Abstract
Irpex lacteus F17 is well-known for its ability to degrade recalcitrant aromatic pollutants, which mainly results from the action of the manganese peroxidase (MnP) that it is able to produce. Recently, the genome sequencing and annotation of this strain provided comprehensive picture of the ligninolytic peroxidase gene family. In addition to revealing the presence of 13 MnPs, genes for five dye-decolorizing peroxidases (DyPs) were also discovered in the I. lacteus F17 genome, which are unrelated to the fungal class II peroxidases. In the present study, amino acid sequences of five DyPs and 13 MnPs, representing two different families of heme peroxidases, were analyzed. Of these, two enzymes, a DyP (Il-DyP4) and a MnP (Il-MnP6) were expressed respectively in Escherichia coli, and were characterized by comparing their molecular models, substrate specificities, and catalytic features. The results showed that Il-DyP4 possessed a higher catalytic efficiency for some representative substrates, and a stronger decolorizing ability to a wide range of synthetic dyes in acidic conditions. Based on electrochemical measurements, Il-DyP4 was found to have a high redox potential of 27 mV at pH 3.5, which was superior to that of Il-MnP6 (− 75 mV), thereby contributing to its ability to oxidize high redox potential substrates, such as veratryl alcohol and polymeric dye Poly R-478. The results highlighted the potential of Il-DyP4 for use in industrial and environmental applications.
Electronic supplementary material
The online version of this article (10.1186/s13568-018-0648-6) contains supplementary material, which is available to authorized users.
Keywords: Irpex lacteus F17, White-rot fungus, Dye-decolorizing peroxidase, Manganese peroxidase, Redox potential, Biotechnological applications
Introduction
Lignocellulose is a structural component of the plant cell wall, mainly comprising cellulose, hemicellulose, and lignin. It is the largest renewable resource in nature and the most promising feedstock for the production of many valuable substances, such as biofuels, chemicals, and materials, from plant sources (Balat 2011). Both cellulose and hemicellulose are carbohydrates, whereas lignin is a highly irregular and insoluble polymer comprising phenylpropanoid units, chemically bonded by mostly ether linkage and carbon–carbon bonds, which are both physically stable and chemically inert (Park et al. 2018). In woody plants, lignin accounts for 20–35% in woody biomass and forms lignin–carbohydrate complexes with cellulose and hemicellulose, resulting in xylem that is extremely hard; however, these complexes are difficult to separate and degrade, thus hindering the conversion and utilization of lignocelluloses (Cheng et al. 2012).
Wood-decaying fungi, one of several groups of lignocellulose decomposers in nature, have an important role in the terrestrial carbon cycle (Kellner et al. 2014). They are common inhabitants of forest litter and fallen trees and have typically been classified as white-rot fungi and brown-rot fungi, according to their ability to biodegrade lignin. Brown-rot fungi are capable of degrading carbohydrates, leaving polyaromatic lignin intact because of their lack of ligninolytic enzymes, whereas white-rot fungi have a ligninolytic system, which can degrade lignin as well as cellulose and hemicelluloses (Morgenstern et al. 2008; Ruiz-Dueñas et al. 2013). Therefore, white-rot fungi have received considerable research because of their extracellular lignolytic enzymes, with a focus on secreted fungal class II peroxidases (PODs), such as lignin peroxidase (LiP; EC 1.11.1.14), manganese peroxidase (MnP; EC 1.11.1.13), and versatile peroxidase (VP; EC 1.11.1.16), as well as other classes of enzyme, such as copper-dependent laccases (Fernández-Fueyo et al. 2014). Thus, two types of fungi can be distinguished by the presence or absence of PODs, which can affect the mode of wood decay adopted by white-rot or brown-rot fungi. However, recently published work, based on the genomic data from 33 species of fungi, suggests that a more nuanced categorization of wood decay modes between white-rot and brown-rot fungi is necessary, because numerous other classes of enzyme were found to be involved in lignin degradation in addition to PODs (Riley et al. 2014). For example, genes encoding PODs are absent in the genomes of Botryobasidium botryosum and Jaapia argillacea genomes, although both species show modes of lignin degradation that are similar to those of some white-rot fungi. Moreover, the secondary metabolites reducing polyketide synthases (R-PKSs) are abundant in white-rot fungi but reduced in brown-rot fungi. In addition, the dye-decolorizing peroxidases (DyPs), a second new heme peroxidase superfamily, were identified in fungi that have been shown to degrade model lignin compounds, and were also widespread in white-rot fungi, such as Auricularia delicate and Trametes versicolor, whereas brown-rot fungi lack DyPs (Floudas et al. 2012, 2015).
DyPs are a newly discovered family of heme peroxidases that are unrelated to well-known peroxidases, such as fungal class II PODs, in terms of their amino acid sequence, tertiary structure, and catalytic residues (Colpa et al. 2014). They have a unique protein structure, as well as a distinct amino acid sequence, with a substrate preference for anthraquinone dyes and high peroxidase activity toward a variety of organic compounds (Sugano 2009). The ability of DyPs to oxidize lignin-related compound and nonphenolic lignin model dimers and their low catalytic efficiency on Mn2+ and veratryl alcohol have also been reported (Kinne et al. 2011; Colpa et al. 2014; Fernández-Fueyo et al. 2015; Linde et al. 2015; Loncar et al. 2016). All DyPs contain an iron protoporphyrin IX (heme cofactor) as a prosthetic group and the GXXDG motif in their primary sequence as a part of the heme-binding region (Colpa et al. 2014). They also share a conserved spatial structure and a unique ferredoxin-like fold containing α-helices and antiparallel β-sheets (Linde et al. 2015). Nevertheless, the physiological function of these enzymes is unclear, although they exhibit the biotechnological potential (Colpa et al. 2014; van Bloois et al. 2010).
Irpex lacteus F17 is an indigenous white-rot basidiomycete isolated by our laboratory that efficiently decolorizes and degrades different synthetic dyes, some of which are recalcitrant for their structural diversity (Zhao et al. 2015; Yang et al. 2016). Initially, this strain was named Schizophyllum sp. F17 based on the morphological characteristics of its fruiting body (Cheng et al. 2007) and recognized as I. lacteus F17 after identifying the internal transcribed spacer in its nucleotide sequence (GenBank: JQ403610.3.2) and analyzing its phylogenetic tree (Chen et al. 2015). Recently, genome sequencing of I. lacteus F17 revealed that the genome encodes 14 Class II PODs and five DyPs. The 14 PODs enzymes include one LiP and 13 putative MnPs. Phylogenetic analysis based on 18S rRNA sequences showed that I. lacteus F17 was closely related to Ceriporiopsis subvermispora and Phanerochaete chrysosporium (Yao et al. 2017). Interestingly, the genome of C. subvermispora also encodes 13 MnPs, whereas it has no genes encoding DyPs, similar to P. chrysosporium (Fernández-Fueyo et al. 2012; Floudas et al. 2012).
Research has focused mainly on MnPs from I. lacteus, probably because of their abundance in the genome of this species (Yao et al. 2017). MnPs from I. lacteus are important in the degradation of polycyclic aromatic hydrocarbons and are able to efficiently decolorize different groups of dye (Novotný et al. 2001; Kasinath et al. 2003; Baborová et al. 2006). Recent research indicated that two MnPs from I. lacteus CD2 were able to degrade veratryl alcohol, a nonphenolic lignin compound (Qin et al. 2017). The ability of MnP purified from I. lacteus F17 to degrade synthetic dyes has also been shown (Chen et al. 2015). However, little is known about the catalytic properties of DyP from I. lacteus. To the best of our knowledge, only a wild DyP from I. lacteus grown in liquid medium, has been isolated and characterized (Salvachúa et al. 2013). Thus, we selected a DyP gene (No. 8293, GenBank: MG 209114) from the I. lacteus F17 genome and expressed it in Escherichia coli, and compared it with a MnP gene (No. 6398, GenBank: MG 209112) from the same genome. Any similarities and differences between the two enzymes in terms of their sequence homologies, molecular structures, biochemical and spectroscopic properties, as well as their ability to decolorize different types of dye, were investigated. The results of this study will further reveal the catalytic characteristics of DyPs from I. lacteus F17 to provide a better understanding of the lignin degradation potential of this strain.
Materials and methods
Regents and expression vectors
Dithiothreitol (DTT), glutathione (GSSG), phenylmethanesulfonyl fluoride (PMSF), and isopropyl β-d-thiogalactopyranoside (IPTG) were from Sigma-Aldrich (St. Louis, MO, USA). Reactive Blue 19 (RBlue 19), reactive black 5 (RBlack 5), veratryl alcohol (VA), 2,6-dimethoxyphenol (DMP), ethylene diamine tetraacetic acid (EDTA), and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Aladdin industrial corporation (Shanghai, China).
The E. coli expression vector pET28a(+) and the E. coli expression host Rosetta (DE3) were purchased from Invitrogen (Carlsbad, CA, USA) and TransGen Biotech (Beijing, China), respectively.
All restriction enzymes and DNA polymerases were purchased from TaKaRa (Ostu, Japan). Unless otherwise specified, all other reagents used were from Macklin (Shanghai, China) and were of analytical grade.
Strain and genome
The strain of I. lacteus F17 was cultured on potato dextrose agar (PDA) medium (200 g L−1 of potato extract, 20 g L−1 of glucose, and 20 g L−1 of agar) for 5 days at 28 °C and then preserved at 4 °C.
I. lacteus F17 was deposited at the China Center for Type Culture Collection (CCTCC) under the accession number of CCTCC AF 2014020. The whole-genome shotgun project has been submitted to GenBank under the accession number MQVO00000000 (http://www.ncbi.nlm.nih.gov).
DyP and MnP screening of the I. lacteus F17 genome
The DyP and MnP coding genes in I. lacteus F17 were screened as follows. First, the annotated genome was automatically searched in the NCBI database; second, multiple alignments were performed using Clustal X to compare the deduced amino acid sequences with related peroxidases; finally, the presence of characteristic residues at the heme pocket and substrate oxidation sites were confirmed.
Five DyP genes and 13 MnP genes were identified in the genome of I. lacteus F17. The cDNA sequences encoding the 18 mature proteins were submitted to GenBank: Il-DyP1 (MH120197), Il-DyP2 (MH120198), Il-DyP3 (MH120199), Il-DyP4 (MG209114), Il-DyP5 (MH120196), as well as Il-MnP1 (KC811382), Il-MnP2 (MH120200), Il-MnP3 (MH120201), Il-MnP4 (MH120202), Il-MnP5 (MH120203), Il-MnP6 (MG209112), Il-MnP7 (MH120204), Il-MnP8 (MH120205), Il-MnP9 (MH120206), Il-MnP10 (MH120207), Il-MnP11 (MH120208), and Il-MnP12 (MH120209), Il-MnP13 (MH120210).
Based on Protein Blast on NCBI, sequence identity analyses of five DyPs and 13 MnPs in the I. lacteus F17 were performed. In addition, homology modeling was performed on the basis of the SWISS MODEL server to obtain the templates of crystal structure with the highest identity in the protein database. The templates were then applied to PyMOL to simulate the three-dimensional structure of the proteins and to superimpose the heme, important amino acids, and ions.
Gene clone and expression vector construction
Mycelia of I. lacteus F17 were cultured in compound potato dextrose agar (CPDA) liquid medium at 28 °C at 120 rpm and harvested on the fourth day. Total RNA was extracted as reported by Koo et al. (1998). The total RNA was then reverse transcribed to cDNA.
Il-DyP4 primers DyPf and DyPr (Additional file 1: Table S1) were designed. The above cDNA of I. lacteus F17 was used as a template and polymerase chain reactions (PCR) were performed with Taq DNA polymerase. The PCR products were detected by gel electrophoresis in 0.8% agarose gels, stained using SuperRed/GelRed, and subsequently sequenced. Two primers were designed at the 5′ and 3′ ends of the Il-DyP4 gene with the following sequences: NcoI-DyPf and XhoI-DyPr, which contained the restriction sites NcoI and XhoI (underlined). The resulting PCR product was gel-purified, digested with NcoI and XhoI, and cloned into the corresponding site of the pET28a(+) vector to construct the recombinant plasmid pET28a-Il-DyP4. The plasmid was transformed into chemically competent E. coli Rosetta (DE3) cells and then confirmed by DNA sequencing. The gene clone and expression strain of Il-MnP6 were constructed using the same method (see Additional file 1: Table S1 for primers).
Il-DyP4 and Il-MnP6 expression in E. coli
A positive transformant harboring pET28a-DyP4 was isolated as a single colony for gene expression. The transformant was cultured overnight at 37 °C in 5 mL LB medium containing 50 μg mL−1 kanamycin and 34 μg mL−1 chloramphenicol. The culture was then inoculated into fresh LB medium 400 mL containing kanamycin and chloramphenicol and grown at 37 °C and 200 rpm to an OD600 of approximately 0.6. IPTG was then added to a final concentration of 0.5 mM for induction for 4 h, bacterial pellets were obtained by centrifugation at 4 °C at 12,000×g for 10 min, and were resuspended in lysis buffer (0.02 mM PMSF, 50 mM Tris–HCl, and pH 7.5) in a quarter of the original culture volume. Bacteria were then lysed by sonication for 45 min. The lysed cells were centrifuged at 4 °C at 12,000×g for 30 min to obtain precipitation.
The Il-MnP6 protein was expressed according to the method of Chen et al. (2015).
In vitro activation of Il-DyP4 and Il-MnP6
Il-DyP4 was expressed in the form of inclusion bodies and was solubilized in 10 mL 50 mM Tris–HCl (pH 8.0) containing 8 M urea, 1 mM EDTA, for 3 h at 4 °C to complete solubilization of the Il-DyP4 polypeptide. The refolding reaction system included 2 mM EDTA, 20 μM hemin, and 1 M urea, and had a pH of 6.0.
To investigate the optimal activation conditions for Il-DyP4 protein refolding, 0.2 mL protein (1.2 mg mL−1) was taken and optimized in 5-mL eppendorf tubes. Variable parameters include pH (1.0–8.0), EDTA (0–8 mM), hemin (0–60 μM), and urea (0.1–2.5 M). Refolding was performed at 4 °C for 24 h.
Larger-scale refolding assays were performed using the optimized conditions found in the small-scale experiments. The supernatant, including active Il-DyP4, was dialyzed against 10 mM sodium acetate (pH 6) for subsequent purification. The insoluble material was eliminated by centrifugation at 12,000×g for 30 min.
The refolding results were evaluated by measuring the Il-DyP4 residual enzyme activity using ABTS as reducing substrate according to the enzyme activity assay. Similarly, recombinant Il-MnP6 was expressed in the form of inclusion bodies. In vitro refolding was performed according to the method of Wang et al. (2016).
Il-DyP4 and Il-MnP6 activity assays
Il-DyP4 activity was estimated spectrophotometrically by the oxidation of 1.25 mM ABTS to its cation radical (ε418 = 36,000 M−1 cm−1) in 100 mM sodium tartrate buffer at pH 3.5 at 25 °C.
Il-MnP6 activity was determined using two substrates. The first was determined based on the oxidation of Mn2+ to Mn3+ at 25 °C and 240 nm (ε240 = 6500 M−1 cm−1) at pH 4.5 in 0.11 M sodium lactate buffer (Salvachúa et al. 2013). The second was followed by the oxidation of 1.25 mM DMP to its cation radical (ε469 = 27,500 M−1 cm−1) in 100 mM sodium tartrate buffer containing 1 mM MnSO4, at pH 4 at 55 °C.
In all cases, peroxidase activity assays were carried out in the presence of 0.1 mM H2O2 and were carried out in triplicate. Control treatments without H2O2 and/or without enzyme were also performed. One unit (U) of peroxidase oxidized 1 μmol of substrate/min, and units were calculated based upon U mg−1 of protein per mL−1 of enzyme solution.
Purification of DyP and MnP and mass characterization
Refolded solutions containing recombinant Il-DyP4 and Il-MnP6 were purified using Ni–NTA affinity chromatography. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a 12% Tris–HCl separation gel, and samples were then stained with Coomassie Brilliant Blue R-250.
Furthermore, purified proteins were identified by mass spectrometry. First, purified recombinant Il-DyP4 and Il-MnP6 proteins were excised from the gel and digested with trypsin. Subsequently, the resulting peptide mixtures were analyzed by liquid chromatography–mass spectrometry (LC–MS) using a Proteome X-LTQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). All obtained peptides were compared with the predicted amino acid sequence of Il-DyP4 and Il-MnP6.
Effect of pH and temperature on DyP and MnP activity and stability
The optimum pH for substrate oxidation activity of Il-DyP4 and Il-MnP6 was estimated at 25 °C in 0.11 M citrate–phosphate buffer (pH 2.2–8.0) or Tris–HCl buffer (pH 8.6–9.0). To evaluate the pH stability, the enzymes were incubated at 4 °C for 12 h at different pH (2.2–9.0).
The optimum temperature for Il-DyP4 activity was measured from 0 to 60 °C in appropriate increments in 0.1 M sodium tartrate buffer (pH 3.5). The optimum temperature for Il-MnP6 activity was measured from 0 to 85 °C in appropriate increments in 0.11 M sodium lactate buffer (pH 4.5). To evaluate the thermal stability of Il-DyP4 and Il-MnP6, the enzymes were incubated at temperatures ranging from 4 to 65 °C in appropriate increments for 12 h.
The residual enzyme activities of Il-DyP4 and Il-MnP6 were determining using ABTS and Mn2+, respectively, as substrates.
Substrate specificities
Eight different substrates [H2O2, ABTS (ε418 = 36,000 cm−1 M−1), Mn2+ (ε240 = 6500 cm−1 M−1), DMP (ε469 = 27,500 cm−1 M−1), guaiacol (ε456 = 12,100 cm−1 M−1), VA (ε310 = 9300 cm−1 M−1), RBlue19 (ε595 = 10,000 cm−1 M−1), RBlack 5 (ε598 = 30,000 cm−1 M−1)] were used to study the substrate specificities of Il-DyP4 and Il-MnP6 in 0.1 M sodium tartrate. The reactions were initiated by the addition of 0.1 mM H2O2. In the MnP reaction system, 1 mM Mn2+ was added. The oxidation activity for these substrates was measured based on the molar absorbance of the corresponding reaction product at the specific wavelength.
Steady-state kinetic constants of Il-DyP4 or Il-MnP6 were determined spectrophotometrically using a UV–visible spectrophotometer (Shanghai, China). First, the effect of pH and temperature on the activity for oxidation of eight different substrates by both enzymes were investigated, respectively, and then the optimal values were used to determine their kinetic constants (Km, kcat and kcat/Km) by the hyperbolic, non-linear least squares method.
H2O2 stability and the effects of compounds on enzyme activity
The H2O2 stability of both enzymes was determined by incubating 25 nM of enzyme for 30 min at 25 °C in the presence of increasing H2O2 enzyme concentrations (0–10 mM). The residual activities were then monitored using the method for assaying enzyme activity.
Additionally, under the specified enzyme activity measurement conditions, Il-DyP4 used ABTS as a substrate, whereas Il-MnP6 used DMP as a substrate. Residual enzyme activity was determined in the presence and absence of three different concentrations (0.1, 1, and 10 mM) of different cations (Co2+, Ba2+, Cd2+, Mn2+, Na+, Ca2+, Mg2+, Zn2+, Ni2+, Cu2+, Fe2+, Fe2+, and Al3+), thiourea, EDTA, DTT, and β-mercaptoethanol. Enzymes were incubated with these compounds for 5 min before the activity measurement.
Spectroscopic characterization of Il-DyP4 and Il-MnP6
Far-UV CD spectra measurements were implemented with a MOS-500 CD spectrometer (Bio-Logic, Grenoble, France), with a 1-mm light path cell. The protein concentration was 0.1 mg mL−1 in 0.15 M phosphate buffer at pH 6.5. The CD spectra were recorded using a 2-mm bandwidth in the far-UV region (190–250 nm) at room temperature.
UV–Vis spectra were obtained in the 200–700 nm region at room temperature using a DU 730 UV spectrophotometer (Beckman Coulter, Brea, CA, USA); the Il-DyP4 concentration was 0.58 mg mL−1 in 0.1 M phosphate buffer at pH 6.0 and that of Il-MnP6 was 1.26 mg mL−1 in 0.1 M sodium acetate buffer at pH 5.9.
Il-DyP4 and Il-MnP6 electrochemical experiments
Cyclic voltammetry (CV) was used to determine the redox potential of Il-DyP4 and Il-MnP6. Pyrolytic graphite electrode (PGE), platinum wire, and Ag/AgCl were used as the working electrode, the counter electrode, and reference electrode, respectively (Mendes et al. 2015). The first step the PGE surface was polished with Al2O3 on sandpaper (2000 mesh); secondly, the electrode was thoroughly ultrasonicated with water and ethanol for 5 min each; thirdly, 5 μg protein was dropped onto the PGE, and maintained at 4 °C for 20 h to allow the protein to fully adsorb to the electrode surface. Finally, the electrode was cleaned with distilled water, measured for a scan rate at 50 mV s−1, and the potential was then cycled between − 1.0 and + 0.6 V.
Dye decolorization
The decolorization ability of Il-DyP4 or Il-MnP6 towards five different classes of dye (anthraquinone dyes, azo dyes, phenazine dyes, triphenylmethane dyes, and aniline dyes) was measured using a spectrophotometer. Reaction mixtures contained 100 mM sodium tartrate buffer (pH 3.5, 4.0 or 4.5), 25 nM of purified enzyme and dyes (at a concentration of 25–200 μM); the concentration ratio of H2O2/dye was 4:1. Besides, 1 mM Mn2+ was supplemented in the Il-MnP6 reaction system. Reactions were subsequently performed for 30 min at 35 °C, and were then examined for the level of decolorization by the two enzymes.
Decolorization assays of polymeric dye Poly R-478 were performed to reveal the ability of Il-DyP4 or Il-MnP6 to degrade lignin. The reaction mixture contained sodium tartrate buffer (100 mM), Poly R-478 dye (0.01%), and Il-DyP4 (100 nM) or Il-MnP6 (100 nM) in a total volume of 1 mL (MnP reaction system added 1 mM Mn2+). The reaction was initiated by the addition of H2O2 (0.2 mM) and the mixture was incubated at different pH conditions at 35 °C for 30 min. Dye decolorization was measured using a spectrophotometer at 520 nm, which is the maximum visible absorbance of Poly R-478. Control samples, without enzyme or H2O2, were done in parallel under identical conditions.
The decolorization percentage was calculated according to Eq. 1.
| 1 |
where A0 is the initial absorbance at λmax (nm) and At refers to the absorbance at λmax (nm) at reaction time t. The data were the mean values of triplicate experiments.
Results
DyP and MnP cDNAs analyses
Amino acid sequence alignments of five DyPs (Il-DyP1–5: accession numbers MH120197, MH120198, MH120199, MG209114, and MH120196, respectively) from I. lacteus F17 displayed typical characteristic GXXDG conserved motifs (Additional file 1: Fig. S1). Some crucial role of the conserved amino acid residues were shown in colors, such as the proximal histidine residue as well as the distant aspartic acid and arginine residues. There was a 57–83% shared sequence identity among the five DyPs (Additional file 1: Table S2). Moreover, alignment analysis of conserved amino acid sites in 13 MnPs (Il-MnP1–13: KC811382, MH120200, MH120201, MH120202, MH120203, MG209112, MH120204, MH120205, MH120206, MH120207, MH120208, MH120209, and MH120210, respectively) from I. lacteus F17 was also performed (Additional file 1: Fig. S2). Likewise, some crucial role of the conserved amino acid residues was shown in colors, including Mn2+-binding and Ca2+-binding amino acid ligands etc. There was a 49–99% shared sequence identity among the 13 MnPs (Additional file 1: Table S3).
To understand the essential characteristics and biochemical properties of these two different types of enzyme, we tested DyPs and MnPs expression levels in I. lacteus F17 by culturing this organism in CPDA liquid shake cultures (28 °C). Genes encoding Il-DyP4 and Il-MnP6 were found to be the predominant expressed genes. Therefore, Il-DyP4 (MG209114) and Il-MnP6 (MG209112) were selected for further research.
According to the ExPASy (Gasteiger et al. 2003) server prediction online, Il-DyP4 contained 502 amino acids, with a molecular weight of 54.5 kDa and a theoretical pI of 5.06. The signal peptide of Il-DyP4 was confirmed to contain 52 amino acids (Salvachúa et al. 2013). In addition, Il-DyP4 showed 58% identity with BadDyP (GenBank: BAA77283.1) from Bjerkandera adusta, 42% identity with Pleurotus ostreatus DyP (GenBank: CAK55151.1), 40% identity with T. versicolor DyP (GenBank: EIW57847.1), and < 33% identity with DyPs from bacteria, such as E. coli (PDB 5GT2_A), Shewanella oneidensis (PDB 2HAG_A), and Bacteroides thetaiotaomicron (PDB 2GVK_A). According to the ExPASy server, Il-MnP6 contained 359 amino acids, with a molecular weight of 38.2 kDa and a theoretical pI of 4.94. Using SignalP 4.1, the signal peptide of Il-MnP6 was predicted to be 23 amino acids long. In addition, Il-MnP6 showed 73% identity with MnP3 (GenBank: CAD92855.1) from Phlebia radiata, 72% identity with MnP (GenBank: CAG33918.4) from T. versicolor, and 55% identity with MnP (GenBank: AAB30859.1) from P. chrysosporium. Moreover, Il-MnP6 contained eight cysteines, and formed four disulfide bonds, and its amino acid sequence revealed an 89% identity with previously reported Il-MnP1 (GenBank:AGO86670.2) from I. lacteus F17, indicating that it is a short-type MnP (Chen et al. 2015). As such, the other 11 MnPs in I. lacteus F17 genome also belonged to the short MnP subfamily, based on the analyses of their amino acid sequences.
To analyze the structural properties of the two enzymes, molecular models of the deduced mature proteins were built (Additional file 1: Fig. S3). For Il-DyP4, the crystal structure of B. adusta DyP (PDB 3afv) (sequence identity 62.19%) was used as a template. For Il-MnP6, the crystal structure of Pleurotus eryngii VP (PDB 2boq) was used as a template (sequence identity 70.61%). In addition, the presence of the amino acids residues mentioned above was confirmed in the two structural models (Additional file 1: Fig. S3).
Expression and in vitro activation of Il-DyP4 and Il-MnP6
The cDNA sequences encoding Il-DyP4 and Il-MnP6 were amplified by PCR and inserted into the expression vector pET28a(+). The resulting recombinant constructs were then transformed into E. coli Rosetta (DE3) cells for heterologous expression.
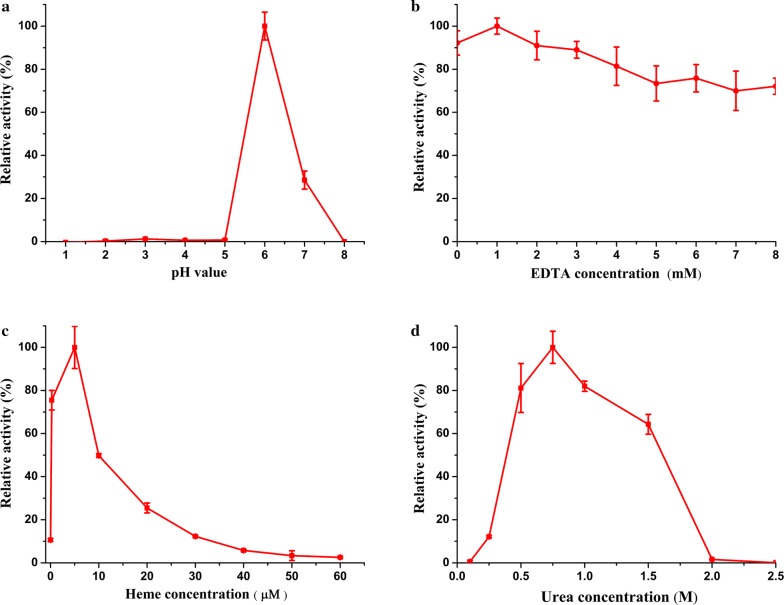
The optimum enzyme activity achieved for Il-DyP4 was 10.8 U mg−1, using the following optimal refolding conditions: 1 mM EDTA, 5 μM hemin, 0.75 M urea in 50 mM phosphate (pH 6) (Fig. 1a–d).
Fig. 1.
The effect of several parameters on the refolding of recombinant Il-DyP4. a pH, b EDTA concentration, c hemin concentration, and d urea concentration were systematically examined. All reactions were performed with 1.2 mg mL−1 protein in 50 mM phosphate buffer. The reactions took place in the dark for 24 h at 4 °C. The residual activity of Il-DyP4 was measured according to enzyme activity assay. Deviation values are standard deviations based on triplicate determinations
The optimum enzyme activity of Il-MnP6 was 2 U mg−1, using the following optimal refolding conditions: 1.5 M urea, 150 mM CaCl2, 25 μM hemin, 10% glycerol, 0.5 mM GSSG, 0.05 mM MnSO4, 20 mM KCl in 50 mM Tris–HCl (pH 8.5).
Purification of Il-DyP4 and Il-MnP6 and mass characterization
Two recombinant proteins with six His-tags at the C terminus were purified using Ni–NTA affinity chromatography, and exhibited a single band on SDS-PAGE (Additional file 1: Fig. S4). Purified Il-DyP4 had a molecular weight of 54.0 kDa, close to the 54.5 kDa predicted. The final recovery, which was obtained from the total activity, was approximately 85% (Table 1). The specific activity of purified Il-DyP4 was 140 U mg−1. Purified Il-MnP6 had a molecular weight of 44.6 kDa, close to the 38.2 kDa predicted (Additional file 1: Fig. S4). The final recovery of Il-MnP6 was almost 60% (Table 1). The specific activity of purified Il-MnP6 was 23.8 U mg−1.
Table 1.
Isolation and purification of Il-DyP4 and Il-MnP6 from inclusion bodies
| Sample | Protein concentration (mg mL−1) | Protein (mg) | Specific activity (U mg−1) | Total activity (U) | Yield (%) | |
|---|---|---|---|---|---|---|
| DyP | Inclusion body | 8.78 | 87.8 | |||
| Dialyzed | 0.24 | 28.2 | 10.8 | 305 | 100 | |
| Ni–NTA | 0.0093 | 1.87 | 140 | 261 | 86 | |
| MnP | Inclusion body | 4.45 | 22.2 | |||
| Dialyzed | 0.23 | 8.1 | 2 | 16.2 | 100 | |
| Ni–NTA | 0.0082 | 0.41 | 23.8 | 9.8 | 60 |
The purified Il-DyP4 was analyzed by mass spectrometry. These peptides accurately matched the deduced amino acid sequence of Il-DyP4 (see Additional file 1: Table S4). The first fragment (MGSAGNDSLPFENIQGDILVGMKK) was the N-terminus amino acid sequence, which has 100% identity with the native I. lacteus DyP (Salvachúa et al. 2013).
Similarly, the purified Il-MnP6 was also analyzed by mass spectrometry. These peptides accurately matched the deduced amino acid sequence of Il-MnP6 (see Additional file 1: Table S5). Thus, these data confirmed the successful expression of Il-DyP4 and Il-MnP6 in E. coli Rosetta (DE3).
Effects of pH and temperature on the activity and stability of Il-DyP4 and Il-MnP6
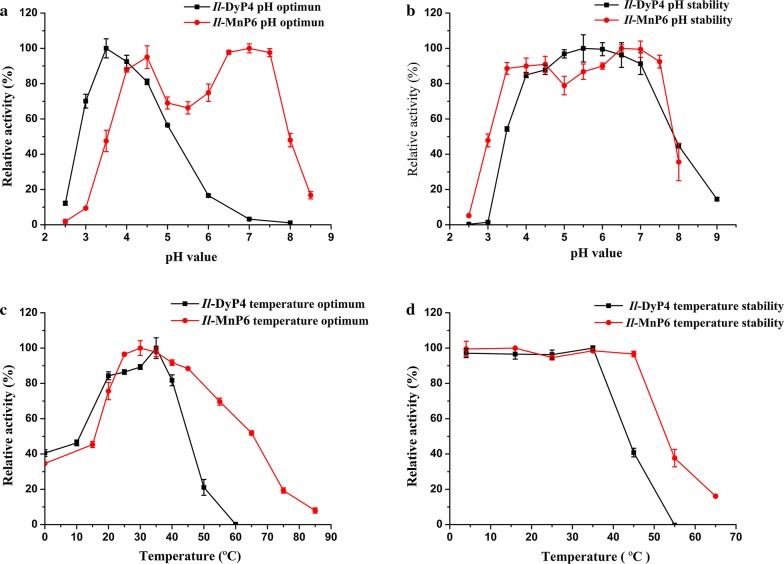
The optimal pH of Il-DyP4 for ABTS oxidation was 3.5 (Fig. 2a). During incubation of Il-DyP4 in different pH buffers for 12 h, the enzyme retained more than 80% of its enzymatic activity at pH 4–7 and was most stable at pH 5.5 (Fig. 2b). Unlike Il-DyP4, the optimum pH of Il-MnP6 showed double peaks (4.5 and 7.0), and used Mn2+ as a substrate. Il-MnP6 also retained > 75% of its enzymatic activity at a wider range of pH (3.5–7.5), indicating that the pH stability of Il-MnP6 was superior to that of Il-DyP4.
Fig. 2.
Effects of pH and temperature on the activity and stability of Il-DyP4 and Il-MnP6. And Il-DyP4 and Il-MnP6 activities at the beginning were considered to be 100%. Deviation values are standard deviations based on triplicate determinations. a The pH optimum of Il-DyP4 and Il-MnP6. The Il-DyP4 and Il-MnP6 activities were determined in the citrate–phosphate buffer, pH (2.2–8.0) and Tris–HCl buffer (pH 8.5) at 25 °C, respectively. b The pH stability of Il-DyP4 and Il-MnP6. Il-DyP4 and Il-MnP6 was incubated for 12 h at 25 °C in various pH values in citrate–phosphate (2.2–8.0) or Tris–HCl buffer (9.0). The residual activity of Il-DyP4 and Il-MnP6 was measured according to their enzyme activity assay. c The temperature optimum of Il-DyP4 and Il-MnP6. The enzyme reaction of Il-DyP4 was performed in 0.1 M sodium tartrate buffer, pH 3.5 at 0–60 °C. The enzyme reaction of Il-MnP6 was performed in 0.11 M sodium lactate buffer, pH 4.5 at 0–85 °C. d The temperature stability of Il-DyP4 and Il-MnP6. Il-DyP4 and Il-MnP6 were incubated for 12 h at 4–65 °C. The residual activity of Il-DyP4 and Il-MnP6 was measured according to their enzyme activity assay
Il-DyP4 exhibited the highest enzymatic activity at 35 °C and retained 90% of its enzymatic activity after incubation at temperatures below 35 °C for 12 h (Fig. 2c, d). Il-MnP6 showed maximal enzymatic activity at 30 °C and retained > 90% of its enzymatic activity below 45 °C for 12 h.
Substrate specificities of Il-DyP4 and Il-MnP6
Significant differences were recorded in the substrate specificities of Il-DyP4 and Il-MnP6 on eight representative substrates (Table 2). For heme-containing peroxidases, H2O2 was used as an electron-accepting substrate to oxidize a variety of compounds. The results showed that the catalytic efficiency of Il-DyP4 in the reduction of H2O2 was an order of magnitude higher than that of Il-MnP6. For ABTS oxidation, the catalytic efficiency (kcat/Km) of Il-DyP4 was up to 1.3 × 108 s−1 M−1, two orders of magnitude higher than that of Il-MnP6. For Mn2+ oxidation, which is often used as a substrate for MnP, the catalytic efficiency (kcat/Km) of Il-DyP4 was 3.0 × 105 s−1 M−1, which was an order of magnitude lower than that of Il-MnP6. Phenolic substrates, such as DMP and guaiacol, which were commonly used as universal substrates for heme peroxidase, displayed similar catalytic efficiency for Il-DyP4 and Il-MnP6. VA, a nonphenolic compound, was also assayed, revealing the catalytic efficiency of Il-DyP4 to be 5.2 × 103 s−1 M−1, although no activity was detected for Il-MnP6 in 0.1 M sodium tartrate, pH 3.5. For two dyes tested, the catalytic efficiency of Il-DyP4 oxidizing anthraquinone dye RBlue 19 was 4.0 × 107 s−1 M−1, which was three orders of magnitude higher than that of Il-MnP6. The catalytic efficiency of Il-DyP4 oxidizing azo dyes RBlack 5 was 1.7 × 106 s−1 M−1, which was two orders of magnitude higher that of Il-MnP6.
Table 2.
Kinetic parameters—Km (μM), kcat (s−1), and kcat/Km (s−1M−1) of Il-DyP4 and Il-MnP6 for substrates oxidation obtained based on the optimal pH and temperature (°C)
| Il-DyP4 | Il-MnP6 | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | Temperature | Kinetic constants | pH | Temperature | Kinetic constants | |||
| H2Oa2 | 3.5 | 35 | K m | 163 ± 20.8 | 4.5 | 30 | K m | 194 ± 18 |
| k cat | (1.2 ± 0.08) × 104 | k cat | 879 ± 48 | |||||
| kcat/Km | (7.6 ± 0.5) × 107 | kcat/Km | (4.5 ± 0.3) × 106 | |||||
| ABTS | 3.5 | 35 | K m | 62 ± 11 | 3.5 | 45 | K m | 83 ± 6.5 |
| k cat | 8356 ± 747 | k cat | 245 ± 7 | |||||
| kcat/Km | (1.3 ± 0.1) × 108 | kcat/Km | (3.0 ± 0.09) × 106 | |||||
| Mn2+ | 4.5 | 35 | K m | 2687 ± 463 | 4.5 | 30 | K m | 129 ± 8 |
| k cat | 806 ± 94 | k cat | 369 ± 6.1 | |||||
| kcat/Km | (3.0 ± 0.4) × 105 | kcat/Km | (2.9 ± 0.3) × 106 | |||||
| DMP | 4 | 35 | K m | 58 ± 3 | 4.5 | 55 | K m | 53 ± 8.6 |
| k cat | 4896 ± 131 | k cat | 989 ± 100 | |||||
| kcat/Km | (8.4 ± 0.2) × 107 | kcat/Km | (1.9 ± 0.2) × 107 | |||||
| Guaiacol | 4 | 45 | K m | 24 ± 7.5 | 4 | 45 | K m | 2.6 ± 0.6 |
| k cat | 413 ± 34 | k cat | 184 ± 7 | |||||
| kcat/Km | (1.7 ± 0.1) × 107 | kcat/Km | (7.1 ± 0.3) × 107 | |||||
| VA | 3.5 | 45 | K m | (2.1 ± 0.98) × 104 | –b | – | K m | –c |
| k cat | 108 ± 47 | k cat | – | |||||
| kcat/Km | (5.2 ± 2.3) × 103 | kcat/Km | – | |||||
| RBlue 19 | 4.5 | 35 | K m | 133 ± 34 | 3 | 35 | K m | 81 ± 39.5 |
| k cat | 5345 ± 921 | k cat | 7.3 ± 2.6 | |||||
| kcat/Km | (4.0 ± 0.7) × 107 | kcat/Km | (9.0 ± 3.2) × 104 | |||||
| RBlack 5 | 4 | 35 | K m | 159 ± 61 | 3 | 35 | K m | 4.4 ± 0.7 |
| k cat | 267 ± 96 | k cat | 0.06 ± 0.003 | |||||
| kcat/Km | (1.7 ± 0.6) × 106 | kcat/Km | (1.5 ± 0.07) × 104 | |||||
Means and 95% confidence limits
aActivities were measured with 1.25 mM ABTS and 1 mM Mn2+ by Il-DyP4 and Il-MnP6, respectively
bNot detected
cExperimental condition: [VA] = 1 mM, [MnP] = 25 nM, [H2O2] = 0.1 mM, [MnSO4] = 1.0 mM, [Tartrate buffer] = 100 mM, pH = 3.5, temperature = 45 °C, reaction time = 60 min
Effects of H2O2 and chemicals on Il-DyP4 and Il-MnP6 activity
In general, fungal heme-containing peroxidases depend on H2O2 to start the catalytic cycle of the reaction, although the enzymes are prone to inactivation in the presence of excess H2O2. The H2O2 stability of Il-DyP4 and Il-MnP6 showed that the enzyme activity of Il-DyP4 was reduced by 60% as the H2O2 concentration increased from 0.1 to 4 mM, whereas the enzyme activity of Il-MnP6 was reduced by 10%. However, when the H2O2 concentration was increased from 4 to 10 mM, the enzyme activity of Il-DyP4 was reduced by only 10%, whereas 80% of the enzymatic activity of Il-MnP6 was lost rapidly. Therefore, Il-DyP4 was less resistant to low H2O2 concentrations, and more resistant to high H2O2 concentrations, and vice versa for Il-MnP6.
The effects of three concentrations of several metal ions and other compounds on Il-DyP4 and Il-MnP6 were also assayed (Table 3). For Mn2+, Na+, Mg2+, Zn2+, Ni2+, Cu2+, and Al3+, no loss of enzyme activity was observed for Il-DyP4 and Il-MnP6 within the tested concentrations. When the concentrations of Co2+ and Ca2+ were increased to 10 mM, Il-DyP4 was significantly inhibited, whereas no significant effect was observed on Il-MnP6. Both Fe3+ and Fe2+ significantly inhibited the activity of both enzymes, except at low concentrations of Fe3+ (0.1 mM).
Table 3.
Effects of different chemicals on the activity of Il-DyP4 and Il-MnP6
| Chemicals | Il-DyP4a | Il-MnP6b | ||||
|---|---|---|---|---|---|---|
| Relative enzyme activity (%) | Relative enzyme activity (%) | |||||
| 0.1 mM | 1 mM | 10 mM | 0.1 mM | 1 mM | 10 mM | |
| Co2+ | 97 | 76 | 21 | 107 | 107 | 111 |
| Ba2+ | 101 | 103 | 88 | 106 | 95 | 115 |
| Cd2+ | 100 | 103 | 87 | 103 | 98 | 51 |
| Mn2+ | 101 | 100 | 102 | 67 | 100 | 106 |
| Na+ | 99 | 93 | 96 | 103 | 96 | 98 |
| Ca2+ | 87 | 79 | 47 | 94 | 97 | 90 |
| Mg2+ | 102 | 100 | 102 | 93 | 105 | 108 |
| Zn2+ | 88 | 86 | 72 | 91 | 93 | 85 |
| Ni2+ | 100 | 106 | 102 | 98 | 97 | 92 |
| Cu2+ | 104 | 109 | 98 | 96 | 98 | 50 |
| Fe2+ | 8 | 9 | 1 | 19 | 0 | 0 |
| Fe3+ | 104 | 13 | 2 | 100 | 37 | 0 |
| Al3+ | 107 | 107 | 69 | 100 | 91 | 22 |
| Thiourea | 99 | 93 | 33 | 95 | 82 | 19 |
| EDTA | 99 | 107 | 87 | 93 | 8 | 4 |
| DTT | 99 | 1 | 0 | 25 | 0 | 0 |
| β-Mercaptoethanol | 92 | 45 | 0 | 15 | 0 | 0 |
aABTS oxidation by the Il-DyP4
bDMP oxidation by the Il-MnP6
DTT, thiourea, and β-mercaptoethanol had little effect on Il-DyP4 activity at lower concentrations (0.1 mM), whereas Il-MnP6 activity was completely or partially inhibited when using 0.1 mM and 1 mM of these compounds. There was no change in Il-DyP4 activity at the three EDTA concentrations tested, whereas Il-MnP6 activity declined rapidly.
Spectroscopic characterization of Il-DyP4 and Il-MnP6
Circular dichroism (CD) spectra measurement in the far-UV region is often used to observe the secondary structures of proteins. The far-UV CD spectra of Il-DyP4 and Il-MnP6 exhibited two negative ellipticity bands at 208 and 222 nm, respectively (Additional file 1: Fig. S5), indicating α-helical structure characteristics, but with significant differences in content. The α-helix and β-sheet contents of Il-DyP4 and Il-MnP6 were calculated using the K2D program. The results showed that the α-helical content of Il-DyP4 (≥ 9%) was lower than that of Il-MnP6 (≥ 17%), whereas the β-sheet content of Il-DyP4 (≥ 43%) was higher than that of Il-MnP6 (≥ 31%).
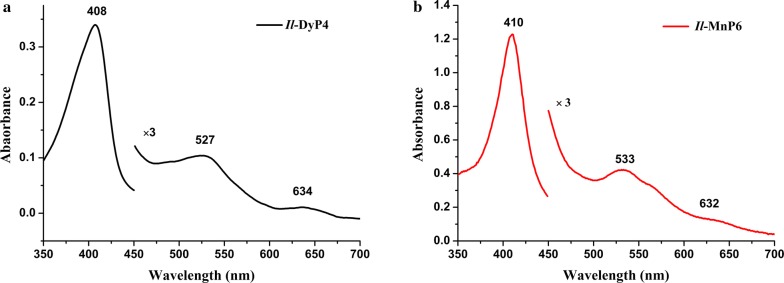
The UV–Vis spectrum of Il-DyP4 (Fig. 3a) was similar to that of Il-MnP6 (Fig. 3b). Il-DyP4 exhibited a Soret peak at 408 nm, a Q band at 527 nm, and a charge transfer (CT) band at 634 nm, while Il-MnP6 showed a Soret peak at 410 nm, a Q band at 533 nm, with a narrow CT band at 632 nm. The Reinheitszahl (RZ) values (calculated from the A408/A280 and A410/A280 absorption ratios) for Il-DyP4 and Il-MnP6 were 1.5 and 3.17, respectively.
Fig. 3.
a The UV–Vis absorbance spectra of Il-DyP4. b The UV–Vis absorbance spectra of Il-MnP6. The region between 450 and 700 nm has been expanded (×3 absorbance)
Redox properties of Il-DyP4 and Il-MnP6
The midpoint redox potential of the Fe3+/Fe2+ couple () at different pH values (3.5, 6.5, and 8) were estimated by CV for Il-DyP4 and Il-MnP6 (Table 4). Il-DyP4 obtained the highest midpoint redox potential (= 27 ± 10 mV) at pH 3.5, decreasing to a lower potential (= − 232 ± 10 mV) at pH 8.0. Similarly, Il-MnP6 obtained the highest midpoint redox potential (= − 75 ± 10 mV) at pH 3.5, decreasing to a lower potential (= − 168 ± 10 mV) at pH 8.0. When the pH increased from 3.5 to 8.0, the midpoint redox potential of Il-DyP4 was decreased by 259 mV, whereas that of Il-MnP6 decreased by 93 mV.
Table 4.
Midpoint redox potentials of Il-DyP4 and Il-MnP6 (vs. Ag/AgCl)
| (mV) | |||
|---|---|---|---|
| pH | 3.5 | 6.5 | 8.0 |
| Il-DyP4 | 27 ± 10 | − 87.5 ± 10 | − 232 ± 10 |
| Il-MnP6 | − 75 ± 10 | − 100 ± 10 | − 168 ± 10 |
Dye decolorization
Dyes are colorful organic compounds that are widely used in the food, textile, drug, and paper industries, often resulting in water pollution. The first DyP discovered was named because of its ability to decolorize various dyes (Kim and Shoda 1999). Accordingly, we selected 16 different dyes to examine the decolorization capability of Il-DyP4 and Il-MnP6 under pH 3.5, 4.0, and 4.5. The results revealed that the ability of Il-DyP4 to oxidize most of the anthraquinone dyes, azo dyes, phenazine dyes, triphenylmethane dyes, and aniline dyes was stronger than that of Il-MnP6 (Table 5), and also greater than that of TfuDyP from Thermobifida fusca (van Bloois et al. 2010). In addition, the results revealed that Il-DyP4 was able to decolorize anthraquinone dyes more quickly than was Il-MnP6. Interestingly, direct yellow 8 did not show any significant decolorization in our experiments. Further decolorization experiments using this dye are in progress.
Table 5.
Decolorization (%) of different kinds of synthetic dyes by Il-DyP4 and Il-MnP6
| Nr. | Dye | λmax (nm) | Concentration (μM) | Il-DyP4a | Il-MnP6a | ||||
|---|---|---|---|---|---|---|---|---|---|
| pH 3.5 | pH 4 | pH 4.5 | pH 3.5 | pH 4 | pH 4.5 | ||||
| Anthraquinone dyes | |||||||||
| 1 | Reactive blue 4 | 598 | 200 | 79.28 | 81.92 | 82.01 | 1.87 | 7.24 | 0.96 |
| 2 | Reactive blue 5 | 600 | 100 | 74.06 | 82.73 | 83.61 | 12.40 | 18.07 | 8.26 |
| 3 | Reactive blue 19 | 595 | 100 | 78.39 | 74.97 | 70.01 | 2.78 | 5.71 | 5.90 |
| Azo dyes | |||||||||
| 4 | Direct sky blue 5B | 598 | 100 | 80.62 | 81.45 | 77.91 | 16.95 | 59.72 | 63.75 |
| 5 | Reactive black 5 | 598 | 50 | 60.57 | 59.30 | 40.86 | 0.22 | 0 | 0.69 |
| 6 | Acid red 18 | 506 | 100 | 43.07 | 43.73 | 38.04 | 6.09 | 7.14 | 3.53 |
| 7 | Reactive violet 5 | 570 | 100 | 92.16 | 90.81 | 80.96 | 19.97 | 21.32 | 1.10 |
| 8 | Methyl orange | 464 | 50 | 33.12 | 30.21 | 29.60 | 11.21 | 9.32 | 1.95 |
| 9 | Direct yellow 8 | 392 | 25 | 2.62 | 4.66 | 2.04 | 0 | 0 | 0 |
| 10 | Orange G | 478 | 50 | 18.38 | 21.28 | 25.39 | 33.80 | 34.72 | 22.90 |
| 11 | Orange yellow II | 484 | 50 | 28.65 | 27.94 | 36.59 | 7.58 | 4.68 | 2.18 |
| 12 | Orange yellow IV | 445 | 50 | 62.55 | 60.07 | 60.16 | 13.23 | 10.40 | 4.26 |
| 13 | Congo red | 498 | 10 | 0 | 38.69 | 52.77 | 0 | 0 | 0 |
| Phenazine dyes | |||||||||
| 14 | Neutral red | 550 | 100 | 22.50 | 24.15 | 31.09 | 0 | 2.40 | 5.68 |
| Triphenylmethane dyes | |||||||||
| 15 | Malachite green | 618 | 50 | 84.80 | 82.43 | 72.53 | 24.74 | 28.03 | 24.69 |
| Aniline dyes | |||||||||
| 16 | Basic fuchsin | 542 | 25 | 45.10 | 39.75 | 23.86 | 3.46 | 3.63 | 0.25 |
aPercentage of dye decolorization after 30 min is based on the observed decrease in absorbance at λmax
In addition, we selected Poly R-478 to determine the ability of Il-DyP4 and Il-MnP6 to degrade lignin. The maximum efficiency of Il-DyP4 to decolorize Poly R-478 at an enzyme concentration of 100 nM was 15.76%, which was higher than the 0.66% of Il-MnP6 under pH 4.0 at 35 °C (Additional file 1: Fig. S6), indicating the Il-DyP4 had a better ligninolytic activity compared with Il-MnP6.
When the concentration of Il-DyP4 increased from 100 to 1000 nM, and the H2O2 concentration increased from 0.2 to 0.4 mM, the efficiency of Il-DyP4 to oxidize 0.01% Poly R-478 at pH 4 increased to 24%. When the concentration of Il-DyP4 was 1000 nM, and H2O2 concentration increased from 0.4 to 1.2 mM, the efficiency of Il-DyP4 to oxidize 0.01% Poly R-478 at pH 4 increased to 45%. The maximum decolorization percentage was 50% for Il-DyP4 for 1 h of reaction, and no increase in decolorization efficiency was observed 3 h after the initiation of the reaction.
Discussion
I. lacteus is a cosmopolitan white-rot species that has become a model ligninolytic basidiomycete. The fungus can produce LiP, MnP, and laccase simultaneously, which is similar to P. radiata and T. versicolor (Novotný et al. 2009).
Recently, we published the analysis of the genome sequence of I. lacteus F17 (Yao et al. 2017) and updated five cDNAs encoding DyPs and 13 cDNAs encoding MnPs to GeneBank. Herein, we used the available information to further analyze the amino acid sequence consensus and important residues in these DyPs and MnPs, which belong to two different heme peroxidase families. Furthermore, among these enzymes, Il-DyP4 and the Il-MnP6 were selected and characterized to further investigate the basis of the ligninolytic system of I. lacteus F17.
Both recombinant enzymes were expressed as inclusion bodies in E. coli Rosetta (DE3) and active enzymes were achieved via in vitro refolding. For optimizing of Il-DyP4, the refolding solution needed the supplement of optimal concentrations of EDTA, hemin, and urea. Compared with Il-DyP4, the in vitro refolding process of Il-MnP6 was complicated and active Il-MnP6 was obtained by the refolding solution containing various factors, including urea, hemin, GSSG, CaCl2, glycerin, MnSO4, KCl, hemin, and Ca2+ at pH 8.0. Among these, a key factor of Ca2+ affected the reconstitution of this fungal MnP. These refolding differences could be ascribed to the different amino acid compositions and structures of the two proteins.
SDS-PAGE analyses indicated that purified Il-DyP4 was monomeric, and its molecular weight was consistent with that of reported DyPs (50–60 kDa) (Colpa et al. 2014). In addition, comparison of the N-terminal sequence of Il-DyP4 with that of native DyP from I. lacteus revealed their similarity. Likewise, Il-MnP6 purified by Ni–NTA affinity chromatography was also a monomer, and its molecular weight was almost identical to that of purified native MnP from I. lacteus F17 (Zhao et al. 2015).
In this study, the biochemical properties of the two heme peroxidases were investigated. Under acidic conditions at pH 3.5, Il-DyP4 was more robust for ABTS oxidation, which fell within the optimal pH range (pH 3–4.5) of most of the DyPs reported from fungi (Linde et al. 2014; Fernández-Fueyo et al. 2015; Behrens et al. 2016), and was similar to the optimal pH of native DyP from I. lacteus (Salvachúa et al. 2013). Intriguingly, compared with the acidic pH of fungi DyPs, the optimal pH of bacteria DyPs was 5.0–7.0 (Yu et al. 2014; Ramzi et al. 2015; Rahmanpour et al. 2016). The most distinguishing feature of DyP peroxidases is their powerful catalytic properties (Colpa et al. 2014). In the current study, Il-DyP4 exhibited a broad substrate range and a strong ability to oxidize different structures of compounds.
Although all the substrates tested except VA were oxidized by the two enzymes, the kinetic constants and the catalytic efficiencies of Il-DyP4 differed markedly from those of Il-MnP6. Il-DyP4 was able to oxidize Mn2+ with a kcat/Km value only an order of magnitude lower than that of the Il-MnP6, but higher than that of Pleos-DyP1 and Pleos-DyP4 reported from P. ostreatus (Fernández-Fueyo et al. 2015). Remarkably, Il-DyP4 exhibited a lower kcat/Km for VA, a nonphenolic lignin model compound, similar to that of native DyP from I. lacteus and both AjPs from Auricularia auricula-judae, showing a certain ligninolytic activity (Salvachúa et al. 2013; Linde et al. 2014). However, the VA-oxidizing activities of Il-MnP6 was not detected under our test conditions, this was different from a previous report which shows IlMnP1 and IlMnP2 from I. lacteus CD2 that are able to oxidize VA in the presence of Mn2+ (Qin et al. 2017). Qin et al. (2017) indicated that either malonate or oxalate buffer was essential in the VA degradation by the two MnPs. Therefore, the sodium tartrate buffer was changed to the malonate buffer in order to re-examine the ability of VA oxidation in Il-MnP6 from I. lacteus F17. Interestingly, the assay revealed the activity of Il-MnP6 for VA oxidation in 50 mM malonate buffer (pH 3.5), and that the activity had improved with the increase in enzyme concentration (data not shown). Such results supports the findings from Qin et al. (2017), who showed that the Mn3+-malonate complexes may mediate oxidation of VA through the action of radicals. Yoshida and Sugano reviewed the characteristics of DyP-type peroxidases and showed that the kcat/Km of bacteria DyPs for anthraquinone compounds was 102–105 s−1 M−1, whereas the kcat/Km of basidiomycota DyPs reached 106–107 s−1 M−1 (Yoshida and Sugano 2015). In the present study, the recombinant Il-DyP4 from I. lacteus F17 exhibited higher catalytic efficiency (4.0 × 107 s−1 M−1) for RBlue 19 than did basidiomycota DyPs from I. lacteus, A. auricula-judae, and P. ostreatus (Salvachúa et al. 2013; Linde et al. 2014; Fernández-Fueyo et al. 2015). Compared with Il-MnP6, Il-DyP4 showed a lower affinity for azo dye RBlack 5, although its catalytic efficiency was almost two orders of magnitude higher than that of Il-MnP6, and was similar to that of native DyP from I. lacteus and superior to that of other DyPs (Salvachúa et al. 2013). Therefore, these results revealed the remarkable ability of Il-DyP4 to catalyze these substrates. These data also demonstrated that anthraquinone dye RBlue 19 and ABTS were the preferential substrates for Il-DyP4, which was consistent with the report by Linde et al. (2015). For Il-MnP6, Mn2+ was reconfirmed as its preferred substrate.
The effect of H2O2 and other compounds at different concentrations on the enzymatic activity of the two enzymes was also examined. Although, the catalytic efficiency of Il-DyP4 for H2O2 was an order of magnitude higher than that of Il-MnP6, it appeared to be more susceptible than Il-MnP6 at lower H2O2 concentrations (< 7.0 mM). This could be attributed to the different amino acid residues in the heme pocket influencing the enzymatic stability. Ogola et al. (2010) increased the H2O2 resistibility of a DyP-type peroxidase from Anabaena sp. by the site-directed mutagenesis of methionine residues. By contrast, reducing agents, such as DTT, thiourea, and β-mercaptoethanol (1 and 10 mM), significantly affected the enzymatic activity of Il-MnP6, resulting in the reduction of disulfide bonds and inactivation of the enzyme; by contrast, Il-DyP4 only contains a cysteine residue and lacks disulfide bonds, explaining the only small effect of this enzyme on these compounds. Interestingly, 1 and 10 mM of Fe2+ and Fe3+ caused a dramatic decrease in activities of both enzymes, compared with other metal ions. To the best of our knowledge, the reason for this inhibition is unclear. However, it has been known that the two types of heme peroxidase share the same catalytic cycle, using H2O2 as an electron acceptor to form two oxidized intermediates, compound I and II, which are then reduced back to the native enzymes. In both native proteins, heme iron (protoporphyrin IX) is a penta-coordinated high-spin state Fe3+, bound to the imidazole ring N of the conserved proximal histidine (His), which has been proposed to be a key amino acid residue. The distance between the heme iron and proximal His influences the redox potential of ligninolytic peroxidases (Martínez 2002). In our assay, the high concentration of exogenous Fe ions might have disturbed and altered the formation of covalent bonds between the heme iron and His, leading to enzyme inactivation. Exogenous irons might be able to interact with the imidazole groups at a high concentration, and affect the bonding state of the active center Fe3+ of the heme porphyrin ring. Thus, more detailed studies are needed to further characterize the inhibition mechanism of Fe ions on the activity of these two enzymes. Guo et al. (2009) proposed a possible inhibition mechanism of lanthanum ions on the activity of horseradish peroxidase (HRP). They found that La3+ can combine with amide groups of the polypeptide chain and destroy the native structure of HRP. Tayefi-Nasrabadi et al. (2006) also reported conformational changes and activity alterations induced by Ni2+ ions in HRP.
Differences were observed in the CD spectra between Il-DyP4 and Il-MnP6, both in terms of the α-helix and β-sheets contents, in agreement with the predicted molecular models. The UV–Vis absorption spectrum of Il-DyP4 had features similar to that of Il-MnP6. A characteristic CT band at 634 nm was observed in the spectrum of Il-DyP4, indicating a high-spin heme Fe3+ state. Also, the RZ value was similar to that of native DyP from I. lacteus (Salvachúa et al. 2013), thus, it appears that the in vitro refolding conditions used in this study were optimized. A similar pattern was observed in Il-MnP6: a characteristic CT band at 632 nm was obvious, and the RZ value (3.17) was almost identical to that native MnP from the white-rot fungus P. chrysosporium (Whitwam and Tien 1996). Hence, despite the tedious refolding process, Il-MnP6 was reconstituted successfully from inclusion bodies.
Further investigations of the midpoint redox potential of the two enzymes were carried out, and the values of indicated that Il-DyP4 was superior to Il-MnP6, particularly at pH 3.5. Nevertheless, the two enzymes were differentially affected by changes in pH. The impact of pH on the redox potential of Il-DyP4 was bigger than that of Il-MnP6 (Table 4), indicating that Il-DyP4 was more sensitive to changes in pH. Moffeta and colleagues indicated that the redox potential of heme peroxidases is influenced by several factors, including the electronic nature of the amino acids ligating the heme, and the electrostatic interactions with residues surrounding the heme, as well as the solvent accessibility of the heme (Moffeta et al. 2003).
To determine the potential application ability of the two enzymes, five different structures of 16 synthetic dyes were selected and a lower enzyme concentration of 25 nM was used to decolorize these dyes in the two same enzymatic reaction systems. The differences in decolorization percentage of 15 synthetic dyes were obvious between the two enzymes, except for Orange G. Il-DyP4 was superior to Il-MnP6 in all the structure types of dye tested and showed high oxidation capacities at pH values of 3.5–4.5, which were optimal pH values for most reported DyPs and MnPs (Hofrichter et al. 2010). This was suggested to be because of the increased redox potential of the oxidized heme at low pH (Petruccioli et al. 2009). Our voltammetric experiment data illustrated the strongest oxidization ability of Il-DyP4 at pH 3.5, and showed that the redox potential declined dramatically with the increase in pH. Thus, this result explained why DyPs required acidic pH conditions to complete substrate oxidization. In addition, in the case of the Il-DyP4 system, dye decolorization was carried out directly by the addition of enzyme and H2O2, whereas MnPs catalyzed the Mn2+-mediated oxidation reaction, and an optimal concentration of Mn2+ was required for the MnP decolorization system. Thus, the biotechnological application of MnPs would be limited because of the heavy metal contamination in treated industrial wastes, whereas DyPs are only robust under acidic conditions, which would not be appropriate in some industrial applications, such as the treatment of neutral and alkaline waste in pulping and bleaching. Therefore, there is still much research to be done to enhance the industrial applicability of these enzymes.
Poly R-478, a structurally complicated and recalcitrant anthraquinone derivative, is a suitable indicator of the ability of white-rot fungi to degrade lignin, and it was used as a model compound for the measurement of ligninolytic activity (Zhao et al. 2015). The results of Il-DyP4 to decolorize Poly R-478 highlighted its ligninolytic activity and its ability to oxidize lignin and related recalcitrant compounds. The precise structure of Il-DyP4 and its key roles in lignin degradation also need to be clarified in future studies.
Additional file
Additional file 1. Additional tables and figures.
Authors’ contributions
ZHD performed the experiments and wrote the paper. RS participated in part of the work and wrote the paper. ZHD and RS contributed equally to this work. BJL carried out the part of the MnP experiment. MWY participated in the sequence analysis of DyPs and MnPs. RJ conceived of the study, and participated in its design and helped to revise the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Ping Chen for his assistance to the electrochemical experiment. We thank Yinliang Zhang for her help to structural simulation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data sets supporting the results of this manuscript are included within the article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This research is supported by the National Natural Science Foundation of China (31570102, 31070109).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- DyP
dye decolorizing peroxidase
- MnP
manganese peroxidase
- I. lacteus F17
Irpex lacteus F17
- E. coli
Escherichia coli
- Rz
Reinheitszahl
- H2O2
hydrogen peroxide
- GSSG
glutathione
- DTT
dithiothreitol
- DMP
2,6-dimethoxyphenol
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- VA
veratryl alcohol
- PMSF
phenylmethanesulfonyl fluoride
- IPTG
isopropyl-β-d-thiogalactopyranoside
- RBlue 19
reactive blue 19
- RBlack 5
reactive black 5
- EDTA
ethylene diamine tetraacetic acid
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PGE
pyrolytic graphite electrode
- CD
circular dichroism
Footnotes
Zihong Duan and Rui Shen contributed equally to this work
Electronic supplementary material
The online version of this article (10.1186/s13568-018-0648-6) contains supplementary material, which is available to authorized users.
Contributor Information
Zihong Duan, Email: 1294643776@qq.com.
Rui Shen, Email: 2322546049@qq.com.
Binjie Liu, Email: 1985397650@qq.com.
Mengwei Yao, Email: 1033605128@qq.com.
Rong Jia, Phone: +86 551 63861063, Email: ahdxjiarong@126.com.
References
- Baborová P, Möder M, Baldrian P, Cajthamlová K, Cajthaml T. Purification of a new manganese peroxidase of the white-rot fungus Irpex lacteus, and degradation of polycyclic aromatic hydrocarbons by the enzyme. Res Microbiol. 2006;157:248–253. doi: 10.1016/j.resmic.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Balat M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manage. 2011;52:858–875. doi: 10.1016/j.enconman.2010.08.013. [DOI] [Google Scholar]
- Behrens CJ, Zelena K, Berger RG. Comparative cold shock expression and characterization of fungal dye-decolorizing peroxidases. Appl Biochem Biotechnol. 2016;179:1404–1417. doi: 10.1007/s12010-016-2073-0. [DOI] [PubMed] [Google Scholar]
- Chen WT, Zheng LL, Jia R, Wang N. Cloning and expression of a new manganese peroxidase from Irpex lacteus F17 and its application in decolorization of reactive black 5. Process Biochem. 2015;50:1748–1759. doi: 10.1016/j.procbio.2015.07.009. [DOI] [Google Scholar]
- Cheng XB, Jia R, Li PS, Tu SQ, Zhu Q, Tang WZ, Li XD. Purification of a new manganese peroxidase of the white-rot fungus Schizophyllum sp. F17, and decolorization of azo dyes by the enzyme. Enzyme Microb Tech. 2007;41:258–264. doi: 10.1016/j.enzmictec.2007.01.020. [DOI] [Google Scholar]
- Cheng SN, Wilks C, Yuan ZS, Leitch M, Xu C. Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water–ethanol co-solvent. Polym Degrad Stabil. 2012;97:839–848. doi: 10.1016/j.polymdegradstab.2012.03.044. [DOI] [Google Scholar]
- Colpa DI, Fraaije MW, van Bloois E. DyP-type peroxidases: a promising and versatile class of enzymes. J Ind Microbiol Biot. 2014;41:1–7. doi: 10.1007/s10295-013-1371-6. [DOI] [PubMed] [Google Scholar]
- Fernández-Fueyo E, Ruiz-Dueñas FJ, Miki Y, Martinez MJ, Hammel KE, Martinez AT. Lignin-degrading peroxidases from genome of selective ligninolytic fungus Ceriporiopsis subvermispora. J Biol Chem. 2012;287:16903–16916. doi: 10.1074/jbc.M112.356378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Fueyo E, Ruiz-Dueñas FJ, Martínez MJ, Romero A, Hammel KE, Medrano FJ, Martínez AT. Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol Biofuels. 2014;7:2. doi: 10.1186/1754-6834-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Fueyo E, Linde D, Almendral D, Lopez-Lucendo MF, Ruiz-Dueñas FJ, Martinez AT. Description of the first fungal dye-decolorizing peroxidases oxidizing manganese (II) Appl Microbiol Biotechnol. 2015;99:8927–8942. doi: 10.1007/s00253-015-6665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar T, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, John FS, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young A, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336:1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- Floudas D, Held BW, Riley R, Nagy LG, Koehler G, Ransdell AS, Younus H, Chow J, Chiniquy J, Lipzen A, Tritt A, Sun H, Haridas S, LaButti K, Ohmc RA, Kües U, BlanchetteRA Grigoriev IV, Minto RE, Hibbett DS. Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genet Biol. 2015;76:78–92. doi: 10.1016/j.fgb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy—the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SF, Wang LH, Lu BH, Lu TH, Ding XL, Huang XH. Inhibition mechanism of lanthanum ion on the activity of horseradish peroxidase in vitro. Spectrochim Acta. 2009;75:936–940. doi: 10.1016/j.saa.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T. New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol. 2010;87:871–897. doi: 10.1007/s00253-010-2633-0. [DOI] [PubMed] [Google Scholar]
- Kasinath A, Novotný Č, Svobodová K, Patel KC, Šašek V. Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed-bed bioreactor. Enzyme Microb Tech. 2003;32:167–173. doi: 10.1016/S0141-0229(02)00279-X. [DOI] [Google Scholar]
- Kellner H, Luis P, Pecyna MJ, Barbi F, Kapturska D, Krüger D, Zak DR, Marmeisse R, Vandenbol M, Hofrichter M. Widespread occurrence of expressed fungal secretory peroxidases in forest soils. PLoS ONE. 2014;9:e95557. doi: 10.1371/journal.pone.0095557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Shoda M. Purification and characterization of a novel peroxidase from Geotrichum candidum dec 1 involved in decolorization of dyes. Appl Environ Microbiol. 1999;65:1029–1035. doi: 10.1128/aem.65.3.1029-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne M, Poraj-Kobielska M, Ullrich R, Nousiainen P, Sipilä J, Scheibner K, Hammel KE, Hofrichter M. Oxidative cleavage of nonphenolic β-O-4 lignin model dimers by an extracellular aromatic peroxygenase. Holzforschung. 2011;65:673–679. doi: 10.1515/hf.2011.057. [DOI] [Google Scholar]
- Koo K, Foegeding PM, Swaisgood HE. Isolation of RNA and DNA fragments using diatomaceous earth. Biotechnol Tech. 1998;12:549–552. doi: 10.1023/A:1008859632378. [DOI] [Google Scholar]
- Linde D, Coscolín C, Liers C, Hofrichter M, Martínez AT, Ruiz-Dueñas FJ. Heterologous expression and physicochemical characterization of a fungal dye-decolorizing peroxidase from Auricularia auricula-judae. Protein Expres Purif. 2014;103:28–37. doi: 10.1016/j.pep.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Linde D, Ruiz-Dueñas FJ, Fernández-Fueyo E, Guallar V, Hammel KE, Pogni R, Martinez AT. Basidiomycete DyPs: genomic diversity, structural- functional aspects, reaction mechanism and environmental significance. Arch Biochem Biophys. 2015;574:66–74. doi: 10.1016/j.abb.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Loncar N, Colpa DI, Fraaije MW. Exploring the biocatalytic potential of a DyP-type peroxidase by profiling the substrate acceptance of Thermobifida fusca DyP peroxidase. Tetrahedron. 2016;72:7276–7281. doi: 10.1016/j.tet.2015.12.078. [DOI] [Google Scholar]
- Martínez AT. Molecular biology and structure-function of lignin-degrading heme peroxidases. Enzyme Microb Technol. 2002;30:425–444. doi: 10.1016/S0141-0229(01)00521-X. [DOI] [Google Scholar]
- Mendes S, Brissos V, Gabriel A, Catarino T, Turner DL, Todorovic S, Martins LO. An integrated view of redox and catalytic properties of B-type PpDyP from Pseudomonas putida MET94 and its distal variants. Arch Biochem Biophys. 2015;574:99–107. doi: 10.1016/j.abb.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Moffeta DA, Foleyb J, Hechta MH. Midpoint reduction potentials and heme binding stoichiometries of de novo proteins from designed combinatorial libraries. Biophys Chem. 2003;105:231–239. doi: 10.1016/S0301-4622(03)00072-3. [DOI] [PubMed] [Google Scholar]
- Morgenstern I, Klopman S, Hibbett DS. Molecular evolution and diversity of lignin degrading heme peroxidases in the agaricomycetes. J Mol Evol. 2008;66:243–257. doi: 10.1007/s00239-008-9079-3. [DOI] [PubMed] [Google Scholar]
- Novotný Č, Rawal B, Bhatt M, Patel M, Šašek V, Molitoris HP. Capacity of Irpex lacteus and Pleurotus ostreatus for decolorization of chemically different dyes. J Biotechnol. 2001;89:113–122. doi: 10.1016/S0168-1656(01)00321-2. [DOI] [PubMed] [Google Scholar]
- Novotný Č, Cajthaml T, Svobodova K, Šušla M, Šašek V. Irpex lacteus, a white-rot fungus with biotechnological potential—review. Folia Microbiol. 2009;54:375–390. doi: 10.1007/s12223-009-0053-2. [DOI] [PubMed] [Google Scholar]
- Ogola HJO, Hashimoto N, Miyabe S, Ashida H, Ishikawa T, Shibata H, Sawa Y. Enhancement of hydrogen peroxide stability of a novel Anabaena sp. DyP-type peroxidase by site-directed mutagenesis of methionine residues. Appl Microbiol Biotechnol. 2010;87:1727–1736. doi: 10.1007/s00253-010-2603-6. [DOI] [PubMed] [Google Scholar]
- Park J, Riaz A, Insyani R, Kim J. Understanding the relationship between the structure and depolymerization behavior of lignin. Fuel. 2018;217:202–210. doi: 10.1016/j.fuel.2017.12.079. [DOI] [Google Scholar]
- Petruccioli M, Frasconi M, Quaratino D, Covino S, Favero G, Mazzei F, Federici F, D’Annibale A. Kinetic and redox properties of MnP II, a major manganese peroxidase isoenzyme from Panus tigrinus CBS 577.79. J Biol Inorg Chem. 2009;14:1153–1163. doi: 10.1007/s00775-009-0559-8. [DOI] [PubMed] [Google Scholar]
- Qin X, Sun XH, Huang HQ, Bai YG, Wang Y, Luo HY, Yao B, Zhang XY, Su XY. Oxidation of a non-phenolic lignin model compound by two Irpex lacteus, manganese peroxidases: evidence for implication of carboxylate and radicals. Biotechnol Biofuels. 2017;10:103. doi: 10.1186/s13068-017-0787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanpour R, Rea D, Jamshidi S, Fulop V, Bugg TDH. Structure of Thermobifida fusca DyP-type peroxidase and activity towards Kraft lignin and lignin model compounds. Arch Biochem Biophys. 2016;594:54–60. doi: 10.1016/j.abb.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Ramzi AB, Hyeon JE, Han SO. Improved catalytic activities of a dye-decolorizing peroxidase (DyP) by overexpression of ALA and heme biosynthesis genes in Escherichia coli. Process Biochem. 2015;50:1272–1276. doi: 10.1016/j.procbio.2015.05.004. [DOI] [Google Scholar]
- Riley R, Salamov AA, Brown DW, Nagy LG, Floudas D, Held BW, Levasseur A, Lombard V, Morin E, Otillar R, Lindquist EA, Sun H, LaButti KM, Schmutz J, Jabbour D, Luo H, Baker SE, Pisabarro AG, Walton JD, Blanchette RA, Henrissat B, Martin F, Cullen D, Hibbett DS, Grigoriev IV. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci USA. 2014;111:9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Dueñas FJ, Ludell T, Floudas D, Nagy LG, Barrasa JM, Hibbett DS, Martínez AT. Lignin-degrading peroxidases in Polyporales: an evolutionary survey based on 10 sequenced genomes. Mycologia. 2013;105:1428–1444. doi: 10.3852/13-059. [DOI] [PubMed] [Google Scholar]
- Salvachúa D, Prieto A, Martínez ÁT, Martinez MJ. Characterization of a novel dye-decolorizing peroxidase (DyP)-type enzyme from Irpex lacteus and its application in enzymatic hydrolysis of wheat straw. Appl Environ Microbiol. 2013;79:4316–4324. doi: 10.1128/AEM.00699-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano Y. DyP-type peroxidases comprise a novel heme peroxidase family. Cell Mol Life Sci. 2009;66:1387–1403. doi: 10.1007/s00018-008-8651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayefi-Nasrabadi H, Keyhani E, Keyhani J. Conformational changes and activity alterations induced by nickel ion in horseradish peroxidase. Biochime. 2006;88:1183–1197. doi: 10.1016/j.biochi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- van Bloois E, Torres Pazmiño DE, Winter RT, Fraaije MW. A robust and extracellular heme-containing peroxidase from Thermobifida fusca as prototype of a bacterial peroxidase superfamily. Appl Microbiol Biot. 2010;86:1419–1430. doi: 10.1007/s00253-009-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ren K, Jia R, Chen WT, Sun RR. Expression of a fungal manganese peroxidase in Escherichia coli: a comparison between the soluble and refolded enzymes. BMC Biotechnol. 2016;16:2–15. doi: 10.1186/s12896-016-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwam R, Tien M. Heterologous expression and reconstitution of fungal Mn peroxidase. Arch Biochem Biophys. 1996;333:439–446. doi: 10.1006/abbi.1996.0413. [DOI] [PubMed] [Google Scholar]
- Yang XT, Zheng JZ, Lu YM, Jia R. Degradation and detoxification of the triphenylmethane dye malachite green catalyzed by crude manganese peroxidase from Irpex lacteus F17. Environ Sci Pollut Res. 2016;23:9585–9597. doi: 10.1007/s11356-016-6164-9. [DOI] [PubMed] [Google Scholar]
- Yao MW, Li WM, Duan ZH, Zhang YL, Jia R. Genome sequence of the white-rot fungus Irpex lacteus F17, a type strain of lignin degrader fungus. Stand Genomic Sci. 2017;12:55. doi: 10.1186/s40793-017-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Sugano Y. A structural and functional perspective of DyP-type peroxidase family. Arch Biochem Biophys. 2015;574:49–55. doi: 10.1016/j.abb.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Yu WN, Liu WN, Huang HQ, Zheng F, Wang XY, Wu YY, Li KJ, Xie XM, Jin Y. Application of a novel alkali-tolerant thermostable DyP-type peroxidase from Saccharomonospora viridis DSM 43017 in biobleaching of eucalyptus kraft pulp. PLoS ONE. 2014;9:e110319. doi: 10.1371/journal.pone.0110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XS, Huang XJ, Yao JT, Zhou Y, Jia R. Fungal growth and manganese peroxidase production in a deep tray solid-state bioreactor, and in vitro decolorization of Poly R-478 by MnP. J Microbiol Biotechnol. 2015;25:803–813. doi: 10.4014/jmb.1410.10054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional tables and figures.
Data Availability Statement
The data sets supporting the results of this manuscript are included within the article.