Abstract
Phyllodes tumor is a benign breast cancer with a malignant potential. It is very rare in Saudi Arabia and also rare around the world. Malignant phyllodes tumors originate from the connective tissue of the breast, so they are histologically sarcomas. We report the largest phyllodes tumor ever seen in Saudi Arabia, a 41-year-old female who presented with a huge breast mass occupying the whole breast with areas of ulceration. Her history had started 14 months previously with a slowly growing left breast swelling, ultimately the lump ulcerated and became necrotic. Mastectomy with partial resection of the pectoral muscles was done. The tumor specimen measured exactly 30×20×13 cm in size, and weight of 5.4 kg, and with the closest margin of resection 0.5 cm away from the tumor the histopathology report came back as malignant phyllodes tumor. The patient was sent for radiotherapy and chemotherapy. We concluded that accurate preoperative pathological diagnosis is very important for management of phyllodes tumor, and allows correct surgical planning and avoidance of reoperation.
Keywords: Malignant, Phyllodes tumor, Prognosis, Breast
1. Introduction
Phyllodes tumor is a rare tumor of the breast in comparison to other histologic subtypes, however, itself, is not a rare tumor, accounting for <1% of all breast malignancies (1, 2 ), and has an incidence of about 2.1 per million. Most of these tumors are benign, but some have a malignant potential. These tumors commonly occur in females during the 4th or 5th decade of life. They can grow rapidly and the associated symptoms can mimic other types of breast carcinoma, particularly if the mass ulcerates and bleeds. Phyllodes tumor usually compresses the surrounding tissue from which it is usually well demarcated. The bulk of this tumor is connective tissue with mixed gelatinous cystic and solid areas (3). Malignant tumors usually have rhabdomyosarcoma and liposarcoma rather than fibrosarcomas, the number of mitoses may help in the diagnosis of the malignant subtype (4–6). The only treatment option for these tumors is surgical removal (3). Phyllodes tumors often present a diagnostic and treatment dilemma. The World Health Organization classified phyllodes tumors into benign, borderline, and malignant categories based on the degree of stromal cellular atypia, mitotic activity per 10 high-power fields, degree of stromal overgrowth (these three are main), tumor necrosis, and margin appearance. Borderline tumors have the greatest tendency for local recurrence (6, 7). All forms of phyllodes tumors have malignant potential and can behave like sarcomas with blood-borne metastasis to various organs, commonly the lungs, bone, and abdominal viscera (5). The majority of phyllodes tumors have been described as benign (35% to 64%), with the remainder divided between the borderline and malignant subtypes. A five-year survival rate was observed in almost 100% of patients with benign tumors, 98% with borderline, and about 88% with malignant (8). The cutoff point for giant phyllodes tumor is 10cm, this size presents management problems to the surgeon, and although the surgical management of phyllodes tumors had been previously addressed in the literature, few reports have commented on the giant phyllodes tumor (9). Depending on malignant potential, bulky tumor, recurrence, and status of resection margins, the treatment may vary between wide local excision with 1cm breast tissue or radiotherapy (10). Revision surgery may be required for a high percentage of tumors with inadequate margin removal, and radiotherapy after breast surgery may significantly reduce the local recurrence rate for borderline and malignant tumors (9, 11). What was fascinating about our case was not so much the initial presentation, but the aggressiveness of this variation of phyllodes, because the mass had been excised two months previously in another hospital, then it aggressively reoccurred again.
2. Case presentation
2.1. Clinical presentation
A 41-year-old single female presented to us in King Khalid Hospital in Tabuk, Saudi Arabia in December 2015, with a lump in the left breast that had been gradually increasing for 14 months. Two months before the patient sought medical advice, the mass had ulcerated and had started to bleed with severe pain. Excision of the mass was done in another hospital then it aggressively reoccurred again, there was no family history of the same illness. On presentation, the patient was alert, conscious and her vital signs were stable. On breast examination, giant ulcerating mass (>30 cm in diameter) occupied the entire left breast with areas of necrosis. With bleeding from different points, no other masses were palpable as were the axillary lymph nodes.
2.2. Laboratory and imaging findings
The hemoglobin level was 7 g/dl and all other investigations were within normal limits. A Trucut biopsy showed a malignant phyllodes tumor. Computer tomography scanning for chest, abdomen and pelvis was normal, also isotope scan was normal.
2.3. Post-operative findings and follow-up
The patient underwent total mastectomy without axillary dissection.
2.3.1. Macroscopic findings
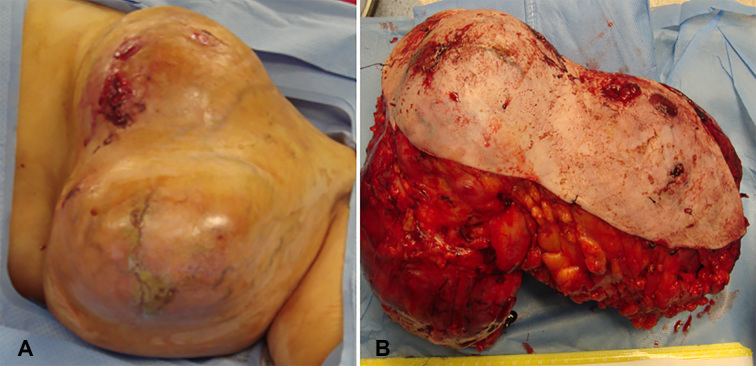
A breast tissue measuring 30×20×13 cm in dimensions was resected (Figure 1A, 1B). Near total surface of the specimen is covered by tan colored skin 30×17. Deep margin displays butt out smooth surfaced nodules, cut surface is multinodular and shows variable sized fleshy, soft to firm, off-white to ash colored leafy nodules.
Figure 1.
Post-operative findings (Macroscopic). A) Phyllodes tumor pre-operative; B) Phyllodes tumor post-operative
2.3.2. Microscopic findings
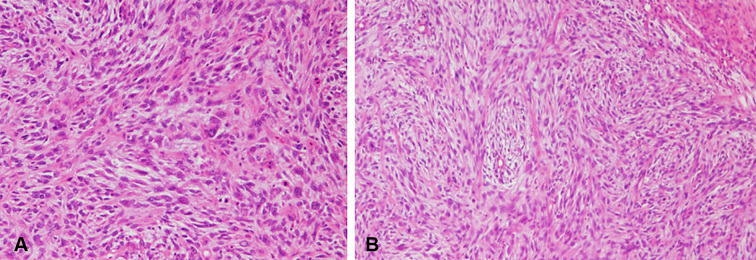
Fragments of breast tissue and several circumscribed nodular masses consistent with fibro adenomatous changes, poorly organized variably arranged stroma and gland with stromal dominance were seen. Areas of hemorrhage, thrombosis, and necrosis were also evident. Moreover, an increased mitosis with stromal (Figure 2A), and epithelial proliferation and leaf pattern epithelial component of the tumor growth was also observed (Figure 2B). No remarkable changes were evident on the underlying skin. A final histological diagnosis was malignant phyllodes tumor.
Figure 2.
Post-operative findings (Microscopic).
2.3.3. Follow-up
The patient had an uneventful recovery and received radiotherapy. She was free of recurrence one year after surgery
3. Discussion
One of the challenges facing the treating physician is the finding of the patients who will develop local recurrence or metastasis. As is well-known, phyllodes tumors are usually found as an incidental finding during the examination of a female breast. These tumors are usually well circumscribed and painless with an average size of 5 cm. But lesions measuring more than 30 cm have also been reported. While primarily a disease of females, reports of these tumors have been published in a few cases of male breasts (12, 13). Phyllodes tumors’ peak incidence is between 30 to 40 years, but can occur at any age (14). Etiology of these tumors remains unknown. The left breast is more commonly affected than the right one as we see in this case and other cases which we treated. These tumors grow radially and compress the surrounding breast parenchyma, a false capsule is created, through which the tumor extends and grows into the rest of the healthy mammary tissue (15). The overlying skin is usually shiny and translucent enough to reveal underlying veins at its initial presentation (3). Ultimately, the tumor can cause an ulcer or open wound on the skin. These tumors represent a character of sizeable malignant sarcoma, taking a leaf-like appearance on gross examination and cystic spaces on histological examination (16). In most cases, it mimics a benign breast condition such as fibroadenoma, making the diagnosis more challenging unless it grows to a large, ulcerative, hemorrhagic lesion. The tumor differentiates from other benign breast disorders by the increased mitotic activity, cellular atypia, and stromal proliferation. Although the malignant potential is very rare, lungs are the most common metastatic site, followed by the skeleton, heart and liver (17). Mammography and breast ultrasound cannot differentiate phyllodes from other benign breast conditions such as fibroadenomas (18). The incisional and excisional biopsies are the definitive methods for diagnosing the phyllodes tumor, although, core cut biopsy is a reliable investigation for diagnosis. Complete surgical resection is the treatment of choice; however, particularly in the borderline and malignant phyllodes tumors, the extent of the resection is controversial as they penetrate in the surrounding healthy tissues (19). For this reason, a wide local excision is done that must include a healthy breast tissue. No proven curative or palliative role in the management of these tumors was confirmed for neoadjuvant and adjuvant therapy, due to the lack of tumor-free margins during the surgical resection, a recurrence rate of less than 13% is usually observed (20). A close follow-up with frequent breast examinations and imaging tests is recommended after surgery. Positive surgical margins, tumor size, high mitotic count stromal overgrowth, and necrosis are factors which have shown an increase in the local recurrence. If the tumor size is >10 cm, then the prevalence of local recurrence is four times greater than smaller tumors whereas surgical margin of less than one cm, the risk is increased by five-fold and stromal overgrowth increases the probability of local recurrence by seven-fold (21). Our patient had most of the common factors for a high likelihood of local recurrence, including the first recurrence within two months of the previous excision and with a tumor size of >30 cm, high mitotic rate, poorly organized variably arranged stroma, marked pleomorphism, the surgical margin less than one cm and focal tumor necrosis. In spite of all of these findings, she received post-operative radiotherapy and on close follow up for one year, there was no recurrence. The preoperative diagnosis and proper management are crucial in phyllodes tumors because of their tendency to recur and also the malignant potential in some of these tumors.
4. Conclusions
Accurate preoperative pathological diagnosis allows correct surgical planning and avoidance of reoperation. The value of FNAC in the diagnosis of phyllodes tumor remains controversial, but core needle biopsy has high sensitivity value. Surgical management is the mainstay, and local recurrence in phyllodes tumors has been associated with inadequate local excision.
Acknowledgments
The author would like to thank Dr. Mohammad Maari and Dr. Hadeer Meir for their participation in the management of this case.
Footnotes
iThenticate screening: May 23, 2018, English editing: May 25, 2018, Quality control: May 25, 2018
This article has been reviewed/commented by three experts
Conflict of Interest:
There is no conflict of interest to be declared.
References
- 1.Yohe S, Yeh IT. “Missed” diagnosis of Phyllodes tumor on breast biopsy: pathological clues to its recognition. Int J Surg Pathol”. 2008;16(2):137–42. doi: 10.1177/1066896907311378. [DOI] [PubMed] [Google Scholar]
- 2.Farias-Eisner GT, Small K, Swistel A, Ozerdem U, Talmor M. Immediate Implant Breast Reconstruction with Acellular Dermal Matrix for Treatment of a Large Recurrent Malignant Phyllodes Tumor. Aesthetic Plast Surg. 2014;38(2):373–8. doi: 10.1007/s00266-014-0283-9. [DOI] [PubMed] [Google Scholar]
- 3.Lannin DR, Geibel J. Cystosarcoma Phyllode’s. 2014. Available from: http://emedicine.medscape.com/article/188728-overview.
- 4.Kumar T, Patel MD, Bhargavan R, Kumar P, Patel MH, Kothari K, et al. Largest Phyllodes Tumor- Case Report and Brief Review Article. Indian J Surg Oncol. 2011;2(2):141–4. doi: 10.1007/s13193-011-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moffat CJ, Pinder SE, Dixon AR, Elston CW, Blamey RW, Ellis IO. Phyllodestumour of the breast: a clinicopathological review of the thirty-two cases. Histopathology. 1995;27(3):205–18. doi: 10.1111/j.1365-2559.1995.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 6.Rowell MD, Perry RR, Jeng-Gwang H, Barranco SC. Phyllodes tumors. Am J Surg. 1993;165:376–9. doi: 10.1016/S0002-9610(05)80849-9. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Badve S. Phyllodes tumors of breast. Curr Treatoptions Oncol. 2001;2:139–47. doi: 10.1007/s11864-001-0056-y. [DOI] [PubMed] [Google Scholar]
- 8.Ye John Shangming Seven Lakes High School, Katy, TX. Statistical analysis on the behavior and recurrence of breast phyllodes tumors. J Clin Oncol. 2016;34(suppl 3) [Google Scholar]
- 9.Tan PH1, Thike AA, Tan WJ, Thu MM, Busmanis I, Li H, et al. Predicting clinical behaviour of breast phyllodestumours: a nomogram based on histological criteria and surgical margins. J Clin Path. 2012;65(1):69–76. doi: 10.1136/jclinpath-2011-200368. [DOI] [PubMed] [Google Scholar]
- 10.Liang MI, Ramaswamy B, Patterson CC, McKelvey MT, Cordillo G, Nuovo GJ, et al. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and review of literature. World J Surg Oncol. 2008;6:117. doi: 10.1186/1477-7819-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth RJ, Jr, Wells WA, Mitchell SE, Cole BF. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009;16(8):2288–94. doi: 10.1245/s10434-009-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bapat K, Oropeza R, Sahoo S. Benign phyllodes tumor of the male breast. Breast J. 2002;8(2):115–6. doi: 10.1046/j.1524-4741.2002.08209.x. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen VT, Andreasen C. Phyllodes tumors of the male breast. Histopathology. 1987;11:761–2. doi: 10.1111/j.1365-2559.1987.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 14.Salvadori B, Cusumano F, Del Bo R, Delledonne V, Grassi M, Rovini D, et al. Surgical treatment of the phyllodes tumors of the breast. Cancer. 1989;63(12):2532–6. doi: 10.1002/1097-0142(19890615)63:12<2532::AID-CNCR2820631229>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Adamietz B. Differentiation Between Phyllodes Tumor and Fibroadenoma Using Real-Time Elastography. Ultraschall Med. 2011;32(Suppl 2):E75–9. doi: 10.1055/s-0031-1282024. [DOI] [PubMed] [Google Scholar]
- 16.Parker SJ, Harries SA. Phyllodes tumors. Postgrad Med J. 2001;77(909):428–35. doi: 10.1136/pmj.77.909.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe M, Miyata S, Nishimura S, Iijima K, Makita M, Akiyama F, et al. Malignant transformation of breast fibroadenoma to malignant phyllodes tumor: long term outcome of 36 malignant phyllodes tumors. Breast Cancer. 2011;18(4):268–72. doi: 10.1007/s12282-009-0185-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim JG, Kim SY, Jung HY, Lee DY, Lee JE. Extremely rare borderline phyllodes tumor in the male breast: a case report. Clin Imaging. 2015;39(6):1108–11. doi: 10.1016/j.clinimag.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Ben Hassouna J, Damak T, Gamoudi A, Chargui R, Khomsi F, Mahjoub S, et al. Phyllodes tumors of the breast: a case series of 106 patients. Am J Surg. 2006;192(2):141–7. doi: 10.1016/j.amjsurg.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Belkacémi Y, Bousquet G, Marsiglia H, Ray-Coquard I, Magné N, Malard Y, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70(2):492–500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Asoglu O1, Ugurlu MM, Blanchard K, Grant CS, Reynolds C, Cha SS, et al. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann Surg Oncol. 2004;11:1011–7. doi: 10.1245/ASO.2004.02.001. [DOI] [PubMed] [Google Scholar]