Abstract
Induced pluripotent stem cell derived cardiomyocytes (iPSC-CMs) provide a human source of cardiomyocytes for use in cardiovascular research and regenerative medicine. However, attempts to use these cells in vivo have resulted in drastic cell death caused by mechanical, metabolic, and/or exogenous factors. To explore this issue, we designed a Biomimetic Cardiac Tissue Model (BCTM) where various parameters associated with heart function including heart rate, peak-systolic pressure, end-diastolic pressure and volume, end-systolic pressure and volume, and ratio of systole to diastole can all be precisely manipulated to apply hemodynamic loading to culture cells. Using the BCTM, two causes of low survivability in current cardiac stem cell therapies, mechanical and metabolic, were explored. iPSC-CMs were subject to physiologically relevant mechanical loading (50 mmHg systolic, 10% biaxial stretch) in either a low- or high-serum environment and mechanical loads were applied either immediately or gradually. Results confirm that iPSC-CMs subject to mechanical loading in low-serum conditions experienced widespread cell death. The rate of application of stress also played an important role in adaptability to mechanical loading. Under high-serum conditions, iPSC-CMs subject to gradual imposition of stress were comparable to iPSC-CMs maintained in static culture when evaluated in terms of cell viability, sarcomeric structure, action potentials and conduction velocities. In contrast, iPSC-CMs that were immediately exposed to mechanical loading had significantly lower cell viability, destruction of sarcomeres, smaller action potentials and lower conduction velocities. We report that iPSC-CMs survival under physiologically relevant hemodynamic stress requires gradual imposition of mechanical loads in a nutrient-rich environment.
Keywords: induced pluripotent stem cells, cardiac tissue engineering, hemodynamic loading
For TOC Only
INTRODUCTION
Cardiovascular disease (CVD) affects nearly 70 million Americans, resulting in an economic burden of > $300 billion and accounting for nearly 40% of all deaths in the US1. Myocardial injuries occur primarily due to heart attacks or myocardial infarctions and can result in long term complications that result in heart failure, atrial fibrillation and the increased risk of subsequent myocardial infarctions. Conventional treatments of cardiac tissue injury focus on management of the condition rather than repair and regeneration of injured tissue. Two major shortcomings limiting cardiovascular regeneration efforts are (a) the limited availability of appropriate human cell types and (b) the lack of physiologically relevant cell culture models to culture cardiac cells.
The breakthrough in somatic cell reprogramming in mouse cells 2 and human cells 3 has ushered in a new era in pluripotent stem cell based therapeutics and regenerative medicine. Spontaneously beating cardiomyocytes have been generated from iPS cells (iPSC-CMs) using established protocols 4,5 and have been shown to share electrophysiological, transcriptional and biochemical markers of adult cardiomyocytes 6–8. While iPSC-CMs hold significant promise and are already being used to screen drugs in patients, they are markedly different from adult cardiomyocytes 7–9. Adult cardiomyocytes do not survive for extended durations in culture and experience rapid de-differentiation and loss of function whereas iPSC-CMs can be maintained in culture for long durations10–12. iPSC-CMs share similarities with adult cardiomyocytes such as presence of sarcomeric proteins, all major calcium ion channels, resting membrane potentials, robust ion channel currents and calcium stores in the sarcoplasmic reticulum 7–9. However, they differ from adult cardiomyocytes as they are smaller in size, exhibit underdeveloped and relatively disorganized sarcomeres, lack t-tubules, possess large pacemaker currents and beat spontaneously, indicating that they still remain embryonic in phenotype due to incomplete reprogramming and residual epigenetic memory 13.
To date, iPSC-CMs have been cultured exclusively using standard cell culture techniques in static conditions. While these techniques can provide genetic and biochemical stimuli, they fail to adequately replicate critical mechanical stresses experienced by cardiac cells in vivo. In reviewing literature, we found that there were several groups that acknowledged the importance of mechanical stress in cardiac development and function14–16. However, available systems for culture of cardiac cells under conditions of mechanical stress primarily involve the FlexerCell systems that subject cultured cells to either uniaxial or biaxial stretch16–18,19. While this mimics the stretch seen during the filling phase; fluid filling, systolic pressure, end-diastolic pressure and subsequent relaxation during the normal cardiac cycle have not been reproduced. Other groups have combined electrical stimulation 20 and perfusion 21 with stretch but these do not mimic the pressure-volume changes seen within the left ventricle. Our group has previously developed cardiac cell culture models (CCCMs) for culture of cardiac cells under pressure-volume changes seen in the left ventricle22–24. However, the CCCM is non-physiological as it utilizes a pump to induce fluid flow within the system and various parameters of cardiac function are coupled and cannot be varied independently. To overcome these shortcomings and enable culture of cells in 3D, we developed the Biomimetic Cardiac Tissue Model. Cardiac cells are cultured within a cell culture chamber on 3D gels. This chamber mimics ventricular function by pumping fluid within a circulatory loop. Using the BCTM, pressure-volume changes associated with any chamber of the heart at any stage of development can be accurately reproduced.
Current iPS cell based therapies have shown limited success after in vivo implantation following myocardial infarction25. One such experiment injected stem cells encapsulated within Matrigel26. These cells were unable to survive the rigors of the heart without extensive use of pro-survival factors that inhibit cellular death mechanisms26. In another example, a stem cell patch was preconditioned and placed within a porcine myocardial infarction model25. The preconditioning of the cells improved cell survivability but patch integration was nonexistent due to a layer of matrix that formed between the heart and the patch. The buffer layer most likely helped to shield the cells from the highest level of mechanical strain thus limiting cell death. Both studies concluded that the significant cell death was caused by the limited nutrient supply, excessive mechanical forces, and/or exogenous factors. Small improvements in heart function following cardiac patch therapy have been suggested to result from structural support and cytokine production from the patch and not from direct integration into the infarct region27. Given the benefit observed from cardiac patches where there was significant cell loss and poor integration, it is worth exploring methods of maximizing cellular survival in the face of hemodynamic loads.
The BCTM therefore provides unique opportunities to recreate the in vivo cardiac environment to evaluate iPSC-CMs in vitro. In this study, we used the BCTM to investigate the effects of the nutrients (serum) on survival and the adaptability of iPSC-CMs under conditions of hemodynamic loading with the goal of identifying protocols that enable high percentage of survival of iPSC-CMs under physiologically relevant pressure-volume changes seen in the left-ventricle.
MATERIALS AND METHODS
Cell Culture Chamber Fabrication
Cell culture chambers were fabricated using standard soft-lithography using (poly) dimethyl siloxane (PDMS) (Sylgard 184, Dow Corning, Midland, MI) as previously described28. Figure 1A shows the cell culture chamber within the BCTM. The chamber was created using a two-step process of bonding a thin PDMS membrane to the bottom of a square PDMS block with a 19mm diameter hole through the center. The PDMS block used a base to cross-linker ratio of 10:1 while the thin membrane used a ratio of 15:1. The sub 50μm thin membrane was formed by spinning 15:1 PDMS on a silicon wafer. Both components were cured overnight at 60°C. Once cured, both pieces were cleaned and bonded using oxygen plasma (Harrick plasma systems, Ithaca, NY) optimized to create a permanent bond using 700mTorr pressure, 30W, and 30s exposure.
Figure 1:
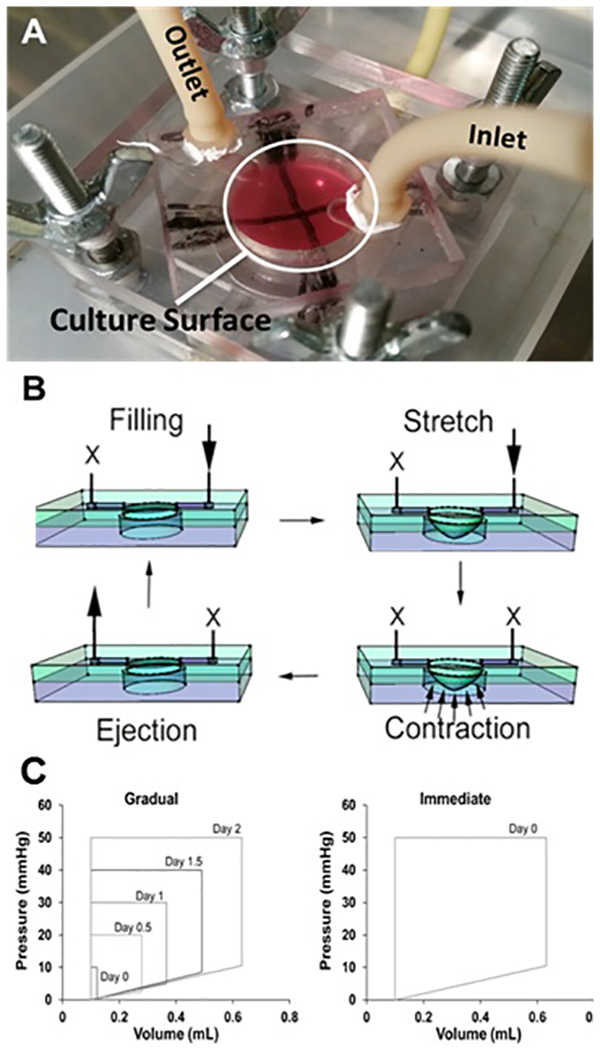
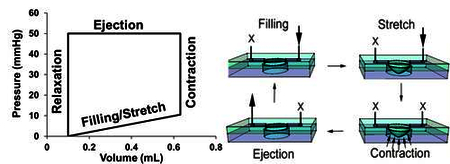
(A) Image of the BCTM culture chamber within the flow loop, the X drawn along the bottom of the thin membrane allows for easy verification of the stretch mechanics. (B) Schematic diagram representing how the BCTM reproduces the cardiac cycle. Two one ways valves on both sides of the stimulation chamber prevent retrograde flow during contractions and create flow in one direction. The arrows represent the direction of flow while the X’s represent where flow is prevented during each stage of the cycle. (C) Pressure-volume loops illustrating changes between the gradual and immediate application of hemodynamic load over the total 72-hour stimulation course. Each loop represents the filling of the chamber, isovolumetric contractions, ejection and relaxation. In the gradual samples, the filling volume and ejection pressure were gradually increased whereas the immediate samples experienced maximal filling and ejection pressures instantly. The device was designed to have the end diastolic pressure of each curve correspond to the percent biaxial stretch at the center of the membrane. For example, an end diastolic pressure of 5 mmHg corresponds to a biaxial stretch of 5% at the center.
Chamber Surface Modification
After autoclave sterilization, the chambers were again exposed to oxygen plasma using 700mTorr pressure, 30W, and one minute exposure time. Immediately, a 5% solution of APTES (Thermo Scientific) was added to the activated surface for 30 minutes at room temperature. The solution was then removed and the chamber was washed with 95% ethanol and then deionized (DI) water. The chambers were then baked at 80°C for 30 minutes. After baking, the chambers were washed with 95% ethanol and then DI water. A 2.5% glutaraldehyde (Sigma) solution was added to the chambers and allowed to incubate overnight at 4°C. The next day chambers were washed three times with DI water. This APTES/Glutaraldehyde surface modification protocol was adapted from published protocols29–31. Neutralized rat tail collagen I solution (Corning) was mixed with growth factor reduced Matrigel (Corning), ~1:4 matrigel/collagen, and added to the chambers and incubated for one hour at 37°C. Chambers were then washed with medium before seeding.
Cell Culture
H9c2 rat myoblast cells were cultured in DMEM-HG supplemented with 10% FBS and 1× pen/strep within a 37°C, 5% CO2 incubator. Cells were seeded at a density of 50,000 cells/cm2 and were grown to confluence before placing within the BCTM for stimulation. Human iPSC-CMs (Cellular Dynamics CMC-100–010-001) were seeded at 63,000 cells/cm2 in manufacturer supplied plating media. After two days iPSC-CMs were switched to manufacturer supplied maintenance media (Cellular Dynamics) and cultured for at least one week to ensure recovery from thawing and formation of a synchronously contracting monolayer. Media for cells under static conditions was refreshed every other day. There are no ethical concerns with the use of iPSC-CMs.
Working of the BCTM
The BCTM has no pump to move the media and instead relies on the pumping action of the cell culture chamber’s thin membrane. The result is a system that closely mimics the ventricle during the cardiac cycle. The BCTM (Fig. 1A) consists of a cell culture chamber integrated within a flow loop. Fluid filling is achieved by creating a pressure difference and pumping is achieved by causing compression of fluid within the pumping chamber using a programmable pressure generator (LB Engineering). The working of the BCTM is schematically explained in Figure 1B.
Strain Characterization
Elastic modulus varies with different ratios of PDMS monomer to cross linker therefore; different ratios and thicknesses were evaluated until a membrane with the ideal elastic modulus was found to create 10% radial and circumferential stretch at the center with 10mmHg pressure applied to its face. In these experiments we focus on biaxial stretch located at the center of the circular membrane, in contrast to the outside edge where only uniaxial radial strain is observed.
Stimulation Conditions
To test the effect of serum concentration on cell survivability both H9c2 and iPSC-CMs were stimulated at less than 1% biaxial strain at 1Hz, 10mmHg ES, and 1mmHg ED in the presence of low (1% FBS or less) or high (10%) serum for 48 hours. To determine the effect of gradual versus immediate subjection to mechanical load the end diastolic and systolic pressures are time variant in the gradual sample to produce a steady increase in mechanical stresses (Fig. 1C). The sample that receives the immediate stress is placed at the end point condition at day 0 as shown in Figure 1C. Both gradual and immediate samples concluded at 1Hz, 50mmHg systolic, and 10mmHg end diastolic and remained there for 24 hours before ending the experiment. Before stimulation media is changed for all samples to DMEM-HG supplemented with 10% FBS and 1× pen/strep. Following stimulation the gel attached to the thin PDMS membrane is cut from the device for analysis.
Live/Dead Staining
Cells were removed from the loop and washed with media before using the live/dead staining kit (Thermo Scientific L3224). Ethidium homodimer (4μM) and Calcein (2μM) were added to plain DMEM-HG and incubated for 30 minutes at room temperature. After imaging samples were fixed with 4% paraformaldehyde for 10 minutes at room temperature.
Immunofluorescence Microscopy
Cells are removed from the system and washed with phosphate buffered saline (PBS) and fixed using 4% paraformaldehyde for 10 minutes at room temperature. Cells are washed before permeabilizing using 0.5% Triton-X100 for 10 minutes at room temperature. Blocking was done using a 2% BSA solution for one hour at room temperature. Mouse anti-rabbit troponin (DSHB, RV-C2) at 5μg/mL in 2% BSA was added to the cells and incubated under gentle rocking for one hour at room temperature. The samples were then washed with PBS and TBST for 5 minutes at a time. Secondary goat anti-mouse-TR (Santa Cruz, sc-2781) was added at 1:200 for one hour at room temperature under gentle rocking followed by washing with PBS and TBST. Samples were then incubated with a 1:30 phalloidin (Invitrogen, 21833) solution in 2% BSA for 30 minutes at room temperature. Cells are then washed and mounted using hard set mounting medium with DAPI (Vectashield, H-1500) and imaged the next day.
Conduction Velocity Measurements
An iPSC-CM monolayer was transferred into a perfusion chamber mounted on an inverted microscope and perfused with Hanks balanced salt solution (HBSS) at 37oC. Cells were stained with fluorescent voltage-sensitive dye RH237 at 5μM for 5 minutes. To eliminate motion artifact from optical recordings, cell contractions were inhibited by supplementing staining and perfusion solutions with 5μM blebbistatin. Optical action potentials were recorded using a 16×16 photodiode array and an optical mapping system with a spatial resolution of 110 μm/diode as described previously 32. Activation times were determined at the 50% level of action potential upstrokes and used to construct activation maps and calculate conduction velocity. We verified that acquired action potential recordings and conduction velocity maps without blebbistatin were similar to that acquired with blebbistatin, with the exception of motion artifacts, confirming the fidelity of the recordings.
Statistical Analysis
A paired t-test was used for the analysis of the live/dead statistics and to compare the conduction velocities of gradual vs immediate stress samples with two-tailed significance set at p ≤ 0.05 for both experiments.
RESULTS
Metabolic Factors – Insights from H9c2 Rat Myoblast Cells
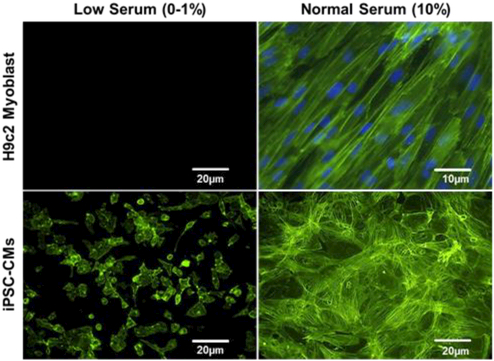
In preliminary studies with the BCTM we sought to determine the effect of low serum conditions (1% FBS) on H9c2 cells subjected to mechanical loading but they were unable to survive even low levels of stress. Low serum stimulation was attempted because literature suggests that H9c2 cells subject to culture under low serum conditions form myotubes and we sought to evaluate this differentiation process under hemodynamic loading 33. Various degrees of stress were attempted starting from 10mmHg/1mmHg systolic/diastolic pressure and 1% biaxial strain up to 50mmHg/10mmHg systolic/diastolic pressure, and 10% biaxial strain at 1Hz (healthy resting human heart rate). All of these conditions resulted in widespread cellular death. However, when cultured in the presence of 10% serum, H9c2 survived both low (10mmHg/1mmHg systolic/diastolic pressure, 1% biaxial strain) and high levels (50mmHg/10mmHg systolic/diastolic pressure, 10% biaxial strain) of mechanical stimulation (Fig. 2, top). Also significant was the ability of the H9c2 cells to align perpendicular to the primary direction of stretch near the edge where uniaxial stress dominates. It was the H9c2 cell death under low serum conditions that gave us the insight to evaluate if serum could be playing a similar role in the cell death of iPSC-CMs during stimulation.
Figure 2:
Fluorescence microscopy to visualize f-actin staining (green) in H9c2 myoblasts and Human iPSC-CMs (bottom) stimulated within the BCTM under 10mmHg/1mmHg systolic/diastolic pressure and 1% biaxial strain stimulated at 1Hz for 48 hours under conditions of low (0–1%) (left) and high (10%) serum (right).
Metabolic Factors – Translating observations from H9c2 cells to Human iPSC-CMs
The recommended medium for culture of Human iPSC-CMs involves a basic medium composed of RPMI-1640 supplemented with B27 serum-free supplement to prevent dedifferentiation of differentiated iPSC-CMs 5. In initial experiments, human iPSC-CMs exposed to the similar levels of hemodynamic stress as the H9c2 cells (10mmHg/1mmHg systolic/diastolic pressure, 1% biaxial strain) resulted in widespread cell death. To determine if the medium rather than serum resulted in cell death, various serum free media formulation were tested including RPMI-1640 supplemented with B27 (Gibco), Cardiac Maintenance Media (Stem Cell Theranostics), and DMEM-HG supplemented with B27 serum-free supplement. However, results confirm that the absence of serum significantly affected iPSC-CM survival under mechanical loading. Similar to results obtained with H9c2 cells, addition of serum (10% FBS) to any medium (DMEM or RPMI-1640) significantly enhanced their ability to survive (Fig. 2, bottom). Despite drastically increased cell viability, iPSC-CMs unlike H9c2 cells, failed to align or reorganize in response to the 48-hours of BCTM stimulation.
iPSC-CM Preconditioning: Gradual vs Immediate Application of Stress
Using the BCTM, iPSC-CMs can be subject to physiologically relevant mechanical loads in either an immediate or time variant (gradual) fashion (Fig. 1C). Considering that the endocardial layer of the left ventricular chamber experiences a pressure of ~120 mmHg and the epicardial layer does not experience any pressure, a reasonable estimate of pressure experienced by cardiomyocytes within the ventricular wall is ~ 50–60 mmHg. With the gradual samples, Peak Systolic Pressure (PSP in mmHg) and End Diastolic Pressure (EDP in mmHg) were adjusted to 10PSP/~1EDP, 20PSP/2.5EDP, 30PSP/5EDP, 40PSP/8.5EDP, and 50PSP/10.5EDP at time points of 0, 24, 32, 48, and 56 hours respectively and then maintained at 50PSP/10.5EDP for an additional 24 hours. In contrast, the immediate samples were instantly subject to 50PSP/10.5EDP and maintained at that level for the entire 72 hours of the experiment. In these experiments the EDP pressure correlates with the filling pressure (diastolic pressure) required to impose percent stretch associated with filling of the chamber. All samples were stimulated cyclically at 1Hz throughout the 72 hour stimulation. These results demonstrate the utility of the BCTM to precondition iPSC-CMs gradually to attain pressure and stretch associated with locations within the myocardial tissue (gradual samples) or simulate the effect of direct delivery of iPSC-CMs intra-myocardially where they immediately experience physiological pressure and stretch (immediate samples).
Gradual vs Immediate Stress – Cell Survival
Viability of iPSC-CMs subject to either time variant (gradual) or immediate mechanical loading was evaluated using Live-Dead assays (Fig. 3, top). Cell viability following 72 hours of stimulation within the BCTM was significantly different for each of these stress application protocols. Immediate subjection of the iPSC-CMs to mechanical stress caused significant cell death, whereas, if the stress was applied over 2 days cell death was minimal and statistically indistinguishable from the static control samples (Fig. 3, bottom).
Figure 3:
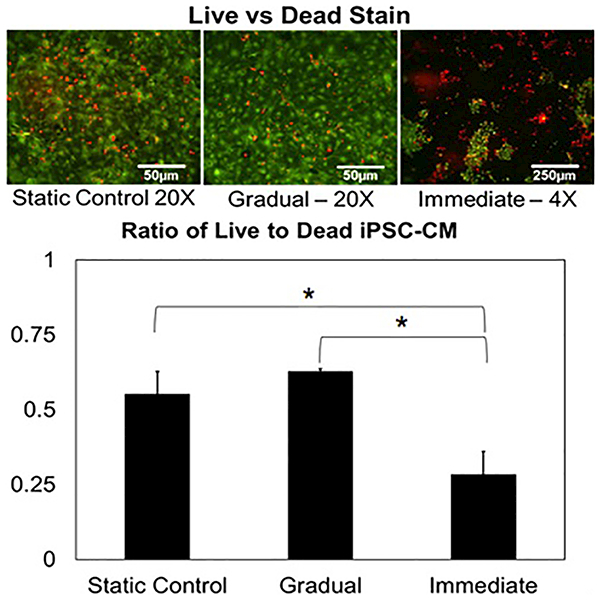
(Top) Live (green)/dead (red) staining performed on iPSC-CMs following gradual and immediate application of hemodynamic load. The lower magnification image of the immediate sample was required to reliably quantify the increased cell loss experienced within the sample (Bottom) Ratio of live to dead cells shows statistical significance between immediate and other samples (p<0.05, N=3). No statistical significance was found between the static and gradual samples (p>0.05, N=3).
Gradual vs Immediate Stress – Cytoskeletal Integrity
iPSC-CMs cultured for 72 hours under conditions where the rate of stress application was either gradual or immediate and iPSC-CMs cultured under static conditions were evaluated for cytoskeletal (f-actin) and sarcomeric (cardiac troponin T) proteins (Fig. 4). Instant application of 10% biaxial stress caused noticeable destruction of the cytoskeleton as noted from the discontinuous f-actin and complete loss of cardiac troponin in the immediate samples. However, gradual application of stress caused no significant effect on the cytoskeleton when compared to static controls. However, even in areas close to the edge where uniaxial stress dominates, no cellular alignment via stretch induced reorganization was seen.
Figure 4:
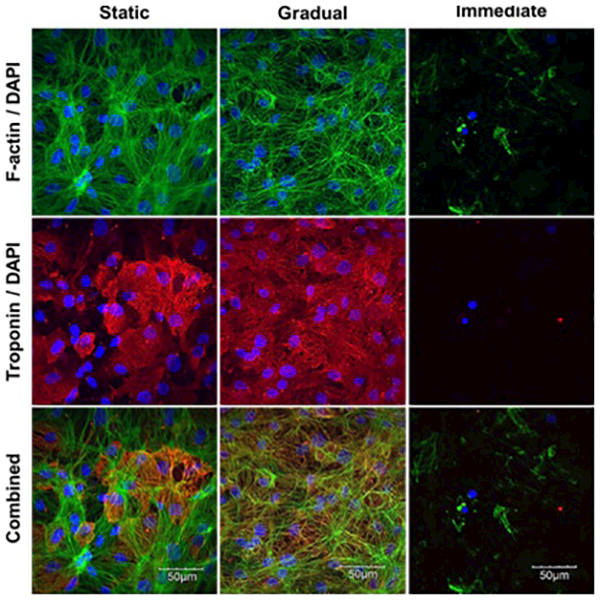
Immunofluorescence microscopy to image cytoskeleton: f-actin (green) (top) and cardiac troponin T (red) (middle), with DAPI (blue) counterstain highlighting differences between static, gradual and immediate (left-right) application of hemodynamic load. Images were taken at the center of the device where biaxial stretch dominates.
Gradual vs Immediate Stress – Electrophysiological Behavior
Finally, the effect of gradual or immediate imposition of stress on iPSC-CMs action potential generation and inter-cellular coupling was evaluated using optical mapping of activation spread (Fig. 5). The conduction velocity maps (Fig. 5, middle) shows that propagation of action potentials was faster and smoother in cells maintained in static culture and in the culture with gradually applied stress compared to the culture with immediate stress (Fig. 5, bottom). Due to the widespread cell death in the immediate samples paceable regions were isolated to the outer peripheral of the device where strain is minimized and uniaxial stretch dominates. However, the difference in the effect of stress application rate on the conduction velocity between the gradual and immediate samples (Fig. 5, Graph Below) was statistically significant regardless of recording location. The differences in conduction velocities between the static controls and immediate samples were also statistically significant whereas the difference between static controls and gradual samples was not (Fig. 5, Graph Below).
Figure 5:
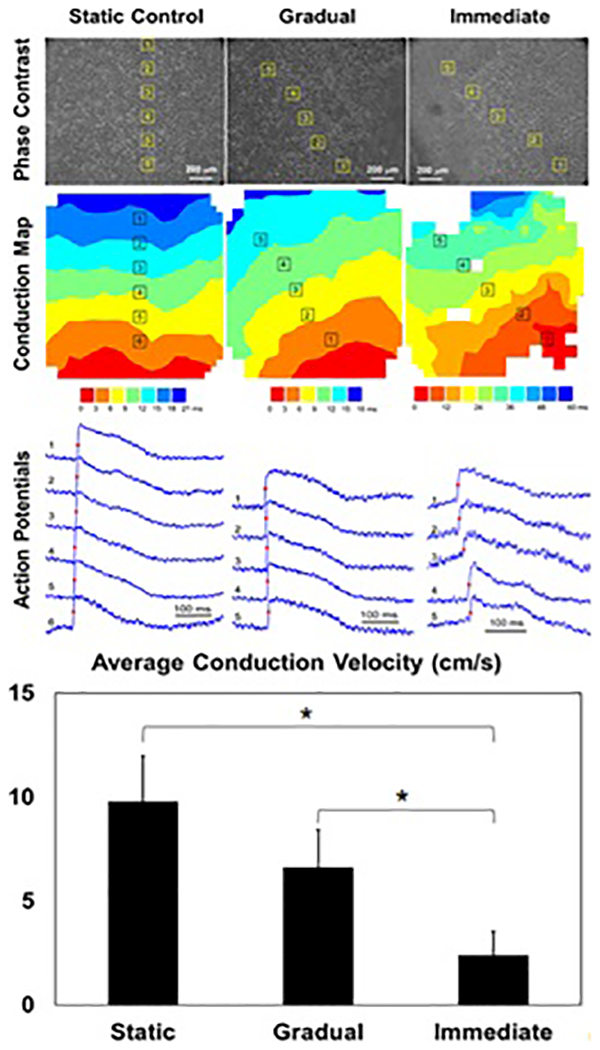
Electrophysiological evaluation of representative samples: static (left), gradual (middle), and immediate samples (right) showing phase contrast image (top), local conduction map (middle), and generated action potentials (bottom). Paceable regions of the immediate samples were localized to the peripheral of the device where uniaxial stretch dominates. (Graph Below) Conduction velocity statistics showing significant difference between gradual and immediate samples as well as between static and immediate samples (p<0.05, N=5).
DISCUSSION
These results are relevant to in vivo studies since low serum/nutrient environments are characteristic of the post myocardial infarction region of the heart. Most studies have attempted to integrate iPSC-CMs into these regions without additional steps to precondition cells or increase metabolic supply to the infarct region. We have shown that without additional nutrient supply the cells are unable to survive mechanical stress typical of the heart, in stark contrast to static cultures which have no cell loss. It is highly possible that cells under mechanical load require additional nutrients to repair and reorganize following mechanical damage.
Differences between the H9c2 cell line and iPSC-CMs also vary the response to hemodynamic loads. H9c2 cells are not terminally differentiated, have an active cell cycle, and an unorganized cytoskeletal network that allows for easier cytoskeletal reorganization and realignment with the stretch. However, iPSC-CMs are terminally differentiated and have a complex organization of sarcomeric proteins, indicated by the striated f-actin and troponin. These sarcomeres are randomly organized due to the fact that iPSC-CMs are differentiated in static conditions in the absence of mechanical stimuli conducive to alignment, which may adversely impact how they transduce stress and thus disrupt their ability to align. Considering the complex organization of the cardiac sarcomeric apparatus and its close proximity to the mitochondria it is possible that excessive damage to the cytoskeleton may lead to mitochondrial damage and thus release of mitochondrial apoptotic factors 34. A possible explanation for the moderate improvement in survivability seen in experiments using iPSC-CMs in conjunction with pro-survival cocktails may be the fact that mitochondrial death pathways are inhibited26.
Deterioration of conduction velocity following hemodynamic stress suggest a possible effect on cell-cell coupling or reduction in cell excitability, which can have important consequences in cell-based therapies due to the potential creation of arrhythmias at cell injection or patch site. It is likely that mechanical loading causes irreversible damage to various components of intercalated discs that facilitate strong inter-cellular coupling. Mechanical stretch induced loss of cell-cell coupling was commonly observed in the immediate samples where there were noticeably few paceable regions within the biaxial region of stretch even if living/beating cells were observed. The paceable regions in immediate samples were more prevalent on the outer periphery where uniaxial stretch dominates, suggesting that iPSC-CMs may be more sensitive to biaxial stretch compared to uniaxial stretch.
The BCTM represents enabling technology for culture of cardiac cells under physiologically relevant hemodynamic loading and creates a viable alternative to expensive animal models which have limited relevance to the human etiology. The major advantage of the BCTM is the ability to independently modulate variables associated with the hemodynamic loading of the heart. The central region within the BCTM cell culture chamber experiences biaxial stretch which is representative of the type of stretch seen in the left ventricular wall. In our studies, the gradual condition represents a conditioning regimen where stretch and pressure can be gradually increased to levels seen in the adult heart whereas the immediate condition represents naïve iPSC-CMs placed within or on the surface of a beating heart. In this study we used the BCTM to isolate the causes of cellular death in iPSC-CMs that were directly delivered into the beating heart. Both mechanical and metabolic factors were identified as contributing factors that limit cell survivability and adaptability under conditions of hemodynamic loading. The dysfunction seen in these experiments was also closely related to the magnitude and rate of application of mechanical loads. Based on our findings, we suggest that the BCTM has potential to evaluate methods to enhance iPSC-CMs survivability and adaptability following transplantation within regions that are constantly subject to high levels of hemodynamic stress.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Ding Li and Dr. Jianbo Wang for their expertise with confocal imaging. This work was funded by a National Institute of Health R21 grant # 11675980 and an Alabama Institute of Medicine Seed Grant. AJR was supported by NIH T32 training grant # 5T32HL007918–18.
Non-Standard Abbreviations
- BCTM
Biomimetic Cardiac Tissue Model
REFERENCES
- (1). Mozaffarian D; Benjamin EJ; Go AS; Arnett DK; Blaha MJ; Cushman M; Das SR; de Ferranti S; Despres JP; Fullerton HJ; Howard VJ; Huffman MD; Isasi CR; Jimenez MC; Judd SE; Kissela BM; Lichtman JH; Lisabeth LD; Liu S; Mackey RH; Magid DJ; McGuire DK; Mohler ER 3rd; Moy CS; Muntner P; Mussolino ME; Nasir K; Neumar RW; Nichol G; Palaniappan L; Pandey DK; Reeves MJ; Rodriguez CJ; Rosamond W; Sorlie PD; Stein J; Towfighi A; Turan TN; Virani SS; Woo D; Yeh RW; Turner MB Circulation 2016, 133, 447–454. [DOI] [PubMed] [Google Scholar]
- (2). Takahashi K; Yamanaka S Cell 2006, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- (3). Takahashi K; Tanabe K; Ohnuki M; Narita M; Ichisaka T; Tomoda K; Yamanaka S Cell 2007, 131, 861–872. [DOI] [PubMed] [Google Scholar]
- (4). Mummery CL; Zhang J; Ng ES; Elliott DA; Elefanty AG; Kamp TJ Circ Res 2012, 111, 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5). Zhang J; Wilson GF; Soerens AG; Koonce CH; Yu J; Palecek SP; Thomson JA; Kamp TJ Circ Res 2009, 104, e30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6). Zhang J; Wilson GF; Soerens AG; Koonce CH; Yu J; Palecek SP; Thomson JA; Kamp TJ Circ Res 2009, 104, e30–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7). Martins AM; Vunjak-Novakovic G; Reis RL Stem Cell Rev 2014, 10, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8). Iglesias-Garcia O; Pelacho B; Prosper F J Mol Cell Cardiol 2013, 62, 43–50. [DOI] [PubMed] [Google Scholar]
- (9). Nakamura K; Hirano K; Wu SM J Cardiovasc Transl Res 2013, 6, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10). Graham EL; Balla C; Franchino H; Melman Y; del Monte F; Das S J Vis Exp 2013, e50289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11). Li D; Wu J; Bai Y; Zhao X; Liu L J Vis Exp 2014. [DOI] [PMC free article] [PubMed]
- (12). Parameswaran S; Kumar S; Verma RS; Sharma RK Can J Physiol Pharmacol 2013, 91, 985–998. [DOI] [PubMed] [Google Scholar]
- (13). Knollmann BC Circ Res 2013, 112, 969–976; discussion 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14). Yang X; Pabon L; Murry CE Circ Res 2014, 114, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15). Zimmermann WH Stem Cell Res Ther 2013, 4, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16). Ye F; Yuan F; Li X; Cooper N; Tinney JP; Keller BB Physiol Rep 2013, 1, e00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17). Lu L; Ravens U Organogenesis 2013, 9, 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18). Mihic A; Li J; Miyagi Y; Gagliardi M; Li SH; Zu J; Weisel RD; Keller G; Li RK Biomaterials 2014, 35, 2798–2808. [DOI] [PubMed] [Google Scholar]
- (19). Boerboom RA; Rubbens MP; Driessen NJ; Bouten CV; Baaijens FP Ann Biomed Eng 2008, 36, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20). Xiao Y; Zhang B; Liu H; Miklas JW; Gagliardi M; Pahnke A; Thavandiran N; Sun Y; Simmons C; Keller G; Radisic M Lab Chip 2014, 14, 869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21). Brown MA; Iyer RK; Radisic M Biotechnol Prog 2008, 24, 907–920. [DOI] [PubMed] [Google Scholar]
- (22). Nguyen MD; Giridharan G; Prabhu SD; Sethu P Conf Proc IEEE Eng Med Biol Soc 2009, 2009, 1060–1063. [DOI] [PubMed] [Google Scholar]
- (23). Nguyen MD; Tinney JP; Ye F; Elnakib AA; Yuan F; El-Baz A; Sethu P; Keller BB; Giridharan GA Anal Chem 2015. [DOI] [PMC free article] [PubMed]
- (24). Nguyen MD; Tinney JP; Yuan F; Roussel TJ; El-Baz A; Giridharan G; Keller BB; Sethu P Anal Chem 2013, 85, 8773–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25). Wendel JS; Ye L; Tao R; Zhang J; Zhang J; Kamp TJ; Tranquillo RT Stem Cells Transl Med 2015, 4, 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26). Laflamme MA; Chen KY; Naumova AV; Muskheli V; Fugate JA; Dupras SK; Reinecke H; Xu C; Hassanipour M; Police S; O’Sullivan C; Collins L; Chen Y; Minami E; Gill EA; Ueno S; Yuan C; Gold J; Murry CE Nat Biotechnol 2007, 25, 1015–1024. [DOI] [PubMed] [Google Scholar]
- (27). Zhang J Curr Treat Options Cardiovasc Med 2015, 17, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28). Patibandla PK; Rogers AJ; Giridharan GA; Pallero MA; Murphy-Ullrich JE; Sethu P Anal Chem 2014, 86, 10948–10954. [DOI] [PubMed] [Google Scholar]
- (29). Kuddannaya S; Chuah YJ; Lee MH; Menon NV; Kang Y; Zhang Y ACS Appl Mater Interfaces 2013, 5, 9777–9784. [DOI] [PubMed] [Google Scholar]
- (30). Louise Meyer R; Zhou X; Tang L; Arpanaei A; Kingshott P; Besenbacher F Ultramicroscopy 2010, 110, 1349–1357. [DOI] [PubMed] [Google Scholar]
- (31). Sandison ME; Cumming SA; Kolch W; Pitt AR Lab Chip 2010, 10, 2805–2813. [DOI] [PubMed] [Google Scholar]
- (32). Sowell B, Fast VG Heart Rhythm 2012, 9, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33). Pagano M; Naviglio S; Spina A; Chiosi E; Castoria G; Romano M; Sorrentino A; Illiano F; Illiano G J Cell Physiol 2004, 198, 408–416. [DOI] [PubMed] [Google Scholar]
- (34). Zhao J; Yin M; Deng H; Jin FQ; Xu S; Lu Y; Mastrangelo MA; Luo H; Jin ZG Cell Death Differ 2016, 23, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.