt Baker (Phillipsburg, NJ, USA) and HPLC-grade water, from Fisher Scientific (Pittsburgh, PA, USA). We analyzed archived spot urine samples collected from 30 healthy adult volunteers (15 females and 15 males) with a mean age of ~ 30 years in Albany, New York, USA, in 2017 to demonstrate the feasibility of the developed method. The volunteers did not have any known history of occupational exposure to the target chemicals. The samples were stored at −20°C until analysis. The study was approved by the Institutional Review Board of the New York State Department of Health.
2.2 Preparation of standard solutions and mixtures
Analytical standards of mMTP, mTBTP, mETP, and TPA were dissolved in acetonitrile to prepare stock solutions at a concentration of 1000 μg/mL. TPA and 2D4-TPA were poorly soluble in many organic solvents, and these two compounds were first dissolved in 0.5 mL of dimethyl sulfoxide and then diluted in acetonitrile. mEHTP and 13C6-mEHTP were purchased (dissolved in MTBE) at a concentration of 1000 μg/mL. The native standard solutions of 6 TPhMs and 7 PhMs and isotopically labeled ISs of 2 TPhMs and 7 PhMs were serially diluted with acetonitrile and used in calibration and spiking experiments.
2.3 Sample preparation/extraction
Urine samples and quality assurance/quality control (QA/QC) samples were extracted using a solid-phase extraction (SPE) method. The extraction protocol was modified from a previously reported method for PhMs [14]. A total of 250 μL of urine were transferred into a 15-mL polypropylene (PP) tube. The QA/QC samples, including reagent blank, matrix blank, and matrix spike, and urine samples were spiked with 40 ng each of ISs prior to extraction. To evaluate the matrix effects and the recovery of target chemicals, we spiked 40 ng each of ISs into a matrix match sample prior to injection (after the SPE step). The samples were buffered with 250 μL of 1 M ammonium acetate (adjusted to pH 4.5 with acetic acid), containing 50 units of β-glucuronidase (prepared by spiking 20 μL of β-glucuronidase in 20 mL of 1 M ammonium acetate solution), and incubated at 37° C for 12–15 h in an incubator shaker. After enzymatic digestion, the samples were diluted with 1 mL of phosphate buffer (pH 2; prepared by dissolving 20 g sodium phosphate monobasic monohydrate in 990 mL of HPLC-grade water and 10 mL of ortho-phosphoric acid).
Solid phase extraction was performed with an ABS Elut-NEXUS (Agilent Technologies, Inc., Folsom, CA, USA; 60 mg, 3.0 mL) cartridge similar to that reported earlier for PhMs [14]. The cartridge was conditioned with 1.5 mL of acetonitrile and 1.2 mL of phosphate buffer. The diluted samples were then loaded onto the SPE cartridge. The cartridge was washed with 2.0 mL of 0.1 M formic acid and 2.0 mL of HPLC-grade water and dried under vacuum for approximately 5 min. The target analytes were eluted with 1.5 mL of acetonitrile, followed by 1.5 mL of ethyl acetate and then 0.5 mL of methanol. The eluate was collected into a 15-mL PP tube and concentrated to near-dryness under a gentle nitrogen stream. Finally, the residues were reconstituted with 250 μL of the solvent mixture (20 μL of acetonitrile: 180 μL of HPLC-grade water: 50 μL of methanol), vortexed, and transferred into amber glass vials for HPLC–MS/MS analysis.
2.4 HPLC-MS/MS analysis
The chromatographic separation of TPhMs and PhMs was accomplished using an Agilent 1260 Series HPLC system (Agilent Technologies Inc., Santa Clara, CA, USA). Identification and quantification of TPhMs and PhMs were performed with an ABSCIEX 4500 QTRAP mass spectrometer (Applied Biosystems, Foster City, CA, USA) under electrospray ionization (ESI) negative ion mode. Chromatographic separation was achieved using an Ultra AQ C18 column (100 mm × 2.1 mm, 3 μm; Restek, Bellefonte, PA, USA) connected to a Javelin guard column (Betasil C18, 2.1 mm × 20 mm, 5 μm; Thermo Electron Corp., Waltham, MA, USA). The mobile phase comprised of 0.4% (v/v) acetic acid in HPLC-grade water (A) and 0.4% (v/v) acetic acid in acetonitrile (B). The target compounds were separated by gradient elution at a mobile phase flow rate of 250 μL/min, starting at 90% A, held for 1 min, decreased to 70% A within 3 min (4th min), then decreased to 55% A within 2 min (6th min), held for 4 min (10th min), then decreased to 35% A within 2 min (12th min), held for 3 min (15th min), followed by a decrease to 10% A in 1 min (16th min), held for 4 min (20th min), then increased to 40% A within 2 min (22nd min), and reverted to 90% A at the 24th min and held for 4 min (for the column equilibration), with a total run time of 28 min (Table S1).
The mass spectrometric parameters were optimized for all target chemicals by infusing standard solutions using an in-built syringe pump. Electrospray voltage was set at −4.5kV; curtain gas flow rate was set at 10 psi; collision gas was set at medium level (8–10 psi); and source heater was set at 550°C. The nebulizer gas/ion source gas 1 was set at 60 psi, and the heater gas/ion source gas 2 was set at 45 psi. The injection volume was 5.0 μL with draw and eject speeds at 200 μL/min. Two mass transitions (a quantifier and a qualifier) were selected for each analyte. The ionization parameters for each target compound are summarized in Table 1. The MS/MS was operated in a multiple reaction monitoring (MRM) mode.
Table 1.
MRM transitions of target terephthalate metabolites and their optimized mass spectrometric parameters. Q1 and Q3 resolutions were set to unity. MRM detection window for each compound was set at 100 (msec).
| Q1 Mass (Da) | Q3 Mass (Da) | RT (min) | DP | EP | CE | CXP | |
|---|---|---|---|---|---|---|---|
| TPA | |||||||
| Quantifier | 164.9 | 120.9 | 9.06 | −35.0 | −15.0 | −22.0 | −6.0 |
| Qualifier | 164.9 | 148.8 | 9.06 | −50.0 | −12.0 | −22.0 | −6.0 |
| 2D4-TPA | |||||||
| Quantifier | 169.1 | 124.9 | 9.03 | −35.0 | −5.0 | −22.0 | −8.0 |
| Qualifier | 169.1 | 81.0 | 9.03 | −35.0 | −5.0 | −25.0 | −6.0 |
| mMTP | |||||||
| Quantifier | 179.0 | 134.8 | 11.76 | −50.0 | −5.0 | −22.0 | −10.0 |
| Qualifier | 179.0 | 119.8 | 11.76 | −28.0 | −15.0 | −28.0 | −10.0 |
| mETP | |||||||
| Quantifier | 192.9 | 119.9 | 13.35 | −60.0 | −5.0 | −28.0 | −4.0 |
| Qualifier | 192.9 | 76.1 | 13.35 | −50.0 | −15.0 | −40.0 | −6.0 |
| mTBTP | |||||||
| Quantifier | 221.0 | 119.8 | 16.70 | −35.0 | −15.0 | −30.0 | −12.0 |
| Qualifier | 221.0 | 75.9 | 16.70 | −40.0 | −15.0 | −40.0 | −4.0 |
| mBzTP | |||||||
| Quantifier | 255.3 | 119.9 | 17.12 | −50.0 | −15.0 | −25.0 | −8.0 |
| Qualifier | 255.3 | 210.8 | 17.12 | −40.0 | −12.0 | −22.0 | −6.0 |
| mEHTP | |||||||
| Quantifier | 276.9 | 232.9 | 21.01 | −35.0 | −12.0 | −22.0 | −4.0 |
| Qualifier | 276.9 | 119.7 | 21.01 | −60.0 | −10.0 | −35.0 | −8.0 |
| 13C6-mEHTP | |||||||
| Quantifier | 283.1 | 238.9 | 21.00 | −35.0 | −12.0 | −25.0 | −8.0 |
| Qualifier | 283.1 | 126.1 | 21.00 | −35.0 | −15.0 | −30.0 | −8.0 |
RT- Retention time in minutes; DP – Declustering potential; EP – Entrance potential; CE – Collision energy; and CXP – Collision cell exit potential; MRM – multiple reaction monitoring
2.5. Instrumental calibration and QA/QC
Contamination that can arise from laboratory materials and solvents was evaluated by the analysis of reagent/procedural blanks (RB). Matrix blanks (MB) also were analyzed to monitor for the matrix effects on the response of target analytes as well as the background levels (that ranged between 0.04 and 5.2 ng/mL) that affected recoveries from matrix spike (MS) experiments. Among the target analytes, mEHTP existed at the highest background levels (3.6 ng/mL), followed by TPA (1.2 ng/mL). The rest of the target TPhMs had negligible background levels (0.02–0.15 ng/mL). The background concentrations of target chemicals found in RB and MB were subtracted from the real urine sample concentrations and matrix spike experiments, respectively. A calibration standard (midpoint) and methanol were injected periodically to monitor for drift in instrumental sensitivity and carry-over of target chemicals between samples, respectively. Linear calibration (1/x weighted regression) curves were constructed for each analyte at 11 different concentrations (0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10, 20, 40, 100 and 200 ng/mL) of standards in solvent (water:acetonitrile; 9:1 ratio) as well as in pooled urine (Fig. S2). The regression coefficients (r) of calibration curves ranged from 0.995 to 0.999.
2.6 Accuracy and precision
Due to the lack of certified reference materials for TPhMs, accuracy and precision of the analytical method were evaluated by the analysis of urine matrix spiked at four known concentrations of target chemicals (0.5, 20, 40 and 100 ng/mL). Accuracy or trueness was evaluated based on the recovery (%) of the target chemicals from the matrix spike experiments and precision was expressed as percent relative standard deviation (% RSD) from the true concentration. Both accuracy and precision were assessed by intra-day (n=5) and inter-day (n=5) measurements. The isotope dilution method was used for quantification. For mMTP, mETP, mTBTP and mBzTP, for which no corresponding IS was available, ISs of the corresponding phthalate isomers were used for quantification.
3 Results and Discussion
3.1 ESI-MS/MS
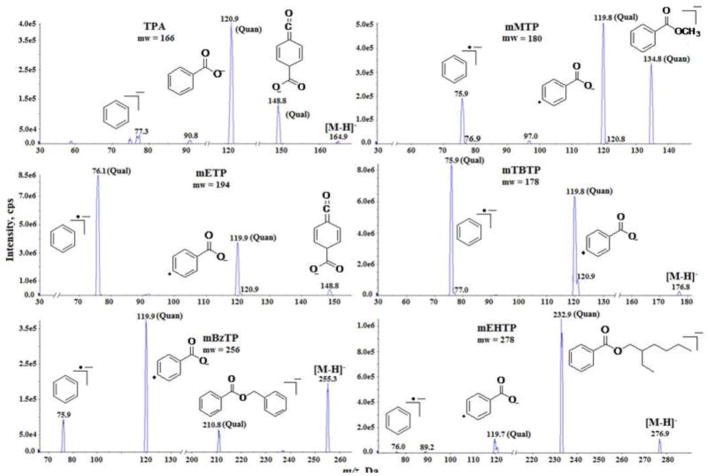
The ESI-MS/MS spectra of the target analytes are shown in Fig. 1. We selected the two most abundant product ions as quantifier and qualifier ions (Table 1). The ESI-MS/MS spectra showed that the preferred fragmentation of target chemicals in negative ionization mode was the cleavage of alkyl side chain, and, thus, the product ions retained the aromatic ring. It is noteworthy that all TPhMs produced two common fragments at m/z 76 and m/z 120, except for TPA. These two fragments can be used for precursor ion scan experiments for selective identification of TPhMs in any given matrix. The overall ionization pattern was unique for each analyte (Fig. 1). Further, the fragmentation patterns were different from that of the isomeric phthalate metabolites (PhMs). This enabled selective and positive identification of TPhMs and PhMs without any bias in the identification of these isomers.
Fig. 1.
Mass spectrometric fragmentation of terephthalate metabolites (TPhMs) analyzed in this study. Quan: Quantifier transition; Qual: Qualifier transition.
3.2 Chromatographic separation of target chemicals
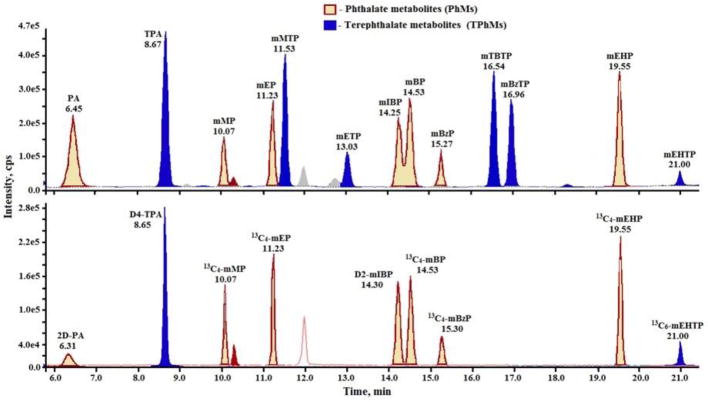
The Ultra AQ C18 column enabled complete separation of TPhMs from PhMs (Fig. 2). Although, all target chemicals were eluted within 22 min, the total run time was maintained for 28 min to enable equilibration of the LC column, and to reduce the possibility of drift in retention times. Needle wash was performed between injections with a solvent mixture of 50:50 methanol and water to minimize analyte carryover. The retention and separation of organic compounds in the reverse-phase C18 column is largely determined by their differences in polarities [15]. In general, phthalates are more polar than are terephthalates and, thus, are less strongly retained by the stationary phase of chromatographic column. Further, the short chain esters of PhMs and TPhMs have lower molecular weights and are more polar than are the long chain esters [16]. Therefore, the short chain esters of both PhMs and TPhMs have short retention times (Table 1). In general, TPhMs eluted later than did their corresponding PhMs isomers (Fig. 2). We did not observe any coelution of TPhMs and PhMs. In addition to different mass spectrometric fragmentation, chromatographic separation of TPhMs and PhMs allowed for simultaneous identification and quantification of both of these classes of compounds in urine samples. The method was more sensitive (~2 times) for TPhMs than for the corresponding PhMs, except for mETP and mEHTP, that they had a lower instrumental response than those of mEP and mEHP, respectively (Fig. 2).
Fig. 2.
Chromatograms of target terephthalate metabolites (TPhMs) and corresponding phthalate metabolites (PhMs). Blank urine was spiked at 40 ng/mL of native standards (NS) and internal standards (IS) and extracted.
3.3 Method validation
The method was validated by repeated analysis of urine matrix fortified with native standards and ISs at four different concentrations and analyzed on different days. We also included duplicate analysis and procedural reagent blank (RB), matrix blank (MB), and matrix spike (MS) samples as a measure for QA and QC.
Excellent accuracy and precision were found for all TPhMs fortified in urine at four different levels (Table 2). The results showed no significant differences between individual measurements (at four different concentrations) made intra- or inter-day. Accuracy or recovery (%) for all TPhMs ranged between 86 and 117% and 88 and 112%, respectively, for intra- and inter-day measurements. Similarly, precision (RSD %) ranged between 1.4 and 12.2% and 0.6 and 12.1%, respectively, for intra- and inter-day measurements. This confirmed the repeatability of the method to precisely analyze the target compounds.
Table 2.
Accuracy and precision (intra and inter) of the method for the analysis of terephthalate metabolites, calculated from the analysis (n=5) of urine spiked at 4 different concentrations (0.5, 20, 40 and 100 ng/mL).
| TPA | mMTP | mETP | mTBTP | mBzTP | mEHTP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Spiked (ng/mL) |
Mean (ng/mL) |
RSD (%) | Accur. (%) |
Mean (ng/mL) |
RSD (%) |
Accur. (%) |
Mean (ng/mL) |
RSD (%) | Accur. (%) |
Mean (ng/mL) |
RSD (%) | Accur. (%) |
Mean (ng/mL) |
RSD (%) | Accur. (%) |
Mean (ng/mL) |
RSD (%) |
Accur. (%) |
| Intra-day (n=5) | ||||||||||||||||||
| 0.5 | -* | - | - | 0.47 | 2.7 | 94 | 0.58 | 2.1 | 116 | 0.56 | 4.3 | 112 | 0.43 | 5.1 | 86 | -* | - | - |
| 20 | 21.2 | 6.2 | 106 | 22.7 | 9.6 | 114 | 18.1 | 1.4 | 90.5 | 17.3 | 3.2 | 86.5 | 20.2 | 3.4 | 101 | 23.4 | 4.8 | 117 |
| 40 | 43.1 | 2.8 | 108 | 36.2 | 6.2 | 90.5 | 39.1 | 5.8 | 97.8 | 36.8 | 4.1 | 92 | 35.5 | 3.1 | 88.8 | 41.9 | 2.2 | 105 |
| 100 | 96.4 | 12.2 | 96.4 | 98.1 | 5.1 | 98.1 | 108 | 4.2 | 108 | 92.6 | 9.4 | 92.6 | 98.2 | 2.3 | 98.2 | 109 | 3.6 | 109 |
| Inter-day (n=5) | ||||||||||||||||||
| 0.5 | -* | - | - | 0.44 | 3.6 | 88 | 0.56 | 0.7 | 112 | 0.53 | 1.2 | 106 | 0.49 | 0.6 | 98 | -* | - | - |
| 20 | 20.8 | 3.7 | 104 | 21.9 | 4.7 | 110 | 18.8 | 5.4 | 94 | 18.2 | 3.9 | 91 | 19.1 | 2.7 | 95.5 | 22.1 | 3.7 | 111 |
| 40 | 41.5 | 2.1 | 104 | 36.4 | 5.9 | 91 | 40.2 | 6.6 | 101 | 38.5 | 5.4 | 96.3 | 36.9 | 6.4 | 92.3 | 43.7 | 6.2 | 109 |
| 100 | 94.6 | 8.4 | 94.6 | 97.5 | 11 | 97.5 | 104 | 1.9 | 104 | 88.7 | 6.9 | 88.7 | 101 | 3.5 | 101 | 105 | 12.1 | 105 |
|
| ||||||||||||||||||
| LOD | 0.85 | 0.12 | 0.05 | 0.03 | 0.04 | 1.25 | ||||||||||||
| LOQ | 2.8 | 0.4 | 0.17 | 0.12 | 0.15 | 3.75 | ||||||||||||
Where,
-not measured due to high background;
RSD: Relative standard deviation; Accur.: Accuracy (or recovery); LOD: Limit of detection; LOQ: Limit of quantification.
3.4 Matrix effects
Matrix effect was evaluated by spiking known concentrations of target analytes (a full calibration range; 0.1–200 ng/mL) in HPLC water (solvent calibration) and pooled urine (matrix matched calibration). The spiked samples were passed through the entire analytical procedures and calibration curves were constructed based on the response (peak areas) ratio of analyte/IS vs spiked concentration (ng/mL). The slopes obtained for spiking experiments (n=3) in water and pooled urine for all TPhMs are presented in Fig. S2. The regression coefficients ranged from 0.995 to 0.999 and 0.996 to 0.999 for solvent and matrix matched calibrations, respectively. The slopes obtained for matrix matched calibrations for all TPhMs were lower than those of pure solvent-based calibrations, except for mMTP. These results suggest that, although 4 of 6 TPhMs did not have corresponding labeled ISs, use of labeled ISs of isomeric PhMs provided an account for matrix suppression in real urine samples. Furthermore, the magnitude of ion-suppression observed for TPhMs can be accounted for by solvent or matrix matched calibration standards.
3.5 Stability and other critical parameters
We performed sample digestion at 37 °C (optimum temperature for enzyme activity) to mimic the physiological temperature of 36.8±0.7 °C. A number of studies that measured environmental chemicals in urine used similar conditions and temperature for the measurement of total concentrations (both free and conjugated forms) of contaminants [1, 12, 18]. We also digested samples at 20 °C, but the recoveries of TPhMs were poor. The stability of analytical standards was examined by storing them (1 ppm mixture) at −20 °C and 4 °C and periodically measuring the concentrations (for up to 4 weeks). We found that there was no remarkable degradation of standards at these temperatures. We tested the effect of enzyme concentrations (25, 50, 100 and 200 units) and found that the recoveries of target chemicals were optimal at 50 units of enzyme activity. Similarly, pH of ammonium acetate buffer played a crucial role in efficient extraction of target chemicals. Among the tested conditions (pH= 4.5, 5.0, 5.5 and 6.0), pH 4.5 was found to yield excellent recoveries of the target chemicals. Furthermore, addition of methanol as an elution solvent enhanced the recoveries of target TPhMs, especially for mBzTP. We also observed that preventing samples from exposure to light (by wrapping with aluminum foil) during digestion overnight, improved the recoveries (by ~4–6%).
3.6 Limit of detection (LOD) and Limit of quantification (LOQ)
The LODs and LOQs of the target analytes were determined based on the standard deviation (SD) of five measurements obtained from the analysis of urine spiked at the lowest concentration. The LOD was calculated as 3 times the SD of the lowest calibrant concentration divided by the slope of the calibration curve and the LOQ as 10 times the SD of the lowest calibrant concentration divided by the slope of the calibration curve [17]. The calculated LOD and LOQ values for TPhMs ranged 0.03–1.25 ng/mL and 0.1–3.75 ng/mL, respectively (Table 2). The LOQs of mEHTP (3.75 ng/mL) and TPA (2.8 ng/mL) were higher due to the presence of background levels of contamination, which probably originated from solvents, tubings and other HPLC components. However, these two TPhMs can be measured reliably in samples owing to their existence at notable concentrations (as reported below). Thus, the method is sensitive and suitable for biomonitoring of human exposure to terephthalates at sub-nanogram per milliliter urinary concentrations.
3.7 Urine sample analysis
Using the developed method, we analyzed 30 spot urine samples collected in 2017 from adult donors (15 females and 15 males) in Albany, New York, USA, for the target compounds. Both TPA and mMTP were detected in 100% of the samples. The detection frequencies of other TPhMs were in the following decreasing order: mEHTP> mETP> mTBTP≥mBzTP (Table 3). Dimethyl terephthalate, the parent compound of mMTP, is a starting material in the synthesis of polyesters, such as PET, the most commonly used terephthalate [19], which supports ubiquitous detection of mMTP in urine samples. Similarly, TPA is a common metabolite (non-specific) of several terephthalates, which can result from exposure to any terephthalate. In fact, all target TPhMs had relatively high detection rates, reflecting the widespread application of these compounds in consumer products. Terephthalates are thought to be less toxic [10]. Owing to the toxicity of phthalates, as reported in several studies [20–23], the application of terephthalates as a replacement for phthalates in consumer products is projected to increase. Robust analytical methods are fundamental to the accurate assessment of human exposure to this group of chemicals. Overall, the average concentrations (mean) of target TPhMs in urine samples were 249, 5.9, 0.5, <LOQ, 1.8 and 9.5 ng/mL for TPA, mMTP, mETP, mTBTP, mBzTP, and mEHTP, respectively. The corresponding levels of phthalate isomers (PhMs) in urine were 190, 6.6, 64.2, 14.5, 6.8, 4.2 and 2.6 ng/mL for PA, mMP, mEP, mBP, mIBP, mBzP, and mEHP, respectively. The average urinary concentration of mEHTP was higher than that of mEHP (9.5 vs. 2.6 ng/mL), and TPA was higher than that of PA (249 vs. 190 ng/mL). Although the sample size was small, results suggest that terephthalates exposures can be comparable to or higher than those of phthalate exposures, especially DEHTP. The concentrations mMTP were comparable to those of mMP (5.9 vs. 6.6 ng/mL), which further alludes to the ongoing use of terephthalates.
Table 3.
Concentrations and detection rates of terephthalate metabolites in urine samples (n=30) collected from Albany, New York, USA in 2017.
| TPA | mMTP | mETP | mTBTP | mBzTP | mEHTP | |
|---|---|---|---|---|---|---|
| Female (n = 15) | ||||||
|
|
||||||
| Mean±SD (ng/mL) | 272 ± 210 | 6.37 ± 2.6 | 0.42 ± 0.98 | < LOQ | 1.2 ± 1.8 | 8.7 ± 5.2 |
| Median (ng/mL) | 74 | 3.9 | < LOQ | < LOQ | 0.57 | 5.6 |
| Conc. range (ng/mL) | 12.4–1250 | 1.20–11.5 | < LOQ–3.2 | < LOQ–0.58 | < LOQ–3.4 | < LOQ–14.1 |
| Detection rate - df (%) | 100 | 100 | 80 | 26.6 | 33.3 | 86.6 |
|
|
||||||
| Male (n = 15) | ||||||
|
|
||||||
| Mean±SD (ng/mL) | 225 ± 174 | 5.2 ±4.7 | 0.58 ± 0.24 | < LOQ | 2.3 ± 1.5 | 10.2 ± 4.8 |
| Median (ng/mL) | 63.5 | 2.2 | 0.32 | <LOQ | 0.90 | 6.2 |
| Conc. range (ng/mL) | 8.8–940 | 0.8–7.5 | < LOQ–2.4 | < LOQ–0.64 | < LOQ–4.8 | < LOQ–18.9 |
| Detection rate - df (%) | 100 | 100 | 66.5 | 53.3 | 40 | 73.3 |
4 Conclusions
We successfully developed an analytical method for extraction and analysis of TPhMs in human urine. Excellent chromatographic separations and distinct mass spectrometric fragmentation of TPhMs (from PhMs) as well as great sensitivity with excellent accuracy and precision were obtained. The method can be used concurrently for the analysis of phthalate metabolites. TPhMs were found in urine samples at high detection rates and at concentrations comparable to or higher than those of PhMs. A significant increase in human exposure to terephthalates is expected with the increasing application of these compounds in consumer products. Hence, our method will be useful for assessment of human exposure patterns for terephthalates. Development of labeled internal standards of terephthalate metabolites will further enhance the quality of biomonitoring results.
Supplementary Material
Highlights.
HPLC-MS/MS method was developed for the analysis of six terephthalate metabolites in human urine.
Excellent chromatographic separation with greater sensitivity at sub-ppb concentrations was achieved.
The method enables concurrent analysis of terephthalate and phthalate metabolites in urine.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number U2CES026542-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interest: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silva MJ, et al. Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicology in Vitro. 2015;29(4):716–721. doi: 10.1016/j.tiv.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirnitzer U, et al. Systemic toxicity of di-2-ethylhexyl terephthalate (DEHT) in rodents following four weeks of intravenous exposure. Toxicology Letters. 2011;205(1):8–14. doi: 10.1016/j.toxlet.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 3.de Jong E, et al. Biobased Monomers, Polymers, and Materials. American Chemical Society; 2012. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters; pp. 1–13. [Google Scholar]
- 4.Kurokawa H, et al. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polymer Degradation and Stability. 2003;79(3):529–533. [Google Scholar]
- 5.Paszun D, Spychaj T. Chemical Recycling of Poly(ethylene terephthalate) Industrial & Engineering Chemistry Research. 1997;36(4):1373–1383. [Google Scholar]
- 6.Sheehan JR. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. Terephthalic Acid, Dimethyl Terephthalate, and Isophthalic Acid. [Google Scholar]
- 7.Statista. [accessed on 28th March, 2018];Polyethylene terephthalate (PET) production worldwide in 2014 and 2020 (in million metric tons) 2017 Available from: https://www.statista.com/statistics/650191/global-polyethylene-terephthalate-production-outlook/
- 8.Nagorka R, et al. Diisononyl 1,2-cyclohexanedicarboxylic acid (DINCH) and Di(2-ethylhexyl) terephthalate (DEHT) in indoor dust samples: Concentration and analytical problems. International Journal of Hygiene and Environmental Health. 2011;214(1):26–35. doi: 10.1016/j.ijheh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.ECC. [accessedon 15th November, 2017];Eastman Chemical Company (ECC) - Eastman™non-phthalate plasticizers and applications. 2017 Available from: http://www.eastman.com/Brands/Eastman_plasticizers/Pages/Overview.aspx.
- 10.ECC. Eastman Chemical Company (ECC) - Why Eastman 168 is a non-phthalate plasticizer. A regulatory memo. 2014. [Google Scholar]
- 11.Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Molecular Nutrition & Food Research. 2007;51(7):899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- 12.Lessmann F, et al. Determination of metabolites of di(2-ethylhexyl) terephthalate (DEHTP) in human urine by HPLC-MS/MS with on-line clean-up. Journal of Chromatography B. 2016;1011:196–203. doi: 10.1016/j.jchromb.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Stein TP, et al. Autism and Phthalate Metabolite Glucuronidation. Journal of Autism and Developmental Disorders. 2013;43(11):2677–2685. doi: 10.1007/s10803-013-1822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Wu Q, Kannan K. Phthalate metabolites in urine from China, and implications for human exposures. Environment International. 2011;37(5):893–898. doi: 10.1016/j.envint.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Mehta A. [accessed on 16th November,2017];Principle of Reversed-Phase Chromatography HPLC/UPLC (with Animation) 2012 Available from: http://pharmaxchange.info/press/2012/12/principle-of-reversed-phase-chromatography-hplcuplc-with-animation/
- 16.Mihucz VG, Záray G. Occurrence of antimony and phthalate esters in polyethylene terephthalate bottled drinking water. Applied Spectroscopy Reviews. 2016;51(3):183–209. [Google Scholar]
- 17.Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis: Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. Journal of Chromatography A. 2003;987(1):57–66. doi: 10.1016/s0021-9673(02)01536-4. [DOI] [PubMed] [Google Scholar]
- 18.Silva MJ, et al. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Archives of Toxicology. 2017;91(10):3287–3291. doi: 10.1007/s00204-017-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach C, et al. Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: Chemical analysis and potential toxicity. Food Chemistry. 2013;139(1):672–680. doi: 10.1016/j.foodchem.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Bareum K, Kyunghee J. Estrogenic and Androgenic Potential of Phthalates and Their Alternatives. Korean Journal of Environmental Health. 2016;42(3):169–188. [Google Scholar]
- 21.Carbone S, et al. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Hormones and Behavior. 2013;63(5):692–699. doi: 10.1016/j.yhbeh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Boberg J, et al. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reproductive Toxicology. 2011;31(2):200–209. doi: 10.1016/j.reprotox.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Christiansen S, et al. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reproductive Toxicology. 2010;30(2):313–321. doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.