Abstract
Understanding how pesticide exposure to non-target species influences toxicity is necessary to accurately assess the ecological risks these compounds pose. To assess the potential metabolic activation of broad use pesticides in amphibians, in vitro and in vivo metabolic rate constants were derived from toad (Anaxyrus terrestris) livers in experiments measuring the depletion of atrazine (ATZ), triadimefon (TDN), and fipronil (FIP) as well as formation of their metabolites. To determine the predictability of these in vitro derived rate constants, Fowler’s toads (Anaxyrus fowleri) were exposed to soil contaminated with each of the pesticides at maximum application rate. Desethyl atrazine (DEA) and deisopropyl atrazine (DIA), both metabolites of ATZ, exhibited similar velocities (Vmax) while the KM constant for DIA was two times higher than DEA. TDN was metabolized into two diastereomers of triadimenol (TDL A and TDL B), where TDL B had a Vmax around two times higher than TDL A. The metabolite fipronil sulfone’s Vmax and KM were 150 pmol min−1 mg−1 and 29 μM, respectively. While intrinsic clearance rates for the pesticides ranged from 0.54 to 38.31 mL min−1 kg−1. Thus, gaining knowledge on differences in metabolism of pesticides within amphibians is important in estimating risk to these non-target species since the inherent toxicity of metabolites can differ from the parent compound.
Keywords: Microsomes, Metabolism, Pesticides, Amphibians
Graphical abstract
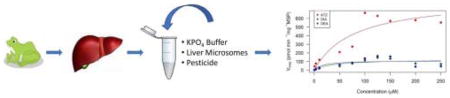
1. Introduction
Over 2.4 billion kilograms of pesticides have been used worldwide in preventing diseases, dealing with nuisance animals, and aiding in crop management (Stokstad and Grullón, 2013). Worldwide, herbicides have the most prevalent usage and compromise approximately 40% of all pesticides applied for both commercial and industrial use. The percentages of the total global use of pesticides are 33% for insecticides and 10% for fungicides (Stokstad and Grullón, 2013). In the United States, pesticide use surpassed 1 billion pounds in 2007 and their use continues to increase (Grube et al., 2011). Of note, 80% of pesticides being applied in the U.S. are for agricultural purposes (Stokstad and Grullón, 2013). Atrazine, triadimefon, and fipronil are broad use pesticides in the U.S. where atrazine is one of the most commonly used herbicides to control weeds in agricultural crops (Lang et al., 1996), triadimefon is a broad-spectrum fungicide used in both fruit and evergreen farming (Kenneke et al., 2008), and fipronil is an insecticide with both industrial and consumer applications (Hainzl et al., 1998). Although pesticides are used to control insects, diseases and general nuisance organisms, exposure to non-target species frequently occurs. Amphibians are important sentinel environmental species to pesticide exposure because they have the ability to integrate stressors from both aquatic and terrestrial ecosystems. Numerous species begin early life stages in aquatic environments and then migrate to land. It has been demonstrated that amphibian populations are in decline and pesticide exposure, in these non-target species, has been identified as one of the primary causative factors (Wake, 1991; Davidson et al., 2002; Mann et al., 2009). Amphibians are often exposed to pesticides during direct and indirect agricultural application and also during rain events when water sources become contaminated with pesticides where amphibians breed and deposit broods (Hayes et al., 2002; Houlahan and Findlay, 2003).
Through investigating the consequence of pesticide exposure in amphibians, Brühl et al. (2013) observed in an agricultural overspray scenario, that mortality of the frogs exposed to pesticides ranged from 100% after one hour to 40% after seven days. Reynaud et al. (2012) observed that fipronil was found in various organs throughout the female green frog (Pleophylax kl. esculentus) after one day of exposure to contaminated water, while the gall bladder had the highest bioconcentration factor after eight days of exposure. Additionally, fipronil sulfone, a biomarker of fipronil exposure, was the most prominent metabolite found in the female green frogs exposed to fipronil contaminated water (Reynaud et al., 2012). Hayes et al. (2003) reported atrazine concentrations in both water and amphibians that were collected from the same sites, along with its main metabolites. Previous work in our laboratory has shown that seven species of amphibians exposed to five different pesticides bioaccumulated atrazine in the greatest concentrations; however, when application rates were taken into account, fipronil was the most permeable to amphibian skin (Van Meter et al., 2014).
Amphibian skin is well known for being extremely permeable to both respiratory gases and water. Thus many anurans lack a hydrophobic barrier, due to the absence of epidermal scales and other protective layers (thick stratum corneum) observed in other terrestrial species (Hillman et al., 2009). Since amphibians need to stay hydrated and they do not physically imbibe water, they have developed a highly vascularized seat patch. This vascularized seat patch is located in the posteroventral region of anurans, and is where the primary route of absorption of water occurs (Bentley and Main, 1972). Pesticides and metals are known to be absorbed through the skin of amphibians (James et al., 2004; Willens et al., 2006; Van Meter et al., 2014, 2015, 2016, 2018). Therefore, the skin is presumed to be the major route of exposure for contaminants in amphibians compared to pulmonary and oral routes (Smith et al., 2007).
After entering the body, pesticide metabolism is commonly initiated by the liver, where phase I proteins such as cytochrome P450 enzymes are used to help with the oxidation, reduction, and hydrolysis of xenobiotics (Lang et al., 1996; Hanioka et al., 1999a). In in vitro exposures, liver degraded atrazine into two major metabolites: desethyl atrazine and deisopropyl atrazine in rats, guinea pigs, goats, rabbits and humans (Adams et al., 1990; Lang et al., 1996; Hanioka et al., 1999b). Hanioka et al. (1998) observed that rats exposed to atrazine formed deisopropyl atrazine ten times faster than desethyl atrazine. Hepatic metabolism of triadimefon can only result in triadimenol formation through 11β-hydroxysteroid dehydrogenase type 1 (Kenneke et al., 2008). Triadimenol is considered more toxic than the parent in an array of species examined and interestingly, is also used as a pesticide (Roberts and Huston, 1999; Kenneke et al., 2010). Fipronil can degrade into fipronil sulfone, fipronil sulfide, and fipronil desulfinyl depending on different chemical processes such as metabolic oxidation or photolysis (Hainzl et al., 1998; Reynaud et al., 2012). In human liver microsomes, fipronil sulfone was the major metabolite observed through oxidation (Tang et al., 2004). Fipronil sulfone is also known to be more toxic than its parent fipronil in many marine invertebrates, fish, and avian species (U.S. EPA, 1996; Baird et al., 2013).
Although data on pesticide metabolism exists for many species, limited data are available for amphibians. To better understand the role of amphibian hepatic metabolism following pesticide exposure, the objective of this study was two-fold: (1) to obtain values of the maximum velocity (Vmax) and Michaelis constant (KM) for both parent and metabolites in vitro in toad microsomes and (2) to compare in vitro data and their predictive abilities to estimate body burdens determined through in vivo exposure studies using amphibians.
2. Materials and methods
Pesticide active ingredients and their metabolites were obtained from the U.S. Environmental Protection Agency’s National Pesticides Standard Repository (Fort Meade, MD, USA). Active ingredients (AI) and metabolites analyzed in the study were ≥96.5% purity for atrazine (ATZ; CAS 1912-24-9), deisopropyl atrazine (DIA; CAS 1007-28-9), desethyl atrazine (DEA; CAS 6190-65-4), triadimefon (TDN; CAS 43121-43-3), triadimenol (TDL; CAS 55219-65-3), fipronil (FIP; CAS 120068-37-3), fipronil sulfone (F. sulfone; CAS 120068-36-2) and tetraconazole (CAS 112281-77-3). RapidStart NADPH (nicotinamide adeninedinucleotide phosphate) regenerating system along with all solvents used for pesticide extraction and analysis were of highest grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). Southern toads (n = 46) were purchased from Backwater Reptiles (FL, USA) with half female and half male and shipped live to Celsis In Vitro Technologies where they were immediately euthanized for liver microsome preparation.
2.1. In vitro studies
Pooled southern toad (Anaxyrus terrestris) liver microsomes were purchased from Celsis In Vitro Technologies (Baltimore, MD, USA) and stored in a −80 °C freezer until used for metabolic assays. The total P450 concentration for the toad liver microsomes was 1.249 nmol/mg provided by the vendor. Metabolism assays were performed based on the method of Mazur et al. (2007) with slight modifications and done in triplicate. Briefly, microsomes were pre-incubated at 30 °C for 10 min in potassium phosphate buffer (100 mM, pH 7.42) in microcentrifuge tubes prior to the addition of substrate (total volume 500 μL). Concentrations of AI used in the study ranged from 0.7–200 μM (organic solvent in reaction medium was less than 1%) with a final microsomal protein concentration of 0.2 mg/mL. The reaction was initiated by the addition of RapidStart NADPH regenerating system for a final yield of 500 μM. After concurrent addition of both AI and NADPH, the assays were vortexed and incubated for a specified time (0–90 min). Reactions were quenched using 0.5 mL of 60% MeOH:H2O (v:v) with internal standard (tetraconazole), vortexed and then immediately placed on ice. The samples were centrifuged at 4 °C for 10 min at 13500 rpm. Following centrifugation and protein precipitation, aliquots of the assay were placed in 2 mL vials and analyzed by liquid chromatography coupled to mass spectrometry (LC–MS).
2.2. Vmax and KM values
Utilizing the data analysis described in Crowell et al. (2010), the maximum velocity (Vmax, pmol min−1 mg−1) and Michaelis constant (KM, μM) were calculated using the Michaelis-Menten equation:
Briefly, substrate concentration ([S], μM) for each pesticide was plotted against time to calculate the initial reaction rates (pmol min−1). These values were then normalized to microsomal protein (MSP) to obtain an initial reaction velocity (V, pmol min−1 mg−1). Vmaxand KM parameters along with standard error for each parent and corresponding metabolite(s) were calculated by plotting initial reaction rates versus substrate concentration in RStudio (version 3.2.3, 2015).
2.3. Intrinsic clearance, CLint
Intrinsic clearance was similarly calculated based on the formula in Crowell et al. (2010):
For toads, 5.4 mg of MSP per gram of liver was used due to taxonomic relations (Noshiro and Omura, 1984) and the liver weight used for toads was 52 g/kg body (based on liver weight being 5.2% of body weight).
2.4. In vivo studies
Exposure studies were carried out based on the methods in Van Meter et al. (2014). Fowler’s toads (Anaxyrus fowleri) were reared at U.S. EPA in Athens, GA from egg masses collected at University of Georgia’s Whitehall Forest. Toads were reared in 375 L outdoor wading pools and fed Tetra Fin fish food ad libitum until metamorphosis. As toad metamorphs emerged they were transferred to 600 L polyethylene tanks padded with sphagnum moss and leaf litter to simulate a terrestrial environment. All juvenile toads were fed purchased crickets and cultured fruit flies until 60–90 days post-metamorphosis. Rearing, housing and animal studies were all done in accordance with approved animal use and care protocols (IACUC protocol (A2012 05-018-Y1-A0) received approval from the University of Georgia Institutional Animal Care and Use Committee). Soil collected from a reference study site in Newton, GA in June 2013 was processed through a 2 mm sieve and stored at <4 °C until used. Soil was applied to the bottom of an aquarium (10 gallon) and each pesticide was applied to the surface of the soil using a compressed air propellant Spray Gun® canister attached to a graduated glass jar.
Maximum labeled application rates were scaled down to the size of the aquarium and final rates were 22.9, 2.7, and 1.1 μg/cm2 for ATZ, TDN, and FIP, respectively. Fowler’s toads (n = 5) were then placed into aquaria (n = 4) where replicate individuals were separated by PVC pipes (10 cm internal diameter) for a total of 20 toads per pesticide. One replicate from each tank was randomly removed and placed in a pre-weighed 50 mL centrifuge tube at each time step: 2 h, 4 h, 12 h, 24 h, and 48 h and euthanized in a −80 °C freezer. Additionally, three soil samples were collected at each time point from each tank into pre-weighed 15 mL centrifuge tubes and stored at −20 °C freezer until analyzed. Prior to pesticide extraction, amphibians were brought to room temperature to obtain final weights and 5 mL of water (18.2 MΩ) was added to facilitate homogenization with a tissue grinder. All samples were spiked with 10 μL of 1000 ppm tetraconazole and placed on a freeze drier overnight. Next, 5 mL of methanol (MeOH) was added to each 50 mL centrifuge tube and placed into a sonicator (Model Branson B-22-4) for 30 min. Samples were centrifuged for 20 min at 3250 rpm and the supernatant was transferred to a 20 mL scintillation vial. The above procedure was repeated to ensure that all the pesticide was extracted. The pooled supernatants were placed under a steady stream of nitrogen gas and blown down until approximately 1 mL of MeOH remained. Next, 10 mL of water (18.2 MΩ) and 3 mL of methyl tert-butyl ether (MTBE) were added to each vial along with <1 g of sodium sulfate to remove any emulsion. After complete separation of the two phases, the top organic layer was transferred to a 2 mL centrifuge tube and centrifuged for 15 min at 13500 rpm. From this sample, a 1 mL aliquot was taken and placed into a 2 mL vial to be blown dry under nitrogen and finally reconstituted in 1 mL 30% MeOH (v:v) and analyzed via liquid chromatography mass spectrometry (LC–MS).
2.5. LC–MS instrumentation
All analytical conditions have been previously reported in Van Meter et al. (2014). Active ingredients and metabolites were quantified on an Agilent 1100 Series HPLC coupled to a 6120 mass spectrometer equipped with an Eclipse XDB-C18 (3.5 μm particle size, 3.0 × 150 mm; Agilent Technologies, CA, USA). Initial conditions were held for 2 min at 70% water with 0.1% formic acid (A) and 30% acetonitrile with 0.1% formic acid (B). Ramped to 90% B over 16 min and held there for 4 min before returning to starting conditions. The flow rate was 0.3 mL min−1 for the entire run. Quantification of the pesticides and their corresponding metabolites were based on calibration curves of authentic standards that ran at the beginning of a run with QAQC samples analyzed intermittently. FIP and F. sulfone were analyzed in negative electrospray ionization (ESI) while all other active ingredients and metabolites were analyzed in positive ESI in selected ion monitoring mode (SIM; Table 1).
Table 1.
LC–MS analytical parameters for active ingredients and metabolites in in vitro and in vivostudies.
| Compound | Retention Time | Mass 1 (m/z) | Mass 2 (m/z) | Ionization Mode |
|---|---|---|---|---|
| DIA | 4.3 | 174 | 176 | (+) |
| DEA | 6.2 | 188 | 146 | (+) |
| ATZ | 13.8 | 216 | 174 | (+) |
| TDL A | 15.8 | 296 | 227 | (+) |
| TDL B | 16.3 | 296 | 227 | (+) |
| TDN | 17.6 | 294 | 225 | (+) |
| Tetraconazole | 17.7 | 372 | (+) | |
| FIP | 19.7 | 435 | 330 | (−) |
| F. Sulfone | 20.1 | 451 | 415 | (−) |
2.6. Statistical analysis
Vmax and KM constants were calculated using RStudio (version 3.2.3, 2015) all values presented in Table 2 are average ± standard error. For TDL B the 200 μM data point was an outlier and excluded from all calculations.
Table 2.
Michaelis-Menten parameters for depletion and formation of pesticides in mammals and mixed gender southern toad (Anaxyrus terrestris) liver microsomes, intrinsic clearance rates, and extrapolation of Vmaxin vitro to Vmaxin vivo (values are average ± standard error, ND = not determined).
| Compound | Depletion/Formation | Species | Vmax(pmol min−1 mg−1) | KM (μM) | CLint(mL min−1 kg−1) | Vmax (in vivo) (mg h−1 kg−1)0.75 | Reference |
|---|---|---|---|---|---|---|---|
| ATZ | Depletion | Sprague-Dawley rats | 720 | 27.5 | Adams et al. (1990) | ||
| Fischer rats | 1160 | 27.8 | Adams et al. (1990) | ||||
| Toads | 834 ± 210 | 77 ± 52 | 3.04 | 2.3 | This study | ||
| DIA | Formation | Rats | 1459 ± 266 | 50.4 ± 7.4 | Hanioka et al. (1999b) | ||
| Guinea pigs | 413 ± 31 | 28.4 ± 1.7 | Hanioka et al. (1999b) | ||||
| Mouse | 805 ± 50 | 29.5 ± 1.9 | Hanioka et al. (1999b) | ||||
| Humans | 220.8 ± 8.7 | 440.6 ± 40.2 | Joo et al. (2010) | ||||
| Toads | 124 ± 32 | 36 ± 37 | 1.86 | 0.46 | This study | ||
| DEA | Formation | Rats | 135 ± 27 | 44.5 ± 4.9 | Hanioka et al. (1999b) | ||
| Guinea pigs | 109 ± 10 | 16.2 ± 1.7 | Hanioka et al. (1999b) | ||||
| Mouse | 277 ± 23 | 52.5 ± 4.4 | Hanioka et al. (1999b) | ||||
| Humans | 224.5 ± 6.7 | 79.7 ± 9.5 | Joo et al. (2010) | ||||
| Toads | 112 ± 31 | 17 ± 26 | 0.97 | 0.47 | This study | ||
| TDN | Depletion | Rats | 2860 ± 100 | 2.17 ± 1.6 | Barton et al. (2006) | ||
| Rats (male) | 7452 ± 493 | 47 ± 8 | 245 | Crowell et al. (2010) | |||
| Mouse (male) | 2824 ± 261 | 45 ± 11 | 155 | Crowell et al. (2010) | |||
| Toads | 639 ± 71 | 5 ± 4 | 38.31 | 2.38 | This study | ||
| TDL A | Formation | Rats (male)a | 5703 ± 149 | 61 ± 4 | 142 | Crowell et al. (2010) | |
| Rats (female)a | 344 ± 15 | 78 ± 8 | 7 | Crowell et al. (2010) | |||
| Toads | 281 ± 192 | 147 ± 201 | 0.54 | 1.29 | This study | ||
| TDL B | Formation | Rats (male)a | 5703 ± 149 | 61 ± 4 | 142 | Crowell et al. (2010) | |
| Rats (female)a | 344 ± 15 | 78 ± 8 | 7 | Crowell et al. (2010) | |||
| Mouse (male)a | 1052 ± 38 | 13 ± 2 | 196 | Crowell et al. (2010) | |||
| Mouse (female)a | 1341 ± 52 | 6 ± 1 | 531 | Crowell et al. (2010) | |||
| Toads | 1712 ± 2441 | 239 ± 566 | 2.02 | 5 | This study | ||
| FIP | Depletion | Toads | ND | ND | ND | ND | This study |
| F. Sulfone | Formation | Rats | 390.4 | 19.9 | Tang et al. (2004) | ||
| Human | 107.4 | 27.2 | Tang et al. (2004) | ||||
| Toads | 150 ± 27 | 29 ± 23 | 1.47 | 1.11 | This study |
Triadimenol is not divided into its chiral forms.
3. Results and discussion
3.1. Vmax and KM values
To determine pesticide metabolic rate constants in amphibians, a series of toad microsomal experiments were conducted to determine both the depletion rates of ATZ, TDN, FIP, and formation rates for their corresponding metabolites. In all the microsomal experiments the formation of the metabolites plateaued in the concentration range of 0.7–250 μM.
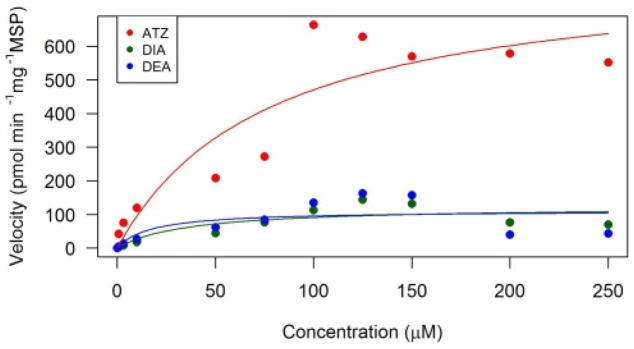
In the toad microsomes, ATZ depletion rate was 834 pmol min−1 mg−1 while the KM was 77 μM (Fig. 1; Table 2). Compared to other species, the Vmax for Sprague-Dawley and Fischer rats were 720 and 1160 pmol min−1 mg−1 (respectively), resulting in toads’ metabolic parameters falling between the two species (Adams et al., 1990). However, the KM constant for Sprague-Dawley and Fischer rats were 27.5 and 27.8 μM, which was significantly lower than the toads.
Fig. 1.
Michaelis-Menten kinetic analyses of atrazine (ATZ), deisopropyl atrazine (DIA), and desethyl atrazine (DEA) in southern toad (Anaxyrus terrestris) liver microsomes. Atrazine is the parent compound in red, DIA and DEA are metabolites in green and blue, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
DEA and DIA were metabolized at equal rates with a Vmax of 112 and 124 pmol min−1 mg−1, respectively (Fig. 1; Table 2). The velocity for DEA in toads was similar to the one observed by Hanioka et al. (1999b) in male rats at 135 pmol min−1 mg−1. Furthermore, Hanioka et al. (1999b) exposed microsomes from mice and guinea pigs to atrazine, where the DEA Vmaxfor guinea pigs at 109 pmol min−1 mg−1 was slightly lower than toads, while the mouse was slightly higher at 277 pmol min−1 mg−1. On the other hand, DIA was produced ten times faster in rats at a rate of 1459 pmol min−1 mg−1 compared to toads (Hanioka et al., 1999b). The toad had the lowest Vmax by 3–6.5-fold for DIA when compared to mice and guinea pigs, 805 and 413 pmol min−1 mg−1, respectively (Hanioka et al., 1999b). However, Joo et al. (2010) found that human liver microsomes produced DEA and DIA at 224 and 220 pmol min−1 mg−1, respectively, which appears two times faster than toads. A major difference observed in the in vitro study was that the metabolite diaminochlorotriazine (DACT) was not found in the toad microsomes even though it was a major metabolite found in vivo (rats) and a minor one in vitro (rat microsomes) (Brzezicki et al., 2003; Ross and Filipov, 2006).
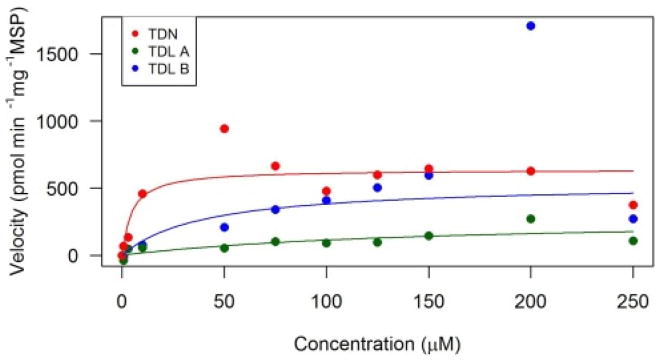
The depletion rate for TDN was 639 pmol min−1 mg−1, which was several fold slower compared to both rats and mice as studied in Crowell et al. (2010). Furthermore, the KM was 5 μM for toads and this was found to be at least 7 times lower compared to rats and mice (Crowell et al., 2010). In Barton et al. (2006) the Vmax was only 4.5 times higher for rats compared to toads, however, when the rats were pretreated with a high dose of TDN the Vmaxapp increased, resulting in a 5-fold difference.
Approximately 30% of the pesticides in commercial use are chiral enantiomers of one another, leading to racemic mixtures often being used in pesticide formulations (Ulrich et al., 2009). However, with chiral pesticides, one enantiomer is often more toxic than the other, and information on the concentrations of each enantiomer are essential to protect non-target species (Garrison, 2006). For instance, TDL consists of two diastereomers A and B (A: 1S,2R; 1R,2S; B: 1S,2S; 1R,2R). The 1S,2R TDL enantiomer was the most toxic to rats; furthermore, in rainbow trout it was produced at the slowest rate out of all the TDL enantiomers (Kenneke et al., 2010). Additionally, in Kenneke et al. (2008) the diastereomer TDL B was formed at a greater rate than the TDL A diastereomer in rats. This was also observed in toad microsomes, where the enantiomer TDL B was metabolized faster than TDL A (Fig. 2). The TDL B formation velocity was around two times higher at 537 pmol min−1 mg−1 than TDL A which was 281 pmol min−1 mg−1 in toads (Fig. 2; Table 2). The KM concentrations for TDL A was three times higher than TDL B in toads with values at 147 and 40 μM, respectively. However, TDL A formation was similar to TDL total in female rats with a velocity of 344 pmol min−1 mg−1, while being at least an order of magnitude slower than the male rat (Crowell et al., 2010). While for TDL B, the toad microsomes were slightly higher than the female rats, but half the velocity of the male and female mouse (Crowell et al., 2010).
Fig. 2.
Michaelis-Menten kinetic analyses of triadimefon (TDN), triadimenol A (TDL A), and triadimenol B (TDL B) in southern toad (Anaxyrus terrestris) liver microsomes. Triadimefon is the parent compound in red, TDL A and TDL B are metabolites in green and blue, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
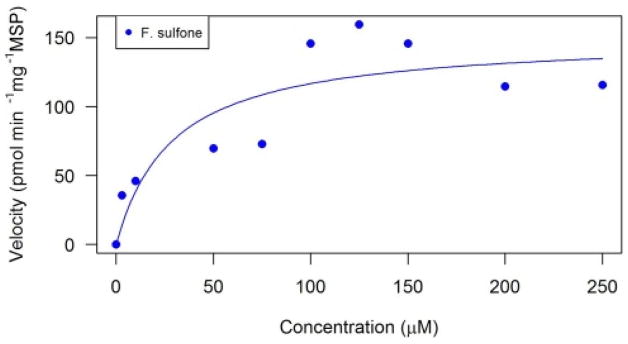
There was only formation of fipronil sulfone in the microsomal systems of amphibians, which was consistent with the in vivo study (Fig. 3). Additionally, this same observation was noted in Tang et al. (2004) where only F. sulfone was quantified in both human and rat liver microsomes. Fipronil sulfone formation has been determined in vivo in green frogs(Pelophylax kl. esculentus), barking treefrogs (Hyla gratiosa), and green treefrogs (H. cinerea) (Reynaud et al., 2012; Van Meter et al., 2015). In the current study, toad microsomes metabolized FIP into F. sulfone with a Vmax of 150 pmol min−1 mg−1 and a KM of 29 μM (Table 2). Rat microsomes were shown to metabolize FIP 3.8 times faster than human liver microsomes with a Vmax of 0.39 and 0.11 nmol min−1 mg−1, respectively (Tang et al., 2004). Toad microsomes again fall intermittent to these two values, however, the toads had a KM that was three times higher than either rats or humans (27.2 and 19.9 μM, respectively; Tang et al., 2004).
Fig. 3.
Michaelis-Menten kinetic analyses of fipronil sulfone (F. sulfone) in southern toad (Anaxyrus terrestris) liver microsomes. Fipronil (parent) appeared to be immediately depleted in these exposures.
3.2. Intrinsic clearance, CLint
The intrinsic clearance rates were calculated for the depletion of the parent compounds and the formation of the metabolites (Table 2). In this study, intrinsic clearances ranged from 0.54 to 38.31 mL min−1 kg−1 with those calculated for TDL A and TDN being the lowest and highest rates, respectively. The metabolites of atrazine, DIA and DEA, had the lowest clearance rates at 0.97 and 1.86 mL min−1 kg−1, compared to ATZ which was 3.04 mL min−1 kg−1. Of all the pesticides tested, TDN had the highest intrinsic clearance at 38.31 mL min−1 kg−1, while TDN−s metabolites were much lower at 0.54 and 3.77 mL min−1 kg−1 for TDL A and TDL B, respectively. The intrinsic clearance for F. sulfone was 1.47 mL min−1 kg−1. Intrinsic clearance can be a flow-limited system if the metabolism and clearance exceeds the hepatic blood flow rate, meaning the rate-limiting step becomes the transportation of the substrate and its product to that tissue (Crowell et al., 2010). In female rats exposed to TDN, the CLint values were calculated to be near the hepatic blood flow rate of rodents suggesting that metabolism may not be blood-flow limited (Crowell et al., 2010). The hepatic blood flow for toads (Bufo arenarum) was calculated to be 25 mL min−1 kg−1 by Uranga (1969), (where the hepatic blood flow was divided by the average weight of the toads in kg) thus most of the intrinsic clearances for the pesticides, both parents and metabolites, were below this value, except TDN. This suggests that the metabolism and clearance for these pesticides are not flow-limited and can be problematic for amphibians. In this study, the microsomes were of a mixed sex, while examining the differences between sexes could be further investigated. Additionally, examining liver microsomes from toads at various developmental stages could result in different biotransformation abilities.
Rats appear to be acceptable surrogates for predicting the Vmax for DEA. However, all other analytes examined in this study were several-fold to an order of magnitude different than other published kinetic rate values for various species. Within the select few species (rats, mice and humans) that are mainly examined for in vitro studies, the kinetic rates are different and this could be due to differences in species, sex or function/abundance of the cytochrome P450 enzymes (Hanioka et al., 1999a; Crowell et al., 2010). However, there is very little research that examines these kinetic rates in amphibians. These kinetic parameters can be used for physiologically based pharmacokinetic models for pesticide exposure in amphibians, which can aid in creating amphibian models for risk assessment purposes.
3.3. In vivo studies
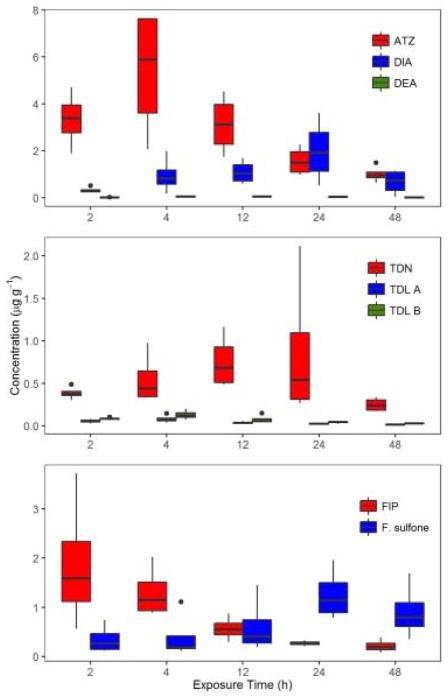
For ATZ the maximum concentration was reached at 4 h in this study, and decreased at 48 h while for DIA and DEA both were observed at the earliest time tested (Fig. 4; Table 3). This is comparable to Storrs Méndez et al. (2009), who observed that atrazine rapidly crossed the seat patch in toads and equilibrated at 5 h. However, DEA concentrations were the same throughout the entire exposure, while DIA concentrations increased up to 24 h. This resulted in the combined DEA and DIA concentration being 10–130% of the total atrazine body burden. TDN was observed to increase in concentration until 24 h and then it decreased at 48 h (Fig. 4; Table 3). It appears that TDN could have a slower absorption rate compared to the other two pesticides examined in this study because it took longer to reach its maximum threshold. The metabolites of TDN reached maximum concentration at 4 h then decreased, which could be due to further metabolism of these metabolites, however, the degradates of TDL were not analyzed in this experiment. Throughout the entire in vivo exposure, TDL B had higher concentrations than TDL A, which corresponds to the in vitro experiments where TDL B was formed at a faster rate than TDL A. Fipronil decreased slowly over the entire study, while F. sulfone increased, even surpassing fipronil’s concentration at the 24 and 48 h time points (Fig. 4; Table 3). While these same pesticides were examined in Van Meter et al. (2014); Van Meter et al. (2016), the exposure length was only 8 h and the body burden for pesticides was a summation of parent and metabolites, making it difficult to draw inferences about metabolism across data sets. Reynaud et al. (2012) observed F. sulfone to be the primary metabolite in female green frogs exposed to FIP, which was similar to the current in vivo study. Furthermore, Reynaud et al. (2012) observed an increase in bioconcentration factor for FIP over six days in green frogs, which was the opposite of the current study.
Fig. 4.
Pesticide tissue concentrations (μg g−1) in Fowler−s toads (Anaxyrus fowleri) exposed for 2, 4, 12, 24, or 48 h on pesticide contaminated soil.
Table 3.
Tissue and soil concentrations (μg g−1) of parent pesticide and corresponding metabolites in Fowler’s Toads (Anaxyrus fowleri) and soil following exposure on contaminated soil. Standard errors are in parentheses.
| Tissue | Soil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | 2 h | 4 h | 12 h | 24 h | 48 h | 0 h | 2 h | 4 h | 12 h | 24 h | 48 h |
| ATZ | 3.34 (0.59) | 5.36 (1.38) | 3.12 (0.63) | 1.55 (0.30) | 1.00 (0.18) | 22.59 (2.11) | 22.71 (2.16) | 23.12 (1.75) | 20.84 (1.75) | 34.42 (2.33) | 33.12 (3.17) |
| DIA | 0.33 (0.06) | 0.94 (0.38) | 1.08 (0.25) | 1.99 (0.67) | 0.66 (0.27) | 0.004 (0.004) | 0.00 (0.00) | 0.02 (0.01) | 0.07 (0.03) | 0.06 (0.02) | 0.05 (0.03) |
| DEA | 0.01 (0.002) | 0.04 (0.01) | 0.05 (0.01) | 0.04 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.01 (0.01) | 0.06 (0.01) | 0.11 (0.04) | 0.06 (0.01) | 0.06 (0.01) |
| TDN | 0.39 (0.03) | 0.55 (0.15) | 0.75 (0.16) | 0.87 (0.43) | 0.25 (0.04) | 4.68 (0.61) | 5.21 (0.85) | 8.54 (0.52) | 9.43 (0.87) | 6.50 (0.40) | 5.10 (0.35) |
| TDL A | 0.06 (0.01) | 0.08 (0.02) | 0.04 (0.008) | 0.02 (0.004) | 0.02 (0.002) | 0.01 (0.001) | 0.01 (0.000) | 0.01 (0.000) | 0.01 (0.001) | 0.01 (0.001) | 0.02 (0.002) |
| TDL B | 0.08 (0.01) | 0.13 (0.03) | 0.08 (0.02) | 0.04 (0.01) | 0.03 (0.002) | 0.02 (0.001) | 0.02 (0.001) | 0.02 (0.001) | 0.03 (0.003) | 0.02 (0.004) | 0.05 (0.005) |
| FIP | 1.87 (0.67) | 1.30 (0.26) | 0.57 (0.12) | 0.27 (0.03) | 0.21 (0.07) | 0.72 (0.09) | 0.86 (0.07) | 1.20 (0.12) | 1.08 (0.12) | 0.94 (0.12) | 0.56 (0.07) |
| F. sulfone | 0.35 (0.14) | 0.40 (0.24) | 0.61 (0.29) | 1.25 (0.26) | 0.91 (0.28) | 0.01 (0.002) | 0.02 (0.004) | 0.03 (0.005) | 0.02 (0.005) | 0.03 (0.005) | 0.02 (0.003) |
3.4. In vitro to in vivo extrapolations
Extrapolation from Vmaxin vitro liver microsomes to Vmaxin vivo for risk assessments was similar to the method in Lipscomb et al. (1998) (Table 2). DIA and DEA had the lowest values at 0.47 and 0.46 mg h−1 kg−1, respectively, while the highest value was for TDN at 2.38 mg h−1 kg−1. Atrazine had a Vmaxin vivo value of 2.30 mg h−1 kg−1, which was slightly higher than TDL B (2.10 mg h−1 kg−1). TDL A (1.29 mg h−1 kg−1) was lower than TDL B, but higher than DEA, DIA and F. sulfone (1.11 mg h−1 kg−1). One difference between the in vitroand in vivo studies was the in vivo study had used only one concentration, which was the maximum application rate for each pesticide. Even though the in vitro and in vivo study were similar for TDL A and B, where higher concentrations were predicted for TDL B. This was not true for DIA and DEA, where DIA was much higher than DEA for in vivo studies. However, we did not examine the depletion rates for any of the metabolites, but the clearance rate for DIA was two times higher than DEA, which could result in different tissue concentrations. It is also possible that enzyme induction could cause differences in tissue concentration considering the in vivo exposures lasted 48 h and other studies have noted an increase in P450 content after exposure to ATZ (Dong et al., 2009; Fu et al., 2013). Overall, these Vmaxin vivo values can also be used for physiologically based pharmacokinetic models to determine pesticide tissue concentrations in amphibians for risk assessment.
4. Conclusions
Estimating kinetic rate constants for chemicals is a well-established method for estimating the metabolism rates for compounds. Identifying the main metabolites of pesticides utilizing in vitro microsomes can aid in estimating the kinetic rate constants for broad-spectrum use pesticides that are ubiquitous in the environment. Obtaining a better understanding for these kinetic rate parameters, Vmax and KM, can help elucidate the fate and toxicity of these chemicals in vivo. Even though in vitro exposures minimize time and expense that are required for testing and estimating potential toxicity, little research has been done on amphibians and other non-targeted species in the environment. This study shows that species extrapolation cannot always be viable considering that most of the pesticides examined in this study were different when compared to common laboratory species.
Acknowledgments
Thanks to Candice Lavelle and Matt Etterson for manuscript review and edits. This IACUC protocol (A2012 05-018-Y1-A0) received approval from the University of Georgia Institutional Animal Care and Use Committee. This research was supported in part by an appointment to the Research Participation Program for the U.S. Environmental Protection Agency, Office of Research and Development, administered by the Oak Ridge Institute for Science and Education through interagency agreement between the U.S. Department of Energy and EPA. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
Footnotes
Conflict of interest
None.
References
- Adams NH, Levi PE, Hodgson E. In vitro studies of the metabolism of atrazine, simazine, and terbutryn in several vertebrate species. Journal of Agricultural and Food Chemistry. 1990;38:1411–1417. [Google Scholar]
- Baird S, Garrison A, Jones J, Avants J, Bringolf R, Black M. Enantioselective toxicity and bioaccumulation of fipronil in fathead minnows (Pimephales promelas) following water and sediment exposures. Environmental Toxicology and Chemistry. 2013;32:222–227. doi: 10.1002/etc.2041. [DOI] [PubMed] [Google Scholar]
- Barton HA, Tang J, Sey YM, Stanko JP, Murrell RN, Rockett JC, Dix DJ. Metabolism of myclobutanil and triadimefon by human and rat cytochrome P450 enzymes and liver microsomes. Xenobiotica. 2006;36:793–806. doi: 10.1080/00498250600821292. [DOI] [PubMed] [Google Scholar]
- Bently, Main Zonal differences in permeability of the skin of some anuran Amphibia. Am J Physiol. 1972;223:361–363. doi: 10.1152/ajplegacy.1972.223.2.361. [DOI] [PubMed] [Google Scholar]
- Brühl CA, Schmidt T, Pieper S, Alscher A. Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Scientific Reports. 2013;3:1135. doi: 10.1038/srep01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezicki JM, Andersen ME, Cranmer BK, Tessari JD. Quantitative identification of atrazine and its chlorinated metabolites in plasma. Journal of Analytical Toxicology. 2003;27:569–573. doi: 10.1093/jat/27.8.569. [DOI] [PubMed] [Google Scholar]
- Crowell SR, Henderson WM, Fisher JW, Kenneke JF. Gender and species differences in triadimefon metabolism by rodent hepatic microsomes. Toxicology Letters. 2010;193:101–107. doi: 10.1016/j.toxlet.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Davidson C, Shaffer HB, Jennings MR. Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conservation Biology. 2002;16:1588–1601. [Google Scholar]
- Dong X, Zhu L, Wang J, Wang J, Xie H, Hou X, Jia W. Effects of atrazine on cytochrome P450 enzymes of zebrafish (Danio rerio) Chemosphere. 2009;77:404–412. doi: 10.1016/j.chemosphere.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Fu Y, Li M, Liu C, Qu JP, Zhu WJ, Xing HJ, Xu SW, Li S. Effect of atrazine and chlorpyrifos exposure on cytochrome P450 contents and enzyme activities in common carp gills. Ecotoxicology and Environmental Safety. 2013;94:28–36. doi: 10.1016/j.ecoenv.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Garrison AW. Probing the enantioselectivity of chiral pesticides. Environmental Science & Technology. 2006;40:16–23. doi: 10.1021/es063022f. [DOI] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides industry sales and usage. US EPA; Washington, DC: 2011. [Google Scholar]
- Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chemical Research in Toxicology. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Jinno H, Tanaka-Kagawa T, Nishimura T, Ando M. Changes in rat liver cytochrome P450 enzymes by atrazine and simazine treatment. Xenobiotica. 1998;28:683–698. doi: 10.1080/004982598239263. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Jinno H, Tanaka-Kagawa T, Nishimura T, Ando M. In vitro metabolism of chlorotriazines: Characterization of simazine, atrazine, and propazine metabolism using liver microsomes from rats treated with various cytochrome P450 inducers. Toxicology and Applied Pharmacology. 1999a;156:195–205. doi: 10.1006/taap.1999.8648. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Jinno H, Tanaka-Kagawa T, Nishimura T, Ando M. In vitro metabolism of simazine, atrazine and propazine by hepatic cytochrome P450 enzymes of rat, mouse and guinea pig, and oestrogenic activity of chlorotriazines and their main metabolites. Xenobiotica. 1999b;29:1213–1226. doi: 10.1080/004982599237895. [DOI] [PubMed] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Herbicides: feminization of male frogs in the wild. Nature. 2002;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): Laboratory and field evidence. Environmental Health Perspectives. 2003;111:568. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman SS, Withers PC, Drewes RC, Hillyard SD. The effects of adjacent land use on wetland amphibian species richness and community composition. Can J Fish Aquat Sci. 2003;60:1078–1094. [Google Scholar]
- Houlahan JE, Findlay CS. The effects of adjacent land use on wetland amphibian species richness and community composition. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1078–1094. [Google Scholar]
- James S, Little E, Semlitsch R. Effects of multiple routes of cadmium exposure on the hibernation success of the American toad (Bufo americanus) Arch Environ Contam Toxicol. 2004;46(2004):518–527. doi: 10.1007/s00244-003-3005-x. [DOI] [PubMed] [Google Scholar]
- Joo H, Choi K, Hodgson E. Human metabolism of atrazine. Pesticide Biochemistry and Physiology. 2010;98:73–79. [Google Scholar]
- Kenneke JF, Ekman DR, Mazur CS, Konwick BJ, Fisk AT, Avants JK, Garrison AW. Integration of metabolomics and in vitro metabolism assays for investigating the stereoselective transformation of triadimefon in rainbow trout. Chirality. 2010;22:183–192. doi: 10.1002/chir.20725. [DOI] [PubMed] [Google Scholar]
- Kenneke JF, Mazur CS, Ritger SE, Sack TJ. Mechanistic investigation of the noncytochrome P450-mediated metabolism of triadimefon to triadimenol in hepatic microsomes. Chemical Research in Toxicology. 2008;21:1997–2004. doi: 10.1021/tx800211t. [DOI] [PubMed] [Google Scholar]
- Lang D, Criegee D, Grothusen A, Saalfrank RW, Bocker RH. In vitro metabolism of atrazine, terbuthylazine, ametryne, and terbutryne in rats, pigs, and humans. Drug Metabolism and Disposition. 1996;24:859–865. [PubMed] [Google Scholar]
- Lipscomb JC, Fisher JW, Confer PD, Byczkowski JZ. In vitro to in vivo extrapolation for trichloroethylene metabolism in humans. Toxicology and Applied Pharmacology. 1998;152:376–387. doi: 10.1006/taap.1998.8485. [DOI] [PubMed] [Google Scholar]
- Mann RM, Hyne RV, Choung CB, Wilson SP. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environmental Pollution. 2009;157:2903–2927. doi: 10.1016/j.envpol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Mazur CS, Kenneke JF, Tebes-Stevens C, Okino MS, Lipscomb JC. In vitro metabolism of the fungicide and environmental contaminant trans-bromuconazole and implications for risk assessment. Journal of Toxicology and Environmental Health, Part A. 2007;70:1241–1250. doi: 10.1080/15287390701380914. [DOI] [PubMed] [Google Scholar]
- Noshiro M, Omura T. Microsomal monooxygenase system in frog livers. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1984;77:761–767. doi: 10.1016/0305-0491(84)90310-9. [DOI] [PubMed] [Google Scholar]
- Reynaud S, Worms IA, Veyrenc S, Portier J, Maitre A, Miaud C, Raveton M. Toxicokinetic of benzo[a]pyrene and fipronil in female green frogs (Pelophylax kl. esculentus) Environmental Pollution. 2012;161:206–214. doi: 10.1016/j.envpol.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Roberts T, Huston D. Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides. The Royal Society of Chemistry; Cambridge, UK: 1999. pp. 1090–1097. [Google Scholar]
- Ross MK, Filipov NM. Determination of atrazine and its metabolites in mouse urine and plasma by LC-MS analysis. Analytical Biochemistry. 2006;351:161–173. doi: 10.1016/j.ab.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Smith PN, Cobb GP, Godard-Codding C, Hoff D, McMurry ST, Rainwater TR, Reynolds KD. Contaminant exposure in terrestrial vertebrates. Environ Pollut. 2007;150(2007):41–64. doi: 10.1016/j.envpol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Stokstad E, Grullón G. Infographic: pesticide planet. Science. 2013;341:730–731. doi: 10.1126/science.341.6147.730. [DOI] [PubMed] [Google Scholar]
- Storrs Méndez SI, Tillitt DE, Rittenhouse TAG, Semlitsch RD. Behavioral response and kinetics of terrestrial atrazine exposure in American toads (Bufo americanus) Archives of Environmental Contamination and Toxicology. 2009;57:590–597. doi: 10.1007/s00244-009-9292-0. [DOI] [PubMed] [Google Scholar]
- Tang J, Amin Usmani K, Hodgson E, Rose RL. In vitro metabolism of fipronil by human and rat cytochrome P450 and its interactions with testosterone and diazepam. Chemico-Biological Interactions. 2004;147:319–329. doi: 10.1016/j.cbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ulrich E, Morrison C, Goldsmith M, Foreman W. The need to identify and analyze chiral pesticides. Poster presented at the annual meeting of the Society of Environmental Toxicology and Chemistry.2009. [Google Scholar]
- Uranga J. The hepatic production of a glomerular pressure substance in the toad (Bufo arenarum) General and Comparative Endocrinology. 1969;13:179–184. doi: 10.1016/0016-6480(69)90236-6. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Fipronil Pesticide Fact Sheet. Washington, DC: 1996. Report no.: EPA 737-F-96-005. [Google Scholar]
- Van Meter RJ, Glinski DA, Henderson WM, Garrison AW, Cyterski M, Purucker ST. Pesticide uptake across the amphibian dermis through soil and overspray exposures. Archives of Environmental Contamination and Toxicology. 2015;69:545–556. doi: 10.1007/s00244-015-0183-2. [DOI] [PubMed] [Google Scholar]
- Van Meter RJ, Glinski DA, Henderson WM, Purucker ST. Soil organic matter content effects on dermal pesticide bioconcentration in American toads (Bufo americanus) Environmental Toxicology and Chemistry. 2016;35:2734–2741. doi: 10.1002/etc.3439. [DOI] [PubMed] [Google Scholar]
- Van Meter RJ, Glinski DA, Hong T, Cyterski M, Henderson WM, Purucker ST. Estimating terrestrial amphibian pesticide body burden through dermal exposure. Environmental Pollution. 2014;193:262–268. doi: 10.1016/j.envpol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Van Meter RJ, Glinski DA, Purucker ST, Henderson WM. Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans) Sci Total Environ. 2018;624:1348–1359. doi: 10.1016/j.scitotenv.2017.12.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake DB. Declining amphibian populations. Science. 1991;253:860–861. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- Willens S, Stoskopf MK, Baynes RE, Lewbart GA, Tayler SK, Kennedy-Stoskopf S. Percutaneous malathion absorption by anuran skin in flow-through diffusion cells. Environ Toxicol Pharmacol. 2006;22:255–262. doi: 10.1016/j.etap.2006.04.010. [DOI] [PubMed] [Google Scholar]