Summary
Overexpression and/or gene amplification of HER2, a crucial member of the HER family of four receptors, occur in about 15–20% of breast cancers and define an aggressive subtype of the disease. Activated HER homo and heterodimers govern a complex and redundant downstream signaling network that regulates cell survival and metastasis. Despite treatment with effective HER2-targeted therapies, many HER2-positive tumors fail to respond, or initially respond but eventually develop resistance. One of the upfront reasons for this treatment failure is failure to accurately select the tumors that are truly dependent on HER2 for survival and so would benefit the most from HER2-targeted therapy. In these truly HER2-addicted tumors (i.e. physiologically dependent), resistance could be the result of an incomplete inhibition of signaling at the HER receptor layer. In this regard, preclinical and clinical studies have documented the superiority of combination anti-HER2 therapy over single agent therapy to achieve a more comprehensive inhibition of the various HER receptor dimers. HER2 can be further activated or reactivated by mutations or other alterations in HER2 itself, or in other HER family members. Even when a complete and sustained HER inhibition is achieved, resistance to anti-HER therapy can arise by other somewhat dominant mechanisms, including preexisting or emerging alternative signaling pathways such as the estrogen receptor, deregulated downstream signaling components, especially of the PI3K pathway, and the tumor immune microenvironment. Most of the clinical trials that have investigated the efficacy of anti-HER2 therapies took place in the background of aggressive chemotherapy regimens, thus confounding the identification of key factors of resistance to the anti-HER2 treatments. Recent studies, however, have suggested that some HER2-amplified tumors may benefit from anti-HER2 therapy combined with only single chemotherapy agent or in the absence of any chemotherapy. This de-escalation approach, a promising therapeutic strategy, is currently being explored in the clinic. In this review, we summarize the major molecular determinants that play a crucial role in influencing tumor response and resistance to HER2-targeted therapy, and discuss the growing need for patient stratification in order to facilitate the development of de-escalation strategies using HER2-targeted therapy alone with no chemotherapy.
Keywords: HER2-positive breast cancer, HER2-targeted therapy resistance, oncogenic addiction, PI3K/PTEN, estrogen receptor, tumor microenvironment
Introduction
The human epidermal growth factor receptor 2 (HER2/ERBB2) is a key member of the HER family consisting of four receptor tyrosine kinases (HER1-4). This family together governs a complex and redundant signaling process to regulate cell proliferation, survival, and metastasis(1, 2). The HER family receptors are activated by multiple ligands with the exception of HER2, which has no known ligand but can be activated by heterodimerization with other ligand-bound HER receptors or by homodimerization in tumors expressing high levels of HER2. HER2 overexpression, largely due to gene amplification, is observed in about 15–20% of breast cancers, known as HER2-positive, and accounts for their aggressive and metastatic behavior. Multiple HER2-targeted therapies, including humanized monoclonal antibodies such as trastuzumab and pertuzumab, and tyrosine kinase inhibitors such as lapatinib, have been developed, with trastuzumab being the first to be FDA-approved(1, 2). Though highly effective in patients, especially in combination with aggressive chemotherapy, intrinsic and acquired resistance to trastuzumab and other HER2-targeted drugs still occurs and remains clinically challenging. Trastuzumab by itself, with no chemotherapy is much less effective(1) as it only partially inhibits HER2 signaling, although it also activates antibody-dependent cell-mediated cytotoxicity (ADCC)(1). One major plausible mechanism responsible for resistance is incomplete inhibition of the HER receptor layer, particularly considering the functional redundancy of signaling from multiple HER receptor dimers and compensatory signaling within the pathway(3). This suggests that dual inhibition using the combination of pertuzumab or lapatinib with trastuzumab might achieve a more complete blockade of HER signaling. To that end, the superiority of dual anti-HER2 therapy over single-agent treatment has been established in the clinic in both the neoadjuvant and metastatic settings, although most of the clinical trials were done in the presence of chemotherapy(4, 5). We and others have proved the concept that combining HER2-targeted agents without concomitant chemotherapy can produce complete tumor eradication in preclinical HER2-positive breast cancer mouse models(6–8). These findings led to several neoadjuvant clinical trials with similar treatments of dual HER2 inhibition with no chemotherapy, which have yielded substantial pathologic complete response (pCR)(9–11). These results suggest that a subset of patients with HER2 amplified tumors may not need chemotherapy at all. It is therefore essential to identify upfront those patients who can be spared chemotherapy, as well as to understand the mechanisms of resistance in order to devise more effective tailored treatments. The various mechanisms of resistance to anti-HER2 therapy have been comprehensively reviewed in the last few years(12–15). In this review, our focus is to summarize the major molecular determinants that play a crucial role in influencing tumor response and resistance to HER2-targeted therapy, and to discuss the rationale and clinical significance of patient stratification for successful HER2-targeted therapy without chemotherapy.
Major contributing factors of response and resistance to HER2-targeted therapy
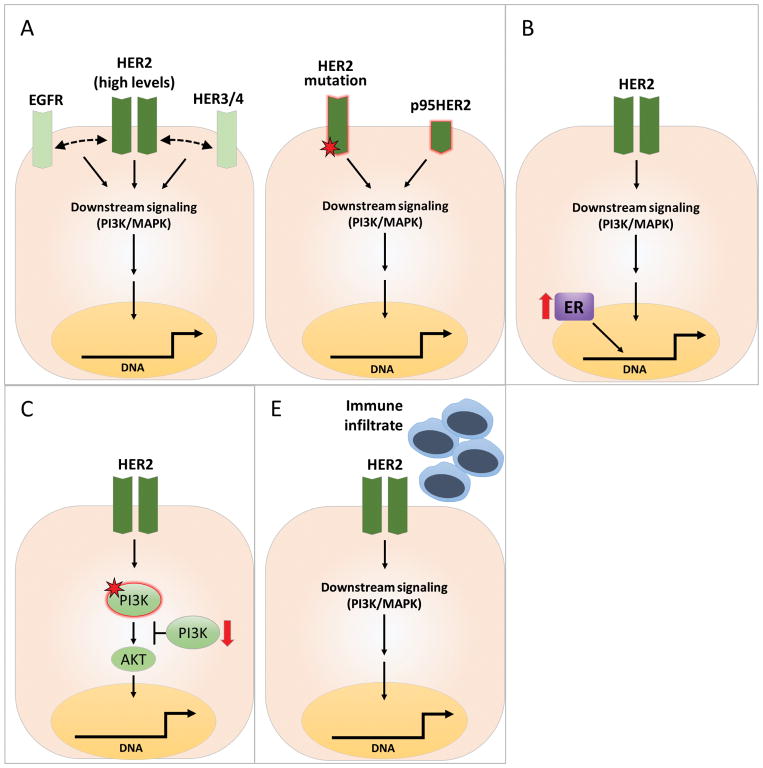
The response of a tumor to HER2-targeted therapy primarily depends on HER2 expression level and how much the tumor is dependent on HER2 for its existence. Emerging evidence further suggests that the HER2 therapy response is also governed by a multitude of other factors. The principal determinants of HER2 therapy response and resistance can be largely grouped into four major categories (Figure 1). The first category is HER2 itself, since a tumor will best respond to anti-HER2 therapy, especially without chemotherapy, only when it exhibits absolute dependence (Oncogene addiction) on HER2 for sustained proliferation and survival, which is associated with high levels of HER2 gene amplification, RNA expression, and downstream signaling (Figure 1A). Despite successful HER2-targeted therapy, HER2-addicted tumors can develop resistance due to reactivation of the signaling pathway via HER2 itself or other compensatory mechanisms within the HER receptor layer, which may be pre-existing at the time of treatment or acquired in due course. The second category includes additional alternative signaling pathways, pre-existing or acquired, that provide compensatory signaling to offset and overcome the inhibitory effects of HER2-targeted therapy. One leading member of this category is estrogen receptor (ER) signaling (Figure 1B). This is particularly crucial in HER2-positive tumors that are also hormone receptor (HR)-positive. The third category is deregulation of downstream signaling components in the HER signaling pathway, especially the PI3K/PTEN pathway, one of the major downstream components of HER signaling (Figure 1C). The fourth and most recently revisited category is the tumor immune microenvironment (Figure 1D). Each of the categories mentioned above is comprised of multiple eminent players. However, in this review, we will focus on discussing the significance of a key and more prevalent component in each category that has been characterized preclinically and suggested to play a role in the clinical setting.
Figure 1. Determinants of response and mechanisms of resistance to HER2-targeted therapies in HER2-positive breast cancer.
In HER2-positive breast cancer, despite effective anti-HER2 regimens with mono or dual agents to more completely inhibit all the HER dimers, intrinsic and acquired resistance is considerable. The prevalent determinants of response and mechanisms of resistance include the following categories: A) Optimized response to anti-HER2 therapy requires HER2 oncogenic addiction, which is mostly associated with high and homogeneous levels of HER2 gene copy number, and gene and protein expression (left). However, sustained activation of HER2 signaling through alterations in HER2 such as mutations or protein cleavage may result in resistance to therapy (right); B) Activation of alternative survival pathways (e.g., upregulation of ER levels and activity); C) Deregulation of the downstream PI3K/AKT pathway, mainly due to activating mutations of the PIK3CA gene encoding the p110α catalytic subunit of PI3K or to partial or complete loss of PTEN, leading to PI3K hyperactivation; D) Tumor immune microenvironment and immune response.
Oncogenic HER2 addiction
One of the principal determining factors of response to anti-HER2 therapies is the extent to which the cancer cells within a HER2-positive breast cancer are dependent on HER2 itself for the maintenance of their malignant phenotype. This dependency is termed as oncogene-addiction, and such tumors respond best to drugs targeting the oncogene they are addicted to(16). High and homogeneous levels of HER2 gene amplification, expression of HER2 mRNA and protein, and HER2 downstream signaling are necessary for true HER2 addiction, and identification of HER2-addicted tumors is essential to reap the complete therapeutic benefit of HER2-targeted drugs. In this regard, methods and defined cutoffs to accurately identify the true HER2-addicted tumors with high HER2 expression and signaling activity are critical. Indeed, recent studies suggest that high HER2 mRNA levels and the PAM50-classfied HER2-enriched subtype are associated with a better response to HER2 therapy both with(17) and without chemotherapy(10). Additionally, the key role of intra-tumor HER2 amplification heterogeneity in HER2-targeted therapy resistance has been recently shown(18), which further emphasizes the need for homogeneous HER2 amplification to achieve an effective response to HER2-targeted therapy. It is expected that the cutoff needed to identify truly HER2-addicted tumors that will benefit from HER2-targeted therapy without chemotherapy may be higher than the current guidelines in place, which aim to identify those patients who may benefit from adding HER2-targeted treatment to chemotherapy(19). In this regard, we need to investigate and compare several leading methods and cutoffs to redefine HER2 status at various levels including HER2 copy number, mRNA, and protein, as well as by intrinsic HER2-enriched subtype by the PAM50 molecular subtyping classification. It is important to understand that the tumors that do not meet this higher cutoff may still benefit from and need HER2-targeted therapy but these will not be considered as the true HER2-addicted tumors.
Even in these HER2-addicted tumors, intrinsic and acquired resistance to HER2-targeted therapy is still common. This can occur at various molecular levels including HER2 itself or other HER family members. HER2 mutations, mostly activating, have been reported in both HER2-positive and negative tumors in about ~3% of breast cancer [The Cancer Genome Atlas Study (TCGA) by cBioPortal](20, 21). These activating mutations play a key role in activating HER2 signaling, including in HER2-negative tumors, but also influence response and resistance to HER2-targeted therapy, and therefore may nullify the therapeutic effect of various HER2-targeted agents(22). Two recent studies by Boulbes et al. and Zuo et al. reported the presence of multiple novel HER family mutations in both primary and metastatic HER2-positive breast tumors(23, 24). Boulbes et al. identified 12 novel or previously reported HER family mutations in primary breast tumors, and showed that patients whose primary tumors harbor these mutations demonstrated poor response to trastuzumab-based chemotherapy in the metastatic setting(23). Importantly, in our very recent study, we have shown in preclinical HER2-positive breast cancer models that the most common HER2 mutation, L755S residing in the kinase domain of the receptor, reactivates HER2 signaling and thereby constitutes an endogenous mechanism of acquired resistance to lapatinib and trastuzumab-containing HER2-targeted therapies(25). Interestingly, the Zuo et al. study that analyzed 18 pairs of primary and metastatic lesions, which included 16 pairs from patients who received one year of trastuzumab, showed that the L755S and the nearby K753E HER2 mutations appeared in three and two metastatic lesions, respectively(24). Collectively, these intriguing results suggest that rare pre-existing or acquired HER2 mutations emerge as a mechanism of acquired resistance, similar to the events of ESR1 constitutive active mutations recently described in endocrine resistant metastatic breast cancer(26). The suggested HER2 L755S-conferred conformational change in the HER2 kinase domain renders it unable to bind lapatinib. In this regard, our recent study suggests that the HER2 L755S-mediated resistance to various anti-HER2 regimens can be partly overcome by irreversible HER1/2 inhibitors, such as afatinib and neratinib(25). Thus, the significance of these HER2 mutations in primary and acquired resistance, the implementation of more sensitive methods to identify them in HER2-positive tumors with HER2 gene amplification, and the therapeutic efficacy of the irreversible inhibitors in this setting need to be further explored.
An additional mechanism of resistance to HER2-targeted therapy at the level of the HER2 protein itself is the p95HER2. It is a truncated form of HER2 generated by cleavage of the extracellular domain of the full-length HER2 by matrix metalloproteases(27). Expression of p95HER2 observed in more than 30% of HER2 overexpressing breast tumors, has been shown to be associated with a poor response to trastuzumab(28–31) since it lacks the trastuzumab binding site and contains a hyperactive kinase domain. Finally, beyond HER2 itself, increased or altered compensatory signaling by additional HER family members, such as HER3(1) may abrogate the therapeutic benefit of HER2 inhibition. Accordingly, incorporation of inhibitors directed against HER3 to HER2-targeted therapy is currently under investigation(32).
Alternative signaling pathways: The role of ER
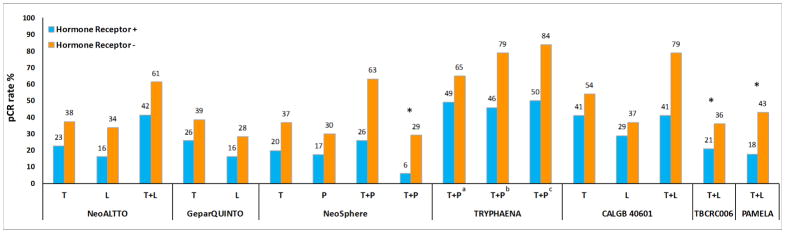
Approximately half of the HER2-positive tumors are estrogen receptor (ER) positive. Over the past decade, studies have suggested that HER2+ ER+ and HER2+ ER− tumors represent distinct subtypes with different patient outcomes under HER2-targeted therapy(33). At the molecular level, multiple studies have shown the existence of bidirectional crosstalk, with positive and negative feedback loops between HER2 and ER signaling pathways. In the preclinical setup, we and others have shown that the expression of ER and its downstream targets is increased in cells with acquired resistance to anti-HER2 therapy(8, 34, 35). Reactivation of ER expression and signaling, including a switch from ER-negative to ER-positive status, were observed in clinical HER2-positive tumors after neoadjuvant lapatinib treatment(34, 35). These results suggest the significance of ER expression and signaling as an alternative survival mechanism in these tumors. In this setting, an ab initio unblocked, re-expressed, and/or reactivated ER signaling can function as a compensatory escape pathway by providing an alternative proliferative and survival signaling to evade sustained HER2 blockade. Interestingly, the neoadjuvant NeoALTTO trial, which compared the efficacy of lapatinib vs. trastuzumab vs. their combination, all with paclitaxel, reported that high RNA levels of ESR1, the gene encoding ER, were associated with lower pCR rates(36). In our preclinical study using ER+ HER2+ xenograft tumor models, only a transient tumor regression was observed with anti-HER2 therapy when ER is left uninhibited. On the other hand, treatment with concurrent anti-HER2 and endocrine therapy resulted in complete tumor eradication in these models(6–8). Indeed, across multiple clinical trials, using mono and dual anti-HER2 drugs, response rates were inferior in HR-positive tumors compared to HR-negative tumors(4, 9–11, 37, 38) (Figure 2). Among published clinical trials, TBCRC 006, PAMELA, and one arm in NeoSphere trials did not include chemotherapy. However, TBCRC 006 and PAMELA combined anti-HER2 therapy with endocrine therapy, if tumors were ER-positive, but NeoSphere did not include endocrine therapy. With the limitation of cross-study comparison and small patient cohorts, the outcome of patients treated with endocrine therapy compared to no endocrine therapy suggests that adding concomitant endocrine therapy is needed for HER2-positive/HR-positive tumors. Finally, in both the PAMELA trial (after 14 days of dual HER2 blockade without chemotherapy) and the CALGGB 40601 trial (at the completion of single or dual anti-HER2 treatment plus chemotherapy), a substantial percentage of HER2-enriched tumors at baseline switched to the Luminal A subtype, an observation further indicating the reactivation of ER signaling in response to anti-HER2 therapy(10, 17). Together, these data suggest that ER levels and signaling may predict outcome to anti-HER2 therapy and that these two pathways should be concomitantly blocked.
Figure 2. Rates of pathological complete response (pCR) by hormone receptor status of the primary tumor in published neoadjuvant trials of single or dual anti-HER2 therapy.
Abbreviations: pCR, pathological complete response; L, lapatinib; P, pertuzumab; T, trastuzumab. *, treatment arms without chemotherapy; a,b, treatment arms included 5-fluorouracil, epirubicin, cyclophosphamide, and docetaxel with different schedules; c, treatment arm included docetaxel and carboplatin. Numbers on top of the bars indicate pCR rate (%).
Activation of HER2-downstream signaling: Deregulation of the PI3K/AKT pathway
The HER2 downstream PI3K/AKT pathway is a master regulator of cell growth and survival(39, 40). Deregulation that hyperactivates the PI3K/AKT pathway include activating mutations in PIK3CA, the gene encoding the p110α catalytic subunit of PI3K, or partial/complete loss of the tumor suppressor PTEN that negatively regulates the PI3K pathway(39, 41). Several studies have suggested that constitutive activation of the PI3K/AKT pathway is associated with HER2 therapy resistance(42–47). Gain-of-function mutations in PIK3CA are observed in about 20% of HER2-positive breast cancer cases(48–52).
Multiple neoadjuvant(51, 53), adjuvant(54), and metastatic(52) studies that analyzed patient response to anti-HER2 therapy in combination with chemotherapy reported that patients with PIK3CA mutations showed response rates inferior to those with wild type PIK3CA tumors. A recent pooled analysis of >950 HER2-positive patients from 5 neoadjuvant trials that tested the efficacy of single or dual anti-HER2 therapy with chemotherapy reported that PIK3CA mutant tumors are associated with lower pCR rates compared to wild-type tumors, especially within the HR-positive subgroup(51). In the metastatic setting, an association between PIK3CA mutation status and inferior patient outcome was observed in the CLEOPATRA trial(52). Nevertheless, conflicting results have also been reported regarding the correlation between tumor PIK3CA mutation status and response to HER2 therapy, especially in the adjuvant setting. The adjuvant FINHER(50) and NSABP B-31(49) trials, which randomized patients to test the efficacy of trastuzumab in the adjuvant setting, failed to show association between PIK3CA mutation status and anti-HER2 therapy response. If chemotherapy used in these trials is effective in HER2-positive tumors containing PIK3CA mutations, then any correlation between the presence of this mutation and resistance to the HER2-targeted therapy will be diluted or lost entirely.
Lost or significantly low PTEN expression is observed in about 20–25% of HER2-positive breast cancers(42, 45, 55, 56). Loss of PTEN expression has been shown to be associated with reduced response to trastuzumab-containing regimens(55, 57, 58). In the neoadjuvant GeparQuattro study, PTEN levels assessed by quantitative immunofluorescence assay predicted pCR after anti-HER2 treatment combined with chemotherapy(59). However, several other studies in the neoadjuvant, adjuvant, and metastatic settings failed to show associations between PTEN loss and anti-HER2 therapy response(45, 47, 55, 59–68). In two of our previous neoadjuvant trials that combined anti-HER2 therapy with chemotherapy, it was reported that PTEN loss is associated with resistance to trastuzumab but not lapatinib(69), with the latter finding confirmed by another similar study(70) and also in the preclinical setting(70). A retrospective analysis of the neoadjuvant NeoALTTO trial failed to demonstrate an association between PTEN protein levels and pCR rates(71). In the adjuvant trastuzumab setting, retrospective analyses of the HER2-positive tumors from N9831 and BCIRG-006 trials revealed no association between PTEN loss and trastuzumab resistance(66, 68). However, as mentioned above, most of these studies were done in the presence of chemotherapy, which can confound the association and mask resistance to pure anti-HER2 therapy since tumors with PIK3CA mutations and perhaps also PTEN loss are sensitive to chemotherapy. Therefore, to truly understand the role of PIK3CA mutations and PTEN loss in anti-HER2 therapy response, these analyses should be performed in clinical trials without chemotherapy. In addition to the confounding effects of chemotherapy, these conflicting results could also be related to differences in the patient cohorts, in the PTEN antibody, or in the expression cutoffs used across studies as well as the lack of standardized methods to quantify PTEN. We have recently shown that even a partial (and not necessarily a complete) loss of PTEN is sufficient to activate the PI3K pathway, further suggesting the possibility that the arbitrary cut-off of complete loss of the PTEN protein might not be correct(41). To fully comprehend the predictive values of PI3KCA mutations and PTEN levels in response to anti-HER2 therapy, these markers should be assessed in tumors from trials of HER2-targeted therapy alone without chemotherapy.
In addition to the PI3K/AKT pathway, increasing evidence suggests the role of Src kinases in HER2 therapy resistance. Several studies using pre-clinical models(72, 73) and human tumors(55, 74, 75) have suggested Src as a key modulator of trastuzumab response and a common downstream node of multiple trastuzumab-resistance pathways. More importantly, Src inhibition has been shown to re-sensitize trastuzumab-resistant cells to trastuzumab both in vitro and in vivo(75). In addition, an increase in the copy number of Yes1, a proto-oncogene and member of the Src family, was observed in the trastuzumab+lapatinib-resistant cells and, more importantly, pharmacological inhibition of Yes1 re-sensitized the resistant cells to trastuzumab(76). These results suggest the potential clinical implications of these mechanisms in overcoming trastuzumab resistance. In addition, the presence and clinical significance of other relevant alterations in the HER signaling network need to be explored.
Tumor microenvironment: Significance of immune response
The role of the immune microenvironment in modulating tumor response to treatment has been well studied over the years. In HER2-positive breast cancer, a high level of tumor infiltrating lymphocytes (TILs) has been shown to be associated with a more favorable prognosis and better pCR with neoadjuvant therapy(59, 77, 78). This is not surprising considering the significant involvement of tumor-host immune interaction in accomplishing the therapeutic efficacy of anti-HER2 therapy, particularly the monoclonal antibodies trastuzumab and pertuzumab. However, in the neoadjuvant and adjuvant setting, somewhat contradictory results have been reported across multiple clinical trials. In the neoadjuvant setting, the GeparQuattro and GeparQuinto(59) studies reported a positive correlation between TILs and HER2 therapy response. In the neoadjuvant NeoALTTO(77) and NeoSphere(78) trials, only low levels of TILs, less than 5%, were found to be associated with lower pCR rates. In the adjuvant setting, high TILs were found to be associated with better response to trastuzumab treatment in the FINHER study(65). In contrast, in the large trastuzumab adjuvant trial N9831, there was a lack of association between high TILs and treatment benefit with trastuzumab(79). However, gene expression analysis of tumors from this trial showed that patients with “immune gene-enriched tumors” had better outcomes if they received trastuzumab(80). Similarly, in multiple other neoadjuvant trials, CALGB 40601(17), CherLOB(81) and GeparSixto(82), expression of immune genes and immune gene signatures showed an independent predictive value in response to dual HER2 blockade and chemotherapy. Moreover, in HER2-positive metastatic breast cancer, recent analysis of tumors from the CLEOPATRA trial revealed that higher levels of TILs correlated with improved overall survival in patients treated with anti-HER2 therapy and chemotherapy(83).
Most clinical trials that studied the correlation of TILs and response to HER2-targeted therapy are in the background of chemotherapy, which could be a confounding factor in judging the predictive value of TILs. Therefore, an assessment of the predictive value of TILs in HER2-therapy response without the influence of chemotherapy is essential to determine the role of the immune infiltrates in predicting the response to anti-HER2 therapy. Further studies in larger patient populations and more quantitative and qualitative methods are needed to establish appropriate cutoffs and investigate whether the quantification of TILs can add predictive and/or prognostic value. New multiplex techniques to better understand the complexity of the immune infiltrates and its association with patient outcome is warranted.
Conclusions and future directions: De-escalation approaches for the treatment of HER2-positive breast cancer
The HER2 signaling network is an attractive therapeutic target for treatment of HER2-positive tumors. Enormous success has been made in the treatment of these tumors through the development of highly effective targeted therapies. The therapeutic progress over the last few decades has revolutionized the treatment of HER2-positive breast cancer and drastically improved patient outcome. The overall strategy so far has been to escalate the treatment by adding more HER2-targeted treatments, such as the addition of HER2 dual inhibition regimens to aggressive chemotherapy regimens. Unfortunately, this treatment escalation is accompanied by significant toxicity and high cost. With the success attained thus far, revisiting the current treatment strategies is absolutely essential and de-escalation, which can be achieved either by reducing or eliminating chemotherapy, to mitigate the adverse effects without impacting patient outcome ought to be considered. The promise of a de-escalation approach in reducing the toxicity without affecting patient outcome has been demonstrated in a recent adjuvant trial using only paclitaxel as the chemotherapy backbone with trastuzumab, a low-toxicity regimen, in HER2-positive patients with favorable prognosis(84). Recent neoadjuvant clinical trials TBCRC 006(11), TBCRC 023(85), PAMELA(10), and NeoSphere(9) (Table 1) investigated the efficacy of dual HER2 inhibition without chemotherapy and demonstrated impressive pCR rates (20–30%) in patients. These results suggest that treatment escalation may not always be beneficial or even essential. In fact, there is burgeoning evidence to demonstrate that a significant fraction of patients with HER2-amplified tumors may not need chemotherapy at all. If these patients could be identified upfront, optimal response could potentially be achieved with HER2-targeted therapy alone. The tumors that do not benefit from this approach may be treated with chemotherapy or other strategies to overcome resistance. The success of a de-escalation approach is dependent upon the discovery of predictive biomarkers for an accurate stratification of patients who will benefit from anti-HER2 therapy alone without chemotherapy. Not less important is the accurate identification of patients who need more therapy, and the determination of mechanisms of resistance is essential to improve treatment strategies. The unique cohort of tumor samples from these neoadjuvant trials without chemotherapy will be critical to identify key determinants and cutoffs of response and resistance to anti-HER2 therapy without the confounding effects of chemotherapy. This will help guide a new generation of clinical trials to test this paradigm for molecular triage of patients.
TABLE 1.
Neo-adjuvant clinical trials of dual HER2 blockade without chemotherapy
| Clinical trial | Phase | Number of patients | HER2-Targeted therapy | Concomitant Endocrine therapy | Duration (weeks) | pCR |
|---|---|---|---|---|---|---|
| TBCRC 006 | II | 64 | Trastuzumab+Lapatinib | Yes | 12 | 27% |
| TBCRC 023 | II | 33 | Trastuzumab+Lapatinib | Yes | 12 | 12% |
| 61 | Trastuzumab+Lapatinib | Yes | 24 | 28% | ||
| PAMELA | II | 150 | Trastuzumab+Lapatinib | Yes | 18 | 31% |
| NeoSphere | II | 107 | Trastuzumab+Pertuzumab | No | 16 | 17% |
Intrinsic and acquired resistance to HER2-targeted therapy occurs through several mechanisms. The more prevalent cause for failure of anti-HER2 treatments is the failure to accurately select the true HER2-addicted tumors that would benefit most from this therapy and can be spared from chemotherapy. These HER2-addicted tumors, in the presence of potent anti-HER2 therapy, can still reactivate the HER2 signaling pathway due to mutation of the HER2 gene itself or other alterations in HER2 or other members of the HER family. Resistance can also emanate from other molecular mechanisms such as alternative signaling pathways, deregulated downstream signaling components, and the tumor immune microenvironment. Our understanding of the molecular determinants of intrinsic and acquired resistance is not complete and there is still much more to be explored. However, it is time for a de-escalation approach by building new predictive tools to stratify patients and launching new clinical trials to triage patients based on these multi-parameter molecular predictors. Overall, a more accurate identification of patients that will benefit from targeted therapy, and a better understanding of the mechanisms of resistance and ways to circumvent them, are essential to optimize patient outcomes.
Acknowledgments
Funding
This work was partly supported by the NIH: SPORE Grants P50 CA058183 and CA186784 (to R.S, C.K.O, and M.F.R); Cancer Center Grant P30 CA125123; the Breast Cancer Research Foundation (to R.S and C.K.O); the Cancer Prevention & Research Institute of Texas CPRIT RP 140102 (to C.D.A.); The Conquer Cancer Foundation - Gianni Bonadonna Breast Cancer Research Fellowship (to C.D.A.); Pas a Pas (to A.P.); Instituto de Salud Carlos III - PI16/00904 (to A.P.); Career Catalyst Grant from the Susan Komen Foundation (to A.P.); the Breast Cancer Research Foundation Grant (to J.S.R-F) and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest statement
R. Schiff, and C. K. Osborne have received research funding from AstraZeneca and GlaxoSmithKline. M. Rimawi has received research funding from GlaxoSmithKline and Genentech, C. K. Osborne is a member of advisory boards for Pfizer and AstraZeneca. A. Prat has received research funding from Nanostring Technologies.
References
- 1.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–28. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135(1):55–62. doi: 10.1043/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimawi MF, Wiechmann LS, Wang YC, Huang C, Migliaccio I, Wu MF, et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res. 2011;17(6):1351–61. doi: 10.1158/1078-0432.CCR-10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99(9):694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 8.Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13(6):R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 10.Llombart-Cussac A, Cortés J, Paré L, Galván P, Bermejo B, Martínez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. The Lancet Oncology. 18(4):545–54. doi: 10.1016/S1470-2045(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 11.Rimawi MF, Mayer IA, Forero A, Nanda R, Goetz MP, Rodriguez AA, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013;31(14):1726–31. doi: 10.1200/JCO.2012.44.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17(1):1–16. doi: 10.1615/critrevoncog.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortora G. Mechanisms of resistance to HER2 target therapy. J Natl Cancer Inst Monogr. 2011;2011(43):95–8. doi: 10.1093/jncimonographs/lgr026. [DOI] [PubMed] [Google Scholar]
- 14.Kessler ER, Elias AD. Resistance to HER2-Targeted Therapy in HER2+ Breast Cancer. Clinical Medicine Reviews in Women’s Health. 2012;4:15–26. (Resistance-to-HER2-Targeted-Therapy-in-HER2+-Breast-Cancer2) [Google Scholar]
- 15.Puglisi F, Minisini AM, De Angelis C, Arpino G. Overcoming treatment resistance in HER2-positive breast cancer: potential strategies. Drugs. 2012;72(9):1175–93. doi: 10.2165/11634000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.I.B. Weinstein Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297(5578):63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 17.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. J Clin Oncol. 2016;34(6):542–9. doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng CK, Martelotto LG, Gauthier A, Wen HC, Piscuoglio S, Lim RS, et al. Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol. 2015;16:107. doi: 10.1186/s13059-015-0657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 20.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–37. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulbes DR, Arold ST, Chauhan GB, Blachno KV, Deng N, Chang WC, et al. HER family kinase domain mutations promote tumor progression and can predict response to treatment in human breast cancer. Mol Oncol. 2015;9(3):586–600. doi: 10.1016/j.molonc.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo WJ, Jiang YZ, Wang YJ, Xu XE, Hu X, Liu GY, et al. Dual Characteristics of Novel HER2 Kinase Domain Mutations in Response to HER2-Targeted Therapies in Human Breast Cancer. Clin Cancer Res. 2016;22(19):4859–69. doi: 10.1158/1078-0432.CCR-15-3036. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, De Angelis C, Burke KA, Nardone A, Hu H, Qin L, et al. HER2 Reactivation through Acquisition of the HER2 L755S Mutation as a Mechanism of Acquired Resistance to HER2-targeted Therapy in HER2+ Breast Cancer. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12(10):573–83. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, et al. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58(22):5123–9. [PubMed] [Google Scholar]
- 28.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744–9. [PubMed] [Google Scholar]
- 29.Molina MA, Saez R, Ramsey EE, Garcia-Barchino MJ, Rojo F, Evans AJ, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8(2):347–53. [PubMed] [Google Scholar]
- 30.Saez R, Molina MA, Ramsey EE, Rojo F, Keenan EJ, Albanell J, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12(2):424–31. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- 31.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N, Chang Y, Rios A, An Z. HER3/ErbB3, an emerging cancer therapeutic target. Acta Biochim Biophys Sin (Shanghai) 2016;48(1):39–48. doi: 10.1093/abbs/gmv103. [DOI] [PubMed] [Google Scholar]
- 33.Vaz-Luis I, Winer EP, Lin NU. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol. 2013;24(2):283–91. doi: 10.1093/annonc/mds286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103(20):7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giuliano M, Hu H, Wang YC, Fu X, Nardone A, Herrera S, et al. Upregulation of ER Signaling as an Adaptive Mechanism of Cell Survival in HER2-Positive Breast Tumors Treated with Anti-HER2 Therapy. Clin Cancer Res. 2015;21(17):3995–4003. doi: 10.1158/1078-0432.CCR-14-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fumagalli D, Venet D, Ignatiadis M, Azim HA, Jr, Maetens M, Rothe F, et al. RNA Sequencing to Predict Response to Neoadjuvant Anti-HER2 Therapy: A Secondary Analysis of the NeoALTTO Randomized Clinical Trial. JAMA Oncol. 2016;3(2):227–234. doi: 10.1001/jamaoncol.2016.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 38.Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13(2):135–44. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 39.Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 40.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu X, Creighton CJ, Biswal NC, Kumar V, Shea M, Herrera S, et al. Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase. Breast Cancer Res. 2014;16(5):430. doi: 10.1186/s13058-014-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18(24):6784–91. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujita T, Doihara H, Kawasaki K, Takabatake D, Takahashi H, Washio K, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94(2):247–52. doi: 10.1038/sj.bjc.6602926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9(6):1489–502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 47.Faratian D, Goltsov A, Lebedeva G, Sorokin A, Moodie S, Mullen P, et al. Systems biology reveals new strategies for personalizing cancer medicine and confirms the role of PTEN in resistance to trastuzumab. Cancer Res. 2009;69(16):6713–20. doi: 10.1158/0008-5472.CAN-09-0777. [DOI] [PubMed] [Google Scholar]
- 48.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pogue-Geile KL, Song N, Jeong JH, Gavin PG, Kim SR, Blackmon NL, et al. Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. J Clin Oncol. 2015;33(12):1340–7. doi: 10.1200/JCO.2014.56.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loi S, Michiels S, Lambrechts D, Fumagalli D, Claes B, Kellokumpu-Lehtinen PL, et al. Somatic mutation profiling and associations with prognosis and trastuzumab benefit in early breast cancer. J Natl Cancer Inst. 2013;105(13):960–7. doi: 10.1093/jnci/djt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016;27(8):1519–25. doi: 10.1093/annonc/mdw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–61. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 53.Cizkova M, Dujaric ME, Lehmann-Che J, Scott V, Tembo O, Asselain B, et al. Outcome impact of PIK3CA mutations in HER2-positive breast cancer patients treated with trastuzumab. Br J Cancer. 2013;108(9):1807–9. doi: 10.1038/bjc.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen JD, Knoop A, Laenkholm AV, Grauslund M, Jensen MB, Santoni-Rugiu E, et al. PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab. Ann Oncol. 2012;23(8):2034–42. doi: 10.1093/annonc/mdr546. [DOI] [PubMed] [Google Scholar]
- 55.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65(7):2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 57.Pandolfi PP. Breast cancer--loss of PTEN predicts resistance to treatment. N Engl J Med. 2004;351(22):2337–8. doi: 10.1056/NEJMcibr043143. [DOI] [PubMed] [Google Scholar]
- 58.Crowder RJ, Lombardi DP, Ellis MJ. Successful targeting of ErbB2 receptors-is PTEN the key? Cancer Cell. 2004;6(2):103–4. doi: 10.1016/j.ccr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, et al. Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clin Cancer Res. 2016;22(23):5747–54. doi: 10.1158/1078-0432.CCR-15-2338. [DOI] [PubMed] [Google Scholar]
- 60.Gallardo A, Lerma E, Escuin D, Tibau A, Munoz J, Ojeda B, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106(8):1367–73. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–56. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Zhang Q, Zhang J, Sun S, Guo H, Jia Z, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gori S, Sidoni A, Colozza M, Ferri I, Mameli MG, Fenocchio D, et al. EGFR, pMAPK, pAkt and PTEN status by immunohistochemistry: correlation with clinical outcome in HER2-positive metastatic breast cancer patients treated with trastuzumab. Ann Oncol. 2009;20(4):648–54. doi: 10.1093/annonc/mdn681. [DOI] [PubMed] [Google Scholar]
- 64.Yonemori K, Tsuta K, Shimizu C, Hatanaka Y, Hirakawa A, Ono M, et al. Immunohistochemical expression of HER1, HER3, and HER4 in HER2-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. J Surg Oncol. 2010;101(3):222–7. doi: 10.1002/jso.21486. [DOI] [PubMed] [Google Scholar]
- 65.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 66.Stern HM, Gardner H, Burzykowski T, Elatre W, O’Brien C, Lackner MR, et al. PTEN Loss Is Associated with Worse Outcome in HER2-Amplified Breast Cancer Patients but Is Not Associated with Trastuzumab Resistance. Clin Cancer Res. 2015;21(9):2065–74. doi: 10.1158/1078-0432.CCR-14-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park YH, Jung HA, Choi MK, Chang W, Choi YL, Do IG, et al. Role of HER3 expression and PTEN loss in patients with HER2-overexpressing metastatic breast cancer (MBC) who received taxane plus trastuzumab treatment. Br J Cancer. 2014;110(2):384–91. doi: 10.1038/bjc.2013.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez EA, Dueck AC, McCullough AE, Chen B, Geiger XJ, Jenkins RB, et al. Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 trial. J Clin Oncol. 2013;31(17):2115–22. doi: 10.1200/JCO.2012.42.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29(2):166–73. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67(3):1170–5. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 71.Nuciforo PG, Aura C, Holmes E, Prudkin L, Jimenez J, Martinez P, et al. Benefit to neoadjuvant anti-human epidermal growth factor receptor 2 (HER2)-targeted therapies in HER2-positive primary breast cancer is independent of phosphatase and tensin homolog deleted from chromosome 10 (PTEN) status. Ann Oncol. 2015;26(7):1494–500. doi: 10.1093/annonc/mdv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278(41):40057–66. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- 73.Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69(2):475–82. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell. 2010;18(5):423–35. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17(4):461–9. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeda T, Yamamoto H, Kanzaki H, Suzawa K, Yoshioka T, Tomida S, et al. Yes1 signaling mediates the resistance to Trastuzumab/Lap atinib in breast cancer. PLoS One. 2017;12(2):e0171356. doi: 10.1371/journal.pone.0171356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1(4):448–54. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bianchini G, Pusztai L, Pienkowski T, Im YH, Bianchi GV, Tseng LM, et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol. 2015;26(12):2429–36. doi: 10.1093/annonc/mdv395. [DOI] [PubMed] [Google Scholar]
- 79.Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, et al. Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016;2(1):56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33(7):701–8. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dieci MV, Prat A, Tagliafico E, Pare L, Ficarra G, Bisagni G, et al. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann Oncol. 2016;27(10):1867–73. doi: 10.1093/annonc/mdw262. [DOI] [PubMed] [Google Scholar]
- 82.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 83.Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–41. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rimawi MF, Niravath PA, Wang T, Rexer B, editors. TBCRC023: a randomized multicenter phase II neoadjuvant trial of lapatinib, trastuzumab, with or without endocrine therapy for 12 weeks vs. 24 weeks in patients with HER2 overexpressing breast cancer. Cancer Res; Proc of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2014; 2015. Abstract nr S6–02. [Google Scholar]