Abstract
“See one, do one, teach one” remains an unofficial, unsanctioned framework for procedural skill learning in medicine. Appropriately, medical educators have sought alternative simulation venues for students to safely learn their craft. With the end goal of ensuring competence, educational programming will require the use of valid simulation with appropriate fidelity. While cadavers have been used for teaching anatomy for hundreds of years, more recently they are being repurposed as a “high‐fidelity” procedural skill learning simulation resource. Newly deceased, previously frozen, and soft‐preserved cadavers, such as those used in Baltimore and Halifax, produce clinical cadavers with high physical and functional fidelity that can serve as simulators for performing many high‐acuity procedures for which there is otherwise limited clinical or simulation opportunities to practice. While access and cost may limit the use of cadavers for simulation, there are opportunities for sharing resources to provide an innovative procedural learning experience using the oldest of medical simulation assets, the human body.
In 1890 William Halsted, Chief of Surgery at Johns Hopkins introduced residency training and coined the phrase “see one, do one, teach one.”1 Although criticized for concerns of patient safety, this mentorship model of teaching procedural skills has essentially remained unchanged. Pedagogic concerns of the phrase mostly surrounds the literal interpretation of the numerator “one”; however, similar to Miller's pyramid, Halsted was describing a staged progression toward competent independent practice.1, 2, 3 For various reasons, but perhaps most importantly concerns regarding patient safety, procedural medicine learning is gradually moving away from the bedside as the sole, primary learning venue.
Previously, accrediting bodies in the United States and Canada listed between 80 and 150 procedures that were considered within the domain of the specialty for emergency medicine.4, 5, 6 Establishing consensus over which procedures should be deemed necessary can be challenging and often revolves around the likelihood of being exposed to the procedure both during training and as a practicing physician. Even when examining a more focused list of key indicator procedures there is considerable variability in the numbers of procedures residents will be exposed to during their training period.5, 7 In a recent study only half of surveyed residents reported adequate procedural skills during training.8
Simulation is taking on a rapidly growing role that is predicted to increase significantly with the implementation of competency‐based medical education (CBME).9, 10, 11, 12 The translational evidence supporting simulation for procedural learning is certainly not overwhelmingly positive; however, its role is indisputable where traditional experiences are either inadequate or unavailable.13, 14, 15, 16, 17, 18, 19 For high‐acuity, low‐opportunity procedural scenarios, simulation learning should be considered mandatory as opposed to the unacceptable alternative of lowering the competence bar or excluding certain potentially lifesaving interventions declaring them as unnecessary because of their infrequent occurrence in clinical practice. Procedural medicine learning requires physically and functionally appropriate simulators to meet the educational needs of learners. Clinical cadavers can potentially provide an invaluable experience for learners, serving as a simulation recourse when either there are no simulators or existing ones are inadequate for the procedural task at hand.
How Real Does Simulation Need to Be?
Medical simulation represents a diverse range of educational learning experiences meant to simulate “real‐life” clinical situations. In discussing the educational role of simulators and simulation, issues of validity and fidelity are commonly referred to. For CBME, simulation validity will carry significant weight in educational programming as we attempt to assess milestone progression (construct validity) and ultimately entrust the learner as competent to perform their clinical activities independently (predictive validity).20 Fidelity is a more commonly used (and misused) term in simulation.21 “High”‐fidelity simulation has historically referred to more elaborate, technologically advanced scenarios and expensive models compared to lower cost, simpler “low”‐fidelity approaches. This distinction has been challenged and there remains controversy on an appropriate definition of fidelity as it relates to simulation to the point that some have even called for abandoning the term.21, 22, 23 Acknowledging that there is active debate on the definition and role, most would agree that fidelity describes “the extent to which the appearance and behavior of the simulator/simulation match the appearance and behavior of the simulated system or task.21, 22, 23, 24 It is useful to further differentiate physical (structural, engineering) fidelity describing appearance and functional (psychological) fidelity which reflects the behavior of the simulator or simulation.21, 22, 23, 24 When discussing procedural skill learning it is also important that we distinguish the simulator (materials, models, manikins) from the simulation, the latter of which may include use of a simulator but in general refers to the contextual experience of a clinical scenario.
The current state of evidence supporting the relationship between simulation fidelity and outcomes (educational and clinical) is far from clear.25 The reason for the questionable relationship between simulation fidelity and educational outcomes is multifactorial but in part may relate to inconsistent definitions and descriptions of simulator/simulation fidelity. To realize the educational impact of fidelity it is important to look beyond the physical interface of the simulator and the learner. For fidelity to influence change in behavior, other factors must be considered such as ensuring objectives are well aligned with the simulation experience, the complexity of the skill is matched with an appropriate simulator, and the learner's stage of training has been considered in the simulation design.24, 26
As stated, for task‐related procedural learning it is important to understand the role of the simulator in the simulation. The psychomotor technical hands‐on interaction between learner, simulator, and instructor potentially has very different educational objectives than the learning that takes place in a context focused clinical scenario simulation.26, 27 It is not surprising that using complex, high‐fidelity manikins for a simulation exercise meant to assess nontechnical skills is not likely to add value.28 While these high‐tech, expensive manikins may talk, blink, and accommodate various procedures, these feature‐rich actions are often not very realistic and have the potential to distract participants from the intended learning objectives of the simulation.21, 29 Similarly, using a cadaver as a high‐fidelity resource to learn basic suturing techniques would not be expected to demonstrate benefit compared to learning using a ham hock.
In contrast, what about learning to repair an extensor tendon in the hand or suturing a lip laceration involving the vermillion border? What about performing a resuscitative thoracotomy, lateral canthotomy, burr hole, or pericardiocentesis? Do we stop teaching these relatively uncommonly needed procedures, use animal labs, and/or develop potentially expensive new simulator technologies? There may be simulators available for each of these procedures but can they produce superior realism to that using a single cadaveric human body?
Manikin simulators for airway management training have been available for several decades. Attempts to increase their fidelity have again produced expensive products that are more technologically complex and dynamically responsive, that breathe and talk, and that can present anatomic challenges for the learner, but often cost tens of thousands of dollars. Despite simulators being anatomically correct (physical fidelity) their materials may lack functional fidelity in the way the materials respond to manipulation and therefore may pose the risk of inconsistently reproducing conditions necessary to reinforce core emergency skills required for airway management on real patients.30, 31, 32, 33, 34, 35, 36, 37 To date no single airway manikin can provide realistic, reinforcing conditions for bag‐mask ventilation, optimal laryngoscopy (head lift, laryngeal manipulation, bougie use), accept an array of supraglottic airways, and present realistic anatomy for a surgical airway. Clinical cadavers have the potential to provide excellent physical and functional fidelity for learning airway management and countless other procedures.
Change, adaption, and ultimately improvement of a psychomotor skill are dependent on having accurate sensory information (tactile, visual, auditory) that must be perceived and then interpreted by the learner as relevant (Figure 1).26, 38 These micro feedback loops are dynamic and may illicit adaptive responses very rapidly (within seconds). Using laryngoscopy as an example, performing a lift on the tongue too proximal, without placing the tip of the blade tip correctly at the base of the vallecula (engaging the hyoepiglottic ligament), will not move the epiglottis out of the way to allow a view the glottic inlet. One hand mask ventilation in an edentulous patient may not produce adequate ventilation, recognized by sensory stimuli such as inadequate chest rise and the sounds and feel of a mask seal leak from a functional obstruction that requires corrective placement of an OPA and a two‐hand, two‐person mask ventilation approach. Most cricothyrotomy training models, while anatomically correct, fail to reproduce the significant lateral mobility of the larynx within the neck in an apneic patient (and clinical cadavers). Stabilizing this mobility is critical to success and perhaps one of the key causes of failure in accessing the cricothyroid space in a rescue “can't intubate, can't oxygenate” scenario. Producing accurate, real sensory input from our peripheral receptors to the brain and back, to elicit an adaptive, appropriate motor response, is essential for psychomotor learning.26, 38 If one believes these motor learning principles, then fidelity should matter.
Figure 1.
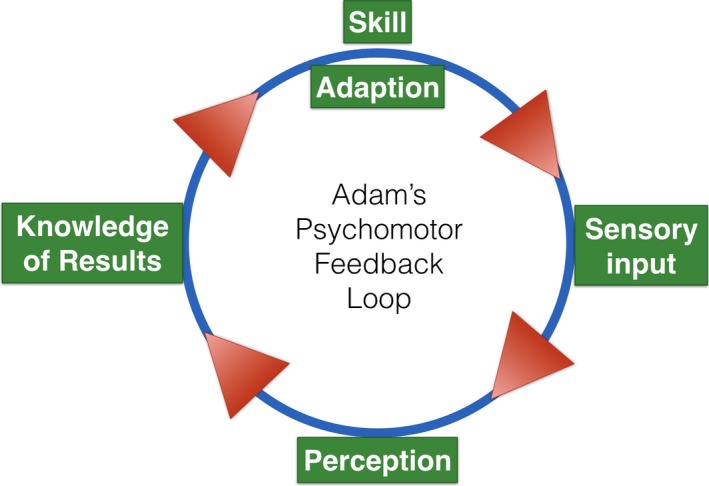
Adams psychomotor feedback loop.23
Cadavers as Simulators
The process of embalming began around the time of the American Civil War nearly 150 years ago with the intent of preserving and disinfecting the body for funeral purposes.39, 40 Modern embalming came with the discovery of formaldehyde which by the late 1800s was in common use throughout medical schools in Europe to preserve bodies for dissection.40 Numerous embalming recipes have since been developed, using various concentrations of formaldehyde mixed with other chemical agents, and remain in use today.40 All of these techniques, while preserving the anatomic integrity of the body for years of use, render the specimens rigid and without natural coloration.
While the anatomic sciences had become an important and well‐established academic discipline by the early 1900s, the academic role of dissection had reached a peak as the opportunity of new gross anatomic discovery declined. Surgery gradually became the responsibility of the surgeon specialist and, combined, these factors marked the beginning of a decline in the role of anatomic dissection. The number of hours of gross anatomy teaching in medical schools have been falling dramatically over the past five decades.41 With the steep decline in hands‐on anatomy teaching, academic anatomy departments in many university settings began to struggle and, in many cases, were falling victim to budget cuts. Dissection is being replaced by alternative less hands‐on learning with the use of prosections and, in some cases, new technologies that attempt to visually virtualize the human body.42
Meanwhile, examples of anatomic innovation were appearing as clinicians searched for safe venues to learn and practice procedural skills. While others had been experimenting with and using alternative preservation methods to produce more functional clinically relevant specimens (R. Wade, personal communication, June 3, 2017), Thiel43 in 1992 published his technique that produced cadavers with more natural color and which more closely resembled a living body.
The Clinical Cadaver
Few would argue with the face validity of using the human body as a simulator for teaching procedure skills. The use of cadavers for learning clinical procedures has quietly and informally emerged as a potential new, realistic (high‐fidelity) simulation resource. Cadavers used for learning medical education can be classified as:
-
1
Unpreserved newly deceased cadavers
-
a
Consented: used clinically in situ
-
b
Donated: “fresh” or frozen.
-
a
-
2
Preserved cadavers
-
a
Hard‐fixed (formalin)
-
b
Soft preserved (i.e., Thiel, saturated salts, Baltimore and Halifax prep).
-
a
Ethics and legal issues surrounding consent, and a general feeling of discomfort that surveyed health care providers have expressed, have made practicing on the newly deceased in situ, in the immediate clinical environment, an uncommonly practiced method for procedural learning.44, 45, 46
Cadavers prepared for anatomy learning and dissection traditionally use formaldehyde as the primary fixative. This hard‐fixation anatomic preservation technique creates long‐lasting specimens that can be stored at room temperature. However, their general appearance, color, relative immobility, and haptic experience is not realistic, making their use for leaning clinical procedures very limited.39, 47 Opportunities to learn procedures on the newly deceased (a.k.a. fresh cadavers) may occur when specimens are used soon after they are received in a human body donation program. Use of newly deceased cadavers for clinical purposes will be limited by a period of rigor mortis and thereafter decomposition significantly constrains their availability for learning.48 Freezing the bodies and then thawing them for use provides more flexibility in accessing specimens for planned educational events; however, once thawed decomposition will again ensue.47
After years of experimentation Thiel49 published his method of preservation in 1992 that produces lasting specimens that both appear and respond in manner that more closely resembles a recently deceased human. Since this publication, the Thiel method has been used around the world but mostly in Europe for procedural learning where it is recognized as a valuable soft‐preservation technique.32, 47, 50 This preparation involves an initial perfusion with a specific embalming solution followed by immersion in a mixture of preservation chemicals for 2 or more months. Once prepared these specimens have a distinct advantage of retaining most of their favorable properties for upwards of a year and do not require refrigeration, although skin sloughing will occur.39 This technique involves large tanks with large volumes of potentially hazardous chemicals and may result in cost and safety challenges, especially in educational settings where there may be a high turnover of specimens used for invasive procedures.47, 51
Numerous other soft‐preservation techniques have been described in an attempt to produce specimens that look, feel, and respond to manipulations in a manner that closely resembles what would be expected in an anesthetized patient.39, 40, 47, 48, 51, 52, 53 Each techniques have reported advantages and disadvantages and issues related to cost, preparation complexity, storage requirements, longevity, and quality. In contrast to hard‐fixed cadavers, where the primary goal is to produce specimens that are suitable for anatomic dissection, soft‐preserved specimens are primarily sought as a realistic simulation resource for learning clinical procedures and collectively referred to as clinical cadavers.
The translational evidence for cadaver use as a simulation resource remains limited.54 Current literature has demonstrated that learners perceive clinical cadavers as a high‐fidelity, more realistic simulation resource and improve both learner and instructor confidence in procedural performance.54, 55, 56, 57, 58, 59, 60 Comparisons of cadavers to available simulators has again produced mixed results; however, particularly for high‐acuity emergency procedures such as advanced airway management including cricothyrotomy, clinical cadaver experience seems to provides a superior learning experience compared to other nonbiologic simulators.32, 37, 56, 60, 61, 62 For countless other procedures there simply are no simulators available for deliberate practice and it is difficult to argue against the use of the human body for skills learning (Figure 2).
Figure 2.
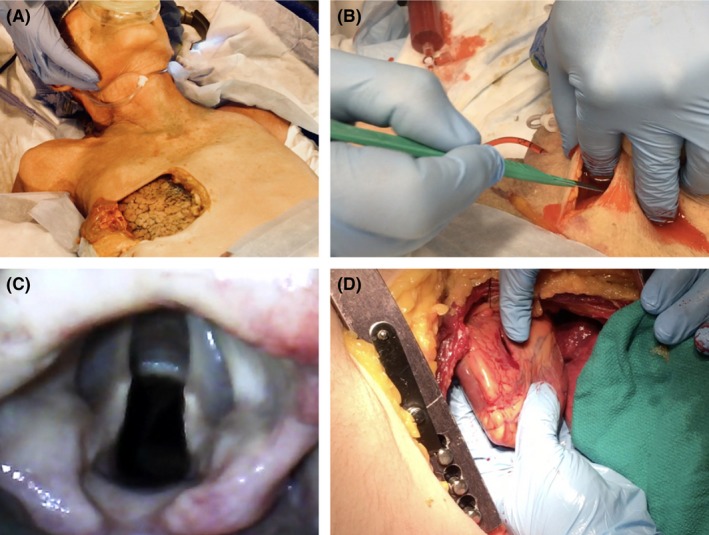
Examples of clinical cadaver use for procedural learning. (A) Lung window used for ventilation feedback; (B) bleeding cricothyrotomy; (C) view of glottis by video laryngoscopy; (D) thoracotomy with a penetrating right ventricle injury.
The Baltimore and Halifax Experience
Ron Wade is the Director of the State Anatomy Board of Maryland, which currently has thousands of living registered donors who upon death have committed to gift their bodies to advance medical science. Over 2,700 bodies are received into their program annually of which close to two‐thirds are prepared as clinical cadavers primarily to teach procedural skills. To date, the Baltimore clinical cadaver preparation and experience has not been published in the peer‐reviewed literature despite the tens of thousands of learners and even more patients who since 1982 have likely benefited from the practice of countless potentially lifesaving skills. Dr. Levitan's airway course has provided a hands‐on learning experience to over 3,000 participants since 2001, and cadaveric specimens from the same laboratory have been the basis of numerous practice‐changing research publications.63, 64, 65
In 2006, after visiting Baltimore to evaluate Ron Wade's clinical cadaver preparation, the then Dalhousie Department of Anatomy and Neurobiology prepared their first clinical cadaver. Since this first preparation a reinvigorated anatomic sciences program was soon academically rebranded as a first of its kind, a funded formal entity, the Dalhousie Clinical Cadaver Program (CCP). Serving undergraduate, postgraduate, CME, and other groups including prehospital care and the military, the Dalhousie CCP has grown from its first year, when it was used as a resource seven times, to 2017 where clinical cadavers were used for more than 180 educational sessions by well over a 1,000 learners performing several hundred different procedures. The Dalhousie University Human Body Donation Program has been in existence for over 150 years and has approximately 3,000 registered living donors. Of the approximately 150 bodies that are accepted annually approximately 60% are being prepared using a method based on that developed in Baltimore (Halifax Clinical Cadaver Preparation).
The growth of the program has been facilitated by the dedicated anatomic services staff committed to innovation and a willingness to adapt to growing demand. The modified preparation used in Halifax allows prepared clinical cadavers to be used for up to 4 weeks (while refrigerated) and still retain their physical and functional fidelity. This longevity is critical to the daily operations of the program allowing staged and coordinated access to body regions for progressively more invasive procedures performed by various specialty group learners. Emergency medicine, for example, can use a cadaver for advanced airway management, lateral canthotomy, chest tube placement, ultrasound, vascular access, intraosseous access, thoracotomy, and complex wound and tendon repair. Neurosurgery and ENT can then follow with more invasive procedures of the head and neck region. Similarly, thoracics can follow in the chest and concurrently general surgery can use the abdomen and orthopedics the limbs. This shared, multidisciplinary coordinated approach to access preserves what is still considered a limited recourse and ultimately allows for a more manageable shared funding model.
Limitations of Clinical Cadavers Use for Simulation
The majority of clinical cadavers donated through human body donation programs are elderly and therefore may not accurately represent a cross‐section of the population on whom the procedures will ultimately be performed. Like all simulators, clinical cadavers lack a dynamic circulation although they can be realistically ventilated and their circulation perfused for vascular access and to create bleeding.62, 66, 67, 68, 69 Pathology can be created to simulate real, clinically relevant challenges that must be overcome but for which there is no safe alternative learning opportunities.58 Most clinical cadaver programs screen donations and/or test for more common communicable infectious diseases. The infectious risk will be dependent on the preparation (or lack of in the newly deceased) technique used and in general soft preserved cadavers may pose an unknown theoretical risk as a host for potentially infectious organisms. Attaining procedural competence for high‐acuity, time‐sensitive, and relatively rare procedures such as a thoracotomy in the controlled setting of a cadaver laboratory is critical for both for both patient and clinician safety reasons where risk for a sharps injury is not insignificant.70 It is advised that when using clinical cadavers for procedural skills learning, all users should practice universal precautions and report any exposures as they would in a clinical setting.
The most commonly reported limitation to the use of clinical cadavers is access and cost.56, 71 While this may be an unsurmountable obstacle for some, historically many medical schools around the world would have or have had access to anatomic services and a human body donation program. As discussed departments of anatomy have been cut by many medical education institutions deemed as less relevant opting for alternative methods of learning anatomy. Unfortunately, this potential loss of resources may threaten a repurposed use for donated bodies as a clinical cadaver recourse to support CBME simulation programing. Maryland and Dalhousie are two examples of programs that have captured this opportunity and facilitated a growing demand for clinical cadavers for procedural learning, simulation, and research. Our hope is that this access is improving patient safety and clinical outcomes, particularly for high‐acuity skills for which there is little alternative opportunities to practice or perform research. While there is a growing body of literature describing cadaver use in simulation, as stated previously, the translational evidence supporting improved clinical and patient safety outcomes is limited.37, 54, 55, 57, 59, 62, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 Additionally, there is a growing number of researchers who are using cadavers for noneducational research.63, 64, 65, 82, 83 Much of this scientific work, in particular, studies evaluating new procedural approaches, would neither be feasible nor ethical to perform on patients.
Reported costs for cadaveric simulation have varied from 200 to upwards of 5,000 dollars and will obviously depend on the existing infrastructure, most importantly access to a human body donation program.60, 84 Using the Halifax Clinical Cadaver preparation technique, cadavers can be used for up to 4 weeks when maintained and kept in a refrigerated (4°C) environment between use. The equipment needs, the cost of chemicals and time to prepare specimens are no more than that required for traditionally hard‐fixed formalin cadavers. Most additional costs relate to the need for refrigerated storage and the cost of transportation if required. Increasingly, programs operate at least in part using a cost‐recovery funding model. In Halifax the cost to users is scaled providing subsidized low‐cost access for undergraduate and postgraduate users. CME and research users pay more and external users such as the military or EMS provider groups pay a moderately higher amount. Program oversight is critical and allows the coordination of progressively invasive uses that avoid anatomic overlap, allowing multiple learner groups to access each cadaver. The list of procedures that can be performed on clinical cadavers is limitless and it may be easier to compile a list of procedures that cannot be performed instead of the reverse.
Final Thoughts
One‐hundred twenty‐five years after Halstead's initial proposed hierarchy of procedural learning Sawyer et al.27 in 2015 published a competency‐based pedagogic follow‐up framework—learn, see, practice, prove, do, and maintain. Simulation will continue to play a growing role in all stages of this progression. However, most significantly, simulation will support the learner in the practice phase and for evaluators and accrediting bodies to prove that expected levels of independent competence/performance have been achieved. While institutional clinical experiences should make many learning milestones achievable, ensuring that valid procedural maintenance of competence educational opportunities are reliably available for learners and practicing clinicians is more challenging.
Medical educators have an immense responsibility of ensuring that the thousands of procedures required for physicians to perform competently will be done well beyond a minimal level of “safe” by all graduates. Success of such procedural programing is in part dependent on the predictive validity of the simulation used to train learners to a level of skill mastery. To do this effectively and adapt to the ever‐changing and costly technical advances in clinical medicine we must look for alternative learning venues. It is simply not feasible to keep pace with clinical advances by creating matching simulator technologies that can address the fidelity needs of learners as they progress in their training. Life can come after death by continued support of human body donation programs and their anatomic science partners as key assets that can support access to clinical cadavers for simulation with high physical and functional fidelity. There is much to learn on optimizing preparation techniques, enhancing both static and dynamic fidelity and conducting both educational and procedural science research.
The authors acknowledge Ronald Wade, Director of the Anatomical Services, Division of the University of Maryland School of Medicine, and Director of the Maryland State Anatomy Board and the Dalhousie University Human Body Donation Program, Halifax, Nova Scotia, Canada: To those who have given after life so that others may benefit during life.
AEM Education and Training 2018;2:239–247
The authors have no relevant financial information or potential conflicts to disclose.
References
- 1. William Cameron J, Halsted Stewart. Our surgical heritage. Ann Surg 1997;225:445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller GE. The assessment of clinical skills/competence/performance. Acad Med 1990;65:S63–7. [DOI] [PubMed] [Google Scholar]
- 3. Lockyer J, Carraccio C, Chan MK, et al. Core principles of assessment in competency‐based medical education. Med Teach 2017;39:609–16. [DOI] [PubMed] [Google Scholar]
- 4. Farion K, Morrison LJ. Redefining emergency medicine procedures: Canadian competence and frequency survey. Acad Emerg Med 2001;8:731–8. [DOI] [PubMed] [Google Scholar]
- 5. Hayden SR, Panacek EA. Procedural competency in emergency medicine: The current range of resident experience. Acad Emerg Med 1999;6:728–35. [DOI] [PubMed] [Google Scholar]
- 6. Allison J, Aghababian RV, Barsan WG, et al. Core content for emergency medicine. Ann Emerg Med 1997;29:792–811. [DOI] [PubMed] [Google Scholar]
- 7. Langdorf MI, Montague BJ, Bearie B, Sobel CS. Quantification of procedures and resuscitations in an emergency medicine residency. J Emerg Med 1998;16:121–7. [DOI] [PubMed] [Google Scholar]
- 8. Petrosoniak A, Herold J, Woolfrey K. Emergency medicine procedural skills: what are residents missing? Can J Emerg Med 2013;15:241–8. [DOI] [PubMed] [Google Scholar]
- 9. Hart D, Bond W, Siegelman J, et al. Simulation for assessment of milestones in emergency medicine residents. Acad Emerg Med 2018;25:205–20. [DOI] [PubMed] [Google Scholar]
- 10. Colmers‐Gray IN, Walsh K, Chan TM. Assessment of emergency medicine residents: a systematic review. Can Med Educ J 2017;8:e106–22. [PMC free article] [PubMed] [Google Scholar]
- 11. Russell E, Hagel C, Petrosoniak A, Howes D, Dagnone D, Hall AK. Simulation in Canadian postgraduate emergency medicine training ‐ a national survey. CJEM 2016;18(S1):S52. [DOI] [PubMed] [Google Scholar]
- 12. Dagnone JD, Hall AK, Sebok‐syer S, et al. Competency‐based simulation assessment of resuscitation skills in emergency medicine postgraduate trainees ‐ a Canadian multi‐centred study. Can Med Educ J 2016;7:e57–67. [PMC free article] [PubMed] [Google Scholar]
- 13. Henriksen K, Rodrick D, Grace EN, Brady PJ. Challenges in health care simulation: are we learning anything new? Acad Med 2018;93:705–8. [DOI] [PubMed] [Google Scholar]
- 14. Higham H, Baxendale B. To err is human: use of simulation to enhance training and patient safety in anaesthesia. BJA Br J Anaesth 2017;119(Suppl 1):i106–14. [DOI] [PubMed] [Google Scholar]
- 15. Motola I, Devine LA, Chung HS, Sullivan JE, Issenberg SB. Simulation in healthcare education: a best evidence practical guide. AMEE Guide No. 82. Med Teach 2013;35:e1511–30. [DOI] [PubMed] [Google Scholar]
- 16. Griswold‐Theodorson S, Ponnuru S, Dong C, Szyld D, Reed T, McGaghie WC. Beyond the simulation laboratory: a realist synthesis review of clinical outcomes of simulation‐based mastery learning. Acad Med 2015;90:1553–60. [DOI] [PubMed] [Google Scholar]
- 17. Dawe SR, Pena GN, Windsor JA, et al. Systematic review of skills transfer after surgical simulation‐based training. Br J Surg 2014;101:1063–76. [DOI] [PubMed] [Google Scholar]
- 18. Buckley CE, Kavanagh DO, Traynor O, Neary PC. Is the skillset obtained in surgical simulation transferable to the operating theatre? Am J Surg 2014;207:146–57. [DOI] [PubMed] [Google Scholar]
- 19. Zevin B, Aggarwal R, Grantcharov TP. Surgical simulation in 2013: why is it still not the standard in surgical training? J Am Coll Surg 2014;218:294–301. [DOI] [PubMed] [Google Scholar]
- 20. McDougall EM. Validation of surgical simulators. J Endourol 2007;21:244–7. [DOI] [PubMed] [Google Scholar]
- 21. Hamstra SJ, Brydges R, Hatala R, Zendejas B, Cook DA. Reconsidering fidelity in simulation‐based training. Acad Med 2014;89:387–92. [DOI] [PubMed] [Google Scholar]
- 22. Schoenherr JR, Hamstra SJ. Beyond fidelity: deconstructing the seductive simplicity of fidelity in simulator‐based education in the health care professions. Simul Healthc 2017;12:117–23. [DOI] [PubMed] [Google Scholar]
- 23. Grierson LE. Information processing, specificity of practice, and the transfer of learning: considerations for reconsidering fidelity. Adv Heal Sci Educ 2014;19:281–9. [DOI] [PubMed] [Google Scholar]
- 24. Maran NJ, Glavin RJ. Low‐ to high‐fidelity simulation ‐ a continuum of medical education? Med Educ 2003;37(Suppl 1):22–8. [DOI] [PubMed] [Google Scholar]
- 25. Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ 2012;46:636–47. [DOI] [PubMed] [Google Scholar]
- 26. Kovacs G. Procedural skills in medicine: linking theory to practice. J Emerg Med 1997;15:387–91. [DOI] [PubMed] [Google Scholar]
- 27. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain. Acad Med 2015;90:1025–33. [DOI] [PubMed] [Google Scholar]
- 28. Gu Y, Witter T, Livingston P, et al. The effect of simulator fidelity on acquiring non‐technical skills: a randomized non‐inferiority trial. Can J Anaesth 2017;64:1182–93. [DOI] [PubMed] [Google Scholar]
- 29. Dahlsstrom N, Dekker S, Van Winsen R, Nyce J. Fidelity and validity of simulator training. Theor Issues Ergon Sci 2009;10:305–14. [Google Scholar]
- 30. Hesselfeldt R, Kristensen MS, Rasmussen LS. Evaluation of the airway of the SimMan full‐scale patient simulator. Acta Anaesthesiol Scand 2005;49:1339–45. [DOI] [PubMed] [Google Scholar]
- 31. Jordan GM, Silsby J, Bayley G, Cook TM. Evaluation of four manikins as simulators for teaching airway management procedures specified in the Difficult Airway Society guidelines, and other advanced airway skills. Anaesthesia 2007;62:708–12. [DOI] [PubMed] [Google Scholar]
- 32. Szűcs Z, László CJ, Baksa G, et al. Suitability of a preserved human cadaver model for the simulation of facemask ventilation, direct laryngoscopy and tracheal intubation: a laboratory investigation. Br J Anaesth 2016;116:417–22. [DOI] [PubMed] [Google Scholar]
- 33. Rai MR, Popat MT. Evaluation of airway equipment: man or manikin? Anaesthesia 2011;66:1–3. [DOI] [PubMed] [Google Scholar]
- 34. Myatra SN, Kalkundre RS, Divatia JV. Optimizing education in difficult airway management: meeting the challenge. Curr Opin Anaesthesiol 2017;30:748–54. [DOI] [PubMed] [Google Scholar]
- 35. Schebesta K, Spreitzgrabner G, Hörner E, Hüpfl M, Kimberger O, Rössler B. Validity and fidelity of the upper airway in two high‐fidelity patient simulators. Minerva Anestesiol 2015;81:12–8. [PubMed] [Google Scholar]
- 36. Schebesta K, Hüpfl M, Rössler B, Ringl H, Müller MP, Kimberger O. Degrees of reality: airway anatomy of high‐fidelity human patient simulators and airway trainers. Anesthesiology 2012;116:1204–9. [DOI] [PubMed] [Google Scholar]
- 37. Takayesu JK, Peak D, Stearns D. Cadaver‐based training is superior to simulation training for cricothyrotomy and tube thoracostomy. Intern Emerg Med 2017;12:99–102. [DOI] [PubMed] [Google Scholar]
- 38. Adams JA. Historical review and appraisal of research on the learning, retention, and transfer of human motor skills. Psychol Bull 1987;101:41. [Google Scholar]
- 39. Balta JY, Cronin M, Cryan JF, O'Mahony SM. Human preservation techniques in anatomy: a 21st century medical education perspective. Clin Anat 2015;28:725–34. [DOI] [PubMed] [Google Scholar]
- 40. Brenner E. Human body preservation ‐ old and new techniques. J Anat 2014;224:316–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dyer GS, Thorndike ME. Quidne mortui vivos docent? The evolving purpose of human dissection in medical education. Acad Med 2000;75:969–79 [DOI] [PubMed] [Google Scholar]
- 42. BioDigital . A Better Way to Understand Health and The Human Body. 2018. Available at: https://www.biodigital.com/product. Accessed Nov 3, 2017.
- 43. Thiel W. An arterial substance for subsequent injection during the preservation of the whole corpse. Ann Anat 1992;174:197–200. [PubMed] [Google Scholar]
- 44. Makowski AL. The ethics of using the recently deceased to instruct residents in cricothyrotomy. Ann Emerg Med 2015;66:403–8. [DOI] [PubMed] [Google Scholar]
- 45. Morag RM, DeSouza S, Steen PA, et al. Performing procedures on the newly deceased for teaching purposes: what if we were to ask? Arch Intern Med 2005;165:92–6. [DOI] [PubMed] [Google Scholar]
- 46. Council on Ethical and Judicial Affairs of the American Medical Association . Performing procedures on the newly deceased. Acad Med 2002;77(12 Pt 1):1212–6. [PubMed] [Google Scholar]
- 47. Hayashi S, Naito M, Kawata S, et al. History and future of human cadaver preservation for surgical training: from formalin to saturated salt solution method. Anat Sci Int 2016;91:1–7. [DOI] [PubMed] [Google Scholar]
- 48. Anderson SD. Practical light embalming technique for use in the surgical fresh tissue dissection laboratory. Clin Anat 2006;19:8–11. [DOI] [PubMed] [Google Scholar]
- 49. Thiel W. The preservation of the whole corpse with natural color. Ann Anat 1992;174:185–95. [PubMed] [Google Scholar]
- 50. Benkhadra M, Gérard J, Genelot D, et al. Is Thiel's embalming method widely known? A world survey about its use. Surg Radiol Anat 2011;33:359–63. [DOI] [PubMed] [Google Scholar]
- 51. Hammer N, Löffler S, Bechmann I, Steinke H, Hädrich C, Feja C. Comparison of modified Thiel embalming and ethanol‐glycerin fixation in an anatomy environment: potentials and limitations of two complementary techniques. Anat Sci Educ 2015;8:74–85. [DOI] [PubMed] [Google Scholar]
- 52. Jaung R, Cook P, Blyth P. A comparison of embalming fluids for use in surgical workshops. Clin Anat 2011;24:155–61. [DOI] [PubMed] [Google Scholar]
- 53. Messmer C, Kellogg RT, Zhang Y, et al. A technique to perfuse cadavers that extends the useful life of fresh tissues: the Duke experience. Anat Sci Educ 2010;3:191–4. [DOI] [PubMed] [Google Scholar]
- 54. Yiasemidou M, Gkaragkani E, Glassman D, Biyani CS. Cadaveric simulation: a review of reviews. Ir J Med Sci 2017. Nov 14. [DOI] [PubMed] [Google Scholar]
- 55. Gunst M, O'Keeffe T, Hollett L, et al. Trauma operative skills in the era of nonoperative management: the trauma exposure course (TEC). J Trauma 2009;67:1091–6. [DOI] [PubMed] [Google Scholar]
- 56. Ferguson IM, Shareef MZ, Burns B, Reid C. A human cadaveric workshop: one solution to competence in the face of rarity. Emerg Med Australas 2016;28:752–4. [DOI] [PubMed] [Google Scholar]
- 57. Ferrada P, Anand RJ, Amendola M, Kaplan B. Cadaver laboratory as a useful tool for resident training. Am Surg 2014;80:408–9. [PubMed] [Google Scholar]
- 58. Bowyer MW, Kuhls DA, Haskin D, et al. Advanced surgical skills for exposure in trauma (ASSET): the first 25 courses. J Surg Res 2013;183:553–8. [DOI] [PubMed] [Google Scholar]
- 59. Kim SC, Fisher JG, Delman KA, Hinman JM, Srinivasan JK. Cadaver‐based simulation increases resident confidence, initial exposure to fundamental techniques, and may augment operative autonomy. J Surg Educ 2016;73:e33–41. [DOI] [PubMed] [Google Scholar]
- 60. Tabas JA, Rosenson J, Price DD, Rohde D, Baird CH, Dhillon N. A comprehensive, unembalmed cadaver‐based course in advanced emergency procedures for medical students. Acad Emerg Med 2005;12:782–5. [DOI] [PubMed] [Google Scholar]
- 61. Yang JH, Kim YM, Chung HS, et al. Comparison of four manikins and fresh frozen cadaver models for direct laryngoscopic orotracheal intubation training. Emerg Med J 2010;27:13–6. [DOI] [PubMed] [Google Scholar]
- 62. Reihsen TE, Alberti L, Speich J, Poniatowski LH, Hart D, Sweet RM. Feasibility of a perfused and ventilated cadaveric model for assessment of lifesaving traumatic hemorrhage and airway management skills. J Trauma Acute Care Surg 2016;80:799–804. [DOI] [PubMed] [Google Scholar]
- 63. Levitan RM, Kinkle WC. Initial anatomic investigations of the I‐gel airway: a novel supraglottic airway without inflatable cuff. Anaesthesia 2005;60:1022–6. [DOI] [PubMed] [Google Scholar]
- 64. Levitan RM, Kinkle WC, Levin WJ, Everett WW. Laryngeal view during laryngoscopy: a randomized trial comparing cricoid pressure, backward‐upward‐rightward pressure, and bimanual laryngoscopy. Ann Emerg Med 2006;47:548–55. [DOI] [PubMed] [Google Scholar]
- 65. Levitan RM, Bortle CD, Snyder TA, Nitsch DA, Pisaturo JT, Butler KH. Use of a battery‐operated needle driver for intraosseous access by novice users: skill acquisition with cadavers. Ann Emerg Med 2009;54:692–4. [DOI] [PubMed] [Google Scholar]
- 66. Willaert W. On‐pump Vascular Reperfusion of Thiel Embalmed Cadavers [Dissertation]. Ghent: Ghent University, 2015. [Google Scholar]
- 67. Carey JN, Minneti M, Leland HA, Demetriades D, Talving P. Perfused fresh cadavers: method for application to surgical simulation. Am J Surg 2015;210:179–87. [DOI] [PubMed] [Google Scholar]
- 68. Minneti M, Baker CJ, Sullivan ME. The development of a novel perfused cadaver model with dynamic vital sign regulation and real‐world scenarios to teach surgical skills and error management. J Surg Educ 2017. Oct 13. [DOI] [PubMed] [Google Scholar]
- 69. Delpech PO, Danion J, Oriot D, Richer JP, Breque C, Faure JP. SimLife a new model of simulation using a pulsated revascularized and reventilated cadaver for surgical education. J Chir Viscerale 2017;154:15–20. [DOI] [PubMed] [Google Scholar]
- 70. Sikka R, Millham FH, Feldman JA. Analysis of occupational exposures associated with emergency department thoracotomy. J Trauma 2004;56:867–72. [DOI] [PubMed] [Google Scholar]
- 71. Reznick RK, Macrae H. Teaching surgical skills–changes in the wind. N Engl J Med 2006;355:2664–9. [DOI] [PubMed] [Google Scholar]
- 72. Amini R, Ho H, Ng V, et al. Introducing a fresh cadaver model for ultrasound‐guided central venous access training in undergraduate medical education. West J Emerg Med 2016;17:362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tomlinson JE, Yiasemidou M, Watts AL, Roberts DJ, Timothy J. Cadaveric spinal surgery simulation: a comparison of cadaver types. Global Spine J 2016;6:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nematollahi S, Kaplan SJ, Knapp CM, et al. Introduction of a fresh cadaver laboratory during the surgery clerkship improves emergency technical skills. Am J Surg 2015;210(401–3):e2. [DOI] [PubMed] [Google Scholar]
- 75. Fonseca AL, Evans LV, Gusberg RJ. Open surgical simulation in residency training: a review of its status and a case for its incorporation. J Surg Educ 2013;70:129–37. [DOI] [PubMed] [Google Scholar]
- 76. Gilbody J, Prasthofer AW, Ho K, Costa ML. The use and effectiveness of cadaveric workshops in higher surgical training: a systematic review. Ann R Coll Surg Engl 2011;93:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cosman P, Hemli JM, Ellis AM, Hugh TJ. Learning the surgical craft: a review of skills training options. ANZ J Surg 2007;77:838–45. [DOI] [PubMed] [Google Scholar]
- 78. Sidhu RS, Park J, Brydges R, MacRae HM, Dubrowski A. Laboratory‐based vascular anastomosis training: a randomized controlled trial evaluating the effects of bench model fidelity and level of training on skill acquisition. J Vasc Surg 2007;45:343–9. [DOI] [PubMed] [Google Scholar]
- 79. Reed AB, Crafton C, Giglia JS, Hutto JD. Back to basics: use of fresh cadavers in vascular surgery training. Surgery 2009;146:757–62; discussion 762–3. [DOI] [PubMed] [Google Scholar]
- 80. Anastakis DJ, Regehr G, Reznick RK, et al. Assessment of technical skills transfer from the bench training model to the human model. Am J Surg 1999;177:167–70. [DOI] [PubMed] [Google Scholar]
- 81. Martin M, Vashisht B, Frezza E, et al. Competency‐based instruction in critical invasive skills improves both resident performance and patient safety. Surgery 1998;124:313–7. [PubMed] [Google Scholar]
- 82. Winkelmann A, Heinze AK, Hendrix S. Acknowledging tissue donation: human cadaveric specimens in musculoskeletal research. Clin Anat 2016;29:65–9. [DOI] [PubMed] [Google Scholar]
- 83. Benninger B. Formally acknowledging donor‐cadaver‐patients in the basic and clinical science research arena. Clin Anat 2013;26:810–3. [DOI] [PubMed] [Google Scholar]
- 84. Aboud ET, Aboud G, Aboud T. “Live cadavers” for practicing airway management. Mil Med 2015;180(3 Suppl):165–70. [DOI] [PubMed] [Google Scholar]


