Summary:
The aim of this study was to differentiate normal and scarred hamster cheek pouch samples by applying a quantitative image analysis technique for determining collagen fiber direction and density in second-harmonic generation microscopy images. This paper presents a collagen tissue analysis of scarred cheek pouches of four adult male Golden Syrian hamsters as an animal model for vocal fold scarring. One cheek pouch was scarred using an electrocautery unit and the other cheek was used as a control for each hamster. A home-built upright microscope and a compact ultrafast fiber laser were used to acquire depth resolved epi-collected second-harmonic generation images of collagen fibers. To quantify the average fiber direction and fiber density in each image, we applied two-dimensional Fourier analysis and intensity thresholding at five different locations for each control and scarred tissue sample, respectively. The resultant depth-resolved average fiber direction variance for scarred hamster cheek pouches (0.61 ± 0.03) was significantly lower (p < 0.05) than control tissue (0.73 ± 0.04), indicating increased fiber alignment within the scar. Depth-resolved average voxel density measurements indicated scarred tissues contained greater (p < 0.005) fiber density (0.72 ± 0.09) compared to controls (0.18 ± 0.03). In the present study, image analysis of both fiber alignment and density from depth-resolved second-harmonic generation images in epi-detection mode enabled the quantification of the increased collagen fiber deposition and alignment typically observed in fibrosis. The epi-detection geometry is the only viable method for in vivo imaging as well as imaging thick turbid tissues. These quantitative endpoints, clearly differentiating between control and scarred hamster cheek pouches, provide an objective means to characterize the extent of vocal fold scarring in vivo in preclinical and clinical research. In particular, this non-invasive method offers advantages for monitoring scar treatments in live animals and following the effects of scarring-related treatments such as application of steroids or drugs targeting pathways involved in fibrosis.
Keywords: hamster cheek pouch, vocal fold scarring, collagen fiber alignment, collagen fiber density, second-harmonic generation microscopy, scar remodeling, image analysis, Fourier transform, ultrafast fiber lasers
Introduction
Vocal fold scarring is one of the predominant causes of voice disorders, affecting an estimated 2–6 million people in the United State alone (Ramig and Verdolini, ’98; Roy et al., 2004, 2005; Best and Fakhry, 2011; Cohen et al., 2012; Bhattacharyya, 2014). Vocal fold scarring arises as a wound healing response to injury or inflammation and results in collagenous scar tissue which impairs vibration (Rousseau et al., 2004; Tateya et al., 2005, 2006; Hirano et al., 2009) by reducing the viscoelasticity of the vocal fold. During the wound healing response, scar tissue can replace the superficial lamina propria (SLP) along with deeper parts of the lamina propria (LP). The scar tissue predominantly consists of collagen and fibronectin, both of which increase the stiffness of the mucosa and can lead to severe impairment in voice production or dysphonia (Hirano, 2005). Unfortunately, current methods for treating vocal fold scarring are inconsistent and frequently ineffective, and there is currently no accepted treatment for restoring phonation to scarred vocal folds (Woo et al., ’94; Benninger et al., ’96; Kriesel et al., 2002; Thibeault et al., 2002; Zeitels and Healy, 2003; Hirano, 2005; Hansen and Thibeault, 2006; Zeitels et al., 2007).
In scarred vocal folds, collagen fibers are found to be more organized and densely packed compared to normal vocal folds (Rousseau et al., 2003, 2004; Yildirim et al., 2013; Heris et al., 2015). The collagen fiber orientation and density are important in determining biomechanical properties of biological tissues (Provenzano et al., 2006; Thomopoulos et al., 2006; Hadian et al., 2007; Bayan et al., 2009; Robinson and Tranquillo, 2009). The gold standard method to determine collagen fiber density and direction is to stain tissue sections with picrosirius red or Masson’s trichrome. This analysis requires complex and destructive sample preparation to stain collagen fibers, and analysis is typically based on subjective scoring systems, which limits comparisons among different studies. Thus, there is a need to develop a nondestructive technique to resolve collagen fibers in three-dimensional (3D) tissues and automatically quantify fiber density and directionality to provide objective endpoints for evaluating the treatment of vocal fold scarring.
The hamster cheek pouch model has been widely used as a model for mucosal disease processes such as carcinogenesis (White et al., ’81; Kingsbury et al., ’97; Adams et al., 2000; Driver et al., 2005; Meier et al., 2007; Vairaktaris et al., 2008). Like the vocal fold mucosa, there is a squamous epithelium, an underlying lamina propria (which is thinner than specialized vocal fold lamina propria) and then a thin layer of striated muscle. The layered structure is analogous to the vocal fold although the thickness of the constituent layers is thinner in the cheek pouch. The cheek pouch is much more accessible and has a larger area than the vocal fold. While the surface area of one vocal fold is a few mm2 in laboratory rodents, the hamster cheek pouch area that can be everted through the mouth is a fold of mucosa that is about 100 mm2 on each side. Thus, the size of the cheek pouch, the fact that it can be everted for treatment or imaging, and the analogous anatomical organization of the tissue layers are the main advantages of this model. Using this model, one could track the development of scar in vivo or follow the effects of scarring-related treatments such as application of steroids or drugs targeting pathways involved in fibrosis.
Multi-photon nonlinear imaging microscopies such as two-photon autofluorescence microscopy (TPAF) and second-harmonic generation (SHG) microscopy can perform noninvasive and 3D deep tissue imaging with subcellular resolution using tightly focused ultrashort pulses (Zipfel et al., 2003a,b). Second-harmonic generation microscopy is a coherent two-photon process necessitating intense ultrashort laser pulses passing through a highly polarizable material with a non-centrosymmetric molecular organization, such as collagen fibers (Campagnola and Loew, 2003). Second-harmonic generation microscopy has shown to be a highly functional and noninvasive tool to obtain 3-D resolved collagen distribution and density in fibrotic tissues (Strupler et al., 2007), human skin (Cicchi et al., 2008), human cadaver vocal folds (Miri et al., 2012), porcine vocal folds (Yildirim et al., 2013), and rat vocal folds (Heris et al., 2015).
Different computational methods have been suggested to quantify the average fiber direction and fiber density in an image obtained by different imaging modalities. The Fourier transform is most commonly applied to compute the two-dimensional (2D) power spectral density (PSD) of an image (Levitt et al., 2007; Ayres et al., 2008; Sander et al., 2009; Cicchi et al., 2010; Sivaguru et al., 2010). Sampling the average power of the PSD at pixels corresponding to different angles can yield an accurate measure of the total fiber orientation distribution in the image (Bayan et al., 2009). More complex fiber orientation measurements also have been demonstrated, such as defining and tracking fiber objects through energy minimization or line propagation algorithms (Mori and Van Zijl, 2002; Rodriguez et al., 2009; Bas and Erdogmus, 2010). Also, a set of zero-crossing maps was generated to form a stability map from which significant linear patterns (fiber directions) were detected (Liu, ’91; Heris et al., 2015). In terms of the fiber density, the common method is to set a threshold intensity manually for forming a binary image and to count the number of pixels whose intensities are equal to 1 (Strupler et al., 2007; Medyukhina et al., 2011; Heris et al., 2015). In addition to determine collagen fiber direction and density, geometrical organization (Medyukhina et al., 2011), and geometrical properties (Cicchi et al., 2010) of the collagen fibers were determined to differentiate normal and scarred tissue samples.
In a parallel effort, we have developed an algorithm to rapidly and accurately detect fiber orientation (Quinn and Georgakoudi, 2013) and applied it to quantify fiber organization in cutaneous scar tissue (Quinn et al., 2015). For the fiber density calculations, we select appropriate intensity thresholds determined by the Otsu’s method, which calculates the optimal threshold intensity by dividing the signal and background values so that their combined variance is minimal (Otsu, ’79; Provenzano et al., 2006; D’Amore et al., 2010). Thus, our method is less susceptible to uncertainties in collagen fiber density measurement due to manually determined intensity threshold values, which have been commonly used, in previous studies (Strupler et al., 2007; Medyukhina et al., 2011; Heris et al., 2015).
The aim of the present work is to compare control and scarred hamster cheek pouch samples by quantifying collagen direction and density with an automated SHG image analysis technique to see if this method can differentiate between these two tissue states. This technique is based on taking the Fourier transform (FT) and calculating the 2D power spectral density of depth-resolved SHG images to determine collagen fiber direction. Additionally, our technique utilizes Otsu method for automatic intensity thresholding of the SHG signal to calculate any differences in collagen fiber density. Overall, this work illustrates the feasibility of utilizing SHG imaging and automated image analysis techniques as pragmatic approaches easily applicable in vivo to non-invasively quantify collagen organization in scarred vocal folds and guide surgical interventions.
Materials and Methods
Experimental Setup
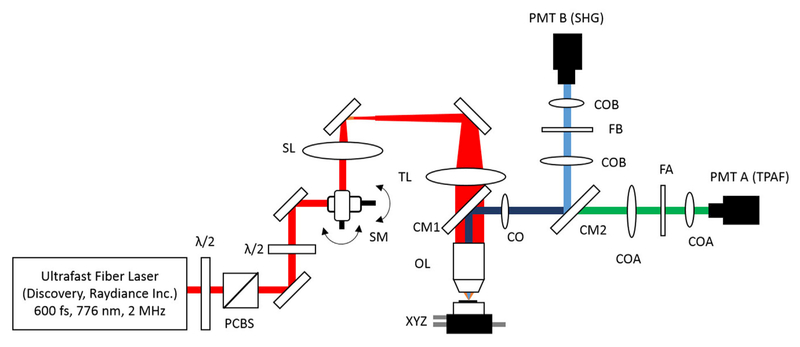
We used our home-built, upright laser-scanning microscope to perform second-harmonic generation (SHG) imaging (Fig. 1) using an ultrafast Er-doped fiber laser (Discovery, Raydiance Inc.). This laser provides 3 W average power at 1,552 nm (1.5 μJ pulse energy) and 1 W when frequency doubled to 776 nm (0.5 μJ pulse energy) at 2 MHz with a 600 fs pulse width. Since the number of photons generated in two-photon based microscopies is inversely proportional with duty cycle (multiplication of repetition rate and pulse width), moderate repetition rates in the range of 1–10 MHz are optimal for SHG imaging with reasonable imaging speeds (Wang et al., 2014). Thus, having 2 MHz repetition rate enabled us to image using approximately an order of magnitude less average power compared to those studies using standard high repetition rate Ti: sapphire lasers (Heris et al., 2015).
Fig. 1.
Schematic of the upright microscope system for nonlinear imaging. (a) Ultrafast laser pulses at 776 nm from a compact fiber laser system are attenuated using a combination of half-wave plate (λ/2) and polarizing cube beam splitter (PCBS). Another half-wave plate is used to adjust the polarization state of the laser. Laser pulses are scanned by a pair of galvanometric scanning mirrors (SM), which is imaged on the back aperture of the 0.75 NA, 20× objective by a pair of scan lens (SL) and tube lens (TL). The samples are placed on a three-axis motorized stage (XYZ) for nonlinear imaging. Emitted light (either TPAF or SHG signal) is collected by a cold mirror (CM1) and collection optics (CO). A second cold mirror (CM2) separates SHG and TPAF signals into different collection paths.
We focused 776 nm excitation wavelength with a 0.75-NA, 20× air objective (Nikon Plan Apo) to perform SHG imaging in epi-detection mode. The epi-detection geometry is the only viable method for in vivo imaging as well as imaging thick turbid tissues. Thus, the same objective was used for excitation and collection. By collecting in the epi-direction, from a thick turbid tissue, our signal was likely a combination of backward SHG and backscattered SHG, and thus lacked any sensitivity to the directionality of the SHG signal. We compensated the laser power attenuation due to absorption and scattering coefficients of the samples, as we imaged deeper in the tissue. The laser power was adjusted at each depth according to the optical properties of specific tissue type and its SHG signal histogram in a way that only 0.1% of overall pixels were saturated in each image.
Emitted light is collected by a cold mirror (CM1–HT-1.00, CVI Laser, Carlsbad, CA) and collection optics (CO) placed right behind the objective. A second cold mirror (CM2–Di01- R405, Semrock, Lake Forest, IL) separates the SHG and two-photon autofluorescence (TPAF) signals into different collection paths. We collect the TPAF signal through collection optics A (COA) and a laser-blocking filter (FA) into the PMT A (H10770PA-40, Hamamatsu, Japan) and the SHG signal through collection optics B (COB) and a laser-blocking filter B (FB) into the PMT B (R3896, Hamamatsu, Japan). In our experiments, we adjusted linear polarization angle of the incoming laser beam with using another half wave plate (HWP) so that we obtained maximum SHG signal for each sample to maximize our sensitivity to collagen fiber density. Further details of the experimental setup can be found in our previous study (Yildirim et al., 2013).
To measure the resolution of our system, we suspended 100 nm fluorescent beads (Invitrogen, F8803) in agar gel which replicated the expected tissue scattering lengths (30–35 μm), and measured the point spread function (PSF) at imaging depths ranging from 50 to 500 μm. The average lateral and axial full width half maximum (FWHM) of the two-photon PSF were 0.58 ± 0.07 μm and 2.51 ± 0.35 μm, respectively. We scanned the laser beam in the x- and y-axes using a pair of galvanometric mirrors (Cambridge Technologies, Inc.) and swept the focal spot over a 150 × 150 μm2 field of view (FOV) for 3.3 s to take 10 images of the same FOV. The axial displacement between consecutive SHG images was 2 μm, and the imaging depth was increased until the signal to background ratio (SBR) became 1. The maximum SHG imaging depth was approximately 120 μm for all control and scarred tissue samples, reaching the theoretical maximum imaging depths as will be discussed below. The computer software (MPScan) was used to reconstruct these signals into 512 × 512 pixel images at 3.05 frame per second (fps) with pixel dwell time of 1.25 μs. Since the laser repetition rate was 2 MHz, each pixel received at least two laser pulses. We therefore averaged 10 images for each plane to smoothen pixel-to-pixel fluctuations.
Automated SHG Image Analysis Method
Collagen fiber direction in the SHG images was determined in a similar manner to previous Fourier-based analysis approaches (Bayan et al., 2009; Sander and Barocas, 2009; Quinn and Georgakoudi, 2013). Each 8-bits, gray scale image is composed of pixels that vary spatially in intensity. Rapid changes in intensity are indicative of object edges and thus fiber orientation can be quantitatively analyzed through a 2D Fourier transformation of the image into frequency space. Prior to transformation, SHG images were apodized using a Hann window to eliminate discontinuities at the edges of the image. A power spectral density (PSD) was computed from real and imaginary parts of the 2D discrete Fourier transform and provided the relative magnitudes of the underlying frequency components of the image. Rapid changes in intensity across an image at a given orientation are reflected by greater power density values in the orthogonal direction in Fourier space. Because the PSD map is symmetrical about the origin located in the center of the map, we assigned each PSD pixel location with polar coordinates relative to the origin. Average PSD values were computed in discrete increments of 1˚ within spatial frequencies ranging from 0.05 to 0.5 pixels−1 to obtain orientation distribution histograms for each image. The average collagen fiber direction and directional variance were calculated from the orientation distribution of each image through vector addition (Sander and Barocas, 2009). Directional variance values range from 0 (perfectly aligned fibers) to 1 (isotropic/random organization). Thus, our method could estimate the level of isotropy of collagen fibers independent of their absolute direction which may be affected by the laser polarization.
Collagen fiber density in the SHG images was obtained by computing the number of pixels with intensities exceeding an optimum threshold intensity relative to the total number of pixels. To automate the determination of this optimum threshold intensity, we used Otsu’s method (Otsu, ’79). Otsu’s method is a nonparametric and unsupervised method for automatic threshold selection for 2-D images using the zeroth- and the first-order cumulative moments of the gray-level histograms.
To perform a reliable image analysis in terms of the selections of the region of interest (ROI) without any bias, we chose five different locations in a 1.7 mm2 region for all control and scarred samples. Each ROI consisted of 150 × 150 × 120 μm3 tissue block. To reduce bias in the selection of the ROI, the center of the first tissue block location was randomly selected and the center of other four ROIs were selected 1 mm apart from the center of the first tissue block in four directions (east, west, north, and south) (Yildirim et al., 2013). For each tissue block, average fiber direction and density results were calculated by averaging corresponding 2-D results over the whole tissue block between depths where collagen fibers first appeared and signal to background ratio became one. Then, we averaged all five tissue block results to calculate overall fiber direction and density for each control and scarred sample.
Animal Model
We used four adult male Golden Syrian hamsters (Charles River Labs, Wilmington, MA) of 100 to 120 g body weight, in which scars could be created within an easily accessible mucosal surface. To scar the cheek pouches, we first anesthetized the animals by injecting a mixture of ketamine (200 mg/kg) and xylazine (8 mg/kg) intraperitoneally. We then cauterized 5–10 mm diameter circular areas of one cheek pouch with an electrocautery unit (Conmed Saber 2400, Utica, NY). The hamsters were euthanized after a survival period of 1 month using 0.5 mL intraperitoneal euthasol. The scarred and contralateral normal cheek pouches were removed and mounted on rubber test-tube stoppers with fine needles to hold the cheek pouch mucosa flat. After rinsing with saline, the tissue was frozen in isopentane and cooled in liquid nitrogen. The cheek pouch tissue was prepared at Massachusetts General Hospital Voice Laboratory (MGH) in Boston and shipped on dry ice to the Ben-Yakar Laboratory at the University of Texas at Austin for bench-top testing. After delivery, the cheek pouches were stored at −80˚C. For each experiment, we thawed the cheek pouches in saline solution and covered them with a glass cover slip to flatten their epithelial surface to compensate aberrations with the objective. To ensure the proper identification of the tissue surface with TPAF, 5 μL of a solution of 100 nm fluorescent beads (F-8823, Invitrogen) in saline was deposited onto the tissue surface prior to placement of the cover slip. All experimentation followed an animal use protocol that was approved by the Committee on Animal Care and the Institutional Animal Care and Use Committee of the Massachusetts General Hospital and The University of Texas at Austin. All surgical procedures and housing of animals took place at the Massachusetts General Hospital.
Statistical Analysis
Paired Student’s t-tests were used to compare the collagen fiber directional variance and density between scarred and controlled cheek pouches with α = 0.05.
Results
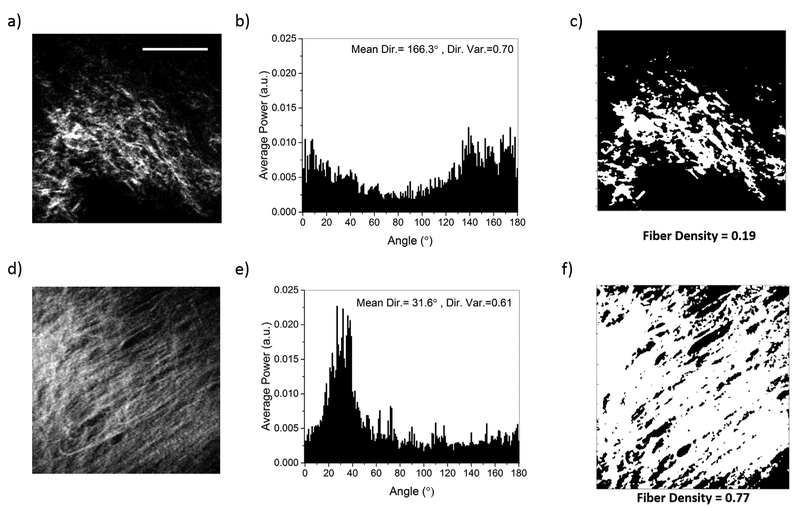
Figure 2 represents SHG nonlinear images used to characterize the direction and density of collagen fibers in normal and scarred hamster cheek pouches. Qualitative interpretation of the SHG images of normal (Fig. 2a) and scarred (Fig. 2d) tissue samples revealed that collagen fibers were denser and more aligned in scarred samples than control samples. We performed a quantitative analysis for collagen fiber direction and density using the methods described above. In a representative example, the FT results showed that scarred tissue sample (Fig. 2e) had a directional variance of 0.61 and the amplitude of the dominant direction was at least fourfold greater than other directional angles. On the other hand, control tissue sample (Fig. 2b) had a higher variance of 0.70 and more importantly the amplitude of the dominant direction was comparable to most of the other directional angles. After determining fiber direction in the SHG images, we performed automated intensity thresholding to obtain the collagen fiber density. A representative example of this analysis showed that the fiber density in the scarred tissue sample (Fig. 2f) was fourfold higher than the fiber density in the control tissue sample (Fig. 2c).
Fig. 2.
Representative SHG images, collagen fiber direction histograms, and collagen fiber density plots for control (a–c) and scarred (d–f) hamster cheek pouch samples. The scale bar represents 50 μm.
To reveal the distribution of collagen fiber direction as a function of imaging depth, we report a depth-resolved plot of collagen fiber orientation for the normal and scarred tissue samples discussed above in Figure 3. Here, the corresponding average power is also included as a color bar on the right hand side of the figure. The normal tissue sample (Fig. 3a) had no dominant fiber direction for the entire 120 μm imaging depth. On the other hand, the scarred tissue sample (Fig. 3b) showed significant fiber alignment between 40 and 100 μm imaging depth. The average fiber direction was 130 ± 5˚ which remained approximately constant in the range of 40–100 μm imaging depth. As expected collagen fibers did not appear in the epithelial layer of the tissue between 0 and 40 μm depths. Beyond 120 μm, the SHG imaging quality degraded substantially as the SBR approached to 1.
Fig. 3.
Representative results for the calculations of depth-resolved collagen fiber directions for control (a) and scarred (b) hamster cheek pouch samples.
To evaluate the utility of these quantitative metrics and understand inter- and intra-sample variations in fiber density and direction, we performed depth-resolved fiber direction and fiber density analysis at five different locations with four different normal and scarred tissue samples as shown in Figure 4. The collagen fiber directional variance (p < 0.005 for Samples 1, 3, and 4 and p < 0.05 for Sample 2) was lower in scarred tissues than control ones, indicating all scarred tissue samples had more aligned collagen fibers than the controls (Fig. 4a). In addition, the fiber densities in scarred tissues were three to fivefolds higher (p < 0.005) than control tissues for all four samples (Fig. 4b). Overall, both fiber direction and fiber density analysis revealed a statistically significant difference between control and scarred tissue samples.
Fig. 4.
Collagen fiber directional variance and fiber density for four different samples as averaged at five different locations per sample and for 30–35 axial planes for each location. (a) In all samples, directional variance was lower in scarred samples than control samples in a statistically significant manner. The resultant average fiber direction variance was 0.73 ± 0.04 for control and 0.61 ± 0.03 for scarred hamster cheek pouch tissues. (b) Collagen fiber density was three to fivefolds higher in scarred samples than in control samples. The resultant average fiber density was 0.18 ± 0.03 for control and 0.72 ± 0.09 for scarred hamster cheek pouch tissues. The error bars in the plots represent the mean standard deviation for each sample as averaged over 150 FOVs. (**p < 0.005, *p < 0.05).
Discussion
The development and evaluation of effective treatments necessitate understanding and objectively characterizing structural changes during vocal fold scarring. Density and organization of the extracellular matrix (ECM) components determine the biomechanical function of the lamina propria (Billiar and Sacks, 2000; Sander et al., 2009). Several previous studies have addressed the orientation and density of collagen fibers in the lamina propria in relation to vocal fold scarring. In some of these studies, collagen density has been determined by performing immunohistology and non-linear imaging. These studies have shown that collagen became thicker, more organized, and denser (two to fivefolds) in mature scarred vocal folds (Rousseau et al., 2003, 2004; Yildirim et al., 2013). Early stages of vocal fold scarring, on the other hand, showed low or high density and disorganized collagen fibers (Thibeault et al., 2002; Rousseau et al., 2003). These differences in collagen density among previous studies may be attributed to the fact that there is a different timeline for remodeling stages of vocal fold scarring where there is an ongoing process of collagen synthesis. Similarly, wound-healing studies suggest that as remodeling progresses (20 days to 1 year), collagen fibers become thicker and more organized (parallel arrays) with eventual crosslinking (Ehrlich, 2000).
Unlike collagen fiber density, fiber direction has not been thoroughly evaluated in previous vocal fold scarring studies. One recent study, however, characterized the microstructure of scarred rat vocal folds using forward collected SHG microscopy of thin slices and found the dispersion of the collagen fiber directionality to be lower than the one in uninjured control tissues (Heris et al., 2015). Similarly, in our study, the average fiber directional variance values were significantly lower (p < 0.05) in scarred tissues (0.61 ± 0.03) compared to control ones (0.73 ± 0.04), indicating increased fiber alignment in the scar. Lower fiber directional variance values in scar tissues are also consistent with other measurements in mature scars from a rat model of cutaneous burns (Quinn et al., 2015).
In general, intensity threshold based techniques to calculate collagen fiber density, depend on the chosen intensity threshold. To minimize the effects of intensity threshold, our approach counts all the collagen pixels above a certain threshold rather than taking the mean intensity of all those pixels. Therefore, it is less susceptible to intensity variations among fibers related to their fibril organization. Furthermore, we adjusted the laser excitation power to maximize the SHG signal with saturating 0.1 % of the pixels and to compensate for loses due the optical properties of samples at each depth. This approach consequently reduced the effect of intensity variations due to scattering and absorption in our calculations. While forward to backward ratio and polarization-sensitive SHG methods can provide additional context regarding the molecular organization within fibers, these are however time consuming measurements and difficult to perform in vivo and 3D thick tissues. Most frequently, they are performed on thin sections or ex vivo samples. With epi-detected SHG imaging method, we offer a more pragmatic approach that is easily applicable in vivo.
Since routine histology is a destructive method requiring complex processing steps, SHG microscopy is a valuable alternative to non-invasively determine collagen fiber direction and density for assessing the progression of vocal fold scarring. The analysis of SHG microscopy has the ability to provide results that are consistent with the histological evaluation of tissues. In our previous work, for example, we found that the scarred tissue samples showed regular and dense pattern of collagen fibers as compared to control samples when they were stained with Masson–Trichrome (Yildirim et al., 2013). This high directional and high density collagen fiber trend was consistent in both histology and SHG microscopy results for all four samples. When combined with the presented analysis metrics, SHG imaging could provide a means to non-destructively monitor vocal fold scarring in research studies and, given future development of endoscopic probes, in the clinic.
Nonlinear imaging modalities such as TPAF and SHG are well suited for high-resolution in vivo imaging. Second-harmonic generation microscopy specifically resolves collagen fibers because of their non-centro-symmetric characteristics. On the other hand, both elastin and collagen fibers in the extracellular matrix autofluoresce, reducing the specificity of TPAF for collagen fibers. Since SHG is an inherent nonlinear imaging modality, it can provide deep tissue imaging of unstained tissues. SHG microscopy provided approximately 120 μm imaging depth within hamster cheek pouches in this study. The maximum imaging depth in SHG microscopy is highly dependent on the optical properties of tissues. It has been shown that the maximum imaging depth for TPAF can vary between 3–4 scattering lengths near 800 nm excitation wave-lengths for different tissue types (Theer and Denk, 2006; Durr et al., 2011). Since the scattering length of hamster cheek pouches has been found to be 30–35 μm at 776 nm wavelength (Yildirim et al., 2013), the maximum imaging depth should be in the 100–150 μm range, corresponding well with our experimental results. Thus, SHG microscopy is a good candidate for clinical evaluation of scarring in the superficial lamina propria of the vocal fold. Even though optical coherence tomography (OCT) has been used in the clinical setting, there remains a need for new tools such as SHG microscopy with higher specificity to collagen, and finer resolution to discriminate individual fibers within the scar.
On the other hand, SHG has a fundamental imaging depth limit due to gradually increasing out-of-focus (background) signal when increasing imaging depth to compensate for the losses due to scattering. Recently, the growing interest in performing high-resolution, deep tissue imaging has galvanized the use of longer excitation wavelengths and three-photon based techniques to improve maximum imaging depth of SHG. Our group showed that the maximum imaging depth in porcine vocal folds can be improved by three times by performing third-harmonic generation (THG) microscopy at 1552 nm excitation wavelength compared to SHG microscopy at 776 nm wavelength (Yildirim et al., 2015). While providing the ability to image at depths beyond 400 μm, THG signal can originate from both collagen and elastin fibers and thus provides a limited specificity to extract 3-D collagen fiber orientation.
To summarize, we utilized Fourier-based orientation analysis and intensity thresholding to determine collagen fiber direction and density, respectively, in 3D SHG image stacks of control and scarred hamster cheek pouches. Our results are promising in that both fiber direction variance (p < 0.05) and fiber density (p < 0.005) are significantly different between control and scarred hamster cheek pouches. We believe that this automatized SHG image analysis method can provide significant feedback on fiber density and alignment in normal, scarred, and surgically or medically treated vocal folds. Also, one could test scar treatments such as biomaterials, drugs or growth factors which will eventually be used to measure treatment outcome in humans.
Conclusion
The aim of this study was to develop an automatized SHG image analysis method to differentiate normal and scarred hamster cheek pouch samples by quantifying collagen fiber direction and density. We utilized Fourier-based methods and intensity thresholding to determine collagen fiber directional variance and density, respectively. The resultant depth-resolved average fiber direction variance was 0.73 ± 0.04 for control and 0.61 ± 0.03 for scarred hamster cheek pouches. This analysis showed that the fiber direction in scarred samples was less random than in control samples. Depth-resolved collagen fiber density was 0.18 ± 0.03 for control and 0.72 ± 0.09 for scarred tissues, respectively. This fourfold difference in collagen fiber density demonstrated, as expected, that the average fiber densities in scarred tissues were remarkably higher than that in control tissues. The method we presented here will guide development of turn-key ultrafast fiber laser-assisted treatment methods for sub-epithelial image guided surgeries, similar to proposed scarred vocal folds treatments currently under development in our lab (Hoy et al., 2008, 2011, 2012, 2014; Yildirim et al., 2013; Ferhanoglu et al., 2014).
Acknowledgments
The authors would like to thank Raydiance Inc., for the use of their Discovery fiber laser, Kaushik Subramanian and Chris Martin for editing the text, and Dr. David Kleinfeld and his research group for the use of the MPScan.
Contract grant sponsor: National Science Foundation; Contract grant number: (IDR: CBET-1014953 and Career Award: CBET-0846868); Contract grant sponsor: Cancer Prevention Research Institute of Texas (CPRIT); Contract grant number: RP130412; Contract grant sponsor: National Institutes of Health; Contract grant number: K99EB017723.
Footnotes
Conflicts of interest: There is no conflict of interest between the authors.
This work was performed at the Mechanical Engineering Department of The University of Texas at Austin.
References
- Adams J, Heintz P, Gross N, et al. 2000. Acid/pepsin promotion of carcinogenesis in the hamster cheek pouch. Arch Otolaryngol Head Neck Surg 126:405–409. [DOI] [PubMed] [Google Scholar]
- Ayres CE, Jha BS, Meredith H, et al. 2008. Measuring fiber alignment in electrospun scaffolds: a user’s guide to the 2D fast Fourier transform approach. J Biomater Sci Polym Ed 19:603–621. [DOI] [PubMed] [Google Scholar]
- Bas E, Erdogmus D. 2010. Piecewise linear cylinder models for 3-dimensional axon segmentation in brainbow imagery I S Biomed Imaging, IEEE: New York: p 1297–1300. [Google Scholar]
- Bayan C, Levitt JM, Miller E, Kaplan D, Georgakoudi I. 2009. Fully automated, quantitative, noninvasive assessment of collagen fiber content and organization in thick collagen gels. J Appl Phys 105:102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger MS, Alessi D, Archer S, et al. 1996. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg 115:474–482. [DOI] [PubMed] [Google Scholar]
- Best SR, Fakhry C. 2011. The prevalence, diagnosis, and management of voice disorders in a National Ambulatory Medical Care Survey (NAMCS) cohort. Laryngoscope 121: 150–157. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya N 2014. The prevalence of voice problems among adults in the United States. Laryngoscope 124: 2359–2362. [DOI] [PubMed] [Google Scholar]
- Billiar KL, Sacks MS. 2000. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: part II-a structural constitutive model. J Biomech Eng 122: 327–335. [DOI] [PubMed] [Google Scholar]
- Campagnola PJ, Loew LM. 2003. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol 21:1356–1360. [DOI] [PubMed] [Google Scholar]
- Cicchi R, Sestini S, De Giorgi V, et al. 2008. Nonlinear laser imaging of skin lesions. J Biophotonics 1:62–73. [DOI] [PubMed] [Google Scholar]
- Cicchi R, Kapsokalyvas D, De Giorgi V, et al. 2010. Scoring of collagen organization in healthy and diseased human dermis by multiphoton microscopy. J Biophotonics 3: 34–43. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Kim J, Roy N, Asche C, Courey M. 2012. Prevalence and causes of dysphonia in a large treatment-seeking population. Laryngoscope 122:343–348. [DOI] [PubMed] [Google Scholar]
- Driver M, Upadhyay UD, Shapshay SM, Wang Z. 2005. Laser-assisted low-dose retinoic acid in oral cancer chemoprevention. Laryngoscope 115:283–286. [DOI] [PubMed] [Google Scholar]
- Durr NJ, Weisspfennig CT, Holfeld BA, Ben-Yakar A. 2011. Maximum imaging depth of two-photon autofluorescence microscopy in epithelial tissues. J Biomed Opt 16:026008–026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amore A, Stelia JA, Wagner WR, Sacks MS. 2010. Characterization of the complete fiber network topology of planar fibrous tissues and scaffolds. Biomaterials 31: 5345–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP. 2000. Collagen considerations in scarring and regenerative repair. Basic Clin Dermatol 19:99–114. [Google Scholar]
- Ferhanoglu O, Yildirim M, Subramanian K, Ben-Yakar A. 2014. A 5-mm piezo-scanning fiber device for high speed ultrafast laser microsurgery. Biomed Opt Express 5: 2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadian M, Corcoran BM, Han RI, Grossmann JG, Bradshaw JP. 2007. Collagen organization in canine myxomatous mitral valve disease: an X-ray diffraction study. Biophys J 93: 2472–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JK, Thibeault SL. 2006. Current understanding and review of the literature: vocal fold scarring. J Voice 20: 110–120. [DOI] [PubMed] [Google Scholar]
- Heris HK, Miri AK, Ghattamaneni NR, et al. 2015. Microstructural and mechanical characterization of scarred vocal folds. J Biomech 48:708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Minamiguchi S, Yamashita M, et al. 2009. Histologic characterization of human scarred vocal folds. J Voice 23: 399–407. [DOI] [PubMed] [Google Scholar]
- Hirano S 2005. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg 13:143–147. [DOI] [PubMed] [Google Scholar]
- Hoy CL, Everett WN, Yildirim M, et al. 2012. Towards endoscopic ultrafast laser microsurgery of vocal folds. J Biomed Opt 17:0380021–0380028. [DOI] [PubMed] [Google Scholar]
- Hoy CL, Ferhanoglu O, Yildirim M, et al. 2014. Clinical ultrafast laser surgery: recent advances and future directions. IEEE J Sel Top Quant 20:242–255. [Google Scholar]
- Hoy CL, Durr NJ, Chen PY, et al. 2008. Miniaturized probe for femtosecond laser microsurgery and two-photon imaging. Opt Express 16:9996–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy CL, Ferhanoglu O, Yildirim M, et al. 2011. Optical design and imaging performance testing of a 9.6-mm diameter femtosecond laser microsurgery probe. Opt Express 19:10536–10552. [DOI] [PubMed] [Google Scholar]
- Kingsbury JS, Cecere W, Mang TS, Liebow C. 1997. Photodynamic therapy for premalignant lesions in DMBA-treated hamsters: a preliminary study. J Oral Maxillofac Surg 55:376–381. [DOI] [PubMed] [Google Scholar]
- Kriesel KJ, Thiebault SL, Chan RW, et al. 2002. Treatment of vocal fold scarring: rheological and histological measures of homologous collagen matrix. Ann Otol Rhinol Laryngol 111:884–889. [DOI] [PubMed] [Google Scholar]
- Levitt JM, Hunter M, Mujat C, et al. 2007. Diagnostic cellular organization features extracted from autofluorescence images. Opt Lett 33:3305–3307. [DOI] [PubMed] [Google Scholar]
- Liu ZQ. 1991. Scale space approach to directional analysis of images. Appl Opt 30:1369–1373. [DOI] [PubMed] [Google Scholar]
- Medyukhina A, Vogler N, Latka I, et al. 2011. Automated classification of healthy and keloidal collagen patterns based on processing SHG images of human skin. J Biophotonics 4:627–636. [DOI] [PubMed] [Google Scholar]
- Meier JD, Enepekides DJ, Poirier B, et al. 2007. Treatment with 1-alpha,25-dihdroxyvitamin D3 (vitamin D3) to inhibit carcinogenesis in the hamster buccal pouch model. Arch Otolaryngol Head Neck Surg 133:1149–1152. [DOI] [PubMed] [Google Scholar]
- Miri AK, Tripathy U, Mongeau L, Wiseman PW. 2012. Nonlinear laser scanning microscopy of human vocal folds. Laryngoscope 122:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Van Zijl PCM. 2002. Fiber tracking: principles and strategies-a technical review. NMR Biomed 15: 468–480. [DOI] [PubMed] [Google Scholar]
- Otsu NA. 1979. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9:63–66. [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, et al. 2006. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KP, Georgakoudi I. 2013. Rapid quantification of pixel-wise fiber orientation data in micrographs. J Biomed Opt 18:046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KP, Golberg A, Broelsch GF, et al. 2015. An automated image processing method to quantify collgen fibre organization within cutaneous scar tissue. Exp Dermatol 24:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Verdolini K. 1998. Treatment efficacy: voice disorders. J Speech Lang Hear Res 41:S101–S116. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Tranquillo RT. 2009. Planar Biaxial behaviour of fibrin-based tissue-engineered heart valve leaflets. Tissue Eng Part A 15:2763–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Hof BR, Wearne SL. 2009. Three-dimensional neuron tracing by voxel scooping. J Neurosci Meth 184:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau B, Hirano S, Scheidt TD, et al. 2003. Characterization of vocal fold scarring in a canine model. Laryngoscope 113:620–627. [DOI] [PubMed] [Google Scholar]
- Rousseau B, Hirano S, Chan RW, et al. 2004. Characterization of chronic vocal fold scarring in a rabbit model. J Voice 18:116–124. [DOI] [PubMed] [Google Scholar]
- Roy N, Merrill RM, Thibeault S, et al. 2004. Prevalence of voice disorders in teachers and the general population. J Speech Lang Hear Res 47:281–293. [DOI] [PubMed] [Google Scholar]
- Roy N, Merrill RM, Gray SD, Smith EM. 2005. Voice disorders in the general population: prevalence, risk factors, and occupational impact. Laryngoscope 115: 1988–1995. [DOI] [PubMed] [Google Scholar]
- Sander EA, Barocas VH. 2009. Comparison of 2D fiber network orientation measurement methods. J Biomed Mater Res A 88:322–331. [DOI] [PubMed] [Google Scholar]
- Sander EA, Stylianopoulos T, Tranquillo RT, Barocas VH. 2009. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc Natl Acad Sci USA 106: 17675–17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Durgam S, Ambekar R, et al. 2010. Quantitative analysis of collagen fiber organization in injured tendons using Fourier transform-second harmonic generation imaging. Opt Express 18:24983–24993. [DOI] [PubMed] [Google Scholar]
- Strupler M, Pena AM, Hernest M, et al. 2007. Second harmonic imaging and scoring of collagen in fibrotic tissues. Opt Express 15:4054–4065. [DOI] [PubMed] [Google Scholar]
- Tateya T, Tateya I, Sohn JH, Bless DM. 2005. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol 114:183–191. [DOI] [PubMed] [Google Scholar]
- Tateya T, Tateya I, Sohn JH, Bless DM. 2006. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol 115:285–292. [DOI] [PubMed] [Google Scholar]
- Theer P, Denk W. 2006. On the fundamental imaging-depth limit in two-photon microscopy. J Opt Soc Am A Opt Image Sci Vis 23:3139–3149. [DOI] [PubMed] [Google Scholar]
- Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. 2002. Histologic and rheologic characterization of vocal fold scarring. J Voice 16:96–104. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Marquez JP, Wienberger B, Birman V, Genin GM. 2006. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech 39:1842–1851. [DOI] [PubMed] [Google Scholar]
- Vairaktaris E, Spyridonidou S, Papakosta V, et al. 2008. The hamster model of sequential oral oncogenesis. Oral Oncol 44:315–324. [DOI] [PubMed] [Google Scholar]
- Wang K, Horton NG, Charan K, Xu C. 2014. Advanced fiber soliton sources for nonlinear deep tissue imaging in biophotonics. IEEE J Sel Top Quant 20:50–60. [Google Scholar]
- White FH, Gohari K, Smith CJ. 1981. Histological and ultrastructural morphology of 7,12 demethylbenz(alpha)-anthracene carcinogenesis in hamster-cheek pouch epithelium. Diagn Histopathol 4:307–333. [PubMed] [Google Scholar]
- Woo P, Casper J, Colton R, Brewer D. 1994. Diagnosis and treatment of persistent dysphonia after laryngeal surgery: a retrospective analysis of 62 patients. Laryngoscope 104:1084–1091. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Ferhanoglu O, Kobler J, Zeitels SM, Ben-Yakar A. 2013. Parameters affecting ultrafast laser microsurgery of subepithelial voids for scar treatment in vocal folds. J Biomed Opt 18:118001–118001. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Durr NJ, Ben-Yakar A. 2015. Tripling the maximum imaging depth with third-harmonic generation microscopy. J Biomed Opt 20:096013–096013. [DOI] [PubMed] [Google Scholar]
- Zeitels SM, Blitzer A, Hillman RE, Anderson RR. 2007. Foresight in laryngology and laryngeal surgery: a 2020 vision. Ann Otol Rhinol Laryngol Suppl 198:2–16. [DOI] [PubMed] [Google Scholar]
- Zeitels SM, Healy GB. 2003. Laryngology and phonosurgery. N Engl J Med 349:882–892. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW. 2003. a. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21:1368–1376. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, et al. 2003. b. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA 100:7075–7080. [DOI] [PMC free article] [PubMed] [Google Scholar]