Abstract
Survivin (coding gene BIRC5) is a dual functional protein acting as a critical inhibitor of apoptosis (IAP) and key regulator of cell cycle progression. It is usually produced in embryonic tissues during development and undetectable in most adult tissues. Overexpression of Survivin frequently occurs in various human cancers and increased Survivin correlates with poor clinic outcome, tumor recurrence, and therapeutic resistance. Because of its selective expression in tumor, but not normal tissues, Survivin has been recognized as an attractive target for cancer treatment. Although several therapeutic approaches targeting Survivin are actively under clinical trials in human cancers, to date no Survivin-targeted therapy has been approved for cancer treatment. Numerous studies have devoted to uncover the underlying mechanism resulting in Survivin dysregulation at multiple levels, such as transcriptional and post-transcriptional regulation. The current article provides a literature review on the transcriptional and epigenetic regulation of Survivin expression in human cancers. We focus on the impact of DNA methylation and histone modifications, including specific lysine methylation, demethylation, and acetylation on the expression of Survivin. The latest development of epigenetic approaches targeting Survivin for cancer treatment are also discussed.
Keywords: Survivin, epigenetics, DNA methylation, histone modification, cancer therapy
Introduction
Survivin (encoded by the gene BRIC5) is a member of the inhibitor of apoptosis (IAP) family (Sah et al., 2006). It is distinguished from other IAPs because of its bi-functionality as a regulator of both apoptosis and cell mitosis. Survivin is frequently overexpressed in a wide variety of human malignancies, but is scarce in the majority of adult normal tissues (Altieri, 2003; Ambrosini et al., 1997). Aberrant Survivin expression is associated with tumor cell proliferation, progression, angiogenesis, therapeutic resistance, and poor prognosis (Hirano et al., 2015; Li et al., 2015; Liu et al., 2008; Sokolowska et al., 2015). Thus, Survivin has been considered as a diagnostic and prognostic marker. It has also been identified as an excellent molecular target for cancer therapy (Pennati et al., 2008; Ryan et al., 2009; Sato et al., 2009; Weiss et al., 2008). Regulatory mechanisms of BIRC5 gene expression are not fully understood. It is well known that expression of a gene can be regulated at the transcriptional and post-translational levels, whereas the expression of Survivin is thought to be regulated primarily at the transcriptional level. Thus, how to develop a potent therapeutic method targeting Survivin poses a challenge. One strategy is to elucidate the precise mechanisms controlling BIRC5 gene expression and identify prevalent control factors. The aim of the current article is to provide a literature review on the transcriptional and epigenetic regulation of Survivin expression in human cancers. The latest advance of epigenetic targeting of Survivin for cancer treatment are also discussed.
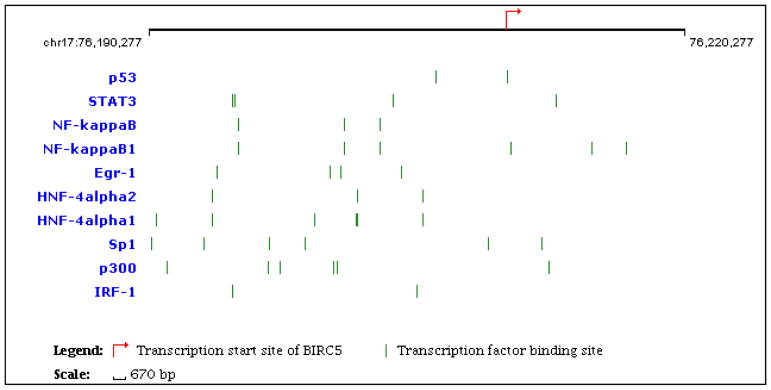
1. Transcriptional factors binding to the promoter region of BRIC5 gene
The gene coding Survivin is BIRC5, which locates at chromosome 17q25 in humans and consists of three introns and four exons. Expression of BIRC5 is high during fetal development and in most tumors, yet low in adult tissues. Alternatively spliced transcript variants encoding distinct Survivin isoforms are described (Altieri, 1994a, b). Numerous transcription factors have been predicted binding to specific sequences in the promoter region of BIRC5 and are involved in its transcriptional regulation. The majority of binding sites for transcription factors are clustered in a proximal area of the promoter (–250…+70 from the transcription initiation point). The 10 most studied transcription factors and their relevant binding sites are shown in figure 1. The functional binding sites for Sp1 (Chen et al., 2011; Cheng et al., 2012; Zhang et al., 2015), NF-κB (Angileri et al., 2008; Papanikolaou et al., 2011; Wang et al., 2010), Stat3 (Huang et al., 2014; Kim et al., 2016), Rb (Raj et al., 2008; Yang et al., 2008), p53 (Vaziri et al., 2005; Zhou et al., 2002), and Egr1 (Wagner et al., 2008b) have been found in BIRC5 gene promoter, suggesting a possible involvement of these factors in direct control of BIRC5 gene expression. Among them, Sp1, Stat3 and NF-κB activate expression of BIRC5 gene. For example, eight possible Sp1 binding sites with canonical or close to canonical sequence (G/T)(G/A)GGCG- (G/T)(G/A)(G/A)(C/T) were found in the promoter region of BIRC5 (Li and Altieri, 1999; Mityaev et al., 2008). The binding site called “Sp1-complex” relative to the transcription initiation point is actually a cluster composed of two overlapping putative Sp1 binding sites. Simultaneous introduction of several mutations into the sequence of the Sp1-complex cluster leads to a sharp decrease in BIRC5 gene promoter activity (Xu et al., 2007). Some transcriptional factors inhibit BIRC5 expression. The tumor suppressor p53, as well as the transcription factor Egr1 are involved in repression of BIRC5 gene promotor (Nakano et al., 2005). P53-mediated inhibition of BIRC5 gene expression has been verified in a number of studies (Chang et al., 2013). It has also been shown that BIRC5 is transcriptionally repressed upon DNA damage by wild type p53. Pradhan et al have published a serial of articles to elucidate the molecular basis of doxorubicin-induced down-regulation of BIRC5 transcription (Esteve et al., 2005, 2007; Smallwood et al., 2007). Expectedly, these different transcriptional factors can work together to regulate BIRC5 gene expression. For example, in colorectal cancer HCT116 cells treated with doxorubicin, the transcriptional factor Sp1 retains on BIRC5 promoter, acting as an anchor to recruit p53. Furthermore, coordination among the transcriptional factors is necessary because of overlaps of their binding sites. For instance, p53 may inhibit activation of BIRC5 expression by preventing the BIRC5 promoter from HIF (hypoxia inducing factor) binding. This can be achieved via competition for binding with overlapping sites. Moreover, p53 may counteract the binding of Sp1 factor, thereby suppressing BIRC5 promoter activity (Esteve et al., 2007; Smallwood et al., 2007).
Fig 1.
Diagram depicting the 10 most relevant transcription factors and their predicted binding sites in 20kb upstream and 10kb downstream of the transcription start site of BIRC5 (Adapted from http://www.sabiosciences.com/chipqpcrsearch.php).
2. Methylation status of BRIC5 promoter in human cancers
Abnormal DNA methylation status is frequently observed in a variety of human cancers. Hypermethylation of CpG islands in the promoter regions of tumor suppressive genes generally mediates loss of the gene expression. In contrast, DNA hypomethylation is widely spread over chromosomes, hence loosening the intrinsic heterochromatin regions, activating the latent retrotransponson’s, causing genomic instability, and subsequently resulting in activation of gene transcription (Baylin and Jones, 2011; Das and Singal, 2004). This section summarizes the current knowledge of BIRC5 promoter methylation in human cancers.
Sequence analysis of the 5′ flanking region of BIRC5 gene reveals a GC-rich region ranges from -254nt (from starting codon ATG) to +110nt in exon 1. The percentage of GC di-nucleotides in this region ranges between 65% to 80%, with a CG:GC ratio of 0.98, hence reaching the length and base composition criteria of a canonical CpG island (Ambrosini et al., 1997; Li and Altieri, 1999). However, the relationship between BIRC5 promoter methylation and its expression is complicated. Higher expression levels of Survivin may not always correlate with less methylated promoter. Several studies show no difference between normal and neoplastic tissues regarding the methylation status in the CpG island of BIRC5 promoter. DNAs extracted from fetal liver, normal breast, colon and uterus, as well as breast and lung cancer tissues are all unmethylated at the CpG island (Li and Altieri, 1999). The methylation status of BIRC5 promoter in various tumors can be divided into the following three scenarios.
2.1 Promoter methylation irrelevant to BIRC5 expression
In cervical carcinomas (Chaopatchayakul et al., 2010), esophageal cancers (Yang et al., 2009), and acute myeloid leukemia (AML) (Wagner et al., 2008a), the methylation patterns of BIRC5 promoter have been shown to have no relationship to Survivin expression and tumor progression. Analyses of the CpG island in BIRC5 promoter reveal no methylation in normal tissues and cervical carcinoma (Chaopatchayakul et al., 2010). In esophageal cancers, BIRC5 mRNA is overexpressed. However, this overexpression is not due to its promoter hypomethylation, as it is unmethylated in both cancer and non-cancerous tissues, regardless of Survivin expression profile (Yang et al., 2009). Overexpression of Survivin is common in AML and the potential involvement of BIRC5 promoter methylation has been studied. Results show that BIRC5 promoter is unmethylated in both peripheral blood mononuclear cells and AML blasts, suggesting that the methylation status is not a major contributor of Survivin re-expression during leukemogenesis (Wagner et al., 2008a).
No BIRC5 promoter methylation has been found in astrocytoma and non-astrocytoma tissues (Yu et al., 2004), whereas a study with 27 glioblastoma multiforme samples shows BIRC5 promoter methylation in 8 samples (Hervouet et al., 2010). Although statistical analysis suggests a positive correlation between methylation and the expression levels of DNMT1 (DNA methyltransferase 1) in glioblastoma, clinical outcome analyses indicate that the methylation profile of BIRC5 has no association with patient survival (Hervouet et al., 2010). Despite there is no relevance of BIRC5 promoter methylation with its expression in glioma, folate supplementation induces downregulation of BIRC5 mRNA via increasing its DNA methylation (Hervouet et al., 2009). Folate treatment enhances temozolomide-induced apoptosis and inhibits proliferation of glioma cells through methylation of apoptosis-related genes, among them, BIRC5-encoded Survivin is considered as a molecular determinant in the reduction of cell proliferation and gain of sensitivity in apoptosis (Hervouet et al., 2009). Similar results are also reported in rodent models of glioma showing that folate supplementation is able to limit tumorigenesis (Cartron et al., 2012).
2.2 Hypomethylation correlated with high expression of BIRC5
Unmethylated promoter (hypomethylation) may contribute to the elevated expression of Survivin in oral squamous cell carcinomas (OSCCs). Tanaka et al (Tanaka et al., 2003) compared the expression of Survivin in OSCCs and oral pre-malignant lesions. While 41 of 71 (58%) OSCC cases were positive immunohistochemical staining for Survivin and 14 of 38 (37%) oral pre-malignant lesion cases were positive for Survivin staining. There was no positive staining for Survivin in the corresponding normal tissues. Methylation of BIRC5 promoter was studied in 9 OSCCs and their matched normal tissues. Among them, 4 normal tissues had methylated BIRC5 promoter and all tumor tissues had no evidence of BIRC5 promoter methylation. Despite of the low sample number, the data provided an insight of Survivin expression possibly regulated by DNA hypomethylation in OSCCs (Tanaka et al., 2003).
2.3 Hypermethylation correlated with high expression of BIRC5
Different from aforementioned, the expression levels of Survivin are positively correlated with its promoter methylation in endometrial cancers. In normal endometrial tissues, BIRC5 promoter is completely unmethylated, however, the methylation levels increase from low grade to high grade endometrial cancers correlated with elevated expression of Survivin protein (Nabilsi et al., 2009). The hypermethylation of BIRC5 promoter in endometrial cancers is found to block the binding of p53, a repressor of BIRC5 gene transcription, to its promoter region, hence increase Survivin expression (Nabilsi et al., 2009). Similar phenomenon of the high grade tumors companying with higher levels of methylated BIRC5 promoter often occurs in patients with bladder cancer (Berrada et al., 2012). Although hypermethylation of BIRC5 promoter is more frequently observed in high grade tumors and increases from low to high stages, there is no significant correlation between stages/grades and the methylation status of BIRC5 promoter (Berrada et al., 2012). Additional studies are warranted to determine whether the underlying mechanism is the same as that in endometrial cancers.
3. Histone modifications contributing to Survivin dysregulation
A histone modification is a covalent post-translational modification of histone proteins, which mainly includes specific residue methylation/demethylation, phosphorylation, acetylation, ubiquitylation, sumoylation, etc. These modifications can influence gene expression by altering chromatin structure or recruiting different histone modifiers. In this section, we discuss the effect of histone H3 methylation and acetylation on Survivin expression in human cancers.
3.1 Histone H3 methylation and demethylation in regulating BIRC5 expression
During cellular differentiation, the ratio between permissive and repressive epigenetic modifications is either maintained or swiftly changed to create cell-type-specific patterns of gene expression. In general, methylation on both histone H3 lysine 9 (H3K9) and histone H3 lysine 27 (H3K27) mediates heterochromatin formation and participates in silencing gene expression at euchromatic sites. On the other hand, methylation on histone H3 lysine 4 (H3K4) and lysine 79 (H3K79) usually leads to activation of gene transcription. During this regulatory process, both histone methylases and demethylases play important roles to control gene expression. These epigenetic modulators are also involved in regulation of Survivin expression. Some transcriptional factors cooperated with the epigenetic modulators to regulate BIRC5 gene expression. It has been shown that p53 and DNA methyltransferase I (DNMT1) play a pivotal role in the epigenetic suppression of BIRC5 (Esteve et al., 2007). P53 recruits DNMT1, which may facilitate the promoter hypermethylation, and p53 may also recruit other epigenetic modulators, including the histone lysine methyltransferase G9a. G9a is able to catalyze methylation reactions on H3K9 to generate dimethylation of H3K9 (H3K9me2), which provides a binding platform for heterochromatin protein 1 (HP1). Coordination of the epigenetic regulatory proteins creates a suppressive chromatin complex in the proximal promoter region of BIRC5, and thereby results in BIRC5 gene repression upon doxorubicin treatment (Esteve et al., 2007; Smallwood et al., 2007). A recent study suggests that Bmi1 represses Survivin expression in a cell type specific manner via increasing trimethylation of H3K27 (H3K27me3) and direct binding of Bmi1 to the promoter region of BIRC5 (Acquati et al., 2013).
3.2 Histone H3 acetylation and deacetylation in regulating BIRC5 expression
Histone acetylation and deacetylation are essential parts of gene regulation. Histone hyper-acetylation is generally associated with chromatin decondensation, allowing DNA to be accessible to binding proteins, and thereby increases transcriptional activity. In contrast, histone hypo-acetylation contributes to chromatin condensation and transcriptional repression. These reactions are typically catalyzed by enzymes with “histone acetyltransferase” (HAT) or “histone deacetylase” (HDAC) activities. Tsai et al (Tsai et al., 2015) have shown that increased histone acetyltransferase p300 (p300HAT) activity in human melanoma A375 cell line and murine colon adenocarcinoma C26 cell line may enhance Survivin expression. Others report that liver cancer initiation is controlled by the transcription factor AP-1 through SIRT6-dependent inhibition of Survivin, because SIRT6 represses Survivin expression by reducing histone H3K9 acetylation and inhibiting NF-κB activity (Min et al., 2012).
4. Epigenetic strategies targeting Survivin for cancer treatment
Different types of cancer may have slight or significant differences in the way how the BIRC5 gene expression is regulated. Distinct methylation status of BIRC5 promoter is observed in different tissues and cancers. Thus, further investigations assessing the detailed epigenetic regulation of BIRC5 gene transcription in certain cancer types are necessary before we may apply novel epigenetic strategies targeting of Survivin for cancer treatment. If elevated expression of Survivin is positively related to the unmethylated BIRC5 promoter, then inducing methylation of BIRC5 promoter seems to be a reasonable approach to downregulate Survivin. Following this thought, Li and colleagues (Li and Ma, 2010) have tested a novel method to induce hypermethylation of BIRC5 promoter in non-small cell lung cancer (NSCLC) NCI-H460 cell line. They develop a short methylated oligonucloetide called SurKex, which is complementary to the BIRC5 promoter. After treatment with SurKex, the BIRC5 mRNA and its encoded protein (Survivin) were dramatically decreased in NCI-H460 cells. The growth of tumor xenografts derived from NCI-H460 cells in mice was also significantly inhibited. Detailed analyses confirmed that downregulation of Survivin was attributed to the following three mechanisms: 1) SurKex induced site-specific de novo methylation on CpG islands in the complementary region of BIRC5 promoter, which involved activation and participation of DNMT1. 2) SurKex induced histone hypermethylation at its target region, which increased levels of H3K9me2 and H3K27me3. 3) SurKex also triggered histone H4 lysine 16 (H4K16) deacetylation at its target region. This histone deacetylation was demonstrated to guide DNA methylation in BIRC5 silencing upon SurKex treatment (Li and Ma, 2010; Ma et al., 2010; Ma et al., 2011). Thus, the novel technology using methylated oligonucloetide to induce site-specific methylation at hypomethylated “site-of-interest” seems to be a promising approach for cancer therapy.
Similarly, detailed analyses of histone modifications not only improve our understanding of epigenetic regulation of BIRC5 gene transcription during cancer progression, they may also facilitate the development of histone modifying enzyme-targeted therapeutic agents. To this end, a number of recent studies suggest that HDAC inhibitors (HDACis) may be added into cancer treatment regimens because of their inhibitory effects on Survivin expression in a wide variety of human cancer cells. The class I HDACi, MS-275 (also known as SNDX-275 or entinostat) suppressed proliferation and induced apoptosis in human myeloma U266 cells, which might be associated with the downregulation of Survivin expression (Ma et al., 2009). We have shown that entinostat is able to effectively inhibit Survivin expression via induction of the Survivin-targeting miRNAs in NSCLC cells, and thereby significantly enhance the antitumor activity of paclitaxel against NSCLC (Wang et al., 2016). Feng et al examined the cytotoxic effects of a pan-HDACi, TSA in combination with silibinin on two pancreatic cancer cell lines (Panc1 and Capan2), and investigated the possible mechanisms (Feng et al., 2015). Their studies found that combinatorial treatment of TSA and silibinin exerted an additive growth inhibitory effect on the pancreatic cancer cells by inducing cell cycle G2/M arrest and apoptosis. Moreover, treatment with TSA and silibinin resulted in a profound reduction in the expression of cyclin A2, cyclin B1, Cdk1, and Survivin. Sakai T et al. reported that the combinations of a novel HDACi OBP-801 (also known as YM753) and the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 synergistically inhibited cell growth and induced apoptosis in renal cell carcinoma, which was resistant to traditional cancer therapies. It was partly due to markedly decreased Survivin protein levels (Yamada et al., 2013).
Perspectives
Survivin is selectively overexpressed in tumors, but undetectable in normal tissues (Altieri, 2008; Kanwar et al., 2013), and increased Survivin correlates with poor prognosis, tumor recurrence, and drug resistance in various human cancers (Coumar et al., 2013; Zaffaroni and Daidone, 2002). Thus, inhibition of Survivin is likely an efficacious therapy in reducing relapse risk and improving the survival of cancer patients. Yet, there is currently no FDA-approved Survivin-targeted therapy for cancer treatment. Recent studies on the epigenetic mechanisms of Survivin dysregulation in human cancers provides us a new avenue to identify novel epigenetic strategy to downregulate Survivin. In addition, it has been suggested that epigenetic alteration is one of the major mechanisms influencing expression of miRNAs, and the miRNAs binding to 3′-UTR of BIRC5 mRNA play an important role in controlling Survivin expression. Thus, the specific changes of miRNA expression profiles in cancer cells present unique opportunities to manipulate Survivin expression epigenetically (Lu et al., 2005). Our recent studies show that the “sister” miRNAs work cooperatively to inhibit their common targets (Wahdan-Alaswad and Liu, 2013; Wang et al., 2013), inspiring us to believe that those miRNAs with multiple binding sites on 3′-UTR of BIRC5 mRNA should be more specific and effective than the miRNAs with single binding sites to downregulate Survivin. Therefore, in addition to the epigenetic approaches targeting BIRC5 transcription discussed above, specific miRNAs may represent exciting tools to inhibit Survivin for cancer therapy (Huang et al., 2015). We are currently testing whether miR-542-3p, which has three binding sites on the 3′-UTR of BIRC5 mRNA, may be potentially developed as a unique anti-Survivin agent to enhance chemotherapy for cancer treatment. Nonetheless, the molecular basis of Survivin dysregulation in human cancers is far from well understood. Further investigations on the underlying mechanisms will certainly facilitate the development of novel Survivin-targeted therapy for cancer treatment.
Acknowledgments
This work was supported in part by the NIH/NCI (R01CA201011) and the National Natural Science Foundation of China (81472763) (to BL).
Footnotes
Conflict of interests
The authors declare that they have no conflict of interests.
References
- Acquati S, Greco A, Licastro D, Bhagat H, Ceric D, Rossini Z, Grieve J, Shaked-Rabi M, Henriquez NV, Brandner S, Stupka E, Marino S. Epigenetic regulation of survivin by Bmi1 is cell type specific during corticogenesis and in gliomas. Stem cells. 2013;31:190–202. doi: 10.1002/stem.1274. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Molecular cloning of effector cell protease receptor-1, a novel cell surface receptor for the protease factor Xa. J Biol Chem. 1994a;269:3139–3142. [PubMed] [Google Scholar]
- Altieri DC. Splicing of effector cell protease receptor-1 mRNA is modulated by an unusual retained intron. Biochemistry. 1994b;33:13848–13855. doi: 10.1021/bi00250a039. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Angileri FF, Aguennouz M, Conti A, La Torre D, Cardali S, Crupi R, Tomasello C, Germano A, Vita G, Tomasello F. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112:2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrada N, Amzazi S, Ameziane El Hassani R, Benbacer L, El Mzibri M, Khyatti M, Chafiki J, Abbar M, Al Bouzidi A, Ameur A, Attaleb M. Epigenetic alterations of adenomatous polyposis coli (APC), retinoic acid receptor beta (RARbeta) and survivin genes in tumor tissues and voided urine of bladder cancer patients. Cell Mol Biol (Noisy-le-grand) 2012;(Suppl 58):OL1744–1751. [PubMed] [Google Scholar]
- Cartron PF, Hervouet E, Debien E, Olivier C, Pouliquen D, Menanteau J, Loussouarn D, Martin SA, Campone M, Vallette FM. Folate supplementation limits the tumourigenesis in rodent models of gliomagenesis. Eur J Cancer. 2012;48:2431–2441. doi: 10.1016/j.ejca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Chang HL, Chen CY, Hsu YF, Kuo WS, Ou G, Chiu PT, Huang YH, Hsu MJ. Simvastatin induced HCT116 colorectal cancer cell apoptosis through p38MAPK-p53-survivin signaling cascade. Biochim Biophys Acta. 2013;1830:4053–4064. doi: 10.1016/j.bbagen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Chaopatchayakul P, Jearanaikoon P, Yuenyao P, Limpaiboon T. Aberrant DNA methylation of apoptotic signaling genes in patients responsive and nonresponsive to therapy for cervical carcinoma. Am J Obstet Gynecol. 2010;202:281e281–289. doi: 10.1016/j.ajog.2009.11.037. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang X, Li W, Zhang H, Zhao C, Li Y, Wang Z, Chen C. Sp1 upregulates survivin expression in adenocarcinoma of lung cell line A549. Anat Rec (Hoboken) 2011;294:774–780. doi: 10.1002/ar.21378. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Ling X, Haller A, Nakahara T, Yamanaka K, Kita A, Koutoku H, Takeuchi M, Brattain MG, Li F. Suppression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoter. Int J Biochem Mol Biol. 2012;3:179–197. [PMC free article] [PubMed] [Google Scholar]
- Coumar MS, Tsai FY, Kanwar JR, Sarvagalla S, Cheung CH. Treat cancers by targeting survivin: just a dream or future reality? Cancer Treat Rev. 2013;39:802–811. doi: 10.1016/j.ctrv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- Esteve PO, Chin HG, Pradhan S. Human maintenance DNA (cytosine-5)-methyltransferase and p53 modulate expression of p53-repressed promoters. P Natl Acad Sci USA. 2005;102:1000–1005. doi: 10.1073/pnas.0407729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve PO, Chin HG, Pradhan S. Molecular mechanisms of transactivation and doxorubicin-mediated repression of survivin gene in cancer cells. J Biol Chem. 2007;282:2615–2625. doi: 10.1074/jbc.M606203200. [DOI] [PubMed] [Google Scholar]
- Feng W, Cai DW, Zhang B, Lou GC, Zou XP. Combination of HDAC inhibitor TSA and silibinin induces cell cycle arrest and apoptosis by targeting survivin and cyclinB1/Cdk1 in pancreatic cancer cells. Biomed Pharmacother. 2015;74:257–264. doi: 10.1016/j.biopha.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Hervouet E, Debien E, Campion L, Charbord J, Menanteau J, Vallette FM, Cartron PF. Folate Supplementation Limits the Aggressiveness of Glioma via the Remethylation of DNA Repeats Element and Genes Governing Apoptosis and Proliferation. Clin Cancer Res. 2009;15:3519–3529. doi: 10.1158/1078-0432.CCR-08-2062. [DOI] [PubMed] [Google Scholar]
- Hervouet E, Vallette FM, Cartron PF. Impact of the DNA methyltransferases expression on the methylation status of apoptosis-associated genes in glioblastoma multiforme. Cell Death Dis. 2010;1:e8. doi: 10.1038/cddis.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H, Maeda H, Yamaguchi T, Yokota S, Mori M, Sakoda S. Survivin expression in lung cancer: Association with smoking, histological types and pathological stages. Oncol Lett. 2015;10:1456–1462. doi: 10.3892/ol.2015.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lyu H, Wang J, Liu B. MicroRNA regulation and therapeutic targeting of survivin in cancer. Am J Cancer Res. 2015;5:20–31. [PMC free article] [PubMed] [Google Scholar]
- Huang K, Li LA, Meng YG, You YQ, Fu XY, Song L. Arctigenin promotes apoptosis in ovarian cancer cells via the iNOS/NO/STAT3/survivin signalling. Basic Clin Pharmacol Toxicol. 2014;115:507–511. doi: 10.1111/bcpt.12270. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Kamalapuram SK, Kanwar RK. Survivin signaling in clinical oncology: a multifaceted dragon. Med Res Rev. 2013;33:765–789. doi: 10.1002/med.21264. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim HA, Seong MK, Seol H, Oh JS, Kim EK, Chang JW, Hwang SG, Noh WC. STAT3-survivin signaling mediates a poor response to radiotherapy in HER2-positive breast cancers. Oncotarget. 2016;7:7055–7065. doi: 10.18632/oncotarget.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Altieri DC. Transcriptional analysis of human survivin gene expression. Biochem J. 1999;344(Pt 2):305–311. doi: 10.1042/0264-6021:3440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Ma AN. Induction of apoptosis of non-small cell lung cancer by a methylated oligonucleotide targeting survivin gene. Cancer Gene Ther. 2010;17:441–446. doi: 10.1038/cgt.2009.82. [DOI] [PubMed] [Google Scholar]
- Li JY, Shi J, Sang JF, Yao YZ, Wang XC, Su L. Role of survivin in the pathogenesis of papillary thyroid carcinoma. Genet Mol Res. 2015;14:15102–15111. doi: 10.4238/2015.November.24.19. [DOI] [PubMed] [Google Scholar]
- Liu J, Du WQ, Fan DM. Survivin, the promising target in hepatocellular carcinoma gene therapy. Cancer Biol Ther. 2008;7:555–556. doi: 10.4161/cbt.7.4.5891. [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Ma AN, Huang WL, Wu ZN, Hu JF, Li T, Zhou XJ, Wang YX. Induced epigenetic modifications of the promoter chromatin silence survivin and inhibit tumor growth. Biochem Biophys Res Commun. 2010;393:592–597. doi: 10.1016/j.bbrc.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Ma AN, Lu J, Zhou XJ, Wang YX. Histone deacetylation directs DNA methylation in survivin gene silencing. Biochem Biophys Res Commun. 2011;404:268–272. doi: 10.1016/j.bbrc.2010.11.105. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhao M, Yu XD, Wang ZH. MS-275, a histone deacetylase inhibitor, induces apoptosis and alters survivin gene expression in human myeloma cell line U266. Ai Zheng. 2009;28:466–471. [PubMed] [Google Scholar]
- Min LH, Ji Y, Bakiri L, Qiu ZX, Cen J, Chen XT, Chen LL, Scheuch H, Zheng H, Qin LX, Zatloukal K, Hui L, Wagner EF. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- Mityaev MV, Kopantzev EP, Buzdin AA, Vinogradova TV, Sverdlov ED. Functional significance of a putative sp1 transcription factor binding site in the survivin gene promoter. Biochemistry (Mosc) 2008;73:1183–1191. doi: 10.1134/s0006297908110035. [DOI] [PubMed] [Google Scholar]
- Nabilsi NH, Broaddus RR, Loose DS. DNA methylation inhibits p53-mediated survivin repression. Oncogene. 2009;28:2046–2050. doi: 10.1038/onc.2009.62. [DOI] [PubMed] [Google Scholar]
- Nakano J, Huang CL, Liu D, Ueno M, Sumitomo S, Yokomise H. Survivin gene expression is negatively regulated by the p53 tumor suppressor gene in non-small cell lung cancer. Int J Oncol. 2005;27:1215–1221. [PubMed] [Google Scholar]
- Papanikolaou V, Iliopoulos D, Dimou I, Dubos S, Kappas C, Kitsiou-Tzeli S, Tsezou A. Survivin regulation by HER2 through NF-kappaB and c-myc in irradiated breast cancer cells. J Cell Mol Med. 2011;15:1542–1550. doi: 10.1111/j.1582-4934.2010.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Tar. 2008;12:463–476. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- Raj D, Liu T, Samadashwily G, Li F, Grossman D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis. 2008;29:194–201. doi: 10.1093/carcin/bgm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BM, O’Donovan N, Duffy MJ. Survivin: A new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Sato A, Asano T, Ito K, Sumitomo M, Asano T, Hayakawa M. A potential novel combination therapy targeting survivin in renal cancer cells: Inhibition of survivin expression by topotecan and hexamethylene bisacetamide. Mol Med Rep. 2009;2:423–428. doi: 10.3892/mmr_00000116. [DOI] [PubMed] [Google Scholar]
- Smallwood A, Esteve PO, Pradhan S, Carey M. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev. 2007;21:1169–1178. doi: 10.1101/gad.1536807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska J, Urbanska K, Gizinski S, Wysocka A, Cywinska A, Lechowski R. Survivin expression in canine lymphomas in relation with proliferative markers. Pol J Vet Sci. 2015;18:113–122. doi: 10.1515/pjvs-2015-0015. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Uzawa K, Shibahara T, Yokoe H, Noma H, Tanzawa H. Expression of an inhibitor of apoptosis, survivin, in oral carcinogenesis. J Dent Res. 2003;82:607–611. doi: 10.1177/154405910308200807. [DOI] [PubMed] [Google Scholar]
- Tsai YJ, Tsai TM, Peng PC, Li PT, Chen CT. Histone acetyltransferase p300 is induced by p38MAPK after photodynamic therapy: the therapeutic response is increased by the p300HAT inhibitor anacardic acid. Free Radical Bio Med. 2015;86:118–132. doi: 10.1016/j.freeradbiomed.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Vaziri SA, Hill J, Chikamori K, Grabowski DR, Takigawa N, Chawla-Sarkar M, Rybicki LR, Gudkov AV, Mekhail T, Bukowski RM, Ganapathi MK, Ganapathi R. Sensitization of DNA damage-induced apoptosis by the proteasome inhibitor PS-341 is p53 dependent and involves target proteins 14-3-3sigma and survivin. Mol Cancer Ther. 2005;4:1880–1890. doi: 10.1158/1535-7163.MCT-05-0222. [DOI] [PubMed] [Google Scholar]
- Wagner M, Schmelz K, Dorken B, Tamm I. Epigenetic and genetic analysis of the survivin promoter in acute myeloid leukemia. Leuk Res. 2008a;32:1054–1060. doi: 10.1016/j.leukres.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Wagner M, Schmelz K, Dorken B, Tamm I. Transcriptional regulation of human survivin by early growth response (Egr)-1 transcription factor. Int J Cancer. 2008b;122:1278–1287. doi: 10.1002/ijc.23183. [DOI] [PubMed] [Google Scholar]
- Wahdan-Alaswad R, Liu B. “Sister” miRNAs in cancers. Cell cycle (Georgetown, Tex) 2013;12:3703–3704. doi: 10.4161/cc.26875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Brems JJ, Gamelli RL, Holterman AX. Survivin signaling is regulated through nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim Biophys Acta. 2010;1803:1368–1375. doi: 10.1016/j.bbamcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wang S, Huang J, Lyu H, Lee CK, Tan J, Wang J, Liu B. Functional cooperation of miR-125a, miR-125b, and miR-205 in entinostat-induced downregulation of erbB2/erbB3 and apoptosis in breast cancer cells. Cell Death Dis. 2013;4:e556. doi: 10.1038/cddis.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhu L, Zuo W, Zeng Z, Huang L, Lin F, Lin R, Wang J, Lu J, Wang Q, Lin L, Dong H, Wu W, Zheng K, Cai J, Yang S, Ma Y, Ye S, Liu W, Yu Y, Tan J, Liu B. MicroRNA-mediated epigenetic targeting of Survivin significantly enhances the antitumor activity of paclitaxel against non-small cell lung cancer. Oncotarget. 2016;7:37693–37713. doi: 10.18632/oncotarget.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Heinz C, Groner B. Survivin - An inhibitor of apoptosis as a target for peptide based therapy for human breast cancer. J Pept Sci. 2008;14:125–125. [Google Scholar]
- Xu R, Zhang P, Huang J, Ge S, Lu J, Qian G. Sp1 and Sp3 regulate basal transcription of the survivin gene. Biochem Biophys Res Commun. 2007;356:286–292. doi: 10.1016/j.bbrc.2007.02.140. [DOI] [PubMed] [Google Scholar]
- Yamada T, Horinaka M, Shinnoh M, Yoshioka T, Miki T, Sakai T. A novel HDAC inhibitor OBP-801 and a PI3K inhibitor LY294002 synergistically induce apoptosis via the suppression of survivin and XIAP in renal cell carcinoma. Int J Oncol. 2013;43:1080–1086. doi: 10.3892/ijo.2013.2042. [DOI] [PubMed] [Google Scholar]
- Yang J, Song K, Krebs TL, Jackson MW, Danielpour D. Rb/E2F4 and Smad2/3 link survivin to TGF-beta-induced apoptosis and tumor progression. Oncogene. 2008;27:5326–5338. doi: 10.1038/onc.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xiong G, Chen X, Xu X, Wang K, Fu Y, Yang K, Bai Y. Survivin expression in esophageal cancer: correlation with p53 mutations and promoter polymorphism. Dis Esophagus. 2009;22:223–230. doi: 10.1111/j.1442-2050.2008.00885.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang HY, Gu J, Lin S, Li JH, Lu W, Wang YF, Zhu JD. Methylation profiles of thirty four promoter-CpG islands and concordant methylation behaviours of sixteen genes that may contribute to carcinogenesis of astrocytoma. BMC Cancer. 2004;4:65. doi: 10.1186/1471-2407-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffaroni N, Daidone MG. Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug Resist Updat. 2002;5:65–72. doi: 10.1016/s1368-7646(02)00049-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen HX, Zhou SY, Wang SX, Zheng K, Xu DD, Liu YT, Wang XY, Wang X, Yan HZ, Zhang L, Liu QY, Chen WQ, Wang YF. Sp1 and c-Myc modulate drug resistance of leukemia stem cells by regulating survivin expression through the ERK-MSK MAPK signaling pathway. Mol Cancer. 2015;14:56. doi: 10.1186/s12943-015-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Gu L, Li F, Zhu Y, Woods WG, Findley HW. DNA damage induces a novel p53-survivin signaling pathway regulating cell cycle and apoptosis in acute lymphoblastic leukemia cells. J Pharmacol Exp Ther. 2002;303:124–131. doi: 10.1124/jpet.102.037192. [DOI] [PubMed] [Google Scholar]