Abstract
The Martian surface is cold, dry, exposed to biologically harmful radiation and apparently barren today. Nevertheless, there is clear geological evidence for warmer, wetter intervals in the past that could have supported life at or near the surface. This evidence has motivated National Aeronautics and Space Administration and European Space Agency to prioritize the search for any remains or traces of organisms from early Mars in forthcoming missions. Informed by (1) stratigraphic, mineralogical and geochemical data collected by previous and current missions, (2) Earth's fossil record, and (3) experimental studies of organic decay and preservation, we here consider whether, how, and where fossils and isotopic biosignatures could have been preserved in the depositional environments and mineralizing media thought to have been present in habitable settings on early Mars. We conclude that Noachian‐Hesperian Fe‐bearing clay‐rich fluvio‐lacustrine siliciclastic deposits, especially where enriched in silica, currently represent the most promising and best understood astropaleontological targets. Siliceous sinters would also be an excellent target, but their presence on Mars awaits confirmation. More work is needed to improve our understanding of fossil preservation in the context of other environments specific to Mars, particularly within evaporative salts and pore/fracture‐filling subsurface minerals.
Keywords: Mars, astrobiology, fossils
Key Points
Noachian‐Hesperian Fe‐bearing clay‐rich fluvio‐lacustrine siliciclastic sediments are favored in the search for ancient Martian life
There is insufficient confidence in the nature of reported silica sinters on Mars or the possibility of preservation in the deep biosphere
Experimental taphonomy approaches from paleontology should now be adapted to understand limits on preservation under Martian conditions
1. Introduction
The search for evidence of life on Mars is one of the outstanding scientific challenges of our time. Low temperatures and pressures, intense ionizing radiation, oxidizing soil chemistry, and low levels of thermodynamic water activity greatly reduce the possibility of life at the Martian surface today. However, extensive ancient valley networks and sedimentary rocks laid down in the Noachian and Hesperian Periods of Martian history (respectively, ~4.0–3.6 billion years ago [Ga] and ~3.6–3.0 Ga; Fassett & Head, 2011; Werner & Tanaka, 2011) are suggestive of much more clement global conditions, including widespread liquid water at the surface. The uncertain timing and duration of water availability are still debated and have important implications for the potential viability, complexity, distribution, and preservation potential of any life that arose on early Mars. Nevertheless, Noachian and Hesperian rocks have long been recommended as a target for fossils, that is, the physical or chemical remains of organisms and their activities (e.g., McKay & Stoker, 1989). On Earth, these remains are preserved as casts or molds in sediment, as mineral coatings or replacements, and as surviving biominerals, organic matter, or stable isotopic signatures. Locating samples on Mars that are likely to preserve such signals and studying them with appropriate instrumentation, both in situ and possibly after returning them robotically to Earth, are now strategic priorities for both NASA (National Aeronautics and Space Administration) and ESA (the European Space Agency). One of the primary objectives of NASA's Mars 2020 rover is to cache a variety of drill‐core rock samples from astrobiologically promising paleoenvironments; some of these samples may be returned to Earth by subsequent missions for analysis (Farley & Williford, 2017; Mustard et al., 2013). The ESA‐Roscosmos ExoMars 2020 rover will also be equipped to test technologies required for subsequent sample‐return missions (Vago et al., 2017). Understanding how, why, and where morphological, molecular, or isotopic biosignatures might have survived on Mars will significantly enhance the success of these missions by informing the selection of landing sites, rover traverse pathways, and sampling strategies.
There is no compelling evidence for life on Mars, now or in the geological past. However, there is now a very strong case that the surface of early Mars was habitable. Orbital data reveal fluvial valley networks draining thousands of square kilometers, exhumed meandering and branched distributary channels, paleolake deposits in topographic depressions, and alluvial fans/deltas entering these lakes, all of which reflect sustained precipitation and subaqueous sediment transport during the Noachian and Hesperian periods (Cabrol & Grin, 1999; Fassett & Head, 2008; Goudge et al., 2016; Grant et al., 2008; Grotzinger, Gupta, et al., 2015; Malin & Edgett, 2003; Metz et al., 2009; Moore & Howard, 2005). Some fan‐shaped deposits, possibly deltaic, have been interpreted as aligned along the shoreline of a large ocean (Di Achille & Hynek, 2010; DiBiase et al., 2013) that would have covered the northern lowlands, although this is controversial. At Gale Crater, however, the Mars Science Laboratory mission (Curiosity rover) has explored the sedimentary record of a Late Noachian/Early Hesperian paleolake that persisted for thousands to millions of years, with evidence for mild salinity, moderate pH, and local redox gradients (Grotzinger et al., 2014; Grotzinger, Gupta, et al., 2015; Hurowitz et al., 2017). The presence of liquid water at the surface over this length of time implies a denser atmosphere, which should also have minimized the radiation flux. Tantalizingly, these rocks contain organic molecules (Freissinet et al., 2015) preserved under relatively reducing conditions (Grotzinger et al., 2014). Such Noachian‐Hesperian water bodies could have hosted microbial life, which was present on Earth during this time interval (Grotzinger et al., 2014). There is experimental support for the long‐standing idea that meteorites could have transported viable microbial cells between the two planets, although the ecological obstacles to continued growth on arrival may be considerable (Fajardo‐Cavazos et al., 2005; Foucher et al., 2010; Mileikowsky et al., 2000; Shuster & Weiss, 2005; Weiss et al., 2000).
The Martian surface has been cold and predominantly dry for at least the last three billion years (i.e., the Amazonian Period, immediately following the Hesperian), but the subsurface could have sustained stable reservoirs of geothermally heated liquid water for much of this time, representing a long‐lived habitat that could have exchanged living cells with shorter‐lived habitats at the surface (Boston et al., 1992; Clifford et al., 2010; Ehlmann et al., 2011; Fisk & Giovannoni, 1999; Thomas et al., 2017; Travis et al., 2003). Transport from the surface would probably have been required to provide the initial inoculum or at least chemical precursors to life (e.g., Patel et al., 2015). Once established, however, a “deep biosphere” would have been protected from the deteriorating conditions at the surface and able to persist far longer given indigenous sources of energy and nutrients (Des Marais, 2010; Westall, Foucher, et al., 2015). Potentially analogous subterranean ecosystems have been studied most intensively in the Witwatersrand Basin of South Africa, where anaerobic microbial populations achieve densities of at least 103–104 cells/mL at depths of 3–4 km (Lin et al., 2006; Moser et al., 2005). Life in this setting appears to have been isolated from the surface for millions of years (Lin et al., 2006) and relies largely on abiotic carbon sources and electron donors, the latter dominated by molecular hydrogen supplied by water‐rock reactions (Lin et al., 2005; Sherwood Lollar et al., 2006). There is clear evidence that such reactions occurred on Mars, including the presence of the mineral serpentine at the Martian surface and in Martian meteorites (Blamey et al., 2015; Changela & Bridges, 2011; Ehlmann et al., 2010). The putative detection of intermittent traces of methane in the Martian atmosphere—which could be a product of water‐rock reactions or even of hydrogen‐oxidizing microbes themselves—may imply the persistence of a habitable environment into geologically recent time (Krasnopolsky et al., 2004; Mumma et al., 2009; Webster et al., 2015).
It is thus highly likely that various Martian paleoenvironments were habitable, and plausible that they were inhabited, but could they have preserved fossils? On Earth, most organisms fail to fossilize because their remains are physically destroyed, chemically oxidized or dissolved, digested by their own enzymes, or consumed by other organisms. Fossilization only occurs when processes of preservation outpace degradation (Allison, 1988). Fossilization usually begins when organisms are buried in sediment or entombed in minerals, fixing their remains in place, and reducing their exposure to degradative processes including heterotrophic consumption (animal, fungal, or bacterial). Minerals may replicate the morphology of the buried organism, cement the sediment around it, or fill the remaining void following decay. Microbes commonly self‐fossilize by entombing themselves in the mineral byproducts of their own metabolic activity (see section 4). Organic cells and tissues are usually short lived, but they can serve as a template for the nucleation of more permanent minerals or their precursors, which in turn can stabilize the original organic matter, particularly when clay minerals form (Gabbott, 1998; Naimark, Kalinina, Shokurov, Markov, et al., 2016). Organic material can also survive when passively entombed by rapid precipitation, as in Precambrian marine cherts and Phanerozoic hydrothermal sinters (e.g., Campbell et al., 2015; Pancost et al., 2006; Schultze‐Lam et al., 1995; Yee et al., 2003). Organic molecules with robust chemical backbones can be indicative of general or specific biological origins (i.e., “biomarkers” or “molecular fossils”) and are common in fine‐grained sedimentary rocks (Peters et al., 2005; Summons & Lincoln, 2012).
The same high‐radiation and oxidizing conditions that limit the habitability of the Martian surface today would also destroy exposed organic remains. The average galactic cosmic ray dose rate measured in Gale Crater was ~0.2 mGy/day (Hassler et al., 2014); energetic particles associated with solar flares and coronal mass ejections can occasionally produce dose rates orders of magnitude higher, and the UV flux combined with strong oxidants also destroys organic matter (e.g., ten Kate et al., 2005; Wadsworth & Cockell, 2017). Although the exact nature of the early Martian climate is still uncertain, the Noachian‐Early Hesperian radiation flux was attenuated by a thicker atmosphere that supported stable liquid water at the surface (Grotzinger et al., 2014; Mahaffy et al., 2015), and potentially also by an Earth‐like magnetic field, although this may have been lost by the early Noachian (e.g., Lillis et al., 2013). The strong oxidants presently in Martian soil may have been less concentrated or absent in the wetter conditions and milder radiation environment, although chlorine isotope measurements by the Curiosity rover in Gale Crater suggest perchlorate production during the Hesperian (Carrier & Kounaves, 2015; Farley et al., 2016). Any organisms dwelling on early Mars would therefore presumably have been degraded primarily as a result of biological heterotrophy and enzymatic autolysis, as on Earth. Although low temperatures and a lack of burrowing or grazing animals would have inhibited decay and favored preservation, any fossil record on Mars would be biased toward robust, decay‐resistant biological materials, such as biominerals/organominerals (Perry et al., 2007) or thick microbial sheaths, and toward environments that promoted preservation. Favorable circumstances for preservation would have included rapid burial, a high accumulation rate of organic remains, the presence of mineralizing fluids, and the inhibition of decay. Research on the nature, distribution, and quality of fossils representing both microsoft‐ and macrosoft‐bodied organisms on Earth, supported by decay experiments on cells and tissues and the environmental conditions required to preserve them (“experimental taphonomy”; Briggs & McMahon, 2016), provides essential guidelines to the minerals, lithologies, and facies most likely to host microbial fossils on Mars (Table 1).
Table 1.
Lithofacies and Depositional Environments on Mars and Their Potential to Preserve Biosignatures
| Lithofacies | Reported occurrence of this facies in Noachian and/or Hesperian terrains on Mars | Potential to preserve biosignatures | |||||
|---|---|---|---|---|---|---|---|
| Rock type | Paleoenvironment of deposition | Organic cells | Mineralized cells | Organic carbon | Molecular fossils | MISS | |
| Siliciclastic sandstone/siltstone | Fluvial systems | Abundant | − | ++ | − | − | − |
| Detrital claystone | Inferred; some candidate instances (Milliken & Bish, 2010; Ehlmann et al., 2009, 2013) | + | + | ++ | ++ | − | |
| Evaporative sulfates and chlorides | Shoreface or ephemeral lakes | Some probable instances, for example, “bathtub ring” in Terra Sirenum crater (Wray et al., 2011); Meridiani Planum sulfates are interpreted as ex situ playa salts (Grotzinger et al., 2005) | +++ (<) | − | +++ (<) | − | − |
| Siliciclastic sandstone/siltstone | Distal deltaic, lacustrine and marine | Abundant | − | +++ | + | + | +++ |
| Claystone of detrital origin | Abundant | ++ | ++ | +++ | +++ | +++ | |
| Chert or fine‐grained rock with high amorphous silica content | Globally distributed silica of indeterminate origin; amorphous silica present in “silicilyte”‐like rock at Gale Crater (Morris et al., 2016) | +++ | +++ | +++ | +++ | +++ | |
| Bedded or authigenic carbonates | Scarce possible instances (Ehlmann, Mustard, Murchie, et al., 2008; Michalski & Niles, 2010) | − | + | − | − | +++ | |
| Authigenic phosphate | Possible component of amorphous phase at Gale Crater (Forni et al., 2015) | +++ | +++ | +++ | + (<) | +(<) | |
| Carbonate/travertine | Calcareous springs | One disputable instance (Morris et al., 2010) | −(<) | ++ (<) | − (<) | − (<) | ++ (<) |
| Siliceous sinter | Siliceous springs | Globally distributed occurrences of silica of indeterminate origin; Gusev Crater deposit may be sinter‐like (Ruff & Farmer, 2016) but it may alternatively be a fumarolic acid‐leachate less favorable to habitability or preservation. | +++ | +++ | +++ | +++ | +++ |
| Sulfate veins | Groundwater 100–103 m below surface | Abundant and frequently encountered by rovers | − (X) | − (X) | − (X) | − (X) | − (X) |
| Authigenic clays | May be globally widespread depending on interpretation of observed phyllosilicates (Ehlmann et al., 2011, 2013) | – | – | – | – | – | |
| Other pore‐ and vein‐filling secondary minerals of low‐temperature origin (e.g., calcite, zeolite, goethite, chalcedony, and chlorite) | No unequivocal reports but predicted by Ehlmann et al. (2011) and Michalski et al. (2013); difficult to resolve at spatial resolution of orbital data | ++ (<) | ++ | ++ (<) | + (<) | – | |
Note. Potential to preserve biosignatures given the former presence of organisms is rated: − low (though not zero); + moderate; ++high; +++ very high. Caveats are enclosed in parentheses: total absence of any reported examples of preservation from Earth's rock record is indicated by “X,” absence of any reported examples older than 1 Ga is indicated by “<.” MISS = microbially induced sedimentary structures.
2. Potentially Fossiliferous Rocks and Minerals on Mars
2.1. Secondary Minerals in Basalt
The likelihood that the surface of Mars has been dry for the last several billion years has directed attention to the habitability of fractures and pores in deep basaltic rocks and sediments, where liquid water may have been sustained by geothermal heat (e.g., Cockell, 2014a, 2014b; Hays et al., 2017; Michalski et al., 2013). However, the potential for preserving fossils within the secondary minerals that form in such rock‐hosted habitats remains unclear. Microscopic textures in basalt, ranging from simple filled or unfilled pits to elaborate helical “tunnels,” are widely reported as “bio‐alteration” features or microbial “trace fossils” (e.g., McLoughlin et al., 2009; Staudigel et al., 2008; Thorseth, 2011), but in most cases an abiotic origin cannot be excluded (Fisk et al., 2013). More compelling are reports of filamentous and rod‐shaped structures mineralized in basalt void space on Earth by low‐temperature submarine or subterranean hydrothermal activity (e.g., Cavalazzi et al., 2011; Hofmann & Farmer, 2000; Ivarsson et al., 2016; McKinley et al., 2000; Peckmann et al., 2008; Schumann et al., 2004). Such structures range from micrometer to millimeter in scale and are typically preserved three‐dimensionally in clay, iron oxides, and other minerals within open‐ or mineral‐filled vesicles and fractures. Some appear to have been mineralized in anoxic conditions (e.g., Bengtson et al., 2017; Ivarsson et al., 2016; Schumann et al., 2004). Unfortunately, it has not been demonstrated that any of these structures represent Mars‐relevant chemoautotrophs. Indeed, subsurface productivity on Earth is strongly dependent on the heterotrophic remineralization of carbon originally fixed at the surface by photosynthesis (Kallmeyer et al., 2012; McMahon & Parnell, 2014). As such, the bulk of Earth's massive deep biosphere, and presumably also its fossil record, is a poor analog for any ancient or modern Martian equivalent which, in the absence of a productive surface biosphere, would be much smaller and dominated by chemoautotrophs, not heterotrophs.
Lithoautotrophic communities in deep groundwaters in South Africa and the Canadian Shield are more relevant to scenarios for life on Mars (e.g., Moser et al., 2005; Onstott et al., 2009). However, these stagnant aquifers have chemically equilibrated with their mineralogical environment following long isolation from the surface (Lippmann et al., 2003; Holland et al., 2013) and are therefore unlikely to mineralize actively except during rare tectonic events (McNutt et al., 1990; Sherwood Lollar et al., 2007). To our knowledge, there are no reports of mineralized cells found at these sites nor any definitive fossil record from elsewhere of ancient microbial ecosystems analogous to these. Mineralized fractures and void spaces are difficult to resolve and interpret at the spatial scale of orbital data from Mars, and even on Earth probably do not usually yield robust indigenous biosignatures. A better understanding of the distribution, degradation, and preservation of Mars‐relevant microbes in Mars‐relevant subsurface environments is therefore needed before landing sites can be selected to search for a fossil deep biosphere on Mars.
2.2. Evaporite Salts
Evaporite salts, including Ca/Mg/Fe/Al‐sulfates and chlorides, are widespread in basins on Mars, commonly occurring in Noachian and Hesperian‐aged terrains (Ehlmann et al., 2016; Gendrin et al., 2005; Glotch et al., 2010; Murchie et al., 2009; Osterloo et al., 2008; Wray et al., 2011). In some cases, these salts are associated with paleoenvironments inferred to be playa like or lacustrine with strong potential for habitability (Ehlmann & Edwards, 2014; Grotzinger et al., 2005), although salinities may have been so high in Mg‐sulfate brines as to render them uninhabitable (Knoll & Grotzinger, 2006; Tosca et al., 2008). Such evaporite minerals have the potential to trap organisms and preserve them as organic fossils which, like those in chert (see below), are readily visible in transmitted light. Gypsum‐permineralized carbonaceous microfossils interpreted as algae and bacteria, the latter comparable to anaerobic nitrate‐reducing sulfide oxidizers, have recently been found in Permian, Miocene, and recent evaporites (Pierre et al., 2015; Schopf et al., 2012). Calcified organic‐rich microbial filaments have also been found in Miocene gypsiferous stromatolites (Allwood et al., 2013; see section 2.3 below for discussion of stromatolites). It has been suggested that fluid inclusions in halite may preserve viable microbial cells over ~100 Myr, although this is controversial because of well‐founded doubts that such cells could repair DNA damage induced by natural radiation over these timescales (Fish et al., 2002; Jaakkola et al., 2016; Satterfield et al., 2005; Vreeland et al., 2000). More research needs to be done on potential controls on the presence and long‐term persistence of morphological and molecular fossils in evaporites before prioritizing these minerals in landing‐site selection. However, vertical and lateral patterns in sulfates and other salts in lacustrine environments can record important information about paleoenvironmental and physiochemical conditions (Bristow & Milliken, 2011). Thus, lacustrine evaporites would be a target for sampling if encountered in the course of a rover traverse, especially if associated with other evidence for habitable water chemistry.
Both Curiosity and Opportunity have also encountered sulfate veins in postburial fractures (Caswell & Milliken, 2017; Vaniman et al., 2014), representing diagenetic precipitates from briny groundwater rather than bottom‐nucleated growth at the sediment‐water interface, the common style of evaporitic deposition on Earth. The potential for such vein sulfates to yield biosignatures is not well understood.
2.3. Phosphate and Carbonate
Laminated and nodular phosphates, and to a lesser extent carbonates, are important sources of well‐preserved fossil microbes and organic matter on Earth (e.g., Figure 1a; Knoll et al., 1993; Morais et al., 2017; Xiao et al., 2014). Unfortunately, similar deposits have not yet been identified on Mars. Small amounts of phosphorus have been detected at Gale Crater, both as igneous‐sourced detrital crystalline apatite in sandstone and as a component of an amorphous/poorly crystalline phase in mudstone, which may be secondary (Forni et al., 2015; Rampe et al., 2017). Magmatic and metasomatic phosphate also occur in Martian meteorites, as do trace amounts of carbonate. Carbonate in the soil at the Phoenix landing site is considered to have formed in situ recently from CO2 dissolved in thin water films (Boynton et al., 2009); it is absent or below detection limits at Gale Crater (Bristow et al., 2017). Climate models indicate that temperatures would have been too low under the “faint young Sun” to sustain large volumes of water on early Mars without abundant atmospheric CO2, conditions that would have promoted widespread carbonate mineralization, particularly as basalt would have buffered pH (Niles et al., 2013; Wordsworth, 2016). Thus, the lack of bedded carbonate on the Martian surface is difficult to reconcile with the abundant evidence for wet conditions in the Noachian‐Hesperian. The early Martian atmosphere may have been warmed by other greenhouse gases with minimal contribution from CO2 and hence insignificant carbonate formation (e.g., Bristow et al., 2017). Alternatively, large carbonate deposits may be concealed beneath alteration assemblages, lava flows, or soil (Clark, 1999) and have yet to be discovered; indeed, isolated carbonates formerly buried to several kilometers have been detected in craters (Michalski & Niles, 2010; Wray et al., 2016). However, the origin of these carbonates is not clear, and impact metamorphism might have damaged biosignatures in such exposures.
Figure 1.

Terrestrial fossils that inform the search for life on Mars: (a) Calcified cyanobacterial sheaths (Girvanella) in limestone, upper Cambrian Campbell's Member, western Newfoundland. Image courtesy of S. Pruss, Smith College. (b) Stromatolites in chert, Archean Strelley Pool Formation, Western Australia. (c) Stromatolites in limestone, Paleoproterozoic Rocknest Formation, Wopmay Orogen, northwest Canada. (d) Stromatolites in sandstone, Neoproterozoic Witvlei Group, Namibia. (e) Filamentous and coccoidal microfossils in chert, Paleoproterozoic Gunflint Formation, Ontario, Canada. Image courtesy of A. H. Knoll, Harvard University. (f) Mat‐forming colonial coccoidal cyanobacteria in chert, Neoproterozoic Min'yar Formation. (g) Wrinkle structures in siltstone draped over conglomerate, middle Cambrian March Point Formation, western Newfoundland. Image courtesy of S. Pruss, Smith College. (h) Organically preserved cyanobacteria (Symplassosphaeridium sp.) macerated from shale, upper Mesoproterozoic Iqqittuq Formation, Arctic Canada. Image courtesy of H. Agić, University of California, Santa Barbara. Scale bar: (a) 200 μm, (e) 75 μm, (f) 625 μm, (g) 60 mm, and (h) 120 μm. The scale of Figures 1c and 1d is indicated by a Swiss army knife, hammer, and lens cap, respectively.
Despite the apparent lack of bedded carbonate on Mars, carbonates formed at low temperatures (~18°C) are present in the ~4.1 Ga Martian meteorite ALH84001 (Halevy et al., 2011). In addition, carbonates of possible hydrothermal origin offer an alternative target for biosignature detection. The Mars Reconnaissance Orbiter identified magnesium carbonate associated with olivine and clays in the Nili Fossae region (Ehlmann, Mustard, Murchie, et al., 2008), and Spirit discovered carbonate‐rich (16–34 wt %) outcrops (named the Comanche outcrops) of similar composition in Gusev Crater (Morris et al., 2010). These carbonates probably formed through the aqueous alteration of mafic precursors by hydrothermal activity. The evidence for hydrothermal activity in Gusev Crater may indicate a genetic similarity between the carbonates there and volcanism‐related, nonmarine, Mg‐rich travertines on Earth. Some young travertines yield organic biomarkers (e.g., Jorge‐Villar et al., 2007) and microbial microfabrics (Riding, 1991). Submarine carbonate vent chimneys can likewise preserve molecular fossils as well as isotopic biosignatures (e.g., Brazelton et al., 2006; Lincoln et al., 2013; Méhay et al., 2013;).
Molecular, microfossil, and isotopic biosignatures in carbonates are vulnerable to damage by fluid throughflow, chemical alteration, and recrystallization over geological time. Young hydrothermal carbonates contain cellular and molecular fossils (e.g., Zhang et al., 2004), and cellular preservation by iron and carbonate minerals has been reported from Jurassic travertines where Ostwald ripening of calcite seems to have inhibited diagenetic alteration (Potter‐McIntyre et al., 2017). Precambrian travertines lack such biosignatures, which may reflect sustained alteration processes on Earth that would be less severe on Mars (Brasier et al., 2013; see section 5 below). However, these rocks do commonly contain stromatolites, that is, layered conical, domal, columnar, or branching macroscopic growth structures attached to a surface and formed by carbonate precipitation and/or the trapping and binding of sediment (Figures 1b–1d; Bosak et al., 2013; Grotzinger & Knoll, 1999; Riding, 1999). Microbes are commonly implicated in these processes, but it has long been clear that not all stromatolite‐like features are necessarily biological, especially those formed by precipitation (rather than trapping and binding). This complicates the interpretation of Precambrian precipitated stromatolites and those that have undergone substantial diagenesis (Allwood et al., 2009; Grotzinger & Knoll, 1999; Grotzinger & Rothman, 1996). Triangular structures exposed perpendicular to bedding on a weathered, heavily metamorphosed carbonate in the Isua Supracrustal Belt in Greenland, for example, which were interpreted by Nutman et al. (2016) as Earth's earliest stromatolites, are morphologically ambiguous (their 3‐D structure is unreported) and lack organic carbon or other evidence to confirm biogenicity.
Although microfossils are rare in carbonate stromatolites, studies of Precambrian examples and modern analogs have identified structures and morphologies with a high potential to record biological activity (e.g., Allwood et al., 2006; Beukes & Lowe, 1989; Bosak et al., 2009, 2010; Dupraz et al., 2004; Grey, 1994; Hoffman, 1976; Jones et al., 1997, 1998; Komar et al., 1965; Reid et al., 2000; Sim et al., 2012; Sumner, 1997). Only recently, however, through a combination of theory, experiment, and field observations, have we begun to understand the processes that produce robust morphological biosignatures in macroscopic stromatolite‐like structures as old as three billion years (Batchelor et al., 2000; Batchelor et al., 2004; Batchelor et al., 2005; Bosak et al., 2013; Cuerno et al., 2012; Dupraz et al., 2006; Mariotti, Perron, et al., 2014, Mariotti, Pruss, et al., 2014; Petroff et al., 2010, 2013; Sim et al., 2012; Walter et al., 1976) or in microscopic textures (Bosak et al., 2009; Bosak et al., 2010; Bosak et al., 2013; Mata et al., 2012). Although most stromatolites are too small to be identified remotely, they would be readily observable by a rover on Mars and would be a prime target for astrobiological sampling. More generally, however, further research is needed to clarify the potential for biosignature preservation in carbonates similar to those so far encountered on Mars.
2.4. Hydrothermal Silica
Hydrothermal systems, both at and below the paleosurface, have long been recognized as likely habitable sites with the potential to preserve fossils (e.g., Farmer & Des Marais, 1999; McKinley et al., 2000; Walter & Des Marais, 1993). Some Noachian terrains are inferred to record mineral alteration by hydrothermal fluids that passed through the Martian upper crust prior to excavation by impact cratering (Ehlmann et al., 2009, 2011; Michalski et al., 2013). The thermal afterglow of impacts themselves can drive hydrothermal circulation in the vicinity of craters (Osinski et al., 2013), which may produce postimpact silica and sulfate veins, as well as Al‐rich clays (e.g., as revealed in Endeavour Crater by the Opportunity rover; Arvidson et al., 2014). A recent global survey of crater central peaks using Mars Reconnaissance Orbiter data has shown that ~22% of those with hydrated minerals show spectral evidence for hydrated (opaline) silica associated with uplifted materials, possible impact melt deposits, and various unconsolidated materials (Sun & Milliken, 2015).
Silica dissolved and mobilized at depth stays in solution at high temperatures, but the expression of hydrothermal systems at the cool sediment‐water or sediment‐atmosphere interface induces rapid, massive surface precipitation. The resulting sinter deposits typically preserve microbial filaments as silicified casts, molds, and coatings, often in such density and abundance that they constitute a large fraction of the rock and determine its macroscopic texture (e.g., Cady & Farmer, 1996; Munoz‐Saez et al., 2016; Trewin et al., 2003). Young examples yield a wide range of lipid biomarkers representative of hot spring organisms (e.g., Gibson et al., 2008; Kaur et al., 2008; Pancost et al., 2006). Other biosignatures can include fenestrae representing silicified bubbles of microbially generated gases (Bosak et al., 2009, 2010; Mata et al., 2012) and millimeter‐scale laminated fabrics arising from the interplay of biofilms and silicifying fluids (e.g., Konhauser et al., 2004). Opaline siliceous sinter transforms to solid microcrystalline or cryptocrystalline forms (cristobalite, tridymite, and quartz, i.e., chert) during burial, which may preserve the organic remains of eukaryotes and prokaryotes at submicron resolution. The best‐known example is the Devonian (~410 Ma) Rhynie Chert in Scotland, which preserves plants, animals, fungi, and bacteria entombed and permineralized with silica (Trewin, 1993, 1996) and yields well‐preserved organic biomarkers (e.g., Preston & Genge, 2010; Qu et al., 2015). Finely laminated Archean cherts containing hydrothermally silicified biofilms also preserve some organic matter, but the high metamorphic grades of these rocks (Westall, Campbell, et al., 2015) ensure that any molecular biosignatures have been erased. Curie point pyrolysis of an Archean chert from the Warrawoona Formation of the Pilbara Craton in Western Australia yielded alkane/alkene doublets with a slight odd/even preference (Derenne et al., 2008), an indisputable biosignature. However, given the metamorphic grade of rocks from the locality (Flannery et al., 2018) and the possibility of contamination from contemporary surface‐dwelling microbes, results such as this should be viewed with caution. Nevertheless, cherts formed on early Mars would not have been subjected to such intense metamorphism and may retain biosignatures in contrast to similar rocks on Earth (see section 5).
Silicification of organic remains involves the bonding of silicic acid to organic cell walls or envelopes, ensuring long‐term stability (e.g., Knoll, 1985). Experiments have confirmed that diverse archaeal and bacterial extremophiles and even viruses silicify readily in silica saturated solutions, with minimal dependence on cell/substrate type, pH, or salinity (Orange et al., 2009, 2013, 2014; Westall et al., 1995; Westall, 1997). Such results suggest that silicification could outpace cell lysis and degradative processes in brines on early Mars (e.g., Harrison et al., 2016; Toporski et al., 2002; Yee et al., 2003).
Besides occurring in hydrothermal settings, amorphous silica is expected to be present on Mars as a result of low‐temperature chemical weathering of basalt (McLennan, 2003; Tosca et al., 2004), and orbital and in situ observations have shown it to be widespread (Milliken et al., 2008; Squyres et al., 2008; Sun & Milliken, 2015). It may be challenging to differentiate hydrothermal silica from silica enrichment by in situ weathering processes (potentially including “acid fog”; Tosca et al., 2004); such weathered rocks may be aggressively altered and are not expected to preserve biosignatures. Silica‐rich deposits near the remotely observed Nili Patera caldera have been suggested to represent ancient hydrothermal systems, although not necessarily formed at the surface, based on their setting and distribution (Skok et al., 2010). Siliceous material examined by the Spirit rover in Gusev Crater has also been interpreted as a hydrothermal deposit, although other explanations of its chemical composition are possible (Squyres et al., 2008). Stratiform, “rubbly” nodules of opaline silica in Gusev Crater have been considered morphologically comparable to digitate, biologically influenced sinter nodules produced in shallow water at the El Tatio volcanic spring in Chile, which are rich in preserved microbial filaments (Ruff & Farmer, 2016). However, the simple digitate appearance of these nodules could arise from abiotic processes (aggregation, concretionary growth, sedimentation, and/or weathering) and is not in itself a biosignature or even definitive evidence of hot spring deposition (Anderson, 1930; Grotzinger & Knoll, 1999; McLoughlin et al., 2008). Nevertheless, true hot spring sinters on Mars would represent an excellent search target for silicified microfossils and organic matter from a paleoenvironment likely to have been habitable. Perhaps, the most promising location for preservation in silica identified to date on Mars occurs at Gale Crater, where a 5–10‐m‐thick interval of lacustrine strata in the Murray Formation is enriched in silica (see below).
2.5. Chert and Silicified Sediments
Bedded and nodular marine cherts on Earth, which are a diagenetic product of amorphous silica precipitated at or just below the seafloor, represent a major source of well‐preserved microfossils, particularly of Precambrian age (e.g., Barghoorn & Tyler, 1965; Schopf, 1968; Schopf et al., 2008) when pore waters became silica saturated in the absence of silica‐secreting organisms (Maliva et al., 2005; Siever, 1992). This led to rapid, early (perhaps syndepositional) silica precipitation that formed a rigid, impermeable solid material, resistant to later fluid alteration (Bartley et al., 2000; Ramseyer et al., 2013; Stolper et al., 2017). Such silica‐rich rocks on Earth, especially where amorphous content is high, can result in good or even spectacular preservation of cells and colonies (Figures 1e and 1f); dozens of examples are known from the Proterozoic (Schopf & Klein, 1992), including the iconic, densely packed assemblages of filamentous and coccoidal bacteria in the Gunflint Formation (Ontario, Canada, ~1.9 Ga) and the Bitter Springs Formation (Central Australia, ~850 Ma). The Archean record is more haphazardly preserved, probably because of ubiquitous hydrothermal and metamorphic overprinting and recrystallization. Chert (including early replacive chert after carbonate) is the dominant preserving medium of most purported microfossils older than 2.5 Ga (Schopf, 2006, and references therein), although most of these are controversial (Brasier et al., 2006). Historically, however, there has been a lack of attention to siliciclastic lithologies, which have recently begun to prove fruitful (Javaux et al., 2010; Wacey et al., 2011).
On Mars, the aqueous alteration of basaltic crust is thought to have supplied abundant silica to rivers and lakes (McLennan, 2003; McLennan & Grotzinger, 2008). As on Earth, this silica could have solidified very early, providing an ideal medium for the preservation of any microorganisms living in the water column, on the lake floor, or in shallow subsurface sediment. Opaline silica has been observed from orbit in laterally continuous, well‐stratified deposits adjacent to the Valles Marineris canyon system, and in some cases these deposits occur as inverted channel systems (Milliken et al., 2008; Weitz, Milliken, et al., 2008; Weitz et al., 2010). Silica has also been observed in strata associated with potential sublacustrine fans within Melas Chasma (Metz et al., 2009) and in closed basins in the Noctis Labyrinthus region (Thollot et al., 2012).
More recently, the Curiosity rover recovered evidence for sedimentary silicification in silica‐rich mudstones in the Marias Pass area of Gale Crater where the lower Murray Formation forms a thick sequence of lacustrine mudstones that interfingers and overlies fluvial‐deltaic sandstones and conglomerates (Grotzinger, Gupta, et al., 2015; Morris et al., 2016; Hurowitz et al., 2017; Rampe et al., 2017). The grain size is below the limit of resolution (<60–70 μm) of the Mars Hand Lens Imager, and parallel stratification with a mean lamina thickness of about ~0.5 mm extends laterally for at least several tens of centimeters (Figure 2). Quiescent subaqueous deposition is further evidenced by the absence of cross stratification, mudcracks, or any evidence for transport, erosion, or reworking. The “Buckskin” rock drilled by the Curiosity rover is characterized by ~40 wt % crystalline and ~60 wt % X‐ray diffraction amorphous material, and a bulk composition of ~74 wt % SiO2. The crystalline silica comprises trydimite and cristobalite with a bulk rhyolite‐like composition, suggesting a felsic volcanic provenance for the sediment (Morris et al., 2016). The amorphous material is silica rich, ~39 wt % opal‐A and/or silica glass and opal‐CT, and most likely represents an authigenic lacustrine precipitate or diagenetic alteration product (Hurowitz et al., 2017; Morris et al., 2016).
Figure 2.
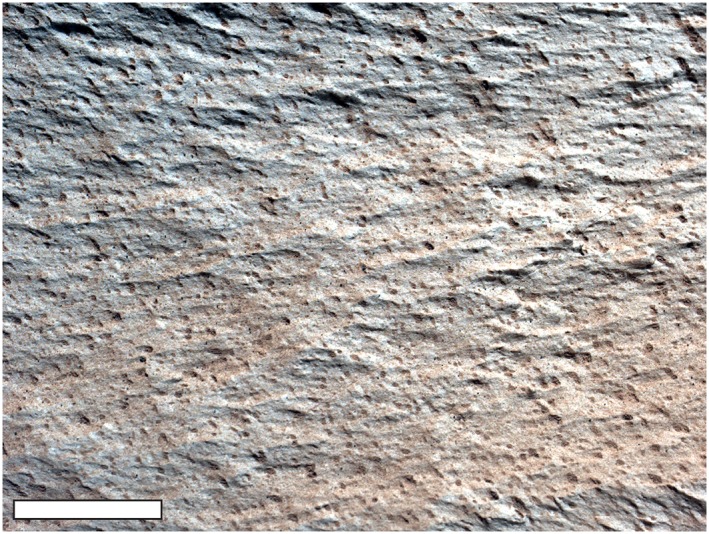
Photograph taken by the Curiosity rover of “Lamoose” target, a float block from the Murray Formation, Gale Crater, Mars. It is minimally dust covered (reddish tone) and sculpted by wind to reveal very fine lamination (here oriented upper left to lower right) and fine grain size. Wind‐induced surface striations trend obliquely to the primary depositional lamination. Scale bar: 1 cm.
Such a facies of finely laminated, fine‐grained silica‐rich mudstone, with substantial amorphous silica, may represent a favorable context for microfossil preservation. Early lithification would have sealed the rock (and any contained fossils) from later fluids that oxidized other parts of the Murray Formation (Hurowitz et al., 2017; Rampe et al., 2017). The presence of magnetite of probable authigenic origin in one drill hole (rather than hematite as in 14 others spread over ~150 m of section) signifies a lower degree of oxidation in either the primary or diagenetic environment, or both (Grotzinger, Crisp, et al., 2015; Hurowitz et al., 2017; Morris et al., 2016; Rampe et al., 2017; Vaniman et al., 2014). Indeed, magnetite as well as some of the hematite in Gale Crater could have formed by redox‐related primary precipitation, a process conducive to the preservation of cellular fossils (Fraeman et al., 2016; Hurowitz et al., 2017). Iron oxides can also adsorb silica and enhance silica precipitation, further strengthening the potential for preservation (Meister et al., 2014). The occurrence of a silica‐magnetite mudstone facies in part of the Murray formation demonstrates the potential for finding similar types of sedimentary rocks in lacustrine settings elsewhere on Mars, providing a strong candidate for sample return.
2.6. Siliciclastic Sediments
Orbiter‐obtained geomorphological evidence for siliciclastic facies on Mars indicates alluvial fan, fluvio‐deltaic, sublacustrine fan, and aeolian deposits (e.g., Dromart et al., 2007; Malin & Edgett, 2003; Metz et al., 2009; Milliken et al., 2014; Moore & Howard, 2005). Rover observations confirmed the presence of proximal to distal fluvial, deltaic, lacustrine, and aeolian facies (Grotzinger et al., 2005; Grotzinger et al., 2014; Lewis et al., 2008; Williams et al., 2013). The organization of fluvial systems at Gale Crater shows facies transitions analogous to terrestrial “source‐to‐sink” networks leading to accumulation of lacustrine mudstones hundreds of meters thick (Grotzinger, Gupta, et al., 2015; Szabó et al., 2015; Fedo et al., 2017). Fluvial channel sediments represent high‐energy depositional environments where the chances of preserving fossils are poor, although allochthonous biosignatures may result from reworking, transport, and sometimes concentration, for example, in fossiliferous clasts, by fluvial processes. Distal deltaic, lacustrine, shoreline and subtidal deposits, on the other hand, could preserve a wide range of sedimentary microbialites (Figures 1d and 1g), morphological fossils (Figure 1h), and/or organic biosignatures (e.g., Ehlmann, Mustard, Fassett, et al., 2008; Summons et al., 2011; Grotzinger et al., 2014).
Siliciclastic sediments are texturally and chemically diverse and preserve fossils in a variety of modes. Fine‐grained and clay‐rich siliciclastic lithologies are associated with some of the best preservation of microbes and soft‐bodied eukaryotes on Earth (e.g., Butterfield, 1990, 1995; Callow & Brasier, 2009; Farmer & Des Marais, 1999; Javaux & Knoll, 2017; Yuan et al., 2011), including those of Archean age (Javaux et al., 2010). The low permeability of fine‐grained sediments limits diffusion away from decaying remains once they are buried and favors the precipitation of authigenic minerals such as carbonate, pyrite, and phosphate. These minerals can replicate cells and tissues and/or cement the grains around them into concretions or high‐resolution molds, a process that has been studied experimentally (e.g., McCoy et al., 2015). The charged surface area of sedimentary clay minerals adsorbs and retains organic matter; the organic carbon content of marine mud and ancient shales correlates strongly with the total surface area of clay minerals within these lithologies (e.g., Hedges & Keil, 1995; Kennedy et al., 2002), particularly in sediments rich in smectite (Ransom et al., 1998). Cyanobacteria become coated with clay minerals in less than a week in experiments with sand, silt, dissolved silica, and suspended clays (Newman et al., 2016, 2017), ultimately resembling Precambrian‐Cambrian fossil filaments composed of aluminosilicates (e.g., Callow & Brasier, 2009). However, the relative importance of trapping suspended clays versus clay precipitation in natural environments remains unknown, and trapping is thought to dominate (Konhauser et al., 1998; Konhauser & Urrutia, 1999; Newman et al., 2016, 2017).
The late Precambrian and early Paleozoic fossil record includes a large number of clay‐hosted Konservat‐Lagerstätten that preserve soft tissues as carbonaceous compressions, commonly with a secondary coating of authigenic clays that appears to track the original organic matter (Briggs, 2003). The role of preexisting clay in retarding decay appears to be more important for this style of preservation than the precipitation of early authigenic minerals (Gaines et al., 2008). Al3+ and Fe2+ ions, for example, may stabilize organic matter by promoting the crosslinking (“tanning”) of proteins or inhibiting the activity of autolytic enzymes (Butterfield, 1995; Petrovich, 2001; Wilson & Butterfield, 2014). Experiments have shown that polychaetes and crustaceans buried in the aluminum‐rich clay kaolinite for months‐to‐years are better preserved than those buried in other minerals (Wilson & Butterfield, 2014; Naimark, Kalinina, Shokurov, Boeva, et al., 2016). Clays rich in Al3+ and Fe2+ have likewise been shown to suppress the growth of various heterotrophic bacteria, including representatives of the microbial community typically involved in tissue decay, providing the first clear evidence of how clays might inhibit decay (McMahon et al., 2016; Morrison et al., 2016). Such interactions may explain why particular clay mineralogies correlate with the presence or absence of fossils in some stratigraphic sections (e.g., Anderson et al., 2014, 2018).
More generally, taphonomic factors identified as favorable for preservation in siliciclastics include reduced microbial activity in sediments, the presence of iron, the type of clay, the activity of microbes in photosynthetic mats, elevated concentrations of silica, redox gradients, and the activity of sulfate reducing microbes that ultimately produce pyrite (Darroch et al., 2012; Gehling, 1999; Laflamme et al., 2011; Naimark, Kalinina, Shokurov, Boeva, et al., 2016; Tarhan et al., 2016; Wilson & Butterfield, 2014). The effects of most of these factors need to be investigated further before we can infer their likely impact on Mars. However, our current understanding suggests that Fe/Mg‐rich detrital smectites in fluvio‐deltaic and lacustrine deposits in ancient lake basins on Mars provide a promising context for fossil and organic preservation, especially where Fe‐rich end‐members can be identified (e.g., Ehlmann, Mustard, Fassett, et al., 2008; Hurowitz et al., 2017; Milliken & Bish, 2010; Rampe et al., 2017; Vaniman et al., 2014).
Textural evidence of microbial activity occurs in siliciclastic lithologies from mudstone to sandstone and ranges from patterned textures on bedding planes (“microbially induced sedimentary structures” or “MISS”; Noffke et al., 2001) to three‐dimensional stromatolites (Schieber et al., 2007). In the absence of direct evidence of biogenicity, some MISS are difficult to distinguish visually from structures formed by sediment loading, shrinkage or shearing, or by the interaction of sediment with currents, escaping pore water, or early cements (Davies et al., 2016; Schieber et al., 2007). Others can probably only form via the growth of microbial mats on sandy surfaces, which may wrinkle (Figure 1g) or crack subaqueously at scales that indicate microbial aggregation or biostabilization (Gehling & Droser, 2009; Mariotti, Perron, et al., 2014; McMahon et al., 2017). Interactions between microbial surfaces, clay minerals, and microbial sulfide or silica at the surface of some MISS can preserve organic matter and replicate ~100‐μm‐scale filamentous microfossils in clay minerals or pyrite, even in relatively coarse lithologies (Callow & Brasier, 2009). MISS could be recognizable at distances of several meters and would warrant investigation if detected. However, the hypothesis that such structures may be visible in Curiosity images from the Gillespie Lake sandstone member within Gale Crater (Noffke, 2015) is not compelling. In common with Davies et al. (2018), we interpret the photographed features as erosional/fracture surfaces, not bedding planes; where dust‐free bedding planes are exposed on nearby outcrops, they show no textural features attributable to microbial mats. Indeed, MISS may be difficult to recognize on Mars because exposed bedding planes tend to be effaced by aeolian weathering.
2.7. Sedimentary and Diagenetic Iron Oxides
Iron oxide minerals are widespread on Mars. Remote sensing data have shown that hematite occurs in sedimentary outcrops at Meridiani Planum, chaos terrains around Valles Marineris, and various locations associated with interior layered deposits within Valles Marineris (Bibring et al., 2007; Christensen et al., 2000, 2001; Glotch & Christensen, 2005; Glotch & Rogers, 2007; Weitz, Lane, et al., 2008; Weitz et al., 2012). Detailed orbital geologic mapping and in situ measurements at Meridiani Planum by the Opportunity rover led to a consensus view that hematite in these locations formed through secondary diagenetic processes, likely associated with regional groundwater upwelling (Chojnacki & Hynek, 2008; Le Deit et al., 2008; Lichtenberg et al., 2010; Mangold et al., 2008; Massé et al., 2008; Murchie et al., 2009; Noe Dobrea et al., 2008; Poulet et al., 2008; Roach et al., 2010; Squyres et al., 2004; Sowe et al., 2012; Wendt et al., 2011). Hematite was recently identified within the sedimentary rocks on Mount Sharp in Gale Crater by both orbital (Fraeman et al., 2013; Milliken et al., 2010) and in situ Curiosity data (Rampe et al., 2017). In contrast to other hematite‐bearing localities on Mars, hematite appears to be intimately associated with primary sedimentary structures here, suggesting that it reflects pervasive secondary oxidation or authigenesis of hematite (or a ferric precursor phase) in an oxidizing lacustrine environment (Hurowitz et al., 2017). Detailed measurements by Curiosity are currently being used to test these end‐member hypotheses.
Environments where hematite and other ferric iron minerals are precipitated have been considered thermodynamically inimical to organic matter preservation because of a variety of complex mechanisms known as Fenton reactions (Fenton, 1894). Fenton chemistry involves the reaction of peroxides with ferrous or ferric iron to form free radicals, which rapidly oxidize organic molecules (Pignatello et al., 2006). On this basis hematite‐bearing rocks explored by the Mars Exploration Rover Opportunity in Meridiani Planum have been considered to have poor organic preservation potential (Sumner, 2004). If peroxides (Encrenaz et al., 2012) and perchlorates (Hecht et al., 2009) observed on the modern surface of Mars were similarly abundant early in the planet's history, then Fenton chemistry may have strongly limited organic preservation (and habitability) in iron‐rich environments. “Photo‐Fenton” chemistry resulting from strong fluxes of UV radiation in combination with peroxide, perchlorate, and hematite has been shown to be lethal to microorganisms (Wadsworth & Cockell, 2017). However, molecular and morphological microbial biosignatures preserved in association with iron minerals on Earth imply that the taphonomic influence of iron is not straightforward. Hematite can aid the preservation of sedimentary organic matter under certain circumstances (Adhikari & Yang, 2014). Evaporatively concentrated iron oxides in the acidic Rio Tinto fluvial system in Spain entomb diverse microbes at the present day and have done so for millions of years, preserving cells, molecular fossils, and microbial laminations in ferruginous siliciclastic sediments (Fernández‐Remolar & Knoll, 2008; Preston et al., 2011). Modern lipid biomarkers and permineralized microbial body fossils are also preserved by iron minerals at Yellowstone hot springs (Parenteau et al., 2014; Parenteau & Cady, 2010).
Iron formations (sedimentary rocks with >15% Fe content) are abundant in Earth's rock record from ~2.5 to 4 Ga (e.g., Trendall, 2002). Although the mechanisms that formed these lithologies are debated (including the role of bacterial iron oxidation), it is widely thought that iron formations were deposited under a reducing terrestrial atmosphere that permitted the dissolution, transport, and dissemination of ferrous iron across sedimentary basins. The majority of Martian sedimentary outcrops known to yield ferric oxide do not resemble terrestrial iron formations, but several studies have theorized their presence on Mars (Bridges et al., 2008; Burns, 1993; Fallacaro & Calvin, 2006; Schaefer, 1996). The total organic carbon content of banded‐iron formations on Earth is typically low compared to that of adjacent shales or carbonates (e.g., Klein & Beukes, 1989), although carbonaceous material is associated with iron minerals in well preserved banded‐iron formations as old as 3.2 Ga (Bontognali et al., 2013). Hematite coatings have been observed intimately associated with organic‐walled microfossils in the ~1.9 Gunflint and Biwabik Iron Formations and attributed to postdepositional precipitation from oxidizing fluids (Alleon et al., 2016; Shapiro & Konhauser, 2015), and iron minerals have also been observed within Gunflint microfossil cell lumina (Lepot et al., 2017), demonstrating that iron mineralization does not preclude cellular preservation in kerogen on billion year timescales.
3. Preservation of Molecular Fossils and Other Organics on Mars
Organic molecules were recently detected at the parts per billion level on the Martian surface, consistent with the expectation that trace amounts (Flynn & McKay, 1990) should be present in the soil as a result of meteorite falls throughout Martian history. Nevertheless, the Martian surface is a harsh environment for the survival of organic compounds. Ionizing particles penetrate the surface and cause cascades of secondary particles (Dartnell et al., 2007; Kminek & Bada, 2006). Curiosity's Radiation Assessment Detector instrument revealed that present‐day radiation flux, penetrating rock and soil, is sufficient to destroy 99.9% of 100‐atomic mass unit biomolecules in the uppermost 4–5 cm within 650 million years (Hassler et al., 2014). The dose of radiation received probably does not vary greatly with lithology (Kim et al., 1998), but molecules stabilized by diagenetic processes and mineral interactions may survive for longer (as does organic matter in carbonaceous chondrite meteorites, which are irradiated in space for millions of years; Hassler et al., 2014). Measurement of cosmogenic nuclides by Mars rovers reveals the exposure age of rocks, enabling the search for organic biomarkers to be focused on the most recently uncovered materials (e.g., Farley et al., 2014). An alternative approach is to extract samples from beneath the irradiated zone; the ESA/Roscosmos ExoMars rover will carry a 2‐m drill for this purpose (Vago et al., 2017). Drilling to much greater depths (~1,000 m) would be necessary to allow access to ancient permafrost water‐ice unaffected by past warming events, which might contain cryopreserved biomolecules or even cells. Such an enterprise is not currently planned and would face significant engineering and planetary protection challenges (McKay et al., 2013; Smith & McKay, 2005).
UV‐promoted chemical oxidation, as well as ionizing radiation, removes organic matter from Martian regolith. In an attempt to rationalize the Viking experimental findings, Benner et al. (2000) hypothesized that the chemical environment on Mars was not conducive to the preservation of organic compounds. In particular, peroxides and hydroxyl radicals were likely continuously produced on Mars via Fenton chemistry that, today, is widely used to clean up waste water. Further, these authors hypothesized that these strong oxidants would progressively attack any organic matter on the Martian surface leaving only a residue of organic acid salts including acetate, oxalate, and benzene carboxylates. Such salts would not be sufficiently volatile to be detected by the thermal desorption/pyrolysis experiments conducted by Viking and Curiosity.
The Sample Analysis at Mars instrument suite on Curiosity detected limited organic compounds via pyrolysis‐gas chromatography‐mass spectrometry and identified small aliphatic and aromatic organic fragments in pyrolysis‐evolved gas analysis experiments (Freissinet et al., 2015; Glavin et al., 2013; Ming et al., 2014), results consistent with the survival of some macromolecular organic matter in Martian sediments, possibly residues of ancient carbonaceous meteoritic infall (Flynn & McKay, 1990). The detection of chlorinated compounds in these sediments may be a result of the reaction during pyrolysis of organic compounds indigenous to mudstone with highly oxidizing oxychlorine compounds also present in the environment (Freissinet et al., 2015; Glavin et al., 2013; Ming et al., 2014). Measurements of cosmogenic nuclides revealed that the host mudstone was exposed only 78 ± 30 million years ago (by scarp retreat) even though crater counting indicates that the sediment was deposited 4.1–3.5 Ga (Farley et al., 2014). Thus, recent exposure may account for the survival of the organics.
4. Preservation Potential of Plausible Mars Analog Microbes
The paleontological term “preservation potential” conventionally refers to the likelihood that a particular taxon, tissue type, or molecule will enter the fossil record given suitable environmental conditions; thus, the primary influence on preservation potential in this sense is the nature of the organism. Earth's macroscopic fossil record is dominated by high‐preservation‐potential biomineralized animal skeletons, which originated in the Neoproterozoic and rapidly diversified during the Cambrian and Ordovician Periods (Porter, 2007). However, the window of opportunity for such complex life to arise on Mars apparently closed early, and the required innovations seem improbable given that eukaryotes appear to have arisen only once on Earth (Summons et al., 2011; Westall, Foucher, et al., 2015). Crater count‐based ages for valley networks on Mars and the paucity of valleys in younger terrains suggest that the availability of surface water had diminished by middle‐to‐late Hesperian time (Fassett & Head, 2011), although results from the Curiosity rover show that local‐scale in situ observations are necessary to ground truth such global inferences (Grotzinger et al., 2014, Grotzinger, Gupta, et al., 2015; Hurowitz et al., 2017). The period of peak water availability at the Martian surface may thus have been 1–2 billion years before the origin of eukaryotes on Earth (Dacks et al., 2016), and habitable environments on the Martian surface may have been more restricted than on Earth since that time (Ehlmann & Edwards, 2014; Grotzinger & Milliken, 2012). Moreover, the low abundance of free oxygen in the Martian atmosphere at any time in its history probably precluded the evolution of large, multicellular organisms with differentiated tissues (e.g., Erwin, 2015). For these reasons, scenarios for potential life on Mars tend to involve anaerobic microbial models (e.g., Rothschild, 1990; King, 2015; Westall, Foucher, et al., 2015). Anaerobic microbes thought to be capable of living in the conditions of early Mars include methanogens, sulfate and sulfur reducers, photosynthesizers, fermenters, iron reducers, and phototrophic or denitrifying iron oxidizers (Cockell, 2014b; Nealson & Conrad, 1999; Nixon et al., 2013). Favorable conditions for these kinds of organisms have been demonstrated for mudstones deposited in the lacustrine environment of Gale Crater (Grotzinger et al., 2014).
The intrinsic preservation potential of microbial groups depends first on their resistance to physicochemical decay, including degradation by heterotrophic microbes, autolytic enzymes, osmotic stress, heat, and irradiation, and second on their tendency to serve as templates for the nucleation of minerals from the environment or resulting from their own cellular metabolism. Research into the preservation potential of microbial groups that could have lived on early Mars is at an early stage.
4.1. Methanogens and Sulfate‐/Sulfur‐Reducing Bacteria
Microbial methanogenesis and sulfate/sulfur reduction are inferred to be among the oldest metabolic strategies on Earth (e.g., Bontognali et al., 2012; Shen et al., 2009; Ueno et al., 2006), but microbes that carry out these metabolisms do not produce diagnostic morphological fossils because their cells are simple in shape and lack robust sheaths. However, these metabolisms can generate strong isotopic fractionations in minerals, organic matter, and fluids (Bontognali et al., 2012; Ueno et al., 2006; Williford et al., 2016). Microbial methane is strongly depleted in carbon‐13 relative to carbon‐12. This signature has been reported in Archean sedimentary organic matter (e.g., Eigenbrode & Freeman, 2006; Hayes, 1994; see Slotznick & Fischer, 2016 for a discussion of other metabolisms that could have produced this signal), in Archean basalt fluid inclusions (~3.5 Ga; Ueno et al., 2006), and in Phanerozoic carbonate generated by methane oxidation (Bristow & Grotzinger, 2013; Campbell, 2006; Peckmann & Thiel, 2004). Sulfate and sulfur reduction can also result in the formation of iron sulfide minerals that coat or replicate cells; when sulfate does not limit the rates of sulfate reduction, the resulting pyrite may be depleted in sulfur‐34 relative to sulfur‐32. Syntrophic consortia of sulfate‐reducing bacteria and anaerobic methane oxidizing archaea have also been found to mediate the encrustation of cells with clay minerals in marine sediments (Chen et al., 2014).
4.2. Photosynthetic Bacteria
Other microbes—most importantly cyanobacteria, which use water as an electron donor—produce extracellular sheaths that are robust against degradation and also provide a favorable locus for mineral nucleation (e.g., Bartley, 1996). Although cyanobacteria‐lacking sheaths also have a long fossil record, those that possess them can promote their own preservation by precipitating and trapping clay minerals (Newman et al., 2016, 2017). In environments where iron(II) is abundant, the sheaths can become coated by iron oxides when iron(II) is oxidized by photosynthetic oxygen (e.g., Parenteau & Cady, 2010). Cyanobacteria also trap and bind carbonate grains that are cemented into characteristic coarse laminae; they are the primary builders of modern stromatolites (e.g., Reid et al., 2000). The degradation of exopolymeric material in the zones of sulfate reduction also promotes the precipitation of finer, micritic laminae and cements these structures (Dupraz et al., 2009; Vasconcelos et al., 2006; Visscher et al., 2000). However, textural differences between modern stromatolites and most Archean and Proterozoic examples (Grotzinger & Knoll, 1999), differences in the seawater chemistry now and in the past (Bosak & Newman, 2003), and the late inferred timing of the evolution of cyanobacteria (e.g., Magnabosco et al., 2018) make modern marine stromatolites and microbialites imperfect analogs for structures from the early Earth or Noachian‐Hesperian Mars.
Earlier photosynthesizers on Earth are thought to have used reduced sulfur, hydrogen, iron(II), hydrogen peroxide, or organic acids as electron donors (Blankenship & Hartman, 1998; Konhauser et al., 2005). Microbial communities enriched on these electron donors have been found to promote mineralization in experiments. Like cyanobacteria, anaerobic iron(II) oxidizing phototrophs can become encrusted with iron minerals (Posth et al., 2013; our Figures 3a–3c). These minerals include iron carbonate, silica, and microcrystalline iron minerals (Figure 3d), illustrating a fossilization mechanism that could occur in an anoxic environment rich in iron and silica, potentially characteristic of early Mars. Similar experimental cultures enriched on hydrogen or methane develop micrometer‐thick mineral layers comprising 10‐nanometer‐sized particles after 1–4 months (Figures 4a–4c). These precipitates include calcite, dolomite, and minor amounts of clay minerals (Figure 4d).
Figure 3.
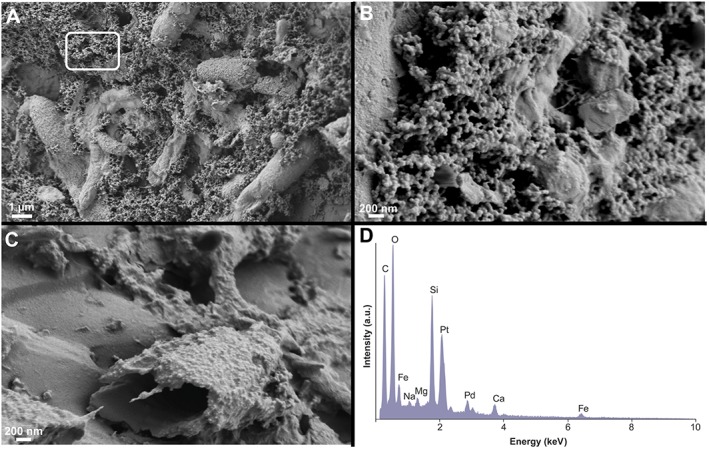
Scanning electron micrographs of cells and precipitates in cultures of anoxygenic photosynthetic microbes enriched from the anoxic mud of Fayetteville Green Lake, NY. The cultures were enriched in a photosynthetic minimal medium with the concentrations of major ions that matched those in Fayetteville Green Lake. The biofilms grew on quartz sand in the presence of 1 mM Fe(II) under an atmosphere of 20% CO2 and 80% N2 at pH 7 on a 12‐h day:12‐h night cycle. Green sulfur bacteria (Chlorobium sp.) are the main photosynthetic organisms in these cultures; other strictly anaerobic microbes such as Geobacter, Acholeplasma, and Desulfomicrobium sp. are also present. (a) Cells heavily encrusted by microcrystalline minerals. (b) Close‐up of the nanometer‐scale minerals covering surfaces of cells. (c) Remnants of a rod‐shaped cell encased in minerals. (d) Energy dispersive X‐ray spectrum of precipitates marked by the white rectangle in Figure 3a shows the presence of Fe, Si, Ca, and O. The dissolved silica was not added to the culture medium: the silica in the precipitates around cells was derived from the quartz sand.
Figure 4.
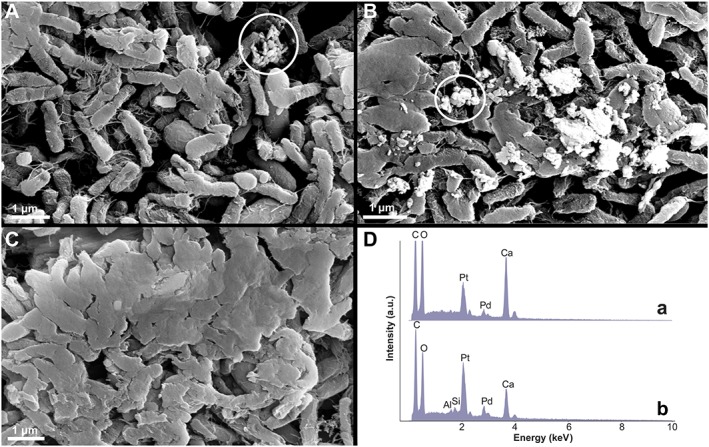
Scanning electron micrographs of photosynthetic cultures enriched from the anoxic mud of Fayetteville Green Lake, NY in the presence of methane (CH4) and hydrogen (H2). The cultures were enriched and grown on aragonite sand in a photosynthetic minimal medium with concentrations of major ions that matched those in Fayetteville Green Lake. The medium was reduced by 50‐mM Na2S. All cultures were grown at pH 7, on a 12‐h day:12‐h night cycle. Cultures enriched in the presence of H2 were grown under an atmosphere of 5% H2, 15% CO2, and 80% N2, those enriched on CH4 were grown under an atmosphere of 5% CH4, 15% CO2, and 80% N2. The cultures contain anoxygenic photosynthetic microbes; cultures enriched on hydrogen also produce methane. (a) One‐month old culture enriched on CH4. (b) One‐month old culture enriched on H2. (c) Four‐month‐old H2 culture. (d) Energy Dispersive X‐ray Spectroscopy spectrum (a) of the precipitates marked by the circle in Figure 4a. The cultures were grown in the absence of added clay minerals, but the Energy Dispersive X‐ray Spectroscopy spectrum (b) of the area marked by the circle in Figure 4b and X‐ray diffraction analyses suggests that clay minerals form in these cultures.
More generally, although anoxygenic photosynthetic microbes lack thick cyanobacterial‐like sheaths, they can form cohesive, sturdy microbial mats replete with extracellular polymeric substances that trap sediment grains and stabilize sediments (Bosak et al., 2013). The interaction of anoxygenic photosynthetic communities with sediments and flowing water can therefore be expected to produce different morphological signals at the sediment‐water interface. The precipitation of authigenic clays and iron minerals within anoxygenic photosynthetic communities requires more investigation in experiments and also hints at favorable conditions for fossilization.
4.3. Other Iron Oxidizers
Other iron oxidizers can thrive in diverse settings where Fe2+ is brought into contact with electron acceptors (including O2, NO3 −, ClO3 −, and ClO4 −). This metabolism fuels chemosynthesis by bacteria that form distinctive, mineralized tubular sheaths and twisted or branching stalks of iron oxyhydroxides within centimeter‐thick mats (Chan et al., 2009, 2011; Edwards et al., 2004, 2011; Emerson et al., 2010). This bacterial iron oxyhydroxide transforms into hematite during diagenesis, and well‐preserved examples have been described from hydrothermal jaspers ranging in age from Ordovician to Cretaceous (Little et al., 1999; Little et al., 2004; Little & Thorseth, 2002). Probable iron oxidizing organisms are represented by hematite microfossils in rocks at least as old as 1.74 Ga (Little & Thorseth, 2002; Slack et al., 2007). The bacteria‐like microfossils of the ~1.9 Ga Gunflint Iron Formation are preserved on a compositional spectrum from organic carbon to hematite, but it is debated whether the iron oxide represents an original metabolic product or later diagenetic replacement (Planavsky et al., 2009; Shapiro & Konhauser, 2015; Lepot et al., 2017). Hematite microstructures in the Archean Nuvvagittuq Supracrustal Belt in Canada, constrained to 4.3–3.8 Ga, have been interpreted as autofossilized iron oxidizers and thus as the oldest known microfossils (Dodd et al., 2017). Their simple tubular morphology and parallel arrangement, however, are compatible with abiotic alternatives (e.g., Garcia‐Ruiz et al., 2003, 2009; Wacey et al., 2018), and a lack of information about field relationships undermines the reported age and biogenicity. Furthermore, the carbon isotopic data provided by Dodd et al. (2017) in support of claims for biogenicity are not distinguishable from abiotic alternatives (Bottinga, 1969; van Zuilen et al., 2002).
The capacity of hydrothermal Fe‐Si systems to preserve organic matter in association with inorganic elemental, mineral and morphological biosignatures is poorly understood. Also unclear is whether the organic carbon associated with these systems preserves diagnostic isotopic biosignatures. One study suggests that autotrophic iron oxidation yields organic matter that is >15‰ lower in δ13C than organic compounds preserved in association with abiotic iron oxides precipitated in submarine hydrothermal settings (Kennedy et al., 2010). This signature could be preserved in organic matter associated with iron oxides and has been reported from iron‐rich Jurassic concretions from a nonhydrothermal setting, which yielded δ13Corg values of −20.55‰ (Weber et al., 2012).
Microbial oxidation of iron could have occurred on early Mars, given the abundance of reduced iron. Electron acceptors may have been limiting; plausible candidates include nitrate (Stern et al., 2015) and perchlorate (Glavin et al., 2013; Hecht et al., 2009), although the latter has not been shown convincingly to support the growth of iron oxidizers (Nixon et al., 2012). If Martian iron oxidizing microbes were preserved in a similar fashion to those on Earth, such structures might even be observable in situ by rover‐mounted instruments. Unlike silica‐hosted carbonaceous microfossils that commonly occur as filaments and spheroids <10 μm in diameter, microfossils preserved in iron oxide on Earth commonly exhibit textures with dimensions closer to tens to hundreds of micrometers, which is the observational scale of Mars rover‐based instrumentation (Williams et al., 2015).
4.4. Iron Reducers
Microbial iron reduction has been implicated in the precipitation of authigenic clays in silica‐rich deltaic sediments (Michalopoulos & Aller, 1995, 2004). Enhanced by the trapping of suspended clays, this process may also have contributed to the preservation of some unicellular Neoproterozoic eukaryotes in Fe‐rich berthierine (Mus & Moczydłowska, 2000). Some iron reducers actively dissolve ferric clay minerals, liberating both iron and silica, which can generate aggregates of authigenic clay and quartz around the metabolizing cells (Metcalfe et al., 2013; Vorhies & Gaines, 2009). Future work may elucidate whether such processes could have preserved fossils on Mars.
5. Long‐Term Preservation on Mars
Entry into the fossil record is no guarantee of long‐term preservation; fossils can be destroyed by weathering, diagenetic alteration, and metamorphism in the deep subsurface. Heat and pressure result in the recrystallization of mineral grains and the transformation of organic compounds into kerogen and ultimately graphite. Almost all rocks on Earth of equivalent age to Noachian terrains on Mars have been recycled by Earth processes including subduction. The isolated vestiges that remain have undergone severe, and sometimes multiple, metamorphism but could contain metasedimentary graphite, highly disordered kerogens (e.g., Ohtomo et al., 2014; van Zuilen et al., 2003), or even graphitic inclusions in zircon (Bell et al., 2015). Although the C‐isotopic signature of these Eoarchean carbonaceous remains is similar to some imparted by biological processes, abiotic interpretations are also consistent with the data (Bottinga, 1969; van Zuilen et al., 2003).
Mars, however, currently lacks global plate tectonics and may always have done so. A crust tens of kilometers thick and very sporadic volcanism ensure that crustal materials are recycled very slowly; sedimentary rocks older than any on Earth survive as evidence of this lack of recycling (Grotzinger & Milliken, 2012). Older Martian crust has been pervasively fractured by impacts, baked by volcanism, and/or altered by groundwater‐rock reactions. The lack of tectonic subsidence, however, results in lower rates of burial, and the temperature gradient with depth is lower than that on Earth, such that metamorphism is generally less severe. The abundance of Fe‐Mg smectites in Gale Crater mudstones (Rampe et al., 2017; Vaniman et al., 2014) indicates burial temperatures of less than 100°C, above which these minerals would have been diagenetically transformed. Low geothermal gradients in Martian sedimentary basins are also reflected in the long‐term persistence of opaline silica (Frydenvang et al., 2017; Yen et al., 2017), which recrystallizes on Earth in a few million years in the presence of water (Tosca & Knoll, 2009). Likewise, aqueous alteration assemblages, although widespread, are chemically juvenile, indicating that interaction with aqueous fluids was limited (Tosca & Knoll, 2009), probably because of the long‐term persistence of cold and dry conditions (Shuster & Weiss, 2005). Nevertheless, this process could have dissolved and reprecipitated evaporites, potentially destroying any fossils within them, and the inferred acidic composition of these fluids may have resulted in the loss of some carbonate and phosphate minerals.
Physical weathering proceeds very slowly on Mars compared to rates on Earth, as evidenced by the survival of Noachian craters, channels, and other topographic features that have been exposed since their formation. Aeolian and freeze‐thaw weathering, as well as water‐related dissolution and leaching of mobile elements, are prevalent but result only in surficial damage (Grotzinger et al., 2013; McLennan & Grotzinger, 2008). In general, fossiliferous lithologies from early Mars would have a good chance of surviving to the present.
6. Finding and Verifying Fossils
The search for microbial biosignatures in rocks more than a billion years old on Earth typically follows a three‐step protocol. First, promising outcrops are explored at macroscopic scales to establish a stratigraphic context and identify sedimentary structures indicative of interactions between biological processes, sediment transport, and mineral precipitation. Second, these are sampled for further analysis, usually beginning with the study of petrographic thin sections (i.e., microscope slides), which may reveal candidate microbial fabrics or even preserved cells and tissues. Third, microscale and nanoscale chemical and isotopic information is acquired from candidate biosignatures in order to confirm and reconstruct the biological processes that contributed to their formation.
An important goal of such work is to exclude the possibility that putative biosignatures are either recent contaminants or abiotic phenomena that merely resemble fossils. Most prokaryotes are morphologically simple spheroids and filaments, and the interplay of abiotic mineral growth, geochemical reactions, and sediment dynamics can generate convincing pseudofossils (e.g., Grotzinger & Rothman, 1996; Garcia‐Ruiz et al., 2003, 2009; Hofmann, 2004). Abiotic processes can also fractionate carbon and sulfur isotopes, and the behavior of these isotope systems in Martian environments is not yet fully understood. Robust detection of morphological, isotopic, or any other biosignatures on Mars would therefore require evidence of a paleoenvironmental context and alteration history consistent with biology and incompatible with nonbiological processes alone (Summons et al., 2011). Several authors have attempted to establish criteria for evaluating the syngenicity, antiquity, and biogenicity of putative microbial biosignatures from Mars or Archean Earth, emphasizing that similar protocols apply on both planets (e.g., Brasier & Wacey, 2012). As noted elsewhere in this review, these protocols raise critical questions about purported 3.7‐Gyr‐old stromatolites and 3.8‐Gyr‐old filamentous microfossils on Earth (Dodd et al., 2017; Nutman et al., 2016), and about recently hypothesized MISS in Mars surface imagery (Noffke, 2015).
7. Recommendations for Future Experimental Work
Future taphonomic investigations should incorporate our current knowledge of Martian (paleo)environments to better constrain Mars‐specific conditions that can preserve or destroy biosignatures and organic matter. There is a particular need for laboratory studies to investigate the impact of likely conditions in habitable environments on early Mars upon the taphonomy of microbial groups that could have been present, such as those represented in Earth's Archean record. Relevant environmental conditions include anoxic CO2‐rich atmospheres, sulfur‐ and iron‐based redox gradients, high‐salinity fluids, sulfates and other evaporites, basaltic soil, Fe/Mg‐rich detrital clays, oxychlorine compounds, and temperatures and pressures close to the edge of the liquid water stability field. Experiments are necessary to explore microbially mediated clay authigenesis, carbonate precipitation, and other drivers of fossil preservation under such conditions, and to reveal which water‐rock interactions in Martian subsurface environments are likely to be conducive to fossil preservation. Postdepositional processes on Mars must also be considered, that is, the long‐term preservation of organic molecules in organo‐mineral associations in the presence of abundant chlorine, perchlorate, and peroxide (Benner et al., 2000; Glavin et al., 2013; Navarro‐Gonzalez et al., 2010). Although the degradation of microbial cells and lipid biomarkers on the Martian surface has been investigated in simulated atmospheres and radiation environments (e.g., de la Vega et al., 2007; Johnson et al., 2011; Kminek & Bada, 2006), such experiments have little relevance to biosignatures entombed within rocks and buried under a radiation shielding but oxidizing soil layer for most of Martian history.
A new program of Mars‐centric taphonomic experiments would reduce our reliance on imperfect analogies between paleoenvironments on Mars and Earth. Experimental and theoretical considerations of processes that operate in the absence of biology would also help us to avoid false positives by exploring the potential of water‐rock interactions to form abiotic pseudofossils under Mars‐relevant conditions, including life‐like morphologies, mineral alteration textures, and porous‐layered textures within silica sinters.
8. Conclusions
Previous studies highlighted numerous preservational processes, minerals, lithologies, and microbial groups as targets in the search for life on Mars (e.g., Hays et al., 2017; Summons et al., 2011; Westall, Foucher, et al., 2015). However, planning for forthcoming rover missions requires that this search be optimized. Here we have considered current understanding of (1) the occurrence and distribution of lithologies and minerals on Mars; (2) the habitability of the environments they represent; and (3) the potential of these environments to preserve the remains of organisms most likely to have lived in them, as revealed by studies of fossils on Earth and experiments in the laboratory. On this basis, we recommend that iron‐rich lacustrine mudstones, especially those rich in silica, should be prioritized for biosignature exploration. These rocks are present on Mars, represent aqueous environments with a range of redox states suitable for anaerobic metabolisms, and offer the possibility of preservation by silicification, clay authigenesis, clay‐organic adsorption, and iron mineralization. Hot spring sinters are also an attractive target based on likely habitability and rapid mineral precipitation, but their presence on Mars remains to be confirmed (Squyres et al., 2008; Skok et al., 2010; Ruff & Farmer, 2016). Pores and fractures mineralized in the subsurface could represent a geologically younger target, but more research is needed to determine whether they can be confidently identified on Mars and whether appropriate Mars‐analog organisms in such settings preserve reliable biosignatures, even on Earth. Likewise, it has yet to be determined whether evaporites can rival lacustrine mudstones in their potential to retain biosignatures for billions of years under Martian conditions.
Acknowledgments
The data drawn upon for this study are available in the figures and cited references. We are grateful for helpful comments from C. P. McKay, A. H. Knoll, and two anonymous reviewers. Financial support was provided by the NASA Astrobiology Institute NNA13AA90A, Foundations of Complex Life, Evolution, Preservation, and Detection on Earth and Beyond. The Simons Collaboration on Origins of Life provided funding to T. B., J. P. G., and R. E. S. S. M. acknowledges support from the European Union's Horizon 2020 Research and Innovation Programme under Marie Skłodowska‐Curie grant agreement 747877. K. H. W. and A. F. acknowledge support of a grant from the National Aeronautics and Space Administration for work performed at the Jet Propulsion Laboratory, California Institute of Technology.
McMahon, S. , Bosak, T. , Grotzinger, J. P. , Milliken, R. E. , Summons, R. E. , Daye, M. , et al. (2018). A field guide to finding fossils on Mars. Journal of Geophysical Research: Planets, 123, 1012–1040. 10.1029/2017JE005478
References
- Adhikari, D. , & Yang, Y. (2014). Selective stabilization of aliphatic organic carbon by iron oxide. Scientific Reports, 5, 11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleon, J. , Bernard, S. , Le Guillou, C. , Marin‐Carbonne, J. , Pont, S. , Beyssac, O. , et al. (2016). Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. Nature Communications, 7, 11977 10.1038/ncomms11977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison, P. A. (1988). The decay and mineralization of proteinaceous macrofossils. Paleobiology, 14, 39–154. [Google Scholar]
- Allwood, A. C. , Burch, I. W. , Rouchy, J. M. , & Coleman, M. (2013). Morphological biosignatures in gypsum: Diverse formation processes of Messinian (∼6.0 Ma) gypsum stromatolites. Astrobiology, 13(9), 870–886. 10.1089/ast.2013.1021 [DOI] [PubMed] [Google Scholar]
- Allwood, A. C. , Grotzinger, J. P. , Knoll, A. H. , Burch, I. W. , Anderson, M. S. , Coleman, M. L. , & Kanik, I. (2009). Controls on development and diversity of Early Archean stromatolites. Proceedings of the National Academy of Sciences, 106(24), 9548–9555. 10.1073/pnas.0903323106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood, A. C. , Walter, M. R. , Kamber, B. S. , Marshall, C. P. , & Burch, I. W. (2006). Stromatolite reef from the Early Archaean era of Australia. Nature, 441(7094), 714–718. 10.1038/nature04764 [DOI] [PubMed] [Google Scholar]
- Anderson, C. A. (1930). Opal stalactites and stalagmites from a lava tube in Northern California. American Journal of Science, 20(115), 22–26. [Google Scholar]
- Anderson, R. P. , Macdonald, F. A. , Tosca, N. J. , Bosak, T. , Bold, U. , & Briggs, D. E. G. (2014). Taphonomy of eukaryotic microfossils between Cryogenian ice ages explored in the Zavkhan terrane, southwestern Mongolia. Geological Society of America Abstracts with Programs, 46(6), 542. [Google Scholar]
- Anderson, R. P. , Tosca, N. J. , Gaines, R. R. , Mongiardino Koch, N. , & Briggs, D. E. G. (2018). A mineralogical signature for Burgess Shale‐type fossilization. Geology. 10.1130/G39941.1 [DOI] [Google Scholar]
- Arvidson, R. E. , Squyres, S. W. , Bell, J. F. , Catalano, J. G. , Clark, B. C. , Crumpler, L. S. , et al. (2014). Ancient aqueous environments at Endeavour crater, Mars. Science, 343(6169), 1248097 10.1126/science.1248097 [DOI] [PubMed] [Google Scholar]
- Barghoorn, E. S. , & Tyler, S. A. (1965). Microorganisms from the Gunflint chert. Science, 147(3658), 563–575. 10.1126/science.147.3658.563 [DOI] [PubMed] [Google Scholar]
- Bartley, J. K. (1996). Actualistic taphonomy of cyanobacteria: Implications for the Precambrian fossil record. Palaios, 11(6), 571–586. 10.2307/3515192 [DOI] [Google Scholar]
- Bartley, J. K. , Knoll, A. H. , Grotzinger, J. P. , & Sergeev, V. N. (2000). Lithification and fabric genesis in precipitated stromatolites and associated peritidal carbonates, Mesoproterozoic Billyakh Group, Siberia In Grotzinger J. P. & James N. P. (Eds.), Carbonate sedimentation and diagenesis in the evolving Precambrian world (Vol. 67, pp. 59–73). Tulsa, OK: SEPM (Society for Sedimentary Geology), Special Publication. [Google Scholar]
- Batchelor, M. T. , Burne, R. V. , Henry, B. I. , & Jackson, M. J. (2004). A case for biotic morphogenesis of coniform stromatolites. Physica A: Statistical Mechanics and its Applications, 337(1), 319–326. 10.1016/j.physa.2004.01.065 [DOI] [Google Scholar]
- Batchelor, M. T. , Burne, R. V. , Henry, B. I. , & Slatyer, T. (2005). Statistical physics and stromatolite growth: New perspectives on an ancient dilemma. Physica A: Statistical Mechanics and its Applications, 350(1), 6–11. 10.1016/j.physa.2005.01.014 [DOI] [Google Scholar]
- Batchelor, M. T. , Burne, R. V. , Henry, B. I. , & Watt, S. D. (2000). Deterministic KPZ model for stromatolite laminae. Physica A: Statistical Mechanics and its Applications, 282(1), 123–136. 10.1016/S0378-4371(00)00077-7 [DOI] [Google Scholar]
- Bell, E. A. , Boehnke, P. , Harrison, T. M. , & Mao, W. L. (2015). Potentially biogenic carbon preserved in a 4.1 billion‐year‐old zircon. Proceedings of the National Academy of Sciences, 112(47), 14518–14521. 10.1073/pnas.1517557112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson, S. , Rasmussen, B. , Ivarsson, M. , Muhling, J. , Broman, C. , Marone, F. , et al. (2017). Fungus‐like mycelial fossils in 2.4‐billion‐year‐old vesicular basalt. Nature Ecology & Evolution, 1, 0141 10.1038/s41559-017-0141 [DOI] [PubMed] [Google Scholar]
- Benner, S. A. , Devine, K. G. , Matveeva, L. N. , & Powell, D. H. (2000). The missing organic molecules on Mars. Proceedings of the National Academy of Sciences, 97, 2425–2430. 10.1073/pnas.040539497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, N. J. , & Lowe, D. R. (1989). Environmental control on diverse stromatolite morphologies in the 3000 Myr Pongola Supergroup, South Africa. Sedimentology, 36(3), 383–397. 10.1111/j.1365-3091.1989.tb00615.x [DOI] [Google Scholar]
- Bibring, J.‐P. , Arvidson, R. E. , Gendrin, A. , Gondet, B. , Langevin, Y. , Le Mouelic, S. , et al. (2007). Coupled ferric oxides and sulfates on the Martian surface. Science, 317, 5842. [DOI] [PubMed] [Google Scholar]
- Blamey, N. J. , Parnell, J. , McMahon, S. , Mark, D. F. , Tomkinson, T. , Lee, M. , et al. (2015). Evidence for methane in Martian meteorites. Nature Communications, 6, 7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship, R. E. , & Hartman, H. (1998). The origin and evolution of oxygenic photosynthesis. Trends in Biochemical Sciences, 23(3), 94–97. 10.1016/S0968-0004(98)01186-4 [DOI] [PubMed] [Google Scholar]
- Bontognali, T. R. R. , Fischer, W. W. , & Föllmi, K. B. (2013). Siliciclastic associated banded iron formation from the 3.2 Ga Moodies Group, Barberton Greenstone Belt, South Africa. Precambrian Research, 226, 116–124. 10.1016/j.precamres.2012.12.003 [DOI] [Google Scholar]
- Bontognali, T. R. R. , Sessions, A. L. , Allwood, A. C. , Fischer, W. W. , Grotzinger, J. P. , Summons, R. E. , & Eiler, J. M. (2012). Sulfur isotopes of organic matter preserved in 3.45‐billion‐year‐old stromatolites reveal microbial metabolism. Proceedings of the National Academy of Sciences, 109(38), 15146–15151. 10.1073/pnas.1207491109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosak, T. , Bush, J. W. M. , Flynn, M. R. , Liang, B. , Ono, S. , Petroff, A. P. , & Sim, M. S. (2010). Formation and stability of oxygen-rich bubbles that shape photosynthetic mats. Geobiology, 8, 45–55. 10.1111/j.1472-4669.2009.00227.x [DOI] [PubMed] [Google Scholar]
- Bosak, T. , Knoll, A. H. , & Petroff, A. P. (2013). The meaning of stromatolites. Annual Review of Earth and Planetary Sciences, 41, 21–44. 10.1146/annurev-earth-042711-105327 [DOI] [Google Scholar]
- Bosak, T. , Liang, B. , Sim, M. S. , & Petroff, A. P. (2009). Morphological record of oxygenic photosynthesis in conical stromatolites. Proceedings of the National academy of Sciences of the United States of America, 106(27), 10,939–10,943. 10.1073/pnas.0900885106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosak, T. , & Newman, D. K. (2003). Microbial nucleation of calcium carbonate in the Precambrian. Geology, 31(7), 577–580. [DOI] [Google Scholar]
- Boston, P. J. , Ivanov, M. V. , & McKay, C. P. (1992). On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus, 95, 300–308. 10.1016/0019-1035(92)90045-9 [DOI] [PubMed] [Google Scholar]
- Bottinga, Y. (1969). Calculated fractionation factors for carbon and hydrogen isotope exchange in the system calcite‐carbon dioxide‐graphite‐methane‐hydrogen‐water vapor. Geochimica et Cosmochimica Acta, 33, 49–64. 10.1016/0016-7037(69)90092-1 [DOI] [Google Scholar]
- Boynton, W. V. , Ming, D. W. , Kounaves, S. P. , Young, S. M. M. , Arvidson, R. E. , Hecht, M. H. , et al. (2009). Evidence for calcium carbonate at the Mars Phoenix landing site. Science, 325(5936), 61–64. 10.1126/science.1172768 [DOI] [PubMed] [Google Scholar]
- Brasier, A. T. , Salminen, P. E. , Melezhik, V. A. , & Fallick, A. E. (2013). Earth's earliest travertines In Melezhik V. A., et al. (Eds.), Reading the archive of Earth's oxygenation. Volume 3: Global events and the Fennoscandian Arctic Russia ‐ Drilling Early Earth Project(pp. 1435–1456). Berlin, Germany: Springer; 10.1007/978-3-642-29670-3_9 [DOI] [Google Scholar]
- Brasier, M. D. , McLoughlin, N. , Green, O. , & Wacey, D. (2006). A fresh look at the fossil evidence for early Archaean cellular life. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 361(1470), 887–902. 10.1098/rstb.2006.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier, M. D. , & Wacey, D. (2012). Fossils and astrobiology: New protocols for cell evolution in deep time. International Journal of Astrobiology, 11(04), 217–228. 10.1017/S1473550412000298 [DOI] [Google Scholar]
- Brazelton, W. J. , Schrenk, M. O. , Kelley, D. S. , & Baross, J. A. (2006). Methane‐ and sulfur‐metabolizing microbial communities dominate the lost city hydrothermal field ecosystem. Applied and Environmental Microbiology, 72, 6257–6270. 10.1128/AEM.00574-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges, N. T. , Hook, S. J. , Thomson, B. J. , Crowley, J. K. , Souza Filho, C. R. , Macambira, J. B. , & Lima Pereira Silva, G. (2008). Brazilian analog for ancient marine environments on Mars. Eos, 89(36), 329–330. 10.1029/2008EO360001 [DOI] [Google Scholar]
- Briggs, D. E. G. (2003). The role of decay and mineralization in the preservation of soft‐bodied fossils. Annual Review of Earth and Planetary Sciences, 31(1), 275–301. 10.1146/annurev.earth.31.100901.144746 [DOI] [Google Scholar]
- Briggs, D. E. G. , & McMahon, S. (2016). The role of experiments in investigating the taphonomy of exceptional preservation. Palaeontology, 59(1), 1–11. 10.1111/pala.12219 [DOI] [Google Scholar]
- Bristow, T. F. , & Grotzinger, J. P. (2013). Sulfate availability and the geological record of cold‐seep deposits. Geology, 41(7), 811–814. 10.1130/G34265.1 [DOI] [Google Scholar]
- Bristow, T. F. , Haberle, R. M. , Blake, D. F. , Des Marais, D. J. , Eigenbrode, J. L. , Fairén, A. G. , et al. (2017). Low Hesperian PCO2 constrained from in situ mineralogical analysis at Gale Crater, Mars. Proceedings of the National Academy of Sciences, 114(9), 2166–2170. 10.1073/pnas.1616649114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow, T. F. , & Milliken, R. E. (2011). Terrestrial perspective on authigenic clay mineral production in ancient Martian lakes. Clays and Clay Minerals, 59(4), 339–358. 10.1346/CCMN.2011.0590401 [DOI] [Google Scholar]
- Burns, R. G. (1993). Rates and mechanisms of chemical weathering of ferromagnesian silicate minerals on Mars. Geochemica et Cosmochimica Acta, 57(19), 4555–4574. [Google Scholar]
- Butterfield, N. J. (1990). Organic preservation of non‐mineralizing organisms and the taphonomy of the Burgess Shale. Paleobiology, 16(3), 272–286. [Google Scholar]
- Butterfield, N. J. (1995). Secular distribution of Burgess Shale‐type preservation. Lethaia, 28(1), 1–13. 10.1111/j.1502-3931.1995.tb01587.x [DOI] [Google Scholar]
- Cabrol, N. A. , & Grin, E. A. (1999). Distribution, classification, and ages of Martian impact crater lakes. Icarus, 142(1), 160–172. 10.1006/icar.1999.6191 [DOI] [Google Scholar]
- Cady, S. , & Farmer, J. (1996). Fossilization processes in siliceous thermal springs: Trends in preservation along thermal gradients In Bock G. R., & Goode J. A. (Eds.), Evolution of hydrothermal ecosystems on Earth and Mars, (pp. 150–173). Chichester, UK: John Wiley. [DOI] [PubMed] [Google Scholar]
- Callow, R. H. , & Brasier, M. D. (2009). Remarkable preservation of microbial mats in Neoproterozoic siliciclastic settings: Implications for Ediacaran taphonomic models. Earth‐Science Reviews, 96(3), 207–219. 10.1016/j.earscirev.2009.07.002 [DOI] [Google Scholar]
- Campbell, K. A. (2006). Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: Past developments and future research directions. Palaeogeography, Palaeoclimatology, Palaeoecology, 232(2), 362–407. [Google Scholar]
- Campbell, K. A. , Lynne, B. Y. , Handley, K. M. , Jordan, S. , Farmer, J. D. , Guido, D. M. , et al. (2015). Tracing biosignature preservation of geothermally silicified microbial textures into the geological record. Astrobiology, 15(10), 858–882. 10.1089/ast.2015.1307 [DOI] [PubMed] [Google Scholar]
- Carrier, B. L. , & Kounaves, S. P. (2015). The origins of perchlorate in the Martian soil. Geophysical Research Letters, 42, 3739–3745. 10.1002/2015GL064290 [DOI] [Google Scholar]
- Caswell, T. E. , & Milliken, R. E. (2017). Evidence for hydraulic fracturing at Gale Crater, Mars: Implications for burial depth of the Yellowknife Bay formation. Earth and Planetary Science Letters, 468, 72–84. 10.1016/j.epsl.2017.03.033 [DOI] [Google Scholar]
- Cavalazzi, B. , Westall, F. , Cady, S. L. , Barbieri, R. , & Foucher, F. (2011). Potential fossil endoliths in vesicular pillow basalt, Coral Patch Seamount, eastern North Atlantic Ocean. Astrobiology, 11(7), 619–632. 10.1089/ast.2011.0657 [DOI] [PubMed] [Google Scholar]
- Chan, C. S. , Fakra, S. C. , Edwards, D. C. , Emerson, D. , & Banfield, J. F. (2009). Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochimica et Cosmochimica Acta, 73(13), 3807–3818. 10.1016/j.gca.2009.02.036 [DOI] [Google Scholar]
- Chan, C. S. , Fakra, S. C. , Emerson, D. , Fleming, E. J. , & Edwards, K. J. (2011). Lithotrophic iron‐oxidizing bacteria produce organic stalks to control mineral growth: Implications for biosignature formation. The ISME Journal, 5(4), 717–727. 10.1038/ismej.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changela, H. G. , & Bridges, J. C. (2011). Alteration assemblages in the nakhlites: Variation with depth on Mars. Meteoritics & Planetary Science, 45(12), 1847–1867. 10.1111/j.1945-5100.2010.01123.x [DOI] [Google Scholar]
- Chen, Y. , Li, Y. L. , Zhou, G. T. , Li, H. , Lin, Y. T. , Xiao, X. , & Wang, F. P. (2014). Biomineralization mediated by anaerobic methane‐consuming cell consortia. Scientific Reports, 4, 5696 10.1038/srep05696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki, M. , & Hynek, B. (2008). Geological context of water‐altered minerals in Valles Marineris, Mars. Journal of Geophysical Research, 113, E12005 10.1029/2007JE003070 [DOI] [Google Scholar]
- Christensen, P. R. , Bandfield, J. L. , Clark, R. N. , Edgett, K. S. , Hamilton, V. E. , Hoefen, T. , et al. (2000). Detection of crystalline hematite mineralization on Mars by the Thermal Emission Spectrometer: Evidence for near‐surface water. Journal of Geophysical Research, 105, 9623–9642. 10.1029/1999JE001093 [DOI] [Google Scholar]
- Christensen, P. R. , Morris, R. V. , Lane, M. D. , Banfield, J. L. , & Malin, M. C. (2001). Global mapping of Martian hematite mineral deposits: Remnants of water‐drive processes on early Mars. Journal of Geophysical Research, 106(E10), 23,873–23,885. [Google Scholar]
- Clark, B. C. (1999). On the non‐observability of carbonates on Mars. 5th Mars Conf. Abstr. 6214 (Lunar and Planetary Institute, Houston, Texas).
- Clifford, S. M. , Lasue, J. , Heggy, E. , Boisson, J. , McGovern, P. , & Max, M. D. (2010). Depth of the Martian cryosphere: Revised estimates and implications for the existence and detection of subpermafrost groundwater. Journal of Geophysical Research, 115, E07001 10.1029/2009JE003462 [DOI] [Google Scholar]
- Cockell, C. S. (2014a). The subsurface habitability of terrestrial rocky planets: Mars In Kallmeyer J., & Wagner D. (Eds.), Microbial life of the deep biosphere (pp. 225–260). Berlin: DeGruyter. [Google Scholar]
- Cockell, C. S. (2014b). Trajectories of Martian habitability. Astrobiology, 14(2), 182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuerno, R. , Escudero, C. , Garcia‐Ruiz, J. M. , & Herrero, M. A. (2012). Pattern formation in stromatolites: Insights from mathematical modelling. Journal of the Royal Society Interface, 9(70), 1051–1062. 10.1098/rsif.2011.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks, J. B. , Field, M. C. , Buick, R. , Eme, L. , Gribaldo, S. , Roger, A. J. , et al. (2016). The changing view of eukaryogenesis–fossils, cells, lineages and how they all come together. Journal of Cell Science, 129(20), 3695–3703. 10.1242/jcs.178566 [DOI] [PubMed] [Google Scholar]
- Darroch, S. A. F. , Laflamme, M. , Schiffbauer, J. D. , & Briggs, D. E. G. (2012). Experimental formation of a microbial death mask. Palaios, 27, 293–303. 10.2110/palo.2011.p11-059r [DOI] [Google Scholar]
- Dartnell, L. R. , Desorgher, L. , Ward, J. M. , & Coates, A. J. (2007). Modelling the surface and subsurface Martian radiation environment: Implications for astrobiology. Geophysical Research Letters, 34, L02207 10.1029/2006GL027494 [DOI] [Google Scholar]
- Davies, N. S. , Liu, A. G. , Gibling, M. R. , & Miller, R. F. (2016). Resolving MISS conceptions and misconceptions: A geological approach to sedimentary surface textures generated by microbial and abiotic processes. Earth‐Science Reviews, 154, 210–246. 10.1016/j.earscirev.2016.01.005 [DOI] [Google Scholar]
- Davies, N. S. , Liu, A. G. , Gibling, M. R. , & Miller, R. F. (2018). Reply to comment on the paper by Davies et al. “Resolving MISS conceptions and misconceptions: A geological approach to sedimentary surface textures generated by microbial and abiotic processes” (Earth‐Science Reviews, 154 (2016), 210–246). Earth‐Science Reviews, 176, 384–386. 10.1016/j.earscirev.2017.11.024 [DOI] [Google Scholar]
- de la Vega, U. P. , Rettberg, P. , & Reitz, G. (2007). Simulation of the environmental climate conditions on Martian surface and its effect on Deinococcus radiodurans . Advances in Space Research, 40(11), 1672–1677. 10.1016/j.asr.2007.05.022 [DOI] [Google Scholar]
- Derenne, S. , Robert, F. , Skrzypczak‐Bonduelle, A. , Gourier, D. , Binet, L. , & Rouzaud, J. N. (2008). Molecular evidence for life in the 3.5 billion year old Warrawoona chert. Earth and Planetary Science Letters, 272(1‐2), 476–480. 10.1016/j.epsl.2008.05.014 [DOI] [Google Scholar]
- Des Marais, D. J. (2010). Exploring Mars for evidence of habitable environments and life. Proceedings of the American Philosophical Society, 154(4), 402–421. [Google Scholar]
- Di Achille, G. , & Hynek, B. M. (2010). Ancient ocean on Mars supported by global distribution of deltas and valleys. Nature Geoscience, 3(7), 459–463. [Google Scholar]
- DiBiase, R. A. , Limaye, A. B. , Scheingross, J. S. , Fischer, W. W. , & Lamb, M. P. (2013). Deltaic deposits at Aeolis Dorsa: Sedimentary evidence for a standing body of water on the northern plains of Mars. Journal of Geophysical Research: Planets, 118, 1285–1302. 10.1002/jgre.20100 [DOI] [Google Scholar]
- Dodd, M. S. , Papineau, D. , Grenne, T. , Slack, J. F. , Rittner, M. , Pirajno, F. , et al. (2017). Evidence for early life in Earth's oldest hydrothermal vent precipitates. Nature, 543(7643), 60–64. 10.1038/nature21377 [DOI] [PubMed] [Google Scholar]
- Dromart, G. , Quantin, C. , & Broucke, O. (2007). Stratigraphic architectures spotted in southern Melas Chasma, Valles Marineris, Mars. Geology, 35(4), 363–366. 10.1130/G23350A.1 [DOI] [Google Scholar]
- Dupraz, C. , Pattisina, R. , & Verrecchia, E. P. (2006). Translation of energy into morphology: Simulation of stromatolite morphospace using a stochastic model. Sedimentary Geology, 185(3), 185–203. [Google Scholar]
- Dupraz, C. , Reid, R. P. , Braissant, O. , Decho, A. W. , Norman, R. S. , & Visscher, P. T. (2009). Processes of carbonate precipitation in modern microbial mats. Earth‐Science Reviews, 96(3), 141–162. 10.1016/j.earscirev.2008.10.005 [DOI] [Google Scholar]
- Dupraz, C. , Visscher, P. T. , Baumgartner, L. K. , & Reid, R. P. (2004). Microbe‐mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology, 51(4), 745–765. 10.1111/j.1365-3091.2004.00649.x [DOI] [Google Scholar]
- Edwards, K. J. , Bach, W. , McCollom, T. M. , & Rogers, D. R. (2004). Neutrophilic iron‐oxidizing bacteria in the ocean: Their habitats, diversity, and roles in mineral deposition, rock alteration, and biomass production in the deep‐sea. Geomicrobiology Journal, 21(6), 393–404. 10.1080/01490450490485863 [DOI] [Google Scholar]
- Edwards, K. J. , Glazer, B. T. , Rouxel, O. J. , Bach, W. , Emerson, D. , Davis, R. E. , et al. (2011). Ultra‐diffuse hydrothermal venting supports Fe‐oxidizing bacteria and massive umber deposition at 5000 m off Hawaii. The ISME Journal, 5, 1748–1758. 10.1038/ismej.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlmann, B. L. , Berger, G. , Mangold, N. , Michalski, J. R. , Catling, D. C. , Ruff, S. W. , et al. (2013). Geochemical consequences of widespread clay mineral formation in Mars' ancient crust. Space Science Reviews, 174(1‐4), 329–364. 10.1007/s11214-012-9930-0 [DOI] [Google Scholar]
- Ehlmann, B. L. , & Edwards, C. S. (2014). Mineralogy of the Martian surface. Annual Review of Earth and Planetary Sciences, 42, 291–315. 10.1146/annurev-earth-060313-055024 [DOI] [Google Scholar]
- Ehlmann, B. L. , Mustard, J. F. , Fassett, C. I. , Schon, S. C. , Head, J. W. III , Des Marais, D. J. , et al. (2008). Clay minerals in delta deposits and organic preservation potential on Mars. Nature Geoscience, 1(6), 355–358. 10.1038/ngeo207 [DOI] [Google Scholar]
- Ehlmann, B. L. , Mustard, J. F. , & Murchie, S. L. (2010). Geologic setting of serpentine deposits on Mars. Geophysical Research Letters, 37, L06201 10.1029/2010GL042596 [DOI] [Google Scholar]
- Ehlmann, B. L. , Mustard, J. F. , Murchie, S. L. , Bibring, J. P. , Meunier, A. , Fraeman, A. A. , & Langevin, Y. (2011). Subsurface water and clay mineral formation during the early history of Mars. Nature, 479(7371), 53 10.1038/nature10582 [DOI] [PubMed] [Google Scholar]
- Ehlmann, B. L. , Mustard, J. F. , Murchie, S. L. , Poulet, F. , Bishop, J. L. , Brown, A. J. , et al. (2008). Orbital identification of carbonate‐bearing rocks on Mars. Science, 322(5909), 1828–1832. [DOI] [PubMed] [Google Scholar]
- Ehlmann, B. L. , Mustard, J. F. , Swayze, G. A. , Clark, R. N. , Bishop, J. L. , Poulet, F. , et al. (2009). Identification of hydrated silicate minerals on Mars using MROCRISM: Geologic context near Nili Fossae and implications for aqueous alteration. Journal of Geophysical Research, 114, E00D08 10.1029/2009JE003339 [DOI] [Google Scholar]
- Ehlmann, B. L. , Swayze, G. A. , Milliken, R. E. , Mustard, J. F. , Clark, R. N. , Murchie, S. L. , et al. (2016). Discovery of alunite in Cross crater, Terra Sirenum, Mars: Evidence for acidic, sulfurous waters. American Mineralogist, 101(7), 1527–1542. 10.2138/am-2016-5574 [DOI] [Google Scholar]
- Eigenbrode, J. L. , & Freeman, K. H. (2006). Late Archean rise of aerobic microbial ecosystems. Proceedings of the National Academy of Sciences, 103(43), 15,759–15,764. 10.1073/pnas.0607540103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson, D. , Fleming, E. J. , & McBeth, J. M. (2010). Iron‐oxidizing bacteria: An environmental and genomic perspective. Annual Review of Microbiology, 64, 561–583. 10.1146/annurev.micro.112408.134208 [DOI] [PubMed] [Google Scholar]
- Encrenaz, T. , Greathouse, T. K. , Lefèvre, F. , & Atreya, S. K. (2012). Hydrogen peroxide on Mars: Observations, interpretation and future plans. Planetary and Space Science, 68, 3–17. 10.1016/j.pss.2011.03.019 [DOI] [Google Scholar]
- Erwin, D. H. (2015). Early metazoan life: Divergence, environment and ecology. Philosophical Transactions of the Royal Society, B: Biological Sciences, 370(1684), 20150036 10.1098/rstb.2015.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo‐Cavazos, P. , Link, L. , Melosh, H. J. , & Nicholson, W. L. (2005). Bacillus subtilis spores on artificial meteorites survive hypervelocity atmospheric entry: Implications for lithopanspermia. Astrobiology, 5(6), 726–736. 10.1089/ast.2005.5.726 [DOI] [PubMed] [Google Scholar]
- Fallacaro, A. , & Calvin, W. (2006). Spectral properties of Lake Superior banded iron formation: Applications to Martian hematite deposits. Astrobiology, 6(4), 563–580. 10.1089/ast.2006.6.563 [DOI] [PubMed] [Google Scholar]
- Farley, K. A. , Malespin, C. , Mahaffy, P. , Grotzinger, J. P. , Vasconcelos, P. M. , Milliken, R. E. , et al. (2014). In situ radiometric and exposure age dating of the Martian surface. Science, 343(6169), 1247166 10.1126/science.1247166 [DOI] [PubMed] [Google Scholar]
- Farley, K. A. , Martin, P. , Archer, P. D. Jr. , Atreya, S. K. , Conrad, P. G. , Eigenbrode, J. L. , et al. (2016). Light and variable 37Cl/35Cl ratios in rocks from Gale Crater, Mars: Possible signature of perchlorate. Earth and Planetary Science Letters, 438, 14–24. 10.1016/j.epsl.2015.12.013 [DOI] [Google Scholar]
- Farley, K. A. , & Williford, K. H. (2017). Seeking signs of life, and more: NASA's Mars 2020 mission. Eos, 98 10.1029/2017EO066153 [DOI] [Google Scholar]
- Farmer, J. D. , & Des Marais, D. J. (1999). Exploring for a record of ancient Martian life. Journal of Geophysical Research, 104(E11), 26,977–26,995. 10.1029/1998JE000540 [DOI] [PubMed] [Google Scholar]
- Fassett, C. I. , & Head, J. W. (2008). Valley network‐fed, open‐basin lakes on Mars: Distribution and implications for Noachian surface and subsurface hydrology. Icarus, 198(1), 37–56. 10.1016/j.icarus.2008.06.016 [DOI] [Google Scholar]
- Fassett, C. I. , & Head, J. W. (2011). Sequence and timing of conditions on early Mars. Icarus, 211(2), 1204–1214. 10.1016/j.icarus.2010.11.014 [DOI] [Google Scholar]
- Fedo, C. M. , Grotzinger, J. P. , Gupta, S. , Stein, N. T. , Watkins, J. , Banham, S. , et al. (2017). Facies analysis and basin architecture of the upper part of the Murray formation, Gale Crater, Mars. In: Lunar and Planetary Science Conference XLVIII, abstract 1689.
- Fenton, H. J. H. (1894). Oxidation of tartaric acid in presence of iron. Journal of the Chemical Society, 65, 899–910. [Google Scholar]
- Fernández‐Remolar, D. C. , & Knoll, A. H. (2008). Fossilization potential of iron‐bearing minerals in acidic environments of Rio Tinto, Spain: Implications for Mars exploration. Icarus, 194(1), 72–85. 10.1016/j.icarus.2007.10.009 [DOI] [Google Scholar]
- Fish, S. A. , Shepherd, T. J. , McGenity, T. J. , & Grant, W. D. (2002). Recovery of 16S ribosomal RNA gene fragments from ancient halite. Nature, 417(6887), 432–436. 10.1038/417432a [DOI] [PubMed] [Google Scholar]
- Fisk, M. R. , Crovisier, J. L. , & Honnorez, J. (2013). Experimental abiotic alteration of igneous and manufactured glasses. Comptes Rendus Geoscience, 345(4), 176–184. 10.1016/j.crte.2013.02.001 [DOI] [Google Scholar]
- Fisk, M. R. , & Giovannoni, S. J. (1999). Sources of nutrients and energy for a deep biosphere on Mars. Journal of Geophysical Research, 104, 11805–11815. 10.1029/1999JE900010 [DOI] [Google Scholar]
- Flannery, D. T. , Allwood, A. C. , Summons, R. E. , Williford, K. H. , Abbey, W. , Matys, E. D. , & Ferralis, N. (2018). Spatially‐resolved isotopic study of carbon trapped in ~3.43 Ga Strelley Pool Formation stromatolites. Geochimica et Cosmochimica Acta, 223, 21–35. 10.1016/j.gca.2017.11.028 [DOI] [Google Scholar]
- Flynn, G. J. , & McKay, D. S. (1990). An assessment of the meteoritic contribution to the Martian soil. Journal of Geophysical Research, 95(B9), 14,497–14,509. 10.1029/JB095iB09p14497 [DOI] [Google Scholar]
- Forni, O. , Gaft, M. , Toplis, M. J. , Clegg, S. M. , Maurice, S. , Wiens, R. C. , et al. (2015). First detection of fluorine on Mars: Implications for Gale Crater's geochemistry. Geophysical Research Letters, 42, 1020–1028. 10.1002/2014GL062742 [DOI] [Google Scholar]
- Foucher, F. , Westall, F. , Brandstätter, F. , Demets, R. , Parnell, J. , Cockell, C. S. , et al. (2010). Testing the survival of microfossils in artificial Martian sedimentary meteorites during entry into Earth's atmosphere: The STONE 6 experiment. Icarus, 207(2), 616–630. 10.1016/j.icarus.2009.12.014 [DOI] [Google Scholar]
- Fraeman, A. A. , Arvidson, R. E. , Catalano, J. G. , Grotzinger, J. P. , Morris, R. V. , Murchie, S. L. , et al. (2013). A hematite‐bearing layer in Gale Crater, Mars: Mapping and implications for past aqueous conditions. Geology, 41, 10. [Google Scholar]
- Fraeman, A. A. , Ehlmann, B. L. , Arvidson, R. E. , Edwards, C. S. , Grotzinger, J. P. , Milliken, R. E. , et al. (2016). The stratigraphy and evolution of lower Mount Sharp from spectral, morphological, and thermophysical orbital data sets. Journal of Geophysical Research: Planets, 121, 1713–1736. 10.1002/2016JE005095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissinet, C. , Glavin, D. P. , Mahaffy, P. R. , Miller, K. E. , Eigenbrode, J. L. , Summons, R. E. , et al. (2015). Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. Journal of Geophysical Research: Planets, 120, 495–514. 10.1002/2014JE004737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydenvang, J. , Gasda, P. J. , Hurowitz, J. A. , Grotzinger, J. P. , Wiens, R. C. , Newsom, H. E. , et al. (2017). Diagenetic silica enrichment and late‐stage groundwater activity in Gale Crater, Mars. Geophysical Research Letters, 44, 4716–4724. 10.1002/2017GL073323 [DOI] [Google Scholar]
- Gabbott, S. E. (1998). Taphonomy of the Ordovician Soom Shale Lagerstätte: An example of soft tissue preservation in clay minerals. Palaeontology, 41, 631–668. [Google Scholar]
- Gaines, R. R. , Briggs, D. E. G. , & Zhao, Y. L. (2008). Cambrian Burgess Shale‐type deposits share a common mode of fossilization. Geology, 36(10), 755–758. 10.1130/G24961A.1 [DOI] [Google Scholar]
- Garcia‐Ruiz, J. M. , Hyde, S. T. , Carnerup, A. M. , Christy, A. G. , Van Kranendonk, M. J. , & Welham, N. J. (2003). Self‐assembled silica‐carbonate structures and detection of ancient microfossils. Science, 302, 1194–1197. 10.1126/science.1090163 [DOI] [PubMed] [Google Scholar]
- García‐Ruiz, J. M. , Melero‐García, E. , & Hyde, S. T. (2009). Morphogenesis of self‐assembled nanocrystalline materials of barium carbonate and silica. Science, 323, 362–365. 10.1126/science.1165349 [DOI] [PubMed] [Google Scholar]
- Gehling, J. G. (1999). Microbial mats in terminal Proterozoic siliciclastics; Ediacaran death masks. Palaios, 14(1), 40–57. 10.2307/3515360 [DOI] [Google Scholar]
- Gehling, J. G. , & Droser, M. L. (2009). Textured organic surfaces associated with the Ediacara biota in South Australia. Earth‐Science Reviews, 96(3), 196–206. 10.1016/j.earscirev.2009.03.002 [DOI] [Google Scholar]
- Gendrin, A. , Mangold, N. , Bibring, J. P. , Langevin, Y. , Gondet, B. , Poulet, F. , et al. (2005). Sulfates in Martian layered terrains: The OMEGA/Mars Express view. Science, 307(5715), 1587–1591. 10.1126/science.1109087 [DOI] [PubMed] [Google Scholar]
- Gibson, R. A. , Talbot, H. M. , Kaur, G. , Pancost, R. D. , & Mountain, B. (2008). Bacteriohopanepolyol signatures of cyanobacterial and methanotrophic bacterial populations recorded in a geothermal vent sinter. Organic Geochemistry, 39(8), 1020–1023. 10.1016/j.orggeochem.2008.04.014 [DOI] [Google Scholar]
- Glavin, D. P. , Freissinet, C. , Miller, K. E. , Eigenbrode, J. L. , Brunner, A. E. , Buch, A. , et al. (2013). Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. Journal of Geophysical Research: Planets, 118, 1955–1973. 10.1002/jgre.20144 [DOI] [Google Scholar]
- Glotch, T. D. , Bandfield, J. L. , Tornabene, L. L. , Jensen, H. B. , & Seelos, F. P. (2010). Distribution and formation of chlorides and phyllosilicates in Terra Sirenum, Mars. Geophysical Research Letters, 37, L16202 10.1029/2010GL044557 [DOI] [Google Scholar]
- Glotch, T. D. , & Christensen, P. (2005). Geologic and mineralogic mapping of Aram Chaos: Evidence for a water‐rich history. Journal of Geophysical Research, 110, E09006 10.1029/2004JE002389 [DOI] [Google Scholar]
- Glotch, T. D. , & Rogers, A. D. (2007). Evidence for aqueous deposition of hematite‐ and sulfate‐rich light‐toned layered deposits in Aureum and Iani Chaos, Mars. Journal of Geophysical Research, 112, E06001 10.1029/2006JE002863 [DOI] [Google Scholar]
- Goudge, T. A. , Fassett, C. I. , Head, J. W. , Mustard, J. F. , & Aureli, K. L. (2016). Insights into surface runoff on early Mars from paleolake basin morphology and stratigraphy. Geology, 44(6), 419–422. 10.1130/G37734.1 [DOI] [Google Scholar]
- Grant, J. A. , Irwin, R. P. , Grotzinger, J. P. , Milliken, R. E. , Tornabene, L. L. , McEwen, A. S. , et al. (2008). HiRISE imaging of impact megabreccia and sub‐meter aqueous strata in Holden Crater, Mars. Geology, 36(3), 195–198. 10.1130/G24340A.1 [DOI] [Google Scholar]
- Grey, K. (1994). Stromatolites from the Palaeoproterozoic Earaheedy Group, Earaheedy Basin, Western Australia. Alcheringa, 18(3), 187–218. 10.1080/03115519408619495 [DOI] [Google Scholar]
- Grotzinger, J. P. , Arvidson, R. E. , Bell, J. F. , Calvin, W. , Clark, B. C. , Fike, D. A. , et al. (2005). Stratigraphy and sedimentology of a dry to wet eolian depositional system, Burns formation, Meridiani Planum, Mars. Earth and Planetary Science Letters, 240(1), 11–72. 10.1016/j.epsl.2005.09.039 [DOI] [Google Scholar]
- Grotzinger, J. P. , Crisp, J. A. , & Vasavada, A. R. (2015). Curiosity's mission of exploration at Gale Crater, Mars. Elements, 11(1), 19–26. 10.2113/gselements.11.1.19 [DOI] [Google Scholar]
- Grotzinger, J. P. , Gupta, S. , Malin, M. C. , Rubin, D. M. , Schieber, J. , Siebach, K. , et al. (2015). Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale Crater, Mars. Science, 350, aac7575 10.1126/science.aac7575 [DOI] [PubMed] [Google Scholar]
- Grotzinger, J. P. , Hayes, A. G. , Lamb, M. P. , & McLennan, S. M. (2013). Sedimentary processes on Earth, Mars, Titan, and Venus. Comparative Climatology of Terrestrial Planets, 1, 439–472. [Google Scholar]
- Grotzinger, J. P. , & Knoll, A. H. (1999). Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annual Review of Earth and Planetary Sciences, 27(1), 313–358. 10.1146/annurev.earth.27.1.313 [DOI] [PubMed] [Google Scholar]
- Grotzinger, J. P. , & Milliken, R. E. (2012). The sedimentary rock record of Mars: Distribution, origins, and global stratigraphy. Sedimentary Geology of Mars. SEPM Special Publication, 102, 1–48. [Google Scholar]
- Grotzinger, J. P. , & Rothman, D. H. (1996). An abiotic model for stromatolite morphogenesis. Nature, 383(6599), 423–425. 10.1038/383423a0 [DOI] [Google Scholar]
- Grotzinger, J. P. , Sumner, D. Y. , Kah, L. C. , Stack, K. , Gupta, S. , Edgar, L. , et al. (2014). A habitable fluvio‐lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science, 343(6169), 1242777 10.1126/science.1242777 [DOI] [PubMed] [Google Scholar]
- Halevy, I. , Fischer, W. W. , & Eiler, J. M. (2011). Carbonates in the Martian meteorite Allan Hills 84001 formed at 18±4 C in a near‐surface aqueous environment. Proceedings of the National Academy of Sciences, 108(41), 16895–16899. 10.1073/pnas.1109444108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J. P. , Aggarwal, S. D. , & Cockell, C. S. (2016). Salinity influences the response of Halomonas hydrothermalis to artificial fossilization by evaporative silicification. Geomicrobiology Journal, 33(5), 377–386. [Google Scholar]
- Hassler, D. M. , Zeitlin, C. , Wimmer‐Schweingruber, R. F. , Ehresmann, B. , Rafkin, S. , Eigenbrode, J. L. , et al. (2014). Mars' surface radiation environment measured with the Mars Science Laboratory's Curiosity rover. Science, 343(6169), 1244797 10.1126/science.1244797 [DOI] [PubMed] [Google Scholar]
- Hayes, J. M. (1994). Global methanotrophy at the Archean‐Proterozoic transition In Bengtson S. (Ed.), Early life on Earth, (pp. 220–236). New York, NY: Columbia University Press. [Google Scholar]
- Hays, L. E. , Graham, H. V. , Des Marais, D. J. , Hausrath, E. M. , Horgan, B. , McCollom, T. M. , et al. (2017). Biosignature preservation and detection in Mars analog environments. Astrobiology, 17(4), 363–400. 10.1089/ast.2016.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht, M. H. , Kounaves, S. P. , Quinn, R. C. , West, S. J. , Young, S. M. M. , Ming, D. W. , et al. (2009). Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix lander site. Science, 325(5936), 64–67. 10.1126/science.1172466 [DOI] [PubMed] [Google Scholar]
- Hedges, J. I. , & Keil, R. G. (1995). Sedimentary organic matter preservation: An assessment and speculative synthesis. Marine Chemistry, 49(2‐3), 81–115. 10.1016/0304-4203(95)00008-F [DOI] [Google Scholar]
- Hoffman, P. (1976). Environmental diversity of middle Precambrian stromatolites. Developments in Sedimentology, 20, 599–611. [Google Scholar]
- Hofmann, B. A. , & Farmer, J. D. (2000). Filamentous fabrics in low‐temperature mineral assemblages: Are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planetary and Space Science, 48(11), 1077–1086. 10.1016/S0032-0633(00)00081-7 [DOI] [Google Scholar]
- Hofmann, H. J. (2004). Archean microfossils and abiomorphs. Astrobiology, 4, 135–136. 10.1089/153110704323175115 [DOI] [PubMed] [Google Scholar]
- Holland, G. , Lollar, B. S. , Li, L. , Lacrampe‐Couloume, G. , Slater, G. F. , & Ballentine, C. J. (2013). Deep fracture fluids isolated in the crust since the Precambrian era. Nature, 497(7449), 357–360. 10.1038/nature12127 [DOI] [PubMed] [Google Scholar]
- Hurowitz, J. A. , Grotzinger, J. P. , Fischer, W. W. , McLennan, S. M. , Milliken, R. E. , Stein, N. , et al. (2017). Redox stratification of an ancient lake in Gale Crater, Mars. Science, 356(6341), eaah 6849 10.1126/science.aah6849 [DOI] [PubMed] [Google Scholar]
- Ivarsson, M. , Bengtson, S. , & Neubeck, A. (2016). The igneous oceanic crust—Earth's largest fungal habitat? Fungal Ecology, 20, 249–255. 10.1016/j.funeco.2016.01.009 [DOI] [Google Scholar]
- Jaakkola, S. T. , Ravantti, J. J. , Oksanen, H. M. , & Bamford, D. H. (2016). Buried alive: Microbes from ancient halite. Trends in Microbiology, 24(2), 148–160. 10.1016/j.tim.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Javaux, E. J. , & Knoll, A. H. (2017). Micropaleontology of the lower Mesoproterozoic Roper Group, Australia, and implications for early eukaryotic evolution. Journal of Paleontology, 91(2), 199–229. [Google Scholar]
- Javaux, E. J. , Marshall, C. P. , & Bekker, A. (2010). Organic‐walled microfossils in 3.2‐billion‐year‐old shallow‐marine siliciclastic deposits. Nature, 463(7283), 934–938. 10.1038/nature08793 [DOI] [PubMed] [Google Scholar]
- Johnson, A. P. , Pratt, L. M. , Vishnivetskaya, T. , Pfiffner, S. , Bryan, R. A. , Dadachova, E. , et al. (2011). Extended survival of several organisms and amino acids under simulated Martian surface conditions. Icarus, 211(2), 1162–1178. 10.1016/j.icarus.2010.11.011 [DOI] [Google Scholar]
- Jones, B. , Renaut, R. W. , & Rosen, M. R. (1997). Biogenicity of silica precipitation around geysers and hot‐spring vents, North Island, New Zealand. Journal of Sedimentary Research, 67(1), 88–104. [Google Scholar]
- Jones, B. , Renaut, R. W. , & Rosen, M. R. (1998). Microbial biofacies in hot‐spring sinters: A model based on Ohaaki Pool, North Island, New Zealand. Journal of Sedimentary Research, 68(3), 413–434. 10.2110/jsr.68.413 [DOI] [Google Scholar]
- Jorge‐Villar, S. E. , Benning, L. G. , & Edwards, H. G. (2007). Raman and SEM analysis of a biocolonised hot spring travertine terrace in Svalbard, Norway. Geochemical Transactions, 8(1), 8 10.1186/1467-4866-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallmeyer, J. , Pockalny, R. , Adhikari, R. R. , Smith, D. C. , & D'Hondt, S. (2012). Global distribution of microbial abundance and biomass in subseafloor sediment. Proceedings of the National Academy of Sciences, 109(40), 16,213–16,216. 10.1073/pnas.1203849109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, G. , Mountain, B. W. , & Pancost, R. D. (2008). Microbial membrane lipids in active and inactive sinters from Champagne Pool, New Zealand: Elucidating past geothermal chemistry and microbiology. Organic Geochemistry, 39(8), 1024–1028. 10.1016/j.orggeochem.2008.04.016 [DOI] [Google Scholar]
- Kennedy, C. B. , Gault, A. G. , Fortin, D. , Clark, I. D. , Pedersen, K. , Scott, S. D. , & Ferris, F. G. (2010). Carbon isotope fractionation by circumneutral iron‐oxidizing bacteria. Geology, 38(12), 1087–1090. 10.1130/G30986.1 [DOI] [Google Scholar]
- Kennedy, M. J. , Pevear, D. R. , & Hill, R. J. (2002). Mineral surface control of organic carbon in black shale. Science, 295(5555), 657–660. 10.1126/science.1066611 [DOI] [PubMed] [Google Scholar]
- Kim, M.‐H. Y. , Thibeault, S. A. , Simonsen, L. C. , & Wilson, J. W. (1998). Comparison of Martian meteorites and Martian regolith as shield materials for galactic cosmic rays, NASA Technical Papers, NASA TP‐1998‐208724. https%3A%2F%2Fdoi.org%2F10.1002%2F(SICI)1521-3773(19980518)37%3A9%253C1261%3A%3AAID-ANIE1261%253E3.0.CO%3B2-2
- King, G. M. (2015). Carbon monoxide as a metabolic energy source for extremely halophilic microbes: Implications for microbial activity in Mars regolith. Proceedings of the National Academy of Sciences, 112, 4465–4470. 10.1073/pnas.1424989112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, C. , & Beukes, N. J. (1989). Geochemistry and sedimentology of a facies transition from limestone to iron‐formation deposition in the early Proterozoic Transvaal Supergroup, South Africa. Economic Geology, 84, 1733–1774. 10.2113/gsecongeo.84.7.1733 [DOI] [PubMed] [Google Scholar]
- Kminek, G. , & Bada, J. L. (2006). The effect of ionizing radiation on the preservation of amino acids on Mars. Earth and Planetary Science Letters, 245, 1, (1–1), 5. [Google Scholar]
- Knoll, A. H. (1985). Exceptional preservation of photosynthetic organisms in silicified carbonates and silicified peats. Philosophical Transactions of the Royal Society of London B, 311(1148), 111–122. [Google Scholar]
- Knoll, A. H. , & Grotzinger, J. (2006). Water on Mars and the prospect of Martian life. Elements, 2(3), 169–173. 10.2113/gselements.2.3.169 [DOI] [Google Scholar]
- Knoll, A. H. , Fairchild, I. J. , & Swett, K. (1993). Calcified microbes in Neoproterozoic carbonates; Implications for our understanding of the Proterozoic/Cambrian transition. PALAIOS, 8(6), 512–525. 10.2307/3515029 [DOI] [PubMed] [Google Scholar]
- Komar, V. A. , Raaben, M. E. , & Semikhatov, M. A. (1965). Procedure for studying Conophyton stromatolites and their stratigraphic implications. Akademii Nauk SSSR Doklady.(AGI translation), 161, 96–99. [Google Scholar]
- Konhauser, K. O. , Fisher, Q. J. , Fyfe, W. S. , Longstaffe, F. J. , & Powell, M. A. (1998). Authigenic mineralization and detrital clay binding by freshwater biofilms: The Brahmani River, India. Geomicrobiology Journal, 15(3), 209–222. 10.1080/01490459809378077 [DOI] [Google Scholar]
- Konhauser, K. O. , Jones, B. , Phoenix, V. R. , Ferris, G. , & Renaut, R. W. (2004). The microbial role in hot spring silicification. Ambio: A Journal of the Human Environment, 33(8), 552–558. 10.1579/0044-7447-33.8.552 [DOI] [PubMed] [Google Scholar]
- Konhauser, K. O. , Newman, D. K. , & Kappler, A. (2005). The potential significance of microbial Fe(III) reduction during deposition of Precambrian banded iron formations. Geobiology, 3, 167–177. 10.1111/j.1472-4669.2005.00055.x [DOI] [Google Scholar]
- Konhauser, K. O. , & Urrutia, M. M. (1999). Bacterial clay authigenesis: A common biogeochemical process. Chemical Geology, 161(4), 399–413. 10.1016/S0009-2541(99)00118-7 [DOI] [Google Scholar]
- Krasnopolsky, V. A. , Maillard, J. P. , & Owen, T. C. (2004). Detection of methane in the Martian atmosphere: evidence for life? Icarus, 172(2), 537–547. 10.1016/j.icarus.2004.07.004 [DOI] [Google Scholar]
- Laflamme, M. , Schiffbauer, J. D. , Narbonne, G. M. , & Briggs, D. E. G. (2011). Microbial biofilms and the preservation of the Ediacara biota. Lethaia, 44(2), 203–213. 10.1111/j.1502-3931.2010.00235.x [DOI] [Google Scholar]
- Le Deit, L. , Le Mouelic, S. , Bourgeois, O. , Combe, J. P. , Mège, D. , Sotin, C. , et al. (2008). Ferric oxides in East Candor Chasma, VallesMarineris (Mars) inferred from analysis of OMEGA/Mars Express data: Identification and geological interpretation. Journal of Geophysical Research, 113, E07001 10.1029/2007JE002950 [DOI] [Google Scholar]
- Lepot, K. , Addad, A. , Knoll, A. H. , Wang, J. , Troadec, D. , Béché, A. , & Javaux, E. J. (2017). Iron minerals within specific microfossil morphospecies of the 1.88 Ga Gunflint Formation. Nature Communications, 8, 14890 10.1038/ncomms14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, K. W. , Aharonson, O. , Grotzinger, J. P. , Squyres, S. W. , Bell, J. F. , Crumpler, L. S. , & Schmidt, M. E. (2008). Structure and stratigraphy of home plate from the Spirit Mars exploration rover. Journal of Geophysical Research, 113, E12S36 10.1029/2007JE003025 [DOI] [Google Scholar]
- Lichtenberg, K. A. , Arvidson, R. E. , Morris, R. V. , Murchie, S. L. , Bishop, J. L. , Fernandez Remolar, D. , et al. (2010). Stratigraphy of hydrated sulfates in the sedimentary deposits of Aram Chaos, Mars. Journal of Geophysical Research, 115, E00D17 10.1029/2009JE003353 [DOI] [Google Scholar]
- Lillis, R. J. , Robbins, S. , Manga, M. , Halekas, J. S. , & Frey, H. V. (2013). Time history of the Martian dynamo from crater magnetic field analysis. Journal of Geophysical Research: Planets, 118, 1488–1511. 10.1002/jgre.20105 [DOI] [Google Scholar]
- Lin, L. H. , Hall, J. , Lippmann‐Pipke, J. , Ward, J. A. , Sherwood Lollar, B. , DeFlaun, M. , et al. (2005). Radiolytic H2 in continental crust: nuclear power for deep subsurface microbial communities. Geochemistry, Geophysics, Geosystems, 6, Q07003 10.1029/2004GC000907 [DOI] [Google Scholar]
- Lin, L. H. , Wang, P. L. , Rumble, D. , Lippmann‐Pipke, J. , Boice, E. , Pratt, L. M. , et al. (2006). Long‐term sustainability of a high‐energy, low‐diversity crustal biome. Science, 314(5798), 479–482. 10.1126/science.1127376 [DOI] [PubMed] [Google Scholar]
- Lincoln, S. A. , Bradley, A. S. , Newman, S. A. , & Summons, R. E. (2013). Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in chimneys of the Lost City Hydrothermal Field. Organic Geochemistry, 60, 45–53. 10.1016/j.orggeochem.2013.04.010 [DOI] [Google Scholar]
- Lippmann, J. , Stute, M. , Torgersen, T. , Moser, D. P. , Hall, J. A. , Lin, L. , et al. (2003). Dating ultra‐deep mine waters with noble gases and 36 Cl, Witwatersrand Basin, South Africa. Geochimica et Cosmochimica Acta, 67(23), 4597–4619. 10.1016/S0016-7037(03)00414-9 [DOI] [Google Scholar]
- Little, C. T. S. , Danelian, T. , Herrington, R. J. , & Haymon, R. M. (2004). Early Jurassic hydrothermal vent community from the Franciscan Complex, California. Journal of Paleontology, 78(3), 542–559. https%3A%2F%2Fdoi.org%2F10.1666%2F0022-3360(2004)078%253C0542%3AEJHVCF%253E2.0.CO%3B2 [Google Scholar]
- Little, C. T. S. , Herrington, R. J. , Haymon, R. M. , & Danelian, T. (1999). Early Jurassic hydrothermal vent community from the Franciscan Complex, San Rafael Mountains, California. Geology, 27(2), 167–170. https%3A%2F%2Fdoi.org%2F10.1130%2F0091-7613(1999)027%253C0167%3AEJHVCF%253E2.3.CO%3B2 [Google Scholar]
- Little, C. T. S. , & Thorseth, I. H. (2002). Hydrothermal vent microbial communities: A fossil perspective. Cahiers de Biologie Marine, 43, 317–319. [Google Scholar]
- Magnabosco, C. , Moore, K. R. , Wolfe, J. M. , & Fournier, G. P. (2018). Dating phototropic microbial lineages with reticulate gene histories. Geobiology, 16, 179–189. 10.1111/gbi.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffy, P. R. , Webster, C. R. , Stern, J. C. , Brunner, A. E. , Atreya, S. K. , Conrad, P. G. , et al. (2015). The imprint of atmospheric evolution in the D/H of Hesperian clay minerals on Mars. Science, 347(6220), 412–414. 10.1126/science.1260291 [DOI] [PubMed] [Google Scholar]
- Malin, M. C. , & Edgett, K. S. (2003). Evidence for persistent flow and aqueous sedimentation on early Mars. Science, 302(5652), 1931–1934. 10.1126/science.1090544 [DOI] [PubMed] [Google Scholar]
- Maliva, R. G. , Knoll, A. H. , & Simonson, B. M. (2005). Secular change in the Precambrian silica cycle: Insights from chert petrology. Geological Society of America Bulletin, 117(7‐8), 835–845. [Google Scholar]
- Mangold, N. , Gendrin, A. , Gondet, B. , LeMouelic, S. , Quantin, C. , Ansan, V. , et al. (2008). Spectral and geological study of the sulfate‐rich region of West Candor Chasma, Mars. Icarus, 194, 519–543. 10.1016/j.icarus.2007.10.021 [DOI] [Google Scholar]
- Mariotti, G. , Perron, J. T. , & Bosak, T. (2014). Feedbacks between flow, sediment motion and microbial growth on sand bars initiate and shape elongated stromatolite mounds. Earth and Planetary Science Letters, 397, 93–100. 10.1016/j.epsl.2014.04.036 [DOI] [Google Scholar]
- Mariotti, G. , Pruss, S. B. , Perron, J. T. , & Bosak, T. (2014). Microbial shaping of sedimentary wrinkle structures. Nature Geoscience, 7(10), 736–740. 10.1038/ngeo2229 [DOI] [Google Scholar]
- Massé, M. , Le Mouélic, S. , Bourgeois, O. , Combe, J. P. , Le Deit, L. , Sotin, C. , et al. (2008). Mineralogical composition, structure, morphology, and geological history of Aram Chaos crater fill on Mars derived from OMEGE Mars Express data. Journal of Geophysical Research, 113, E12006 10.1029/2008JE003131 [DOI] [Google Scholar]
- Mata, S. A. , Harwood, C. L. , Corsetti, F. A. , Stork, N. J. , Eilers, K. , Berelson, W. M. , & Spear, J. R. (2012). Influence of gas production and filament orientation on stromatolite microfabric. Palaios, 27(4), 206–219. 10.2110/palo.2011.p11-088r [DOI] [Google Scholar]
- McCoy, V. E. , Young, R. T. , & Briggs, D. E. G. (2015). Factors controlling exceptional preservation in concretions. Palaios, 30(4), 272–280. 10.2110/palo.2014.081 [DOI] [Google Scholar]
- McKay, C. P. , & Stoker, C. R. (1989). The early environment and its evolution on Mars: Implication for life. Reviews of Geophysics, 27, 189–214. 10.1029/RG027i002p00189 [DOI] [Google Scholar]
- McKay, C. P. , Stoker, C. R. , Glass, B. J. , Davé, A. I. , Davila, A. F. , Heldmann, J. L. , et al. (2013). The Icebreaker Life Mission to Mars: A search for biomolecular evidence for life. Astrobiology, 13(4), 334–353. 10.1089/ast.2012.0878 [DOI] [PubMed] [Google Scholar]
- McKinley, J. P. , Stevens, T. O. , & Westall, F. (2000). Microfossils and paleoenvironments in deep subsurface basalt samples. Geomicrobiology Journal, 17(1), 43–54. [Google Scholar]
- McLennan, S. M. (2003). Sedimentary silica on Mars. Geology, 31(4), 315–318. https%3A%2F%2Fdoi.org%2F10.1130%2F0091-7613(2003)031%253C0315%3ASSOM%253E2.0.CO%3B2 [Google Scholar]
- McLennan, S. M. , & Grotzinger, J. P. (2008). The sedimentary rock cycle of Mars In Bell J. (Ed.), The Martian surface: Composition, mineralogy, and physical properties (pp. 541–577.). Cambridge: Cambridge University Press. [Google Scholar]
- McLoughlin, N. , Furnes, H. , Banerjee, N. R. , Muehlenbachs, K. , & Staudigel, H. (2009). Ichnotaxonomy of microbial trace fossils in volcanic glass. Journal of the Geological Society, 166(1), 159–169. 10.1144/0016-76492008-049 [DOI] [Google Scholar]
- McLoughlin, N. , Wilson, L. A. , & Brasier, M. D. (2008). Growth of synthetic stromatolites and wrinkle structures in the absence of microbes—Implications for the early fossil record. Geobiology, 6(2), 95–105. 10.1111/j.1472-4669.2007.00141.x [DOI] [PubMed] [Google Scholar]
- McMahon, S. , Anderson, R. A. , Saupe, E. E. , & Briggs, D. E. G. (2016). Experimental evidence that clay inhibits bacterial decomposers: Implications for the preservation of organic fossils. Geology, 44, 867–870. 10.1130/G38454.1 [DOI] [Google Scholar]
- McMahon, S. , & Parnell, J. (2014). Weighing the deep continental biosphere. FEMS Microbiology Ecology, 87(1), 113–120. 10.1111/1574-6941.12196 [DOI] [PubMed] [Google Scholar]
- McMahon, S. , van Smeerdijk Hood, A. , & McIlroy, D. (2017). The origin and occurrence of subaqueous sedimentary cracks. Geological Society of London, Special Publication, 448(1), 285–309. 10.1144/SP448.15 [DOI] [Google Scholar]
- McNutt, R. H. , Frape, S. K. , Fritz, P. , Jones, M. G. , & MacDonald, I. M. (1990). The 87Sr/86Sr values of Canadian Shield brines and fracture minerals with applications to groundwater mixing, fracture history, and geochronology. Geochimica et Cosmochimica Acta, 54(1), 205–215. 10.1016/0016-7037(90)90208-3 [DOI] [Google Scholar]
- Méhay, S. , Früh‐Green, G. L. , Lang, S. Q. , Bernasconi, S. M. , Brazelton, W. J. , Schrenk, M. O. , et al. (2013). Record of archaeal activity at the serpentinite‐hosted Lost City Hydrothermal Field. Geobiology, 11, 570–592. 10.1111/gbi.12062 [DOI] [PubMed] [Google Scholar]
- Meister, P. , Chapligin, B. , Picard, A. , Meyer, H. , Fischer, C. , Rettenwander, D. , et al. (2014). Early diagenetic quartz formation at a deep iron oxidation front in the Eastern Equatorial Pacific—A modern analogue for banded iron/chert formations? Geochimica et Cosmochimica Acta, 137, 188–207. 10.1016/j.gca.2014.03.035 [DOI] [Google Scholar]
- Metcalfe, K. S. , Gaines, R. R. , Trang, J. , Scott, S. W. , Crane, E. J. , Lackey, J. , et al. (2013). Experimental constraints on microbial liberation of structural iron from common clay minerals in marine sediments. Abstract B13C‐0506 presented at 2013 Fall Meeting, AGU, San Francisco, California, 9–13 Dec. 10.4161/mge.24775 [DOI]
- Metz, J. M. , Grotzinger, J. P. , Mohrig, D. , Milliken, R. , Prather, B. , Pirmez, C. , et al. (2009). Sublacustrine depositional fans in southwest Melas Chasma. Journal of Geophysical Research, 114, E10002 10.1029/2009JE003365 [DOI] [Google Scholar]
- Michalopoulos, P. , & Aller, R. C. (1995). Rapid clay mineral formation in Amazon delta sediments: Reverse weathering and oceanic elemental cycles. Science, 270(5236), 614–614. 10.1126/science.270.5236.614 [DOI] [Google Scholar]
- Michalopoulos, P. , & Aller, R. C. (2004). Early diagenesis of biogenic silica in the Amazon delta: Alteration, authigenic clay formation, and storage. Geochimica et Cosmochimica Acta, 68(5), 1061–1085. 10.1016/j.gca.2003.07.018 [DOI] [Google Scholar]
- Michalski, J. R. , Cuadros, J. , Niles, P. B. , Parnell, J. , Rogers, A. D. , & Wright, S. P. (2013). Groundwater activity on Mars and implications for a deep biosphere. Nature Geoscience, 6(2), 133 10.1038/ngeo1706 [DOI] [Google Scholar]
- Michalski, J. R. , & Niles, P. B. (2010). Deep crustal carbonate rocks exposed by meteor impact on Mars. Nature Geoscience, 3, 751–755. 10.1038/ngeo971 [DOI] [Google Scholar]
- Mileikowsky, C. , Cucinotta, F. A. , Wilson, J. W. , Gladman, B. , Horneck, G. , Lindegren, L. , et al. (2000). Natural transfer of viable microbes in space: 1. From Mars to Earth and Earth to Mars. Icarus, 145(2), 391–427. 10.1006/icar.1999.6317 [DOI] [PubMed] [Google Scholar]
- Milliken, R. E. , & Bish, D. L. (2010). Sources and sinks of clay minerals on Mars. Philosophical Magazine, 90(17‐18), 2293–2308. 10.1080/14786430903575132 [DOI] [Google Scholar]
- Milliken, R. E. , Ewing, R. C. , Fischer, W. W. , & Hurowitz, J. (2014). Wind‐blown sandstones cemented by sulfate and clay minerals in Gale Crater, Mars. Geophysical Research Letters, 41, 1149–1154. 10.1002/2013GL059097 [DOI] [Google Scholar]
- Milliken, R. E. , Grotzinger, J. P. , & Thomson, B. J. (2010). Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophysical Research Letters, 37, L04201 10.1029/2009GL041870 [DOI] [Google Scholar]
- Milliken, R. E. , Swayze, G. A. , Arvidson, R. E. , Bishop, J. L. , Clark, R. N. , Ehlmann, B. L. , et al. (2008). Opaline silica in young deposits on Mars. Geology, 36(11), 847–850. 10.1130/G24967A.1 [DOI] [Google Scholar]
- Ming, D. W. , Archer, P. D. , Glavin, D. P. , Eigenbrode, J. L. , Franz, H. B. , Sutter, B. , et al. (2014). Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science, 343(6169), 1245267 10.1126/science.1245267 [DOI] [PubMed] [Google Scholar]
- Moore, J. M. , & Howard, A. D. (2005). Large alluvial fans on Mars. Journal of Geophysical Research, 110, E04005 10.1029/2004JE002352 [DOI] [Google Scholar]
- Morais, L. , Fairchild, T. R. , Lahr, D. J. , Rudnitzki, I. D. , Schopf, J. W. , Garcia, A. K. , et al. (2017). Carbonaceous and siliceous Neoproterozoic vase‐shaped microfossils (Urucum Formation, Brazil) and the question of early protistan biomineralization. Journal of Paleontology, 91(3), 393–406. [Google Scholar]
- Morris, R. V. , Ruff, S. W. , Gellert, R. , Ming, D. W. , Arvidson, R. E. , Clark, B. C. , et al. (2010). Identification of carbonate‐rich outcrops on Mars by the Spirit rover. Science, 329(5990), 421–424. 10.1126/science.1189667 [DOI] [PubMed] [Google Scholar]
- Morris, R. V. , Vaniman, D. T. , Blake, D. F. , Gellert, R. , Chipera, S. J. , Rampe, E. B. , et al. (2016). Silicic volcanism on Mars evidenced by tridymite in high‐SiO2 sedimentary rock at Gale Crater. Proceedings of the National Academy of Sciences, 113(26), 7071–7076. 10.1073/pnas.1607098113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, K. D. , Misra, R. , & Williams, L. B. (2016). Unearthing the antibacterial mechanism of medicinal clay: A geochemical approach to combating antibiotic resistance. Scientific Reports, 6, 19043 10.1038/srep19043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, D. P. , Gihring, T. M. , Brockman, F. J. , Fredrickson, J. K. , Balkwill, D. L. , Dollhopf, M. E. , et al. (2005). Desulfotomaculum and Methanobacterium spp. dominate a 4‐to 5‐kilometer‐deep fault. Applied and Environmental Microbiology, 71(12), 8773–8783. 10.1128/AEM.71.12.8773-8783.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumma, M. J. , Villanueva, G. L. , Novak, R. E. , Hewagama, T. , Bonev, B. P. , DiSanti, M. A. , et al. (2009). Strong release of methane on Mars in northern summer 2003. Science, 323(5917), 1041–1045. 10.1126/science.1165243 [DOI] [PubMed] [Google Scholar]
- Munoz‐Saez, C. , Saltiel, S. , Manga, M. , Nguyen, C. , & Gonnermann, H. (2016). Physical and hydraulic properties of modern sinter deposits: El Tatio, Atacama. Journal of Volcanology and Geothermal Research, 325, 156–168. 10.1016/j.jvolgeores.2016.06.026 [DOI] [Google Scholar]
- Murchie, S. , Roach, L. , Seelos, F. , Milliken, R. , Mustard, J. , Arvidson, R. , et al. (2009). Evidence for the origin of layered deposits in Candor Chasma, Mars, from mineral composition and hydrologic modeling. Journal of Geophysical Research, 114, E00D05 10.1029/2009JE003343 [DOI] [Google Scholar]
- Mus, M. M. , & Moczydłowska, M. (2000). Internal morphology and taphonomic history of the Neoproterozoic vase‐shaped microfossils from the Visings Group, Sweden. Norsk Geologisk Tidsskrift, 80(3), 213–228. [Google Scholar]
- Mustard, J.F. , Adler, M. , Allwood, A. , Bass, D.S. , Beaty, D. W. , Bell, J. F. III , et al. (2013). Report of the Mars 2020 Science Definition Team, 154, posted July, 2013, by the Mars Exploration Program Analysis Group (MEPAG) at http://mepag.jpl.nasa.gov/reports/MEP/Mars_2020_SDT_Report_Final.pdf
- Naimark, E. , Kalinina, M. , Shokurov, A. , Boeva, N. , Markov, A. , Zaytseva, L. , & Gabbott, S. (2016). Decaying in different clays: Implications for soft‐tissue preservation. Palaeontology, 59(4), 583–595. 10.1111/pala.12246 [DOI] [Google Scholar]
- Naimark, E. B. , Kalinina, M. A. , Shokurov, A. V. , Markov, A. V. , & Boeva, N. M. (2016). Decaying of Artemia salina in clay colloids: 14‐month experimental formation of subfossils. Journal of Paleontology, 90(3), 472–484. [Google Scholar]
- Navarro‐Gonzalez, R. , Vargas, E. , de la Rosa, J. , Raga, A. C. , & McKay, C. P. (2010). Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. Journal of Geophysical Research: Planets, 115, E12010 10.1029/2009JE003343 [DOI] [Google Scholar]
- Nealson, K. H. , & Conrad, P. G. (1999). Life: past, present and future. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 354(1392), 1923–1939. 10.1098/rstb.1999.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, S. A. , Klepac‐Ceraj, V. , Mariotti, G. , Pruss, S. B. , Watson, N. , & Bosak, T. (2017). Experimental fossilization of matforming cyanobacteria in coarse‐grained siliciclastic sediments. Geobiology, 15(4), 484–498. 10.1111/gbi.12229 [DOI] [PubMed] [Google Scholar]
- Newman, S. A. , Mariotti, G. , Pruss, S. , & Bosak, T. (2016). Insights into cyanobacterial fossilization in Ediacaran siliciclastic environments. Geology, 44(7), 579–582. 10.1130/G37791.1 [DOI] [Google Scholar]
- Niles, P. B. , Catling, D. C. , Berger, G. , Chassefière, E. , Ehlmann, B. L. , Michalski, J. R. , et al. (2013). Geochemistry of carbonates on Mars: Implications for climate history and nature of aqueous environments. Space Science Reviews, 174(1‐4), 301–328. 10.1007/s11214-012-9940-y [DOI] [Google Scholar]
- Nixon, S. , Cousins, C. R. , & Cockell, C. (2013). Plausible microbial metabolisms on Mars. Astronomy & Geophysics, 54, 1.13–1.16. [Google Scholar]
- Nixon, S. L. , Cockell, C. S. , & Tranter, M. (2012). Limitations to a microbial iron cycle on Mars. Planetary and Space Science, 72(1), 116–128. 10.1016/j.pss.2012.04.003 [DOI] [Google Scholar]
- Noe Dobrea, E. Z. N. , Poulet, F. , & Malin, M. (2008). Correlations between hematite and sulfates in chaotic terrain east of Valles Marineris. Icarus, 193, 2008. [Google Scholar]
- Noffke, N. (2015). Ancient sedimentary structures in the ~3.7 Ga Gillespie Lake Member, Mars, that resemble macroscopic morphology, spatial associations, and temporal succession in terrestrial microbialites. Astrobiology, (2), 169–192. 10.1089/ast.2014.1218 [DOI] [PubMed] [Google Scholar]
- Noffke, N. , Gerdes, G. , Klenke, T. , & Krumbein, W. E. (2001). Microbially induced sedimentary structures—A new category within the classification of primary sedimentary structures. Journal of Sedimentary Research, 71(5), 649–656. 10.1306/2DC4095D-0E47-11D7-8643000102C1865D [DOI] [Google Scholar]
- Nutman, A. P. , Bennett, V. C. , Friend, C. R. , Van Kranendonk, M. J. , & Chivas, A. R. (2016). Rapid emergence of life shown by discovery of 3,700‐million‐year‐old microbial structures. Nature, 537(7621), 535–538. 10.1038/nature19355 [DOI] [PubMed] [Google Scholar]
- Ohtomo, Y. , Kakegawa, T. , Ishida, A. , Nagase, T. , & Rosing, M. T. (2014). Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks. Nature Geoscience, 7(1), 25–28. 10.1038/ngeo2025 [DOI] [Google Scholar]
- Onstott, T. C. , McGown, D. J. , Bakermans, C. , Ruskeeniemi, T. , Ahonen, L. , Telling, J. , et al. (2009). Microbial communities in subpermafrost saline fracture water at the Lupin Au Mine, Nunavut, Canada. Microbial Ecology, 58(4), 786–807. 10.1007/s00248-009-9553-5 [DOI] [PubMed] [Google Scholar]
- Orange, F. , Dupont, S. , Goff, O. L. , Bienvenu, N. , Disnar, J. R. , Westall, F. , & Le Romancer, M. (2014). Experimental fossilization of the thermophilic Gram‐positive bacterium Geobacillus SP7A: A long duration preservation study. Geomicrobiology Journal, 31(7), 578–589. 10.1080/01490451.2013.860208 [DOI] [Google Scholar]
- Orange, F. , Lalonde, S. V. , & Konhauser, K. O. (2013). Experimental simulation of evaporation‐driven silica sinter formation and microbial silicification in hot spring systems. Astrobiology, 13(2), 163–176. 10.1089/ast.2012.0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, F. , Westall, F. , Disnar, J. R. , Prieur, D. , Bienvenu, N. , Le Romancer, M. , & Défarge, C. (2009). Experimental silicification of the extremophilic Archaea Pyrococcus abyssi and Methanocaldococcus jannaschii: Applications in the search for evidence of life in early Earth and extraterrestrial rocks. Geobiology, 7(4), 403–418. 10.1111/j.1472-4669.2009.00212.x [DOI] [PubMed] [Google Scholar]
- Osinski, G. R. , Tornabene, L. L. , Banerjee, N. R. , Cockell, C. S. , Flemming, R. , Izawa, M. R. , et al. (2013). Impact‐generated hydrothermal systems on Earth and Mars. Icarus, 224(2), 347–363. 10.1016/j.icarus.2012.08.030 [DOI] [Google Scholar]
- Osterloo, M. M. , Hamilton, V. E. , Bandfield, J. L. , Glotch, T. D. , Baldridge, A. M. , Christensen, P. R. , et al. (2008). Chloride‐bearing materials in the southern highlands of Mars. Science, 319(5870), 1651–1654. 10.1126/science.1150690 [DOI] [PubMed] [Google Scholar]
- Pancost, R. D. , Pressley, S. , Coleman, J. M. , Talbot, H. M. , Kelly, S. P. , Farrimond, P. , et al. (2006). Composition and implications of diverse lipids in New Zealand geothermal sinters. Geobiology, 4, 71–92. 10.1111/j.1472-4669.2006.00069.x [DOI] [Google Scholar]
- Parenteau, M. N. , & Cady, S. L. (2010). Microbial biosignatures in iron‐mineralized phototrophic mats at Chocolate Pots hot springs, Yellowstone National Park, United States. Palaios, 25(2), 97–111. 10.2110/palo.2008.p08-133r [DOI] [Google Scholar]
- Parenteau, M. N. , Jahnke, L. L. , Farmer, J. D. , & Cady, S. L. (2014). Production and early preservation of lipid biomarkers in iron hot springs. Astrobiology, 14, 502–521. 10.1089/ast.2013.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, B. H. , Percivalle, C. , Ritson, D. J. , Duffy, C. D. , & Sutherland, J. D. (2015). Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nature Chemistry, 7(4), 301–307. 10.1038/nchem.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckmann, J. , Bach, W. , Behrens, K. , & Reitner, J. (2008). Putative cryptoendolithic life in Devonian pillow basalt, Rheinisches Schiefergebirge, Germany. Geobiology, 6(2), 125–135. 10.1111/j.1472-4669.2007.00131.x [DOI] [PubMed] [Google Scholar]
- Peckmann, J. , & Thiel, V. (2004). Carbon cycling at ancient methane–seeps. Chemical Geology, 205(3), 443–467. [Google Scholar]
- Perry, R. S. , Mcloughlin, N. , Lynne, B. Y. , Sephton, M. A. , Oliver, J. D. , Perry, C. C. , et al. (2007). Defining biominerals and organominerals: direct and indirect indicators of life. Sedimentary Geology, 201(1‐2), 157–179. 10.1016/j.sedgeo.2007.05.014 [DOI] [Google Scholar]
- Peters, K. E. , Walters, C. C. , & Moldowan, J. M. (2005). The biomarker guide (2nd ed.). Cambridge, UK: Cambridge University Press; 10.1109/IEMBS.2005.1616181 [DOI] [Google Scholar]
- Petroff, A. P. , Sim, M. S. , Maslov, A. , Krupenin, M. , Rothman, D. H. , & Bosak, T. (2010). Biophysical basis for the geometry of conical stromatolites. Proceedings of the National Academy of Sciences, 107(22), 9956–9961. 10.1073/pnas.1001973107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff, A. P. P. , Rothman, D. H. , Beukes, N. , & Bosak, T. (2013). Biofilm growth and fossil form. Physical Review X, 3, 041012 10.1103/PhysRevX.3.041012 [DOI] [Google Scholar]
- Petrovich, R. (2001). Mechanisms of fossilization of the soft‐bodied and lightly armored faunas of the Burgess Shale and of some other classical localities. American Journal of Science, 301, 683–726. 10.2475/ajs.301.8.683 [DOI] [Google Scholar]
- Pierre, F. D. , Natalicchio, M. , Ferrando, S. , Giustetto, R. , Birgel, D. , Carnevale, G. , et al. (2015). Are the large filamentous microfossils preserved in Messinian gypsum colorless sulfide‐oxidizing bacteria? Geology, 43(10), 855–858. 10.1130/G37018.1 [DOI] [Google Scholar]
- Pignatello, J. J. , Oliveros, E. , & McKay, A. (2006). Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Critical Reviews in Environmental Science and Technology, 36, 1–84. 10.1080/10643380500326564 [DOI] [Google Scholar]
- Planavsky, N. , Rouxel, O. , Bekker, A. , Shapiro, R. , Fralick, P. , & Knudsen, A. (2009). Iron-oxidizing microbial ecosystems thrived in late Paleoproterozoic redox-stratified oceans. Earth and Planetary Science Letters, 286(1–2), 230–242. 10.1016/j.epsl.2009.06.033 [DOI] [Google Scholar]
- Potter‐McIntyre, S. L. , Williams, J. , Phillips‐Lander, C. , & O'Connell, L. (2017). Taphonomy of microbial biosignatures in spring deposits: A comparison of modern, quaternary, and Jurassic examples. Astrobiology, 17(3), 216–230. 10.1089/ast.2016.1495 [DOI] [PubMed] [Google Scholar]
- Porter, S. M. (2007). Seawater chemistry and early carbonate biomineralization. Science, 316(5829), 1302–1302. 10.1126/science.1137284 [DOI] [PubMed] [Google Scholar]
- Posth, N. R. , Konhauser, K. O. , & Kappler, A. (2013). Microbiological processes in banded iron formation deposition. Sedimentology, 60(7), 1733–1754. 10.1111/sed.12051 [DOI] [Google Scholar]
- Poulet, F. , Arvidson, R. E. , Gomez, C. , Morris, R. V. , Bibring, J.‐P. , Langevin, Y. , et al. (2008). Mineralogy of Terra Meridiani and western Arabia Terra from OMEGA/Mex and implications for their formation. Icarus, 195(1), 106–130. 10.1016/j.icarus.2007.11.031 [DOI] [Google Scholar]
- Preston, L. J. , & Genge, M. J. (2010). The Rhynie Chert, Scotland, and the search for life on Mars. Astrobiology, 10(5), 549–560. 10.1089/ast.2008.0321 [DOI] [PubMed] [Google Scholar]
- Preston, L. J. , Shuster, J. , Fernández‐Remolar, D. , Banerjee, N. R. , Osinski, G. R. , & Southam, G. (2011). The preservation and degradation of filamentous bacteria and biomolecules within iron oxide deposits at Rio Tinto, Spain. Geobiology, 9(3), 233–249. 10.1111/j.1472-4669.2011.00275.x [DOI] [PubMed] [Google Scholar]
- Qu, Y. , Engdahl, A. , Zhu, S. , Vajda, V. , & McLoughlin, N. (2015). Ultrastructural heterogeneity of carbonaceous material in ancient cherts: Investigating biosignature origin and preservation. Astrobiology, 15(10), 825–842. 10.1089/ast.2015.1298 [DOI] [PubMed] [Google Scholar]
- Rampe, E. B. , Ming, D. W. , Blake, D. F. , Bristow, T. F. , Chipera, S. J. , Grotzinger, J. P. , et al. (2017). Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale Crater, Mars. Earth and Planetary Science Letters, 471, 172–185. 10.1016/j.epsl.2017.04.021 [DOI] [Google Scholar]
- Ramseyer, K. , Amthor, J. E. , Matter, A. , Pettke, T. , Wille, M. , & Fallick, A. E. (2013). Primary silica precipitate at the Precambrian/Cambrian boundary in the south Oman salt basin, sultanate of Oman. Marine and Petroleum Geology, 39(1), 187–197. 10.1016/j.marpetgeo.2012.08.006 [DOI] [Google Scholar]
- Ransom, B. , Kim, D. , Kastner, M. , & Wainwright, S. (1998). Organic matter preservation on continental slopes: Importance of mineralogy and surface area. Geochimica et Cosmochimica Acta, 62(8), 1329–1345. 10.1016/S0016-7037(98)00050-7 [DOI] [Google Scholar]
- Reid, R. P. , Visscher, P. T. , Decho, A. W. , Stolz, J. F. , Bebout, B. M. , Dupraz, C. , et al. (2000). The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature, 406(6799), 989–992. 10.1038/35023158 [DOI] [PubMed] [Google Scholar]
- Riding, R. (1991). Classification of microbial carbonates. Calcareous Algae and Stromatolites, 2, 1–51. [Google Scholar]
- Riding, R. (1999). The term stromatolite: Towards an essential definition. Lethaia, 32(4), 321–330. [Google Scholar]
- Roach, L. H. , Mustard, J. F. , Lane, M. D. , Bishop, J. L. , & Murchie, S. L. (2010). Diagenetic haematite and sulfate assemblages in Valles Marineris. Icarus, 207(2), 659–674. 10.1016/j.icarus.2009.11.029 [DOI] [Google Scholar]
- Rothschild, L. J. (1990). Earth analogs for Martian life. Microbes in evaporites, a new model system for life on Mars. Icarus, 88, 246–260. 10.1016/0019-1035(90)90188-F [DOI] [PubMed] [Google Scholar]
- Ruff, S. W. , & Farmer, J. D. (2016). Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nature Communications, 7, 13554 10.1038/ncomms13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield, C. L. , Lowenstein, T. K. , Vreeland, R. H. , Rosenzweig, W. D. , & Powers, D. W. (2005). New evidence for 250 Ma age of halotolerant bacterium from a Permian salt crystal. Geology, 33(4), 265–268. 10.1130/G21106.1 [DOI] [Google Scholar]
- Schaefer, M. W. (1996). Are there abiotically‐precipitated iron‐formations on Mars? In Mineral spectroscopy: A tribute to Roger G. Burns. The Geochemical Society Houston (Vol. 5, pp. 381–393). Washington, DC: The Geochemical Society. [Google Scholar]
- Schieber J., Bose P. K., Eriksson P. G., Banerjee S., Sarkar S., Altermann W., & Catuneanu O. (Eds.) (2007). Atlas of microbial mat features preserved within the siliciclastic rock record (Vol. 2). Amsterdam: Elsevier. [Google Scholar]
- Schopf, J. W. (1968). Microflora of the Bitter Springs formation, late Precambrian, central Australia. Journal of Paleontology, 651–688. [Google Scholar]
- Schopf, J. W. (2006). Fossil evidence of Archaean life. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 361(1470), 869–885. 10.1098/rstb.2006.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf, J. W. , Farmer, J. D. , Foster, I. S. , Kudryavtsev, A. B. , Gallardo, V. A. , & Espinoza, C. (2012). Gypsum‐permineralized microfossils and their relevance to the search for life on Mars. Astrobiology, 12(7), 619–633. 10.1089/ast.2012.0827 [DOI] [PubMed] [Google Scholar]
- Schopf J. W., & Klein C. (Eds.) (1992). The Proterozoic biosphere: A multidisciplinary study. Cambridge, UK: Cambridge University Press; 10.1300/J074v04n01_07 [DOI] [Google Scholar]
- Schopf, J. W. , Tewari, V. C. , & Kudryavtsev, A. B. (2008). Discovery of a new Chert‐Permineralized Microbiota in the Proterozoic Buxa Formation of the Ranjit window, Sikkim, northeast India, and its astrobiological implications. Astrobiology, 8(4), 735–746. 10.1089/ast.2007.0184 [DOI] [PubMed] [Google Scholar]
- Schultze‐Lam, S. , Ferris, F. G. , Konhauser, K. O. , & Wiese, R. G. (1995). In situ silicification of an Icelandic hot spring microbial mat: Implications for microfossil formation. Canadian Journal of Earth Sciences, 32(12), 2021–2026. 10.1139/e95-155 [DOI] [Google Scholar]
- Schumann, G. , Manz, W. , Reitner, J. , & Lustrino, M. (2004). Ancient fungal life in North Pacific Eocene oceanic crust. Geomicrobiology Journal, 21(4), 241–246. 10.1080/01490450490438748 [DOI] [Google Scholar]
- Shapiro, R. S. , & Konhauser, K. O. (2015). Hematite‐coated microfossils: primary ecological fingerprint or taphonomic oddity of the Paleoproterozoic? Geobiology, 13, 209–224. 10.1111/gbi.12127 [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Farquhar, J. , Masterson, A. , Kaufman, A. J. , & Buick, R. (2009). Evaluating the role of microbial sulfate reduction in the early Archean using quadruple isotope systematics. Earth and Planetary Science Letters, 279(3), 383–391. [Google Scholar]
- Sherwood Lollar, B. , Lacrampe‐Couloume, G. , Slater, G. F. , Ward, J. , Moser, D. P. , Gihring, T. M. , et al. (2006). Unravelling abiogenic and biogenic sources of methane in the Earth's deep subsurface. Chemical Geology, 226(3), 328–339. [Google Scholar]
- Sherwood Lollar, B. , Voglesonger, K. , Lin, L. H. , Lacrampe‐Couloume, G. , Telling, J. , Abrajano, T. A. , et al. (2007). Hydrogeologic controls on episodic H2 release from Precambrian fractured rocks—Energy for deep subsurface life on Earth and Mars. Astrobiology, 7(6), 971–986. 10.1089/ast.2006.0096 [DOI] [PubMed] [Google Scholar]
- Shuster, D. L. , & Weiss, B. P. (2005). Martian surface paleotemperatures from thermochronology of meteorites. Science, 309(5734), 594–600. 10.1126/science.1113077 [DOI] [PubMed] [Google Scholar]
- Siever, R. (1992). The silica cycle in the Precambrian. Geochimica et Cosmochimica Acta, 56(8), 3265–3272. 10.1016/0016-7037(92)90303-Z [DOI] [Google Scholar]
- Sim, M. S. , Liang, B. , Petroff, A. P. , Evans, A. , Klepac‐Ceraj, V. , Flannery, D. T. , et al. (2012). Oxygendependent morphogenesis of modern clumped photosynthetic mats and implications for the Archean stromatolite record. Geosciences, 2(4), 235–259. 10.3390/geosciences2040235 [DOI] [Google Scholar]
- Skok, J. R. , Mustard, J. F. , Ehlmann, B. L. , Milliken, R. E. , & Murchie, S. L. (2010). Silica deposits in the Nili Patera caldera on the Syrtis Major volcanic complex on Mars. Nature Geoscience, 3(12), 838–841. 10.1038/ngeo990 [DOI] [Google Scholar]
- Slack, J. F. , Grenne, T. , Bekker, A. , Rouxel, O. J. , & Lindberg, P. A. (2007). Suboxic deep seawater in the late Paleoproterozoic: Evidence from hematitic chert and iron formation related to seafloor‐hydrothermal sulfide deposits, central Arizona, USA. Earth and Planetary Science Letters, 255(1–2), 243–256. 10.1016/j.epsl.2006.12.018 [DOI] [Google Scholar]
- Slotznick, S. P. , & Fischer, W. W. (2016). Examining Archean methanotrophy. Earth and Planetary Science Letters, 441, 52–59. 10.1016/j.epsl.2016.02.013 [DOI] [Google Scholar]
- Smith, H. D. , & McKay, C. P. (2005). Drilling in ancient permafrost on Mars for evidence of a second genesis of life. Planetary and Space Science, 53(12), 1302–1308. 10.1016/j.pss.2005.07.006 [DOI] [Google Scholar]
- Sowe, M. , Wendt, L. , McGuire, P. C. , & Neukum, G. (2012). Hydrated minerals in the deposits of Aureum Chaos. Icarus, 218, 406–419. 10.1016/j.icarus.2011.12.009 [DOI] [Google Scholar]
- Squyres, S. W. , Arvidson, R. E. , Ruff, S. , Gellert, R. , Morris, R. V. , Ming, D. W. , et al. (2008). Detection of silica‐rich deposits on Mars. Science, 320(5879), 1063–1067. 10.1126/science.1155429 [DOI] [PubMed] [Google Scholar]
- Squyres, S. W. , Grotzinger, J. P. , Arvidson, R. E. , Bell, J. F. , Calvin, W. , Christensen, P. R. , et al. (2004). In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science, 306(5702). 10.1126/science.1104559 [DOI] [PubMed] [Google Scholar]
- Staudigel, H. , Furnes, H. , McLoughlin, N. , Banerjee, N. R. , Connell, L. B. , & Templeton, A. (2008). 3.5 billion years of glass bioalteration: Volcanic rocks as a basis for microbial life? Earth‐Science Reviews, 89(3), 156–176. [Google Scholar]
- Stern, J. C. , Sutter, B. , Freissinet, C. , Navarro‐González, R. , McKay, C. P. , Archer, P. D. , et al. (2015). Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale Crater, Mars. Proceedings of the National Academy of Sciences, 112(14), 4245–4250. 10.1073/pnas.1420932112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolper, D. A. , Love, G. D. , Bates, S. , Lyons, T. W. , Young, E. , Sessions, A. L. , & Grotzinger, J. P. (2017). Paleoecology and paleoceanography of the Athel silicilyte, Ediacaran–Cambrian boundary, Sultanate of Oman. Geobiology, 15(3), 401–426. 10.1111/gbi.12236 [DOI] [PubMed] [Google Scholar]
- Summons, R. E. , Amend, J. P. , Bish, D. , Buick, R. , Cody, G. D. , Des Marais, D. J. , et al. (2011). Preservation of Martian organic and environmental records: Final report of the Mars biosignature working group. Astrobiology, 11, 157–181. 10.1089/ast.2010.0506 [DOI] [PubMed] [Google Scholar]
- Summons, R. E. , & Lincoln, S. A. (2012). Biomarkers: Informative molecules for studies in geobiology In Knoll A. H., Canfield D. E., & Konhauser K. O. (Eds.), Fundamentals of geobiology (1st ed., pp. 269–296). Chichester, UK: Blackwell. [Google Scholar]
- Sumner, D. Y. (1997). Late Archean calcite‐microbe interactions; two morphologically distinct microbial communities that affected calcite nucleation differently. Palaios, 12(4), 302–318. 10.2307/3515333 [DOI] [Google Scholar]
- Sumner, D. Y. (2004). Poor preservation potential of organics in Meridiani Planum hematite‐bearing sedimentary rocks. Journal of Geophysical Research, 109, E12007 10.1029/2004JE002321 [DOI] [Google Scholar]
- Sun, V. Z. , & Milliken, R. E. (2015). Ancient and recent clay formation on Mars as revealed from a global survey of hydrous minerals in crater central peaks. Journal of Geophysical Research: Planets, 120, 2293–2332. 10.1002/2015JE004918 [DOI] [Google Scholar]
- Szabó, T. , Domokos, G. , Grotzinger, J. P. , & Jerolmack, D. J. (2015). Reconstructing the transport history of pebbles on Mars. Nature Communications, 6, 8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhan, L. G. , Hood, A.v. S. , Droser, M. L. , Gehling, J. G. , & Briggs, D. E. G. (2016). Exceptional preservation of soft‐bodied Ediacara biota promoted by silica‐rich oceans. Geology, 44(11), 951–954. 10.1130/G38542.1 [DOI] [Google Scholar]
- ten Kate, I. L. , Garry, J. R. , Peeters, Z. , Quinn, R. , Foing, B. , & Ehrenfreund, P. (2005). Amino acid photostability on the Martian surface. Meteoritics & Planetary Science, 40(8), 1185–1193. 10.1111/j.1945-5100.2005.tb00183.x [DOI] [Google Scholar]
- Thollot, P. , Mangold, N. , Ansan, V. , Le Mouelic, S. , Milliken, R. E. , Bishop, J. L. , et al. (2012). Most Mars minerals in a nutshell: Various alteration phases formed in a single environment in Noctis Labyrinthus. Journal of Geophysical Research, 117, E00J06 10.1029/2011JE004028 [DOI] [Google Scholar]
- Thomas, R. J. , Potter‐McIntyre, S. L. , & Hynek, B. M. (2017). Large‐scale fluid‐deposited mineralization in Margaritifer Terra, Mars. Geophysical Research Letters, 44, 6579–6588. 10.1002/2017GL073388 [DOI] [Google Scholar]
- Thorseth, I. H. (2011). Basalt (glass, endoliths) In Reitner J. & Thiel V. (Eds.), Encyclopedia of geobiology (pp. 103–111). Dordrecht: Springer. [Google Scholar]
- Toporski, J. K. , Steele, A. , Westall, F. , Thomas‐Keprta, K. L. , & McKay, D. S. (2002). The simulated silicification of bacteria—New clues to the modes and timing of bacterial preservation and implications for the search for extraterrestrial microfossils. Astrobiology, 2(1), 1–26. 10.1089/153110702753621312 [DOI] [PubMed] [Google Scholar]
- Tosca, N. J. , & Knoll, A. H. (2009). Juvenile chemical sediments and the long term persistence of water at the surface of Mars. Earth and Planetary Science Letters, 286(3‐4), 379–386. 10.1016/j.epsl.2009.07.004 [DOI] [Google Scholar]
- Tosca, N. J. , Knoll, A. H. , & McLennan, S. M. (2008). Water activity and the challenge for life on early Mars. Science, 320(5880), 1204–1207. 10.1126/science.1155432 [DOI] [PubMed] [Google Scholar]
- Tosca, N. J. , McLennan, S. M. , Lindsley, D. H. , & Schoonen, M. A. (2004). Acid‐sulfate weathering of synthetic Martian basalt: The acid fog model revisited. Journal of Geophysical Research, 109, E05003 10.1029/2003JE002218 [DOI] [Google Scholar]
- Travis, B. J. , Rosenberg, N. D. , & Cuzzi, J. N. (2003). On the role of widespread subsurface convection in bringing liquid water close to Mars' surface. Journal of Geophysical Research, 108(E4), 8040 10.1029/2002JE001877 [DOI] [Google Scholar]
- Trendall, A. F. (2002). The significance of iron‐formation in the Precambrian stratigraphic record In Altermann W. & Corcoran P. L. (Eds.), Precambrian sedimentary environments: A modern approach to ancient depositional systems (pp. 33–66). Oxford: Blackwell, International Association of Sedimentologists. [Google Scholar]
- Trewin, N. H. (1993). Depositional environment and preservation of biota in the Lower Devonian hot‐springs of Rhynie, Aberdeenshire, Scotland. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 84, 433–442. [Google Scholar]
- Trewin, N. H. (1996). The Rhynie cherts: An early Devonian ecosystem preserved by hydrothermal activity. Evolution of hydrothermal ecosystems on Earth and Mars, 202, 131–149. [DOI] [PubMed] [Google Scholar]
- Trewin, N. H. , Fayers, S. R. , & Kelman, R. (2003). Subaqueous silicification of the contents of small ponds in an Early Devonian hot‐spring complex, Rhynie, Scotland. Canadian Journal of Earth Sciences, 40, 1697–1712. 10.1139/e03-065 [DOI] [Google Scholar]
- Ueno, Y. , Yamada, K. , Yoshida, N. , Maruyama, S. , & Isozaki, Y. (2006). Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature, 440, 516–519. 10.1038/nature04584 [DOI] [PubMed] [Google Scholar]
- Vago, J. L. , Westall, F. , Pasteur Teams, Landing Site Selection Working Group , et al. (2017). Habitability on early Mars and the search for biosignatures with the ExoMars Rover. Astrobiology, 17, 471–510. 10.1089/ast.2016.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuilen, M. A. , Lepland, A. , & Arrhenius, G. (2002). Reassessing the evidence for the earliest traces of life. Nature, 418, 627–630. 10.1038/nature00934 [DOI] [PubMed] [Google Scholar]
- van Zuilen, M. A. , Lepland, A. , Teranes, J. , Finarelli, J. , Wahlen, M. , & Arrhenius, G. (2003). Graphite and carbonates in the 3.8 Ga old Isua Supracrustal Belt, southern West Greenland. Precambrian Research, 126, 331–348. 10.1016/S0301-9268(03)00103-7 [DOI] [Google Scholar]
- Vaniman, D. T. , Bish, D. L. , Ming, D. W. , Bristow, T. F. , Morris, R. V. , Blake, D. F. , et al. (2014). Mineralogy of a mudstone at Yellowknife Bay, Gale Crater, Mars. Science, 1243480 10.1126/science.1243480 [DOI] [PubMed] [Google Scholar]
- Vasconcelos, C. , Warthmann, R. , McKenzie, J. A. , Visscher, P. T. , Bittermann, A. G. , & van Lith, Y. (2006). Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sedimentary Geology, 185(3‐4), 175–183. 10.1016/j.sedgeo.2005.12.022 [DOI] [Google Scholar]
- Visscher, P. T. , Reid, R. P. , & Bebout, B. M. (2000). Microscale observations of sulfate reduction: correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology, 28(10), 919–922. https%3A%2F%2Fdoi.org%2F10.1130%2F0091-7613(2000)28%253C919%3AMOOSRC%253E2.0.CO%3B2 [Google Scholar]
- Vorhies, J. S. , & Gaines, R. R. (2009). Microbial dissolution of clay minerals as a source of iron and silica in marine sediments. Nature Geoscience, 2(3), 221–225. 10.1038/ngeo441 [DOI] [Google Scholar]
- Vreeland, R. H. , Rosenzweig, W. D. , & Powers, D. W. (2000). Isolation of a 250 million‐year‐old halotolerant bacterium from a primary salt crystal. Nature, 407(6806), 897–900. 10.1038/35038060 [DOI] [PubMed] [Google Scholar]
- Wacey, D. , Kilburn, M. R. , Saunders, M. , Cliff, J. , & Brasier, M. D. (2011). Microfossils of sulphur‐metabolizing cells in 3.4‐billion‐year‐old rocks of Western Australia. Nature Geoscience, 4(10), 698–702. 10.1038/ngeo1238 [DOI] [Google Scholar]
- Wacey, D. , Saunders, M. , & Kong, C. (2018). Remarkably preserved tephra from the 3430 Ma Strelley Pool Formation, Western Australia: Implications for the interpretation of Precambrian microfossils. Earth and Planetary Science Letters, 487, 33–43. 10.1016/j.epsl.2018.01.021 [DOI] [Google Scholar]
- Wadsworth, J. , & Cockell, C. S. (2017). Perchlorates on Mars enhance the bacteriocidal effects of UV light. Scientific Reports, 7, 4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, M. R. , Bauld, J. , & Brock, T. D. (1976). Microbiology and morphogenesis of columnar stromatolites (Conophyton, Vacerrilla) from hot springs in Yellowstone National Park In Walter M. R. (Ed.), Developments in Sedimentology, Stromatolites (pp. 273–310). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Walter, M. R. , & Des Marais, D. J. (1993). Preservation of biological information in thermal spring deposits: Developing a strategy for the search for fossil life on Mars. Icarus, 101(1), 129–143. 10.1006/icar.1993.1011 [DOI] [PubMed] [Google Scholar]
- Weber, K. A. , Spanbauer, T. L. , Wacey, D. , Kilburn, M. R. , Loope, D. B. , & Kettler, R. M. (2012). Biosignatures link microorganisms to iron mineralization in a paleoaquifer. Geology, 40(8), 747–750. 10.1130/G33062.1 [DOI] [Google Scholar]
- Webster, C. R. , Mahaffy, P. R. , Atreya, S. K. , Flesch, G. J. , Mischna, M. A. , Meslin, P. Y. , et al. (2015). Mars methane detection and variability at Gale Crater. Science, 347(6220), 415–417. 10.1126/science.1261713 [DOI] [PubMed] [Google Scholar]
- Weiss, B. P. , Kirschvink, J. L. , Baudenbacher, F. J. , Vali, H. , Peters, N. T. , Macdonald, F. A. , & Wikswo, J. P. (2000). A low temperature transfer of ALH84001 from Mars to Earth. Science, 290(5492), 791–795. 10.1126/science.290.5492.791 [DOI] [PubMed] [Google Scholar]
- Weitz, C. M. , Lane, M. , Staid, M. , & Noe Dobrea, E. Z. (2008). Gray hematite distribution and formation in Ophir and Candor Chasmata. Journal of Geophysical Research, 113, E02016 10.1029/2007JE002930 [DOI] [Google Scholar]
- Weitz, C. M. , Milliken, R. E. , Grant, J. A. , McEwen, A. S. , Williams, R. M. E. , & Bishop, J. L. (2008). Light‐toned strata and inverted channels adjacent to Juventae and Ganges chasmata, Mars. Geophysical Research Letters, 35, L19202 10.1029/2008GL035317 [DOI] [Google Scholar]
- Weitz, C. M. , Milliken, R. E. , Grant, J. A. , McEwen, A. S. , Williams, R. M. E. , Bishop, J. L. , & Thomson, B. J. (2010). Mars Reconnaissance Orbiter observations of light‐toned layered deposits and associated fluvial landforms on the plateaus adjacent to Valles Marineris. Icarus, 205(1), 73–102. 10.1016/j.icarus.2009.04.017 [DOI] [Google Scholar]
- Weitz, C. M. , Noe Dobrea, E. Z. , Lane, M. D. , & Knudson, A. T. (2012). Geologic relationships between gray hematite, sulfates, and clay and Capri Chasma. Journal of Geophysical Research, 117, E00J09 10.1029/2012JE004092 [DOI] [Google Scholar]
- Wendt, L. , Gross, C. , Kneissl, T. , Sowe, M. , Combe, J. P. , LeDeit, L. , et al. (2011). Sulfates and iron oxides in Ophir Chasma, Mars, based on OMEGA and CRISM observations. Icarus, 213(1), 86–103. 10.1016/j.icarus.2011.02.013 [DOI] [Google Scholar]
- Werner, S. C. , & Tanaka, K. L. (2011). Redefinition of the crater‐density and absolute‐age boundaries for the chronostratigraphic system of Mars. Icarus, 215(2), 603–607. 10.1016/j.icarus.2011.07.024 [DOI] [Google Scholar]
- Westall, F. (1997). Influence of cell wall composition on the fossilisation of bacteria and the implications for the search for early life forms In Cosmovici C. S., Bowyer S., & Werthimer D. (Eds.), International Astronomical Union Colloquium (Vol. 161, pp. 491–504). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Westall, F. , Boni, L. , & Guerzoni, E. (1995). The experimental silicification of microorganisms. Palaeontology, 38(3), 495–528. [Google Scholar]
- Westall, F. , Campbell, K. A. , Bréhéret, J. G. , Foucher, F. , Gautret, P. , Hubert, A. , et al. (2015). Archean (3.33 Ga) microbe‐sediment systems were diverse and flourished in a hydrothermal context. Geology, 43(7), 615–618. 10.1130/G36646.1 [DOI] [Google Scholar]
- Westall, F. , Foucher, F. , Bost, N. , Bertrand, M. , Loizeau, D. , Vago, J. L. , et al. (2015). Biosignatures on Mars: What, where, and how? Implications for the search for Martian life. Astrobiology, 15(11), 998–1029. 10.1089/ast.2015.1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, A. J. , Sumner, D. Y. , Alpers, C. N. , Karunatillake, S. , & Hofmann, B. A. (2015). Preserved filamentous microbial biosignatures in the Brick Flat gossan, Iron Mountain, California. Astrobiology, 15(8), 637–668. 10.1089/ast.2014.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, R. M. , Grotzinger, J. P. , Dietrich, W. E. , Gupta, S. , Sumner, D. Y. , Wiens, R. C. , et al. (2013). Martian fluvial conglomerates at Gale Crater. Science, 340(6136), 1068–1072. 10.1126/science.1237317 [DOI] [PubMed] [Google Scholar]
- Williford, K. H. , Ushikubo, T. , Lepot, K. , Kitajima, K. , Hallmann, C. , Spicuzza, M. J. , et al. (2016). Carbon and sulfur isotopic signatures of ancient life and environment at the microbial scale: Neoarchean shales and carbonates. Geobiology, 14(2), 105–128. 10.1111/gbi.12163 [DOI] [PubMed] [Google Scholar]
- Wilson, L. A. , & Butterfield, N. J. (2014). Sediment effects on the preservation of Burgess Shale‐type compression fossils. Palaios, 29(4), 145–154. 10.2110/palo.2013.075 [DOI] [Google Scholar]
- Wordsworth, R. D. (2016). The climate of early Mars. Annual Review of Earth and Planetary Sciences, 44, 381–408. 10.1146/annurev-earth-060115-012355 [DOI] [Google Scholar]
- Wray, J. J. , Milliken, R. E. , Dundas, C. M. , Swayze, G. A. , Andrews‐Hanna, J. C. , Baldridge, A. M. , et al. (2011). Columbus crater and other possible groundwater‐fed paleolakes of Terra Sirenum, Mars. Journal of Geophysical Research, 116, E01001 10.1029/2010JE003694 [DOI] [Google Scholar]
- Wray, J. J. , Murchie, S. L. , Bishop, J. L. , Ehlmann, B. L. , Milliken, R. E. , Wilhelm, M. B. , et al. (2016). Orbital evidence for more widespread carbonate‐bearing rocks on Mars. Journal of Geophysical Research: Planets, 121, 652–677. 10.1002/2015JE004972 [DOI] [Google Scholar]
- Xiao, S. , Muscente, A. D. , Chen, L. , Zhou, C. , Schiffbauer, J. D. , Wood, A. D. , et al. (2014). The Weng'an biota and the Ediacaran radiation of multicellular eukaryotes. National Science Review, 1(4), 498–520. 10.1093/nsr/nwu061 [DOI] [Google Scholar]
- Yee, N. , Phoenix, V. R. , Konhauser, K. O. , Benning, L. G. , & Ferris, F. G. (2003). The effect of cyanobacteria on silica precipitation at neutral pH: Implications for bacterial silicification in geothermal hot springs. Chemical Geology, 199(1), 83–90. [Google Scholar]
- Yen, A. S. , Ming, D. W. , Vaniman, D. T. , Gellert, R. , Blake, D. F. , Morris, R. V. , et al. (2017). Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth and Planetary Science Letters, 471, 186–198. 10.1016/j.epsl.2017.04.033 [DOI] [Google Scholar]
- Yuan, X. , Chen, Z. , Xiao, S. , Zhou, C. , & Hua, H. (2011). An early Ediacaran assemblage of macroscopic and morphologically differentiated eukaryotes. Nature, 470(7334), 390–393. 10.1038/nature09810 [DOI] [PubMed] [Google Scholar]
- Zhang, C. L. , Fouke, B. W. , Bonheyo, G. T. , Peacock, A. D. , White, D. C. , Huang, Y. , & Romanek, C. S. (2004). Lipid biomarkers and carbon‐isotopes of modern travertine deposits (Yellowstone National Park, USA): implications for biogeochemical dynamics in hot‐spring systems. Geochimica et Cosmochimica Acta, 68(15), 3157–3169. 10.1016/j.gca.2004.03.005 [DOI] [Google Scholar]


