Abstract
Background: We investigated the safety and efficacy of the addition of a trust index to enhanced Model Predictive Control (eMPC) Artificial Pancreas (AP) that works by adjusting the responsiveness of the controller's insulin delivery based on the confidence intervals around predictions of glucose trends. This constitutes a dynamic adaptation of the controller's parameters in contrast with the widespread AP implementation of individualized fixed controller tuning.
Materials and Methods: After 1 week of sensor-augmented pump (SAP) use, subjects completed a 48-h AP admission that included three meals/day (carbohydrate range 29–57 g/meal), a 1-h unannounced brisk walk, and two overnight periods. Endpoints included sensor glucose percentage time 70–180, <70, >180 mg/dL, number of hypoglycemic events, and assessment of the trust index versus standard eMPC glucose predictions.
Results: Baseline characteristics for the 15 subjects who completed the study (mean ± SD) were age 46.1 ± 17.8 years, HbA1c 7.2% ± 1.0%, diabetes duration 26.8 ± 17.6 years, and total daily dose (TDD) 35.5 ± 16.4 U/day. Mean sensor glucose percent time 70–180 mg/dL (88.0% ± 8.0% vs. 74.6% ± 9.4%), <70 mg/dL (1.5% ± 1.9% vs. 7.8% ± 6.0%), and number of hypoglycemic events (0.6 ± 0.6 vs. 6.3 ± 3.4), all showed statistically significant improvement during AP use compared with the SAP run-in (P < 0.001). On average, the trust index enhanced controller responsiveness to predicted hyper- and hypoglycemia by 26% (P < 0.005).
Conclusions: In this population of well-controlled patients, we conclude that eMPC with trust index AP achieved nearly 90% time in the target glucose range. Additional studies will further validate these results.
Keywords: : Artificial pancreas, Automated insulin delivery, Glucose prediction, Hyperglycemia, Hypoglycemia, Type 1 diabetes
Introduction
Recent studies of artificial pancreas (AP) in subjects with type 1 diabetes mellitus (T1D) have shown percent time spent in goal glucose range of 70–180 mg/dL as high as 67%–79% in larger studies.1–5 Improvements in glucose control beyond this will require a next-generation AP that works well with exercise, meal boluses, and other events that cause significant disturbances in glucose homeostasis.
We have previously shown that a control to target model-predictive control (MPC) algorithm is capable of achieving similar high percent time in the target glucose range, even when challenged with a 65 g carbohydrate unannounced meal.6 We have updated and enhanced this setpoint-based controller now incorporating exponential weighting on hypoglycemic excursions, named enhanced MPC7 (eMPC), as well as incorporated a trust index module that dynamically adapts future insulin delivery based on past glucose predictions.8
Most AP systems work as a single input/single output system, taking input from the continuous glucose monitor (CGM) and giving output of suggested insulin dosing. The trust index we have introduced works by examining the results of prior future glucose predictions made by the controller and adapts up or down the aggressiveness of the controller's suggested insulin changes based on how accurate the model predictions are.
The concept of controller adaptation has been explored before in the context of automatic control.9 In the context of AP, Hovorka et al. proposed in 2004 an adaptive MPC scheme for the AP that iteratively identified prediction model parameters based on newly acquired data.10 Turksoy et al. proposed a similar frame for their controller, using data-driven models for glucose prediction.11 For bihormonal controllers, El-Khatib used an adaptive meal bolusing strategy to individualize the impact that these boluses have on the postprandial excursions.12 More recently, Dassau et al. presented a 3-month long study on 30 patients using an adaptation scheme that modified the individual insulin sensitivity profile and insulin to carbohydrate ratio (CR) of each patient at the end of a 4-week cycle (three total adaptations).13,14
Our approach innovates over previous adaptive AP work in three major areas: (1) dynamic modification of the controller's parameters occurs frequently (every CGM sample) and only depends on recent glucose prediction history, (2) adaptation modifies the tuning parameters of the controller directly, whereas the prediction model remains unmodified by the trust index module, and (3) the trust index module, which drives the adaptation of the controller parameters, is model and controller agnostic, meaning that it only uses prediction accuracy measurements, and it can be easily applied to any controller or prediction models used in the AP literature.
To evaluate this new system, we admitted 15 adults with T1D over a 48-h admission in a supervised, transitional environment, challenging them with 1 h of unannounced exercise, a minimum of 30 g carbohydrate per meal, and two overnight periods. Our goal was to test the safety and performance of the system in an early single-arm safety and feasibility trial.
Materials and Methods
This clinical trial assessed the performance of an AP system with (1) an eMPC7 controller, (2) a Health Monitoring System15 (HMS) that monitors for hypoglycemia, and (3) a trust index module that weights future insulin delivery based on past glucose predictions.8 The study was conducted at the Sansum Diabetes Research Institute, Santa Barbara, CA. Design of the control algorithms and engineering of the AP device were done at the Harvard John A. Paulson School of Engineering of Applied Sciences, Harvard University, Cambridge, MA. The primary objective was time within the target glucose range of 70–180 mg/dL as assessed by CGM, determining if this combination of eMPC and the trust index could provide safe and effective glucose control. Secondary safety and effectiveness endpoints included: assessment of the trust index weights on insulin delivery versus standard eMPC tuning, time within the tight target range of 80–140 mg/dL; frequency of hypoglycemia below predefined thresholds of 70 and 54 mg/dL; and frequency of hyperglycemia above predefined thresholds of 180 and 250 mg/dL. Additional secondary outcomes included markers of hypo- and hyperglycemia, as well as safety events, treatments for hypoglycemia, outside interventions as needed, and a failure analysis of the devices/connectivity issues that may have occurred.
Subjects and AP system
Eligible subjects were between 18 and 75 years of age with T1D for at least 1 year, using an insulin pump for at least 6 months, HbA1c <10%, and normal renal function. Key exclusion criteria were pregnancy, one or more episodes of hypoglycemia or hyperglycemia requiring an emergency room visit in the past 6 months, known unstable cardiac disease or untreated cardiac disease, and concurrent use of any noninsulin glucose-lowering agents. Informed consent was obtained before all study procedures. The protocol was approved by the FDA and Chesapeake Institutional Review Board and registered at clinicaltrials.gov (NCT03092310).
Before AP use, subjects performed a 1-week run-in phase using sensor-augmented pump (SAP) with a Dexcom G4® Share™ AP (with 505-algorithm) CGM (Dexcom, Inc., San Diego, CA), to review and optimize each subject's open-loop insulin pump settings throughout the week. The sensor was replaced at least 24 h before the AP session.
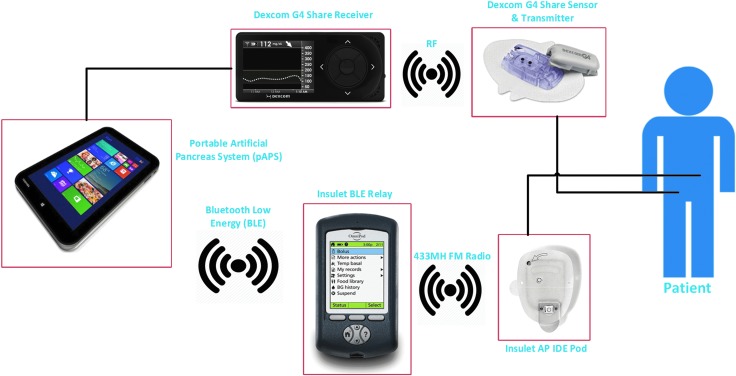
Subjects were then admitted to a supervised transitional environment for the 48-h AP session. Wireless communication among the AP system components occurred with our portable Artificial Pancreas System (pAPS) version 2.0.0.27 running on a Windows 10 tablet computer.16 The pAPS used the subjects' open-loop basal rates, insulin-to-CRs, and insulin sensitivity factors to initialize the closed-loop control (CLC) session. During AP use, subjects wore an OmniPod® Patch Pump with a modified version of the Insulet Personal Diabetes Manager containing a Bluetooth Low Energy (BLE) to Radio Frequency (RF) relay. A diagram of the system is shown in Figure 1.
FIG. 1.
AP system scheme. Closed-loop system parts and communication channels. AP, artificial pancreas.
Study design
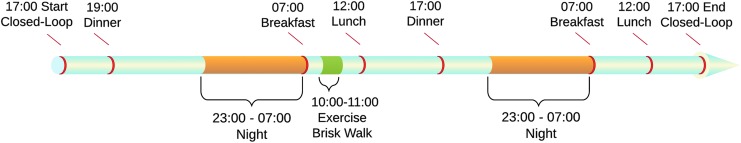
A summary of the 48-h AP session timeline is shown in Figure 2. Boluses for all meals under CLC were given at mealtime based on the subject's CR. Participants were allowed to select the type and carbohydrate content of their meals, according to their dietary preferences, and simulating their everyday lives. Similar to our prior studies,4,6 and validated by others,17 the AP controller modified the mealtime bolus based on CGM value at the time of the meal using both positive and negative corrections to address potential hypoglycemia or rebound hyperglycemia in tandem to the real-time control algorithm as follows: If the CGM glucose value was below the controller setpoint of 110 mg/dL, the meal bolus was reduced by 20%. If the current CGM glucose value was at or above the controller setpoint of 110 mg/dL, but at or below 150 mg/dL, a full meal bolus was given. If the current CGM glucose value was above 150 mg/dL, a full meal bolus was given plus a correction down to 130 mg/dL, with a maximum 2 U correction dose. Recognizing that prolonged suspension of insulin can cause rebound hyperglycemia, the algorithm gave full meal boluses at mealtime if insulin had been suspended for 45 min of the last hour before the meal bolus, even if the current CGM glucose value was below the controller setpoint. This bolus strategy is described as:
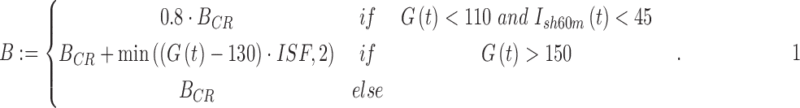 |
FIG. 2.
A 48-h AP session timeline. Subjects started AP use at ∼17:00, then proceeded to the dinner meal at ∼19:00. The next day, ∼3 h after the breakfast meal, subjects did a 1 h outdoor walking session. The lunch meal was served at ∼12:00, with dinner at ∼17:00 on day 2. Subjects ate breakfast and lunch on day 3 while using AP, and were discharged at ∼17:00 day 3. All meals during the study contained between 30 and 90 g carbohydrates, and subjects received a bolus for all meals as per the study protocol.
where B is the bolus given in insulin units, 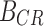 is the bolus as calculated using the participants' CR,
is the bolus as calculated using the participants' CR, 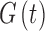 is the glucose reading at current time t, and ISF is the participants' Insulin Sensitivity Factor (IU/mg/dL).
is the glucose reading at current time t, and ISF is the participants' Insulin Sensitivity Factor (IU/mg/dL). 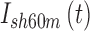 represents the insulin pump suspension history in the past 60 min. It accounts for the amount of minutes that the insulin pump has not delivered any insulin. If insulin delivery was >0 at every sample within the last hour, then
represents the insulin pump suspension history in the past 60 min. It accounts for the amount of minutes that the insulin pump has not delivered any insulin. If insulin delivery was >0 at every sample within the last hour, then 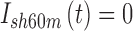 Similarly if insulin delivery was suspended for 40 min, then
Similarly if insulin delivery was suspended for 40 min, then 
A 1-h outdoor exercise session (brisk walk) was performed in the late morning on day 2 of the study. For subjects who had a fingerstick blood sugar below 150 mg/dL at the start of the walk, subjects were allowed to have a 15 g carbohydrate snack before starting the exercise session at their discretion as per their usual exercise routine. Also at anytime while performing the 48-h AP session, subjects were allowed to perform light exercise with study staff present as they would normally do at home.
Capillary fingerstick glucose measurements were performed 30 min before meals, 2 h after meals, at bedtime, and when prompted by the HMS or as requested by the subject.18 Subjects were discharged at ∼17:00 on the third day of the study.
Safety limitations
As previously described, the AP incorporated the HMS, an algorithm that added an independent safety layer.15 The HMS was independent from the AP control algorithms and advised subjects to ingest 16 g of carbohydrate to prevent impending hypoglycemia (glucose <65 mg/dL) that could not be prevented by controller action alone. Subjects performed fingerstick glucose checks with each HMS alert. Hypoglycemia treatments resulting from HMS alerts were counted as hypoglycemic events in our analysis, as previously recommended.19
In addition, controller insulin delivery (besides insulin bolus given to compensate for meals) was bounded. During the day, the maximum insulin delivery allowed by the controller was 1 U every 5 min, whereas at night that limit was 1.6 times the participant's own basal rate, making that constraint time and participant dependent.
Control algorithm design
The controller used was a combination of the trust index module evaluated previously in simulation studies,8 combined with an enhanced setpoint eMPC algorithm published recently.7 The eMPC differs from traditional MPC implementations in the penalization of glucose excursions below the setpoint of the controller, where traditional MPC variants penalize glucose excursions linearly or quadratically, and eMPC implements an exponential penalization. Mathematically, the eMPC calculates insulin delivery at each timestamp by minimizing the following cost function:
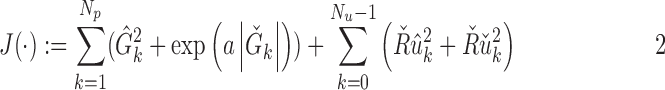 |
 |
 |
 |
 |
where 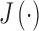 is the cost function to be minimized, Gk is the predicted glucose at sample k, uk is the recommended insulin delivery at the same sample,
is the cost function to be minimized, Gk is the predicted glucose at sample k, uk is the recommended insulin delivery at the same sample,  is the glucose setpoint (110 mg/dL), and
is the glucose setpoint (110 mg/dL), and 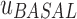 is the basal insulin programmed at each time step. The controller parameters,
is the basal insulin programmed at each time step. The controller parameters, 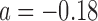 is the exponential weight to hypoglycemic glucose excursions,
is the exponential weight to hypoglycemic glucose excursions, 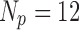 is the prediction horizon, and
is the prediction horizon, and 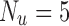 is the horizon for actuation of the controller. The eMPC optimizes
is the horizon for actuation of the controller. The eMPC optimizes 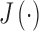 to find the optimal insulin therapy
to find the optimal insulin therapy 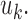
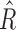 and
and 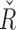 are parameter weights on the insulin deviations from basal, and their values are discussed below.
are parameter weights on the insulin deviations from basal, and their values are discussed below.
The eMPC algorithm functioned as the core decision mechanism on the insulin delivery at every 5-min sample, modulating insulin delivery every 5 min to maintain glucose at a setpoint of 110 mg/dL. The trust index module supervised the tuning parameters of the eMPC, increasing the likelihood of insulin delivery if the eMPC prediction model was accurate in recent history, and reducing that likelihood in case of diverging model predictions. The computation of the trust index follows the next equation:
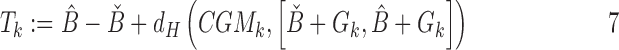 |
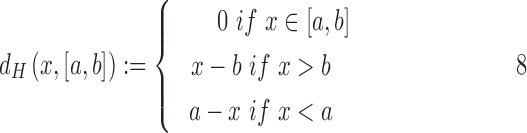 |
where 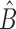 and
and 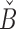 stand for the 5% and 95% boundaries of the pool of prediction errors generated by the prediction model, as explained by Laguna et al.8
stand for the 5% and 95% boundaries of the pool of prediction errors generated by the prediction model, as explained by Laguna et al.8
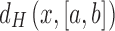 stands for the Hausdorff distance from a point x to an interval
stands for the Hausdorff distance from a point x to an interval  ,
, 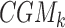 stands for the most current CGM measurement, and Gk stands for the model's prediction of the current CGM sample. Tk is the trust index value at time k. The maximum trust (100%) is defined by design at
stands for the most current CGM measurement, and Gk stands for the model's prediction of the current CGM sample. Tk is the trust index value at time k. The maximum trust (100%) is defined by design at 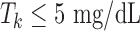 and the lowest possible trusts correspond to an index
and the lowest possible trusts correspond to an index 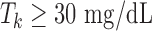 , which is equivalent to a 0% trust. These thresholds are chosen empirically and can be tuned for different prediction horizons and controllers.
, which is equivalent to a 0% trust. These thresholds are chosen empirically and can be tuned for different prediction horizons and controllers.
The two parameters directly controlled by the trust index were 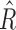 and
and  , which influence controller decisions for glucose predictions above and below setpoint (110 mg/dL), respectively. The baseline numerical values for
, which influence controller decisions for glucose predictions above and below setpoint (110 mg/dL), respectively. The baseline numerical values for 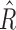 and
and 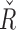 are 6700 and 2500, and their ranges of operation are [4000, 7500] and [300, 4000], respectively. When the trust reaches 100%, the controller parameters reach the range limit
are 6700 and 2500, and their ranges of operation are [4000, 7500] and [300, 4000], respectively. When the trust reaches 100%, the controller parameters reach the range limit 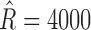 and
and 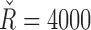 and when it falls to 0%, the tuning is set to
and when it falls to 0%, the tuning is set to  and
and 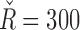 . All values in between are linearly interpolated depending on the value of Tk at each sample k.
. All values in between are linearly interpolated depending on the value of Tk at each sample k.
Statistical analysis
Glycemic outcomes were calculated for the 48-h AP session (averaged per 24-h period) and were compared with the 24-h average daily values of the SAP run-in phase. Although this was a preliminary safety and feasibility study, Power analysis derived from improvements to time in range seen in our previous studies6,13 indicated that a minimum sample size of 14 subjects would be required to achieve 80% power at significance level of 0.05 to detect a change of 13% time in range 70–180 mg/dL. Statistical comparisons were performed using a Wilcoxon rank-sum test. Values are reported as mean ± SD unless otherwise noted.
Results
Subject characteristics
Fifteen subjects completed the study (10 female, 5 male). Mean age was 46.1 ± 17.8 years. Mean HbA1c was 7.2% ± 1.0%. Full subject demographics are listed in Table 1.
Table 1.
Subject Demographics and Baseline Characteristics (n = 15)
| Characteristic | |
|---|---|
| Age (years) mean ± SD | 46.1 ± 17.8 |
| Gender (n) | |
| Female | 10 |
| Male | 5 |
| Weight (kg) mean ± SD | 69.2 ± 12.3 |
| Body mass index (kg/m2) mean ± SD | 24.4 ± 3.5 |
| HbA1c (%) mean ± SD | 7.2 ± 1.0 |
| Duration of diabetes (years) mean ± SD | 26.8 ± 17.6 |
| TDI (U/day) mean ± SD | 35.5 ± 16.4 |
TDI, Total Daily Insulin.
Glycemic control
During the 48-h AP session, mean daily percent time 70–180 mg/dL improved overall from 74.6% ± 9.0% during the open-loop run-in week to 88.0% ± 8.0% during AP use (P = 0.001), and overnight from 73.7% ± 13.4% during the open-loop run-in week to 88.1% ± 21.3% during AP use (P = 0.003). Mean daily percent time 80–140 mg/dL also improved overall from 45.2% ± 10.7% to 60.1% ± 13.9% during AP use (P = 0.004), and overnight from 41.9% ± 12.6% to 67.6% ± 24.1% (P = 0.002).
Hypoglycemia (mean daily percent time <70 mg/dL) improved overall from 7.8% ± 6.0% during the open-loop run-in week to 1.5% ± 1.9% during AP use (P < 0.001), and overnight from 8.1% ± 7.5% during the open-loop run-in week to 1.1% ± 2.0% during AP use (P < 0.001). The mean daily number of hypoglycemic events (CGM <70 mg/dL ≥15 min) improved overall from 6.3 ± 3.4 events/day during the open-loop run-in week to 0.6 ± 0.6 events/day during AP use (P < 0.001).
Although mean glucose did not change (135.1 ± 19.4 mg/dL during open loop; 130.1 ± 15.2 mg/dL AP), glycemic variability (SD) improved from 50.3 ± 10.9 mg/dL during open loop to 32.9 ± 6.4 mg/dL during AP use (P < 0.001).
All glycemic metrics are summarized in Table 2. A summary time-plot of the median and interquartile range of all patients is shown in Figure 3. Figure 4 shows the individual participant evolution from the run-in week to the AP session, both for time in range 70–180 mg/dL and time under 70 mg/dL. Plots for individual subjects are shown in Supplementary Fig. S2 (Supplementary Data are available online at http://online.liebertpub.com/suppl/doi/10.1089/dia.2018.0031).
Table 2.
Glycemic Outcomes (Mean ± SD) for the 48-H Artificial Pancreas Session Versus the Week Prior Sensor-Augmented Pump Use
| 24-H average | Overnight only | |||||
|---|---|---|---|---|---|---|
| AP session | SAP run-in week | P | AP session | SAP run-in week | P | |
| Time in range (80, 140) mg/dL (%) CGM | 60.1 ± 13.9 | 45.2 ± 10.7 | 0.004* | 67.6 ± 24.1 | 41.9 ± 12.6 | 0.002* |
| Time in range (70, 180) mg/dL (%) CGM | 88.0 ± 8.0 | 74.6 ± 9.4 | 0.001* | 88.1 ± 21.3 | 73.7 ± 13.4 | 0.003* |
| Time <54 mg/dL (%) CGM | 0.1 ± 0.3 | 1.7 ± 1.9 | <0.001* | 0.0 ± 0.0 | 1.8 ± 3.3 | <0.001* |
| Time <70 mg/dL (%) CGM | 1.5 ± 1.9 | 7.8 ± 6.0 | <0.001* | 1.1 ± 2.0 | 8.1 ± 7.5 | <0.001* |
| Time >180 mg/dL (%) CGM | 10.5 ± 8.0 | 17.6 ± 10.7 | 0.081 | 7.5 ± 12.9 | 17.4 ± 13.0 | 0.011* |
| Time >250 mg/dL (%) CGM | 0.3 ± 0.8 | 4.1 ± 4.6 | 0.001* | 0.4 ± 1.4 | 4.4 ± 6.7 | 0.002* |
| Mean glucose (mg/dL) CGM | 130.1 ± 15.2 | 135.1 ± 19.4 | >0.5 | 123.7 ± 22.0 | 135.9 ± 22.7 | 0.106 |
| Variability (SD) CGM | 32.9 ± 6.4 | 50.3 ± 10.9 | <0.001* | 24.8 ± 13.0 | 39.7 ± 21.4 | 0.038* |
| Variability (CV) (%) CGM | 0.25 ± 0.04 | 0.37 ± 0.06 | <0.001* | 0.19 ± 0.07 | 0.26 ± 0.10 | 0.028* |
| CGM @6 AM (mg/dL) | 129.7 ± 25.1 | 130.5 ± 22.2 | >0.5 | — | — | — |
| Hypoglycemia: <54 mg/dL, ≥15 min (events/24 h) | 0.0 ± 0.1 | 2.1 ± 2.0 | <0.001* | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.001* |
| Hypoglycemia: <54 mg/dL, ≥30 min (events/24 h) | 0.0 ± 0.0 | 1.1 ± 1.3 | <0.001* | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.018* |
| Hypoglycemia: <54 mg/dL, ≥60 min (events/24 h) | 0.0 ± 0.0 | 0.2 ± 0.3 | 0.008* | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.038* |
| Hypoglycemia: <70 mg/dL, ≥15 min (events/24 h) | 0.6 ± 0.6 | 6.3 ± 3.4 | <0.001* | 0.0 ± 0.1 | 0.5 ± 0.2 | <0.001* |
| Hypoglycemia: <70 mg/dL, ≥30 min (events/24 h) | 0.2 ± 0.5 | 4.6 ± 2.8 | <0.001* | 0.0 ± 0.0 | 0.4 ± 0.2 | <0.001* |
| Hypoglycemia: <70 mg/dL, ≥60 min (events/24 h) | 0.0 ± 0.1 | 2.4 ± 2.1 | <0.001* | 0.0 ± 0.0 | 0.3 ± 0.2 | <0.001* |
Significant change (P < 0.05).
AP, artificial pancreas; CV, coefficient of variation; CGM, continuous glucose monitor; SAP, sensor-augmented pump.
FIG. 3.
Median and interquartile CGM plot. Timeline of the median CGM of every 5-min interval across all 15 participants in the closed-loop ∼48 h study. Quartile lines are calculated as 25% and 75% percentiles of the CGM at every 5-min samples. CGM, continuous glucose monitor.
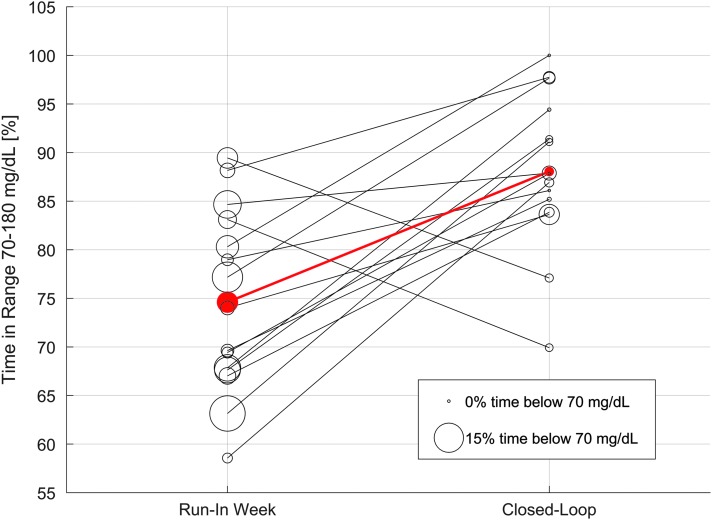
FIG. 4.
Individual metric improvement. Every line represents a participant in the AP study. Progress from the SAP run-in week to the AP system can be read from left to right in the plot. Bubble size represents the time spent below 70 mg/dL. The red line and circles represent mean values. SAP, sensor-augmented pump. (Color graphics available at www.liebertonline.com/dia)
Assessment of trust index performance
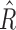 and
and 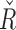 distributions are highly skewed, thus it is more appropriate to depict them using median and interquartile ranges. The distributions of both parameters depending on the time period in the experiment and in the glucose range are shown in Figure 5. As shown in Figure 5, mean
distributions are highly skewed, thus it is more appropriate to depict them using median and interquartile ranges. The distributions of both parameters depending on the time period in the experiment and in the glucose range are shown in Figure 5. As shown in Figure 5, mean 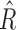 changed from a baseline of 6700 to 4929, a 26% mean change toward a more responsive tuning for hyperglycemia. Similarly,
changed from a baseline of 6700 to 4929, a 26% mean change toward a more responsive tuning for hyperglycemia. Similarly, 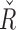 changed from 2500 to 3027, which supposes an increase of 21% toward a more responsive controller response against hypoglycemia. Note that
changed from 2500 to 3027, which supposes an increase of 21% toward a more responsive controller response against hypoglycemia. Note that 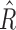 and
and 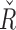 are linearly dependent on the trust index value (
are linearly dependent on the trust index value (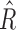 negatively correlated, Ř positively) at each time sample, so the distributions of both parameters are mirrored and shifted, but show similar shapes.
negatively correlated, Ř positively) at each time sample, so the distributions of both parameters are mirrored and shifted, but show similar shapes.
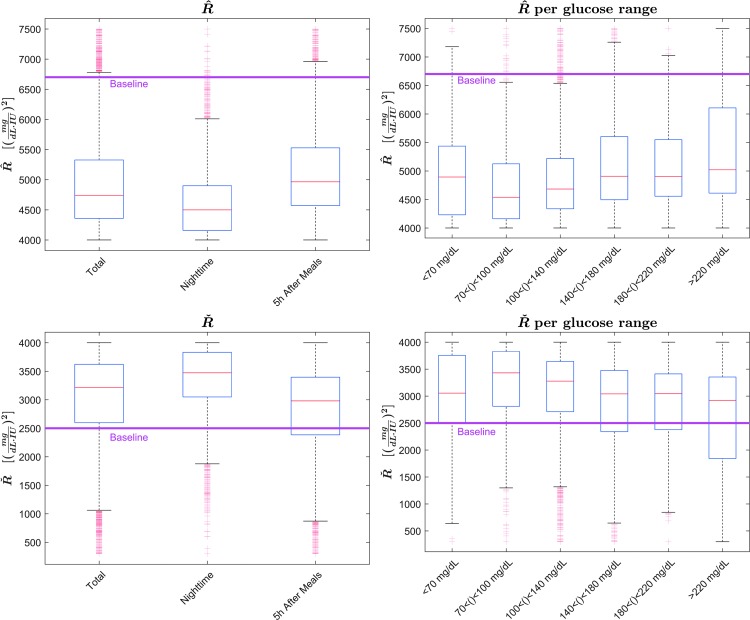
FIG. 5.
Box-plot distributions of 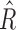 R̂ and
R̂ and 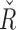 .
. 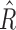 distributions are shown in the top panels, and Ř at the bottom. Distributions separated by time periods are shown in the left panels, whereas distributions depending on the CGM range are displayed at the right. Median values are in red, interquartile ranges are shown as a blue box, minimum and maximum values as black bars, and outliers in red. Baseline values for each parameter are imposed in pink. (Color graphics available at www.liebertonline.com/dia)
distributions are shown in the top panels, and Ř at the bottom. Distributions separated by time periods are shown in the left panels, whereas distributions depending on the CGM range are displayed at the right. Median values are in red, interquartile ranges are shown as a blue box, minimum and maximum values as black bars, and outliers in red. Baseline values for each parameter are imposed in pink. (Color graphics available at www.liebertonline.com/dia)
Median [interquartile range] 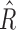 values were 4740 [4358, 5327] and
values were 4740 [4358, 5327] and 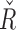 were 3217 [2587, 3621] for the entirety of the dataset, both significantly different than the baseline (P < 0.005). Overnight median
were 3217 [2587, 3621] for the entirety of the dataset, both significantly different than the baseline (P < 0.005). Overnight median 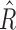 was 4499 [4159, 4900] and
was 4499 [4159, 4900] and 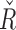 was 3473 [3049, 3832], both significantly shifted from the overall results toward a higher likelihood of insulin delivery (P < 0.005). The values of the tuning parameters in the postprandial periods (5 h after each meal) were, for
was 3473 [3049, 3832], both significantly shifted from the overall results toward a higher likelihood of insulin delivery (P < 0.005). The values of the tuning parameters in the postprandial periods (5 h after each meal) were, for 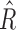 4964 [4572, 5528] and for
4964 [4572, 5528] and for 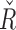 2980 [2385, 3396], both significantly higher than the values of the overall results (P < 0.005) and that those of the overnight period (P < 0.005).
2980 [2385, 3396], both significantly higher than the values of the overall results (P < 0.005) and that those of the overnight period (P < 0.005).
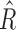 range in the hypoglycemic range (<70 mg/dL) was 4894 [4231, 5436], which is significantly (P < 0.005) higher than the values for the 70–100 mg/dL range 4539 [4162, 5125], but significantly lower than the values for high glucose range 180–220 mg/dL, which was 4904 [4556, 5549] (P < 0.005). The same differences can be observed in the
range in the hypoglycemic range (<70 mg/dL) was 4894 [4231, 5436], which is significantly (P < 0.005) higher than the values for the 70–100 mg/dL range 4539 [4162, 5125], but significantly lower than the values for high glucose range 180–220 mg/dL, which was 4904 [4556, 5549] (P < 0.005). The same differences can be observed in the 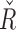 distributions: for the range <70 mg/dL, 3055 [2500, 3756] versus 3431 [2811, 3829] (P < 0.005) for the range 70–100 mg/dL and 3045 [2377, 3412] (P < 0.005) for the 180–220 mg/dL range.
distributions: for the range <70 mg/dL, 3055 [2500, 3756] versus 3431 [2811, 3829] (P < 0.005) for the range 70–100 mg/dL and 3045 [2377, 3412] (P < 0.005) for the 180–220 mg/dL range.
The numeric value of the trust index (0–100) at every time sample (linearly correlated with 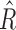 and
and 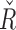 for every subject is shown on the individual subject plots (see Supplementary Data).
for every subject is shown on the individual subject plots (see Supplementary Data).
Connectivity
There were no persistent connectivity issues between device components. Percent time in closed loop was 98.62%. All subjects remained in closed-loop mode for the entire 48-h sessions, except: (1) one subject had her CGM sensor fail on the second day of the study, and was in open loop for 2 h while a new sensor warmed up, and (2) for one subject who was on very low basal rates (0.1 U/h overnight), the limitations on basal rate programmed into the AP system that were designed to prevent hypoglycemia overnight were too restrictive, and he was given a manual bolus on the first night to correct his blood glucose. The closed-loop system was suspended for the second night of the study (Supplementary Data—Individual Subject Plots, Subject RM013) and closed-loop operation was resumed at 5 AM on day 3.
Adverse events
There were no protocol-related adverse events or serious adverse events during the study. The HMS functioned well for predicting glucose levels <65 mg/dL and advised treatment with 16 g of carbohydrate. Although there were multiple alarms for hypoglycemia warnings in the study, treatments for hypoglycemia were few during AP use with a mean of 1.7 ± 1.7 treatments over the 48-h study period.
Conclusions
The majority of AP systems in development work by modulating insulin infusion above or below the patient's specific preprogrammed basal rates,20 based on the CGM glucose level, historical CGM measurements, anticipated CGM trends, historical insulin delivery, and patient-specific information, such as the basal rate profile. The target eMPC algorithm in our study adds a trust index that adjusted the aggressiveness of future insulin delivery based on the accuracy of past glucose predictions, a feature unique to this study that allows the system to attenuate changes to insulin delivery when confidence in recent glucose predictions is low, and then later make more aggressive changes when confidence becomes higher.
This study was the first clinical study to include this intermediate layer,8 which uses no additional information than normally provided to the MPC controller (CGM readings, prior insulin delivery, basal profile, total daily insulin, body weight) but enhances controller aggressiveness of insulin recommendations based on recent prediction error residuals. With this feature enabled in our next-generation AP system, we were able to show it is possible to keep glucose levels in the target range of 70–180 mg/dL nearly 90% of the time in this group of well-controlled patients. This is significantly higher than reported in a recent study of very similar design.21
Additionally, in Figure 4 we observe an overall improvement of the time in range for 13/15 participants, which reinforces the overall efficacy of our controller. Of the two patients that did not improve their time-in-range metric, both of them showed lower time spent in hypoglycemia. Overall, we observed a major reduction of the time spent in hypoglycemia for all patients (7.8% vs. 1.5% time below 70 mg/dL, P < 0.001), as depicted in Figure 4 and Table 2, where all the metrics that measure hypoglycemic impact (frequency of hypoglycemic events, percent time of glucose below 70 mg/dL) are significantly reduced.
Although the study was performed in a supervised transitional environment, we attempted to gauge how the controller would perform under more real-world conditions by adding a number of requirements to the AP session. First, we instructed subjects to consume at least 30 g of carbohydrate per meal, but they were ultimately free to choose the type and size of their meals. The meal sizes were challenging for breakfast, where many of the subjects reported they would normally consume minimal carbohydrates at breakfast (Supplementary Fig. S1). This can also be observed in the summary of Table 3, where two of the participants reported missing breakfast 4 or more days per week. Furthermore, the average breakfast size for the study meals (37.1 g) was larger than the average breakfast at home (32.6 g), although not significantly different. Participants ingested larger meals for lunch and dinner (41.7 and 45.5 g averages, respectively), although this was not significantly different than the reported carbohydrate content of lunch and dinner meals in a normal day for the patients. Additionally, also reported in Table 3, we must highlight that our cohort of patients reported that skipping meals completely was a common practice among some of them (22% of breakfasts, 24% of lunches, and 12% of dinners were missing), and given that no meals were missed by any participants in the AP session, the glycemic disturbances from meals that the AP system was subject to may have been more challenging in some cases than those observed during the SAP run-in week. We therefore can conclude that the AP controller performed significantly better than SAP therapy when challenged with similar meal disturbances in this population of well-controlled patients with these meal sizes. Nevertheless, our protocol did not require larger meals, so it remains to be evaluated that larger meal challenges would yield the same level of glycemic control.
Table 3.
Meal Preferences
| Breakfast | Lunch | Dinner | |
|---|---|---|---|
| Average CHO | |||
| AP session (g CHO) | 37.1 | 41.7 | 45.5 |
| At home (g CHO) | 32.6 | 46.9 | 50.9 |
| P (* if significant) | 0.141 | >0.5 | >0.5 |
| Skipped meals at home | |||
| No. of subjects that reported skipping more than half meals | 2/14 | 3/14 | 0/14 |
| No. of subjects that reported skipping two or more meals/week | 6/14 | 6/14 | 4/14 |
| No. of skipped meals (total) | 22/98 | 23/98 | 12/98 |
First three rows display a summary of carbohydrate quantity for meals in the AP session and reported by the patients at home in an average week of free living. Last three rows depict the prevalence of meals skipped by participants in their daily home routines. No meals were skipped by any participant in the AP 48-h session. One of the participants failed to log meals from the at-home period, thus the numbers are reported on 14/15 patients.
CHO, Carbohydrates.
To further challenge the new design, we included an unannounced exercise challenge in the study, similar to prior studies, where we had up to an hour of exercise, unannounced to the controller.4 During and just after the 1-h brisk walk, we observed no significant difference in average insulin delivery 0.7 ± 0.7 U/h when compared with the whole experiment values 0.9 ± 0.5 U/h (P = 0.097). The system responded to the exercise very well, suspending insulin delivery completely for three participants throughout the duration of the experiments, with only four participants requiring oral carbohydrates during or shortly after the end of the unannounced exercise event. Exercise is a difficult challenge for people with T1D. Management strategies are quite variable, with some people taking extra carbohydrates before, during, and/or post exercise. Insulin doses may be adjusted manually before or during exercise, however, we did not inform the AP controller that exercise was occurring in any way or manually adjust basal profiles. We did allow subjects to take carbohydrates before starting exercise as per their usual routine, and lunch was less than 90 min after the exercise period, all of which contributed to less hypoglycemia during and after exercise. Specific glucose metrics for the exercise period and up to 15 h after the exercise period (to assess latent hypoglycemia) are shown in Supplementary Table S1.
The values of 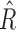 during the night are significantly shifted to a point where greater insulin delivery is more likely than the overall values of
during the night are significantly shifted to a point where greater insulin delivery is more likely than the overall values of 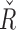 for the whole experiment. This difference is also observed in the values of
for the whole experiment. This difference is also observed in the values of  . This is easily explained due to the night period being more static for glucose (less disturbances), which causes the model predictions to be more accurate, and thus the trust on those predictions is higher. This allows the controller to assertively correct any possible deviations from setpoint during the night. In contrast, the values of the parameters for the postprandial periods are significantly less likely to deliver aggressive doses of insulin, which in addition to Insulin On Board (IOB) constraints on the controller action after meals, reduced the amount of insulin delivered in the period after a meal. We hypothesize that this reduced the number of hypoglycemic episodes throughout the experiment, although the exact impact of the trust index module on this metric is not discernible from other influences in this study.
. This is easily explained due to the night period being more static for glucose (less disturbances), which causes the model predictions to be more accurate, and thus the trust on those predictions is higher. This allows the controller to assertively correct any possible deviations from setpoint during the night. In contrast, the values of the parameters for the postprandial periods are significantly less likely to deliver aggressive doses of insulin, which in addition to Insulin On Board (IOB) constraints on the controller action after meals, reduced the amount of insulin delivered in the period after a meal. We hypothesize that this reduced the number of hypoglycemic episodes throughout the experiment, although the exact impact of the trust index module on this metric is not discernible from other influences in this study.
We recognize other limitations to this study. It was first and foremost designed as a safety and feasibility evaluation of the enhanced controller and additional features, and thus was uncontrolled. Comparisons to the SAP data collection week, which occurred in the unsupervised outpatient setting, compare different clinical settings. During the data collection week subjects were in contact with the study physician, sometimes multiple times, as open-loop settings were adjusted throughout the week. As already mentioned, relatively smaller meal sizes may have positively influenced glycemic control. Nevertheless, this comparison offers a window into the progress AP has undergone over time.
In conclusion, we have shown with a combination of our eMPC algorithm and trust index that weights future insulin delivery based on past glucose predictions, it is possible to increase the percent time in the target glucose range to nearly 90% in this group of well-controlled patients (baseline time in range nearly 75%) without increasing incidence of hypoglycemia. Future larger, controlled outpatient studies with different patient populations are necessary to validate these findings.
Supplementary Material
Acknowledgments
The authors would like to thank Prof. Dale E. Seborg for the valuable insight he provided, and to acknowledge all of the patients who participated in this clinical trial. They would also like to acknowledge the staff at the clinical and engineering centers who helped support this project. Funding for the study was provided by JDRF Grant No. 1-SRA-2016-147-M-R and National Institutes of Health Grant No. DP3DK104057. Product support was provided by Insulet Corporation (Billerica, MA), who provided insulin pumps in kind, and by Dexcom, Inc. (San Diego, CA) who provided research discount pricing on CGM sensors, transmitters, and receivers (G4 Platinum with Share). The funders and device manufacturers had no influence on the design or conduct of the trial and were not involved in data collection or analysis, the writing of the article, or the decision to submit it for publication.
Author Disclosure Statement
E.D. has received consulting fees from Insulet; has received research support from Dexcom, Insulet, Roche, Xeris, and Animas; and receives royalty payments on intellectual property related to the MPC algorithm used in this study. F.J.D. is an advisor to Mode AGC; has received research support from Dexcom, Insulet, Roche, and Xeris; and receives royalty payments on intellectual property related to the MPC algorithm used in this study. J.E.P has conducted AP research sponsored by Insulet Corporation, Tandem Diabetes Care, and Bigfoot Biomedical, and has received product support to his institution from Insulet Corporation, Animas, LifeScan, Roche, and Dexcom. J.B.L. is currently an employee of the Insulet Corporation. A.J.L.S, M.C., C.A., and L.E.L. report no competing financial interests.
References
- 1.Thabit H, Tauschmann M, Allen JM, et al. : Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovatchev B, Cheng P, Anderson SM, et al. : Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 3.Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dassau E, Brown SA, Basu A, et al. : Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: a multicenter randomized trial. J Clin Endocrinol Metab 2015;100:3878–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson SM, Raghinaru D, Pinsker JE, et al. : Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinsker JE, Lee JB, Dassau E, et al. : Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care 2016;39:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JB, Dassau E, Gondhalekar R, et al. : Enhanced model predictive control (eMPC) strategy for automated glucose control. Ind Eng Chem Res 2016;55:11857–11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laguna Sanz AJ, Doyle FJ, III, Dassau E: An enhanced model predictive control for the artificial pancreas using a confidence index based on residual analysis of past predictions. J Diabetes Sci Technol 2017;11:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannou PA, Sun J: Chapter 6: model reference adaptive control. In: Robust Adaptive Control. Vol 1 Upper Saddle River, NJ: PTR Prentice-Hall, 1996:313–433 [Google Scholar]
- 10.Hovorka R, Canonico V, Chassin LJ, et al. : Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 2004;25:905–920 [DOI] [PubMed] [Google Scholar]
- 11.Turksoy K, Quinn L, Littlejohn E, Cinar A: Multivariable adaptive identification and control for artificial pancreas systems. IEEE Trans Biomed Eng 2014;61:883–891 [DOI] [PubMed] [Google Scholar]
- 12.El-Khatib FH, Russell SJ, Magyar KL, et al. : Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab 2014;99:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dassau E, Pinsker JE, Kudva YC, et al. : Twelve-week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: effect on hemoglobin A1c and hypoglycemia. Diabetes Care 2017;40:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiavon M, Dalla Man C, Kudva YC, et al. : Quantitative estimation of insulin sensitivity in type 1 diabetic subjects wearing a sensor-augmented insulin pump. Diabetes Care 2014;37:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey RA, Dassau E, Zisser H, et al. : Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol 2012;6:1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dassau E, Zisser H, Palerm CC, et al. : Modular artificial beta-cell system: a prototype for clinical research. J Diabetes Sci Technol 2008;2:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elleri D, Biagioni M, Allen JM, et al. : Safety, efficacy and glucose turnover of reduced prandial boluses during closed-loop therapy in adolescents with type 1 diabetes: a randomized clinical trial. Diabetes Obes Metab 2015;17:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey RA, Dassau E, Bevier WC, et al. : Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol Ther 2014;16:348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle FJ, III, Huyett LM, Lee JB, et al. : Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 21.Buckingham BA, Forlenza GP, Pinsker JE, et al. : Safety and feasibility of the OmniPod hybrid closed-loop system in adult, adolescent, and pediatric patients with type 1 diabetes using a personalized model predictive control algorithm. Diabetes Technol Ther 2018;20:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.