Abstract
Purpose
Ovarian carcinoma no longer responsive to surgery and chemotherapy remains an incurable disease. Alternative therapeutic options remain desperately needed.
Experimental Design
We describe a heavily pretreated ovarian cancer patient with recurrent disease experiencing a remarkable clinical response to treatment with the anti-PD1 immune check-point inhibitor pembrolizumab. The clinical, pathological, and genomic characteristics of this exceptional ovarian cancer responder were carefully investigated using immunohistochemistry (IHC), quantitative multiplex fluorescence methods (ie, automated quantitative analysis, AQUA) and whole exome sequencing (WES) techniques.
Results
The patient harbored a recurrent/metastatic radiation and chemotherapy-resistant high grade ovarian carcinoma with clear cell features. While progressing on any standard treatment modality she demonstrated a remarkable complete response to the anti-PD1 immune check-point inhibitor pembrolizumab. WES results were notable for the presence a relative low number of mutations (Tumor Mutation Load/Mb = 4.31, total mutations = 164) and a peculiar structural variant disrupting the 3′ region of the PD-L1 gene causing aberrant PD-L1 surface expression as confirmed by immunohistochemistry (IHC) and AQUA technology. Heavy infiltration of the PD-L1-mutated and PD-L1-overexpressing tumor with T cell lymphocytes (ie, CD4+/CD8+ TIL), CD68+ macrophages and CD20+ B cells was detected in the surgical specimen strongly suggesting immune evasion as a key mechanism of tumor growth and survival. Patient’s complete clinical responses remain unchanged at the time of the writing of this report with no significant side-effects reported to date.
Conclusions
Anti-PD1 inhibitors may represent a novel treatment option for recurrent/metastatic human tumors refractory to salvage treatment harboring PD-L1 gene structural variations causing aberrant PD-L1 expression.
Introduction
Ovarian carcinoma remains the cancer with the highest mortality rate among gynecological tumors. In 2017, 22,440 new cases of ovarian carcinoma are predicted in the United States, with 14,080 deaths secondary to this disease (1). While the majority of patients with advanced stage disease (ie, stage III-IV) initially respond to front-line therapy based on cytoreductive surgery and platinum-based chemotherapy, ovarian cancer will recur in the majority of cases. Overall, the 5-year survival rate for FIGO (International Federation of Gynecologists and Obstetricians) stage III disease is 20–25% and for stage IV disease is only 5% (2). These figures illustrate the dire need for the development of novel, more effective approaches for the treatment of chemotherapy-resistant ovarian cancer.
Next generation sequencing (NGS) studies from the cancer genome atlas (TCGA) network and other research groups have recently demonstrated that high grade serous ovarian cancer, the most common histological type of ovarian carcinoma, is characterized by TP53 mutations in over 96% of cases, a relative low number of single nucleotide variants (SNV) when compared to other gynecologic and non-gynecologic tumors and a widespread copy number variants (CNV), a genetic signature correlated with high genomic instability (3,4).
In the last few years the successful treatment of a variety of human cancer patients using antibodies against programmed cell death 1 (PD-1) and its ligand PD-L1 (CD274) has demonstrated the intrinsic immunogenicity of at least a subset of human tumors and the critical importance of PD-1/PD-L1 axis in tumor immune evasion. In some of these trials a high mutational tumor burden and a high expression of PD-L1 protein on the surface of human tumors have been identified as predictive biomarkers for the identification of patients most likely to respond to the immunotherapy treatment (5). However, the mechanisms controlling response to current immune checkpoint inhibitors remain poorly understood as demonstrated by the fact that some human tumors with low mutation burden and/or lacking expression of PD-L1 may still respond to checkpoint blockade (5,6).
Several studies have recently investigated whether PD-L1 expression is associated with tumor infiltrating T cells (TIL) and a favorable prognosis in ovarian cancer patients (7–11) and multiple antibodies directed against PD-1 or PD-L1 are currently being tested in clinical trials (for review see 11). While many of these studies are currently on-going or waiting for results to mature, few have been published. For example, Hamanischi et al., (12) treated twenty patients with platinum-resistant ovarian cancer with an intravenous infusion of nivolumab every 2 weeks at a dose of 1 or 3 mg/kg. The best overall response was 15%, which included two patients who had a durable complete response (in the 3 mg/kg cohort). The disease control rate in all 20 patients was 45%. Of interest, complete responses were seen in ovarian cancer patients with both serous and clear cell histology (12). Varga et al., reported on the activity of pembrolizumab in the ovarian cancer cohort of advanced ovarian epithelial, fallopian tube, or primary peritoneal carcinoma patients with PD-L1+ tumors treated within the KEYNOTE-028 (NCT02054806) study. Out of the 26 patients enrolled, the best overall (confirmed) response was 11.5% with one patient achieving a complete response and 2 patients experiencing partial responses (13). Disis et al., recently reported on 124 patients with recurrent/refractory ovarian cancer treated with Avelumab (ie, anti-PD-L1 humanized antibody)(NCT01772004)(14). Overall response rate was 9.7% based on 12 partial responses (14). Importantly, in all these trials single-agent anti-PD1 or anti-PD-L1 antibodies showed an acceptable safety profile in heavily pretreated ovarian cancer patients (11–14).
Here we describe an ovarian cancer patient treated with neo-adjuvant chemotherapy, followed by surgery and progressing on radiation and multiple regimens of chemotherapy, experiencing an exceptional clinical response to anti-PD-1 therapy (ie, pembrolizumab). An analysis of the pathological and genomic characteristics of the ovarian tumor demonstrated a low/intermediate number of mutations by WES and a PD-L1 gene structural variation causing aberrant PD-L1 surface expression and heavy infiltration of T lymphocytes in the tumor tissue. These results strongly suggest immune evasion as a key mechanism of tumor growth and survival for this chemotherapy/radiation resistant ovarian cancer.
Methods
Immunohistochemistry
Briefly, for immunohistochemistry, 4 μm sections were cut from the formalin-fixed paraffin embedded (FFPE) blocks of the cancer patient collected at the time of cytoreductive surgery and 79 additional high grade serous ovarian carcinoma from a recently described tissue microarray ovarian cancer cohort (15), and stained with the following antibodies according to the manufacturers’ instructions. CD8 (clone 144B, ready to use, DAKO, Carpinteria, CA), CD4 (clone 1F6, 1:40 dilution, Vector, Burlingame CA), CD3 (clone 2GV6, Ventana, Tucson, AZ), CD56 (clone 1B6, 1:200 dilution, Vector, Burlingame CA), CD68 (clone PG-M1, ready to use, DAKO, Carpinteria, CA), CD20 (clone L26, 1:200 dilution, DAKO, Carpinteria, CA), TIA-1 (clone TIA1, ready to use, Biocare, Concord, CA), CK7 (clone OVTL, Dako, Carpinteria, CA) and PD-L1 (clone E1L3N, 1:200 Cell Signaling, Danvers, MA). For antigen retrieval, the sections were pre-treated at low pH for PD-L1 and CD8, CD4, CD20, CD56 and CD68. PD-L1 antibody and membranous immunoreactivity was assessed semi-quantitatively in tumor cells as follows: <1% staining was considered negative, staining in 1–50% of tumor cells was scored as focal, and >50% staining was scored as diffusely positive.
Quantitative Multiplex Fluorescence Analysis of PD-L1 expression
PD-L1 (Spring Bioscience; clone SP142; 1:800), CD68 (DAKO; clone PG-M1; 1:200), CD8 (DAKO; clone C8/144B; 1:250) were used for immunofluorescence multiplex staining using a previously descried method (16). Simultaneously, cytokeratin and DAPI were stained for detection of tumor and nucleus, respectively. Briefly, the patients’ whole tissue section slides and a technical control tumor microarray slides (TMA)(16) were deparaffinized. Antigen retrieval was performed for 20 minutes at 97°C in the PT module from LabVision (Thermo Scientific), in EDTA pH8 buffer. Endogenous peroxidases were blocked for 30 minutes in 2.5% hydrogen peroxide in methanol and subsequently blocked by 0.3% BSA in 0.05% tween with 30-minute incubation. The mixture of primary antibodies (PD-L1, CD68/CD8) was incubated overnight at 40°C in 0.3% BSA in TBST. For the PD-L1/CD68 multiplex panel, primary antibodies incubation was followed by anti-mouse IgG3 secondary antibody (Abcam; ab97260; 1:1000) incubation in 0.3% BSA in TBST for an hour at room temperature to detect CD68. Signal was amplified with Biotinylated Tyramide(Perkin Elmer) at 1:50 dilution and Alexa750-streptavidin (Life Technologies) at 1:100. Following 1-hour incubation of Rabbit EnVisionreagent (Dako) was used for detecting PD-L1 and amplified by Cyanine 5(Cy5) tyramide(Perkin Elmer) at 1:50 dilution. Cytokeratin was detected with 1-hour incubation of anti-rabbit cytokeratin (Dako) at 1:100 and Goat anti-Rabbit secondary antibody (Life Technologies) at 1:100. ProLongmounting medium (Molecular Probes) was the final step to stain nuclei with 4,6-diamidino-2-phenylindole (DAPI). Between each step, slides were washed using TBS/Tween and TBS, each for 2 minutes. For the PD-L1/CD8 multiplex panel, primary antibodies were followed by anti-mouse IgG1 secondary antibody (eBioscience) at 1:100, and signal was amplified with Plus Cyanine 3 (Perkin Elmer) at 1:100. Similar steps were used in this panel for staining PD-L1, cytokeratin and DAPI.
Whole exome sequencing
Briefly, DNA was extracted from patient’s peripheral blood as source of germinal DNA and FFPE tumor samples using a BiOstic® FFPE Tissue DNA Isolation Kit (MO BIO Laboratories #12250-50) with a modified protocol. Genomic DNA was captured on the NimbleGen 2.1M human exome array and subjected to 74 base paired-end reads on the Illumina HiSeq 2000 instrument as described (17). Sequence reads were mapped to the reference genome (hg19) using the ELAND program. Reads outside the targeted sequences were discarded and statistics on coverage were collected from the remaining reads using in-house Perl scripts as previously described (17).
Verification of PD-L1/PLGRKT rearrangement by RT-PCR and Sanger sequencing
Briefly, genomic DNA derived from the patient’s tumor was tested by Real Time PCR using a Custom TaqMan Assay (ID APTZ94T, Life Technologies Inc.) designed to specifically amplify the region of interest. The forward primer (CTTCAAGCAGGGATTCTCAACCT) was designed on the CD274 sequence while the reporter sequence (ATGGAACCCTTTTAGAACCC) and the reverse primer (GCCCCGATACCAGTATGACTTG) were designed to include part of the CD274 sequence and part of the PLGRKT insertion. The detection of the genetic rearrangement in the tumor sample was confirmed by Sanger sequencing.
HLA Typing and Class I Neoantigen Prediction
The patient-specific 4-digit HLA class I allele genotype was assessed by ATHLATES (18) in silico using whole exome sequencing data. All somatic mutations including non-synonymous mutations, frameshift insertions and deletions were translated into 17-mer polypeptides flanking the mutant amino acid. The binding affinity of wild type and mutant nonamers to the patient-specific HLA class I allele was predicted using NetMHCcons (19) algorithms. Nonamers with IC50 below or equal to 500 nM were further evaluated for the recognition by the T-cell receptor using Class I immunogenicity (20) resulting in putative neoantigens. The putative neoantigens were then classified as a strong binder (IC50 < 50nM), intermediate binder (50nM < IC50 < 150nM) or weak binder (150nM < IC50 < 500nM).
Results
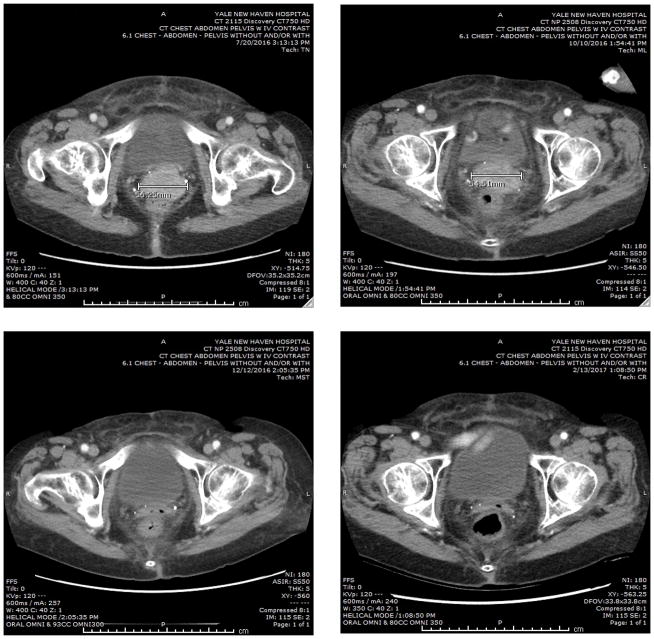
Patient is a 80-year old Caucasian woman originally diagnosed in June 2014 with an advanced stage, high grade, ovarian carcinoma with clear cell features. The initial CAT scan revealed extensive abdominal carcinomatosis, ascites, pelvic lymphadenopathy and a CA125 tumor marker elevated at 1,810 U/mL (normal value less than 35 U/mL). After a diagnostic biopsy she received 6 cycles of neo-adjuvant chemotherapy (ie, carboplatin AUC 5 and paclitaxel 175 mg/m2) followed by a radical surgical procedure. Persistent widespread abdominal carcinomatosis was found at the time of the interval debulking surgery with gross residual disease present in the omentum (nodules up to 2.9 cm), ovaries, fallopian tubes and pelvic lymph nodes. Final pathology was consistent with the pre-chemotherapy biopsy and demonstrated a high grade adenocarcinoma of Mullerian origin with clear cell features most likely started in the ovaries and treatment effect. Because of symptomatic neuropathy, post-operatively she received 6 additional cycles of carboplatin (AUC 5) and Topotecan (2 mg/kg day 1 and 15) in combination with bevacizumab (10 mg/kg, day 1 and 15). The patient remained in remission until March 2015, when a MRI of the pelvic and abdomen followed by a PET/CT imaging revealed the presence of recurrent disease in the pelvis (ie, a mass of 2.6 cm above the vaginal cuff). She was dispositioned to received vaginal cuff brachytherapy followed by pelvic radiation in the form of 28 fractions completed on 7/7/15. Unfortunately, a PET/CT performed on October 2015 demonstrated persistent/recurrent disease with interval increase in size and hyper-metabolism of the pelvic lesion involving the superior aspect of the vaginal cuff. She was therefore dispositioned to receive dose dense paclitaxel protein-bound particles (abraxane), initially at the dose of 100 mg/m2 on day 1, 8 and 15 and subsequently dose-reduced to 80 mg/m2 of a 28 day cycle in combination with bevacizumab (10 mg/kg, day 1 and 15). She did well until June 2016 when a pelvic exam followed by a CAT scan guided biopsy of the enhancing 3.1 × 4.8 cm mass along the superior/cephalad border of the vaginal cuff confirmed the presence of a metastatic/recurrent adenocarcinoma histological consistent with the original high grade mullerian tumor. On August 2016 she was enrolled at Yale university within a Phase II study of pembrolizumab entitled “Efficacy and Safety Study of Pembrolizumab (MK-3475) in Women With Advanced Recurrent Ovarian Cancer” (MK-3475-100/KEYNOTE-100). Pembrolizumab was administered at a flat dose of 200 mg IV on Day 1 of each 3-week cycle. CA125 tumor marker at this time was found elevated at 230 U/mL. A CAT scan performed on October 2016 (ie, after 3 pembrolizumab infusions) demonstrated stable disease (ie, larger diameter of the pelvic/vaginal mass stable at 5.4 cm vs 5.6 cm at baseline) (Figure 1, upper panels). Strikingly, at the time of the second CAT scan imaging obtained 4 months from study initiation (ie, December 2016, Figure 1 left lower panel) a remarkable complete response by RECIST v1.1 criteria was noted to the immune checkpoint inhibitor. Confirmatory CAT scans obtained 8 weeks later (ie, February 2017, Figure 1 right lower panel) and in April and June 2017 (data not shown) demonstrated a sustained complete response of the large pelvic/vaginal tumor with no new lesions. Patient continued to improve in her performance status during treatment with the normalization of the CA125 tumor marker. Per protocol pembrolizumab treatment has been discontinued after the completion of a total of 9 cycles of treatment (24 weeks) and 2 cycles after the confirmatory complete response (ie, CAT scan imaging). The patient’s remarkable clinical response remains unchanged at the time of the writing of this report with no side effects reported to date and the patient experiencing good quality of life.
Figure 1.
Representative CAT scans demonstrating activity (ie, complete response) to pembrolizumab. Left upper panel: Pretreatment images with baseline measurement of the representative metastatic tumor deposit (ie, pelvic/vaginal mass). Right upper Panel; stability of the lesion after 3 pembrolizumab infusions. Left and Right Lower panels: Complete regression of the metastatic tumor deposits after 16 and 24 weeks from treatment initiation with pembrolizumab.
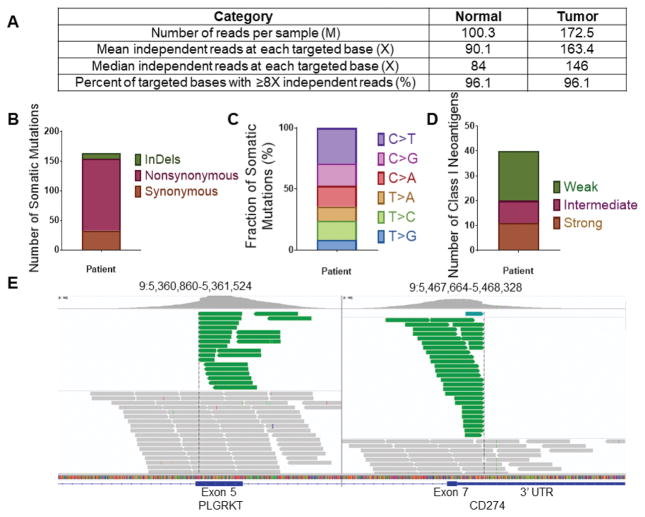
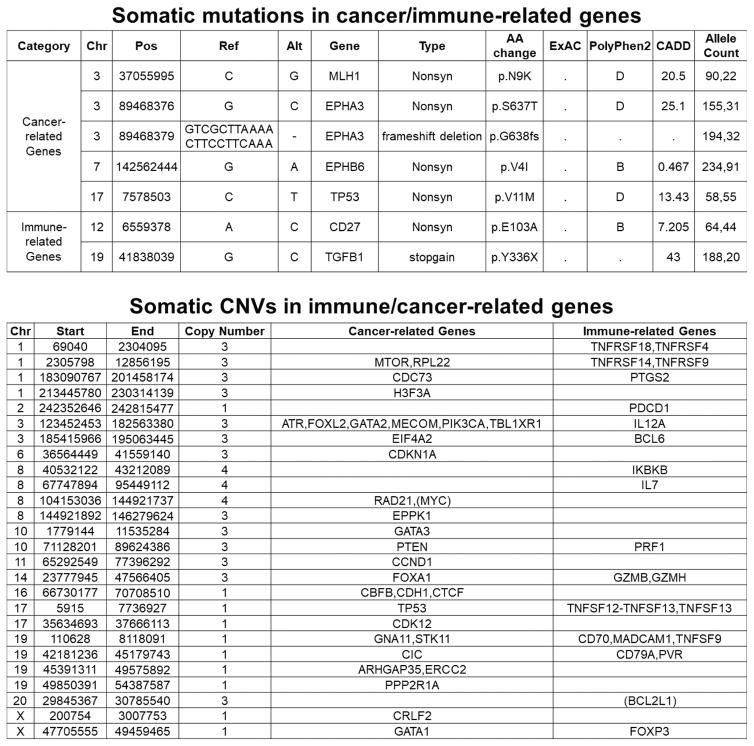
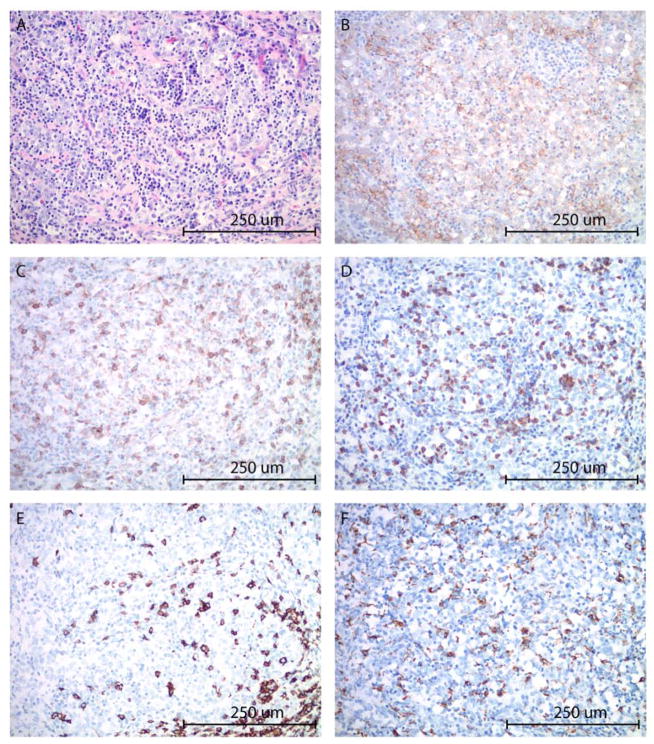
To gain additional knowledge in the genetic and pathologic characteristics of the patient’s tumor demonstrating such exquisite sensitivity to anti-PD1 treatment we initially performed next generation sequencing (NGS) testing using a commercially available platform (Foundation One:, Foundation Medicine, (FM) Inc. Cambridge, MA, a test sequencing the coding region of 315 genes plus introns from 28 genes to a median depth of coverage of greater than 500X)(21) followed by whole exome sequencing (WES)(performed at the Center for Genome Analysis at Yale University School of Medicine). We found the ovarian carcinoma to harbor a low/intermediate number of mutations (ie, tumor mutation burden, TMB = 4.31 mut/Mb, total number of mutations = 164) (Figure 2) when compared to 316 high grade ovarian cancer samples previously reported by the Cancer Genome Atlas Research Network (mean TMB = 1.84 mut/Mb, total number of mutations = 61) (3) and 114 high grade ovarian cancer samples recently reported by Patch et al., (mean TMB = 5.64 mut/Mb, total number of mutations = 271)(4). Using accurate typing of human leukocyte antigen through exome sequencing we predicted a total of 40 potential neoantigens harbored in the tumor including 11 strong, 9 intermediate and 20 weak immunogenic antigens (Figure 2). Somatic mutations (SNV), loss of heterozygosity (LOH) and copy number variations (CNV) in the whole exome sequenced tumor are presented in Figure 3. Nonsynonymous mutations (ie, SNV) in the TP53 gene and MLH1 gene (the later not causing microsatellite instability, data not shown) and a stop mutation in TGF–β (a key player cytokine during host-immune system interaction) amongst other mutations were detected (Figure 3). Gain of function in known oncogenes (ie, the PIK3CA gene on chromosome 3 and in c-MYC on chromosome 8) and in BCL2L1, a gene endowed with anti-apoptotic properties and a crucial role in controlling survival during lymphocyte development and following B- and T-cell activation, were also identified (Figure 3). Of great interest, both FM and WES testing demonstrated a peculiar PD-L1 gene rearrangement (ie, chr9 duplication event affecting the 5′ end of PD-L1, breakpoint in exon 7 (3′ UTR)(Figure 2E). As described in the methods, using primers specific for the predicted fusion, RT-PCR and Sanger sequencing, we confirmed the detection of the genetic rearrangement in the tumor sample (Supplementary Figure 1). The event consists of a translocation/insertion of a 32 nucleotide fragment from the exon 5 of the PLGRKT gene (plasminogen receptor, C-terminal lysine transmembrane protein) in chr9:5361091–5361122 of the hg19 assembly (Figure 2E), into 3′UTR of the PD-L1 gene. PLGRKT, however, remains intact after the tandem duplication event. These genetic characteristics were found to differ from previously demonstrated gain of function of the PD-L1 gene secondary to gene amplification and, importantly, have recently been described in a subset of T cell leukemia and B-cell lymphoma to lead to a marked elevation of PD-L1 extracellular domain secondary to the stabilization of the PD-L1 transcripts through the truncation of the 3′ – untranslated region (UTR) of the PD-L1 gene (22). To confirm the potential high expression of PD-L1 receptor on the surface of the ovarian tumor and thouroughly evaluate the presence or absence of lymphocytic infiltration we next performed PD-L1 immunohistochemistry testing and quantitative multiplex fluorescence analysis in the tumor tissue. We found strong tumor cell expression for PD-L1 by IHC as well as heavy intra-tumoral T lymphocytic (ie, CD8, CD4) and to a lower extent B-cell (CD20) and histiocytic infiltration (ie, CD68) by hematoxylin-eosin (H&E) stain and immunostains (Figures 4). Using an immunofluorescence multiplexing panel (16), we confirmed colocalization of PD-L1 with cytokeratin positive tumor cells and negligible to no PD-L1 expression in infiltrating CD68+ macrophages and CD8+ T lymphocytes (Figure 5). Finally, we compared PD-L1 expression in the patient’s tumor with PD-L1 expression in seventy-nine cases of high grade serous ovarian carcinoma available to our laboratory from a recently characterized tissue microarray (15). As representatively shown in Figure 6, out of the 79 tumors only 15 (19%) showed focal PD-L1 expression in tumor cells while the remaining 64 cases (81%) were negative for PD-L1. None of the cases present in the TMA showed diffuse immunoreactivity for PD-L1 as described in Figure 4 for the ovarian cancer patient harboring the PD-L1-genetic rearrangement.
Figure 2.
Whole exome sequencing results. (A) Quality assessment and quality control of sequencing data for tumor and matched normal samples. (B) Somatic mutation classification. (C) The distribution of six different substitution subtypes. (D) Potential neoantigen classification. Strong: IC50 ≤ 50 nM, Intermediate: 50 nM < IC50 ≤ 150 nM, Weak: 150 nM < IC50 ≤ 500 nM. (E) Integrative Genomics Viewer (IGV) plots showing genomic rearrangement involving the PD-L1 gene. The event consists in a translocation/insertion of a 5′ 32 nucleotide fragment from the exon 5 of the PLGRKT gene, in the 5′ end of the PD-L1 gene, resulting in a breakpoint in exon 7 (3′ UTR). Green reads indicate the tandem duplication. Grey reads display normal sequencing reads.
Figure 3.
Whole exome sequencing results. Somatic mutations (SNV), in cancer or immune-related genes and copy number variations (CNV) in cancer and immune related genes.
Figure 4.
High grade ovarian carcinoma with marked peritumoral inflammatory cell infiltrate (A). The tumor cells and peritumoral inflammatory cells show moderately to intense membranous staining with PD-L1 immunohistochemistry (B). T-lymphocytes represent the predominant component among the immune cells, highlighted by CD4 (C) and CD8 (D) immunostains. B-lymphocytes (E: CD20 immunostain) and macrophages (F: CD68 immunostain) are present in a smaller proportion. CD56 and TIA immunostains were negative for NK-cells (image not shown). (A: hematoxylin-eosin stain, B: PD-L1 immunostain, C: CD4 immunostain, D: CD8 immunostain, E: CD20 immunostain, F: CD68 immunostain; all images at 200x original magnification).
Figure 5.
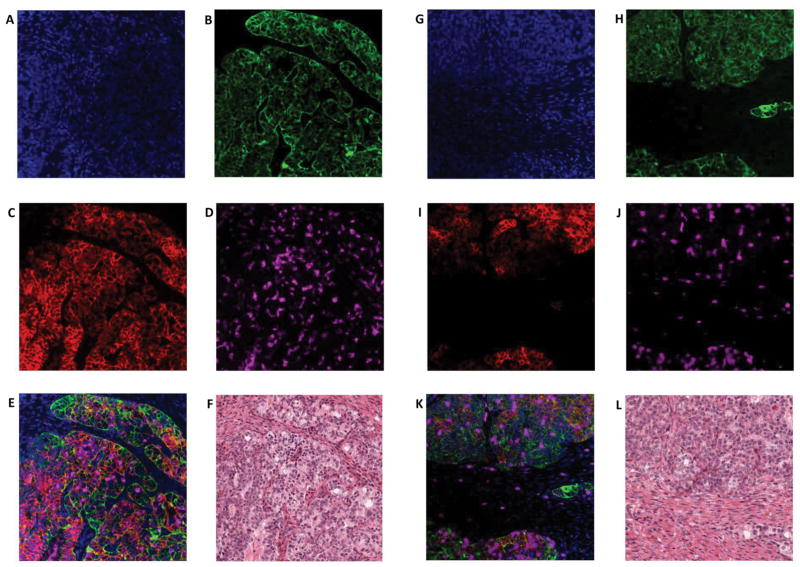
Detection of PD-L1 protein expression in tumor cells using immunofluorescence multiplexing panel. A–F, Multiplex IF panel of PD-L1 (red)/Cytokeratin (green)/DAPI (blue)/CD68 (magenta) of the same region. A–D, Representative images of single channels (DAPI, CK, PD-L1, CD68) of the multiplex IF panel. E, Representative fluorescence image showing the colocalization of PD-L1 and cytokeratin. CD68+ macrophages do not express PD-L1. F, H&E staining. G–L, Multiplex IF panel of PD-L1 (red)/cytokeratin (green)/DAPI (blue)/CD8 (magenta) of the same region. G–J, Representative images of single channels (DAPI, CK, PD-L1, CD8) of the multiplex IF panel. K, Representative fluorescence image showing the colocalization of PD-L1 (red) and cytokeratin (green). CD8 (magenta)+ T cells do not express PD-L1. L, H&E staining.
Figure 6.
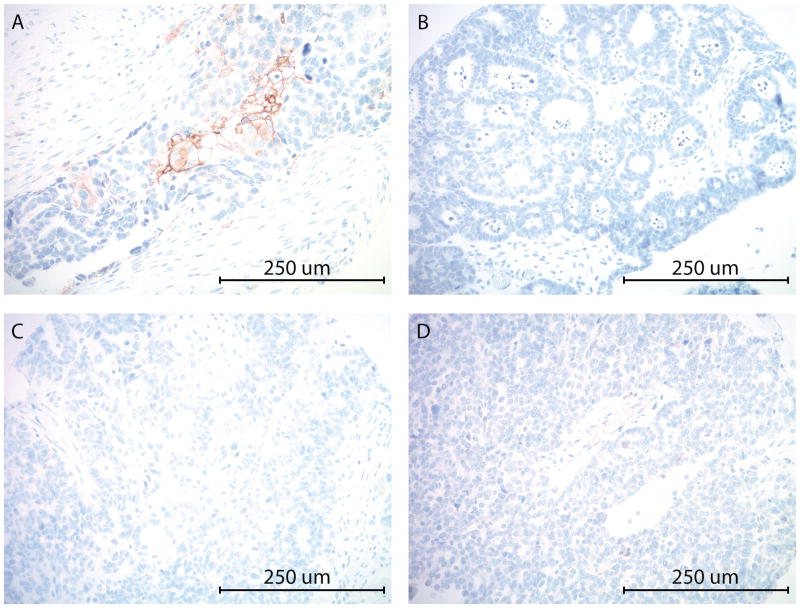
PD-L1 expression by immunohistochemistry in representative high grade ovarian serous carcinomas from the TMA. A: Focal PD-L1 expression (between 1–50%) in tumor cells. C, D, E: No PD-L1 expression is identified in tumor cells. (All images at 200x original magnification).
Discussion
Recurrent ovarian carcinoma resistant to salvage chemotherapy treatments remains an incurable disease. In the last few years multiple preclinical and clinical studies have demonstrated that one mechanism by which multiple human cancers evade host immunity is via upregulation of PD-1 ligand (PD-L1) and its interaction with the PD-1 ligand on antigen specific T-lymphocytes (5,6). Consistent with this view, a variety of human tumors have demonstrated sensitivity to immune check point inhibitor antibodies targeting PD-1 or PD-L1 in vivo and, accordingly, pembrolizumab (Merck & Co., Inc., Kenilworth, N.J., US), nivolumab (Bristol-Myers Squibb Company Princeton, NJ) and atezulumab (Genentech/Roche, San Francisco, CA) have recently received approval by the Food and Drug Administration (FDA) for the treatment of multiple human malignancies. Unfortunately, however, a large number of patients treated do not respond to immune check point treatment and little is currently known about the potential genetic mutations regulating response to the blockade of the PD1/PD-L1 axis.
We describe a heavily pretreated ovarian cancer patient with recurrent disease experiencing a complete and sustained response to pembrolizumab. Remarkably, this ovarian cancer was found exquisitely sensitive to the immune check-point blockade regardless to its high resistance to chemotherapy and radiation as demonstrated by the complete disappearance of a large recurrent tumor mass at 16 weeks from treatment initiation confirmed with multiple serial CAT scans. Of interest WES results demonstrated the tumor to have a relatively low number of mutations when compared to melanoma, lung cancer, ovarian cancer (4) or gynecologic and non-gynecologic tumors characterized by high microsatellite instability (ie, MSI-H endometrial and colorectal tumors)(23). These data combined with our results predicting at least 40 potential neoantigens in the WES tumor suggest that a high tumor mutation burden may not represent a mandatory requirement for clinical response to immune check point inhibitors in ovarian cancer patients.
Of great interest, we found the tumor to harbor a structural variation in the PD-L1 gene recently reported in about 27% of adult T-cell leukemia/lymphoma, 8% of B-cell lymphoma and 2% of stomach cancer patients to lead to marked elevation of PD-L1 transcripts secondary to the truncation of the 3′ – untranslated region (UTR) of the PD-L1 gene (22). Importantly, this rare PD-L1 mutation has recently been reported to provide high sensitivity to immune check-point inhibitor in preclinical animal models and speculated to potentially confer high susceptibility to blockade of the PD-1/PD-L1 axis in human patients (22). Consistent with these preclinical data in xenografted animals, we found our patient tumor to express high levels of the PD-L1 receptor and to be characterized by a high intra-tumoral CD8, CD4, and to a lower extent CD20 B lymphocytic and macrophage (ie, CD68) infiltration by IHC staining. Taken together, the relative low tumor mutation burden, strong PD-L1 surface expression and heavy inflammatory infiltration of this ovarian cancer suggest the PD-L1 structural variation to be the reason of its exquisite sensitivity to immune checkpoint treatment. Mutations in other immunologically relevant genes such as TGF-beta and CD27 were also detected in the tumor. The possible influence of these mutations on the exceptional response of this patient to pembrolizumab are currently unclear.
In conclusion, to our knowledge, this is the first report demonstrating a remarkable clinical activity of an immune checkpoint inhibitor in a patient harboring a rearrangement in the PD-L1 gene. While the PD-L1 structural variation we found in this ovarian cancer patient seems to be a rare event in human tumors with only 31 cases expressing 3′-UTR-trunctated PD-L1 transcript out of 10,210 cancer samples from 33 tumor panel analyzed by TCGA reported to date (22), the exquisite sensitivity of this patient to pembrolizumab suggests that such PD-L1 structural variants may serve as genetic marker for the identification of human cancers selectively upregulating PD-L1 in order to evade anti-tumor immunity. More importantly, these peculiar genetic characteristics may be able to identify human tumors that regardless to their high resistant to surgery, chemotherapy and radiation and a low mutation burden may still be highly susceptible to immune checkpoint PD1 blockade.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Ovarian cancer patients developing chemotherapy-resistant disease have extremely limited therapeutic options. Recent next generation sequencing (NGS) studies demonstrated rare PD-L1 gene rearrangements causing aberrant expression of the extracellular domain of PD-L1 in a subset of human cancers. In preclinical models these PD-L1 variants have been demonstrated to confer high sensitivity to immune check-point inhibitors targeting the PD1/PD-L1 axis. Here we report the first clinical demonstration of the exquisite sensitivity to pembrolizumab of an ovarian cancer patient with recurrent/progressive disease resistant to surgery, chemotherapy and radiation and harboring an aberrant PD-L1 expression secondary to a 3′-UTR disruption of the PD-L1 gene. Our results suggest that PD-L1 rearrangement may be used as a genetic marker to identify cancer patients highly sensitive to anti PD-1 treatment.
Acknowledgments
Funding Informations:
This work was supported in part by grants from NIH U01 CA176067-01A1, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to Alessandro D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from NCI.
Footnotes
Conflicts of interest: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2017. CA: A Cancer Journal for Clinicians. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Creasman DiSaia, DiSaia PJ, Creasman WT., editors. Clinical Gynecologic Oncology. 8. Saunders Elsevier Inc; Philadelphia, PA: 2012. [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 5.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Callahan CL, Wolchock JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–5039. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3360–5. doi: 10.1073/pnas.0611533104. Epub 2007 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecologic Oncology Research and Practice. 2016;3:11. doi: 10.1186/s40661-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 13.Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Berton-Rigaud D, Johnson EA, Cheng JD, Yuan S, Rubin EH, Matei DE. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol. 2015;33(suppl) abstr 5510. [Google Scholar]
- 14.Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J Clin Oncol. 2016;34(suppl) abstr5533. [Google Scholar]
- 15.Carvajal-Hausdorf DE, Schalper KA, Bai Y, Black J, Santin AD, Rimm DL. Objective, domain-specific HER2 measurement in uterine and ovarian serous carcinomas and its clinical significance. Gynecol Oncol. 2017 Apr;145(1):154–158. doi: 10.1016/j.ygyno.2017.02.002. Epub 2017 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non–small cell lung cancer. Lab Invest. 2014;94:107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Choi M, Overton JD, Bellone S, Roque D, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Yang X, Duffy B, Mohanakumar T, Mitra RD, Zody MC, et al. ATHLATES: accurate typing of human leukocyte antigen through exome sequencing. Nucleic Acids Res. 2013;41(14):e142. doi: 10.1093/nar/gkt481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karosiene E, Lundegaard C, Lund O, Nielsen M. NetMHCcons: a consensus method for the major histocompatibility complex class I predictions. Immunogenetics. 2012;64(3):177–86. doi: 10.1007/s00251-011-0579-8. [DOI] [PubMed] [Google Scholar]
- 20.Calis JJ, Maybeno M, Greenbaum JA, Weiskopf D, De Silva AD, Sette A, et al. Properties of MHC class I presented peptides that enhance immunogenicity. PLoS Comput Biol. 2013;9(10):e1003266. doi: 10.1371/journal.pcbi.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013 Nov;31(11):1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534:402–406. doi: 10.1038/nature18294. [DOI] [PubMed] [Google Scholar]
- 23.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.