Abstract
Objective
The purpose of this study was to assess the cross-sectional association between serum/urine biomarkers for osteoarthritis and MR-based cartilage composition and joint morphology of the knee, assessed using MR imaging data from the Osteoarthritis Initiative (OAI).
Design
141 subjects with Kellgren Lawrence (KL) grades 0–3 in the right knee and with available serum/urine biomarker assays were selected from the OAI. Cartilage T2 measurements were performed in the medial femur, lateral femur, medial tibia, lateral tibia, and patella compartments. Compartment-specific knee morphologic grading (WORMS) in the cartilage, meniscus, and bone marrow was also performed. We focused on associations of serum hyaluronan (sHA), serum COMP (sCOMP), serum MMP3 (sMMP3), and Urine CTX-II (uCtX-II)) with MRI parameters (T2, WORMS), assessed using partial correlations adjusted for age, gender, BMI, KL grade in both knees, and diabetes status.
Results
Higher levels of sHA, sMMP3 and sCOMP were correlated (p<0.05) with T2 of the lateral femur (r=0.18 to 0.32) and lateral tibia (r=0.17 to 0.23), and with average T2 of all knee regions (r=0.23). uCTXII was correlated with patellar T2 (r=0.19, P=0.04). Among the morphologic measures, sHA and sMMP3 was positively correlated (r=0.17 to 0.19, p<0.05) with meniscal damage.
Conclusions
This study suggests weak, but statistically significant, correlations of sHA, and sMMP3 with MRI T2 measures of cartilage extra-cellular matrix degeneration.
INTRODUCTION
Osteoarthritis (OA) is a heterogeneous joint disease that affects approximately 250 million people [1], causes severe disability [2], and often leads to total knee replacement (TKR). The rising rates of TKR [3] highlight a need to develop biomarkers that can detect early stages of joint degeneration. Ideally, such biomarkers would identify high-risk subjects that would most benefit from therapeutic intervention, such as lifestyle modification.
Biochemical serum and urine markers of OA are molecules that are released into serum/urine reflecting cartilage or bone turnover and the repair and regeneration processes that occur in joint tissue [4]. These markers are associated with radiographic OA changes, particularly the late stages of disease; Vilim et al. reported that serum COMP is associated with radiographic progression of OA [5], urine CTX-II is associated with progression in joint space narrowing (JSN) [6] and serum hyaluronan is associated with incidence of knee JSN [7]. However, few have studied the relationships of OA serum/urine markers with MR imaging markers of knee cartilage composition and morphologic knee damage which are not appreciated on standard radiographs [8].
Degenerative cartilage compositional change in OA can be quantified using MRI T2 mapping, that identifies abnormalities of the cartilage extracellular matrix including collagen fiber orientation[9]. MRI T2 probes early stages of cartilage degeneration, which are not seen on standard MRI, occurring prior to macroscopic cartilage defects and thinning. Understanding the links between systemic markers of knee joint tissue degeneration/repair expressed in serum/urine and imaging measures of changes in cartilage composition would provide new insights into the role of systemic biomarkers in assessing joint degeneration at early disease stages. Thus, serum/urine biomarkers could potentially provide information on the risk for developing OA at a pre-clinical stage.
The purpose of this study was therefore to assess the relationships of serum/urine biomarkers for osteoarthritis with MR imaging measures of joint structure (cartilage, meniscus, and bone marrow) and cartilage composition, using data from the Osteoarthritis Initiative (OAI).
METHODS
Subject Selection
This study utilizes data from the Osteoarthritis Initiative (OAI; http://www.oai.ucsf.edu/)[10], a multi-center, longitudinal study of persons aged 45–79 years at enrollment, aimed at assessing biomarkers in OA including those derived from MR imaging. The OAI dataset includes both MRI and radiographic images of subjects scanned over eight years. This database can be used to evaluate MRI biomarkers for the development and progression of OA. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards of all participating centers.
Subjects in this study are a subset of those included in the FNIH OARSI Biomarkers Consortium study, a nested case-control study within the OAI of 600 knees (one knee per subject) with structural and/or symptom progression over 4 years and knees that did not progress. Details of the study design are available [11]. Subjects in that study had serum and urine OA biomarkers assessed at baseline, 12 and 24 months. For the present study, we analyzed a convenience sample of these subjects by selecting all subjects that had a Kellgren Lawrence score (KL) ≤ 3 in a right knee from which we had previously obtained both T2 and joint morphology measures from the OAI MR images for other analyses.[12–16]. The OAI exclusion criteria was: (i) inflammatory arthropathies (including rheumatoid arthritis and seronegative spondylarthropathies), (ii) MRI contraindications, (iii) use of ambulatory aids and co-morbid conditions that may affect the ability to participate in the study. For this analysis we further excluded knees with (i) knee injury with deformity of the knee joint, (ii) total joint replacements at the lower extremities, (iii) MRI evidence of fractures or abnormalities, that do not fit into the spectrum of OA and may indicate other severe disease, such as tumor or inflammation.
Serum and Urine Biomarkers
Morning blood and second morning void urine specimens were collected after an overnight fast using a uniform protocol at baseline, 12 month and 24 month clinic visits. Additional details on specimen collection and processing methods can be found in the OAI operations manuals (http://www.oai.ucsf.edu/datarelease/OperationsManuals.asp). Among the eighteen serum and urine biomarkers available from the Biomarkers Consortium study, we focused on four based on previous research studies and recommendations from a consensus document from the Osteoarthritis Research Society International (OARSI) US FDA initiative [17]. These were: serum cartilage oligomeric matrix protein (sCOMP), serum hyaluronan (sHA), serum matrix metalloproteinase-3 (sMMP3), and urine Carboxy-Terminal Telepeptides of Type II Collagen (uCTXII) [7, 17–21]. We performed exploratory analyses of the remaining biomarkers: serum PIIANP (sPIIANP), serum CTXI (sCTXI), Serum CS846 (sCS846), Serum C2C (sC2C), Serum C1,2C (sC12c), Serum CPII (sCPII), Serum NTXI (sNTXI), Serum Coll2 1 NO2 (sColl21NO2), urine NTXI (uNTXI), urine C1,2C (uC12C), and urine Coll2 1 NO2 (uColl21NO2) [17]. The primary and exploratory predictors are summarized in Table 1.
Table 1.
Serum and urine biomarkers analyzed in this study.
| Biomarkers | Manufacturer | Biological Process [22] |
|---|---|---|
| Primary Predictors | ||
| Serum COMP (sCOMP) | Biovendor | Cartilage degradation |
| Serum hyaluronan (sHA) | Corgenix | Osteophyte burden, synovitis |
| Serum MMP-3 (sMMP3) | Invitrogen | Total (active and inactive) metalloprotease involved with joint tissue degradation |
| Urine CTXII (uCTXII) | Biovendor | Type II collagen degradation |
| Exploratory Predictors | ||
| Serum PIIANP (sPIIANP) | Merck Group/Millipore | Type II collagen synthesis |
| Serum CTXI (sCTXI) | IDS | Bone resorption |
| Serum CS846 (sCS846) | IBEX | Cartilage aggrecan synthesis/turnover |
| Serum C2C (sC2C) | IBEX | Type II collagen degradation |
| Serum CPII (sCPIl) | IBEX | Type II collagen synthesis |
| Serum/Urine NTXI (sNTXI/uNTXI) | ALERE - Osteomark | Bone resorption |
| Serum/Urine Coll2 1 NO2 (sColl21 NO2/uColl21 NO2) | Artialis | Type II collagen degradation and inflammation |
| Serum/Urine C1,2C (sC12C/uC12C) | IBEX | Types 1 and II collagen degradation |
Abbreviations: COMP, cartilage oligomeric matrix protein; CTXII, C-terminal crosslinked telopeptide type II collagen; PIIANP, type IIA procollagen amino terminal propeptide; CTXI, C-terminal crosslinked telopeptide of type I collagen; CS846, chondroitin sulfate 846 epitope; C2C, Collagen Type II Cleavage; CPII, C-propeptide of type II collagen; NTXI, N-telopeptide of type I collagen. All biomarker assays were performed by LabCorp Clinical Trials, a Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologists (CAP) certified division within LabCorp, with the exception of urine Col2-1 NO2, which was measured by Artialis, a Good Laboratory Practice-certified facility.
All biomarker assays were performed by LabCorp ClinicalTrials, a Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologists (CAP) certified division within LabCorp, with the exception of urine Col2-1 NO2, which was measured by clinical information. Complete details have been published [22, 23].
MR Imaging
MR images were obtained using four identical 3.0 Tesla (Siemens Magnetom Trio, Erlangen, Germany) scanners in Columbus, Ohio; Baltimore, Maryland; Pittsburgh, Pennsylvania; Pawtucket, Rhode Island. The following sequences were acquired: sagittal 2D intermediate-weighted fast spin-echo sequence (repetition time (TR)/echo time (TE)=3200/30ms, spatial resolution=0.357mm×0.511mm, slice thickness=3.0mm), coronal 2D proton density fast spin-echo sequence (TR/TE=3700/29ms, spatial resolution=0.365mm×0.456mm, slice thickness=3.0mm), and sagittal 3D dual-echo in steady state sequence (TR/TE=16.3/4.7ms, spatial resolution=0.365mmx0.456mm, slice thickness=0.7mm). A sagittal 2D multi-slice multi-echo sequence (MSME, TR=2700ms, TE1-TE7=10-70ms, spatial resolution=0.313mm×0.446mm, slice thickness=3.0mm, and 0.5mm gap) was used for cartilage T2 measurements[24].
Image Analysis
X-ray based KL grade and Joint space Narrowing
Fixed Flexion knee radiographs were obtained at baseline, and radiographic KL grades[25] were provided in the OAI dataset. Subjects with baseline KL grades of 0–3 were selected. Joint space narrowing (JSN) scores at baseline and two years were provided in the OAI dataset. JSN progression (in the right or left knee, individually) was defined as positive if a knee had a ≥ 1 OARSI grade increase in JSN score (medial or lateral) between baseline and two years.
WORMS Scoring
MR images of the right knee obtained at the baseline visit were reviewed on picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA). Three radiologists with 7-, 5- and 5-years of experience graded cartilage lesions. In equivocal cases, a consensus reading was performed with a musculoskeletal radiologist with 24-years of experience. Baseline cartilage and bone marrow lesions were assessed in six regions (patella, trochlea, medial femur, medial tibia, lateral femur and lateral tibia) using a modified semi-quantitative whole-organ magnetic resonance imaging score (WORMS) [26]. The highest score of any lesion was recorded for each region.
Subchondral bone marrow edema pattern was defined as poorly marginated areas of increased T2 signal intensity and graded using a modified 4-point WORMS scale (0, none; 1, diameter 0–5mm; 2, 5–20mm; 3, >20mm) [27]. Meniscal lesions were graded separately in 6 regions (medial/lateral and anterior/body/posterior) using the following 4-point scale: 0-normal; 1-intrasubstance signal; 2-non-displaced tear; 3-displaced or complex tear; 4-complete destruction/maceration.
The maximum (MAX) cartilage, meniscus or bone marrow edema (BME) score was defined as the maximum score in any region. The summation (SUM) cartilage, meniscus or BME score was defined as the summation of WORMS scores in all regions. The reproducibility results for WORMS reading have been previously published [28, 29]: the intra-observer reproducibility in all tissues (meniscus, cartilage, bone marrow) was ≥96%, while the inter-observer reproducibility was ≥97%.
T2 measurements
Semi-automatic cartilage segmentation of lateral/medial femur, lateral/medial tibia, and patella regions was performed as previously described, using an in-house, spline-based software based on MATLAB (MathWorks, Natick, Massachusetts)[29]. Trained investigators segmented the entire cartilage but used rigorous criteria to exclude sections with compromised image quality.
Validated methods for obtaining a T2 map of the cartilage have been published [28, 29]. T2 maps were computed from the MSME images on a pixel-by-pixel basis using 6 echoes (TE=20–70ms) and 3 parameter fittings accounting for noise [30, 31], and averaged over all of the slices in each cartilage region. The first echo (TE=10ms) was not included in the T2 fitting procedure in order to reduce potential errors resulting from stimulated echoes, and a noise-corrected algorithm was implemented [30, 31]. The cartilage T2 reproducibility results have been described previously [28, 29]. The mean T2 values had root mean square (RMS) coefficients of variation (CV) ranging from 0.83% in the medial femur to 3.21% in the patella for intra-reader reproducibility, and from 1.22% in the patella to 1.86% in the lateral tibia for inter-reader reproducibility.
Statistical Analysis
Statistical analysis was performed using STATA version 14 software (StataCorp LP, College Station, TX). Due to large between-subject variations [32], the dynamic nature of the serum markers due to nonlinear or phasic progression [22], and large variations of serum markers over time [32], we averaged the values of the serum and urine biomarkers, respectively, over the three measured timepoints (baseline, 12 and 24 months). For the cross-sectional analysis, partial correlations adjusted for age, gender, BMI, KL grade in both knees, diabetes status were used to evaluate the associations between serum/urine biomarkers and the following outcome variables: cartilage T2, cartilage, meniscus, and bone marrow lesion WORMS scores. The associations between biomarkers and both KL grade and WOMAC pain scores were assessed using partial correlations adjusted for adjusted for age, gender, and BMI. All the assumptions for partial correlation analysis were fulfilled.
We also performed longitudinal analyses of biochemical measures from baseline, 12 months and 24 months, using linear mixed effects models accounting for multiple measurements per subject. We assessed whether annual rates of change in biomarkers differed by baseline measures of (1) presence of a partial thickness cartilage defects (WORMS 0/1 vs. WORMS ≥2) and (2) presence of a meniscus tear (WORMS 0/1 vs. WORMS ≥2), and (3) average T2 subdivided by tertiles, by including an interaction between timepoint and each respective subgroup. The analyses of cartilage defects and T2 tertiles were adjusted for age, gender, BMI, KL grade in both knees, and diabetes status.
RESULTS
Subject Characteristics
The 141 participants in this study had a mean age of 59.7±8.3 years and a mean BMI of 29.9±4.5 kg/m2 at baseline. Sixty-six percent of the subjects were female, and a majority of the subjects had KL2 (n = 66, 46.8%), while 9.2% (n=13) had KL0, 14.9% (n=21) had KL1, and 29.1% (n=41) had KL3. Twelve subjects (8.5%) had JSN in the left knee over two years, while fifteen subjects (10.6%) had JSN in the right knee over two years (Table 2).
Table 2.
Participant Characteristics.
| All Participants | |
|---|---|
| n | 141 |
| Age (years) | 59.7 ± 8.2 |
| BMI (kg/m2) | 29.9 ± 4.5 |
| Gender (female) | 93 (65.9%) |
| WOMAC* pain | 2.6 ± 3.3 |
| Family history of knee replacement | 27 (19.4%) |
| Subjects with type 2 diabetes | 12 (8.7%) |
| Right knee KL | |
| 0 | 13 (9.2%) |
| 1 | 21 (14.9%) |
| 2 | 66 (46.8%) |
| 3 | 41 (29.1%) |
| Left knee KL | |
| 0 | 20 (14.2%) |
| 1 | 25 (17.7%) |
| 2 | 55 (39.0%) |
| 3 | 37 (26.2%) |
| 4 | 4 (2.8%) |
| Right knee JSN over two years | 15 (10.6%) |
| Left knee JSN over two years | 12 (8.5%) |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
No significant associations were found between the primary biomarkers (sHA, sCOMP, sMMP3, uCTXII) and KL grade or WOMAC pain score (adjusted for age, gender, and BMI, Supplementary Table 1) at baseline. No significant associations were found between blood biomarkers and development of joint space narrowing in the right knee over 2 years (n=15, 10.6% of subjects): uCTXII (OR = 1.17; p=0.61, 95% CI: 0.74–1.65), sMMP3 (OR=1.03, p=0.26, 95% CI: 0.97–1.11), sCOMP (OR = 1.01, p=0.71, 95% CI: 0.99–1.02), and sHA (OR =1.01 p=0.11, 95% CI: 0.99–1.04).
Cartilage MRI T2
Serum biomarkers sHA, sCOMP, and sMMP3 were positively correlated with cartilage T2 values (Table 3). For sHA, significant associations were found with T2 averaged over all cartilage compartments (r=0.23, p=0.01, 95% CI: 0.06–0.41), lateral femur T2 (r=0.32, p=0.001, 95% CI: 0.16–0.50) and lateral tibia T2 (r=0.20, p=0.01, 95% CI: 0.03–0.37), Table 3 and Figure 1. sCOMP showed positive associations with cartilage T2 in the lateral femur (r=0.18, p=0.03, 95% CI: 0.01–0.35) and the lateral tibia (r=0.17, p=0.04, 95% CI: 0.00–0.34). sMMP3 showed positive associations with cartilage T2 in the average of all compartments (r=0.23, p=0.006, 95% CI: 0.06–0.41), the lateral femur (r=0.18, p=0.03, 95% CI: 0.01–0.35), and the lateral tibia (r=0.23, p=0.01, 95% CI: 0.06–0.41), Figure 2. uCTXII was positively associated with cartilage T2 in the patella (r=0.19, p=0.04, 95% CI: 0.02–0.36). Many significant associations were found between the exploratory predictors and serum/urine biomarkers, especially Coll21NO2 (Supplementary Table 3).
Table 3.
Partial correlations between the primary serum and urine biomarkers and Cartilage T2, cartilage WORMS scores, and Meniscus WORMS scores (adjusted for age, gender, BMI, diabetes status, KL grade on both knee sides) with p values and 95% confidence intervals (CI).
| Parameter | sHA | sCOMP | sMMP3 | uCTXII | ||||
|---|---|---|---|---|---|---|---|---|
| Corr. | p value (95% CI) |
Corr. | p value (95% CI) |
Corr. | p value (95% CI) |
Corr. | p value (95% CI) |
|
| Cartilage T2 | ||||||||
| Ave | 0.23 |
0.01 (0.06 0.41) |
0.14 | 0.08 (−0.03 0.31) |
0.23 |
0.006 (0.06 0.41) |
0.13 | 0.14 (−0.04 0.30) |
| LFC | 0.32 |
0.001 (0.16 0.50) |
0.18 |
0.03 (0.01 0.35) |
0.18 |
0.03 (0.01 0.35) |
0.04 | 0.69 (−0.13 0.21) |
| LT | 0.20 |
0.01 (0.03 0.37) |
0.17 |
0.04 (0.00 0.34) |
0.23 |
0.01 (0.06 0.41) |
0.16 | 0.06 (−0.01 0.33) |
| MFC | 0.16 | 0.07 (−0.01 0.33) |
0.01 | 0.86 (−0.16 0.18) |
0.12 | 0.15 (−0.05 0.29) |
0.09 | 0.27 (−0.08 0.26) |
| MT | 0.09 | 0.30 (−0.08 0.26) |
0.06 | 0.47 (−0.11 0.23) |
0.15 | 0.07 (−0.02 0.32) |
−0.01 | 0.89 (−0.18–0.16) |
| PAT | 0.13 | 0.13 (−0.04 0.30) |
0.02 | 0.81 (−0.15 0.19) |
0.13 | 0.15 (−0.04 0.30) |
0.19 |
0.04 (0.02 0.36) |
|
| ||||||||
| Cartilage WORMS | ||||||||
| SUM | 0.07 | 0.41 (−0.10 0.24) |
−0.04 | 0.61 (−0.21 0.13) |
−0.01 | 0.87 (−0.18 0.16) |
0.09 | 0.27 (−0.08 0.26) |
| MAX | 0.12 | 0.14 (−0.05 0.29) |
0.01 | 0.86 (−0.16 0.18) |
−0.02 | 0.80 (−0.19 0.15) |
0.07 | 0.41 (−0.10 0.24) |
| LFC | 0.05 | 0.51 (−0.12 0.22) |
0.06 | 0.46 (−0.11 0.23) |
−0.05 | 0.50 (−0.22 0.12) |
0.14 | 0.11 (−0.03 0.31) |
| LT | 0.13 | 0.11 (−0.04 0.30) |
0.07 | 0.44 (−0.10 0.24) |
−0.01 | 0.93 (−0.18 0.16) |
0.13 | 0.12 (−0.04 0.30) |
| MFC | 0.06 | 0.49 (−0.11 0.23) |
0.01 | 0.96 (−0.16 0.18) |
0.03 | 0.69 (−0.14 0.20) |
0.03 | 0.65 (−0.14 0.20) |
| MT | −0.02 | 0.77 (−0.19 0.15) |
−0.08 | 0.35 (−0.25 0.09) |
0.07 | 0.36 (−0.10 0.24) |
−0.09 | 0.27 (0.26 0.08) |
| PAT | 0.01 | 0.88 (−0.16 0.18) |
−0.08 | 0.33 (−0.25 0.09) |
−0.07 | 0.40 (−0.24 0.10) |
0.08 | 0.36 (−0.25 0.09) |
| TRO | 0.05 | 0.54 (−0.12 0.22) |
0.01 | 0.87 (−0.16 0.18) |
−0.10 | 0.25 (−0.27 0.07) |
0.13 | 0.16 (−0.04 0.30) |
|
| ||||||||
| Meniscus WORMS | ||||||||
| SUM | 0.12 | 0.16 (−0.05 0.29) |
−0.11 | 0.21 (−0.28 0.06) |
0.15 | 0.07 (−0.02 0.32) |
−0.09 | 0.28 (−0.26 0.08) |
| MAX | 0.17 |
0.05 (0.00 0.34) |
−0.13 | 0.13 (−0.30 0.04) |
0.19 |
0.02 (0.02 0.36) |
−0.12 | 0.19 (−0.29 0.05) |
| medial anterior | −0.11 | 0.20 (−0.28 0.06) |
−0.10 | 0.24 (−0.27 0.07) |
−0.11 | 0.20 (−0.28 0.06) |
−0.09 | 0.26 (−0.26 0.08) |
| medial body | 0.19 |
0.02 (0.02 0.36) |
−0.03 | 0.69 (−0.20 0.14) |
0.21 |
0.01 (0.04 0.38) |
−0.09 | 0.33 (−0.26 0.08) |
| medial posterior | 0.09 | 0.28 (−0.08 0.26) |
−0.07 | 0.38 (−0.24 0.10) |
0.07 | 0.39 (−0.10 0.24) |
−0.07 | 0.38 (−0.24 0.10) |
| lateral anterior | −0.05 | 0.57 (−0.22 0.12) |
0.02 | 0.75 (−0.15 0.19) |
0.18 |
0.04 (0.01 0.35) |
−0.06 | 0.50 (−0.23 0.11) |
| lateral body | 0.13 | 0.13 (−0.04 0.30) |
−0.10 | 0.22 (−0.27 0.07) |
0.19 |
0.02 (0.02 0.36) |
0.02 | 0.85 (−0.15 0.19) |
| lateral posterior | 0.02 | 0.74 (−0.15 0.19) |
−0.08 | 0.35 (−0.25 0.09) |
−0.06 | 0.44 (−0.23 0.11) |
−0.02 | 0.81 (−0.19 0.15) |
Abbreviations: Corr. (partial correlation), LFC (lateral femoral condyle), LT (lateral tibia), MFC (medial femoral condyle) MT (medial tibia), PAT (patella), TRO (Trochlea), Ave (average T2 in all compartments) SUM (Summation of WORMS scores across all regions), MAX (Maximum WORMS scores in any region).
Figure 1.
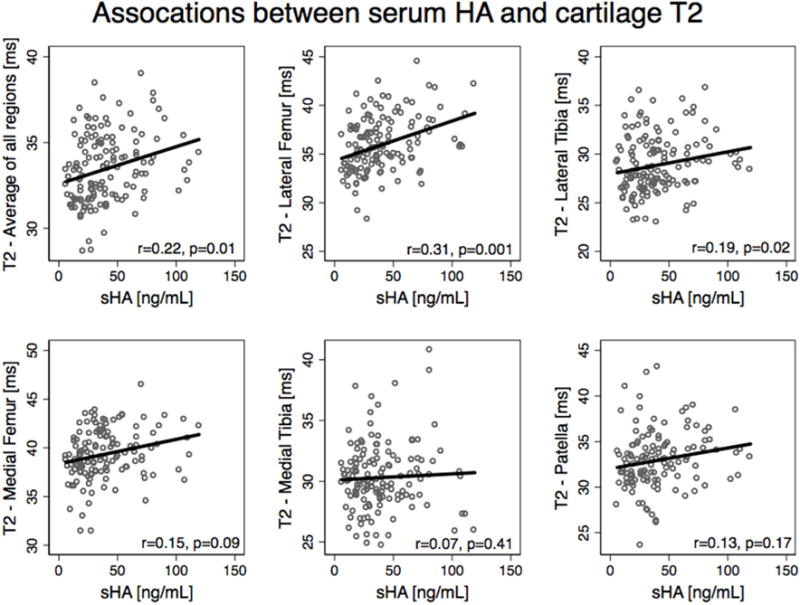
Scatterplots with a line fit using linear regression illustrate the associations between sHA and Cartilage T2. Partial correlations are listed with adjustments for age, gender, BMI, diabetes status, KL grade in both knees, and JSN over 2 years in both knees).
Figure 2.
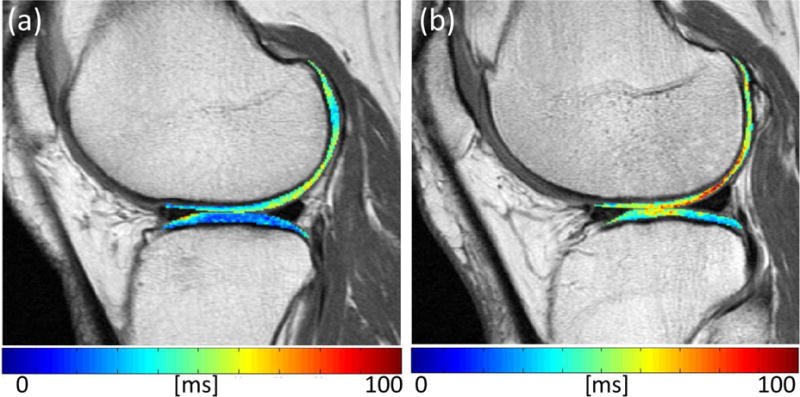
Randomly selected cartilage T2 maps in subject with low sMMP3 of 8.3 ~ 20th percentile (a) and a subject with high sMMP3 29.9 ~ 90th percentile (b). The subject with high sMMP3 values also has high T2 values (in ms), as shown by the red areas in the cartilage.
Interestingly, when subdividing the partial correlations between subjects with KL 0/1 and subjects with KL 2/3: subjects with KL 0 or 1 (n=34) had higher correlations between sMMP3 and cartilage parameters compared to subjects with KL 2 or 3, and compared to the entire cohort. The correlations between sMMP3 and cartilage parameters in subjects with KL 0 or 1 were significant in the average of all compartments: (r=0.52, p=0.004, 95% CI: 0.41–0.75), patella (r=0.41, p=0.03, 95% CI: 0.26–0.61), and medial tibia (r=0.48, p=0.009, 95% CI: 0.35–0.69). In subjects with KL 2 or 3 (n=107), the correlations between sMMP3 and T2 parameters were not significant: average of all compartments: (r=0.14, p=0.14, 95% CI: −0.03–0.31), patella (r=0.02, p=0.80, 95% CI: −0.15–0.19), and medial tibia (r=0.09, p=0.37, 95% CI: −0.08–0.26). However, subjects with KL 2 or 3 had significant correlations between sHA and T2 parameters (average of all compartments r=0.25, p=0.009, 95% CI: 0.08–0.43; lateral femur r=0.30, p=0.002, 95% CI: 0.14–0.48 and the lateral tibia r=0.23, p=0.02, 95% CI: −0.06–0.41). The correlations between T2 and sCOMP were not significantly different when subdivided by KL grade.
Joint Morphology
There were no significant associations between the primary serum/urine biomarkers and cartilage WORMS scores, Table 3. sHA was significantly correlated with meniscus WORMS scores for the medial body (r=0.19, p=0.02, 95% CI: 0.02–0.36), and maximum score of all regions (r=0.17, p=0.05, 95% CI: 0.00–0.34), Table 3. sMMP3 was significantly correlated with meniscus WORMS scores for the medial body (r=0.21, p=0.01, 95% CI: 0.04–0.38), lateral anterior horn (r=0.18, p=0.04, 95% CI: 0.01–0.35), lateral body (r=0.19, p=0.02, 95% CI: 0.02–0.36) and maximum score of all regions (r=0.19, p=0.02, 95% CI: 0.02–0.36), Table 3. sCOMP and uCTXII were not significantly associated with meniscus WORMS scores. The only significant association between serum/urine biomarkers and bone marrow abnormalities graded by the WORMS score was with sMMP3 for the lateral tibia (r=0.20, p=0.02, 95% CI: 0.03–0.37), Supplementary Table 2. For the exploratory predictors, significant associations were found between joint morphology and biomarkers including Coll21NO2, Supplementary Table 3. We also performed a sensitivity analysis in only subjects with medial JSN at baseline (61%) and found similar results to those of the entire sample. There were only 3 subjects with lateral JSN in the sample, thus a sensitivity analysis in these subjects could not be performed.
Longitudinal Changes in Biomarkers
Subjects had significant increases in sHA over 2 years (increase (ng/mL)/year =4.3, p<0.0001, 95% CI: 2.15–6.36). The longitudinal changes in sCOMP (-1.06 ng/mL/year; p=0.87, 95% CI: −13.9–11.8), sMMP3 (−0.07 ng/mL/year; p=0.80, 95% CI: − 0.62–0.49), and uCTXII (1.58 ng/mmol Cr/year; p=0.10, 95% CI: −0.03–0.35) were not significant. Both baseline T2 and presence of a cartilage defect were not significantly associated with the rate of change in sHA over 2 years (p=0.77 respectively p=0.99); however, subjects with meniscus tears showed increases in sHA over time (coeff=3.86, p=0.08, 95% CI: −0.53–8.26) compared to subjects without meniscus tears.
While subjects with cartilage defects at baseline had consistently higher sHA values than subjects without defects over 2 years, the differences were not significant (coeff=8.37, p=0.13, 95% CI: −2.54–19.28). However, subjects with high T2 values (averaged over all compartments) at baseline had significantly greater sHA than subjects with low T2 values (T2 Tertile 3 vs. T2 Tertile 1: coeff=14.82, p=0.01, 95% CI: 3.41–26.19) suggesting that subjects with cartilage biochemical abnormalities at baseline had consistently increased expression of sHA over 2 years, Figure 3. Similarly, subjects with high T2 values (averaged over all compartments) at baseline had significantly greater sMMP3 (T2 Tertile 3 vs. T2 Tertile 1: coeff=4.74, p=0.006, 95% CI: 1.34–8.13), sCOMP (T2 Tertile 3 vs. T2 Tertile 1: coeff=131.51, p=0.01, 95% CI: 26.59–236.43), and uCTXII (T2 Tertile 3 vs. T2 Tertile 1: coeff= 0.72, p=0.04, 95% CI: 0.02–1.43) than subjects with low T2 values.
Figure 3.
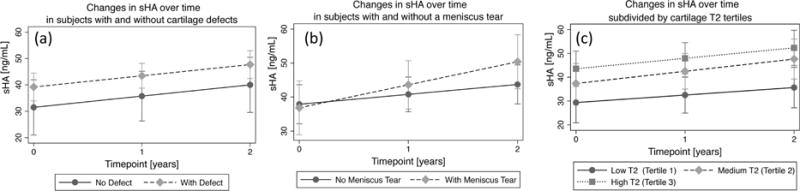
Mixed random effects models of absolute rates of change in sHA (with 95% confidence intervals) subdivided by (a) presence of a partial thickness cartilage defect in a knee, (b) presence of a meniscal tear and (c) T2 values (averaged over all compartments) subdivided into tertiles. Timepoint “0” indicates baseline.
DISCUSSION
Our study has shown significant positive correlations between the serum biomarkers (sHA, sMMP3, sCOMP), and cartilage T2, a measure of early cartilage degeneration. However, no significant associations were found between the primary serum/urine biomarkers and cartilage morphology or KL grade. While the correlations between serum/urine biomarkers and cartilage T2 were relatively low but significant (r~0.20), the results suggest that serum biomarkers may be sensitive to biochemical changes in cartilage that may not be related to morphologic damage and may thus represent early cartilage matrix degeneration.
One of the strengths of the current study is its focus on quantifying early biochemical stages of cartilage degeneration using cartilage T2 mapping. While previous research studies on serum/urine markers in OA have primarily focused on radiographic OA [5–7], few studies have investigated the relationships between biomarkers and cartilage compositional markers. One study targeting early arthroscopic finding in OA [33] reported positive associations between sHA, sCOMP and arthroscopic findings of cartilage softening and swelling (Outerbridge score of 1) and partial thickness defects (Outerbridge score of 2). Our study found similar results showing positive correlations between sHA, sCOMP and early cartilage biochemical degeneration associated with elevated cartilage T2. Interestingly, a high baseline T2 (>66th percentile) was also associated with consistently higher levels of sHA over 2 years of follow-up. One mechanism that may explain the relationship between cartilage degeneration and HA was suggested by Jiao et al.: that cartilage damage may initiate a pathologic inflammatory response, which promotes increases in HA production by the inflamed synovium [33]. Overall, our study demonstrates positive associations between serum marker sHA, suggesting that these markers are associated with early biochemical changes in the cartilage ECM matrix.
Studies have reported varying results on the associations between serum/urine biomarkers and knee morphology. Hunter et al. reported positive correlations with cartilage loss (for a 1-unit increase in COMP, the odds of cartilage loss increased 6.09 times (95% confidence interval [CI] 1.34 to 27.67)[34], while Eckstein et al, found that uCTXII and sCOMP were not associated longitudinal cartilage thinning over 24 months [35]. Another study reported no associations between baseline biochemical markers and cartilage volume or thickness, but changes in CTXII over three months were associated with decreases in cartilage thickness over one year[4]. Our study found no significant correlations between the primary serum/urine biomarkers and cartilage WORMS scores, suggesting that focal cartilage damage is not associated with sCOMP, sHA, sMMP3, and uCTXII, and may potentially be related to focal rather than diffuse abnormality of the cartilage.
Interestingly one of the exploratory, less-well established predictors, Coll21NO2, was associated with cartilage T2, cartilage WORMS, and meniscus WORMS scores n various compartments (Supplementary Table 3). This biomarker is related to collagen Type II degradation and may reflect oxidative-related cartilage degeneration [36]. Studies have shown elevated Coll21NO2 in subjects with OA [36], and increases in these peptides over 1 year were predictive of radiographic knee OA progression[37]. Thus, Coll21NO2 may be an important biomarker that to be considered for future longitudinal evaluation.
In this study, no significant associations between KL score and biochemical markers were found. Previous studies have reported varied results: Golightly et al. [7] showed positive higher baseline COMP and HA levels were associated with incident knee OA over an average follow-up period of 6.3 years, while Bruyere et al. reported no cross-sectional associations between biochemical markers (serum keratan sulphate, serum hyaluronic acid, urine pyridinoline and deoxypyridinoline, serumosteocalcin, and COMP) and femoro-tibial joint space width in more than 200 patients with knee OA [38]. In this study, perhaps, the non-significant associations between biochemical markers and KL score may be attributed to its cross-sectional nature and uneven distribution of KL grade, weighted toward knee KL 2: almost half of the subjects (46.8%, n=66) had a KL 2.
Ideally, biomarkers could be used as diagnostic tools to identify subjects at high risk for the development or progression of OA at which point therapy is most effective, or to test efficacy of therapeutic intervention. Serum/urine biomarkers are dynamic in nature [22, 32], could represent a delayed biological event that may have occurred in the joint, and are affected by injury [39] or exercise [40]. In contrast, MRI and X-ray imaging offer a more stable evaluation of joint structures, and vary more slowly over time. Accordingly, the relatively low correlations in this study (r~0.2) were not unexpected, highlighting the heterogeneity of OA, and that the systemic biomarker changes may only partially explain the biochemical composition captured with MRI T2 during degeneration. Clinically, we believe that both serum biomarkers and MR imaging data provide complementary information comprising both the dynamic changes occurring during joint degeneration and overall disease status, thus highlighting the need for longitudinal evaluations of long-term prognostic value.
There are several limitations of this study: we only analyzed T2 measurements of cartilage composition as provided by the OAI, and it would be beneficial to study other quantitative cartilage assessments such as T1rho mapping. In addition, no significant associations were found between serum/urine biomarkers and knee pain score. These results may be due to the fact that subjects in this study had low knee pain (mean WOMAC knee pain score = 2.6 ± 3.3) based on the WOMAC knee pain scale, which ranges from 0–20. The statistically significant association between uCTXII with patellar disease is unexpected, and this relationship may be due to multiple testing (as we did not adjust for multiple outcomes). A large number of analyses were performed in this study and thus the results should be interpreted with caution. Despite these limitations, we feel that this study is unique: it is the first to assess the relationships between serum/urine biomarkers and MRI cartilage biochemical composition quantified using cartilage T2.
Overall, this study suggests weak but significant associations between serum biochemical markers of OA (sHA, sMMP3, sCOMP) and MRI T2 biochemical degeneration of the cartilage ECM. Based on our results, serum biomarkers and cartilage T2 composition may reflect similar features of the pathophysiology of cartilage matrix degenerative disease.
Supplementary Material
Acknowledgments
Role of Funding Source:
This study was funded by NIH P50-AR060752 and NIH R01-AR064771. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions:
Conception and design: GBJ, MCN, CEM, JN, JAL, UH, NEL TML
Analysis and interpretation of the data: GBJ, MCN, CEM, JN, UH, NEL, TML
Drafting of the article: GBJ, MCN, CEM, JN, JAL, UH, NEL TML
Critical revision of the article for important intellectual content: GBJ, MCN, CEM, JN, JAL, UH, NEL TML
Statistical expertise: GBJ, CEM
Collection and assembly of data: GBJ, JN, JAL, UH
Final approval of the article: GBJ, MCN, CEM, JN, JAL, UH, NEL TML
Competing Interests: None
The absence of a significant interaction with time in the longitudinal analysis suggests the biomarkers are consistently elevated, and supports combining the three timepoints for cross-sectional analysis.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112:S13–19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 3.Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. Lancet. 2012;379:1331–1340. doi: 10.1016/S0140-6736(11)60752-6. [DOI] [PubMed] [Google Scholar]
- 4.Bruyere O, Collette J, Kothari M, Zaim S, White D, Genant H, et al. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65:1050–1054. doi: 10.1136/ard.2005.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–713. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 6.Garnero P, Mazieres B, Gueguen A, Abbal M, Berdah L, Lequesne M, et al. Cross-sectional association of 10 molecular markers of bone, cartilage, and synovium with disease activity and radiological joint damage in patients with hip osteoarthritis: the ECHODIAH cohort. J Rheumatol. 2005;32:697–703. [PubMed] [Google Scholar]
- 7.Golightly YM, Marshall SW, Kraus VB, Renner JB, Villaveces A, Casteel C, et al. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63:2276–2283. doi: 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AlOmran AS. Osteoarthritis of knee: correlation between radiographic and arthroscopic findings. Int Surg. 2009;94:269–272. [PubMed] [Google Scholar]
- 9.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Peterfy C, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016;68:2422–2431. doi: 10.1002/art.39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. Journal of magnetic resonance imaging: JMRI. 2012;35:370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls–data from the osteoarthritis initiative. Arthritis research & therapy. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kretzschmar M, Lin W, Nardo L, Joseph GB, Dunlop DD, Heilmeier U, et al. Association of Physical Activity Measured by Accelerometer, Knee Joint Abnormalities, and Cartilage T2 Measurements Obtained From 3T Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2015;67:1272–1280. doi: 10.1002/acr.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.01.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25:136–144. doi: 10.1097/BOR.0b013e32835a9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnero P. Use of biochemical markers to study and follow patients with osteoarthritis. Curr Rheumatol Rep. 2006;8:37–44. doi: 10.1007/s11926-006-0023-5. [DOI] [PubMed] [Google Scholar]
- 19.Hoch JM, Mattacola CG, Medina McKeon JM, Howard JS, Lattermann C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;19:1396–1404. doi: 10.1016/j.joca.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S, Kumar D, Sharma NR. Role of hyaluronic Acid in early diagnosis of knee osteoarthritis. J Clin Diagn Res. 2014;8:LC04–07. doi: 10.7860/JCDR/2014/11732.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumm J, Tamm A, Lintrop M, Tamm A. The value of cartilage biomarkers in progressive knee osteoarthritis: cross-sectional and 6-year follow-up study in middle-aged subjects. Rheumatol Int. 2013;33:903–911. doi: 10.1007/s00296-012-2463-8. [DOI] [PubMed] [Google Scholar]
- 22.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76:186–195. doi: 10.1136/annrheumdis-2016-209252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus VB, Hargrove DE, Hunter DJ, Renner JB, Jordan JM. Establishment of reference intervals for osteoarthritis-related soluble biomarkers: the FNIH/OARSI OA Biomarkers Consortium. Ann Rheum Dis. 2017;76:179–185. doi: 10.1136/annrheumdis-2016-209253. [DOI] [PubMed] [Google Scholar]
- 24.Peterfy C, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16:1433. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellgren J, Lawrence J. Radiologic assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Stahl R, Jain S, Lutz J, Wyman B, Graverand-Gastineau M, Vignon E, et al. Osteoarthritis of the knee at 3.0 T: comparison of a quantitative and a semi-quantitative score for the assessment of the extent of cartilage lesion and bone marrow edema pattern in a 24-month longitudinal study. Skeletal Radiology. 2011;40:1315–1327. doi: 10.1007/s00256-011-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years–data from the Osteoarthritis Initiative. Osteoarthritis and cartilage. 2012;20:727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging–data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AJ, Joseph PM. The use of power images to perform quantitative analysis on low SNR MR images. Magn Reson Imaging. 1993;11:1051–1056. doi: 10.1016/0730-725x(93)90225-3. [DOI] [PubMed] [Google Scholar]
- 31.Raya J, Dietrich O, Horng A, Weber J, Reiser M, Glaser C. T2 measurement in articular cartilage: Impact of the fitting method on accuracy and precision at low SNR. Magnetic Resonance in Medicine. 2010;63:181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 32.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–2488. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 33.Jiao Q, Wei L, Chen C, Li P, Wang X, Li Y, et al. Cartilage oligomeric matrix protein and hyaluronic acid are sensitive serum biomarkers for early cartilage lesions in the knee joint. Biomarkers. 2016;21:146–151. doi: 10.3109/1354750X.2015.1118547. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DJ, Li J, LaValley M, Bauer DC, Nevitt M, DeGroot J, et al. Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee Study. Arthritis Res Ther. 2007;9:R108. doi: 10.1186/ar2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckstein F, Le Graverand M, Charles H, Hunter D, Kraus V, Sunyer T, et al. Clinical, radiographic, molecular and MRI-based predictors of cartilage loss in knee osteoarthritis. Annals of the Rheumatic Diseases. 2011;70:1223. doi: 10.1136/ard.2010.141382. [DOI] [PubMed] [Google Scholar]
- 36.Deberg M, Labasse A, Christgau S, Cloos P, Bang Henriksen D, Chapelle JP, et al. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2005;13:258–265. doi: 10.1016/j.joca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Deberg MA, Labasse AH, Collette J, Seidel L, Reginster JY, Henrotin YE. One-year increase of Coll 2-1, a new marker of type II collagen degradation, in urine is highly predictive of radiological OA progression. Osteoarthritis Cartilage. 2005;13:1059–1065. doi: 10.1016/j.joca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Bruyere O, Collette JH, Ethgen O, Rovati LC, Giacovelli G, Henrotin YE, et al. Biochemical markers of bone and cartilage remodeling in prediction of longterm progression of knee osteoarthritis. J Rheumatol. 2003;30:1043–1050. [PubMed] [Google Scholar]
- 39.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254) Arthritis Res Ther. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisbie DD, Al-Sobayil F, Billinghurst RC, Kawcak CE, McIlwraith CW. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthritis Cartilage. 2008;16:1196–1204. doi: 10.1016/j.joca.2008.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


