Abstract
Perhaps the most important advance in the field of cell therapy for heart disease has been the recognition that all stem/progenitor cells (both adult and embryonic) fail to engraft in the heart to a significant extent, and thus work via paracrine mechanisms. This fundamental advance has led to four new paradigms that are discussed in this review and that may importantly shape, or even revolutionize, the future of the field: i) repeated cell therapy, ii) intravenous cell therapy, iii) immunomodulatory actions of cell therapy, and iv) new cell types. Since virtually all of our current knowledge of cell therapy is predicated upon the effects of a single cell dose, the idea that the full therapeutic effects of a cell product require repeated doses is disruptive, and has far-reaching implications. For example, inadequate dosing (single-dose protocols) may be responsible, at least in part, for the borderline or disappointing results obtained to date in clinical trials; furthermore, future studies (both preclinical and clinical) may need to incorporate repeated cell administrations. Another disruptive idea, supported by emerging preclinical and clinical evidence, is that intravenously injected cells can produce beneficial effects on the heart, presumably via release of paracrine factors in extracardiac organs or endocrine factors into the systemic circulation. Intravenous administration would obviate the need for direct delivery of cells to the heart, making cell therapy simpler, cheaper, safer, more scalable, and more broadly available, even on an outpatient basis. While the mechanism of action of cell therapy remains elusive, there is compelling in vitro evidence that transplanted cells modulate the function of various immune cell types via release of paracrine factors such as extracellular vesicles, although in vivo evidence is still limited. Investigation of the new paradigms reviewed herein should be a top priority, as it may profoundly transform cell therapy and finally make it a reality.
Keywords: cell therapy, heart failure, repeated administration, intravenous, immune system
Cell therapy is a promising new approach that may transform the management of heart failure (HF), refractory angina, and peripheral artery disease. Many reviews have been published in this burgeoning field. The purpose of the present article is not to repeat what is already in the literature, but rather to discuss novel paradigms that have emerged in recent years and that may importantly shape, or even revolutionize, the future of the field. These include 1) repeated cell therapy, 2) intravenous cell therapy, 3) immunomodulatory actions of cell therapy, and 4) use of new cell types.
Repeated Cell Therapy
The concept of repeated cell therapy was proposed recently1 on the basis of studies in rodents.2–4 It is a major paradigm shift that may fundamentally change the entire field of cell therapy.1 Here, we will briefly recapitulate the salient points.
Intuitively, and as a general consideration, expecting one dose of cells to be enough to bring about a therapeutic benefit seems unrealistic. Is it reasonable to expect that a single administration of cells would be sufficient to correct a chronic process, such as HF, that has taken years or decades to develop? How many therapies are there that can produce a sustained improvement in LV function with a single treatment? It would appear more sensible to assume that just like pharmacologic therapies need to be repeated, so do cell-based therapies. This becomes even more logical when one considers that a major factor limiting the benefits of cell therapy is the poor engraftment of the cells, which disappear rapidly after transplantation, regardless of the type of cell used. In fact, poor engraftment is a universal phenomenon that has been observed with virtually all types of cells studied heretofore5, including embryonic stem cells6. In order for this problem to be fully appreciated, it is critical that survival and engraftment of transplanted cells be carefully quantitated (as opposed to showing anecdotal microphotographs). When such a quantitative analysis is performed, there is no convincing evidence in the literature that any cell type engrafts in significant numbers beyond the first 1–2 months after transplantation. A few cells can persist for 1 year, but their numbers are vanishingly low7. We described this phenomenon a few years ago. Using a highly sensitive assay to measure cell retention8, we found that after either intramyocardial or intracoronary administration of 100,000 c-kit+ cardiac progenitor cells (CPCs) in mice, the number of cells remaining in the heart declined precipitously, so that 35 days later there were only ~1,000 cells left in the entire heart9.
Because of this poor engraftment, it seems self-evident that administering one dose of cells cannot be considered an adequate test of the efficacy of that cell product. However, almost all preclinical and virtually all clinical studies of cell therapy performed heretofore have used one cell administration. There are several reasons for this insistence on single-dose treatments. First, the old paradigm assumed that transplanted cells would engraft, proliferate, and differentiate into new mature myocytes; therefore, it was thought that adequate regeneration could be attained simply by administering one (sufficiently large) dose of cells. However, as mentioned above, we now know that there is minimal, if any, long-term engraftment, regardless of the cell type used. Secondly, in rodents repeated injections of cells (either by the intramyocardial or intracoronary route) are technically difficult and associated with prohibitive mortality. In patients, the use of repeated intramyocardial or intracoronary cell injections is hindered by logistic, safety, financial, and regulatory issues. It is difficult enough to obtain regulatory approval for a single treatment; the hurdles are even greater for multiple treatments.
Several strategies have been attempted to overcome poor engraftment, but these approaches have not been particularly successful in preventing cell loss; although they may prolong survival of transplanted cells, they do not result in significant long-term engraftment. We put forth the idea that the problem of poor engraftment can be overcome, in part, by administering repeated cell doses1. We argued that just as most pharmacologic agents are ineffective when given once but can be highly effective when given repeatedly, so a cell product may be ineffective, or modestly effective, when given as a single treatment, but may turn out to be quite efficacious if given repeatedly1.
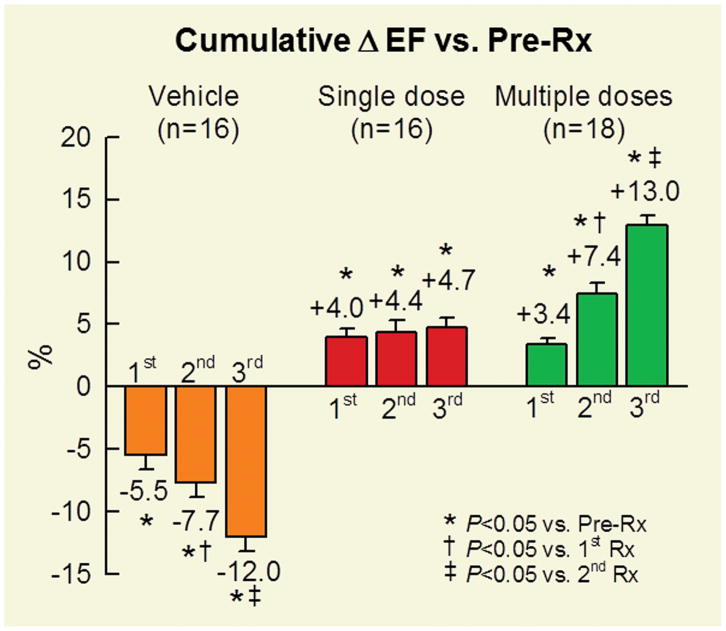
To determine whether repeated treatments are superior to a single treatment, we conducted a study in a rat model of chronic ischemic cardiomyopathy in which c-kit+ CPCs were given either once or three times at 35-day intervals2. We found that each administration of c-kit+ CPCs resulted in an increase in LVEF, such that the total cumulative increase after the 3rd dose was approximately triple that observed after a single dose (Figure 1). We obtained similar results in a mouse model of chronic ischemic cardiomyopathy using a different cell type (cardiac mesenchymal cells)3. In either study, there were no significant differences between three doses and one dose with respect to scar size and amount of viable myocardium. In addition, even after three doses, the number of transplanted cells remaining in the heart at the end of the study was vanishingly low, as was the number of new myocytes derived from transplanted cells (assessed with FISH) and the number of new myocytes derived from endogenous cells (assessed with BrdU/EdU labeling). Thus, the improvement in LV function effected by cell therapy cannot be explained by differentiation of exogenous cells into myocytes or formation of new myocytes from endogenous cells. One possible mechanism whereby cell therapy ameliorated LV function is a reduction in fibrosis (collagen content), particularly in the noninfarcted region2, 3 (the region that is mainly responsible for LV performance). An additional possibility is that cell therapy reduced the content of inflammatory cells in the myocardium and/or inhibited the detrimental actions of these cells3. Much work needs to be done to elucidate the mechanism of action of cell therapy (whether with single or repeated doses).
Figure 1. Cumulative improvement in LV EF after repeated cell doses.
Rats with a 1 month-old MI were given, at 35 day intervals, three injections of vehicle (orange), one injection of c-kit+ CPCs and two of vehicle (red), or three injections of c-kit+ CPCs (green) into the LV cavity. Depicted here are the changes in EF (absolute units) from pretreatment (Pre-Rx), i.e., from before the 1st injection. Data are means±SEM. Reproduced with permission from ref. 1.
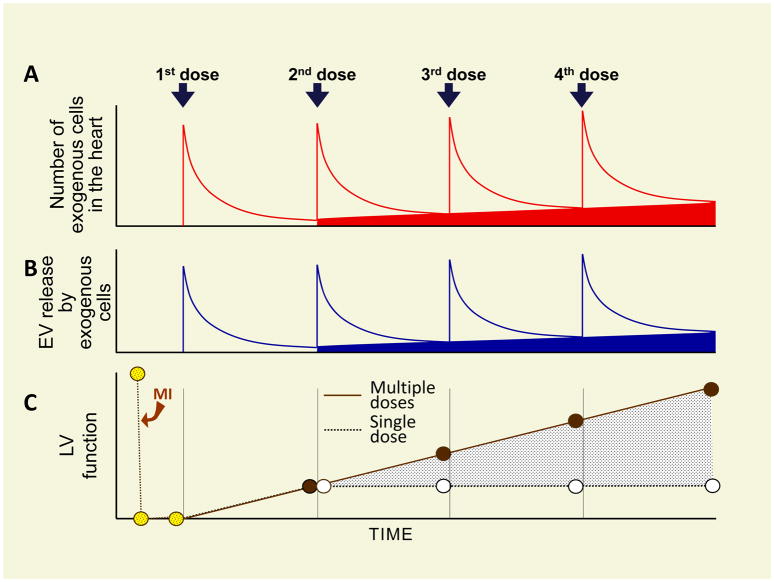
The studies reviewed above2,3 employed two different species and two different cell types and arrived at similar conclusions, which can be summarized as follows (Figure 2). When only one dose of cells is given, the benefits of cell therapy are significantly underestimated. Repeated cell administrations have cumulative beneficial effects and, as a result, are markedly more effective than a single administration. The beneficial effects of repeated cell doses cannot be explained by engraftment and differentiation of transplanted cells, and thus must reflect paracrine mechanisms. Importantly, even repeated doses do not appear to stimulate the formation of new myocytes from endogenous sources, which calls into question the concept that cell therapy promotes true myocardial regeneration. Potential mechanisms of action of transplanted cells include anti-fibrotic and anti-inflammatory effects.
Figure 2. Conceptual paradigm of repeated cell therapy.
(A), After cell administration, the number of transplanted cells in the myocardium falls rapidly, but a small number persists for many weeks (at least 1 year). Repeated cell doses produce a cumulative increase in the number of transplanted cells that persist long term in the myocardium (red area)7. Each cell dose produces a burst of extracellular vesicles (EV) release (B), which is responsible for the cumulative improvement in LV function (C). The cumulative increase in long-lasting exogenous cells (red area) may be associated with a cumulative increase in sustained EV release by these cells (blue area). A and C are based on experimental data whereas B is speculative. Reproduced with permission from ref. 4
In both of the aforementioned studies2, 3, the total number of transplanted cells was three times greater in the multiple-dose group than in the single-dose group. Thus, the superiority of three doses could be caused by the greater total number of cells given rather than by repeated treatments. If this is the case, then the effects of three repeated doses should be recapitulated by one large dose that contains the same total number of cells as the three doses. We tested this concept in our rat model of chronic ischemic cardiomyopathy4. We found that rats given one large dose of CPCs exhibited an improvement in LV function, but the magnitude of that improvement was less than that of rats receiving multiple doses. In addition, the multiple dose-treated animals had less collagen and less infiltration of CD45+ cells in the noninfarcted region, suggesting greater anti-fibrotic and anti-inflammatory actions of repeated doses compared with one large dose. These results support the concept that splitting a dose of CPCs into three smaller doses given a few weeks apart is more effective than giving the same number of cells as a single dose. The fact that the cumulative effects of repeated cell doses are not recapitulated by a single combined dose, even though the total number of cells infused is the same, suggests that the duration of myocardial exposure to the transplanted cells is more important than the intensity of such exposure4.
The idea that the full therapeutic effects of cells cannot be properly evaluated after a single dose and require repeated doses is simple but potentially disruptive. Its implications are far-reaching. For example, the conclusions of previous “negative” studies could be questioned because the benefits of the treatment may have been underestimated or even completely overlooked, due to the use of inadequate treatment protocols. Is it possible that, with repeated dosing, many of these studies would have been “positive”? Is it possible that the traditional single-dose paradigm may be responsible for at least some of the borderline or disappointing results obtained to date in clinical trials of cell therapy? Perhaps the most important implication of this new paradigm is that the protocols of future preclinical and clinical trials of cell therapy need to be changed to incorporate repeated treatments.
Looking ahead, we believe it is important that the concept of repeated dosing be carefully tested in large animal models and in humans. Investigation of these issues should be a top priority in cell therapy, because it may change the way research studies are conducted.
Intravenous route of delivery
The concept of repeated treatments inevitably leads to the question as to whether cells can be delivered intravenously (i.v.), because this route would be by far the most practical for administering multiple doses. Therefore, it is important to discuss i.v. cell delivery in depth.
Until now, every effort has been made to deliver cells (as many as possible) directly to the heart, in the hope that the transplanted cells would engraft and improve LV function. Since i.v. injection results in only a minuscule percentage of cells engrafting in the heart (vide infra),10–14 direct delivery to the myocardium via intracoronary (i.c.), transendocardial, or transepicardial approaches has been used. However, these approaches entail a small but significant risk of adverse events related to the nature of the invasive procedures. For example, i.c. infusion can cause coronary dissection and transendocardial injection can be complicated by cardiac perforation and hemopericardium15. Transendocardial injection cannot be performed in certain patients with prosthetic mechanical valves and devices. Furthermore, i.c., transendocardial, and epicardial delivery of cells necessitates special facilities, expensive equipment, and specialized personnel, making widespread use of cell therapy difficult. On the other hand, the recent recognition that the overwhelming majority of cells die soon after transplantation1, 9, 16, 17,8 and that their beneficial effects are mediated by the release of various factors that act in a paracrine manner to reduce collagen deposition, inhibit apoptosis, augment angiogenesis, inhibit inflammation,18–20 and suppress innate and adaptive immunity18, 21 raises the question as to whether similar beneficial effects can be accrued by i.v. administration of cells and release of paracrine factors into the systemic circulation; if so, this approach would obviate the need for direct delivery of cells to the heart, making cell therapy simpler, cheaper, safer, and more broadly available.
The preclinical and clinical studies that have evaluated i.v. delivery of cells to date are summarized in Table 1.
Table 1.
Preclinical Studies of Intravenous Cell Therapy
| Study | Species | Setting | Cell Type | Cell dose | Follow-up | Outcomes |
|---|---|---|---|---|---|---|
| Nagaya,11 2004 | Rats* | Acute MI | BM MSCs, allogeneic | 5 × 106 | 4 weeks | ↓ IS, ↑ LV EF, ↑ capillary density |
| Ma,12 2005 | Rats* | Acute MI | BM MSCs, allogeneic | 5 × 106 | 28 days | ↑ cardiac function, ↑ angiogenesis |
| Boomsma,22 2007 | Mice* | Acute MI | BM MSCs, allogeneic | 0.5 × 106 | 2 weeks | ↔ infarct morphology, ↑ systolic function |
| Guo49, 2007 | Rats* | Acute MI | BM MSCs, allogeneic | 1 × 107 | 4 weeks | ↑ LV EF ↑ fractional shortening |
| Lee,13 2009 | NOD-SCID mice (immunodeficient) | Acute MI | BM MSCs, human | 2 × 106 | 21 days | ↑ LV EF ↑ fractional shortening |
| Dijk,23 2011 | Rats* | Acute MI | AD SCs, allogeneic | 5 × 106 SVF 1 × 106 AD |
35 days | ↓ IS No adverse events |
| Wang,14 2011 | Rats* | Chronic MI | BM MSCs allogeneic | 5 × 106 | 4 weeks | ↔ LV function |
| Dayan48, 2011 | NOD-SCID mice (immunodeficient) | Acute MI | BM MSCs, HUCPVC allogeneic | 2 × 106 | 4 weeks | ↑ fractional shortening at 4 weeks then ↔ |
| Yang,24 2013 | Rats* | Nonischemic | BM MSCs allogeneic | 5 × 106 5 × 106 +AZA |
4 weeks | ↑ cardiac function with double infusion |
| Yu,25 2014 | Rats* | Nonischemic | BM MSCs, allogeneic | 5 × 106 | 20 weeks | ↑ LV function with repeated infusion |
| Li,26 2015 | Rats* | Acute MI | BM MSCs, allogeneic | 2 × 106 2 × 106 + atorvastatin |
30 days | ↑ LV EF Atorvastatin-pretreated cells homed to the myocardium |
| Mykhaylichenko,27 2016 | Rats* | Acute MI | BM MSCs, allogeneic | 1–5 × 106 1–5 × 106 +AZA |
4 weeks | ↑ LV function and vascularization |
| Luger,20 2017 | Mice* | Acute MI, chronic ischemic cardiomyopathy | BM MSCs, human | 2 × 106 | 6 weeks | ↓ IS ↑ LVEF ↓ Adverse remodeling |
| Price,28 2006 | Swine,* | Acute MI | BM MSCs, allogeneic | 3.2 × 108 | 3 months | ↑ EF, ↔ IS ↓ eccentric hypertrophy |
| Krause,29 2007 | Swine | Acute MI | BM MSCs, autologous | 1 × 106 | 1 month | ↓ IS ↑ hemodynamic |
| Halkos,30 2008 | Swine* | Acute MI | BM MSCs, allogeneic | 1 × 106 3 × 106 10 × 106 |
12 weeks | ↑ LV function |
| Wolf,31 2009 | Swine* | Acute MI | BM MSCs, autologous, allogeneic, and human | 1 × 106 | 4 weeks | ↑ FAS ↓ IS |
| Hong,32 2015 | Swine* | Acute MI | AD SCs, human | 15 × 107 5 × 107 (3 doses) |
28 days | ↑ LV EF ↑ CFR ↓ Perfusion defect area |
| Yamada,164 2018 | Rabbit* | Acute MI | BM MSCs, autologous, allogeneic, and human | 3 × 105 | 2 and 4 weeks | ↑ LV EF ↑ fractional shortening ↑ cardiac remodeling |
Abbreviations: AZA, azacytidine; AD SCs, adipose-derived stem cells; SVF, stromal vascular fraction; BM MSCs, bone marrow mesenchymal stromal cells; UC MSCs, umbilical cord-derived mesenchymal stromal cells; EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction; LV, Left ventricular; IS, infarct size; FAS, fractional area shortening; HUCPVC, Human umbilical cord perivascular cell
non-immunosuppressed
Efficacy in Preclinical Studies
Intravenous delivery of stem or progenitor cells has been investigated in small11–14, 20, 22–27 and large28–32 animal models (Table 1). The earliest report was from Nagaya et al.11 in 2004. These investigators injected mesenchymal stem cells (MSCs) i.v. in isogenic rats 3 h after coronary ligation and found a reduction in infarct size and an increase in capillary density and LV function in treated hearts four weeks later. Shortly thereafter, other studies in rodents also found an improvement in LV function and reported that some MSCs homed to the infarcted myocardium22 and that SDF-1 may play a role in this process.12 This concept is consistent with the finding in mice, also reported in 2004, that SDF-1/CXCR-4 interactions play a crucial role in the recruitment of i.v. injected BM unfractionated cells to the infarcted heart.33 Over the ensuing decade, evidence supporting the utility of i.v. cell therapy has grown considerably. With rare exceptions,14 beneficial effects of i.v. cell therapy on LV function have been observed in a variety of experimental settings (acute MI,11–13, 20, 22, 23, 26–32, 34, 35 chronic MI,14, 20 and nonischemic cardiomyopathy24, 25, 35, 36) and with various cells types, including naïve BM MSCs,11–14, 20, 22, 24–31 stromal vascular fraction from adipose tissue combined with cultured adipose-derived cells,23 and cells pretreated with azacytidine24, 27 or atorvastatin26 (Table 1).
Given the promising results of the studies in rodents, the i.v. route of cell delivery has also been evaluated in large animal models. All studies published so far were conducted in pigs subjected to acute MI (Table 1).28–32 Price et al.28 reported that administration of allogeneic MSCs 30 min after reperfusion resulted in improved LV EF and reduced LV hypertrophy 3 months later. Improved LV function was also observed by Halkos et al.30 12 weeks after infusion of allogeneic MSCs, given 15 min after occlusion/reperfusion, concomitant with augmented vasculogenesis and perfusion in the infarcted region; interestingly, escalating doses of MSCs (1, 3, and 10 million/kg) did not produce dose-dependent effects.30 Consistent with these reports, improved LV function and smaller infarct size were observed 1 month after treatment with autologous MSCs29 and autologous, allogeneic porcine, or human MSCs31, given 48 h after MI. At 4 weeks after MI, MSCs could still be found in the peri-infarct region, although they were more abundant in the lungs and liver29, a distribution that resembles that reported 24 h after MSC injection in mice.20 In non-immunosuppressed pigs, both a single i.v. dose (150 million) and repeated doses (50 million/day for three consecutive days) of human adipose-derived stem cells (hASCs), given immediately after MI, have been reported to improve LV function and coronary flow reserve, promote angiogenesis, and decrease the myocardial perfusion defect 28 days later.32 The hASCs also had systemic immunomodulatory effects, e.g., they prevented the decrease in circulating CD8+ T cells at 7 days after MI. At 2 h after injection, most (>85%) hASCs were trapped in the lungs and only few could be found in the heart (in the peri-infarct area); by 28 days, hASCs disappeared in both tissues.32
Distribution of Cells After i.v. Injection
Do cells home to the heart after i.v. injection? Several studies have examined the distribution and retention of MSCs delivered by the i.v. route. In rat models of acute MI, it has been reported that only ~3% of the transplanted cells are detectable in the myocardium 24 h after delivery,11 and that this number becomes negligible (<200 cells/mm2) by 3 days.12 In this latter study,12 cell retention in the heart at 3 days after injection was greater if MSCs were given at 1 day after MI compared with earlier or later time points, but in all cases retention was minimal.12 Similarly, when MSCs were injected in rats 10–14 days after MI, most were trapped in the lung and <1% could be found in the heart 4 h later (mainly in the infarcted region).37
Lee et al.13 delivered 2×106 human MSCs (hMSCs) i.v. to immunodeficient NOD/SCID mice after acute MI; 99% of the infused cells were cleared from the circulation within 5 min and almost all of them were entrapped in the lungs (from which they disappeared with a half-life of ~24 h). Only a minuscule number (0.04% of injected cells, averaging ~400 cells) were found in the heart 15 min after infusion; this number increased at 1 day to an average of 1,480 cells (0.15% of injected cells) (likely reflecting redistribution from the lungs), but declined to zero by 3 days.13 Despite such low retention in the heart, however, infarct size was reduced and LV function was improved 21 days later. Based on additional studies, the authors attributed this effect to secretion of the TSG-6 - a protein with strong anti-inflammatory actions13, 38 - by hMSCs trapped in the lungs.13 Specifically, transcriptomic analysis of hMSCs recovered from the lungs 10 h after injection showed marked upregulation of the message for TSG-6. The reparative effets of hMSCs were recapitulated in part by recombinanat TSG-6 but not by i.v. injection of hMSCs with TSG-6 knockdown13. Similarly, using a rat model of chronic MI, Wang and colleagues14 found that 1 day after infusion of 5×106 MSCs, most (52%) injected cells were trapped in the lungs and only very few (<1%) were retained in the heart; at 30 days, no MSCs were detected in either the lungs or the heart.
As expected, when i.v. infusion was compared with other delivery routes, myocardial cell retention was found to be much lower in the former. In rats with acute or recent MI, the number of BM MSCS retained in the heart was much less 4 h after i.v. delivery than 4 h after infusion into the LV cavity (in both cases it was <1% of the injected dose); again, most cells infused by the i.v. route were found in the lungs.37 Similarly, in rats with acute MI, no BM MSCs could be detected in the heart 1 week after i.v. injection, whereas 15% of the injected cells were still present after epicardial injection.39 Freyman et al.10 compared i.v., i.c., and transendocardial delivery of allogeneic BM MSCs in pigs immediately after MI; two weeks after cell delivery, 6% and 3% of the injected cells were present in the heart when the MSCs were given via the i.c. and transendocardial route, respectively, but none could be detected after i.v. injection. More than 20% of injected MSCs were in the lung at 14 days after i.v. delivery; interestingly, significant MSC retention in the lung was observed also after i.c. delivery (similar to that seen after i.v. injection) and after transendocardial delivery (approximately one-third of that after i.v. injection), indicating that the lung is a major site of homing for transplanted cells no matter what route is used.10 In a pig model of acute MI, i.c delivery resulted in a 10-fold higher number of cells in the reperfused myocardium compared with i.v. delivery.40
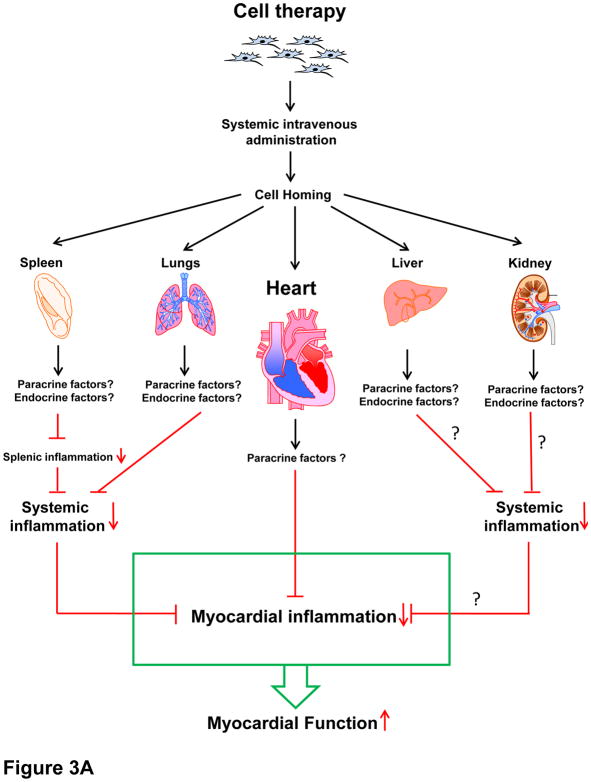
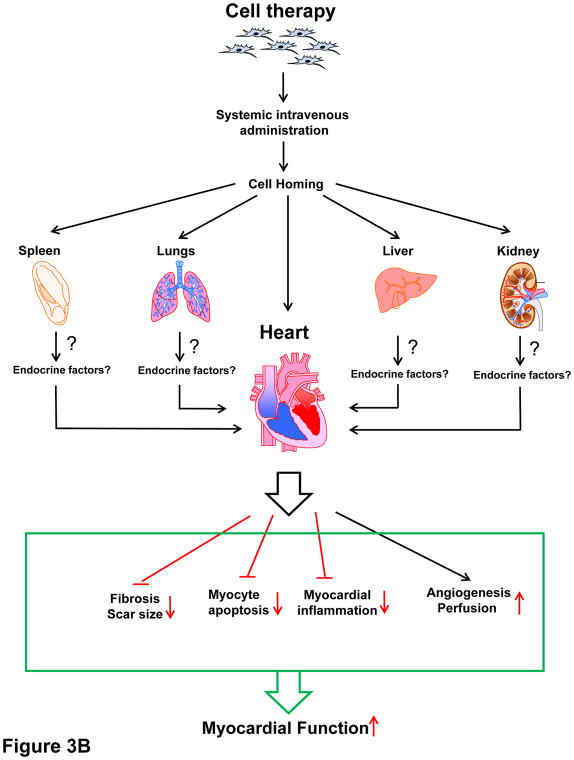
In summary, all available studies indicate that after i.v. administration, the fraction of MSCs that home to the heart within 24 h is minuscule (≤3% of the total number injected) and that it becomes essentially nil by 7 days. All studies have consistently documented significant MSC entrapment in the lungs and, in some cases, 20 in other extracardiac organs (spleen, liver, kidney) (vide infra) (Figure 3A and B).
Figure 3. Potential mechanisms of intravenous cell therapy.
Intravenously injected cells home to various organs and affect the heart in a paracrine and/or endocrine fashion. (A) Paracrine and/or endocrine anti-inflammatory actions. Transplanted cells trapped in extracardiac organs may affect local inflammatory cells (paracrine action) that are released into the circulation and/or they may affect systemic inflammatory cells (endocrine action via release of immunomodulatory factors), ultimately leading to a reduction in myocardial inflammation. For example, cells trapped in the lungs may modulate immune cells circulating through the lungs (paracrine mechanism) and/or release factors (cytokines, miRs, EVs, etc.) that modulate immune cells systemically (endocrine effect). (B). Other endocrine actions. In addition, cells trapped in the lungs may release factors (cytokines, miRs, EVs, etc.) that affect the myocardial milieu at a distance (endocrine effects), resulting in a reduction of scar size, fibrosis in viable tissue, and apoptosis, and in an increase in angiogenesis and myocardial perfusion, thereby leading to improved myocardial function. In theory, transplanted cells that home to the heart may also exert paracrine effects, but the number of such cells is very low.
Mechanism of Action of Cells After i.v. Delivery: Are Cells a New Endocrine Organ?
The mechanism(s) responsible for the salubrious effects of i.v. therapy remains to be elucidated. As detailed above, all studies have demonstrated that cells injected i.v. accumulate preferentially in the lungs,13, 14, 28, 37, 41,29, 32 and that homing to the heart is minimal.13, 14, 29, 37, 41,32 This implies a systemic mechanism of action (Figure 3A and B).
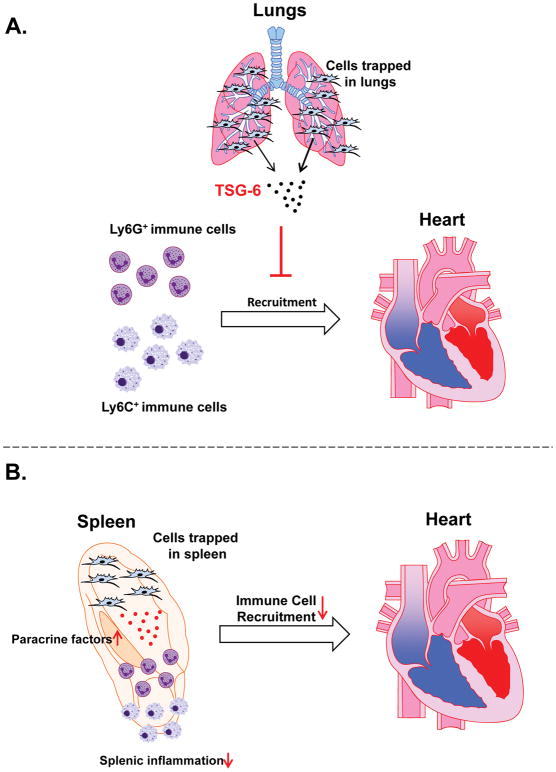
It is possible that transplanted cells trapped in the lungs (and other organs) release immunomodulatory signals that produce systemic anti-inflammatory effects leading to reduced inflammation in the myocardium (Figures 3A and 4A). This hypothesis is supported by multiple lines of evidence. Previous studies have documented the ability of i.v. injected cells (e.g., MSCs) that are trapped in the lungs to affect distant organs 13, 32, 42–44. For example, Noort et al.42 showed that when hMSCs are infused i.v. into mice, they reside in the lungs and promote the engraftment of CD34+ cells into the bone marrow and spleen by mechanisms that do not require hMSC homing in these tissues. Similarly, ASCs delivered by the i.v. route have systemic activity including effects on BM progenitors and adipose tissue mass.43 As mentioned above, Lee et al. reported that the beneficial effects of i.v. hMSCs in mice were mediated in part by the secretion of the anti-inflammatory protein, TSG-6, by hMSCs trapped in the lungs13 (Figure 4A).
Figure 4. Examples of paracrine/endocrine actions of intravenously delivered cells.
(A) Intravenously injected cells trapped in the lungs produce TSG-6, which has anti-inflammatory actions and inhibits myocardial recruitment of Ly6G+ and Ly6C+ immune cells. This is an example of endocrine actions of transplanted cells. (B) Intravenously injected cells home to the spleen and modulate splenic inflammatory cells by secreting paracrine factors; this reduces immune cells recruitment in the myocardium.
Immune cells are known to travel via the blood and lymph between the hematopoietic organs (BM and spleen), lymphoid tissues, and various organs including the heart.45–47 Therefore, a constant turnover of immune cells is part of tissue and organ homeostasis. Since i.v. injected cells, such as BM MSCs, can home to the spleen13, 20 and various lymphoid and non-lymphoid organs,13, 14, 28, 37, 41,29, 32 it is conceivable that they can regulate the locally residing host immune cells, which in turn may affect systemic and local myocardial inflammation (Figure 4B). There is some evidence in support of this concept. In the study by Lee et al. mentioned above,13 i.v. hMSCs, but not hMSCs with TGS-6 knockdown, reduced myocardial infiltration of inflammatory cells, assessed by fluorescent microscopy with Ly6G and Ly6C staining. However, a detailed analysis to identify the specific cell populations in the myocardium was not performed. Dayan et al.48 administered hMSCs or human umbilical cord perivascular cells i.v. 48 h after coronary artery ligation in immunodeficient NOD/SCID mice. They found improved LV function 2 and 4 weeks, but not 16 weeks, later, suggesting that the infused cells had only a short-term effect on myocardial function. Intravenous administration of cells reduced the number of macrophages in the myocardium, and the remaining macrophages had a pro-reparative/anti-inflammatory phenotype; this was accompanied by a decrease in myocardial mRNA expression of pro-inflammatory cytokines (IL-1β and IL-6) and an increase in that of the anti-inflammatory cytokine, IL-10. There was also a reduction in cardiomyocyte apoptosis, suggesting a cardioprotective effect of cell therapy48. Decreased myocardial infiltration by macrophages was also observed when adipose-derived MSCs were injected i.v. at 7 days (but not at 1 day) after a reperfused MI in rats, concomitant with a reduction in scar size49. Hong et al. found that the therapeutic actions of i.v. hASCs in pigs were associated with preservation of circulating CD8+ T cells.32
Further mechanistic insights were recently provided by Luger et al.,20 who examined the effects of i.v. delivery of hMSCs in immunocompetent mice in two models, acute MI and old MI (chronic ischemic cardiomyopathy), in which cells were given 24 h or 4 weeks after coronary artery occlusion/reperfusion, respectively. hMSCs were grown at physiological oxygen levels (5%). When hMSCs were injected 24 h after MI, hMSC concentration 1 day later was much higher (20–40 fold higher) in the kidney, liver, and spleen than in the heart, where hMSCs were found mostly in the infarcted region. (Interestingly, in this model hMSC content in the lung was 25–50% of that in the kidney, liver, and spleen, but still greater than in the heart, which was very low.) Despite minimal myocardial homing, in the acute MI setting hMSCs reduced adverse LV remodeling and dysfunction at 21 days. Similar salubrious effects were observed in the setting of an old MI, in which hMSC-treated mice exhibited a significant improvement in LV end-systolic volume and LV EF. Evidence was provided that these beneficial effects of hMSCs were associated with immunomodulatory actions. Flow cytometric analysis showed that administration of hMSCs 24 h after MI resulted, 7 days later, in a significant reduction in the splenic and myocardial content of NK cells and in the myocardial content of neutrophils; in vitro studies showed that MSCs suppressed NK cell proliferation via secreted factors. In the model of old MI, injection of MSCs lowered the content of myeloid cells in the spleen. A causal role of NK cell depletion in the salubrious effects of hMSCs was supported by the finding that these effects were mimicked when NK cells were depleted systemically with antibodies given prior to MI (interpretation of these data, however, is complicated by the fact that NK cell-depleted mice also exhibited a reduction in infarct size at 21 days; if this reduction occurred early as a result of acute cardioprotection during ischemia/reperfusion, it could explain the improvement in LV function and volumes 21 days later).
Taken together, the observations of Luger et al.20 suggest that i.v. administration of hMSCs improves cardiac function in both the acute MI and the old MI setting, and that these effects are mediated in part by systemic anti-inflammatory actions, specifically, a reduction in the myocardial infiltration of NK cells.20 However, the results must be interpreted with caution because the setting of xenogenic hMSC transplantation in immunocompetent mice differs from the clinical setting of allogeneic MSC transplantation (hMSCs in humans). Further studies need to be performed in allogeneic and syngeneic models to confirm these findings. In this regard, a reduction in circulating NK cells after i.v. injection of MSCs in humans has recently been reported by Butler et al.36 (vide infra).
In summary, the mechanism(s) of i.v. cell therapy remains unclear, but the available evidence suggests that trapping of cells in the lung, spleen, and other extracardiac tissues may result in anti-inflammatory effects, either via paracrine actions of the cells that are lodged in extracardiac tissues or via endocrine actions of these cells, i.e., systemic release of anti-inflammatory factors (Figure 3A and B). In addition, since MSCs and other stem/progenitor cells secrete a panoply of extracellular vesicles, cytokines, non-coding RNAs, etc.,50 it is possible that endocrine release of these factors into the circulation from extracardiac sites may exert other actions that are beneficial to the infarcted heart, e.g., anti-apoptotic, angiogenic, and anti-fibrotic actions. In this scenario, transplanted cells may act as a new (albeit transient) endocrine organ. This is a major area for future investigation, one that may unveil novel cell-free approaches to the treatment of HF.
It is important to note that the systemic and extracardiac actions of cells delivered i.v. could also be operative when cells are delivered via other routes, because many cells home to the lungs even after i.c., epicardial, or transendocardial delivery.51 Using a highly sensitive and accurate PCR-based method to quantify transplanted cells, we have shown that after i.c. infusion of c-kit+ cardiac progenitor cells (CPCs) in mice with acute MI, the number of CPCs present in the lungs 24 h later was more than double that in the heart, and that at 7 days and 35 days, the number of cells found in the lungs and in the heart was similar.9 Even after intramyocardial (epicardial) injection, the number of CPCs present in the lungs is similar to that present in the heart at 5 min and 1 day.8 Therefore, it is conceivable that when cells are delivered locally to the heart, the salubrious effects of cell therapy could be underlain, at least in part, by systemic actions (anti-inflammatory or otherwise) elicited by cells lodged in the lungs, spleen, and other extracardiac tissues. If proven correct, this scenario would be a major shift in our understanding of the mechanism of action of cell therapy in general.
Summary of Preclinical Studies
In summary, a considerable body of preclinical evidence (>15 studies) suggests that i.v. cell therapy exerts beneficial effects on LV function after MI. As shown in Table 1, the data reported in rodents11–14, 20, 22–27 suggest that i.v. infusion of MSCs is effective in alleviating MI-induced LV dysfunction both in the setting of an acute and an old MI. Similarly, the five studies reported in pigs28–32 indicate that i.v. administration of MSCs (autologous, allogeneic, or even xenogeneic) soon (within 48 h) after MI is effective in alleviating subsequent LV dysfunction. One notable gap is the lack of data regarding the effects of i.v. cell therapy in large animal models of chronic ischemic cardiomyopathy (old MI); such data are necessary to translate i.v. cell therapy to the large population of patients with chronic ischemic HF. Filling this gap should be a priority for future investigations. Since almost all studies have used MSCs, further work with other cell types will be important to fully evaluate the utility of i.v. cell therapy. The i.v. route of cell delivery appears to be safe, as no adverse effects have been reported in animals despite infusion of relatively large numbers of cells.
The mechanism of action of i.v. cell therapy remains speculative but clearly does not involve engraftment of exogenous cells in the heart. Rather, the transplanted cells must act by releasing factors that act at a distance (endocrine action) or by modulating cells that circulate in the blood (such as immune cells) (Figure 3A, 3B, 4A, 4B). A few animal studies13, 20, 48 and one small clinical trial36 suggest that, in HF, the improvement in LV function elicited by i.v. administration of MSCs is associated with systemic anti-inflammatory actions in vivo. Whether the functional benefits are caused by the anti-inflammatory actions is unclear. It also remains unclear whether other cells types exhibit similar effects when given by the i.v. route. These are important areas for future investigation. The recognition that the beneficial effects of i.v. cell therapy in HF are mediated by systemic anti-inflammatory actions would usher in a novel strategy for the management of this syndrome based on inhibition of delerious immune responses. Such a concept would be consistent with clinical trials showing systemic anti-inflammatory and immunomodulatory actions of i.v. cells therapy in various conditions, including graft-versus-host disease, multiple sclerosis, systemic lupus erythematosous, chronic obstructive pulmonaty disease, and Crohn’s disease52–58.
Clinical Studies
The encouraging results of the preclinical studies summarized above have motivated investigation of i.v. cell delivery in humans. Three randomized clinical trials have been reported thus far (Table 2).34–36 The first was by Hare et al.,34 who performed a double-blind, placebo-controlled phase I study in which 53 patients with acute MI (either ST-elevation or non-ST-elevation MI occurring 1–10 days prior to randomization) and LV EF ≥ 30% and ≤ 60% received different doses (0.5, 1.6, and 5 million cells/kg) of allogeneic MSCs and were followed for 6 months. The i.v. delivery of MSCs appeared to be safe, with similar adverse event rates between the MSC- and placebo-treated groups. A functional beneficial effect of MSCs was supported by the observation that, in patients who sustained an anterior MI, LV EF (measured by echocardiography) improved significantly at 6 months vs. baseline values in MSC-treated patients but not in in the placebo group. In a subset of patients who underwent cardiac MRI, MSC administration was associated with a significant increase in LV EF at 12 months vs. baseline; furthermore, when plotted relative to EF, MSC-treated patients exhibited evidence of reverse LV remodeling with no increase in LV end-diastolic volume and a decline in LV end-systolic volume, whereas placebo patients demonstrated evidence of LV chamber enlargement. As in the porcine study by Halkos et al.,30 no dose-dependent effects of MSCs were observed. This trial provided evidence of safety, and provisional evidence of efficacy, of allogeneic MSCs in patients with acute MI.34
Table 2.
Clinical Studies of Intravenous Cell Therapy
| Study | Trial Characteristics | Clinical Setting | Cell Type | Cell dose | Follow-up | Outcomes |
|---|---|---|---|---|---|---|
| Hare,34 2009 | Randomized (i.v. MSCs = 34, control = 19) | Acute MI, RWMA EF ≥ 30% to ≤ 60% |
BM MSC, allogeneic | 0.5 × 106 1.6 × 106 5 × 106 |
6 months | ↓ arrhythmia burden ↑ EF (more in AWMI) ↔ wall thickness ↑ global assessment scale |
| Butler,36 2017 | Randomized (i.v. MSCs = 10, control = 12) | Nonischemic EF ≤ 40% |
BM MSC, allogeneic | 1 × 106 | 90 d | ↑ 6MWD ↑ Kansas City Questionnaire |
| Bartolucci,35 2017 | Randomized (i.v. MSCs = 15, control = 15) | HFrEF (ischemic and nonischemic) | UC MSC, allogeneic | 1 × 106 | 12 months | ↑ EF, ↑ NYHA class ↑ MLHFQ |
Abbreviations: AZA, azacytidine; AD SC, adipose-derived stem cells; SVF, stromal vascular fraction; BM MSCs, bone marrow mesenchymal stromal cells; UC MSCs, umbilical cord-derived mesenchymal stromal cells; EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association class; 6MWD, six-minute walk distance; MLHFQ, Minnesota living with heart failure questionnaire; LV, Left ventricular; IS, infarct size; FAS, fractional area shortening.
In 2017 Butler et al.36 reported the results of a single-blind, placebo-controlled, crossover, randomized, phase IIa trial of i.v. allogeneic MSCs (grown under hypoxic conditions [5% O2]) in patients with nonischemic cardiomyopathy (LV EF<40%). Subjects were randomized to i.v. MSCs (n=10, 1.5×106 cells/kg) or placebo (n=12) followed by crossover at 90 days, when each group received the alternative treatment. Although LV EF and LV volumes (measured by MRI) did not differ between the two groups, a small, but statistically significant, decrease in LV volumes and increase in LV EF were observed 90 days after MSC infusion in the treated cohort but not in placebo-treated patients. MSC-treated subjects exhibited a significant increase in the 6-min walk distance and in the Kansas City Cardiomyopathy Questionnaire (KCCQ) clinical summary score and functional status score compared with placebo-treated patients. Administration of MSCs, but not placebo, was associated with a significant decrease in circulating NK cells and a significant increase in CD3 and CD4 cells; the magnitude of the reduction in NK cells was inversely related to the magnitude of the increase on LV EF. There were no discernible differences in serious adverse events between the two groups. This study further supports the concept that i.v. administration of MSCs is safe; in addition, it provides evidence of systemic immunomodulatory effects (as evidenced by the reduction in NK cells, which is consistent with the observations of Luger et al. in mice20) and it suggests that i.v. MSC delivery may improve clinical end-points and functional status in nonischemic cardiomyopathy.36
The RIMECARD trial,35 also published in 2017, was a phase I, randomized, double-blind, and placebo-controlled study in which patients with either ischemic or nonischemic cardiomyopathy were assigned to i.v. infusion of allogeneic umbilical cord (UC)-derived MSCs (1×106 cells/kg) or placebo (n = 15 per group) and were followed for 1 year. No adverse events were reported, suggesting again that i.v. delivery of cells was safe. None of the patients tested developed alloantibodies to the injected cells. Compared with baseline values, patients given UC-derived MSCs, but not those receiving placebo, exhibited a significant improvement in LV EF (assessed by echocardiography as well as MRI) vs. baseline that became apparent at 3 months and was sustained at 6 and 12 months, although LV EF did not differ significantly between the two groups. Throughout the follow-up, the NYHA class, Minnesota Living with Heart Failure questionnaire [MLHFQ] score, and KCCQ score improved in the MSC-treated group, but not in the control group. In vitro studies demonstrated that UC-derived MSCs and BM-derived MSCs produced similar degrees of inhibition of T cell proliferation, suggesting similar immunosuppressive properties. Although the secretomes of the two cell types were similar, UC-MSCs exhibited higher expression of hepatocyte growth factor. This study adds to the evidence that i.v. cell therapy is safe and suggests that UC-derived MSCs may improve LV function, functional status, and quality of life in patients with HFrEF of ischemic or nonischemic origin.35
Taken together, the results of these three trials, 34–36 conducted in three different patient populations (acute MI, ischemic cardiomyopathy, and nonischemic cardiomyopathy) (Table 2), suggest that i.v. infusion of MSCs (allogeneic or autologous) is well-tolerated, and that no humoral immune reaction develops to allogeneic MSCs. The initial results with respect to efficacy are encouraging and warrant further investigation. Since these studies were small and none of the them was designed to assess efficacy, larger phase II trials are in order to determine the impact of i.v. MSC delivery on LV function, LV remodeling, functional status, exercise capacity, and other relevant clinical outcomes as well as to further establish the safety of i.v. cell delivery. It will also be important to compare the i.v. route with other routes of delivery in direct head-to-head comparisons. The i.v. route offers many major potential advantages, including the fact that it is simple, it does not require expensive equipment or specialized training, it could be implemented in almost every medical facility, and perhaps most importantly, it lends itself to repeated cell administrations, which could be performed easily and inexpensively, even as outpatient procedures. Future studies will be necessary to elucidate the optimal protocols for repeated i.v. infusions of cells (e.g., number, frequency, dose, etc.). If i.v. administration of cell products proves to be safe and effective, its use would be a major revolution in the field of cell-based therapies.
Immunomodulatory Actions of Cell Therapy
The recognition that cells do not engraft in the heart to a significant extent1, 9, 16, 17,8 has stimulated a search for new mechanisms underlying their beneficial effects. One emerging mechanism whereby cell therapy (given i.v or by other routes) may improve LV function is via anti-inflammatory actions. In this section we review current knowledge of the effects of cell therapy on the immune system.
Immune response after MI
Cell death resulting from myocardial ischemia induces activation of the complement system, production of ROS, and release of danger-associated molecular patterns 59–64, all of which trigger intense sterile inflammation and infiltration of neutrophils and pro-inflammatory monocytes that digest and clear the dead tissue62, 63, 65–68. This short-lived pro-inflammatory phase, usually lasting a few days, is necessary for a subsequent reparative phase characterized by resolution of inflammation, accumulation of reparative macrophages, removal of apoptotic neutrophils, neovascularization, proliferation of stromal cells, and deposition of extracellular matrix63–65, 69–71. At the end of the reparative phase, immune cells leave the infarcted region and the scar undergoes maturation, with extracellular matrix crosslinking and deactivation and apoptosis of reparative cells63, 72–76. That this inflammatory response plays a crucial role in cardiac repair is demonstrated by the fact that both nonselective inhibition of inflammation after MI with corticosteroids or non-steroidal anti-inflammatory drugs and systemic depletion of neutrophils or macrophages prior to MI have detrimental effects on myocardial repair, leading to rupture and exacerbation of adverse LV remodeling45, 67, 77–79. However, although the early immune response is essential for myocardial repair, infarcted wall stabilization, and preservation of LV function, incomplete resolution of inflammation after the reparative phase contributes to HF progression. Indeed, chronic ischemic HF is associated with accumulation of monocytes/macrophages, dendritic cells, and T cells in the myocardium (including the noninfarcted region) and with systemic expansion of immune cells resulting in their increase in the spleen and in the peripheral blood47, 78, 80, 81. This chronic, non-resolving inflammation contributes to the progression of adverse remodeling and worsens LV function47, 63, 80, 82–84.
Immunomodulatory actions of cell therapy
The discovery that BM MSCs suppress T cell proliferation led to further studies investigating their immunomodulatory properties.85, 86 An extensive body of work has now demonstrated that MSCs can modulate both innate and adaptive immunity, in vitro as well as in vivo87, 88 (Figure 5). Indeed, numerous phase I, II, and III clinical trials are currently testing the immunomodulatory actions of MSCs in the treatment of inflammatory diseases, including graft-versus-host disease (GVHD), Crohn’s disease, ulcerative colitis, multiple sclerosis, and systemic lupus erythematosus (see ClinicalTrials.gov).87, 88 Because the immune system is involved not only in tissue healing acutely after MI, but also in the progression of HF in the chronic phase after MI, the immunomodulatory actions of cell therapy in the treatment of heart disease constitute a new, important avenue for basic and clinical research.63, 73, 77, 89, 90 Cell therapy appears to be a useful tool to identify and harness the salutary components of the immune system that lead to improved outcome in HF.
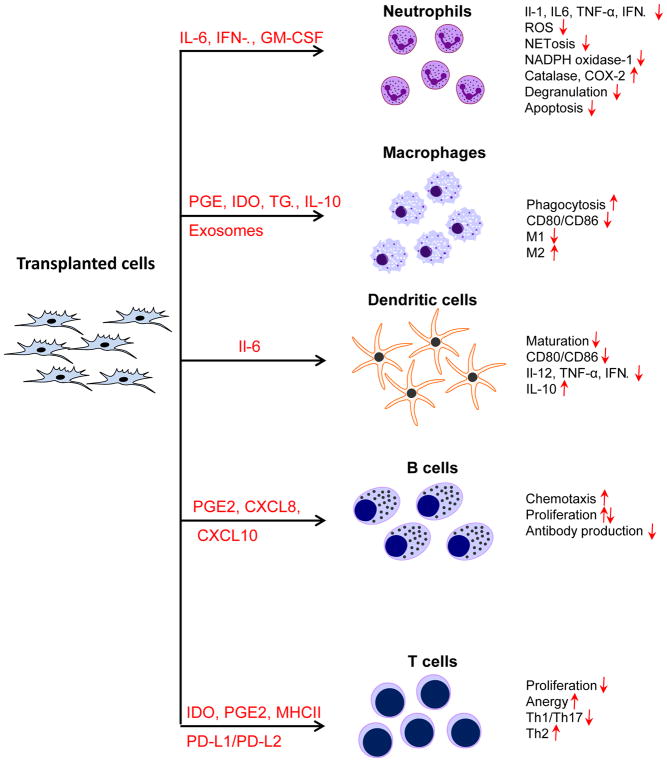
Figure 5. Potential mechanisms whereby transplanted cells modulate the various components of the immune system.
Cells injected into the host secrete a panoply of factors that affect the function of various immune cells, locally (paracrine actions) and potentially at a distance (endocrine actions), as detailed in the text.
Effects on neutrophils
Neutrophils infiltrate infarcted myocardium within minutes after reperfusion in response to DAMPs, cytokines, chemokines, endogenous lipid mediators (e.g. leukotriene B4), histamine, and complement 3 and 5 cleavage components. Extravasated neutrophils secrete pro-inflammatory mediators (IL-1, IL-6, TNF-α), granules containing proteolytic enzymes (e.g., acid hydrolases, elastase, neutral serine proteases, lysozyme, cathepsin G, proteinase 3), neutrophil extracellular traps (NETs), and ROS59, 61, 62, 64, 91–95, which not only perpetuate the inflammatory response, but also contribute to the clearance of dead cells and extracellular matrix debris. Neutrophil depletion prior to MI dysregulates monocyte/macrophage function, promotes adverse remodeling, and worsens LV function74, 86, 87, 91. On the other hand, in the chronic phase of HF, an elevated neutrophil-to-lymphocyte ratio correlates with the severity of the disease and is an indicator of poor prognosis.96–99 Both in vivo and in vitro experiments indicate that neutrophils may have cytotoxic effects and contribute further injury to the myocardium100 Therefore, neutrophils are double-edged swords in myocardial repair.
Little is known regarding the impact of cell therapy on neutrophil function after MI. In vitro studies show that co-culture of neutrophils with BM MSCs extends the lifespan of neutrophils by inhibiting apoptosis, reduces production of ROS and pro-inflammatory cytokines (IL-1, IL-6, TNF-α), inhibits degranulation, NET formation, and expression of NADPH oxidase-1, and increases expression of catalase and cyclooxygenase-2.89, 100–103 Neutralizing experiments have revealed that these effects of BM MSCs on neutrophils are accounted for by production of IL-6, interferon-β (IFN-β), and granulocyte macrophage colony-stimulating factor.89, 100–103 Administration of cord blood mononuclear cells, which contain MSCs, at the time of coronary occlusion reduces neutrophil infiltration and expression of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β) and chemokines (MCP-1, MIP, fractalkine);104 however, the mechanisms responsible for these phenomena are not well defined. For example, it is unknown whether the injected cells regulate neutrophil function directly or indirectly through changes in immune cytokine expression. To date, no systematic studies are available regarding the effects of cell therapy on neutrophil function in vivo, particularly in the setting of myocardial repair.
Effects on monocytes/macrophages
Monocytes and macrophages are part of the innate immunity and it is well established that they play a key role both in the initial insult to the myocardium and in the chronic phase of cardiac injury. They remove dead tissue and apoptotic cells but also produce cytokines and bioactive lipids that regulate fibroblast and endothelial function, immune cells, and resolution of inflammation105–108. Systemic monocyte and macrophage depletion with clodronate liposomes or splenectomy prior to MI impairs myocardial repair and results in LV rupture45, 67. In the chronic phase after MI, macrophages accumulate in the noninfarcted region of the heart, where they contribute to adverse remodeling and progression of contractile dysfunction47, 80. Therefore, macrophages are important players in the pathophysiology of ischemic heart disease.
The immunomodulatory role of BM MSCs in the regulation of macrophage function has been well established in in vitro co-culture systems, where the MSC secretome induces a pro-reparative and anti-inflammatory phenotype in macrophages but also enhances their phagocytic activity. Within this secretome, a number of MSC-derived factors have been identified that modulate macrophage reparative functions, including PGE2, IDO, TGF-β, and IL-1048, 103, 109–111. In addition, the secretome of cardiosphere-derived cells (CDCs) polarizes macrophages to a phenotype distinct from that of classically or alternatively polarized macrophages, enhances their phagocytosis, and decreases expression of CD80 and CD86, two obligatory T cell co-stimulatory receptors112. Data are also available in vivo that support modulation of macrophages by cell therapy delivered shortly after MI. Infusion of BM MSCs 48 h after MI in mice induces a macrophage switch toward a reparative phenotype111. In rat and pig models of MI, injection of allogeneic CDCs soon after reperfusion results not only in improved LV functional recovery, but also in a decrease in the number of myocardial CD68+ macrophages112–115. Adoptive transfer of macrophages preconditioned with CDCs reproduces the reparative effects without CDC administration112, 114, 115. Further mechanistic studies suggested that CDC-derived exosomes transfer miR-181b, which then mediates the reparative macrophage phenotype through a reduction of PKCδ transcript levels113. Taken together, these data111–115 suggest that the reparative effects of CDCs are mediated through modulation of the resident cardiac macrophage population.
In summary, the actions of cell therapy on macrophage function are well recognized in vitro and in vivo. However, whether these actions are important to the beneficial effects of exogenous cells, particularly in the chronic phase of HF, remains unclear; furthermore, our understanding of the mechanism(s) whereby transplanted cells modulate macrophage reparative functions is poor. Elucidating these issues is a top priority for future studies and may bring about major advances in the field of cell therapy.
Effects on dendritic cells (DCs)
DCs are the most potent antigen-presenting cells in the immune system. They are capable of uptake of myocardial peptides generated by MI and presentation, resulting in T cell activation116. This process modulates myocardial healing in the acute phase after infarction by controlling monocyte/macrophage homeostasis and by activating regulatory and helper T cells117–120. However, infiltration of mature DCs in the chronic phase after MI contributes to adverse remodeling, fibrosis, and deterioration of LV function80. Therefore, similar to neutrophils and macrophages, DCs can be viewed as double-edged swords in the pathogenesis of HF.80, 117, 118
Observations in vitro suggest that MSCs have an inhibitory effect on DCs and activate the regulatory T cell response, favoring myocardial repair and resolution of inflammation.103, 121, 122 Co-culture of MSCs with DCs or their precursors inhibits DC maturation and leads to impaired effector function by decreasing CD80 and CD86 expression, which is necessary for T cell activation121, 123–125. This process is at least partially regulated by MSC-derived IL-6. MSCs also suppress expression of pro-inflammatory cytokines (IL-12, TNF-α, IFN-γ) and promote expression of the anti-inflammatory cytokine IL-10 in DCs. However, virtually nothing is known regarding the effect of cell therapy on DC function in the setting of HF in vivo.
Effect on B cells
B cells originate from the BM where hematopoietic progenitor cells differentiate into pro-B cells, which undergo a series of conformational changes to be finally released into the circulation as immature B cells. Next, they home to the secondary lymphoid tissues and/or spleen, where they become transitional B cells. B cell activation and maturation take place once they come in contact with an antigenic stimulus. That causes the cells to either switch isotype and produce antibodies, or to present the foreign molecule to T cells via the major histocompatibility complex class II (MHCII)126–129. B cells may contribute to the auto-antibody production against cardiac specific antigens that has been implicated in the progression of HF, and also regulate T cell subpopulation balance130, 131.
There is considerable evidence that MSCs can regulate B cell function in vitro. B cells have a strong chemotactic activity towards the MSC secretome that is PGE2-, CXCL8-, and CXCL10-independent132. The specific chemotactic factor(s) secreted by MSCs has not been identified. The effects of MSCs on B cell proliferation are more controversial133. Some studies demonstrated that MSCs inhibit B cell proliferation134, 135 while other show an opposite pro-proliferative effect136, 137. Direct contact with MSCs can inhibit caspase 3-mediated apoptosis of peripheral blood B cells through MSC-induced upregulation of VEGF and p-PKBA activation138. MSCs can also regulate the effector function of B cells; that is, they can inhibit IgM, IgG, and IgA production135. Taken together, these studies indicate that locally injected MSCs can affect B cell homeostasis by promoting their recruitment132, survival 138 and proliferation134–137, and also by regulating their effector function by switching the classes of antibodies produced or facilitating their maturation135.
To date, no studies have been reported examining the effect of cell therapy on B cell function in vivo in the context of myocardial repair. Nevertheless, as pointed out above, there is considerable evidence that MSCs can regulate B cell function. This may be another fertile area for future investigation.
Effect on T cells
T lymphocytes are the primary effector cells of the adaptive immune system. Antigen-specific activation and differentiation of naïve T cells lead to the generation of a range of T cell phenotypes that may be defined by the surface markers expression and by the profile of secreted cytokines139–142. T cells expressing CD8, or cytotoxic T cells (Tc), have a direct cytotoxic effect on the targeted cells expressing foreign antigens presented by MHC class I.143, 144 A wide range of CD4-expressing helper T cells (Th) can be identified by cytokine expression profiling. Type one helper T cells (Th1) express the pro-inflammatory cytokines IFNγ, TNF-α, and IL-2, which activate macrophages and perpetuate the immune response.145, 146 Similarly, Th17 lymphocytes (a subpopulation of CD4-expressing Th cells that produce IL-17) are primarily proinflammatory and regulate neutrophil and macrophage recruitment.147 Excessive activation of the Th1 response can lead to uncontrolled tissue damage. Therefore, the proinflammatory response is counterbalanced by Th2 lymphocytes that produce IL-4, IL-13, and IL10, which promote a reparative and anti-inflammatory phenotype in macrophages.148 Regulatory T cells (T regs) expressing CD4, FOXP3, and CD25 are immunosuppressive and inhibit effector T cell function.149
Because T cells are activated in sterile inflammation, including MI, they have been implicated in the processes related to cardioprotection, infarct healing, and progression of HF150. The first evidence for a role of T cells in heart disease came from observations in Rag1 KO mice, which produce no mature T or B cells. In these mice, infarct size was reduced after ischemia-reperfusion151. Adoptive transfer studies indicated that CD4+ but not CD8+ T cells contributed to the cardiotoxic effects. More detailed analyses showed that IFNγ mediates cardiac injury, suggesting that the Th1 subset is responsible for that effect151. This line of evidence is consistent with another study showing that adoptive transfer of regulatory CD4+ T cells induced protection of ischemic myocardium, which was related to ectonuclease (CD39) expression and formation of adenosine151, 152.
In mouse models of MI, T cells become activated and proliferate in the draining lymph nodes; in addition, both CD4+ and CD8+ T cells are elevated in the myocardium within days after MI119. Further analysis indicated that CD8+ T cell deficiency has no effect on infarct healing but lack of CD4+ T cells leads to impaired myocardial repair and worsens myocardial contractile function119, 150. Mechanistically, Th1 CD4+ T cells contribute to a reduction of Ly6CHIGH monocyte recruitment, as deficiency of CD4+ Th1 cells increased recruitment of monocytes and was associated with worsening of LV function119, 150. The absence of CD4+ T reg lymphocytes led to a proinflammatory skewing of the macrophage phenotype, perpetuated proinflammatory response, and impaired LV function153. Therefore, a proper balance between T helper cell subpopulations is necessary for proper myocardial repair.
It is conceivable that stem/progenitor cells can directly interact with T cells to modulate the immune response after MI. Numerous in vitro studies have shown that BM MSCs inhibit T cell proliferation and differentiation.103 Co-culture with MSCs suppresses both CD4+ Th and CD8+ Tc lymphocyte proliferation through cell cycle arrest in the G0/G1 phase.154 Some MSC-expressed factors have been identified that have an antiproliferative effect on T cells; the most studied are IDO, PGE2, and PD-L1/PD-L2.103 Activated MSCs can also express MHC class II antigens and present the antigen to naïve T cells; however, because of the lack of co-stimulatory signals (CD80, CD86, CD40), T cells undergo anergy instead of full activation and proliferation.155, 156 MSCs can also induce an anti-inflammatory response by inhibiting Th1/Th17 cells and activating Th2 polarization of CD4+ Th cells.157 Moreover, MSCs induce T reg differentiation of naïve T cells, which produces immunostasis of T cells and has further anti-inflammatory effects.157, 158 However, no data are currently available in vivo regarding the effects of MSCs or other cell types on T cell function in models of HF. This is another important area for future investigation.
In summary, it is conceivable that the beneficial effects of cell therapy may be mediated by anti-inflammatory actions in the viable (noninfarcted) myocardium. There is compelling in vitro evidence that cell therapy regulates the function of various immune cell types, and some of the paracrine factors involved in these actions have been identified. In particular, exosomes have been identified as a novel immunomodulatory paracrine mechanism for horizontal transfer of RNAs affecting the targeted immune cell transcriptome and function. Further studies will be necessary to identify the specific components of the exosomes that are responsible for their immunomodulatory function and the downstream pathways in the immune cells that are involved in these effects. In the in vivo setting, evidence supporting immunomodulatory actions of cell therapy is still limited; importantly, it is unclear whether these immunomodulatory actions are causally involved in the improvement in LV function and structure after cell therapy. Future research should aim to identify not only the paracrine factors produced by injected cells but also the specific immune cells and signaling pathways responsible for the salutary effects.
New Cell Types
Over the past two decades, several cell types have been studied for the treatment of HF in animal models and in clinical trials16. Although various degrees of therapeutic benefit have been reported with almost all cell types tested, the generally modest improvement in LV function17, the uniformly limited survival of transplanted cells (regardless of cell type17), and their failure to differentiate into the cardiac lineage17 have motivated the search for new, more effective cell types with superior reparative actions on the failing heart. In recent years, three new cell types have been tested in experimental animal models of MI and HF: cortical bone-derived stem cells159–163, multilineage-differentiating stress enduring cells164, and cardiac mesenchymal cells3, 165.
Cortical Bone-Derived Stem Cells
Mesenchymal cells isolated from cortical bone (CB), or cortical bone-derived stem cells (CBSCs), have properties similar to the MSCs isolated from the bone marrow (BM); however, they are more clonogenic and have superior osteogenic potency; thus, they can be considered a more primitive subset of BM MSCs 163, 166–168. CBSCs express classical mesenchymal markers (CD90, CD105, CD29, CD73, and CD29), lack expression of hematopoietic and endothelial markers (CD45 and CD31, respectively), and possess multilineage differentiation potential (adipocytes, osteoblasts, and chondrocytes) and immunomodulatory properties, both of which are typical MSC characteristics160, 161, 163. Compared with BM MSCs and CDCs, CBSCs exhibit greater expression of CD61 and integrin β4, have enhanced proliferative capacity, and are more resistant to apoptotic stimuli161. CBSCs have been reported to exert more potent reparative actions than c-kit+ CPCs in murine models of HF160. However, as is the case with virtually all other cell types, transplanted CBSCs contribute minimally to cardiomyocytes or endothelium, indicating that their superior reparative potential is accounted for by paracrine actions160. This body of evidence suggests that CBSCs represent a distinct and more primitive population of MSCs with a potent paracrine profile. Studies in a porcine model of ischemic cardiomyopathy have shown that CBSCs are safe and exert reparative actions that are similar to those observed in rodents162. Thus, these cells appear to be promising candidates for translation in clinical trials.
Multilineage-differentiating, stress enduring (Muse) cells
Muse cells are stage-specific embryonic antigen-3 (SSEA-3) expressing cells that reside in the BM169–171, adipose tissue172–174, dermis175, and connective tissue of various organs176, but also circulate in the peripheral blood177. They express pluripotency markers such as Sox2, Oct3/4, and Nanog, are stress tolerant, and secrete pro-survival factors that are protective against cells death. Even though Muse cells are a small fraction of the tissues of origin, they are easily expandable in vitro and thus therapeutically relevant170. After i.v. injection, they have been shown to home to the site of organ or tissue injury and contribute to repair176. Muse cells have been reported to repair diabetic skin ulcers178 and brain179–181, liver171, 182, kidney183, and skin injury184.
Recently, these cells have also been tested for their reparative potential in a rabbit model of MI164. Yamada et al.164 reported that BM-derived rabbit and human Muse cells, expanded in vitro and injected i.v. in immunocompetent rabbits, home to infarcted hearts at 3 days and 2 weeks. The migration and homing of i.v. injected Muse cells were toward a sphingosine-1 phosphate (S1P) gradient in the infarcted myocardium via S1P receptor 2 (S1PR2) expressed on the Muse cells. Co-injection of JTE-013 (an S1PR2 antagonist) or knockdown of the receptor with siRNA resulted in reduced recruitment of Muse cell to the heart164. Some of the cells that homed and engrafted in the infarcted heart expressed cardiac-specific markers, such as cardiac troponin I, sarcomeric α-actinin, and connexin 43, as well as vascular markers (CD31 and α-SMA). Rabbits given Muse cells exhibited a reduction in infarct size (~52%) and an increase in LV EF (~17 units) compared with vehicle-treated animals at 2 months. Interestingly, both allogeneic and xenogeneic Muse cells engrafted and induced recovery of LV function in immunocompetent rabbits for up to 6 months without immunosuppression,164 suggesting that they have efficient mechanisms protecting them from immunorejection. This study164 suggests that Muse cells have potential clinical utility, but these results need to be reproduced in other models; furthermore, the mechanism whereby these cells repair the heart remains unclear. Although some of the Muse cells that engrafted after injection expressed cardiac and vascular specific markers, the number of these cells was too small to explain the recovery of LV function. Thus, like all other cell types, Muse cells most likely repair the heart via paracrine or endocrine mechanisms.
Cardiac Mesenchymal Cells
Cardiac mesenchymal cells (CMCs) are the myocardial equivalents of BM MSCs. A population of mesenchymal (or stromal) cells residing in the human heart was described in 2011 by Rossini et al.185, who reported that these cells (termed cardiac mesenchymal or stromal cells) have a phenotype similar to BM MSCs, express mesenchymal markers (CD105, CD73, CD29, CD44, and CD13), and are negative for CD34, CD45, CD14, CD117 (c-kit), CD31, VEGFR2, CD144, and HLA-DR. Expression of CD90 was heterogeneous but lower than in BM MSCs. Transcriptome analysis showed no difference in the expression of pluripotent markers (Oct-4, Nanog, Sox-2, Klf4, and MDR-1) between CMCs and BM MSCs; the expression of cardiac markers (Nkx2.5, alpha-MHC, TnI) was low or absent in both cell populations. However, CMCs had a distinct intracellular miRNA profile compared with BM MSCs. In immunosuppressed rats that received human cells 3 weeks after coronary artery ligation, CMCs, but not BM MSCs, reduced LV end-diastolic pressure and increased dP/dt, although both cells reduced LV weight compared with vehicle-injected rats. Similar to many other cell types, CMC survival after transplantation was negligible; therefore, the functional improvement was most likely related to secretion of paracrine factors185.
We have recently performed a series of studies to evaluate the therapeutic potential of CMCs and to identify their most effective subsets. We have stratified CMCs into two subpopulations based on their adherence to plastic tissue culture dishes. Rapidly adhering cells (RA-CMCs) adhere within hours from the initial plating, whereas slowly adhering cells (SA-CMCs) requires days to attach165, 186. Both cell populations have a similar fibroblast-like morphology, express typical mesenchymal markers (CD90, CD105, CD29, CD73, CD44), and are negative for CD45, CD31, CD34, and c-kit165. Despite their indistinguishable morphology and phenotype, transcriptomic analysis showed striking differences between these two cell types. CMCs were studied in vivo using appropriate protocols and data were analyzed as previously described 187–191. When injected intramyocardially in mice 2 days after MI, SA-CMCs, but not RA-CMCs, improved LV function 1 month later165. However, a negligible number of transplanted cells (either RA-CMCs or SA-CMCs) survived at 1 month after injection. In parallel to the changes in LV function, SA-CMCs, but not RA-CMCs, reduced fibrosis in the noninfarcted region and lowered the number of immune cells detected with CD45 staining at 1 month after cell injection. In vitro experiments showed that compared with RA-CMCs, SA-CMCs exert stronger anti-inflammatory effects on M1 polarized BM macrophages165. Administration of repetitive doses of SA-CMCs in a murine model of chronic ischemic cardiomyopathy resulted in cumulative beneficial effects3, 165, 186 similar to those of c-kit+ CPCs in rats.2
Taken together, these data3, 165, 186 demonstrate that slow adherence to plastic identifies a population of cells with robust therapeutic potential and anti-fibrotic and anti-inflammatory properties. This simple method of CMC stratification has potential clinical application, as SA-CMCs can be isolated and expanded from a small tissue sample, for example, endomyocardial biopsies (EMBs). Indeed, we have recently found that large quantities of CMCs, sufficient for therapeutic administration, can be produced from EMBs harvested in pigs. Compared with c-kit positive CPCs, c-kit negative CMCs offer several advantages. Their expansion from EMBs to generate therapeutically relevant quantities (several million) is simpler (not requiring immunosorting) and thus more consistent and less prone to controversies; it is also faster (because of much higher starting numbers in myocardial tissue), thereby enabling administration of younger cells at low passage numbers. For example, the time required to generate 5 million or more cells would be ~2 weeks for CMCs vs. ~ 2 months for CMCs. For these reasons, the use of CMCs may be cheaper and more widely applicable than c-kit positive CPCs. CMCs are therefore an attractive new option for clinical use. Preclinical studies in pigs are currently ongoing in our lab to confirm the above results in rodents, with a goal toward clinical translation.
Concluding remarks
The field of cell therapy is evolving rapidly. The fundamental shift has been the recognition that all cell types, both adult and embryonic, fail to engraft in the heart, and instead work via paracrine mechanisms1–3, 5–7, 9, 165. This has led to new paradigms and therapeutic approaches, which may revolutionize cell therapy. Since transplanted cells do not persist in the heart more than a few weeks, it seems logical to give repeated doses. Since cells work by releasing factors in the environment, i.v. therapy may also be effective via systemic release of these factors. Since cells do not differentiate into new myocytes, alternative mechanisms are being explored, such as anti-inflammatory and anti-fibrotic actions. Since the cells studied heretofore exhibit limited survival and efficacy, new cell types have been investigated. Future preclinical and clinical research needs to focus on these new strategies, which may finally make cell therapy a reality.
Acknowledgments
This work was supported by NIH Grants P01 HL078825 (to RB and MW) and UM1 HL113530 (to RB).
Non standard abbreviations and acronyms
- BM
bone marrow
- CBSCs
cortical bone-derived stem cells
- CDCs
cardiosphere-derived cells
- CMCs
cardiac mesenchymal cells
- CPCs
cardiac progenitor cells
- EF
ejection fraction
- FISH
fluorescence in situ hybridization
- GVHD
graft-versus-host disease
- hASCs
human adipose-derived stem cells
- IL
interleukin
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- KCCQ
Kansas City Cardiomyopathy questionnaire
- LV
left ventricle/left ventricular
- MI
myocardial infarction
- MLHFQ
Minnesota Living with Heart Failure questionnaire
- MRI
magnetic resonance imaging
- MSCs
mesenchymal stromal cells
- MUSE
multilineage, stress enduring
- NETs
neutrophil extracellular traps
- NK
natural killer
- NYHA
New Your Heart Association
- RA-CMCs
rapidly-adhering CMCs
- ROS
reactive oxygen species
- SA-CMCs
slowly-adhering CMCs
- SDF-1
stromal derived factor 1
- CXCR-4
C-X-C chemokine receptor type 4
- UC
umbilical cord
- TGS-6
tumor necrosis factor – stimulated gene 6
- TNF-α
tumor necrosis alpha
Footnotes
Disclosure
The authors have nothing to disclose.
References
- 1.Bolli R. Repeated Cell Therapy: A Paradigm Shift Whose Time Has Come. Circ Res. 2017;120:1072–1074. doi: 10.1161/CIRCRESAHA.117.310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated Administrations of Cardiac Progenitor Cells Are Markedly More Effective Than a Single Administration: A New Paradigm in Cell Therapy. Circ Res. 2016;119:635–51. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol. 2017;112:18. doi: 10.1007/s00395-017-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang XL, Nakamura S, Li Q, Wysoczynski M, Gumpert AM, Wu WJ, Hunt G, Stowers H, Ou Q, Bolli R. Repeated Administrations of Cardiac Progenitor Cells Are Superior to a Single Administration of an Equivalent Cumulative Dose. J Am Heart Assoc. 2018:7. doi: 10.1161/JAHA.117.007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolli R, Ghafghazi S. Stem cells: Cell therapy for cardiac repair: what is needed to move forward? Nat Rev Cardiol. 2017;14:257–258. doi: 10.1038/nrcardio.2017.38. [DOI] [PubMed] [Google Scholar]
- 6.Zhu K, Wu Q, Ni C, Zhang P, Zhong Z, Wu Y, Wang Y, Xu Y, Kong M, Cheng H, Tao Z, Yang Q, Liang H, Jiang Y, Li Q, Zhao J, Huang J, Zhang F, Chen Q, Li Y, Chen J, Zhu W, Yu H, Zhang J, Yang HT, Hu X, Wang J. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ Res. 2018 doi: 10.1161/CIRCRESAHA.117.311578. [DOI] [PubMed] [Google Scholar]
- 7.Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-Term Outcome of Administration of c-kit(POS) Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Least One Year. Circ Res. 2016;118:1091–105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–22. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–6. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Ge J, Zhang S, Sun A, Shen J, Chen L, Wang K, Zou Y. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol. 2005;100:217–23. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]
- 13.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Jiang Q, Zhang H, Jin P, Yuan X, Wei Y, Hu S. Intravenous administration of bone marrow mesenchymal stromal cells is safe for the lung in a chronic myocardial infarction model. Regen Med. 2011;6:179–90. doi: 10.2217/rme.10.104. [DOI] [PubMed] [Google Scholar]
- 15.Sheng CC, Zhou L, Hao J. Current stem cell delivery methods for myocardial repair. Biomed Res Int. 2013;2013:547902. doi: 10.1155/2013/547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith MC, Bolli R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res. 2015;116:1216–30. doi: 10.1161/CIRCRESAHA.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–34. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Kelkar AA, Butler J, Schelbert EB, Greene SJ, Quyyumi AA, Bonow RO, Cohen I, Gheorghiade M, Lipinski MJ, Sun W, Luger D, Epstein SE. Mechanisms Contributing to the Progression of Ischemic and Nonischemic Dilated Cardiomyopathy: Possible Modulating Effects of Paracrine Activities of Stem Cells. J Am Coll Cardiol. 2015;66:2038–47. doi: 10.1016/j.jacc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, Waksman R, Epstein SE. Intravenously Delivered Mesenchymal Stem Cells: Systemic Anti-Inflammatory Effects Improve Left Ventricular Dysfunction in Acute Myocardial Infarction and Ischemic Cardiomyopathy. Circ Res. 2017;120:1598–1613. doi: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 21.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–97. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17–28. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk A, Naaijkens BA, Jurgens WJ, Nalliah K, Sairras S, van der Pijl RJ, Vo K, Vonk AB, van Rossum AC, Paulus WJ, van Milligen FJ, Niessen HW. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011;7:219–29. doi: 10.1016/j.scr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Piao J, Jin L, Zhou Y. Does pretreatment of bone marrow mesenchymal stem cells with 5-azacytidine or double intravenous infusion improve their therapeutic potential for dilated cardiomyopathy? Med Sci Monit Basic Res. 2013;19:20–31. doi: 10.12659/MSMBR.883737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Q, Li Q, Na R, Li X, Liu B, Meng L, Liutong H, Fang W, Zhu N, Zheng X. Impact of repeated intravenous bone marrow mesenchymal stem cells infusion on myocardial collagen network remodeling in a rat model of doxorubicin-induced dilated cardiomyopathy. Mol Cell Biochem. 2014;387:279–85. doi: 10.1007/s11010-013-1894-1. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Yang YJ, Qian HY, Li Q, Zhang Q, Li XD, Dong QT, Xu H, Song L, Zhang H. Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction: role of CXCR4. Am J Transl Res. 2015;7:1058–70. [PMC free article] [PubMed] [Google Scholar]
- 27.Mykhaylichenko VY, Kubyshkin AV, Samarin SA, Fomochkina, Anisimova LV. Experimental induction of reparative morphogenesis and adaptive reserves in the ischemic myocardium using multipotent mesenchymal bone marrow-derived stem cells. Pathophysiology. 2016;23:95–104. doi: 10.1016/j.pathophys.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–9. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Krause U, Harter C, Seckinger A, Wolf D, Reinhard A, Bea F, Dengler T, Hardt S, Ho A, Katus HA, Kuecherer H, Hansen A. Intravenous delivery of autologous mesenchymal stem cells limits infarct size and improves left ventricular function in the infarcted porcine heart. Stem Cells Dev. 2007;16:31–7. doi: 10.1089/scd.2006.0089. [DOI] [PubMed] [Google Scholar]
- 30.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–36. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 31.Wolf D, Reinhard A, Seckinger A, Gross L, Katus HA, Hansen A. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium-complicated by myocardial tumor formation. Scand Cardiovasc J. 2009;43:39–45. doi: 10.1080/14017430802100280. [DOI] [PubMed] [Google Scholar]
- 32.Hong SJ, Rogers PI, Kihlken J, Warfel J, Bull C, Deuter-Reinhard M, Feng D, Xie J, Kyle A, Merfeld-Clauss S, Johnstone BH, Traktuev DO, Chen PS, Lindner JR, March KL. Intravenous xenogeneic transplantation of human adipose-derived stem cells improves left ventricular function and microvascular integrity in swine myocardial infarction model. Catheter Cardiovasc Interv. 2015;86:E38–48. doi: 10.1002/ccd.25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 34.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartolucci JG, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C, Rojo PG, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial) Circ Res. 2017 doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ, Anderson AS, Wilcox JE, Tankovich NI, Lipinski MJ, Ko YA, Margulies KB, Cole RT, Skopicki HA, Gheorghiade M. Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circ Res. 2017;120:332–340. doi: 10.1161/CIRCRESAHA.116.309717. [DOI] [PubMed] [Google Scholar]
- 37.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 38.Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34:446–50. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- 39.Hale SL, Dai W, Dow JS, Kloner RA. Mesenchymal stem cell administration at coronary artery reperfusion in the rat by two delivery routes: a quantitative assessment. Life Sci. 2008;83:511–5. doi: 10.1016/j.lfs.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grogaard HK, Sigurjonsson OE, Brekke M, Klow NE, Landsverk KS, Lyberg T, Eriksen M, Egeland T, Ilebekk A. Cardiac accumulation of bone marrow mononuclear progenitor cells after intracoronary or intravenous injection in pigs subjected to acute myocardial infarction with subsequent reperfusion. Cardiovasc Revasc Med. 2007;8:21–7. doi: 10.1016/j.carrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 42.Noort WA, Kruisselbrink AB, in’t Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Lowik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–8. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 43.Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M, Fisher AJ, Kamocki K, Brown MB, Presson RG, Jr, Broxmeyer HE, March KL, Petrache I. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183:215–25. doi: 10.1164/rccm.201001-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–37. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132:1880–90. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ Res. 2016;119:853–64. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 49.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 50.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–30. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 52.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–6. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 54.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–94. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23(Suppl 1):S113–22. doi: 10.3727/096368914X685005. [DOI] [PubMed] [Google Scholar]
- 56.Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423–9. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 57.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin F, Battiwalla M, Ito S, Feng X, Chinian F, Melenhorst JJ, Koklanaris E, Sabatino M, Stroncek D, Samsel L, Klotz J, Hensel NF, Robey PG, Barrett AJ. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem Cells. 2014;32:1278–88. doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 60.de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. doi: 10.1155/2013/206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–83. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 65.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–45. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol. 2014;109:444. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 70.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. non-reperfused murine myocardial infarction. Cardiovasc Pathol. 2006;15:83–90. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–53. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–39. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 75.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167:152–66. doi: 10.1016/j.trsl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–197. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 80.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure. Circ Heart Fail. 2017;10:e003688. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–55. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prabhu SD. It takes two to tango: monocyte and macrophage duality in the infarcted heart. Circ Res. 2014;114:1558–60. doi: 10.1161/CIRCRESAHA.114.303933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devaux B, Scholz D, Hirche A, Klovekorn WP, Schaper J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J. 1997;18:470–9. doi: 10.1093/oxfordjournals.eurheartj.a015268. [DOI] [PubMed] [Google Scholar]
- 85.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 86.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 87.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–16. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 89.van Zuylen VL, den Haan MC, Geutskens SB, Roelofs H, Fibbe WE, Schalij MJ, Atsma DE. Post-myocardial infarct inflammation and the potential role of cell therapy. Cardiovasc Drugs Ther. 2015;29:59–73. doi: 10.1007/s10557-014-6568-z. [DOI] [PubMed] [Google Scholar]
- 90.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013;43:986–95. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 92.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–93. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 93.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 95.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 96.Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000;139:94–100. doi: 10.1016/s0002-8703(00)90314-4. [DOI] [PubMed] [Google Scholar]
- 97.Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, Capodilupo R. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–8. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 98.Wasilewski J, Pyka L, Hawranek M, Osadnik T, Kurek A, Skrzypek M, Niedziela J, Desperak P, Kulaczkowska Z, Brzezina M, Krawczyk M, Gasior M. Prognostic value of neutrophiltolymphocyte ratio in predicting long-term mortality in patients with ischemic and nonischemic heart failure. Pol Arch Med Wewn. 2016;126:166–73. doi: 10.20452/pamw.3316. [DOI] [PubMed] [Google Scholar]
- 99.Yu C, Chen M, Chen Z, Lu G. Predictive and prognostic value of admission neutrophil-to-lymphocyte ratio in patients with CHD. Herz. 2016;41:605–613. doi: 10.1007/s00059-015-4399-8. [DOI] [PubMed] [Google Scholar]
- 100.Jiang D, Muschhammer J, Qi Y, Kugler A, de Vries JC, Saffarzadeh M, Sindrilaru A, Beken SV, Wlaschek M, Kluth MA, Ganss C, Frank NY, Frank MH, Preissner KT, Scharffetter-Kochanek K. Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells. 2016;34:2393–406. doi: 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29:1001–11. doi: 10.1002/stem.651. [DOI] [PubMed] [Google Scholar]
- 102.Khan I, Zhang L, Mohammed M, Archer FE, Abukharmah J, Yuan Z, Rizvi SS, Melek MG, Rabson AB, Shi Y, Weinberger B, Vetrano AM. Effects of Wharton’s jelly-derived mesenchymal stem cells on neonatal neutrophils. J Inflamm Res. 2015;8:1–8. doi: 10.2147/JIR.S71987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van den Akker F, Deddens JC, Doevendans PA, Sluijter JP. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2013;1830:2449–58. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 104.Henning RJ, Shariff M, Eadula U, Alvarado F, Vasko M, Sanberg PR, Sanberg CD, Delostia V. Human cord blood mononuclear cells decrease cytokines and inflammatory cells in acute myocardial infarction. Stem Cells Dev. 2008;17:1207–19. doi: 10.1089/scd.2008.0023. [DOI] [PubMed] [Google Scholar]
- 105.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 2016;8:2611–2634. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halade GV, Kain V, Ingle KA, Prabhu SD. Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing. Am J Physiol Heart Circ Physiol. 2017;313:H89–H102. doi: 10.1152/ajpheart.00040.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kain V, Liu F, Kozlovskaya V, Ingle KA, Bolisetty S, Agarwal A, Khedkar S, Prabhu SD, Kharlampieva E, Halade GV. Resolution Agonist 15-epi-Lipoxin A4 Programs Early Activation of Resolving Phase in Post-Myocardial Infarction Healing. Sci Rep. 2017;7:9999. doi: 10.1038/s41598-017-10441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–95. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 110.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marban E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–62. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marban E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation. 2017;136:200–214. doi: 10.1161/CIRCULATIONAHA.116.024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kanazawa H, Tseliou E, Dawkins JF, De Couto G, Gallet R, Malliaras K, Yee K, Kreke M, Valle I, Smith RR, Middleton RC, Ho CS, Dharmakumar R, Li D, Makkar RR, Fukuda K, Marban L, Marban E. Durable Benefits of Cellular Postconditioning: Long-Term Effects of Allogeneic Cardiosphere-Derived Cells Infused After Reperfusion in Pigs with Acute Myocardial Infarction. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marban L, Marban E. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail. 2015;8:322–32. doi: 10.1161/CIRCHEARTFAILURE.114.001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Yu ZX, Fujita S, Yamaguchi ML, Ferrans VJ. Interstitial dendritic cells of the rat heart. Quantitative and ultrastructural changes in experimental myocardial infarction. Circulation. 1993;87:909–20. doi: 10.1161/01.cir.87.3.909. [DOI] [PubMed] [Google Scholar]
- 117.Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, Sano M, Yoshikawa T, Okada Y, Koyasu S, Ogawa S, Fukuda K. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–45. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 118.Naito K, Anzai T, Sugano Y, Maekawa Y, Kohno T, Yoshikawa T, Matsuno K, Ogawa S. Differential effects of GM-CSF and G-CSF on infiltration of dendritic cells during early left ventricular remodeling after myocardial infarction. J Immunol. 2008;181:5691–701. doi: 10.4049/jimmunol.181.8.5691. [DOI] [PubMed] [Google Scholar]
- 119.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–63. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 120.Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L, Ma L, Zhu J, Zou Y, Ge J. Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J Mol Cell Cardiol. 2016;91:123–33. doi: 10.1016/j.yjmcc.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 121.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 122.Zhao ZG, Xu W, Sun L, You Y, Li F, Li QB, Zou P. Immunomodulatory function of regulatory dendritic cells induced by mesenchymal stem cells. Immunol Invest. 2012;41:183–98. doi: 10.3109/08820139.2011.607877. [DOI] [PubMed] [Google Scholar]
- 123.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 124.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 125.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 126.Hardy RR, Li YS, Allman D, Asano M, Gui M, Hayakawa K. B-cell commitment, development and selection. Immunol Rev. 2000;175:23–32. [PubMed] [Google Scholar]
- 127.Hardy RR, Wasserman R, Li YS, Shinton SA, Hayakawa K. Response by B cell precursors to pre-B receptor assembly: differences between fetal liver and bone marrow. Curr Top Microbiol Immunol. 2000;252:25–30. doi: 10.1007/978-3-642-57284-5_3. [DOI] [PubMed] [Google Scholar]
- 128.Melchers F, ten Boekel E, Seidl T, Kong XC, Yamagami T, Onishi K, Shimizu T, Rolink AG, Andersson J. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol Rev. 2000;175:33–46. [PubMed] [Google Scholar]
- 129.Cooper MD. The early history of B cells. Nat Rev Immunol. 2015;15:191–7. doi: 10.1038/nri3801. [DOI] [PubMed] [Google Scholar]
- 130.Sanchez-Trujillo L, Vazquez-Garza E, Castillo EC, Garcia-Rivas G, Torre-Amione G. Role of Adaptive Immunity in the Development and Progression of Heart Failure: New Evidence. Arch Med Res. 2017;48:1–11. doi: 10.1016/j.arcmed.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 131.Caforio AL, Mahon NJ, McKenna WJ. Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmunity. 2001;34:199–204. doi: 10.3109/08916930109007385. [DOI] [PubMed] [Google Scholar]
- 132.Barrio L, Cuevas VD, Menta R, Mancheno-Corvo P, delaRosa O, Dalemans W, Lombardo E, Carrasco YR. Human adipose tissue-derived mesenchymal stromal cells promote B-cell motility and chemoattraction. Cytotherapy. 2014;16:1692–9. doi: 10.1016/j.jcyt.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 133.Fan L, Hu C, Chen J, Cen P, Wang J, Li L. Interaction between Mesenchymal Stem Cells and B-Cells. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, Mullen Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604–15. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 136.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–9. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 137.Rosado MM, Bernardo ME, Scarsella M, Conforti A, Giorda E, Biagini S, Cascioli S, Rossi F, Guzzo I, Vivarelli M, Dello Strologo L, Emma F, Locatelli F, Carsetti R. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24:93–103. doi: 10.1089/scd.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Healy ME, Bergin R, Mahon BP, English K. Mesenchymal stromal cells protect against caspase 3-mediated apoptosis of CD19(+) peripheral B cells through contact-dependent upregulation of VEGF. Stem Cells Dev. 2015;24:2391–402. doi: 10.1089/scd.2015.0089. [DOI] [PubMed] [Google Scholar]
- 139.Zook EC, Kee BL. Development of innate lymphoid cells. Nat Immunol. 2016;17:775–82. doi: 10.1038/ni.3481. [DOI] [PubMed] [Google Scholar]
- 140.Malissen B, Gregoire C, Malissen M, Roncagalli R. Integrative biology of T cell activation. Nat Immunol. 2014;15:790–7. doi: 10.1038/ni.2959. [DOI] [PubMed] [Google Scholar]
- 141.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011;12:467–71. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–80. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tscharke DC, Croft NP, Doherty PC, La Gruta NL. Sizing up the key determinants of the CD8(+) T cell response. Nat Rev Immunol. 2015;15:705–16. doi: 10.1038/nri3905. [DOI] [PubMed] [Google Scholar]
- 145.Lee GR. Transcriptional regulation of T helper type 2 differentiation. Immunology. 2014;141:498–505. doi: 10.1111/imm.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, O’Shea JJ, Laurence A. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011;134:235–45. doi: 10.1111/j.1365-2567.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghoreschi K, Laurence A, Yang XP, Hirahara K, O’Shea JJ. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32:395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wei L. Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation-mediated cardiac fibrosis. Exp Mol Pathol. 2011;90:74–8. doi: 10.1016/j.yexmp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 149.Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103–14. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hofmann U, Frantz S. Role of T-cells in myocardial infarction. Eur Heart J. 2016;37:873–9. doi: 10.1093/eurheartj/ehv639. [DOI] [PubMed] [Google Scholar]
- 151.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–64. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 152.Xia N, Jiao J, Tang TT, Lv BJ, Lu YZ, Wang KJ, Zhu ZF, Mao XB, Nie SF, Wang Q, Tu X, Xiao H, Liao YH, Shi GP, Cheng X. Activated regulatory T-cells attenuate myocardial ischaemia/reperfusion injury through a CD39-dependent mechanism. Clin Sci (Lond) 2015;128:679–93. doi: 10.1042/CS20140672. [DOI] [PubMed] [Google Scholar]
- 153.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 154.Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noel D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–7. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 156.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 157.Ghannam S, Pene J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–12. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 158.Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ong SG, Wu JC. Cortical bone-derived stem cells: a novel class of cells for myocardial protection. Circ Res. 2013;113:480–3. doi: 10.1161/CIRCRESAHA.113.302083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113:539–52. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mohsin S, Troupes CD, Starosta T, Sharp TE, Agra EJ, Smith S, Duran JM, Zalavadia N, Zhou Y, Kubo H, Berretta RM, Houser SR. Unique Features of Cortical Bone Stem Cells Associated With Repair of the Injured Heart. Circ Res. 2015;117:1024–33. doi: 10.1161/CIRCRESAHA.115.307362. [DOI] [PubMed] [Google Scholar]
- 162.Sharp TE, Schena GJ, Hoachlandr-Hobby AR, Starosta T, Berretta RM, Wallner M, Borghetti G, Gross P, Yu D, Johnson J, Feldsott EA, Trappanese DM, Toib A, Rabinowitz JE, George JC, Kubo H, Mohsin S, Houser SR. Cortical Bone Stem Cell Therapy Preserves Cardiac Structure and Function After Myocardial Infarction. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.117.311174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–60. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 164.Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, Baba S, Shigemoto T, Kuroda Y, Kanamori H, Amin M, Kawasaki M, Nishigaki K, Taoka M, Isobe T, Muramatsu C, Dezawa M, Minatoguchi S. S1P-S1PR2 Axis Mediates Homing of Muse Cells into Damaged Heart for Long Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ Res. 2018 doi: 10.1161/CIRCRESAHA.117.311648. [DOI] [PubMed] [Google Scholar]
- 165.Wysoczynski M, Guo Y, Moore JBt, Muthusamy S, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin AA, Zhu X, Hunt G, Gumpert AM, Book MJ, Khan A, Tang XL, Bolli R. Myocardial Reparative Properties of Cardiac Mesenchymal Cells Isolated on the Basis of Adherence. J Am Coll Cardiol. 2017;69:1824–1838. doi: 10.1016/j.jacc.2017.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brouard N, Driessen R, Short B, Simmons PJ. G-CSF increases mesenchymal precursor cell numbers in the bone marrow via an indirect mechanism involving osteoclast-mediated bone resorption. Stem Cell Res. 2010;5:65–75. doi: 10.1016/j.scr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 167.Blashki D, Murphy MB, Ferrari M, Simmons PJ, Tasciotti E. Mesenchymal stem cells from cortical bone demonstrate increased clonal incidence, potency, and developmental capacity compared to their bone marrow-derived counterparts. J Tissue Eng. 2016;7:2041731416661196. doi: 10.1177/2041731416661196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Short BJ, Brouard N, Simmons PJ. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol Biol. 2009;482:259–68. doi: 10.1007/978-1-59745-060-7_16. [DOI] [PubMed] [Google Scholar]
- 169.Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M, Nakahata T, Fujiyoshi Y, Dezawa M. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci U S A. 2011;108:9875–80. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107:8639–43. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Katagiri H, Kushida Y, Nojima M, Kuroda Y, Wakao S, Ishida K, Endo F, Kume K, Takahara T, Nitta H, Tsuda H, Dezawa M, Nishizuka SS. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am J Transplant. 2016;16:468–83. doi: 10.1111/ajt.13537. [DOI] [PubMed] [Google Scholar]
- 172.Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G. Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One. 2013;8:e64752. doi: 10.1371/journal.pone.0064752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Simerman AA, Dumesic DA, Chazenbalk GD. Pluripotent muse cells derived from human adipose tissue: a new perspective on regenerative medicine and cell therapy. Clin Transl Med. 2014;3:12. doi: 10.1186/2001-1326-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, Chazenbalk G, Aiba S, Dezawa M. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014;23:717–28. doi: 10.1089/scd.2013.0473. [DOI] [PubMed] [Google Scholar]
- 175.Tian T, Zhang RZ, Yang YH, Liu Q, Li D, Pan XR. Muse Cells Derived from Dermal Tissues Can Differentiate into Melanocytes. Cell Reprogram. 2017;19:116–122. doi: 10.1089/cell.2016.0032. [DOI] [PubMed] [Google Scholar]
- 176.Dezawa M. Muse Cells Provide the Pluripotency of Mesenchymal Stem Cells: Direct Contribution of Muse Cells to Tissue Regeneration. Cell Transplant. 2016;25:849–61. doi: 10.3727/096368916X690881. [DOI] [PubMed] [Google Scholar]
- 177.Hori E, Hayakawa Y, Hayashi T, Hori S, Okamoto S, Shibata T, Kubo M, Horie Y, Sasahara M, Kuroda S. Mobilization of Pluripotent Multilineage-Differentiating Stress-Enduring Cells in Ischemic Stroke. J Stroke Cerebrovasc Dis. 2016;25:1473–81. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 178.Kinoshita K, Kuno S, Ishimine H, Aoi N, Mineda K, Kato H, Doi K, Kanayama K, Feng J, Mashiko T, Kurisaki A, Yoshimura K. Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers. Stem Cells Transl Med. 2015;4:146–55. doi: 10.5966/sctm.2014-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, Sakata H, Matsuzaka Y, Mushiake H, Tominaga T, Borlongan CV, Dezawa M. Transplantation of Unique Subpopulation of Fibroblasts, Muse Cells, Ameliorates Experimental Stroke Possibly via Robust Neuronal Differentiation. Stem Cells. 2016;34:160–73. doi: 10.1002/stem.2206. [DOI] [PubMed] [Google Scholar]
- 180.Shimamura N, Kakuta K, Wang L, Naraoka M, Uchida H, Wakao S, Dezawa M, Ohkuma H. Neuro-regeneration therapy using human Muse cells is highly effective in a mouse intracerebral hemorrhage model. Exp Brain Res. 2017;235:565–572. doi: 10.1007/s00221-016-4818-y. [DOI] [PubMed] [Google Scholar]
- 181.Uchida H, Niizuma K, Kushida Y, Wakao S, Tominaga T, Borlongan CV, Dezawa M. Human Muse Cells Reconstruct Neuronal Circuitry in Subacute Lacunar Stroke Model. Stroke. 2017;48:428–435. doi: 10.1161/STROKEAHA.116.014950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Iseki M, Kushida Y, Wakao S, Akimoto T, Mizuma M, Motoi F, Asada R, Shimizu S, Unno M, Chazenbalk G, Dezawa M. Muse Cells, Nontumorigenic Pluripotent-Like Stem Cells, Have Liver Regeneration Capacity Through Specific Homing and Cell Replacement in a Mouse Model of Liver Fibrosis. Cell Transplant. 2017;26:821–840. doi: 10.3727/096368916X693662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Uchida N, Kushida Y, Kitada M, Wakao S, Kumagai N, Kuroda Y, Kondo Y, Hirohara Y, Kure S, Chazenbalk G, Dezawa M. Beneficial Effects of Systemically Administered Human Muse Cells in Adriamycin Nephropathy. J Am Soc Nephrol. 2017;28:2946–2960. doi: 10.1681/ASN.2016070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hu MS, Longaker MT. A MUSE for Skin Regeneration. J Invest Dermatol. 2017;137:2471–2472. doi: 10.1016/j.jid.2017.07.825. [DOI] [PubMed] [Google Scholar]
- 185.Rossini A, Frati C, Lagrasta C, Graiani G, Scopece A, Cavalli S, Musso E, Baccarin M, Di Segni M, Fagnoni F, Germani A, Quaini E, Mayr M, Xu Q, Barbuti A, DiFrancesco D, Pompilio G, Quaini F, Gaetano C, Capogrossi MC. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res. 2011;89:650–60. doi: 10.1093/cvr/cvq290. [DOI] [PubMed] [Google Scholar]
- 186.Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, DeMartino AM, Brittian KR, Bolli R, Jones SP. A New Method to Stabilize C-Kit Expression in Reparative Cardiac Mesenchymal Cells. Front Cell Dev Biol. 2016;4:78. doi: 10.3389/fcell.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Triana JF, Li XY, Jamaluddin U, Thornby JI, Bolli R. Postischemic myocardial “stunning”. Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ Res. 1991;69:731–47. doi: 10.1161/01.res.69.3.731. [DOI] [PubMed] [Google Scholar]
- 188.Bennett WR, Yawn DH, Migliore PJ, Young JB, Pratt CM, Raizner AE, Roberts R, Bolli R. Activation of the complement system by recombinant tissue plasminogen activator. J Am Coll Cardiol. 1987;10:627–32. doi: 10.1016/s0735-1097(87)80206-1. [DOI] [PubMed] [Google Scholar]
- 189.Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–89. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- 190.Li XY, McCay PB, Zughaib M, Jeroudi MO, Triana JF, Bolli R. Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest. 1993;92:1025–41. doi: 10.1172/JCI116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–8. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]