Abstract
Background
Tetramic acid, thiophene and hydrazone derivatives were found to exhibit favorable antifungal activity. Aiming to discover novel template molecules with potent antifungal activity, a series of novel 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives containing a hydrazone group were designed, synthesized, and evaluated for their antifungal activity.
Results
The structures of 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives bearing a hydrazone group were confirmed by FT-IR, 1H NMR, 13C NMR, 1H-1H NOESY, EI-MS and elemental analysis. Antifungal assays indicated that some title compounds exhibited antifungal activity against Fusarium graminearum (Fg), Rhizoctorzia solani (Rs), Botrytis cinerea (Bc) and Colletotrichum capsici (Cc) in vitro. Strikingly, the EC50 value of 5e against Rs was 1.26 µg/mL, which is better than that of drazoxolon (1.77 µg/mL). Meanwhile, title compounds 5b, 5d, 5e–5g, 5n–5q and 5t exhibited remarkable anti-Cc activity, with corresponding EC50 values of 7.65, 9.97, 6.04, 6.66, 7.84, 7.59, 9.47, 5.52, 6.41 and 7.53 µg/mL, respectively, which are better than that of drazoxolon (19.46 µg/mL).
Conclusions
A series of 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives bearing a hydrazone group were designed, synthesized and evaluated for their antifungal activity against Fg, Rs, Bc and Cc. Bioassays indicated that some target compounds exhibited obvious antifungal activity against the above tested fungi. These results provide a significant basis for the further structural optimization of tetramic acid derivatives as potential fungicides.
Electronic supplementary material
The online version of this article (10.1186/s13065-018-0452-z) contains supplementary material, which is available to authorized users.
Keywords: Tetramic acid, Hydrazone, Thiophene, Synthesis, Antifungal activity
Background
An emergence of resistant fungi is a huge impetus to the development of agricultural fungicides with novel molecular structures and unique mechanisms [1]. In this regard, the structural optimization of natural heterocycles plays a important role in the searching for bioactive lead compounds [2, 3]. As attractive nitrogenous heterocycles, tetramic acid derivatives are widely researched for some reasons. First, tetramic acid derivatives exist in secondary metabolites from various terrestrial and marine organisms and have favorable compatibility with the environment [4]. Second, tetramic acid derivatives contain a unique pyrroline-2-one or pyrrolidine-2,4-dione substructure that is easy to synthesize to some extent [5]. Third, tetramic acid derivatives are reported to exhibit various agricultural bioactivities including fungicidal [6], herbicidal [7], insecticidal [8], antibacterial and antiviral [9] properties. Encouraged by the above findings, series of tetramic acid derivatives bearing amino [10], strobilurin [6], phenylhydrazine [11], oxime ether [12] and pyrrole [13] groups were synthesized and reported for their antifungal activity against plant fungi in our previous work. However, the potential application of tetramic acid derivatives as agricultural fungicides was greatly limited by their unsatisfactory curative rates [6, 10–13].
Thiophene is an important sulphureous compound that was widely studied for the development of novel fungicides due to their wide and satisfactory antifungal activity [14–17]. As important thiophene derivatives, thicyofen, ethaboxam, silthiopham and penthiopyrad were commercialized as agricultural fungicides in the past decades. Meanwhile, hydrazone is a widely researchful substructure that exists in commercialized agrochemicals including ferimzone, hydramethylnon, diflufenzopyr, pymetrozine, metaflumizone and benquinox [18, 19]. Recently, scholars found introducing a hydrazone group into salicylaldehyde [20], nalidixic acid [21], tetrahydro-β-carboline [22], 1,2,3-triazole [23], benzimidazole [24], diphenyl ether [25], pyrazole amide [26] quinoxaline [27] and carbonic acid ester [28] could effectively improve and broaden their antifungal activity. Obviously, further structural modifications of thiophene and hydrazone derivatives are significant for the development of novel fungicides.
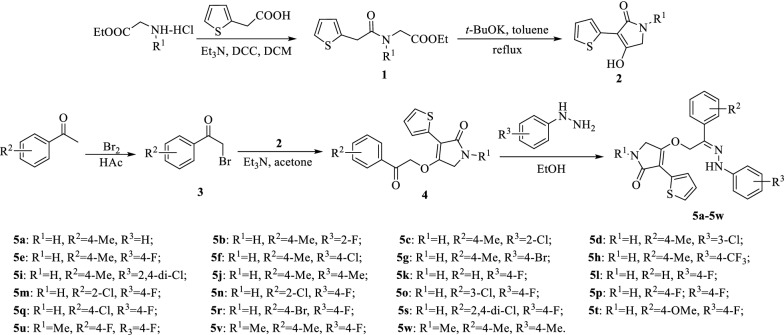
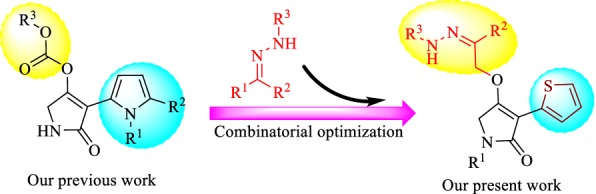
Aiming to extend our previous works on searching for pyrroline-2-one derivatives as agricultural fungicides [6, 10–13, 29], we theorized that introducing a hydrazone group into pyrroline-2-one structure might generate novel lead molecules with better antifungal activity (Fig. 1). Thus, in this study, a thiophene group was firstly neatly combined with pyrroline-2-one scaffold in one molecule by a Dieckmann cyclization. Subsequently, a hydrazone group was introduced into the 4-position of the obtained 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one substructure to generate a series of novel tetramic acid derivatives (Scheme 1). In addition, the fungi Fusarium graminearum (Fg), Rhizoctorzia solani (Rs), Botrytis cinerea (Bc) and Colletotrichum capsici (Cc), which seriously restricted agricultural outputs of wheat, rice, strawberries and pepper, were selected as tested fungi to evaluate the antifungal activity of 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives bearing a hydrazone group. To the best of our knowledge, it is the first report on the synthesis and antifungal activity of 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives bearing a hydrazone group.
Fig. 1.
Design strategy of title compounds
Scheme 1.
Synthesis route to title compounds
Results and discussion
Chemistry
The synthetic route to 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives containing a hydrazone group is shown in Scheme 1. Using a substituted glycine ethyl ester hydrochloride as a starting material, the key intermediate 2 (substituted 4-hydroxy-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one) was synthesized by two steps including amidation and cyclization reactions. The intermediate 2 was reacted with substituted 2-bromo-1-phenylethan-1-one 3 in acetone containing triethylamine to obtain the substituted 4-(2-oxo-2-phenylethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one 4. Subsequently, the obtained intermediate 4 was reacted with substituted phenylhydrazine in acetonitrile to yield the title compound 5 with a good yield. The structures of title compounds were confirmed by FT-IR, 1H NMR, 13C NMR, EI-MS, and elemental analysis. In the IR spectra of title compounds, two obvious peaks at 3294–3447 and 3171–3263 cm−1 are attributed to the N–H stretching vibrations at pyrroline-2-one and phenylhydrazone fragments. The absorption peak of the carbonyl group at 2-position of pyrroline-2-one appears at 1682–1667 cm−1. In 1H NMR spectra, two singlets at δ 9.12–10.35 and 7.83–8.00 ppm are assigned to the NH protons at phenylhydrazone and pyrroline-2-one fragments. Two singlets at δ 4.26–4.49 and 5.36–5.58 ppm mean that the structure of title compounds has two –CH2– fragments. A typical carbon resonance at δ 169.51–172.01 ppm in the 13C NMR spectra confirms the presence of a carbonyl group at 2-position of pyrroline-2-one. Meanwhile, singlets at 43.51–43.77 and 61.73–66.02 ppm confirm the existence of two –CH2– fragments in the molecular structure of title compounds. In the EI-MS spectra of title compounds, the value of [M]+ ion absorption signal is consistent with the calculated value of molecular weight.
Configuration confirmation of title compounds
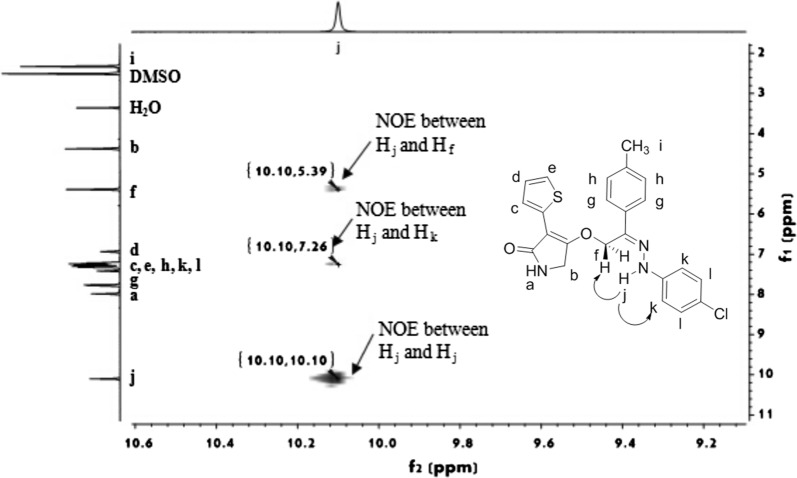
As shown in the 1H NMR and 13C NMR spectra of title compounds, these 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives containing a hydrazone group does present itself via one single molecular structure. Aiming to further understand the structural characteristics of title compounds, the configuration of compound 5f was studied as an example by a 1H-1H NOESY analysis [30]. As shown in Fig. 2, the chemical shifts of Hf, Hj and Hk protons were 5.39, 10.10 and 7.26 ppm in the NOESY spectrum of compound 5f (DMSO-d6), respectively. The obvious NOE phenomena between Hj and Hf, and between Hj and Hk indicated that these protons close with each other, which typically revealed the double bond C=NNH of title compound 5f possesses the cis-configuration.
Fig. 2.
NOESY spectrum of the title compound 5f
Antifungal activity screening of title compounds
Using a mycelial growth rate method [6, 10–13, 21–28], the antifungal effects of title compounds 5a–5w against Rs, Bc, Cc and Fg were evaluated at 10 μg/mL and are shown in Table 1. A agricultural fungicide drazoxolon was used as a positive control of antifungal effects under same conditions. As shown in Table 1, the compounds 5n, 5p and 5u exhibited fine activity against Rs, with inhibitory rates of 91.5, 100.0 and 84.7%, respectively, which are better than that of drazoxolon (84.5%). The compounds 5g, 5p and 5t obviously inhibited the mycelium growth of Bc, with inhibitory rates of 66.4, 61.1 and 51.3%, respectively. The inhibition rates of compounds 5b, 5d–5g, 5m–5r, 5t and 5u against Cc ranged from 48.5 to 100.0%, which are better than that of drazoxolon (46.8%). Table 1 also shown that the anti-Fg effects of target compounds 5e–5g, 5o–5r and 5t at 10 μg/mL were 98.6, 69.0, 67.4, 74.6, 100.0, 68.6, 67.6 and 92.7%, respectively, which are apparently better than that of drazoxolon (67.2%).
Table 1.
Antifungal effects of title compounds 5a–5w at 10 μg/mL
| Compd. | R1 | R2 | R3 | Rs | Bc | Cc | Fg |
|---|---|---|---|---|---|---|---|
| 5a | H | 4-CH3 | H | 10.5 ± 0.5 | 0.0 ± 8.2 | 16.4 ± 2.6 | 7.5 ± 0.5 |
| 5b | H | 4-CH3 | 2-F | 67.7 ± 0.5 | 32.3 ± 0.9 | 57.3 ± 2.7 | 28.4 ± 1.1 |
| 5c | H | 4-CH3 | 2-Cl | 12.6 ± 1.7 | 25.3 ± 1.8 | 21.1 ± 1.3 | 12.2 ± 1.7 |
| 5d | H | 4-CH3 | 3-Cl | 70.6 ± 2.1 | 49.0 ± 1.6 | 61.3 ± 1.8 | 40.6 ± 2.3 |
| 5e | H | 4-CH3 | 4-F | 83.9 ± 1.2 | 49.5 ± 2.2 | 90.7 ± 1.7 | 98.6 ± 0.5 |
| 5f | H | 4-CH3 | 4-Cl | 76.7 ± 1.5 | 34.4 ± 0.5 | 74.7 ± 1.2 | 69.0 ± 0.6 |
| 5g | H | 4-CH3 | 4-Br | 66.7 ± 0.6 | 66.4 ± 2.1 | 74.7 ± 1.9 | 67.4 ± 1.7 |
| 5h | H | 4-CH3 | 4-CF3 | 44.1 ± 3.0 | 38.4 ± 2.4 | 43.2 ± 2.1 | 42.3 ± 1.2 |
| 5i | H | 4-CH3 | 2,4-di-Cl | 47.5 ± 2.4 | 40.2 ± 3.1 | 18.9 ± 2.7 | 10.8 ± 3.5 |
| 5j | H | 4-CH3 | 4-CH3 | 40.7 ± 1.8 | 27.8 ± 0.7 | 38.9 ± 1.8 | 40.5 ± 1.9 |
| 5k | H | 4-CH3 | 4-OCH3 | 20.0 ± 3.5 | 7.3 ± 1.4 | 25.1 ± 1.0 | 9.4 ± 2.8 |
| 5l | H | H | 4-F | 52.8 ± 1.1 | 30.5 ± 3.2 | 40.7 ± 2.1 | 38.1 ± 2.1 |
| 5m | H | 2-Cl | 4-F | 66.3 ± 1.4 | 37.5 ± 2.2 | 50.2 ± 2.8 | 47.3 ± 1.3 |
| 5n | H | 2-Br | 4-F | 91.5 ± 2.0 | 47.2 ± 1.0 | 93.0 ± 1.3 | 53.1 ± 1.1 |
| 5o | H | 3-Cl | 4-F | 70.2 ± 1.8 | 36.1 ± 0.9 | 73.8 ± 1.2 | 74.6 ± 3.2 |
| 5p | H | 4-F | 4-F | 100.0 ± 0.3 | 61.1 ± 3.5 | 100.0 ± 0.2 | 100.0 ± 0.3 |
| 5q | H | 4-Cl | 4-F | 76.4 ± 0.5 | 37.5 ± 1.4 | 83.8 ± 1.6 | 68.6 ± 2.3 |
| 5r | H | 4-Br | 4-F | 55.0 ± 1.8 | 34.7 ± 2.2 | 48.5 ± 1.6 | 67.6 ± 3.0 |
| 5s | H | 2,4-di-Cl | 4-F | 70.6 ± 3.3 | 45.8 ± 1.6 | 43.8 ± 3.0 | 47.8 ± 1.6 |
| 5t | H | 4-OCH3 | 4-F | 67.5 ± 1.2 | 51.3 ± 2.9 | 86.0 ± 3.6 | 92.7 ± 2.5 |
| 5u | 4-CH3 | 4-F | 4-F | 84.7 ± 1.1 | 48.5 ± 2.1 | 53.8 ± 3.3 | 47.9 ± 1.4 |
| 5v | 4-CH3 | 4-CH3 | 4-F | 64.7 ± 2.1 | 36.3 ± 1.8 | 36.1 ± 1.1 | 39.6 ± 3.1 |
| 5w | 4-CH3 | 4-CH3 | 4-CH3 | 37.1 ± 0.1 | 25.8 ± 0.4 | 31.2 ± 1.1 | 35.2 ± 2.1 |
| Drazoxolon a | / | / | / | 84.5 ± 1.8 | 91.2 ± 2.2 | 46.8 ± 1.9 | 67.2 ± 0.9 |
Average of three replicates
a A commercial agricultural fungicide drazoxolon was used for comparison of antifungal activity
Encouraged by the above preliminary bioassays, the EC50 values of some compounds that exhibited fine antifungal activity against Rs, Cc and Fg at 10 μg/mL were determined and are summarized in Table 2. Table 2 shown that the EC50 values of the selected compounds ranged from 1.26 to 9.89 µg/mL against Rs, from 5.52 to 9.97 µg/mL against Cc and from 6.02 to 8.85 µg/mL against Fg. Strikingly, the EC50 value of the title compound 5e against Rs was 1.26 µg/mL, which is better than that of drazoxolon (1.77 µg/mL). Meanwhile, the title compounds 5b, 5d, 5e–5g, 5n–5q and 5t had remarkable EC50 values of 7.65, 9.97, 6.04, 6.66, 7.84, 7.59, 9.47, 5.52, 6.41 and 7.53 µg/mL against Cc, respectively, which are better than that of drazoxolon (19.46 µg/mL). The above results also indicates that 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives containing a hydrazone group can serve as potential structural templates in the search for novel and highly efficient fungicides.
Table 2.
EC50 values of some title compounds against Rs, Cc and Fg
| Compd. | Tested fungus | Regression equation | R | EC50 (µg/mL) |
|---|---|---|---|---|
| 5b | Rs | y = 0.76x + 4.73 | 0.99 | 2.28 ± 3.00 |
| Cc | y = 0.81x + 4.28 | 0.95 | 7.65 ± 5.31 | |
| 5d | Rs | y = 1.42x + 3.57 | 0.98 | 5.23 ± 3.74 |
| Cc | y = 1.60x + 2.95 | 0.98 | 9.97 ± 8.90 | |
| 5e | Rs | y = 0.87x + 4.91 | 0.99 | 1.26 ± 1.12 |
| Cc | y = 1.42x + 3.89 | 0.99 | 6.04 ± 5.35 | |
| Fg | y = 2.32x + 3.17 | 0.97 | 6.13 ± 4.49 | |
| 5f | Rs | y = 0.50x + 4.74 | 0.99 | 3.32 ± 2.74 |
| Cc | y = 1.25x + 3.97 | 0.99 | 6.66 ± 5.33 | |
| Fg | y = 1.74x + 3.54 | 0.99 | 6.90 ± 4.96 | |
| 5g | Rs | y = 0.38x + 4.77 | 0.96 | 4.13 ± 2.83 |
| Cc | y = 1.32x + 3.82 | 0.99 | 7.84 ± 7.03 | |
| Fg | y = 1.25x + 3.87 | 0.96 | 8.03 ± 5.01 | |
| 5n | Rs | y = 1.26x + 4.31 | 0.99 | 3.56 ± 3.16 |
| Cc | y = 1.35x + 3.81 | 0.97 | 7.59 ± 5.12 | |
| 5o | Rs | y = 1.42x + 3.79 | 0.98 | 7.15 ± 5.62 |
| Cc | y = 1.47x + 3.56 | 0.99 | 9.47 ± 8.02 | |
| Fg | y = 1.97x + 3.10 | 0.99 | 7.22 ± 6.01 | |
| 5p | Rs | y = 2.41x + 3.49 | 0.99 | 2.22 ± 1.68 |
| Cc | y = 4.22x + 1.87 | 0.99 | 5.52 ± 5.49 | |
| Fg | y = 3.56x + 2.04 | 0.98 | 6.77 ± 5.14 | |
| 5q | Rs | y = 1.76x + 3.73 | 0.99 | 5.29 ± 4.54 |
| Cc | y = 1.68x + 3.29 | 0.99 | 6.41 ± 4.96 | |
| Fg | y = 3.79x + 1.30 | 0.99 | 7.63 ± 5.81 | |
| 5r | Rs | y = 1.13x + 3.70 | 0.98 | 9.89 ± 7.18 |
| Fg | y = 1.33x + 3.47 | 0.99 | 8.85 ± 8.26 | |
| 5t | Rs | y = 1.27x + 3.81 | 0.99 | 8.62 ± 7.06 |
| Cc | y = 1.39x + 3.24 | 0.98 | 7.53 ± 6.89 | |
| Fg | y = 1.37x + 3.85 | 0.97 | 6.02 ± 5.26 | |
| Drazoxolon a | Rs | y = 2.54x + 4.37 | 0.99 | 1.77 ± 1.62 |
| Cc | y = 0.82x + 3.94 | 0.99 | 19.46 ± 3.93 | |
| Fg | y = 2.04x + 3.88 | 0.99 | 3.53 ± 2.72 |
Average of three replicates
a A commercial agricultural fungicide drazoxolon was used for comparison of antifungal activity
Structure–activity relationships
As indicated in Tables 1 and 2, the antifungal effects of title compounds were greatly affected by structural variations. Some structure–activity relationships (SAR) analyses were discussed as below. First, Tables 1 and 2 show that most of title compounds exhibited better antifungal activity against Rs than that against Bc, Cc and Fg. For example, Table 1 presents that the anti-Rs effects of title compounds 5b, 5d, 5f, 5h, 5i, 5j, 5l, 5m, 5p, 5s, 5u, 5v and 5w are better than the corresponding effects against Bc, Cc and Fg at 10 μg/mL. Table 2 also exhibits that title compounds 5b, 5d, 5e, 5f, 5g, 5n, 5o, 5p and 5q have better EC50 values against Rs than that against Cc and Fg. Second, introducing methyl into the R1 position is disadvantageous for the antifungal activity of title compounds against the tested four fungi. For instance, Table 1 shows that the inhibition rates of compounds 5e, 5j and 5p (R1 = H) are obviously better than that of compounds 5v, 5w and 5u (R1 = Me) against the tested four fungi at 10 μg/mL. Third, when the R2 was substituted by 4-Me, 4-F, 2-Br and 4-OMe groups, the corresponding title compounds 5e, 5n, 5p and 5t exhibited overall better antifungal activity than that of compounds 5l, 5m, 5o and 5q–5s against Rs, Bc and Fg at 10 μg/mL. Finally, a presence of 4-F, 4-Cl and 4-Br groups at the R3 position can effectively enhance the antifungal activity of title compounds against Rs, Bc and Fg. For example, the inhibition effects of compounds 5e, 5f and 5g were overall better than that of compounds 5a–5d and 5h–5k against Rs, Bc and Fg at 10 μg/mL.
Methods and materials
General
Reagents and solvents used without further purification are analytically or chemically pure. Melting points (m.p.) were determined on an uncorrected WRS-1B digital melting point apparatus (Shanghai Precision and Scientific Instrument Corporation, China). The FT-IR spectra were recorded on a Thermo Nicolet 380 FT-IR spectrometer (Thermo Nicolet Corporation, America). 1H NMR, 13C NMR, and 1H-1H NOESY spectra were collected on a Bruker AV 400 MHz spectrometer (Bruker Corporation, Germany) at room temperature with DMSO-d6 as a solvent. Mass spectra were recorded on a TRACE 2000 spectrometer (Finnigan Corporation, America). Elemental analyses were determined on an Elementar Vario EL cube analyzer (Elementar Corporation, German). Reactions were monitored by thin layer chromatography (TLC) on silica gel GF245 (400 mesh). The tested strains Fg, Rs, Bc and Cc were provided by the Laboratory of Plant Disease Control at Nanjing Agricultural University.
General procedures for intermediates 2 and 3
Using glycine ethyl ester hydrochloride or alanine ethyl eater hydrochloride as a starting material, the intermediate 2a (4-hydroxy-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one) or 2b (4-hydroxy-1-methyl-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one) was successfully prepared according a previously procedure [31]. The substituted 2-bromo-1-phenylethan-1-ones 3a–3j were synthesized according to a reported method [32].
General procedures for intermediates 4
A mixture of a intermediate 2 (10 mmol), a intermediate 3 (11 mmol) and triethylamine (11 mmol) in acetone (50 mL) was stirred at room temperature for 4 h. After that, the white solid appeared in the reaction solution was filtered, washed with water and diethyl ether to obtain a intermediate 4.
4-(2-oxo-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4a)
Yellow solid, m.p. 179–181 °C, yield 68%; 1H NMR (400 MHz, DMSO-d6) δ 8.02 (s, 1H, Pyrroline-1-H), 7.77 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.63 (d, J = 3.0 Hz, 1H, Thiophene-3-H), 7.45 (d, J = 5.0 Hz, 1H, Thiophene-5-H), 7.25 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-3.5-2H), 6.93 (t, J = 4.2 Hz, 1H, Thiophene-4-H), 5.38 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H), 2.32 (s, 3H, CH3).
4-(2-oxo-2-phenylethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4b)
Yellow solid, m.p. 172–174 °C, yield 57%; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H, Pyrroline-1-H), 7.86 (d, J = 7.8 Hz, 2H, Ph-2,6-2H), 7.42 (d, J = 3.0 Hz, 1H, Thiophene-3-H), 7.38 (d, J = 5.0 Hz, 1H, Thiophene-5-H), 7.32 (t, J = 6.7 Hz, 2H, Ph-3,5-2H), 7.28–7.21 (m, 1H, Ph-4-H), 6.99–6.94 (m, 1H, Thiophene-4-H), 5.39 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(2-chlorophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4c)
Yellow solid, m.p. 162–164 °C, yield 57%; 1H NMR (400 MHz, DMSO-d6) δ 8.05 (s, 1H, Pyrroline-1-H), 7.62 (dd, J = 5.7, 3.5 Hz, 1H, Ar(2-Cl)-3-H), 7.56 (dt, J = 7.3, 3.7 Hz, 1H, Ar(2-Cl)-4-H), 7.47 (dd, J = 5.7, 3.5 Hz, 2H, Thiophene-3,5-2H), 7.28 (d, J = 4.9 Hz, 1H, Ar(2-Cl)-6-H), 7.16 (d, J = 5,4 Hz, 1H, Thiophene-4-H), 6.87–6.80 (m, 1H, Ar(2-Cl)-5-H), 5.38 (s, 2H, CH2), 4.27 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(2-bromophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4d)
Yellow solid, m.p. 152–154 °C, yield 34%; 1H NMR (400 MHz, DMSO-d6) δ 7.93 (s, 1H, Pyrroline-1-H), 7.68 (d, J = 7.8 Hz, 1H, Ar(2-Br)-6-H), 7.55 (d, J = 7.1 Hz, 1H, Ar(2-Br)-4-H), 7.46 (t, J = 7.4 Hz, 1H, Thiophene-3-H), 7.34 (t, J = 7.6 Hz, 1H, Thiophene-5-H), 7.28 (d, J = 4.8 Hz, 1H, Thiophene-4-H), 7.11 (d, J = 8.5 Hz, 2H, Ar(2-Br)-3,5-2H), 5.37 (s, 2H, CH2), 4.26 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(3-chlorophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4e)
Yellow solid, m.p. 168–170 °C, yield 43%; 1H NMR (400 MHz, DMSO-d6) δ 7.98 (s, 1H, Pyrroline-1-H), 7.93 (s, 1H, Ar(3-Cl)-2-H), 7.84 (d, J = 7.7 Hz, 1H, Ar(3-Cl)-6-H), 7.42 (t, J = 8.5 Hz, 2H, Thiophene-3,5-2H), 7.32 (d, J = 4.9 Hz, 1H, Ar(3-Cl)-4-H), 7.23 (d, J = 8.7 Hz, 2H, Ar(3-Cl)-5-H, Thiophene-4-H), 5.41 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(4-fluorophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4f)
Yellow solid, m.p. 174–176 °C, yield 56%; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H, Pyrroline-1-H), 7.94–7.84 (m, 2H, Ar(4-F)-2,6-2H), 7.41 (d, J = 2.6 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 4.8 Hz, 1H, Thiophene-5-H), 7.12 (t, J = 8.6 Hz, 2H, Ar(4-F)-3,5-2H), 6.99–6.85 (m, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(4-chlorophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4g)
Yellow solid, m.p. 145–147 °C, yield 91%; 1H NMR (400 MHz, DMSO-d6) δ 7.99 (s, 1H, Pyrroline-1-H), 7.89 (d, J = 8.6 Hz, 2H, Ar(4-Cl)-2,6-2H), 7.40 (d, J = 3.3 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 4.9 Hz, 1H, Thiophene-3-H), 7.22 (d, J = 8.8 Hz, 2H, Ar(4-Cl)-3,5-2H), 6.93 (dd, J = 8.8, 4.8 Hz, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(4-bromophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4h)
Yellow solid, m.p. 156–158 °C, yield 71%; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H, Pyrroline-1-H), 7.94–7.86 (m, 2H, Ar(4-Br)-2,6-2H), 7.42 (d, J = 5.9 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 4.9 Hz, 1H, Thiophene-5-H), 7.23 (d, J = 8.7 Hz, 2H, Ar(4-Br)-3,5-2H), 6.94–6.88 (m, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(2,4-dichlorophenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4i)
Yellow solid, m.p. 152–154 °C, yield 44%; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (s, 1H, Pyrroline-1-H), 7.68 (d, J = 1.5 Hz, 1H, Ar(2,4-2Cl)-3-H), 7.61 (d, J = 8.3 Hz, 1H, Thiophene-3-H), 7.51 (dd, J = 8.3, 1.5 Hz, 1H, Thiophene-5-H), 7.30 (d, J = 5.1 Hz, 1H, Ar(2,4-2Cl)-5-H), 7.11 (d, J = 8.7 Hz, 1H, Ar(2,4-2Cl)-6-H), 6.90–6.78 (m, 1H, Thiophene-4-H), 5.37 (s, 2H, CH2), 4.26 (s, 2H, Pyrroline-5-2H).
4-(2-oxo-2-(4-methoxyphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4j)
Yellow solid, m.p. 156–158 °C, yield 57%; 1H NMR (400 MHz, DMSO-d6) δ 7.97 (s, 1H, Pyrroline-1-H), 7.84–7.76 (m, 2H, Ar(4-OCH3)-2,6-2H), 7.42 (d, J = 5.9 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 4.9 Hz, 1H, Thiophene-5-H), 7.23 (d, J = 8.7 Hz, 2H, Ar(4-OCH3)-3,5-2H), 6.97–6.88 (m, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H), 3.78 (s, 3H, CH3).
4-(2-oxo-2-(4-fluorophenyl)ethoxy)-1-methyl-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4k)
Yellow solid, m.p. 166–168 °C, yield 72%; 1H NMR (400 MHz, DMSO-d6) δ 7.90 (dd, J = 8.7, 5.6 Hz, 2H, Ar(4-F)-2,6-2H), 7.40 (d, J = 3.6 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 5.1 Hz, 1H, Thiophene-5-H), 7.29 (d, J = 11.1 Hz, 2H, Ar(4-F)-3,5-2H), 6.94 (dd, J = 5.0, 3.8 Hz, 1H, Thiophene-4-H), 5.39 (s, 2H, CH2), 4.45 (s, 2H, Pyrroline-5-2H), 2.99 (s, 3H, CH3).
4-(2-oxo-2-(4-methylphenyl)ethoxy)-1-methyl-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (4l)
Yellow solid, m.p. 143–145 °C, yield 59%; 1H NMR (400 MHz, DMSO-d6) δ 7.76 (d, J = 7.7 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.41 (d, J = 1.8 Hz, 1H, Thiophene-3-H), 7.31 (d, J = 8.5 Hz, 3H, Thiophene-5-H, Ar(4-CH3)-3,5-2H), 7.00–6.95 (m, 1H, Thiophene-4-H), 5.37 (s, 2H, CH2), 4.45 (s, 2H, Pyrroline-5-2H), 2.99 (s, 3H, CH3), 2.32 (s, 3H, CH3).
General procedures for intermediates 5
A mixture of a intermediate 4 (1.50 mmol) and substituted phenylhydrazine (1.70 mmol) in acetonitrile (35 mL) was stirred under 35 °C. After the reaction was completed, the white solid appeared in the reaction solution was filtered and recrystallized with diethyl ether to obtain a title compound 5.
(Z)-4-(2-(2-phenylhydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5a)
Yellow solid, m.p. 153–155 °C, yield 65%; IR (KBr, cm−1): 3380, 3171, 3063, 1676; 1H NMR (400 MHz, DMSO-d6) δ 9.98 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.78 (s, 1H, Ar(4-CH3)-2-H), 7.76 (s, 1H, Ar(4-CH3)-6-H), 7.42 (d, J = 3.1 Hz, 1H, Thiophene-3-H), 7.33–7.29 (m, 1H, Thiophene-5-H), 7.25 (t, J = 8.4 Hz, 5H, Ph-2,3,5,6-4H, Thiophene-4-H), 7.21 (s, 1H, Ar(4-CH3)-3-H), 6.93 (dd, J = 4.9, 3.8 Hz, 1H, Ar(4-CH3)-5-H), 6.83 (t, J = 6.5 Hz, 1H, Ph-4-H), 5.40 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H), 2.32 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.99, 167.05, 145.68, 137.59, 136.57, 134.91, 132.66, 129.59, 129.51, 126.77, 125.80, 124.57, 124.04, 120.25, 113.44, 103.76, 61.76, 43.65, 21.28; Anal. Calcd for C23H21N3O2S (403.14): C, 68.46; H, 5.25; N, 10.41. Found: C, 68.22; H, 5.27; N, 10.37; EI-MS m/z 403.14 [M]+.
(Z)-4-(2-(2-(2-fluorophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5b)
White solid, m.p. 158–160 °C, yield 51%; IR (KBr, cm−1): 3376, 3177, 3069, 1678; 1H NMR (400 MHz, DMSO-d6) δ 9.54 (s, 1H, Ar–NH=N), 7.95 (s, 1H, Pyrroline-1-H), 7.79 (d, J = 8.2 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.62 (td, J = 8.5, 1.4 Hz, 1H, Thiophene-3-H), 7.43–7.39 (m, 1H, Thiophene-5-H), 7.32 (dd, J = 5.1, 0.9 Hz, 1H, Ar(2-F)-4-H), 7.24 (d, J = 8.1 Hz, 2H, Ar(2-F)-3,6-2H), 7.21–7.13 (m, 2H, Ar(4-CH3)-3,5-2H), 6.93 (dd, J = 5.1, 3.7 Hz, 1H, Ar(2-F)-5-H), 6.91–6.84 (m, 1H, Thiophene-4-H), 5.51 (s, 2H, CH2), 4.35 (s, 2H, Pyrroline-5-2H), 2.33 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.91, 166.71, 151.54, 149.15, 140.53, 138.23, 134.40, 133.83, 133.74, 132.52, 129.53, 126.74, 126.29, 125.52, 125.48, 124.61, 124.07, 120.75, 120.69, 115.87, 103.82, 62.52, 43.58, 21.30; Anal. Calcd for C23H20FN3O2S (421.13): C, 65.54; H, 4.78; N, 9.97. Found: C, 65.12; H, 4.81; N, 9.92; EI-MS m/z 421.13 [M]+.
(Z)-4-(2-(2-(2-chlorophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5c)
White solid, m.p. 160–162 °C, yield 30%; IR (KBr, cm−1): 3376, 3176, 3070, 1679; 1H NMR (400 MHz, DMSO-d6) δ 9.12 (s, 1H, Ar–NH=N), 7.99 (s, 1H, Pyrroline-1-H), 7.82 (d, J = 8.2 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.64 (d, J = 7.2 Hz, 1H, Thiophene-3-H), 7.48 (d, J = 2.9 Hz, 1H, Thiophene-5-H), 7.36 (d, J = 4.1 Hz, 1H, Ar(2-Cl)-3-H), 7.35–7.30 (m, 2H, Thiophene-4-H, Ar(2-Cl)-5-H), 7.26 (d, J = 8.1 Hz, 2H, Ar(4-CH3)-3,5-2H), 6.96 (dd, J = 5.0, 3.7 Hz, 1H, Ar(2-Cl)-6-H), 6.92–6.86 (m, 1H, Ar(2-Cl)-4-H), 5.58 (s, 2H, CH2), 4.35 (s, 2H, Pyrroline-5-2H), 2.34 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.74, 165.99, 141.52, 141.27, 138.68, 133.97, 132.25, 129.80, 129.67, 128.69, 126.76, 126.33, 124.85, 124.45, 121.55, 118.12, 115.32, 104.37, 63.53, 43.56, 21.31; Anal. Calcd for C23H20ClN3O2S (437.1): C, 63.08; H, 4.60; N, 9.60. Found: C, 62.82; H, 4.62; N, 9.57; EI-MS m/z 437.1 [M]+.
(Z)-4-(2-(2-(3-chlorophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5d)
White solid, m.p. 172–174 °C, yield 38%; IR (KBr, cm−1): 3376, 3192, 3069, 1676; 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.77 (d, J = 8.2 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.41 (d, J = 2.8 Hz, 1H, Ar(3-Cl)-3-H), 7.34–7.30 (m, 1H, Thiophene-3-H), 7.27 (t, J = 5.2 Hz, 2H, Thiophene-4,5-2H), 7.25 (s, 1H, Ar(3-Cl)-5-H), 7.23 (s, 1H, Ar(3-Cl)-4-H), 7.18 (d, J = 8.2 Hz, 1H, Ar(4-CH3)-3-H), 6.93 (dd, J = 5.0, 3.7 Hz, 1H, Ar(4-CH3)-5-H), 6.85 (dd, J = 7.8, 1.1 Hz, 1H, Ar(3-Cl)-6-H), 5.39 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H), 2.33 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.94, 166.89, 147.15, 138.39, 138.06, 134.53, 134.24, 132.60, 131.27, 129.57, 126.76, 126.05, 124.63, 124.07, 119.64, 112.79, 112.07, 103.83, 61.84, 43.63, 21.30; Anal. Calcd for C23H20ClN3O2S (437.1): C, 63.08; H, 4.60; N, 9.60. Found: C, 62.81; H, 4.64; N, 9.66; EI-MS m/z 437.1 [M]+.
(Z)-4-(2-(2-(4-fluorophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5e)
White solid, m.p. 149–151 °C, yield 63%; IR (KBr, cm−1): 3368, 3167, 3063, 1676; 1H NMR (400 MHz, DMSO-d6) δ 10.05 (s, 1H, Ar–NH=N), 7.99 (s, 1H, Pyrroline-1-H), 7.76 (d, J = 8.0 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.41 (d, J = 3.3 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 5.0 Hz, 1H, Thiophene-5-H), 7.25 (dd, J = 10.0, 6.3 Hz, 3H, Thiophene-4-H, Ar(4-F)-3,5-2H), 7.21 (s, 1H, Ar(4-CH3)-3-H), 7.12 (t, J = 8.7 Hz, 2H, Ar(4-F)-2,6-2H), 6.95–6.90 (m, 1H, Ar(4-CH3)-5-H), 5.40 (s, 2H, CH2), 4.39 (s, 2H, Pyrroline-5-2H), 2.32 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.97, 167.03, 158.07, 155.74, 142.37, 137.59, 136.71, 134.83, 132.65, 129.49, 126.76, 125.81, 124.56, 124.02, 116.20, 115.98, 114.53, 114.46, 103.74, 61.86, 43.66, 21.27; Anal. Calcd for C23H20FN3O2S (421.1): C, 65.54; H, 4.78; N, 9.97. Found: C, 65.81; H, 4.82; N, 9.89; EI-MS m/z 421.1 [M]+.
(Z)-4-(2-(2-(4-chlorophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5f)
White solid, m.p. 156–157 °C, yield 61%; IR (KBr, cm−1): 3366, 3173, 3071, 1677; 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.77 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.41 (d, J = 2.7 Hz, 1H, Thiophene-3-H), 7.31 (d, J = 8.8 Hz, 3H, Thiophene-5-H, Ar(4-Cl)-3,5-2H), 7.25 (d, J = 10.3 Hz, 3H, Thiophene-4-H, Ar(4-Cl)-2,6-2H), 7.21 (s, 1H, Ar(4-CH3)-3-H), 6.96–6.90 (m, 1H, Ar(4-CH3)-5-H), 5.39 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H), 2.32 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.96, 166.93, 144.64, 137.86, 137.60, 134.66, 132.62, 129.52, 129.41, 126.77, 125.93, 124.60, 124.06, 123.57, 114.90, 103.82, 61.81, 43.64, 21.29; Anal. Calcd for C23H20ClN3O2S (437.1): C, 63.08; H, 4.60; N, 9.60. Found: C, 63.51; H, 4.64; N, 9.67; EI-MS m/z 437.1 [M]+.
(Z)-4-(2-(2-(4-bromophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5g)
White solid, m.p. 160–162 °C, yield 72%; IR (KBr, cm−1): 3364, 3179, 3075, 1677; 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.77 (d, J = 8.1 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.42 (d, J = 8.7 Hz, 3H, Thiophene-3,5-2H, Ar(4-Br)-3-H), 7.31 (d, J = 5.0 Hz, 1H, Ar(4-Br)-5-H), 7.21 (t, J = 8.1 Hz, 4H, Thiophene-4-H, Ar(4-CH3)-3,5-2H, Ar(4-Br)-2-H), 6.96–6.90 (m, 1H, Ar(4-Br)-6-H), 5.38 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H), 2.32 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.95, 166.92, 145.02, 137.89, 137.70, 134.65, 132.62, 132.25, 129.53, 126.77, 125.94, 124.61, 124.06, 115.40, 111.25, 103.82, 61.82, 43.63, 21.29; Anal. Calcd for C23H20BrN3O2S (481.0): C, 57.27; H, 4.18; N, 8.71. Found: C, 57.14; H, 4.21; N, 8.72; EI-MS m/z 481.0 [M]+.
(Z)-4-(2-(2-(2-(4-(trifluoromethyl)phenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5h)
White solid, m.p. 167–169 °C, yield 82%; IR (KBr, cm−1): 3363, 3172, 3074, 1681, 1590; 1H NMR (400 MHz, DMSO-d6) δ 10.35 (s, 1H, Ar–NH=N), 7.98 (s, 1H, Pyrroline-1-H), 7.80 (d, J = 7.7 Hz, 2H, Ar(4-CF3)-3,5-2H), 7.61 (d, J = 8.3 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.40 (s, 2H, Thiophene-3,5-2H), 7.38 (s, 1H, Ar(4-CF3)-2-H), 7.31 (d, J = 4.9 Hz, 1H, Ar(4-CF3)-6-H), 7.24 (d, J = 7.7 Hz, 2H, Ar(4-CH3)-3,5-2H), 6.92 (d, J = 3.7 Hz, 1H, Thiophene-4-H), 5.42 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H), 2.34 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.94, 166.83, 148.76, 139.34, 138.29, 134.42, 132.59, 129.56, 126.98, 126.94, 126.76, 126.20, 124.64, 124.07, 120.13, 119.82, 113.23, 103.87, 61.87, 43.63, 21.30; Anal. Calcd for C24H20F3N3O2S (471.1): C, 61.14; H, 4.28; N, 8.91. Found: C, 61.21; H, 4.31; N, 8.89; EI-MS m/z 471.1 [M]+.
(Z)-4-(2-(2-(2-(2,4-dichlorophenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5i)
White solid, m.p. 172–174 °C, yield 39%; IR (KBr, cm−1): 3363, 3167, 3075, 1679; 1H NMR (400 MHz, DMSO-d6) δ 9.20 (s, 1H, Ar–NH=N), 8.00 (s, 1H, Pyrroline-1-H), 7.82 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.64 (d, J = 8.9 Hz, 1H, Thiophene-3-H), 7.53 (s, 1H, Thiophene-6-H), 7.46 (d, J = 3.0 Hz, 1H, Thiophene-4-H), 7.39 (d, J = 8.8 Hz, 1H, Ar(2,4-2Cl)-6-H), 7.35 (d, J = 5.0 Hz, 1H, Ar(2,4-2Cl)-3-H), 7.26 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-3,5-2H), 6.98–6.93 (m, 1H, Ar(2,4-2Cl)-5-H), 5.58 (s, 2H, CH2), 4.35 (s, 2H, Pyrroline-5-2H), 2.34 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.73, 165.98, 142.51, 140.59, 138.88, 133.78, 132.24, 129.66, 129.09, 128.71, 126.75, 126.46, 124.84, 124.43, 124.26, 118.71, 116.46, 104.34, 63.57, 43.55, 21.32; Anal. Calcd for C23H19Cl2N3O2S (471.1): C, 58.48; H, 4.05; N, 8.90. Found: C, 58.23; H, 4.21; N, 8.86; EI-MS m/z 471.1 [M]+.
(Z)-4-(2-(2-(2-(4-methylphenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5j)
White solid, m.p. 141–143 °C, yield 42%; IR (KBr, cm−1): 3376, 3172, 3069, 1667; 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H, Ar–NH=N), 7.98 (s, 1H, Pyrroline-1-H), 7.75 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.42 (d, J = 3.0 Hz, 1H, Thiophene-3-H), 7.31 (d, J = 5.0 Hz, 1H, Thiophene-5-H), 7.21 (d, J = 7.9 Hz, 2H, Ar(4-CH3)-3.5-2H), 7.11 (dd, J = 27.3, 8.1 Hz, 4H, Ar(4-CH3)-2,3,4,5-4H), 6.93 (t, J = 4.2 Hz, 1H, Thiophene-4-H), 5.38 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H), 2.32 (s, 3H, CH3), 2.23 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.98, 167.09, 143.42, 137.39, 135.82, 135.01, 132.67, 130.02, 129.49, 128.85, 126.76, 125.66, 124.55, 124.02, 113.44, 103.72, 61.73, 43.64, 21.27, 20.75; Anal. Calcd for C24H23N3O2S (417.1): C, 58.48; H, 4.05; N, 8.90. Found: C, 58.23; H, 4.07; N, 8.86; EI-MS m/z 417.1 [M]+.
(Z)-4-(2-(2-(2-(4-methoxyphenyl)hydrazono)-2-(4-methylphenyl)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5k)
White solid, m.p. 140–142 °C, yield 38%; IR (KBr, cm−1): 3376, 3177, 3069, 1679; 1H NMR (400 MHz, DMSO-d6) δ 9.82 (s, 1H, Ar–NH=N), 7.96 (s, 1H, Pyrroline-1-H), 7.74 (d, J = 8.2 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.42 (d, J = 3.4 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 5.0 Hz, 1H, Thiophene-5-H), 7.19 (t, J = 9.0 Hz, 4H, Thiophene-4-H, Ar(4-OCH3)-2,6-2H, Ar(4-CH3)-3-H), 6.96–6.92 (m, 1H, Ar(4-CH3)-5-H), 6.89 (d, J = 9.0 Hz, 2H, Ar(4-OCH3)-3,5-2H), 5.37 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H), 3.70 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 172.01, 167.12, 153.75, 139.63, 137.22, 135.28, 135.10, 132.69, 129.48, 126.76, 125.56, 124.54, 124.03, 115.02, 114.50, 103.73, 61.75, 55.70, 43.65, 21.25; Anal. Calcd for C24H23N3O3S (433.1): C, 66.49; H, 5.35; N, 9.69. Found: C, 66.26; H, 5.33; N, 9.73; EI-MS m/z 433.1 [M]+.
(Z)-4-(2-(2-(2-(4-fluorophenyl)hydrazono)-2-phenylethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5l)
White solid, m.p. 131–133 °C, yield 44%; IR (KBr, cm−1): 3343, 3231, 3060, 1677; 1H NMR (400 MHz, DMSO-d6) δ 10.05 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.86 (d, J = 7.8 Hz, 2H, Ph-2,6-2H), 7.40 (d, J = 7.5 Hz, 3H, Thiophene-3,4,5-3H), 7.32 (t, J = 6.7 Hz, 2H, Ph-3,5-2H), 7.28–7.21 (m, 2H, Ar(4-F)-2,6-2H), 7.12 (t, J = 8.7 Hz, 2H, Ar(4-F)-3,5-2H), 6.95–6.90 (m, 1H, Ph-4-H), 5.40 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.97, 166.96, 158.19, 155.85, 142.23, 137.58, 136.62, 132.63, 128.89, 128.20, 126.76, 125.88, 124.59, 124.04, 116.26, 116.04, 114.64, 114.56, 103.81, 61.81, 43.64; Anal. Calcd for C22H18FN3O2S (407.1): C, 64.85; H, 4.45; N, 10.31. Found: C, 64.78; H, 4.48; N, 10.37; EI-MS m/z 407.1 [M]+.
(Z)-4-(2-(2-chlorophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5m)
Yellow solid, m.p. 125–127 °C, yield 46%; IR (KBr, cm−1): 3312, 3223, 3084, 1682; 1H NMR (400 MHz, DMSO-d6) δ 9.85 (s, 1H, Ar–NH=N), 7.95 (s, 1H, Pyrroline-1-H), 7.58 (dd, J = 5.7, 3.5 Hz, 1H, Ar(2-Cl)-3-H), 7.50 (dt, J = 7.3, 3.7 Hz, 1H, Ar(2-Cl)-4-H), 7.42 (dd, J = 5.7, 3.5 Hz, 2H, Thiophene-3,5-2H), 7.28 (d, J = 4.9 Hz, 1H, Thiophene-4-H), 7.20–7.06 (m, 4H, Ar(4-F)-2,3,6-3H, Ar(2-Cl)-6-H), 7.04 (d, J = 3.1 Hz, 1H, Ar(4-F)-5-H), 6.87–6.80 (m, 1H, Ar(2-Cl)-5-H), 5.38 (s, 2H, CH2), 4.27 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.85, 166.09, 158.18, 155.85, 142.37, 139.22, 136.89, 132.68, 132.34, 131.70, 130.14, 129.95, 127.66, 126.58, 124.56, 124.09, 116.15, 115.93, 114.64, 114.57, 103.88, 65.93, 43.54; Anal. Calcd for C22H17FClN3O2S (441.1): C, 59.80; H, 3.88; N, 9.51. Found: C, 59.78; H, 3.90; N, 9.57; EI-MS m/z 441.1 [M]+.
(Z)-4-(2-(2-bromophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5n)
Yellow solid, m.p. 132–134 °C, yield 35%; IR (KBr, cm−1): 3315, 3219, 3087, 1681; 1H NMR (400 MHz, DMSO-d6) δ 9.94 (s, 1H, Ar–NH=N), 7.96 (s, 1H, Pyrroline-1-H), 7.68 (d, J = 7.9 Hz, 1H, Ar(2-Br)-3-H), 7.55 (d, J = 6.2 Hz, 1H, Ar(2-Br)-4-H), 7.49–7.31 (m, 4H, Thiophene-3,5-2H, Ar(4-F)-2,6-2H), 7.28 (d, J = 4.9 Hz, 1H, Thiophene-4-H), 7.11 (d, J = 8.8 Hz, 2H, Ar(4-F)-3,5-2H), 7.00 (d, J = 3.2 Hz, 1H, Ar(2-Br)-6-H), 6.86–6.79 (m, 1H, Ar(2-Br)-5-H), 5.37 (s, 2H, CH2), 4.26 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.86, 166.06, 142.42, 140.36, 138.78, 133.08, 132.32, 131.85, 130.31, 128.11, 126.58, 124.55, 124.16, 122.75, 116.95, 116.87, 116.28, 116.12, 116.05, 115.90, 114.63, 114.56, 103.90, 66.02, 43.62; Anal. Calcd for C22H17FBrN3O2S (485.0): C, 54.33; H, 3.52; N, 8.64. Found: C, 54.53; H, 3.55; N, 8.57; EI-MS m/z 485.0 [M]+.
(Z)-4-(2-(3-chlorophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5o)
Yellow solid, m.p. 125–126 °C, yield 36%; IR (KBr, cm−1): 3375, 3255, 3067, 1682; 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.89 (s, 1H, Ar(3-Cl)-2-H), 7.83 (d, J = 7.7 Hz, 1H, Ar(3-Cl)-6-H), 7.43 (t, J = 6.9 Hz, 2H, Thiophene-3,5-2H), 7.37 (d, J = 7.7 Hz, 1H, Thiophene-4-H), 7.32 (d, J = 5.0 Hz, 1H, Ar(3-Cl)-4-H), 7.29–7.22 (m, 2H, Ar(4-F)-2,6-2H), 7.14 (t, J = 8.6 Hz, 2H, Ar(4-F)-3,5-2H), 6.97–6.91 (m, 1H, Ar(3-Cl)-5-H), 5.40 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.94, 166.89, 158.39, 156.05, 141.92, 139.73, 135.19, 133.89, 132.62, 130.72, 127.84, 126.77, 125.40, 124.62, 124.50, 123.99, 116.34, 116.11, 114.88, 114.80, 103.84, 61.62, 43.62; Anal. Calcd for C22H17ClBrN3O2S (441.1): C, 59.80; H, 3.88; N, 9.51. Found: C, 59.58; H, 3.85; N, 9.57; EI-MS m/z 441.1 [M]+.
(Z)-4-(2-(4-fluorophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5p)
Yellow solid, m.p. 133–135 °C, yield 67%; IR (KBr, cm−1): 3355, 3229, 3087, 1678; 1H NMR (400 MHz, DMSO-d6) δ 10.07 (s, 1H, Ar–NH=N), 7.98 (s, 1H, Pyrroline-1-H), 7.94–7.85 (m, 2H, Ar(4-F)-2,6-2H), 7.40 (s, 1H, Thiophene-3-H), 7.32 (d, J = 4.7 Hz, 1H, Thiophene-5-H), 7.25 (d, J = 8.4 Hz, 4H, Ar(4-F)-2,6-2H, Ar(4-F)-3,5-2H), 7.12 (t, J = 8.5 Hz, 2H, Ar(4-F)-3,5-2H), 6.94 (s, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.38 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.95, 167.00, 163.52, 161.09, 159.09, 156.73, 142.56, 135.82, 135.76, 134.16, 134.13, 132.68, 128.04, 127.96, 126.72, 124.52, 123.95, 117.04, 116.96, 116.91, 116.11, 115.89, 103.72, 62.11, 43.77; Anal. Calcd for C22H17F2N3O2S (425.1): C, 62.11; H, 4.03; N, 9.88. Found: C, 62.49; H, 4.05; N, 9.86; EI-MS m/z 425.1 [M]+.
(Z)-4-(2-(4-chlorophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5q)
Yellow solid, m.p. 131–133 °C, yield 83%; IR (KBr, cm−1): 3447, 3239, 3123, 1675; 1H NMR (400 MHz, DMSO-d6) δ 10.19 (s, 1H, Ar–NH=N), 7.99 (s, 1H, Pyrroline-1-H), 7.89 (d, J = 8.6 Hz, 2H, Ar(4-Cl)-2,6-2H), 7.46 (d, J = 8.6 Hz, 2H, Ar(4-F)-2,6-2H), 7.41 (d, J = 3.2 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 4.7 Hz, 1H, Thiophene-5-H), 7.27 (dd, J = 9.0, 4.8 Hz, 2H, Ar(4-F)-3,5-2H), 7.13 (t, J = 8.8 Hz, 2H, Ar(4-Cl)-3,5-2H), 6.94 (dd, J = 4.9, 3.8 Hz, 1H, Thiophene-4-H), 5.41 (s, 2H, CH2), 4.39 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.94, 166.89, 158.30, 155.96, 142.05, 136.47, 135.44, 132.69, 132.63, 128.87, 127.56, 126.76, 124.61, 124.02, 116.27, 116.05, 114.76, 114.68, 103.83, 61.60, 43.65; Anal. Calcd for C22H17FClN3O2S (441.1): C, 59.80; H, 3.88; N, 9.51. Found: C, 60.19; H, 3.90; N, 9.46; EI-MS m/z 441.1 [M]+.
(Z)-4-(2-(4-bromophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5r)
White solid, m.p. 136–138 °C, yield 88%; IR (KBr, cm−1): 3294, 3223, 3079, 1679; 1H NMR (400 MHz, DMSO-d6) δ 10.16 (s, 1H, Ar–NH=N), 7.98 (s, 1H, Pyrroline-1-H), 7.91 (dd, J = 8.6, 5.6 Hz, 2H, Ar(4-Br)-2,6-2H), 7.43 (d, J = 8.7 Hz, 2H, Ar(4-F)-2,6-2H), 7.40 (d, J = 3.2 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 4.9 Hz, 1H, Thiophene-5-H), 7.28–7.18 (m, 4H, Ar(4-F)-3,5-2H, Ar(4-Br)-3,5-2H), 6.96–6.90 (m, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.95, 166.88, 163.58, 161.14, 158.19, 155.85, 142.22, 142.21, 135.94, 134.07, 132.62, 128.02, 127.94, 126.76, 124.61, 124.03, 116.25, 116.03, 115.85, 115.63, 114.64, 114.56, 103.84, 61.81, 43.63; Anal. Calcd for C22H17FBrN3O2S (485.0): C, 54.33; H, 3.52; N, 8.64. Found: C, 54.62; H, 3.54; N, 8.62; EI-MS m/z 485.0 [M]+.
(Z)-4-(2-(2,4-dichlorophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5s)
Yellow solid, m.p. 155–157 °C, yield 77%; IR (KBr, cm−1): 3431, 3255, 3103, 1672; 1H NMR (400 MHz, DMSO-d6) δ 9.88 (s, 1H, Ar–NH=N), 7.94 (s, 1H, Pyrroline-1-H), 7.67 (d, J = 2.0 Hz, 1H, Ar(2,4-2Cl)-3-H), 7.61 (d, J = 8.3 Hz, 1H, Thiophene-3-H), 7.51 (dd, J = 8.3, 2.0 Hz, 1H, Thiophene-5-H), 7.30 (d, J = 5.0 Hz, 1H, Ar(2,4-2Cl)-5-H), 7.18–7.06 (m, 4H, Ar(2,4-2Cl)-6-H, Ar(4-F)-2,3,5-3H), 7.04 (d, J = 3.0 Hz, 1H, Ar(4-F)-6-H), 6.85 (dd, J = 5.0, 3.8 Hz, 1H, Thiophene-4-H), 5.36 (s, 2H, CH2), 4.26 (s, 2H, Pyrroline-5-2H); 13C NMR (100 MHz, DMSO-d6) δ 171.81, 165.98, 158.28, 155.94, 142.19, 138.08, 135.88, 133.82, 133.74, 132.93, 132.34, 129.47, 127.85, 126.45, 124.63, 124.01, 116.19, 115.97, 114.70, 114.63, 103.99, 65.77, 43.51; Anal. Calcd for C22H16FCl2N3O2S (475.0): C, 55.47; H, 3.39; N, 8.82. Found: C, 55.42; H, 3.36; N, 8.76; EI-MS m/z 475.0 [M]+.
(Z)-4-(2-(4-methoxyphenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5t)
Yellow solid, m.p. 153–155 °C, yield 58%; IR (KBr, cm−1): 3419, 3251, 3067, 1677; 1H NMR (400 MHz, DMSO-d6) δ 9.91 (s, 1H, Ar–NH=N), 7.97 (s, 1H, Pyrroline-1-H), 7.80 (d, J = 8.8 Hz, 2H, Ar(4-OCH3)-2,6-2H), 7.42 (d, J = 3.6 Hz, 1H, Thiophene-3-H), 7.32 (d, J = 5.0 Hz, 1H, Thiophene-5-H), 7.22 (dd, J = 9.0, 4.8 Hz, 2H, Ar(4-F)-2,6-2H), 7.11 (t, J = 8.8 Hz, 2H, Ar(4-F)-3,5-2H), 6.99–6.92 (m, 3H, Ar(4-OCH3)-3,5-2H, Thiophene-4-H), 5.38 (s, 2H, CH2), 4.37 (s, 2H, Pyrroline-5-2H), 3.78 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 171.97, 166.96, 159.61, 142.49, 136.88, 132.64, 130.14, 127.32, 126.77, 124.59, 124.07, 116.19, 115.97, 114.41, 114.32, 103.80, 61.89, 55.64, 43.64; Anal. Calcd for C23H20FN3O2S (437.1): C, 63.15; H, 4.61; N, 9.61. Found: C, 63.42; H, 4.63; N, 9.66; EI-MS m/z 437.1 [M]+.
(Z)-4-(2-(4-fluorophenyl)-2-(2-(4-fluorophenyl)hydrazono)ethoxy)-1-methyl-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5u)
White solid, m.p. 147–149 °C, yield 80%; IR (KBr, cm−1): 3263, 2987, 1667; 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H, Ar–NH=N), 7.89 (dd, J = 8.3, 5.7 Hz, 2H, Ar(4-F)-2,6-2H), 7.41 (d, J = 2.8 Hz, 1H, Thiophene-5-H), 7.32 (d, J = 4.9 Hz, 1H, Thiophene-3-H), 7.25 (dt, J = 13.7, 6.8 Hz, 4H, Ar(4-F)-3,5-2H, Ar(4-F)-2,6-2H), 7.12 (t, J = 8.8 Hz, 2H, Ar(4-F)-3,5-2H), 6.96–6.91 (m, 1H, Thiophene-4-H), 5.41 (s, 2H, CH2), 4.47 (s, 2H, Pyrroline-5-2H), 2.99 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 169.51, 164.59, 163.57, 161.13, 158.18, 155.84, 142.29, 135.78, 134.15, 132.64, 128.01, 127.93, 126.83, 124.67, 124.06, 116.22, 116.00, 115.83, 115.62, 114.65, 114.58, 103.65, 62.08, 49.70, 29.06; Anal. Calcd for C23H19F2N3O2S (439.1): C, 62.86; H, 4.36; N, 9.56. Found: C, 62.51; H, 4.39; N, 9.52; EI-MS m/z 439.1 [M]+.
(Z)-4-(2-(4-fluorophenyl)-2-(2-(4-methylphenyl)hydrazono)ethoxy)-1-methyl-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5v)
White solid, m.p. 157–159 °C, yield 53%; IR (KBr, cm−1): 3257, 2922, 1670; 1H NMR (400 MHz, DMSO-d6) δ 10.08 (s, 1H, Ar–NH=N), 7.76 (d, J = 7.7 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.41 (s, 1H, Thiophene-5-H), 7.31 (d, J = 8.5 Hz, 3H, Thiophene-3-H, Ar(4-CH3)-3,5-2H), 7.28–7.17 (m, 4H, Ar(4-F)-2,3,5,6-4H), 6.93 (s, 1H, Thiophene-4-H), 5.37 (s, 2H, CH2), 4.45 (s, 2H, Pyrroline-5-2H), 2.99 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 169.53, 164.71, 158.08, 155.74, 142.40, 137.58, 136.59, 134.88, 132.67, 129.49, 126.82, 125.80, 124.63, 124.07, 116.96, 116.88, 116.19, 115.97, 114.55, 114.48, 103.59, 62.06, 49.71, 29.05, 21.26; Anal. Calcd for C24H22FN3O2S (435.1): C, 66.19; H, 5.09; N, 9.65. Found: C, 66.44; H, 5.12; N, 9.71; EI-MS m/z 435.1 [M]+.
(Z)-4-(2-(4-methylphenyl)-2-(2-(4-methylphenyl)hydrazono)ethoxy)-1-methyl-3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one (5w)
White solid, m.p. 175–177 °C, yield 41%; IR (KBr, cm−1): 3230, 2988, 1668; 1H NMR (400 MHz, DMSO-d6) δ 9.99 (s, 1H, Ar–NH=N), 7.74 (d, J = 8.2 Hz, 2H, Ar(4-CH3)-3,5-2H), 7.43–7.39 (m, 1H, Thiophene-5-H), 7.31 (dd, J = 5.1, 0.9 Hz, 1H, Thiophene-3-H), 7.21 (d, J = 8.1 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.16 (d, J = 8.4 Hz, 2H, Ar(4-CH3)-2,6-2H), 7.07 (d, J = 8.4 Hz, 2H, Ar(4-CH3)-3,5-2H), 6.93 (dd, J = 5.1, 3.7 Hz, 1H, Thiophene-4-H), 5.40 (s, 2H, CH2), 4.49 (s, 2H, Pyrroline-5-2H), 2.99 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.23 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 169.56, 164.84, 143.52, 137.32, 135.62, 135.08, 132.70, 129.96, 129.46, 128.77, 126.82, 125.66, 124.57, 124.02, 113.48, 103.49, 62.06, 49.75, 29.06, 21.26, 20.75; Anal. Calcd for C25H25N3O2S (431.1): C, 69.58; H, 5.84; N, 9.74. Found: C, 69.36; H, 5.87; N, 9.77; EI-MS m/z 431.1 [M]+.
Conclusions
A series of 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives bearing a hydrazone group were designed, synthesized and confirmed by FT-IR, 1H NMR, 13C NMR, EI-MS, NOESY and elemental analysis. The antifungal assays indicated that some the title compounds exhibited obvious antifungal activity against Fg, Rs, Bc and Cc. Strikingly, the EC50 value of 5e against Rs was 1.26 µg/mL, which is better than that of drazoxolon (1.77 µg/mL). Meanwhile, title compounds 5b, 5d, 5e–5g, 5n–5q and 5t exhibited remarkable anti-Cc activity, with corresponding EC50 values reached 5.52–9.97 µg/mL, which are better than that of drazoxolon (19.46 µg/mL). These results indicated that 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives containing a hydrazone group can serve as potential structural templates in the search for novel and highly efficient fungicides. Further studies on the antifungal mechanism and structural modification of 3-(thiophen-2-yl)-1,5-dihydro-2H-pyrrol-2-one derivatives containing a hydrazone group are currently underway.
Additional file
Additional file 1. All the copies of FT-IR, 1H NMR, 13C NMR and EI-MS for title compounds 5a–5w.
Authors’ contributions
The current study is an outcome of constructive discussion with CY. ZR, XW, MC and MW carried out the synthesis and characterization experiments of title compounds; ZR, MC, MW and XW tested the antifungal activity of target compounds; XW, ZR, MC and AL performed the FT-IR, 1H NMR, 13C NMR, EI-MS, NOESY and elemental analyses; XW and CY were involved in the drafting of the manuscript and revising the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge the grants from the National Nature Science Foundation of China (No. 31772209) and the Fundamental Research Funds for the Central Universities of China (No. KYTZ201604).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
We have presented all our main data in the form of tables and figures. All the copies of IR, 1H NMR, 13C NMR and EI-MS spectrogram for title compounds 5a–5w were presented in the Additional file 1. The datasets supporting the conclusions of the article are included within the article and the Additional file 1.
Consent for publication
This section are not applicable for this manuscript.
Ethics approval and consent to participate
This section are not applicable for this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13065-018-0452-z) contains supplementary material, which is available to authorized users.
Contributor Information
Xiaobin Wang, Email: bin19910316@sina.com.
Zhengjiao Ren, Email: 2013111013@njau.edu.cn.
Mengqi Wang, Email: 2016111012@njau.edu.cn.
Min Chen, Email: chenmin@njau.edu.cn.
Aiming Lu, Email: luaiming@njau.edu.cn.
Weijie Si, Email: 2015202048@njau.edu.cn.
Chunlong Yang, Email: ycl@njau.edu.cn.
References
- 1.Sparks TC, Lorsbach BA. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag Sci. 2017;73:672–677. doi: 10.1002/ps.4457. [DOI] [PubMed] [Google Scholar]
- 2.Swain T. Secondary compounds as protective agents. Ann Rev Plant Physiol. 1977;28:479–501. doi: 10.1146/annurev.pp.28.060177.002403. [DOI] [Google Scholar]
- 3.Qian X, Lee PW, Cao S. China: forward to the green pesticides via basic research program. J Agric Food Chem. 2010;58:2613–2623. doi: 10.1021/jf904098w. [DOI] [PubMed] [Google Scholar]
- 4.Schobert R, Schlenk A. Tetramic and tetronic acids: an update on new derivatives and biological aspects. Bioorg Med Chem. 2008;16:4203–4221. doi: 10.1016/j.bmc.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 5.Jeong YC, Moloney MG. Tetramic acids as scaffolds: synthesis, tautomeric and antibacterial behaviour. Synlett. 2009;15:2487–2491. [Google Scholar]
- 6.Lu GH, Chu HB, Chen M, Yang CL. Synthesis and bioactivity of novel strobilurin derivatives containing pyrrolidine-2,4-dione moiety. Chin Chem Lett. 2014;25:61–64. doi: 10.1016/j.cclet.2013.10.007. [DOI] [Google Scholar]
- 7.Han BF, Shi QM, Wang XF, Liu JB, Qiang S, Yang CL. Synthesis and bioactivity of novel 3-(1-hydroxyethylidene)-5-substituted-pyrrolidine-2,4-dione derivatives. Chin Chem Lett. 2012;23:1023–1026. doi: 10.1016/j.cclet.2012.07.002. [DOI] [Google Scholar]
- 8.Bruck E, Elbert A, Fischer R, Krueger S, Kuhnhold J, Klueken AM, Nauen R, Niebes JF, Reckmann U, Schnorbach HJ, Steffens R, Waetermeulen XV. Movento, an innovative ambimobile insecticide for sucking insect pest control in agriculture: biological profile and filed performance. Crop Prot. 2009;28:838–844. doi: 10.1016/j.cropro.2009.06.015. [DOI] [Google Scholar]
- 9.Wang PY, Chen L, Zhou J, Fang HS, Wu ZB, Song BA, Yang S. Synthesis and bioactivities of 1-ary-4-hydroxy-1H-pyrrol-2(5H)-one derivatives bearing 1,3,4-oxadiazole moiety. J Saudi Chem Soc. 2017;21:315–323. doi: 10.1016/j.jscs.2016.10.002. [DOI] [Google Scholar]
- 10.Wang XF, Si TF, Li QB, Zhu ZY, Zhu XJ, Qiang S, Yang CL. Synthesis, characterization and biological activity of novel (5-RS, 6S)-5-sec-butyl-3-(1-substitutes amino)ethylidene-1H-pyrrilidine-2,4-diones. ARKIVOC. 2010;2:31–48. [Google Scholar]
- 11.Zhang L, Ren Z, Lu A, Zhao Z, Xu W, Bao Q, Ding W, Yang C. Synthesis, biological activity and 3D-QSAR study of novel pyrrolidine-2,4-dione derivatives containing N-substituted phenylhydrazine moiety. Chem Res Chin Univ. 2015;31:228–234. doi: 10.1007/s40242-015-4348-3. [DOI] [Google Scholar]
- 12.Zhu X, Huang L, Wang X, Zhu Z, Zheng X, Qiang S, Yang C. Synthesis and biological activities of 3-(1′-alkyloxyiminoethyl)-4-hydroxypyrroline-2-one derivatives. Chin J Org Chem. 2009;29:1784–1789. [Google Scholar]
- 13.Xu WQ, Chen M, Wang KY, Ren ZJ, Lu AM, Yang CL. Synthesis, characterization, and antifungal activity of phenylpyrrole-substituted tetramic acids bearing carbonates. Molecules. 2016;21:355. doi: 10.3390/molecules21030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong B, Li ZM, Liu CL, Zhao WG. Synthesis and biological activity of polysubstituted pyridines. Chin J Org Chem. 2004;24:1304–1306. [Google Scholar]
- 15.Harit T, Bellaouchi R, Asehraou A, Rahal M, Bouabdallah I, Malek F. Synthesis, characterization, antimicrobial activity and theoretical studies of new thiophene-based tripodal ligands. J Mol Struct. 2017;1133:74–79. doi: 10.1016/j.molstruc.2016.11.051. [DOI] [Google Scholar]
- 16.Mabkhot YN, Alatibi F, El-Sayed NNE, Kheder NA, Al-Showiman SS. Synthesis and structure–activity relationship of some new thiophene-based heterocycles as potential antimicrobial agents. Molecules. 2016;21:1036. doi: 10.3390/molecules21081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mabkhot YN, Kaal NA, Alterary S, Al-Showiman SS, Farghaly TA, Mubarak MS. Antimicrobial activity of thiophene derivatives derived from ethyl (E)-5-(3-(dimethylamino)acryloyl)-4-methyl-2-(phenylamino)thiophene-3-carboxylate. Chem Cent J. 2017;11:75. doi: 10.1186/s13065-017-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang ZB, Hu DY, Zeng S, Song BA. Novel hydrazone derivatives containing pyridine amide moiety: design, synthesis, and insecticidal activity. Bioorg Med Chem Lett. 2016;26:1161–1164. doi: 10.1016/j.bmcl.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Xie D, Gan X, Zeng S, Zhang A, Yin L, Song B, Jin L, Hu D. Synthesis, antiviral activity, and molecular docking study of trans-ferulic acid derivatives containing acylhydrazone moiety. Bioorg Med Chem Lett. 2017;27:4096–4100. doi: 10.1016/j.bmcl.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Backes GL, Neumann DM, Jursic BS. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg Med Chem. 2014;22:4629–4636. doi: 10.1016/j.bmc.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal N, Kumar R, Srivastva C, Dureja P, Khurana JM. Synthesis of nalidixic acid based hydrazones as novel pesticides. J Agric Food Chem. 2010;58:3056–3061. doi: 10.1021/jf904144e. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Song H, Huang Y, Li J, Zhao S, Song Y, Yang P, Xiao Z, Liu Y, Li Y, Shang H, Wang Q. Design, synthesis, and antiviral, fungicidal, and insecticidal activities of tetrahydro-β-carboline-3-carbohydrazide derivatives. J Agric Food Chem. 2014;62:9987–9999. doi: 10.1021/jf503794g. [DOI] [PubMed] [Google Scholar]
- 23.Dai ZC, Chen YF, Zhang M, Li SK, Yang TT, Shen L, Wang JX, Qian SS, Zhu HL, Ye YH. Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org Biomol Chem. 2015;13:477–486. doi: 10.1039/C4OB01758G. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Chen YF, Yan W, Cao LL, Ye YH. Synthesis and biological evaluation of benzimidazole phenylhydrazone derivatives as antifungal agents against phytopathogenic fungi. Molecules. 2016;21:1574. doi: 10.3390/molecules21111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Cui Z, He H. Synthesis and pesticidal activity of 3-(2-chloro-4-trifluoromethyl)phenoxy benzoylhydrazones. Chin J Org Chem. 2012;32:1925–1929. doi: 10.6023/cjoc201204022. [DOI] [Google Scholar]
- 26.Wu J, Wang J, Hu D, He M, Jin L, Song B. Synthesis and antifungal activity of novel pyrazolecarboxamide derivatives containing a hydrazone moiety. Chem Cent J. 2012;6:51. doi: 10.1186/1752-153X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Dai ZC, Qian SS, Liu JY, Xiao Y, Lu AM, Zhu HL, Wang JX, Ye YH. Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2-phenylhydrazono)methyl)quinoxaline derivatives. J Agric Food Chem. 2014;62:9637–9643. doi: 10.1021/jf504359p. [DOI] [PubMed] [Google Scholar]
- 28.Wang JJ, Si WJ, Chen M, Lu AM, Zhang WH, Yang CL. Synthesis and fungicidal activity of phenylhydrazone derivatives containing two carbonic acid ester groups. J Pestic Sci. 2017;42:84–92. doi: 10.1584/jpestics.D16-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C, Ren Z, Shi S, Xu W, Li L, Wang K (2016) Preparation of thiophene-pyrrolidone compounds for preventing and controlling plant pathogenic fungi. C.N. patent, 105218534
- 30.Hamdi N, Passarelli V, Romerosa A. Synthesis, spectroscopy and electrochemistry of new 4-(4-acetyl-5-substituted-4,5-dihydro-1,3,4-oxodiazol-2-yl)methoxy)-2H-chromen-2-ones as a novel class of potential antibacterial and antioxidant derivatives. C R Chimie. 2011;14:548–555. doi: 10.1016/j.crci.2010.11.001. [DOI] [Google Scholar]
- 31.Lu S, Drees M, Yao Y, Boudinet D, Yan H, Pan H, Wang J, Li Y, Usta H, Facchetti A. 3,6-Dithiophen-2-yl-diketopyrrolo[3,2-b]pyrrole (isoDPPT) as an acceptor building block for organic opto-electronics. Macromolecules. 2013;46:3895–3906. doi: 10.1021/ma400568b. [DOI] [Google Scholar]
- 32.Yurttas L, Duran M, Demirayak S, Gencer HK, Tunali Y. Synthesis and initial biological evaluation of substituted 1-phenylamino-2-thio-4,5-dimethyl-1H-imidazole derivatives. Bioorg Med Chem Lett. 2013;23:6764–6768. doi: 10.1016/j.bmcl.2013.10.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. All the copies of FT-IR, 1H NMR, 13C NMR and EI-MS for title compounds 5a–5w.
Data Availability Statement
We have presented all our main data in the form of tables and figures. All the copies of IR, 1H NMR, 13C NMR and EI-MS spectrogram for title compounds 5a–5w were presented in the Additional file 1. The datasets supporting the conclusions of the article are included within the article and the Additional file 1.