Abstract
Background
Antipyretics reduce fever following childhood vaccinations; after inactivated influenza vaccine (IIV) they might ameliorate fever and thereby decrease febrile seizure risk, but also possibly blunt the immune response. We assessed the effect of antipyretics on immune responses and fever following IIV in children ages 6 through 47 months.
Methods
Over the course of three seasons, one hundred forty-two children, receiving either a single or the first of 2 recommended doses of IIV, were randomized to receive either oral acetaminophen suspension (n=59) or placebo (n=59) (double-blinded) or ibuprofen (n=24) (open-label) immediately following IIV and every 4 to 8 hours thereafter for 24 hours. Blood samples were obtained at enrollment and 4 weeks following the last recommended IIV dose. Responses to IIV were assessed by hemagglutination inhibition assay (HAI). Seroprotection was defined as an HAI titer ≥ 1:40 and seroconversion as a titer ≥ 1:40 if baseline titer < 1:10 or fourfold rise if baseline titer ≥1:10. Participants were monitored for fever and other solicited symptoms on the day of and day following IIV.
Results
Significant differences in seroconversion and post-vaccination seroprotection were not observed between children included in the different antipyretic groups and the placebo group for the vaccine antigens included in IIV over the course of the studies. Frequencies of solicited symptoms, including fever, were similar between treatment groups and the placebo group.
Conclusions
Significant blunting of the immune response was not observed when antipyretics were administered to young children receiving IIV. Studies with larger sample sizes are needed to definitively establish the effect of antipyretics on IIV immunogenicity.
In young children, fever is the mostly commonly reported adverse event following immunization,[1] and is occasionally associated with a febrile seizure (FS). FSs have been reported to occur in children following receipt of measles, mumps and rubella vaccine (MMR), measles, mumps, rubella and varicella vaccine (MMRV), pneumococcal conjugate vaccine (PCV), and inactivated influenza vaccine (IIV).[2, 3] During the 2010–2011 influenza vaccination season in the United States, the first year the 2009 pandemic H1N1 strain (2009pdmH1N1) was included in the seasonal influenza vaccine, an elevated risk of FS was observed in young children on the day of or day following (day 0 to 1) receipt of trivalent IIV (IIV3).[4] The risk was noted to be highest in those receiving IIV3 and 13-valent PCV (PCV13) concomitantly.[4] An observational study performed during the subsequent 2011–2012 season, demonstrated that fever was more common on days 0 to 1 following vaccination among children receiving IIV and PCV13 simultaneously when compared to children receiving either vaccine alone.[5] A separate study, conducted over multiple seasons leading up to 2010–2011, further established that administration of IIV3 on the same day as PCV and/or diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (DTaP) is associated with an increased risk of FS.[6]
Although generally considered to be medically benign, FSs are frightening and anxiety provoking for parents.[7] Therefore, in attempts to reduce fever and potentially FS following immunization, it is thought that administering antipyretics in conjunction with some vaccines might be considered as a potential preventive strategy. Although antipyretics have not been shown to reduce the risk of recurrent FS, their use has not specifically been assessed for prevention of FS after immunization. [8]
While antipyretics reduce fever following infant vaccines,[9, 10] there is concern that they might reduce the immune response to some vaccine antigens.[11] This raises concern about their potential routine use in children receiving childhood vaccines as a FS prevention strategy, which is not currently supported by available evidence.[12] It remains unknown, however, if antipyretics reduce the immune response to IIV in young children. Previous data from controlled studies of seasonal influenza vaccines in adults and one observational study of monovalent 2009pdmH1N1 influenza vaccine in children have shown this not to be the case.[13–16] Therefore, over the course of three seasons we undertook a series of investigations designed to begin assessing the effect of acetaminophen and ibuprofen on immunogenicity and safety outcomes. Our primary objective was to compare the immune response following IIV in children receiving acetaminophen or ibuprofen versus placebo in order to ascertain whether there was evidence that antipyretics blunted the immune response to IIV in children. We also compared the proportions of children with fever and other solicited symptoms following IIV in each antipyretic group versus placebo.
METHODS
Two consecutive randomized, controlled trials were conducted from October 2013 to March 2014 (pilot study) and from September 2014 to April 2015 and September 2015 to March 2016 (expanded study); data were combined for this report. Studies were registered under ClinicalTrials.gov identifiers NCT01946594 and NCT02212990, respectively. The pilot was a randomized (1:1) controlled double-blind comparison of acetaminophen and placebo following IIV. The expanded study was similar in design but the randomization also included an open label ibuprofen arm (3:3:2). The ibuprofen arm was smaller, as it was added to obtain preliminary data on immunogenicity effects, and was open-label as the recommended dosing frequency differs from acetaminophen and because it was rescue therapy for children randomized to receive either acetaminophen or placebo and who developed fever. Protocols were approved by the Duke Institutional Review Board (IRB); the Centers for Disease Control and Prevention (CDC) relied on the determination of the Duke IRB.
Participants
At the time of enrollment, children were required to be between 12 and 35 months and 6 and 47 months of age for the pilot and expanded study, respectively, and could not have previously received the current season’s influenza vaccine. During the pilot, only children needing a single dose of IIV per Advisory Committee on Immunization Practices (ACIP) recommendations were eligible, but during the expanded study, children needing either 1 or 2 doses were enrolled.[17–19] Three children enrolled in the pilot were also enrolled in the expanded study. Children were excluded if they were febrile (≥37.8°C), had a moderate to severe illness, or had already received an antipyretic medication within the prior 72 hours; had a history of a severe allergic reaction to influenza vaccine or any of its components; had a history of Guillain-Barre syndrome within 6 weeks of receipt of a previous influenza vaccine dose; or had a history of immunosuppression. Children could not have received long term high dose oral steroids, any parenteral steroids or high-dose inhaled steroids within the previous 6 months. Children were required to be up-to-date on recommended immunizations and study participation could not cause immunization delay. Participants could not receive concomitant immunizations, or have received an inactivated vaccine within 14 days or a live vaccine within 28 days of a dose of IIV. Children could not be allergic to either acetaminophen or ibuprofen, have underlying conditions precluding their use, and parents could not be planning to routinely administer antipyretics prophylactically. Parents were required to provide written informed consent. The study was conducted at 3 primary care practices in or nearby Durham, NC. We preferentially worked to recruit children with a personal history of FS by performing a search of the patient database for an ICD-9 or ICD-10 coded diagnosis of FS and sending targeted recruitment letters.
Study Drug and Administration
Acetaminophen suspension was compounded to provide 160 mg per 5 mL and to match liquid placebo in appearance and taste. Commercially available ibuprofen suspension containing 100 mg per 5 mL was used. Antipyretic dosing is described in Table 1. Parents were directed to dose placebo similarly as would be recommended for acetaminophen. The first dose of study drug was administered during the clinic visit immediately following IIV receipt. For those children receiving 2 doses of IIV per ACIP recommendation, antipyretics or placebo were only prescribed at the time of the initial IIV dose.[17–19] Parents were instructed to record the time of administration of each dose of study drug on a paper memory aid/diary card.
Table 1: Antipyretic (acetaminophen and ibuprofen) dosing according to participant’s age for pilot and expanded studies.
| Pilot Study |
Expanded Study |
|||
|---|---|---|---|---|
| Acetaminophen Dose (mg) every 4 to 6 hours for 24 hours (maximum of 5 doses) |
Acetaminophen Dose (mg) every 4 to 6 hours for 24 hours (maximum of 5 doses) |
Ibuprofen Dose (mg) Every 6 to 8 hours for 24 hours (maximum of 4 doses) |
||
| Age in months | Participant weight (kg) |
|||
| < 24 | 15 mg/kg (maximum 160) |
5.4–8.1 | 80 | 50 |
| 8.2–10.8 | 120 | 75 | ||
| >10.8 | 160 | 100 | ||
| 24–47 | 160 | — | 160 | 100 |
Influenza Vaccination
IIV was supplied by the clinic, given according to recommended dosing instructions, and administration was not considered a study procedure. During the pilot (2013–2014 influenza season) and first year (2014–2015) of the expanded study, influenza vaccine strains were: A/California/07/2009 X-179A (H1N1), A/Texas/50/2012 X-223A (H3N2) (an A/Victoria/361/2011-like virus), and B/Massachusetts/02/2012 (B Yamagata lineage) for the IIV3 formulation with the addition of the B/Brisbane/60/2008 (B Victoria lineage) strain for the quadrivalent formulation (IIV4).[20, 21] During the second year of the expanded study (20152016), the A/Texas/50/2012 X-223A (H3N2) and B/Massachusetts/02/2012 strains were replaced by an A/Switzerland/9715293/2013 (H3N2)-like virus and the B/Phuket/3073/2013-like virus (B Yamagata lineage). [22] During the pilot study only IIV3 was used, while during the expanded study both IIV3 and IIV4 were used.
Study Procedures
After obtaining written informed consent from the parent or legal guardian, study eligibility criteria were reviewed and the child’s demographic information and medical history including personal history or family history of FSs and influenza vaccination history were obtained. The child’s weight and axillary temperature were measured and the child was randomized to receive either acetaminophen or placebo (both studies) or open-label therapy with ibuprofen (expanded study only). Randomization was done in blocks of 4 (pilot study) or blocks of 8 (expanded study) for each of the practice sites. Randomization schemes were generated by the project statistician and shared with the research pharmacists. The remaining study staff was blinded to the randomization for acetaminophen or placebo.
Parents were provided a thermometer and were instructed to document the child’s temperatures and solicited symptoms on the day of (Day 0) and day following (Day 1) vaccination on the memory aid. Parents measured and documented the child’s axillary temperature prior to administering each dose of study drug and at 24 hours after vaccination. Measurement of axillary temperature was chosen as the preferred method to increase participant acceptability. In addition, parents documented increased fussiness, changes in the child’s sleep patterns and appetite, and use of medical services for the child. Memory aid information was collected by the research team in a follow-up telephone call or by e-mail between 48 to 144 hours following vaccination. Children needing a second dose of IIV were instructed to return to clinic 28 to 42 days following receipt of the first dose.
Blood samples for serologic analysis were obtained at baseline, prior to the first dose of IIV, and 28 days following the last dose. Any serious adverse events, as defined by 21CFR312.32,[23] occurring between enrollment and the final study visit, 28 days following the last dose of IIV, were recorded.
Hemagglutination Inhibition Assays
Influenza viruses were grown in specific-pathogen-free eggs or Madin-Darby canine kidney cells and harvested as pooled and filtered allantoic/amniotic fluid or clarified culture supernatant respectively. Stock titers were quantified using influenza hemagglutination units assay. Antibody titers against the influenza test strains were measured in serum samples by hemagglutination inhibition (HAI) to assays similar as previously described.[24–27] Briefly, 10-fold serial dilutions of RDE (Denka Seiken Co.) treated, heat-inactivated and turkey red blood cell (RBC; Lampire Biologicals) adsorbed sera were reacted with influenza virus, overlayed with a suspension of ~1.0×107 turkey RBCs/mL and RBC agglutination/pelleting visualized.
Outcomes
Immunogenicity
The primary study outcome was to assess seroconversion post-vaccination as denoted by an HAI titer ≥ 1:40 post-vaccination if the baseline titer was < 1:10 or a four-fold rise in HAI titer if the baseline titer was ≥ 1:10. Additional immunogenicity measures were to assess seroprotection as determined by an HAI titer ≥ 1:40 and the geometric mean titer (GMT) at baseline and one month following the last dose of IIV. Seroconversion and seroprotection percentages as well as GMTs with 95% confidence intervals (CIs) are reported.
Reactogenicity
Outcomes included the frequencies of serious adverse events during the period of enrollment and solicited events including: fever (≥ 38°C and ≥ 39°C), increased fussiness, change in appetite and sleep, and receipt of medical attention on day 0 and 1 following IIV.
Statistical Analysis
These exploratory studies were to assess the role of each antipyretic individually on the immune response and fever following IIV, and no formal a priori power calculation was done. Influenza immunity was assessed in the cohort of children who received at least a single dose of study medication and for whom at least one blood sample was obtained while seroconversion was assessed in those with paired blood samples. Reactogenicity was assessed in those who received a dose of study medication and for whom we received any memory aid information. Descriptive statistics including medians, interquartile ranges, counts and percentages were used to summarize study variables. Distribution of categorical variables across study groups were compared using Fisher’s exact tests. Relative risks and 95% CIs for seroconversion and seroprotection were determined for children receiving acetaminophen or ibuprofen separately versus placebo. We considered a p <0.05 statistically significant and conducted all statistical analyses using STATA SE 13.0 or newer (College Station, TX).
RESULTS
Participants
During the three study influenza seasons, 142 children were enrolled and randomized. Participant characteristics are described in Table 2. The greatest percentage (42%) of participants were enrolled during 2014–2015 with similar percentages enrolled during 2013–2014 and 20152016 (28% and 30%, respectively). An equal number of participants were randomized to receive blinded therapy with either acetaminophen (n=59) or placebo (n=59) over three seasons, while 24 children received open label ibuprofen during the last two seasons only. While every child received at least a single dose of study medication shortly after their dose of IIV, most (90%) received 3 or more doses of study medication including the dose received in clinic. There were no differences with respect to the proportion of children receiving 3 or more doses of study medication between either treatment group or the placebo group (p=1.00). Despite instructions for receipt of a maximum of 4 doses of ibuprofen, 3 participants received 5 doses. One child in the acetaminophen group who developed fever, also received ibuprofen for fever relief.
Table 2: Participant characteristics (season of enrollment, demographics, febrile seizure risk, influenza vaccination information and doses of study medication received) according to treatment group.
| TREATMENT GROUP | ||||||
|---|---|---|---|---|---|---|
|
Acetaminophen (n=59) |
Ibuprofen (n=24) |
Placebo (n=59) |
Total (n=142) |
|||
| ENROLLMENT SEASON | ||||||
| n (%) | n (%) | n (%) | n (%) | |||
| 2013–2014a | 20 (33.9%) | 0 (0.0%) | 20 (33.9%) | 40 (28.2%) | ||
| 2014–2015b | 24 (40.7%) | 13 (54.2%) | 23 (39.0%) | 60 (42.2%) | ||
| 2015–2016b | 15 (25.4%) | 11 (45.8%) | 16 (27.1%) | 42 (29.6%) | ||
| DEMOGRAPHICS | ||||||
| Months | p-valuec | Months | p-valuec | Months | Months | |
| Age | ||||||
| Median | 24 | 0.77 | 28 | 0.42 | 24 | 24 |
| n (%) | p-value | n (%) | p-value | n (%) | n (%) | |
| Ethnicity | ||||||
| Hispanic | 9 (15.2%) | 0.39 | 1 (4.2%) | 0.67 | 5 (8.5%) | 15 (10.6%) |
| Race | ||||||
| White only | 23 (39.0%) | 0.35 | 11 (45.8%) | 0.81 | 29 (49.2%) | 63 (44.4%) |
| Black only | 22 (37.3%) | 0.70 | 9 (37.5%) | 0.80 | 19 (32.2%) | 50 (35.2%) |
| Other | 14 (23.7%) | 0.65 | 4 (16.7%) | 1.00 | 11 (18.6%) | 29 (20.4%) |
| Gender | ||||||
| Male | 30 (50.9%) | 1.00 | 13 (54.2%) | 0.35 | 29 (49.2%) | 72 (50.7%) |
| FEBRILE SEIZURE RISK | ||||||
| Participant history of FS |
3 (5.1%) | 0.62 | 2 (8.3%) | 0.20 | 1 (1.7%) | 6 (4.2%) |
| 1st degree relative with FS history |
5 (8.5%) | 0.76 | 1 (4.2%) | 0.43 | 7 (11.9%) | 13 (9.2%) |
| Participant or 1st
degree relative with FS history |
8 (13.6%) | 1.00 | 3 (12.5%) | 1.00 | 8 (13.6%) | 19 (13.4%) |
| INFLUENZA VACCINATION INFORMATION | ||||||
| Number of doses recommended | ||||||
| One dose | 53 (89.8%) | 0.58 | 20 (83.3%) | 1.00 | 50 (84.7%) | 123 (86.6%) |
| Two doses | 6 (10.2%) | ----- | 4 (16.7%) | 9 (15.3%) | 19 (13.4%) | |
| IIV type administered | ||||||
| IIV3 only | 24 (40.7%) | 1.00 | 1 (4.2%) | <0.01 | 23 (39.0%) | 48 (33.8%) |
| IIV4 only | 34 (57.6%) | 1.00 | 23 (95.8%) | <0.01 | 35 (59.3%) | 92 (64.8%) |
| IIV3 and IIV4 | 1 (1.7%) | 1.00 | 0 (0.0%) | 1.00 | 1(1.7%) | 2 (1.4%) |
| Dosage formulation administered | ||||||
| 0.25 mL | 50 (84.7%) | 0.39 | 13 (54.2%) | <0.01 | 54 (91.5%) | 117 (82.4%) |
| 0.5 mL | 9 (15.2%) | 0.39 | 10 (41.7%) | <0.01 | 5 (8.5%) | 24 (16.9%) |
| 0.25 mL and 0.5 mL | 0 (0.0%) | 1.00 | 1(4.2%) | 0.29 | 0 (0.0%) | 1 (0.7%) |
| NUMBER OF DOSES OF ANTIPYRETIC OR PLACEBO ADMINISTERED | ||||||
| <3 | 6 (10.2%) | 1.00 | 2 (8.3%) | 1.00 | 6 (10.2%) | 14 (9.9%) |
| ≥3 | 53 (89.8%) | ----- | 22 (91.7%) | ----- | 53 (89.8%) | 128 (90.1%) |
Pilot Study,
Expanded Study,
Fisher’s exact test for categorical data and Wilcoxon rank sum tests for medians. All statistical comparisons were between acetaminophen and placebo groups or ibuprofen and placebo groups.
When compared to the placebo group, children in both the acetaminophen and ibuprofen groups did not significantly differ with respect to demographic characteristics or risk of FS based on personal or family history. Likewise, the recommended number of IIV doses did not differ between the treatment groups and the placebo group. When compared to the placebo group, a significantly greater proportion of children in the ibuprofen group received IIV4 and the 0.5 mL dose (p<0.001 for both). These differences likely result from the fact that the 2013–2014 pilot study did not include an ibuprofen arm, only included children < 36 months of age, and was conducted in a season in which only IIV3 was used.
Immunogenicity
Seroconversion
Seroconversion for the A strains among placebo recipients varied from of 60% for the A/Texas (H3N2) strain to 100% for the A/Switzerland (H3N2) strain and was 78% for the A/California (H1N1)pdm09 strain, present in IIV during all 3 study seasons (Table 3). Seroconversion percentages for the B strains were in general much lower ranging from 9% for the B/Phuket strain to 32% for the B/Massachusetts strain. There was a non-significant 14 to 15% decrease in seroconversion for the H1N1 strain when antipyretic was used and no statistically significant differences in seroconversion rates for any of the other strains between either of the treatment groups and the placebo group were observed.
Table 3: Seroconversion, seroprotection (baseline and follow-up), and geometric mean titers (GMTS) (baseline and follow-up) as determined by hemagglutination inhibition assay (HAI) according to treatment group.
| Treatment Group | |||||
|---|---|---|---|---|---|
| Acetaminophen | Ibuprofen | Placebo | |||
| n/N (%) | RR (95% CI)a | n/N (%) | RR (95% Cl) a | n/N (%) | |
|
A/California/7/2009
(H1N1)pdm09 [2013–14, 2014–15, and 2015–16 seasons] |
|||||
| Seroconversion (n=117) | 35/52 (67.3%) | RR=0.86 (0.68, 1.10) |
10/15 (66.7%) | RR=0.85 (0.58, 1.26) |
39/50 (78.0%) ----- |
| Baseline Seroprotection (n=132) | 8/57 (14.0%) | RR=0.58 (0.26, 1.28) |
4/17 (23.5%) | RR=0.97 (0.37, 2.58) |
14/58 (24.1%) ----- |
| Follow-up Seroprotection (n=125) | 42/53 (79.2%) | RR=0.88 (0.75, 1.04) |
16/21 (76.2%) | RR=0.84 (0.65, 1.09) |
46/51 (90.2%) ----- |
| Baseline GMT(95%CI) (n=132) | 10.4 (7.5, 14.5) | ----- | 12.3 (6.0, 25.2) | ----- | 13.6 (10.2, 18.3) ----- |
| Follow-up GMT(95%CI) (n=125) | 80.5 (54.2, 119.5) | ----- | 109.5 (50.2, 238.6) | ----- | 105.0 (76.8, 143.5) ----- |
|
A/Texas/50/2012 (H3N2)
[2013–14 and 2014–15 seasons] |
|||||
| Seroconversion (N=85) | 22/39 (56.4%) | RR=0.93 (0.64, 1.36) |
4/8 (50.0%) | RR=0.83 (0.39, 1.73) |
23/38 (60.5%) ----- |
| Baseline Seroprotection (n=91) | 6/42 (14.3%) | RR=0.65 (0.25, 1.66) |
3/8 (37.5%) | RR=1.71 (0.59, 4.95) |
9/41 (22.0%) ----- |
| Follow-up Seroprotection (N=91) | 27/40 (67.5%) | RR=0.88 (0.67, 1.16) |
9/12 (75.0%) | RR=0.98 (0.67, 1.41) |
30/39 (76.9%) ----- |
| Baseline GMT(95%CI) (n=91) | 11.1 (7.2, 17.1) | ----- | 17.6 (3.9, 78.8) | ----- | 12.6 (8.2, 19.2) ----- |
| Follow-up GMT (95%CI)(N=91) | 61.2 (38.5, 97.2) | ----- | 82.3 (34.4, 197.2) | ----- | 79.3 (53.1, 118.5) ----- |
|
A/Switzerland/9715293/2013
(H3N2) [2015–2016 season] |
|||||
| Seroconversion (n=31) | 13/13 (100%) | RR=1 (1, 1) |
5/7 (71.4%) | RR=0.71 (0.45, 1.14) |
11/11 (100%) ----- |
| Baseline Seroprotection (n=40) | 3/15 (20.0%) | RR=1.07 (0.25, 4.49) |
4/9 (44.4%) | RR=2.37 (0.68, 8.31) |
3/16 (18.8%) ----- |
| Follow-up Seroprotection (n=33) | 13/13 (100%) | RR=1 (1, 1) |
8/9 (88.9%) | RR-0.89 (0.71, 1.12) |
11/11 (100%) ----- |
| Baseline GMT(95%CI) (n=40) | 10.0 (4.3, 23.0) | ----- | 52.4 (6.0, 455.7) | ----- | 9.0 (5.0, 16.1) ----- |
| Follow-up GMT(95%CI) (n=33) | 751.0 (377.7, 1493.2) | ----- | 665.1 (119.3, 3708.7) | ----- | 620.2 (249.5, 1541.4) ----- |
|
B/M assachusetts/2/2012
[2013–2014 and 2014–2015 seasons] |
|||||
| Seroconversion (n=85) | 17/39 (43.6%) | RR=1.38 (0.77, 2.49) |
2/8 (25.0%) | RR=0.79 (0.22, 2.87) |
12/38 (31.6%) ----- |
| Baseline Seroprotection (n=91) | 1/42 (2.4%) | RR=0.49 (0.05, 5.18) |
1/8 (12.5%) | RR=1.16 (0.70, 1.90) |
2/41 (4.9%) ----- |
| Follow-up Seroprotection (n=91) | 19/40 (47.5%) | RR=1.16 (0.70, 1.91) |
3/12 (25.0%) | RR=0.61 (0.21, 1.74) |
16/39 (41.0%) ----- |
| Baseline GMT(95%CI) (n=91) | 6.6 (5.6, 7.7) |
----- |
7.7 (3.9, 15.3) |
----- |
7.3 (5.9, 8.9) ----- |
| Follow-up GMT(95%CI) (n=91) | 20.0 (13.3, 30.1) |
----- |
12.2 (5.0, 30.2) |
----- |
19.0 (12.8, 28.2) ----- |
|
B/Brisbane/60/2008b
[20142015 and 2015–2016 seasons] |
|||||
| Seroconversion (n=74) | 5/30 (16.7%) | RR=1.21 (0.36, 4.06) |
4/15 (26.7%) | RR=1.93 (0.56, 6.67) |
4/29 (13.8%) ----- |
| Baseline Seroprotection (n=83) | 0/33 (0.0%) | RR=N/A | 0/17 (0.0%) | RR=N/A | 0/33 (0.0%) ----- |
| Follow-up Seroprotection (n=80) | 5/30 (16.7%) | RR=1.25 (0.37, 4.21) |
4/20 (20.0%) | RR=1.50 (0.42, 5.32) |
4/30 (13.3%) ----- |
| Baseline GMT(95%CI) (n=83) | 5.0 (5.0, 5.0) | ----- | 5.0 (5.0, 5.0) | ----- | 5.0 (5.0, 5.0) ----- |
| Follow-up GMT(95%CI) (N=80) | 8.4 (6.0, 11.8) | ----- | 9.3 (5.5, 15.9) | ----- | 7.9 (5.5, 11.5) ----- |
|
B/Phuket/3073/2013 [2015- 2016 season] |
|||||
| Seroconversion (n=31) | 2/13 (15.4%) | RR=1.69 (0.18, 16.26) |
2/7 (28.6%) | RR=3.14 (0.35, 28.52) |
1/11 (9.1%) ----- |
| Baseline Seroprotection (n=40) | 0/15 (0.0%) | RR=N/A | 0/9 (0.0%) | RR=N/A | 0/16 (0.0%) ----- |
| Follow-up Seroprotection (n=33) | 2/13 (15.4%) | RR=1.69 (0.18, 16.25) |
3/9 (33.3%) | RR=3.67 (0.46, 29.49) |
1/11 (9.1%) ----- |
| Baseline GMT(95%CI) (n=40) | 5.0 (5.0, 5.0) | ----- | 5.0 (5.0, 5.0) | ----- | 5.0 (5.0, 5.0) ----- |
| Follow-up GMT(95%CI) (n=33) | 8.5 (4.4, 16.6) | ----- | 14.1 (4.2, 47.2) | ----- | 10.0 (5.8, 17.3) ----- |
Relative Risk (RR) and 95% CI for seroconversion and seroprotection was determined comparing each treatment group individually versus placebo
Quadrivalent IIV only
Seroprotection
Among all participants baseline seroprotection percentages for the A strains ranged between 20% and 25% and B strains between 0% and 4%. Follow-up seroprotection levels for A strains among placebo recipients ranged from 77% for A/Texas (H3N2) strain to 100% for the A/Switzerland (H3N2) strain. Similar to seroconversion rates, levels of seroprotection for B strains were low, ranging from 9% for the B/Phuket strain to 41% for the B/Massachusetts strain. There were no statistically significant differences in either baseline or follow-up seroprotection percentages for any of the strains between either of the treatment groups and the placebo group.
Geometric Mean Titers
Among all participants, baseline GMTs for the A strains ranged between 12.0 and 13.9 and for the B strains ranged between 5.0 and 7.0. Among placebo recipients follow-up GMTs for the A strains ranged from 79.3 for the A/Texas(H3N2) strain to 620.2 for the A/Switzerland (H3N2) strain. Follow-up GMTs for B strains ranged from 7.9 for the B/Brisbane strain to 19.0 for the B/Massachusetts strain. No statistically significant differences in either baseline or follow-up GMTs were observed for any of the strains between either of the treatment groups and the placebo group.
Reactogenicity
Increases in fever, fussiness, and changes in sleep and appetite did not significantly differ between the two treatment groups and the placebo group on day 0, day 1 and on day 0 or 1 combined (Figure 1). Only two children had a documented elevated axillary temperature. One child in the acetaminophen group had fever ≥39°C on the day following vaccination and one child in the ibuprofen group had fever ≥38°C on the day of vaccination. No child in the placebo group had fever. Additionally, there was no reported use of medical services on the day of or day following vaccination for any child enrolled in the study. There were no serious, unexpected adverse events or FSs reported during the period of enrollment for any of the study participants.
Figure 1. (a-d) Participant solicited symptoms and fever on vaccination day (day 0) the next day (day1) and (days 0 or 1) following inactivated influenza vaccine (IIV) according to treatment group.
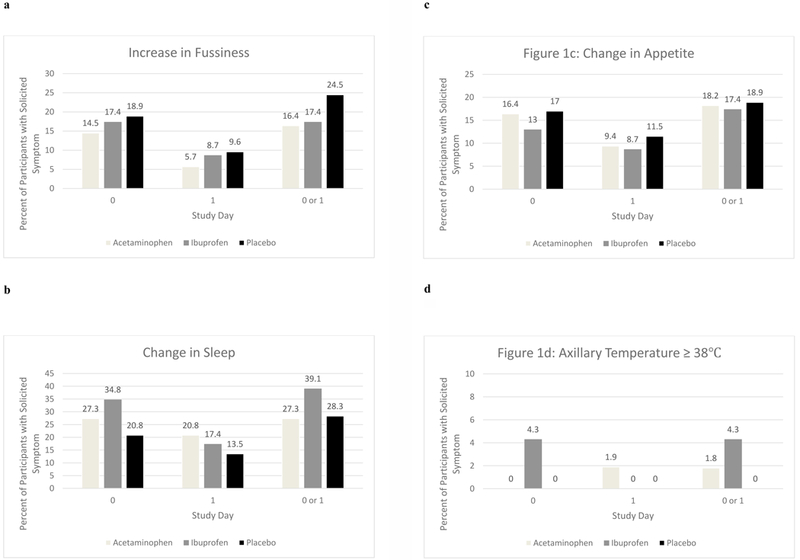
.
DISCUSSION
To our knowledge, this is the first study to prospectively evaluate the role of antipyretics on immune responses and fever following IIV in young children. We assessed the effects of both acetaminophen and ibuprofen separately as they have different mechanisms of action. [28] Our investigations did not suggest significant blunting of the immune responses to influenza A or B antigens by either acetaminophen or ibuprofen. When compared to children receiving placebo, the proportions of children receiving antipyretics who seroconverted following receipt of A/California(HlNl)pdm09 and A/Texas(H3N2) strains were somewhat lower but did not achieve statistical significance. Our study estimates at most a 32–42% decrease in seroconversion for the H1N1 strain when an antipyretic was used; however, given the small sample size these estimates lack precision. This study corroborates findings from studies in adults and a prior observational study of monovalent 2009pdmHlNl influenza vaccine in children which did not detect blunting of the immune response by antipyretics. [13–16]
Only two children in the study had a fever ≥ 38°C including only one with a temperature ≥ 39°C, suggesting that fever was an uncommon occurrence after IIV when administered alone. In a 2011–2012 study assessing temperature during a similar follow-up period after IIV3, 10.3% and 2.6%, respectively, of children were reported to have comparable degrees of fever. [5] These findings were observed during a season in which the IIV product used was noted to have an independent elevated risk of FS.[4] Two differing possibilities may account for the differences in study findings. The 2011–2012 IIV might have induced more fever than the IIV products we assessed over the course of three seasons or the axillary method of assessing temperature elevations was less sensitive than the temporal artery scanner method used in the 2011–2012 investigation.[29] It is also possible that host factors or exposures to other infectious agents contributed to these differences in fever frequency.
Our series of studies had several limitations. These investigations were exploratory and done as a preliminary assessment to evaluate the effect of antipyretics on the immune response following IIV and therefore were not powered to detect differences in seroconversion or seroprotection rates and fever. Although no a-priori power calculation was performed, given the observed seroconversion rate to influenza A vaccine strains in children receiving placebo in our study (74%), a sample size of 471 subjects per group (942 total) would have been required to achieve 80% power at a one-sided alpha=0.05 to detect a 10% decrease in seroconversion rate among those receiving antipyretics. It was also difficult to assess the effects of antipyretics on the response to influenza B strains as we observed low rates of seroconversion and seroprotection to influenza B strains consistent with findings of previously reported studies. [30–32] In addition, the vast majority of children included in this study had received influenza vaccine in the past; the effect of antipyretics on immune response might have been more robust in those IIV naive. Also, we did not assess immunity following the first IIV dose for those receiving two doses. Furthermore, our study did not address the effect of antipyretics on immune responses and fever when IIV is administered concomitantly with either DTaP or PCV13 where there is a known increase in FS risk.[6] Lastly, because our study did not allow for concomitant administration of IIV with other vaccines during well child visits, the health care utilization reported by parents in this study could be different than that of children who receive multiple vaccinations simultaneously.
Although evidence does not support use of antipyretics before or at the time of vaccination, [12] if given by parents for treatment and local discomfort following vaccination our results do not suggest any blunting of the immune response to IIV. In order to address the use of antipyretics as a preventive strategy for children at risk of developing FS following IIV, further research is needed. Our findings could guide the development of larger studies, including analyses that are statistically powered to evaluate both influenza antibody responses and fever and other safety outcomes. Based on our findings, it would be permissible to conduct such a study.
ACKNOWLEDGMENTS
The authors wish to acknowledge the contributions of the following study team members: Beth Patterson, RN, BSN, Lori Hendrickson, RN, BSN, Liz Schmidt, Luis Ballon, Erica Suarez, Stephanie Smith, Beth McLendon Arvik PharmD. and Devindra Sharma, MSN, MPH. In addition, we wish to acknowledge the assistance and support of the following pediatric practices Durham Pediatrics and Duke Children’s Primary Care. The authors also wish to thank Kathryn Edwards MD, Janet Englund MD, and Frank DeStefano MD who reviewed study data as part of an Expert Panel.
Influenza propagation and hemagglutination inhibition assays were performed under the directorship of Dr. Gregory D Sempowski in the Virology Unit of the Duke Regional Biocontainment Laboratory (RBL), which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607). We gratefully acknowledge the technical support of Christopher Sample, Kimberly Parks and Leslee Arwood.
The following reagents were obtained through the NIH Biodefense and Emerging Infectious Research Repository, NIAID, NIH: Influenza A Virus, A/California/07/09 (H1N1)pdm09, Egg Isolate (Produced in Eggs), NR-13663; Influenza A Virus, A/California/07/2009 (HA, NA) x A/Puerto Rico/8/1934 (H1N1)pdm09, Reassortant NYMC X-181, NR-44004 and Ferret Hyperimmune Sera to Influenza A/California/07/2009(H1N1)pdm09, NR-19261.
Influenza B Virus, B/Brisbane/60/2008, FR-177; Influenza B Virus, B/Massachusetts/2/2012 (Yamagata Lineage), FR-1196; Influenza B Virus, B/Phuket/3073/2013 (Yamagata Lineage), FR-1364; Influenza A Virus, A/Texas/50/2012 (H3N2), FR-1210; Influenza A Virus, A/Switzerland/9715293 (H3N2), FR-1368; Ferret Antisera to Influenza B Virus, B/Florida/4/2006 (Yamagata Lineage), FR-391; Ferret Antisera to Influenza B Virus, B/Brisbane/60/2008 (Victoria Lineage), FR-392; Ferret Antisera to Influenza B Virus, B/Massachusetts/2/2012 (Yamagata Lineage), FR-1265; Ferret Antisera to Influenza A Virus, A/Switzerland/9715293/2013 (H3N2), FR-1382; and Ferret Antisera to Influenza A Virus, A/Victoria/361/2011 (H3N2), FR-1079 were obtained through the Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Influenza A Virus, NYMC X-233 + 1P(hy A/Texas/50/2012, H3N2) was kindly provided by Dr. Doris Bucher.
FUNDING
This work was supported by the Centers for Disease Control and Prevention (Clinical Immunization Safety Assessment (CISA) Project Contract [200–2012-53663/0002]).
Footnotes
FINANCIAL DISCLOSURE
Dr. Walter has received funding from bioCSL, GlaxoSmithKline, Merck, Novartis, Novavax, and Pfizer to conduct clinical research studies. He has received support from Novartis as a member of a Data Safety Monitoring Board and from Merck as a consultant.
CONFLICTS OF INTEREST
Dr. Walter has the above noted conflicts.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
PRIOR PRESENTATIONS
This article was presented in part at the 18th Annual Conference on Vaccine Research, April 15, 2016, Bethesda, Maryland.
REFERENCES
- [1].Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)--United States, 1991–2001. MMWR Surveill Summ 2003;52:1–24. [PubMed] [Google Scholar]
- [2].Maglione MA, Das L, Raaen L, Smith A, Chari R, Newberry S, et al. Safety of vaccines used for routine immunization of U.S. children: a systematic review. Pediatrics 2014;134:325–37. [DOI] [PubMed] [Google Scholar]
- [3].Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics 2010;126:e1-8. [DOI] [PubMed] [Google Scholar]
- [4].Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM, Group VSDRCAIW. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine 2012;30:2024–31. [DOI] [PubMed] [Google Scholar]
- [5].Stockwell MS, Broder K, LaRussa P, Lewis P, Fernandez N, Sharma D, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr 2014;168:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Duffy J, Weintraub E, Hambidge SJ, Jackson LA, Kharbanda EO, Klein NP, et al. Febrile Seizure Risk After Vaccination in Children 6 to 23 Months. Pediatrics 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jones T, Jacobsen SJ. Childhood febrile seizures: overview and implications. Int J Med Sci 2007;4:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? A systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neurol 2013;17:585–8. [DOI] [PubMed] [Google Scholar]
- [9].Jackson LA, Peterson D, Dunn J, Hambidge SJ, Dunstan M, Starkovich P, et al. A randomized placebo-controlled trial of acetaminophen for prevention of post-vaccination fever in infants. PLoS One 2011;6:e20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rose MA, Juergens C, Schmoele-Thoma B, Gruber WC, Baker S, Zielen S. An open-label randomized clinical trial of prophylactic paracetamol coadministered with 7-valent pneumococcal conjugate vaccine and hexavalent diphtheria toxoid, tetanus toxoid, 3-component acellular pertussis, hepatitis B, inactivated poliovirus, and Haemophilus influenzae type b vaccine. BMC Pediatr 2013;13:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 2009;374:1339–50. [DOI] [PubMed] [Google Scholar]
- [12].Kroger AT, Duchin J, Vazquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP) 2017. [Google Scholar]
- [13].Andrews NJ, Walker WT, Finn A, Heath PT, Collinson AC, Pollard AJ, et al. Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and non-adjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. Vaccine 2011;29:7913–9. [DOI] [PubMed] [Google Scholar]
- [14].Aoki FY, Yassi A, Cheang M, Murdzak C, Hammond GW, Sekla LH, et al. Effects of acetaminophen on adverse effects of influenza vaccination in health care workers. CMAJ 1993;149:1425–30. [PMC free article] [PubMed] [Google Scholar]
- [15].Gross PA, Levandowski RA, Russo C, Weksler M, Bonelli J, Dran S, et al. Vaccine immune response and side effects with the use of acetaminophen with influenza vaccine. Clin Diagn Lab Immunol 1994;1:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chernesky M, O’Neill D, Pickard L, Castriciano S, Kraftcheck D, Sellors J, et al. Immunogenicity and adverse reactions of influenza vaccination in elderly patients given acetaminophen or placebo. Clin Diagn Virol 1993;1:129–36. [DOI] [PubMed] [Google Scholar]
- [17].Centers for Disease C, Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013–2014. MMWR Recomm Rep 2013;62:1–43. [PubMed] [Google Scholar]
- [18].Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- [19].Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep 2015;64:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Recommended composition of influenza virus vaccines for use in the 2013–2014 northern hemisphere influenza season. Wkly Epidemiol Rec 2013;88:101–14. [PubMed] [Google Scholar]
- [21].Recommended composition of influenza virus vaccines for use in the 2014–2015 northern hemisphere influenza season. Wkly Epidemiol Rec 2014;89:93–104. [PubMed] [Google Scholar]
- [22].Recommended composition of influenza virus vaccines for use in the 2015–2016 northern hemisphere influenza season. Wkly Epidemiol Rec 2015;90:97–108. [PubMed] [Google Scholar]
- [23].Administration FaD. Investigational New Drug Application. In: Administration FaD, editor. 212016. [Google Scholar]
- [24].Cottey R, Rowe CA, Bender BS. Influenza virus. Curr Protoc Immunol 2001;Chapter 19:Unit 19 1. [DOI] [PubMed] [Google Scholar]
- [25].Hsiung GD, Fong CKY, Landry ML, Hsiung GD. Hsiung’s diagnostic virology : as illustrated by light and electron microscopy 4th ed. New Haven: Yale University Press; 1994. [Google Scholar]
- [26].Kendal APPM, Skehel J. Concepts and Procedures for Laboratory Based Influenza Surveillance 1982;Geneva: World Heatlh Organization. [Google Scholar]
- [27].Webster RCN, Stohr K. WHO Manual on Animal Influenza Diagnosis and Surveillance World Health Organization. 2002. [Google Scholar]
- [28].Goodman LS, Brunton LL, Chabner B, Knollmann BrC. Goodman & Gilman’s pharmacological basis of therapeutics 12th ed. New York: McGraw-Hill; 2011. [Google Scholar]
- [29].Reynolds M, Bonham L, Gueck M, Hammond K, Lowery J, Redel C, et al. Are temporal artery temperatures accurate enough to replace rectal temperature measurement in pediatric ED patients? J Emerg Nurs 2014;40:46–50. [DOI] [PubMed] [Google Scholar]
- [30].Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics 2005;115:1039–47. [DOI] [PubMed] [Google Scholar]
- [31].Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290:1608–16. [DOI] [PubMed] [Google Scholar]
- [32].Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. J Infect Dis 2006;194:1032–9. [DOI] [PubMed] [Google Scholar]


