1.0 Background
Alzheimer’s disease (AD) presents a unique challenge. It’s the only top ten cause of disability that doesn’t have a therapy to slow its progression[1]. Several large clinical trials are actively studying individuals in order to discover potential therapies by 2025[1]. The success of these trials, however, depends upon individuals being willing to undergo gene and biomarker testing and learn the related risk of AD dementia. AD stigma presents an obstacle to them doing this[1,2].
Stigma of AD dementia can take the form of one or more of a collection of beliefs, behaviors, and attitudes. The assumptions made about AD and people with the disease often reflect those that confirm stereotypes about symptoms or functional abilities. These stereotypes often depict the later stages of disease when a person is most impaired and fully dependent upon others for care[3–6]. As a result of these negative expectations, stigma can discourage a person from seeking diagnosis, educating themselves about the disease, and participating in research[2,7–9]. It can also lead people to react poorly such as patronizing, isolating, and discriminating against a person with the disease[10–12].
Stigma of AD dementia can differ based upon one’s personal characteristics and beliefs about a disease. As compared to no diagnosis, a diagnosis of AD dementia can mitigate against some forms of stigma, such as harsh judgements about a person’s poor hygiene or aesthetics[13]. But its prognosis – that symptoms are expected to worsen over time— can exacerbate discrimination, pity, and social distance. Belief that AD dementia is a mental illness exacerbates how individuals judge the severity of a person’s symptoms[14]. Other personal characteristics of a person, like age and gender, can also affect how they judge or react to someone with AD dementia[14].
Understanding the composition of beliefs, attitudes, and expectations about AD dementia held by the general public could help inform specific strategies to mitigate stigma and its consequences. If, for example, members of the general public don’t only worry that a person with AD dementia faces discrimination[14] but also worry specifically that a confirmatory genetic test for AD could make a person vulnerable to being discriminated against by health insurance purveyors, this knowledge would be valuable for informing how to direct public education about certain policies, like The Genetic Information Nondiscrimination Act of 2008 (GINA)[15] which offers protections against gene based healthcare insurance discrimination. Alternatively, such information could help identify gaps in current policy protections, whereby concerns among the public are common but policy protections are lacking or insufficient. Together, this information could be useful for understanding factors that deter individuals from seeking care or enrolling in AD research.
The purpose of this study was to understand what features or attributes are most commonly paired with AD dementia by the public. Based on prior studies,[13,14] we hypothesized that concerns about discrimination and the propensity to over-attribute the severity to symptoms would be among the most prevalent. Among those most common, we sought to determine whether their prevalence differed based on characteristics of population subgroups. We expected that older respondents would be more likely to expect a person with AD would encounter employment discrimination and that African American respondents would be less likely than White respondents to expect a person with AD would be excluded from medical decision-making. Understanding which attributes are most often associated with AD dementia by the public may help identify the most overt and wide-reaching concerns related to the disease, which may help inform interventional programs and policy changes to reduce AD stigma.
2.0 Methods
2.1 Study Design
This is a secondary analysis of how a random sample of adults from the general U.S. public reacted to a fictional description of a person with mild stage AD dementia. The data analyzed in the present study are a subset of those used in studies [13] and [14].
2.2 Data Source
Data were obtained from an experimental study that examined whether the cause and prognosis of mild dementia were related to how adults in the general population judged a person with mild AD dementia. The study asked respondents to read a vignette and then complete a survey. Respondents were recruited September 5th through 13th 2013 by an online panel provider. The demographic profiles of online panels have been shown to be representative of the U.S. general population[16].
The survey was distributed to a random sample likely to be adults in the U.S. who were able to provide informed consent and read English. The survey completion rate was 58%. Respondents were asked to provide standard demographic information. The collection of race and ethnicity information was informed by the Census Alternative Questionnaire Experiment[17]. Respondents were asked to self-identify by race or ethnicity or by multiple races.
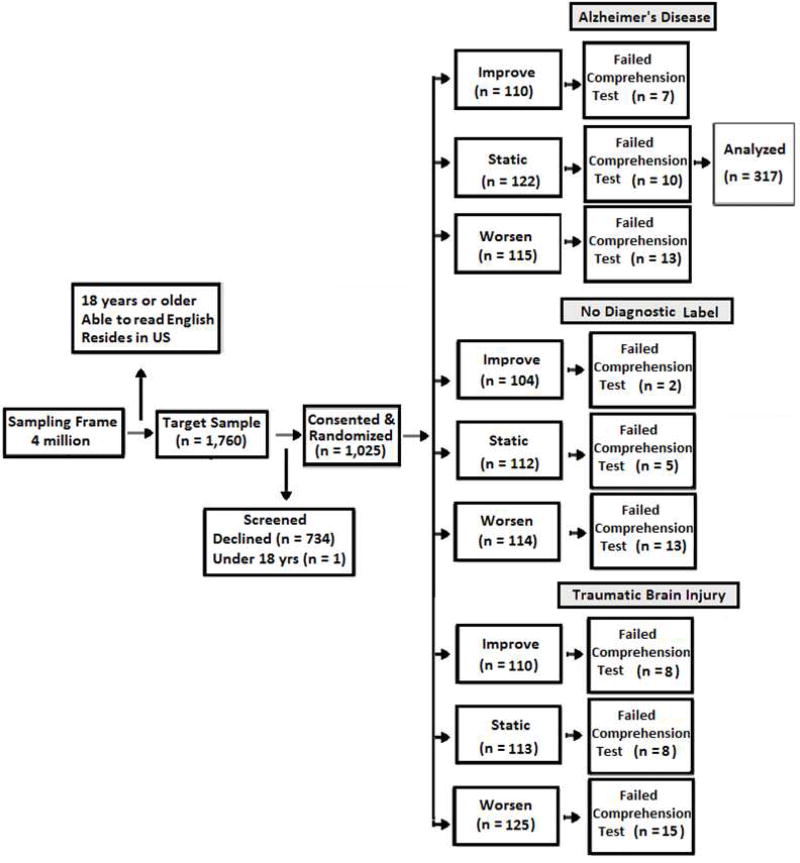
The original study used a 3×3 factorial design whereby consenting adults (N=1,025) were assigned to 1 of 9 conditions using unrestricted simple randomization[18]. In the present study, we analyzed data from 317 of those respondents randomized to 3 of the 9 conditions. All of these respondents were told the cause of the mild stage dementia was AD. The three conditions differed based on whether they were told the person’s condition would 1) worsen, 2) improve, or 3) remain unchanged. A fuller description of the design and randomization is available elsewhere[14].
2.3 Vignette Design
The original study used vignettes to examine how diagnostic label and prognosis contributed to attitudes, emotions, and expectations expressed by the general public. The study was described to participants as being about “health beliefs” and did not mention AD during recruitment or consent.
The vignette described a man suffering from impairments typical of the mild stage of AD dementia. The symptoms described were consistent with observable impairments in six domains of the Clinical Dementia Rating scale[19]: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care.
To personalize the vignette, the character was given a name, Mr. Andrews, and referred to as “he.” Pilot versions of the survey included male and female versions of the vignette, but restrictions in sample size required reducing the number of vignettes. Interest in being consistent with previous research in which vignettes relied on male characters[11], favored retaining the male version of the vignette. Studies of AD dementia that have experimentally varied the gender of non-familial vignette characters have not found appreciable differences in reactions among the general public[20,21]. No other demographic characteristics of the vignette character were given.
After reading the vignette, respondents were given a comprehension test to confirm that they accurately understood the salient details. They had two opportunities to answer correctly. Those who failed on the second attempt were excluded (Appendix Figure A). After this exclusion, the final sample in the present study included data from 317 respondents.
2.4 Study Measures
A modified version of the Family Stigma in Alzheimer’s Disease Scale (FS-ADS[22]) was used to assess the beliefs, feelings, and expectations of respondents. Some items on the original instrument were adapted for understandability and relevance in the context of the current study (See Supplemental materials in [13]). The modified FS-ADS asked respondents how likely they believed the person (described in the vignette) would be to have his healthcare insurance limited due to documentation in his medical record, due to a result from a brain scan, or due to a result from a genetic test. Respondents were also asked the extent to which they expected the person would be discriminated against by employers, excluded from voting or medical decision-making, or exhibit certain symptoms like not remembering recent events, failing at simple tasks, or suffering incontinence. In addition, respondents were asked about their expectations for the person’s aesthetics – like whether they expected the person to have poor hygiene or neglect self-care – and the extent to which they expected people would be disgusted or repulsed by the person. Respondents were asked the extent to which they expected others to feel concern, compassion, sadness, pity, or to behave in ways that ignored, isolated, or helped the person. Responses were given on a scale from 1 to 5 with higher scores indicating stronger endorsement.
A shortened Alzheimer's Disease Knowledge Scale (ADKS[23]) was used to evaluate general knowledge of Alzheimer’s disease. The abbreviated instrument omitted eight items on the original assessment because they could have been answered using information in the vignette[13]. Respondents were also asked to rate the degree that they felt the person’s condition (i.e., Alzheimer’s disease) was a mental illness from “not at all” (1) to “a very great extent” (5). A fuller description of the study’s methods is available elsewhere[13].
2.5 Statistical Analysis
Responses of 4 and 5 on the FS-ADS were considered a positive result in the present study. This cut point was consistent with the 50th percentile or above on all items and the 75th percentile or above on 21 of 44 items. Adjusted generalized linear models (GLMs) with a log link were used to estimate the percentage of the respondents who strongly endorsed each item on the modified FS-ADS. These models adjusted for study prognostic condition (i.e., static, improve, worsen). In order to mitigate family-wise errors of the first kind, a limited number of items were carried forward for further analysis[24]: those endorsed by 60% or more of respondents in the condition where the person’s prognosis was expected to worsen.
In separate bivariate analyses, we used GLMs with a log link to estimate the percentages of respondents strongly endorsing each outcome when they were told the person’s condition would worsen, improve, or remain unchanged over time. In these analyses, estimates with 95% confidence intervals that do not overlap are statistically significant at P<0.05 (Table 3). To build a multivariate model, we used forward step-wise selection in multivariable GLMs to construct statistical models that adjusted for interrelationships among respondent characteristics (Alpha-to-Keep≤0.20). Candidate covariates were all assessed demographic characteristics, general knowledge about AD, and strength of belief that this disease was a mental illness. We then entered the retained covariates together into a multivariate analysis that statistically adjusted for variance shared among the outcomes. We report differences in percentages of respondent endorsement for each outcome and their respective 95% confidence intervals. In these analyses, estimates with 95% confidence intervals that do not include zero are statistically different from zero (P<0.05; Table 4). The multivariable analyses have at least 89% power to detect a difference of 8% or more.
Table 3.
Percentage (95%CI) of Sample of General Public Endorsing Expected Features of Alzheimer's Disease by Study Prognostic Condition (N=317)
| Improve (n=103) |
Static (n=112) |
Worsen (n=102) |
|
|---|---|---|---|
| Doesn't Remember Recent Events | 73.8 (65.8 to 82.7) | 71.4 (63.0 to 79.9) | 79.4 (71.5 to 87.3) |
| Employment Discrimination | 63.4z (54.4 to 72.4) | 55.3z (45.7 to 65.0) | 78.4xy (70.4 to 86.5) |
| Exclusion from Medical Decision-Making | 55.3z (45.7 to 65.0) | 63.4 (54.4 to 72.4) | 73.5x (64.9 to 82.2) |
| Insurance Discrimination due to Medical Record | 46.6z (36.9 to 56.3) | 51.8 (42.5 to 61.1) | 65.7x (56.4 to 75.0) |
| Insurance Discrimination due to Brain Imaging Results | 45.6z (35.9 to 55.3) | 47.3 (38.0 to 56.6) | 62.7x (53.3 to 72.2) |
Note. 95%CI = 95% Confidence Interval. Results derived from Multivariable GLMs with log link.
Statistical significance denoted as:
Statistically different than improve condition;
Statistically different than static condition;
Statistically different than worsen condition.
Table 4.
Adjusted Percent differences (95%CI) in Sample of General Public Endorsing Expected Features of Alzheimer’s Disease (N=317)
| Memory Problems |
Employment Discrimination |
Exclusion from Medical Decision- Making |
Insurance Limited due to data in Medical Record |
Insurance Limited due to Brain Imaging Result |
|
|---|---|---|---|---|---|
| Female vs. Male | 7.9 (−1.8 to 17.7) | −1.3 (−11.8 to 9.2) | 10.7 (−0.04 to 21.4) | 7.0 (−4.0 to 17.9) | −1.0 (−12.0 to 10.1) |
|
| |||||
| Age 50+ vs under age 50 | 11.5* (1.5 to 21.5) | 17.4** (6.6 to 28.1) | 10.9 (−0.07 to 21.9) | 13.7* (2.5 to 25.0) | 8.4 (−3.0 to 19.7) |
|
| |||||
| Black vs White | 0.2 (−18.3 to 18.8) | −20.6* (−40.6 to −0.6) | −26.4** (−46.8 to −5.9) | −30.8** (−51.8 to −9.9) | −33.2** (−54.3 to −12.1) |
| Other vs White | −9.0 (−23.8 to 5.8) | 1.2 (−14.7 to 17.1) | −6.3 (−22.5 to 10.0) | −13.9 (−30.5 to 2.8) | −4.2 (−21.0 to 12.6) |
|
| |||||
| College vs ≤HS | −2.1 (−14.4 to 10.2) | 9.6 (−3.6 to 22.8) | −6.2 (−19.7 to 7.3) | 13.6 (−0.2 to 27.5) | 5.8 (−8.1 to 19.7) |
| More than College vs ≤HS | 1.7 (−11.0 to 14.4) | 4.0 (−9.7 to 17.7) | −11.5 (−25.5 to 2.5) | 5.1 (−9.2 to 19.4) | −2.9 (−17.4 to 11.5) |
|
| |||||
| Strong Belief AD is a Mental Illness | 17.6*** (7.5 to 27.7) | 8.1 (−2.8 to 19.0) | 5.3 (−5.9 to 16.4) | 5.9 (−5.5 to 17.3) | 17.4** (5.9 to 28.9) |
|
| |||||
| Worsen vs improve or static | 4.3 (−5.9 to 14.6) | 16.6** (5.6 to 27.7) | 12.8* (1.5 to 24.0) | 13.2* (1.7 to 24.8) | 14.2* (2.6 to 25.9) |
Note. Results from Multivariate Analysis of Variance that adjust for covariates and variance shared among outcomes. AD = Alzheimer’s disease dementia. ≤HS = High School or less. 95%CI = 95% Confidence Interval. Confidence intervals that do not include zero are statistically significant at p<0.05.
p≤.05
p≤.01
p≤.001
Respondents’ caregiver status was excluded from analysis as small group size prohibited comparisons (n = 19) and its inclusion as a covariate did not substantively alter the main results[25]. All independent variables were screened for multicollinearity (correlation coefficient r >0.7). In analyses that adjusted for multiple comparisons, all independent variables were screened for interactions with study prognostic category (p>5.0). All statistical tests were two-sided. P values ≤0.05 were considered statistically significant. All statistical analyses were performed using Stata 14 (College Station, TX).
3.0 Results
3.1 Respondent Characteristics
In a sample of 317 adults in the general public, respondents’ median age was 49 years (IQR 29), about half (49%) were female, most (80%) self-identified as White (non-Latino), and over half (65%) had less than a 4-year college degree. (Table 1)
Table 1.
Characteristics of Random Sample of Adult General Public (N=317)
| Respondent Characteristic |
Alzheimer's Disease (N=317) |
|---|---|
| Age, median (IQR) | 49 (29) |
| 65+ Years Old, % (n) | 19.1 (61) |
| Females, % (n) | 49.0 (156) |
| Race / Ethnicity, % (n) | |
| White, Non-Latino | 80.4 (255) |
| African American, Non-Latino | 7.3 (23) |
| Othera | 12.3 (39) |
| Education, % (n) | |
| High School/GED or Less | 23.7 (75) |
| Some College or 2-year Degree | 41.6 (132) |
| 4-Year College Degree or Beyond | 34.7 (100) |
| Caregiver (Past or Present), % (n)b | 6.0 (19) |
| Urban/Metro Setting,c % (n) | 78.5 (249) |
| Mental Illness Rating,d median (IRQ) | 3 (3) |
| Alzheimer's Disease Knowledge Scale (ADKS),e median (IQR) | 15 (5) |
Note. Column percentages may not total 100 due to rounding.
Category includes those who identified as Asian, Native American, multiple races, Hispanic or Latino only, other or did not respond (n=4).
Reported past or current primary caregiver of a person with Alzheimer’s disease.
Resides in urban rather than rural area based on Rural Urban Commuting Area (RUCA) classifications. Urban areas included RUCA classes 1 to 3 and rural included classes 4 to 10.
Respondents were also asked to rate the degree the condition described in the vignette was a mental illness from “not at all” (1) to “a very great extent” (5).
Abbreviated version. Maximum possible score = 22.
In analyses that adjusted statistically for study prognostic condition, about three-quarters of respondents expected that a person with mild stage AD dementia would not remember most recent events (73.8%, 95%CI 65.8 to 82.7). In adjusted analyses, over half of respondents expected a person with AD dementia would be discriminated against by employers (55.3%, 95%CI 47.0 to 65.2) and would be excluded from medical decision-making (55.3%, 95%CI 46.9 to 65.4; Table 2). Similarly, high percentages expected the person would have his healthcare insurance limited due to data in the medical record (46.6%, 95%CI 38.0 to 57.2) or have his healthcare insurance limited due to a brain imaging result (45.6%, 95%CI 37.0 to 56.3).
Table 2.
Adjusted Percentage of Respondents by Expected Features of Alzheimer's Disease (N=317)
| Symptom Severity | % (95%CI) | Discrimination | % (95%CI) |
| Doesn't Remember Recent Events | 73.8 (65.8 to 82.7) | Employers Discriminate | 55.3 (47.0 to 65.2) |
| Repeats Self | 32.0 (24.1 to 42.7) | Excluded from Medical Decision-Making | 55.3 (46.9 to 65.4) |
| Fails Simple Tasks | 30.1 (22.4 to 40.4) | Insurance Limited: Medical Record Data | 46.6 (38.0 to 57.2) |
| Speech | 16.5 (10.6 to 25.7) | Insurance Limited: Brain Imaging Result | 45.6 (37.0 to 56.3) |
| Speaks Nonsense | 16.5 (10.4 to 26.3) | Insurance Limited: Genetics | 44.7 (36.0 to 55.4) |
| Disturbs Others | 15.5 (9.6 to 25.2) | Excluded from Voting | 24.3 (16.8 to 35.1) |
| Incontinent | 11.7 (6.3 to 21.4) | Doctors Discriminate | 20.4 (13.6 to 30.6) |
| Pity | % (95%CI) | Dangerousness | % (95 %CI) |
| Sympathy | 48.5 (39.8 to 59.2) | To self | 23.3 (16.4 to 33.1) |
| Pity | 32.0 (23.9 to 43.0) | To Others | 7.3 (4.9 to 10.7) |
| Sadness | 32.0 (23.9 to 42.9) | ||
| Sorrow | 30.1 (22.2 to 40.7) | ||
| Antipathy | % (95%CI) | Social Distance | % (95%CI) |
| Uneasy | 23.3 (16.2 to 33.5) | Hidden | 27.2 (19.8 to 37.3) |
| Fear | 20.4 (14.3 to 29.1) | Social Contracts Limited | 21.4 (14.3 to 31.9) |
| Embarrassed | 11.7 (6.6 to 20.7) | Avoided | 20.4 (13.6 to 30.5) |
| Dread | 11.7 (6.7 to 20.5) | Kept Away from Others | 19.4 (13.0 to 29.1) |
| Repulsed | 10.7 (6.4 to 17.9) | Ignored | 19.4 (13.0 to 29) |
| Guilt | 9.7 (4.1 to 7.1) | Family Contacts Limited | 11.7 (6.7 to 20.4) |
| Disgust | 7.8 (3.9 to 15.6) | ||
| Disgrace | 7.8 (4.0 to 15.2) | ||
| Aesthetics | % (95%CI) | Support | % (95%CI) |
| Unkempt | 11.7 (6.7 to 20.2) | Concern | 46.6 (37.9 to 57.3) |
| Filth | 7.8 (4.1 to 14.6) | Compassion | 42.7 (34.1 to 53.5) |
| Neglected Self Care | 6.8 (3.2 to 14.5) | Willing to Assist | 35.0 (26.9 to 45.5) |
| Looks Disgusting | 5.8 (2.8 to 12.3) | Support | 33.0 (25.1 to 43.4) |
| Bad Odor | 5.8 (2.4 to 14.1) | Willing to Help | 27.8 (20.2 to 38.3) |
Note. 95%CI = 95% Confidence Interval. Results derived from Multivariable GLMs with log link that statistically controlled for prognostic study condition.
3.2 Effects of Alzheimer’s Disease Prognosis
When explicitly informed that the condition of the person with AD dementia would worsen over time, most respondents expected the person would encounter employment discrimination (78.4%, 95%CI 70.4 to 86.5; Table 3). This percentage was substantially higher than when respondents were told that the person’s condition either would improve (63.4%, 95%CI 54.4 to 72.4) or would remain unchanged (55.3%, 95%CI 45.7 to 65.0).
When informed that the condition of the person with AD dementia would worsen over time, most respondents expected the person would have his healthcare insurance limited due to data in the medical record (65.7%, 95%CI 56.4 to 75.0) or due to a brain imaging result (62.7%, 95%CI 53.3 to 72.2). Both estimates were substantially higher than when respondents were told the person’s condition would improve or remain unchanged over time, 46.6% (95%CI 36.9 to 56.3) and 45.6% (95%CI, 35.9 to 55.3) respectively.
About three-quarters of those told the condition of the person with AD dementia would worsen over time expected that the person would be excluded from medical decision-making (73.5%, 95%CI 64.9 to 82.2). This percentage was about 33% higher than that for respondents told the person’s condition would improve over time (55.3%, 95%CI 45.7 to 65.0). No statistically discernable difference was observed between respondents told the person’s condition would worsen and those told the person’s condition would remain unchanged (p>0.05).
3.3 Effects of personal characteristics, knowledge about Alzheimer’s disease dementia, and belief that Alzheimer’s disease is a mental illness
In a multivariate analysis, African American respondents were 20.6% (95%CI −40.6 to −0.6) less likely than White respondents to expect the person with AD dementia would encounter employment discrimination (Table 4). They were also less likely than White respondents to expect the person would have his healthcare insurance limited due to information in the medical record (−30.8%, 95%CI −51.8 to −9.9) or have his healthcare insurance limited due to a brain imaging result (−33.2%, 95%CI −54.3 to −12.1). In addition, they were 26.4% (95%CI −46.8 to −5.9) less likely than White respondents to expect the person would be excluded from medical decision-making.
Respondents age 50 and older were 17.4% (95%CI 6.6 to 28.1) more likely than younger adults to expect that the person with AD dementia would encounter employment discrimination. They were also more likely than those under age 50 to expect the person with AD dementia would have his healthcare insurance limited due to data in the medical record (13.7%, 95%CI 2.5 to 25.0) and to expect that he would not remember most recent events (11.5%, 95%CI 1.5 to 21.5).
Compared to those with weaker beliefs, respondents believing strongly that AD was a mental illness were more likely to expect a person with mild stage AD dementia would not remember most recent events (17.6%, 95%CI 7.5 to 27.7). They were also more likely to expect that the person would have his healthcare insurance limited due to a brain imaging result (17.4%, 95%CI 5.9 to 28.9).
4.0 Discussion
Analyses of a sample of 317 adults in the U.S. general population showed that the most common features attributed to a person with mild stage AD dementia were expecting the person would not remember most recent events (73.8%), would be discriminated against by employers (55.3%), and would be excluded from medical decision-making (55.3%). The least common attributes included expecting the person would be unkempt (11.7%), neglect self-care (6.8%), and have a bad odor (5.8%). These findings were consistent with our earlier study that found the diagnosis of AD dementia mitigates against negative judgments about a person’s aesthetic characteristics[13].
We found that almost half of respondents expected that, as a result of having an AD diagnosis, a person would have his healthcare insurance limited – because of data in the medical record (46.6%) or due to a brain imaging result (45.6%) or due to a genetic test result (44.7%). Our findings have implications for the success of the national Alzheimer’s plan’s goal of developing an effective therapy by 2025[1,2]. To achieve the ambitious goal, individuals with mild or even no symptoms of AD dementia are currently being asked to enroll in prevention trials. These candidates are being identified and enrolled into prevention trials based on results of gene and biomarker testing that places them at elevated risk for developing the disease. Our findings suggest, however, that many people expect that gene and biomarker testing could result in healthcare insurance capitations. These expectations may affect a person’s willingness to learn AD gene and biomarker test results. This could substantially impede the success of prevention trials, which are dependent, for purposes of recruitment and the underlying science, on persons undergoing gene and biomarker testing and learning those results.
Educating the public about The Genetic Information Nondiscrimination Act of 2008 (GINA) [15], which offers protections against gene based healthcare insurance discrimination, may help address some of the public’s concerns. However, our findings suggest the public’s concerns also include issues unaddressed by GINA [26,27]. For example, while GINA offers protections for genetic test results, it does not extend to all types of testing, like brain imaging results[28]. Moreover, it does not offer protections for long-term care insurance, which is often a key factor for persons undergoing AD gene and biomarker testing[29,30].
Reducing public stigma of AD is important for facilitating the success of Alzheimer’s prevention research[1,2]. Reciprocally, research advances may help to mitigate AD stigma. In randomized comparisons, we found that when told the person’s prognosis would improve over time 24% to 41% fewer respondents expected that the person would encounter discrimination or exclusion than when told the person’s prognosis would worsen (Table 3). Thus, advances in therapies that improve the prognosis of AD could help reduce stigma.
Current efforts to discover therapies for AD contrast that of “improving” impaired functioning. These innovations focus on discovering interventions – behavioral and pharmaceutical – that interrupt the underlying neuropathophysiology in persons with no or mild symptoms[31]. In turn, this would slow or prevent cognitive and physical declines. In our study, with one exception, the fact that the person’s condition would remain unchanged showed no differences compared to the current status quo, whereby the person’s condition worsened over time. Our findings suggest that how advances in the science of AD therapies are communicated— as offering improved longevity vs. retaining functioning or preventing declines— could radically affect the social and psychological experience of the disease. Studies are needed to understand how advances in diagnosis, testing, and treatment may shift AD stigma and to help position these advances as opportunities to reduce AD stigma.
We assessed whether respondents reacted differently to a person with AD dementia based on whether they self-identified as White, African American, or another race. Given that as a social group African Americans do not hold the majority’s social power, which is essential for being able to create stigma [9,32], and that they have a higher probability of direct contact with persons with AD dementia, which aids in mitigating stigma[33], we expected AD stigma would be less common among this group than White respondents. In a multivariate analysis, we found support for our hypothesis; African American respondents were less likely than White respondents to endorse all but one of the five attributions. Our findings provide pilot data that suggest further research in this area is warranted. Understanding whether these findings are replicable with larger samples and, if so, how other factors like beliefs about prognosis, moderate them may help inform efforts to address the low participation of African Americans in AD research.
Our results are consistent with prior studies that have found members of the general public can react differently toward a person with AD dementia based on their ages [14,34]. In the current study, we found older age and stronger belief that AD dementia was a mental illness was related to how respondents reacted to the person with AD dementia. These findings suggest that, in addition to ensuring appropriate policy protections exist, it may be necessary to focus on distributing information about these policies within certain population subgroups.
A strength of this study is that the sample was drawn randomly from a large national panel. This type of panel has been found to be representative of the general population[16]. Moreover, respondents were randomized to the diagnosis and prognosis categories. Selection of a random sample and the random assignment of that sample to the study categories lends to the robustness of our findings. However, our sample of 317 is small to adequately reflect the rich diversity of the large U.S. general population. We acknowledge that our sample approximates the age, gender, and race composition of the general population. It also approximates the distribution for educational obtainment when adjusted for race-based disparities. But, particularly given its size, it cannot be fully representative of the nation. Further research with large random samples of the general population is needed to derive more precise estimates and to understand how stigma of Alzheimer’s disease may differ across divergent racial, ethnic, and socioeconomic groups[34–37]. In addition, our vignette described a specific patient with symptoms of mild stage dementia. Results to date from similar studies—particularly those that have experimentally varied the gender of the vignette character— have not found appreciable differences in reactions among the general public based gender of the vignette character. However, they have found evidence that suggests a person’s judgments about social roles, such as the vignette character’s identification as a “mother” or “father” can affect how that person judge’s someone with Alzheimer’s disease[20,21,38]. It’s an area that warrants further investigation. Moreover, although we did not find statistically significant differences based upon respondent gender, our analyses may not have had sufficient statistical power to detect these differences, which ranged from 1% to 10.7% (Table 4).
Implications
Public education and policies are needed to address expectations of employment and insurance discrimination related to gene and biomarker risk data. In addition, it’s unlikely that advances in therapies will serendipitously reduce AD stigma. Studies are needed to understand how advances in diagnosis, testing, and treatment may shift AD stigma and to help position these advances as opportunities to reduce AD stigma.
Research in Context.
Survey of random sample of general public: We surveyed a random sample of the general population to learn what beliefs, attitudes, and expectations are most often associated with AD dementia. This information may help inform strategies to mitigate stigma.
Interpretation of Main Results: People expected a person with AD would be discriminated against by employers, excluded from medical decision-making, and have his health insurance limited due to documentation in the medical record, a brain imaging result, or a genetic test result. Our results suggest that the general public may need education about existing policy protections. In cases where clearly no protections exist, our results support the need for policy development.
Future directions: Our findings call for research to understand how advances in diagnosis, testing, and treatment may shift AD stigma and to help position these advances as opportunities to reduce AD stigma.
Acknowledgments
None.
Funding Sources
This study was supported by a grant from the Alzheimer’s Association (AARF-17-528934) and the Penn Neurodegenerative Disease Ethics and Policy Program and the Healthy Brain Research Center, supported in part by NIA P30 AG01024 and cooperative agreements from the Centers for Disease Control and Prevention (CDC) Prevention Research Centers Program (U48 DP 005006, 005002, 005053, 005000, and 005013). The funding sources had no involvement in the study design, collection, analysis or interpretation of data, writing of the report, or the decision to submit the article for publication.
Appendix
Figure A.
Study Flow through analysis in experiment examining stigmatizing attributions of dementia, Random Sample United States Adult Population 2013
Note. Respondents randomized to the Alzheimer ’s disease condition were included in the present analyses.
Table A.
Wording used in vignette to describe diagnosis and prognosis
| Variable | |||
|---|---|---|---|
| Alzheimer’s disease (AD) | Traumatic brain injury | No disease label | |
| Diagnosis | The doctor does a complete examination of Mr. Andrews. This includes a medical history, memory tests, lab tests, and brain imaging. Based on this information, the doctor diagnoses Mr. Andrews with Alzheimer’s disease | The doctor does a complete examination of Mr. Andrews. This includes a medical history, memory tests, lab tests, and brain imaging. Based on this information, the doctor diagnoses Mr. Andrews with traumatic brain injury. | The doctor does a complete examination of Mr. Andrews. This includes a medical history, memory tests, lab tests, and brain imaging. |
| Symptoms improve with treatment | Symptoms are static with treatment | Symptoms worsen and there’s no treatment | |
| Prognosis | The doctor tells Mr. Andrews that treatment can improve his memory problems and functional difficulties. | The doctor tells Mr. Andrews that treatment can stop the worsening of Mr. Andrews’ memory problems and functional difficulties, but the treatment will not improve these problems. | The doctor tells Mr. Andrews that there are no treatments for Mr. Andrews’ memory problems and functional difficulties and that these problems will get worse. |
Note. Respondents randomized to the conditions that conveyed the Alzheimer’s disease diagnosis were included in current analyses.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts
The authors have no conflicts to disclose.
Human participant protection
The Institutional Review Board of the University of Pennsylvania approved all procedures involving human subjects.
Contributor Information
Shana D. Stites, Department of Medical Ethics and Health Policy, Perlman School of Medicine, University of Pennsylvania.
Jonathan D. Rubright, National Board of Medical Examiners, Philadelphia PA.
Jason Karlawish, Penn Memory Center, Departments of Medicine, Medical Ethics and Health Policy, and Neurology, University of Pennsylvania.
References
- 1. [accessed March 29, 2017];National Plan to Address Alzheimer’s Disease: 2014 Update, ASPE. 2015 https://aspe.hhs.gov/pdf-document/national-plan-address-alzheimers-disease-2014-update.
- 2.Alzheimer’s Association National Plan Milestone Workgroup. Fargo KN, Aisen P, Albert M, Au R, Corrada MM, DeKosky S, Drachman D, Fillit H, Gitlin L, Haas M, Herrup K, Kawas C, Khachaturian AS, Khachaturian ZS, Klunk W, Knopman D, Kukull WA, Lamb B, Logsdon RG, Maruff P, Mesulam M, Mobley W, Mohs R, Morgan D, Nixon RA, Paul S, Petersen R, Plassman B, Potter W, Reiman E, Reisberg B, Sano M, Schindler R, Schneider LS, Snyder PJ, Sperling RA, Yaffe K, Bain LJ, Thies WH, Carrillo MC. 2014 Report on the Milestones for the US National Plan to Address Alzheimer’s Disease. Alzheimers Dement. J. Alzheimers Assoc. 2014;10:S430–452. doi: 10.1016/j.jalz.2014.08.103. [DOI] [PubMed] [Google Scholar]
- 3.Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australas. J. Ageing. 2006;25:74–79. doi: 10.1111/j.1741-6612.2006.00153.x. [DOI] [Google Scholar]
- 4.Pin Le Corre S, Scodellaro C, Arwidson P. The image of patients and caregivers in the social perception of Alzheimer’s disease - results from a literature review and a qualitative study considered with this target. Alzheimers Dement. 2009;5:P233. doi: 10.1016/j.jalz.2009.04.198. [DOI] [Google Scholar]
- 5.Werner P, Goldstein D, Buchbinder E. Subjective Experience of Family Stigma as Reported by Children of Alzheimer’s Disease Patients. Qual. Health Res. 2010;20:159–169. doi: 10.1177/1049732309358330. [DOI] [PubMed] [Google Scholar]
- 6.Van Gorp B, Vercruysse T. Frames and counter-frames giving meaning to dementia: A framing analysis of media content. Soc. Sci. Med. 2012;74:1274–1281. doi: 10.1016/j.socscimed.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 7.Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimers Dement. J. Alzheimers Assoc. 2014;10:S453–456. doi: 10.1016/j.jalz.2014.05.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connell CM, Shaw BA, Holmes SB, Foster NL. Caregivers’ Attitudes Toward Their Family Members’ Participation in Alzheimer Disease Research: Implications for Recruitment and Retention. Alzheimer Dis. Assoc. Disord. 2001;15:137–145. doi: 10.1097/00002093-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Link BG, Cullen FT, Mirotznik J, Struening E. The consequences of stigma for persons with mental illness: Evidence from the social sciences. 1992 [Google Scholar]
- 10.Corner L, Bond J. Being at risk of dementia: Fears and anxieties of older adults. J. Aging Stud. 2004;18:143–155. doi: 10.1016/j.jaging.2004.01.007. [DOI] [Google Scholar]
- 11.Werner P, Giveon SM. Discriminatory behavior of family physicians toward a person with Alzheimer’s disease. Int. Psychogeriatr. 2008;20 doi: 10.1017/S1041610208007060. [DOI] [PubMed] [Google Scholar]
- 12.Wortmann M. Dementia: a global health priority - highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 2012;4:40. doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson R, Harkins K, Cary M, Sankar P, Karlawish J. The relative contributions of disease label and disease prognosis to Alzheimer’s stigma: A vignette-based experiment. Soc. Sci. Med. 2015;143:117–127. doi: 10.1016/j.socscimed.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Stites SD, Johnson R, Harkins K, Sankar P, Xie D, Karlawish J. Identifiable Characteristics and Potentially Malleable Beliefs Predict Stigmatizing Attributions Toward Persons With Alzheimer’s Disease Dementia: Results of a Survey of the U.S. General Public. Health Commun. 2016:1–10. doi: 10.1080/10410236.2016.1255847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genetic Information Nondiscrimination Act of 2008. [accessed July 21, 2017];Natl. Hum. Genome Res. Inst. NHGRI. (n.d.). https://www.genome.gov/24519851/Genetic-Information-Nondiscrimination-Act-of-2008.
- 16.Heen MS, Lieberman JD, Miethe TD. A comparison of different online sampling approaches for generating national samples. Cent. Crime Justice Policy CCJP. 2014;1 [Google Scholar]
- 17.Rastogi S, Jones NA. Oppor. Chall. Appl. Demogr. 21st Century. Springer; Dordrecht: 2012. Overview of the 2010 Census Alternative Questionnaire Experiment: Race and Hispanic Origin Research; pp. 11–26. [DOI] [Google Scholar]
- 18.Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. The Lancet. 2002;359:966–970. doi: 10.1016/S0140-6736(02)08029-7. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br. J. Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 20.Blay SL, Peluso ÉTP. Public Stigma: The Community’s Tolerance of Alzheimer Disease. Am. J. Geriatr. Psychiatry. 2010;18:163–171. doi: 10.1097/JGP.0b013e3181bea900. [DOI] [PubMed] [Google Scholar]
- 21.Low L-F, Anstey KJ. Dementia literacy: Recognition and beliefs on dementia of the Australian public. Alzheimers Dement. 2009;5:43–49. doi: 10.1016/j.jalz.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Werner P, Goldstein D, Heinik J. Development and Validity of the Family Stigma in Alzheimer’s Disease Scale (FS-ADS) Alzheimer Dis. Assoc. Disord. 2011;25:42–48. doi: 10.1097/WAD.0b013e3181f32594. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer’s Disease Knowledge Scale: Development and Psychometric Properties. The Gerontologist. 2009;49:236–247. doi: 10.1093/geront/gnp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox D. Statistical significance tests. Br. J. Clin. Pharmacol. 1982;14:325–331. doi: 10.1111/j.1365-2125.1982.tb01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons; 2013. [Google Scholar]
- 26.Gurwitz D. Biomarkers: better donor protection. Nature. 2011;470:175–175. doi: 10.1038/470175c. [DOI] [PubMed] [Google Scholar]
- 27.Rothstein MA. Currents in Contemporary Ethics. J. Law. Med. Ethics. 2008;36:837–840. doi: 10.1111/j.1748-720X.2008.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Predicting Alzheimer’s: The Social and Policy Implications of Early Diagnosis. [accessed July 21, 2017];Stanford Journal of Public Health. (n.d.). https://www.stanford.edu/group/sjph/cgi-bin/sjphsite/predicting-alzheimer%e2%80%99s-the-social-and-policy-implications-of-early-diagnosis/
- 29.Zick CD, Mathews C, Roberts JS, Cook-Deegan R, Pokorski RJ, Green RC. Genetic Testing for Alzheimer’s Disease and its Impact on Insurance Purchasing Behavior. Health Aff. Proj. Hope. 2005;24:483–490. doi: 10.1377/hlthaff.24.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arias JJ, Karlawish J. Confidentiality in preclinical Alzheimer disease studies When research and medical records meet. Neurology. 2014;82:725–729. doi: 10.1212/WNL.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M, Fratiglioni L, Hooshmand B, Khachaturian AS, Schneider LS, Skoog I, Kivipelto M. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2014;275:229–250. doi: 10.1111/joim.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Link BG, Phelan JC. Labeling and Stigma. In: Aneshensel CS, Phelan JC, Bierman A, editors. Handb. Sociol. Ment. Health. Springer; Netherlands, Dordrecht: 2013. pp. 525–541. [DOI] [Google Scholar]
- 33.Lines L. RTI International. Racial and Ethnic Disparities Among Individuals with Alzheimer’s Disease in the United States: A Literature Review. RTI Press; 2014. [DOI] [Google Scholar]
- 34.Werner P. Social distance towards a person with Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2005;20:182–188. doi: 10.1002/gps.1268. [DOI] [PubMed] [Google Scholar]
- 35.Cheng S-T, Lam LCW, Chan LCK, Law ACB, Fung AWT, Chan W, Tam CWC, Chan W. The effects of exposure to scenarios about dementia on stigma and attitudes toward dementia care in a Chinese community. Int. Psychogeriatr. 2011;23:1433–1441. doi: 10.1017/S1041610211000834. [DOI] [PubMed] [Google Scholar]
- 36.Laforce R, McLean S. Knowledge and Fear of Developing Alzheimer’s Disease in a Sample of Healthy Adults. Psychol. Rep. 2005;96:204–206. doi: 10.2466/pr0.96.1.204-206. [DOI] [PubMed] [Google Scholar]
- 37.Woo BK, Mehta P. Examining the differences in the stigma of dementia and diabetes among Chinese Americans: Stigma of dementia and diabetes. Geriatr. Gerontol. Int. 2017;17:760–764. doi: 10.1111/ggi.12782. [DOI] [PubMed] [Google Scholar]
- 38.Wadley VG, Haley WE. Diagnostic Attributions Versus Labeling: Impact of Alzheimer’s Disease and Major Depression Diagnoses on Emotions, Beliefs, and Helping Intentions of Family Members. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2001;56:P244–P252. doi: 10.1093/geronb/56.4.P244. [DOI] [PubMed] [Google Scholar]