Abstract
Purpose
Primary central nervous system post-transplant lymphoproliferative disorder (PCNS-PTLD) is a complication of solid organ transplantation with a poor prognosis and typically associated with Epstein-Barr virus (EBV). We hypothesized EBV lytic-phase protein expression would allow successful treatment with antiviral therapy.
Experimental Design
Thirteen patients were treated with zidovudine (AZT), ganciclovir (GCV), dexamethasone, and rituximab in EBV+ PCNS-PTLD. Twice-daily, intravenous AZT 1500 mg, GCV 5 mg/kg, and dexamethasone 10 mg were given for 14-days. Weekly Rituximab 375 mg/m2 was delivered for the first four weeks. Twice-daily Valganciclovir 450 mg and AZT 300 mg started day 15. Lytic and latent protein expression was assessed using in situ hybridization and immunohistochemistry. Immunoblot assay assessed lytic gene activation. Cells transfected with lytic kinase vectors were assessed for sensitivity to our therapy using MTS tetrazolium and flow cytometry.
Results
The median time to response was 2 months. Median therapy duration was 26.5 months. Median follow-up was 52 months. The estimated two-year overall survival (OS) was 76.9% (95% CI: 44.2–91.9%). Overall response rate (ORR) was 92% (95% CI: 64–100%). BXLF1/vTK and BGLF4 expression was found in the seven tumor biopsies evaluated. Lytic gene expression was induced in vitro using the four-drug regimen. Transfection with viral kinase cDNA increased cellular sensitivity to antiviral therapy.
Conclusions
EBV+ PCNS-PTLD expressed lytic kinases and therapy with AZT, GCV, rituximab and dexamethasone provided durable responses. Induction of the lytic protein expression and increased cellular sensitivity to antiviral therapy after transfection with viral kinase cDNA provides a mechanistic rationale for our approach.
Keywords: PCNS, PTLD, EBV, AZT, Lytic infection
INTRODUCTION
The Epstein-Barr virus (EBV) is a ubiquitous, lymphotropic gammaherpes virus implicated in nasopharyngeal and gastric carcinomas and lymphoproliferative disorders1,2. Some Hodgkin’s and non-Hodgkin’s B-cell neoplasms are considered “EBV-associated” based on detection of EBV-encoded RNA in tumor tissue by in situ hybridization (EBER-ISH)3,4. Over 90% of PCNS-PTLD cases show EBV-association by immunohistochemistry5. Patients with acquired, iatrogenic, or congenital immunodeficiency are at increased risk of developing an EBV associated B-cell neoplasm6. In several preclinical models including the severe-combined immunodeficiency (SCID) mouse engrafted with human peripheral blood leukocytes, the injection of human leukocytes from a seropositive EBV donor frequently leads to EBV associated lymphoproliferative disorders (LPD) like that seen in PTLD. The spontaneous development of EBV-associated LPD can be treated or prevented with the addition of EBV-specific therapy highlighting the relevance of this virus as a driver of lymphomagenesis7–10.
PCNS-PTLD is a rare complication encountered in patients receiving iatrogenic immunosuppression after solid organ transplantation (SOT) and portends a poor prognosis with estimated three-year survival rates between 32–38% in multi-center retrospective analyses11–13. For decades, the standard treatment of care has included whole brain radiotherapy (WBRT) and methotrexate-based chemotherapy, treatments which lead to severe neurotoxicity, mucositis, myelosuppression, and leukoencephalopathy11–13. Given poor survival, prohibitive treatment toxicity, and an increasing incidence of PCNS-PTLD, better therapies are needed14.
EBV is defined by a discrete viral life cycle with primary infection, latency, and lytic reactivation phases (For a full review see15). Kinases encoded by BXLF1 (thymidine kinase) and BGLF4 (serine/threonine kinase) are expressed after initiation of the lytic phase of viral replication initiated by the BZLF1 gene16–18. Phosphorylation of nucleoside analogs, ganciclovir (GCV) and zidovudine (AZT), occurs by these lytic phase kinases, which can then inhibit viral DNA polymerase. Antiviral therapy targeting lytic gene expression is shown to be an effective treatment in some EBV-associated neoplasms19 with in vitro data showing the additive induction of apoptosis in EBV+ cell lines treated with AZT and GCV20,21. Furthermore, constitutively active lytic-phase gene expression has been demonstrated in EBV+ PCNS-PTLD and systemic PTLDs5,22,23, raising the potential for antiviral therapy as an effective therapeutic option. Roychowdhury and colleagues demonstrated the effectiveness of AZT and GCV plus WBRT in preclinical models of EBV+ PCNS-PTLD and report a patient successfully treated with AZT and GCV without radiation22.
In 1998 a phase I clinical trial of the AZT/GCV combination, based on the Harrington schedule24, opened at The Ohio State University and The University of Miami for patients with PCNS-PTLD. The original Harrington regimen was amended to eliminate the use of IL-2 in recipients of SOT and included a 2-year maintenance phase of oral AZT and GCV, which followed the intravenous 14-day “induction” phase. Unfortunately, this trial was discontinued due to difficulties with patient accrual. Prior to closure of the trial, it was observed, that a subset of patients had remarkable responses and decreased toxicity profiles. In the absence of standard of care for EBV+ PCNS-PTLD, our group continued to use this combination in patients who failed, were not eligible for, or declined primary treatment with high-dose methotrexate (HD-MTX) or radiation therapy. Since reports demonstrated improved outcomes with the use of rituximab in systemic PTLD25,26 and PCNS-PTLD despite poor blood brain barrier penetration27, it was added to our study regimen. We report 13 patients with EBV+ PCNS-PTLD treated with antivirals in combination with rituximab and dexamethasone. We evaluated tumor tissue for expression of BXLF/vTK and BGLF4. We assessed induction of the lytic gene BZLF1 after drug exposure and cellular sensitivity to our proposed antiviral therapy after transfection of BXLF1/vTK and BGLF4 cDNA.
MATERIALS AND METHODS
Eligibility
Patients with biopsy proven EBV+ PCNS-PTLD following SOT were eligible for treatment. Use of this regimen was approved by our institutional IRB. Patients were treated at the The Ohio State University between January 1998 and December 2015. Patient data was collected retrospectively from the electronic medical record. Data collected included demographic, pathology, treatment, response to treatment, treatment toxicities, and survival outcomes (Table 1). Isolated CNS involvement was confirmed by whole body computed tomography and fludeoxyglucose-positron emission tomography (FDG-PET). Patients were required to have an absolute neutrophil count greater than 1,000/mm3 and a platelet count greater than 50,000/mm3 prior to study enrollment. Patients with poor performance status (i.e. ECOG 3+), unable to consent to treatment, inability to tolerate any drug in this regimen, CNS/systemic infection, or cytopenia on presentations were excluded.
Table 1.
Clinical characteristics of 13 PCNS-PTLD patients treated with AZT, GCV, rituximab, and dexamethasone
| ID | Age | Sex | Race | Transplant | Induction | Maintenace | Prior PTLD Treatment |
EBER | Pathology | Viral Kinase Expression (N=7) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | M | W | K ('95, '11) | ATG | Cellcept/Neoral Prednisone | None | Positive | LYG III | Not Tested |
| 2 | 48 | M | W | KP | ATG | Myfortic/Rapamune | None | Positive | LYG III | Yes |
| 3 | 28 | M | W | K ('97, '04) | Cellcept ('97) ATG/Cellcept + pheresis ('04) | Cellcept ('97-'04) Cellcept/Rapamune | None | Positive | DLBCL | Yes |
| 4 | 44 | M | W | K ('77, '02) | Unk | Imuran/Cellcept Prednisone | None | Positive | DLBCL | Yes |
| 5 | 58 | M | W | K | ATG | Myfortic/Neoral | None | Positive | DLBCL | Yes |
| 6 | 77 | F | W | K | ATG | Myfortic/Neoral | None | Positive | LYG III | Yes |
| 7 | 50 | F | W | K | Cellcept | Cellcept/Neoral Predinisone/Prograf | WBRT | Positive | DLBCL | Yes |
| 8 | 61 | F | W | K | ATG | Neoral/Rapamune Prednisone | None | Positive | DLBCL | Yes |
| 9 | 61 | F | B | L | Unk | Cellcept/Neoral Prednisone | None | Unk | LYG III | Not Tested |
| 10 | 42 | M | W | KP | ATG | Myfortic/Prograf Prednisone | None | Positive | LYG III | Not Tested |
| 11 | 72 | F | W | K | ATG | Myfortic/Prograf Prednisone | None | Positive | DLBCL | Not Tested |
| 12 | 31 | M | W | K | Cellcept | Cellcept/Rapamune Prednisone | None | Positive | DLBCL | Not Tested |
| 13 | 72 | F | W | K | Unk | Prograf/Prednisone | None | Positive | DLBCL | Not Tested |
ATG, anti-thymocyte globulin; DLBCL, diffuse large b-cell lymphoma; LyG, lymphomatoid granulomatosis; K, kidney; KP, Kidney-pancreas; WBRT, whole brain radiotherapy
Medication administration
The treatment schema is outlined in Supplemental Figure 1. Immune suppressive medication was reduced in all patients prior to starting treatment for PTLD. Immune suppressive medication was reduced by half and then discontinued one-week later in accordance with recommendations provided by the transplant team. Intravenous GCV and AZT were administered at doses of 5 mg/kg twice daily and 1500 mg twice daily, respectively, along with dexamethasone 10 mg IV twice daily for the first two weeks. On day 15 patients were transitioned to maintenance oral dosing of GCV 1000 mg three times daily or valganciclovir 450 mg twice daily and AZT 300 mg twice daily. Dexamethasone was tapered over a 2-week period after the initial 2-week induction phase. Maintenance therapy was continued until disease progression or development of treatment-limiting toxicities. Maintenance therapy was held for up to 7 days for cytopenias and then restarted at the original dose if resolved. If grade III–IV hematologic toxicities were persistent patients were restarted at once daily dosing for a 50% dose reduction (see Table 2 and Supplemental table 1). GCV and AZT were dose-adjusted for pre-existing renal and hepatic insufficiency. Four doses of rituximab were administered (375 mg/m2) on days 1, 8, 15, and 22.
Table 2.
Hematologic toxicities of 13 patients treated with AZT, GCV, rituximab, and dexamethasone*
| Induction | Maintenance | |||||||
|---|---|---|---|---|---|---|---|---|
|
Grade 1 N (%) |
Grade 2 N (%) |
Grade 3 N (%) |
Grade 4 N (%) |
Grade 1 N (%) |
Grade 2 N (%) |
Grade 3 N (%) |
Grade 4 N (%) |
|
|
|
||||||||
| Anemia | 0 (0) | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 2 (15) | 2 (15) |
| Leukopenia | 3 (23) | 2 (15) | 3 (23) | 0 (0) | 0 (0) | 5 (38) | 2 (15) | 2 (15) |
| Lymphopenia | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 1 (7.7) | 1 (7.7) | 1 (7.7) | 0 (0) |
| Neutropenia | 3 (23) | 0 (0) | 1 (7.7) | 2 (15) | 3 (23) | 0 (0) | 3 (23) | 2 (15) |
| Thrombocytopenia | 3 (23) | 0 (0) | 0 (0) | 0 (0) | 3 (23) | 1 (7.7) | 0 (0) | 2 (15) |
Toxicities were determined according to the National Cancer Institute Common Terminology Criteria Version 4.0
Toxicity assessment
Toxicities were assessed during induction and maintenance therapy as outlined in the National Cancer Institute Common Toxicity Criteria version 4.0. Patients were assessed by routine blood work for hematologic and biochemical toxicities and regular follow-up appointments were scheduled for assessment of all other toxicities. Frequency of follow-up appointments was at the treating physician’s discretion and ranged from 1–3 months.
Response Assessment
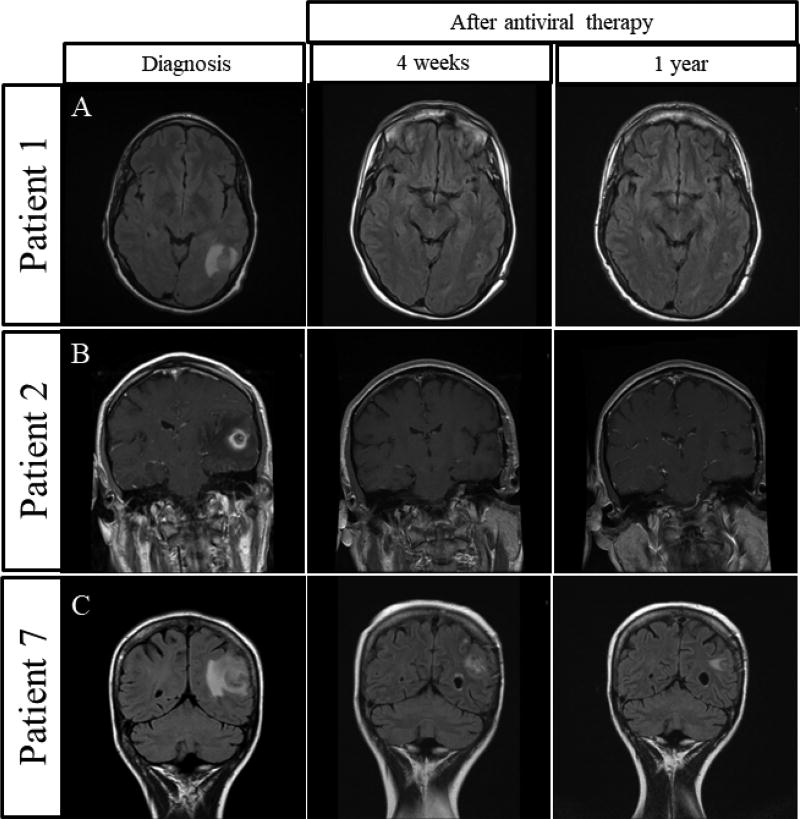
All patients were initially evaluated with magnetic resonance imaging (MRI) of the brain and PET. Response was assessed based on serial brain MRI, beginning within 4 weeks from initiation of induction treatment and reassessed every month thereafter for 4–6 months when possible. Patients then underwent CNS imaging with MRI every 3 months for the first year and followed clinically after stable findings on imaging. No MRIs were performed during steroid therapy. Measurable lesions were required to be at least 2 cm in diameter (Figure 1). Complete response (CR), partial response (PR), and progressive disease (PD) were defined using the criteria published by Cheson et al. A biopsy to confirm progression was not required; however imaging or physical exam documenting progression in accordance with Cheson criteria was required28.
Figure 1.
Three patients with complete response to AZT, GCV, rituximab, and dexamethasone and their corresponding MRIs before treatment and 4 weeks and 1 year after treatment.
In Vitro Analysis
In all cases pathology was reviewed and characterized according to World Health Organization (WHO) criteria29. For each available case, chromogenic in situ hybridization for EBER (EBER-ISH) was performed on formalin-fixed, paraffin-embedded (FFPE) tissue as described previously30. EBER positivity (EBER+) was defined as any number of neoplastic cells with nuclear staining in the 200x field. The remainder of the tissue specimen was used to assess lytic and latent protein expression characterized in Figure 2. Rabbit polyclonal antisera specific for recombinant BXLF1 was prepared as previously described31. EBV+ Akata cells treated with immobilized goat-anti-human IgG express the EBV lytic gene pathway and were used as a positive control for expression of BXLF1 and BGLF4. EBV negative Akata cells were used for a negative control.
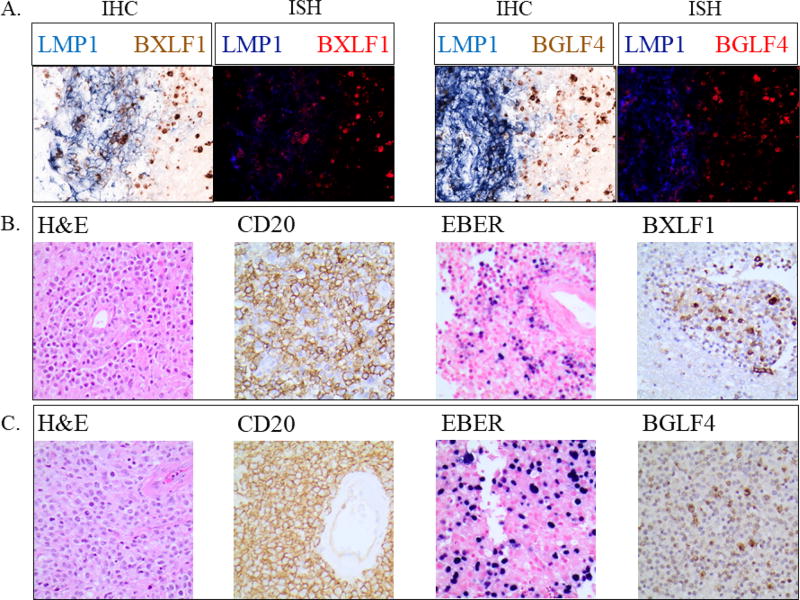
Figure 2.
Representative results from 3 patients showing immunohistochemistry (IHC) and in situ hybridization (ISH) depicting expression of latent and lytic proteins in anatomically distinct regions.
A. Representative IHC (left panels) and ISH (right panels) for latent membrane protein (LMP1) and lytic phase proteins BXLF1 and BGLF4. Results are from patient 2 diagnosed with lymphomatoid granulomatosis (grade III) and illustrate exclusive expression of latent and lytic gene products in distinct regions of the tumor infiltrate.
B/C. Immunohistochemistry showing EBV+ PCNS-PTLD biopsies from two separate patients (B: patient 6: lymphomatoid granulomatosis; C: patient 3: diffuse large B cell lymphoma) co-expressing CD20 and lytic phase protein BXLF1 and BGLF4, respectively.
Immunoblotting in Figure 3 was performed as previously described32. Antibodies for the following proteins were used: β-actin (Cell Signaling Technology, Danvers, MA), BZLF1 (Santa Cruz Biotechnology, Dallas, TX), and BXLF1/vTK (Harvard University, Boston, MA). EBV-infected and uninfected Akata cells were kindly provided by Dr. Richard Ambinder (Johns Hopkins University, Baltimore, MD) and cultured as previously described33. 293T cells in Figure 3 were grown in DMEM obtained from Life Technologies (Grand Island, NY) with fetal bovine serum (10%) obtained from Sigma (St. Louis, MO). MTS assays to measure mitochondrial function were performed using the CellTiter96 assay (Promega, Madison, WI). CMV BXLF1/vTK gene construct and vector control were kindly provided by Dr. Joyce Fingeroth (Harvard University, Boston, MA). pSG5 BGLF4 gene construct and vector control were kindly provided by Dr. Shannon Kenney (University of Wisconsin, Madison, WI). Plasmids were transfected into 293T cells by Lipofectamine 2000 (Life Technologies, Grand Island, NY) according to the manufacturer's instruction. Cells were co-transfected with pmaxGFP (Lonza, Allendale, NJ) and transfection efficiency was analyzed by flow cytometry. Cell viability was measured by flow cytometry using annexin-V-FITC and propidium iodide (PI) (BD Biosciences, San Jose, CA).
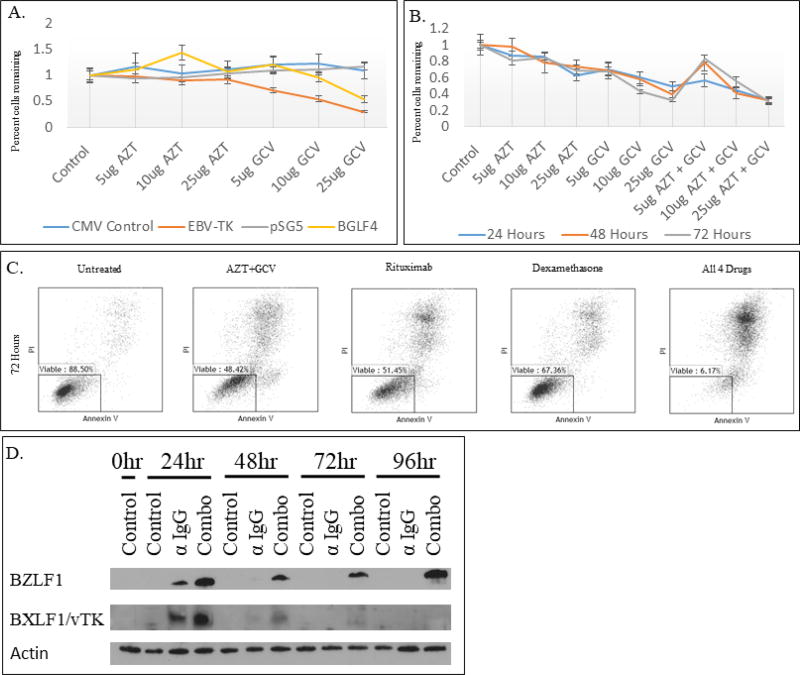
Figure 3.
MTS, flow cytometry, and western blot analysis after transfection of viral kinase vectors and exposure to combinations of AZT, GCV, rituximab, and dexamethasone.
A. 293T cells treated with AZT or GCV at 5, 10, and 25 ug/ml concentrations for 72 hours after transfection with BXLF1 (EBV-TK), BGLF4, or control vector. The y-axis represents percent of cells remaining compared to the untreated, control population. The x-axis is labeled with the varying concentrations of the two antiviral drugs.
B. 293T cells treated with AZT and/or GCV for 24, 48, or 72 hours after transfection with both the BXLF1 and BGLF4 vectors. The y-axis represents percent of cells remaining compared to the transfected, but untreated population. The x-axis is labeled with the treatment cocktail. The concentration is the concentration for both drugs if two drugs were used.
C. Akata cells were treated with 25 ug/ml AZT/GCV, 5 ug/ml rituximab, 200 nM dexamethasone, or a combination of all 4 drugs for 72 hours. Apoptosis was assessed by annexin V/PI and flow cytometry.
D. Expression in Akata cells of the lytic induction gene BZLF1 and lytic viral kinase BXLF1/vTK after exposure to the combination of AZT, GCV, rituximab, and dexamethasone. Combo = AZT (25 ug/ml), GCV (25 ug/ml), Rituximab (1 ug/ml), Dexamethasone (200 nM).
AZT, GCV and dexamethasone were obtained from Sigma (St. Louis, MO), while Rituximab was obtained from Genentech, Inc. (South San Francisco, CA). A range of concentrations for each nucleoside analogue was chosen to evaluate a dose-dependent response. Based on pharmacokinetic data reported in the package inserts GCV reaches a serum concentration of 8–10 mcg/mL and AZT a concentration of ~20 mcg/mL after typical dosing34,35. These concentrations fall within the dosing parameters of the in vitro model. AffiniPure Goat Anti-Human IgG FC Fragment Specific was obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Polyclonal Rabbit Anti-Human IgG was obtained from Dako (Carpinteria, CA).
Statistical analyses
Given the small sample size, statistical analysis was largely descriptive. Overall response rate (ORR) was defined as the proportion of patients achieving CR or PR at the time of best response, and 95% binomial exact confidence interval was provided along with the proportion. Overall survival (OS) and progression free survival (PFS) were defined as the time from start of treatment until progression or death, censoring patient without even at time of last follow-up. One patient was disease free for 10 years and then died from complications of colectomy. This event occurred at a time point later than the last follow-up of all alive patients, so it was decided to censor this patient at 10 years to avoid the potential bias generated by this single event. Estimates of PFS and OS were obtained by the Kaplan-Meier method.
RESULTS
Patient characteristics
Thirteen patients (7M, 6F) with a median age of 50 were treated from 1998–2015. Previous SOT history included kidney (N=13), pancreas (N=2), and liver (N=1). One patient had two chronologically distinct kidney transplants and two patients had simultaneous kidney/pancreas transplants for a total of 16 solid organ transplantations prior to the diagnosis of PCNS-PTLD. Two patients received a second kidney transplant after the successful treatment of PCNS-PTLD. These two kidney transplants were not included in the total number of organs transplanted. Fifty-four percent (7/13) of the patients were EBV seropositive (IgG+) prior to transplantation. As part of their SOT anti-rejection prophylaxis, patients received induction immunosuppressive therapy, which included anti-thymocyte globulin (54%), mycophenylate (23%), and other (23%). All patients were on maintenance immune suppression therapy at diagnosis of PCNS-PTLD. Maintenance immunosuppression at diagnosis is listed in Table 1 along with patient characteristics summary. PCNS-PTLD tumor histologies included diffuse large B-cell lymphoma (DLBCL) (8/13) and grade III lymphomatoid granulomatosis (LyG) (5/13).
Treatment Toxicity
Four patients required dose reduction of GCV and one required reduction of AZT during induction. Patients requiring dose reductions in induction also required reduction in maintenance treatment. Two patients required discontinuation of AZT during maintenance treatment due to persistent transfusion-dependent anemia despite holding AZT for 7 days (grade 4). One patient had AZT discontinued due to nausea and vomiting resulting in acute kidney injury. No other significant non-hematologic toxicities were seen during induction or maintenance therapy. Full summaries of hematologic toxicities and dose reductions are found in Table 2 and Supplemental Table 1 respectively.
Clinical activity
The median duration of follow-up time for all patients who were still alive at last follow-up was 52 months (range 21 – 120 months). Overall response rate (ORR) was 92% (95% CI: 64–100%). Individual responses are summarized in Table 3. Nine patients achieved a complete response with a median time to response of 2 months (range 1 – 6 months). Figure 1 shows three examples of patients who achieved CR and were disease free one year after starting maintenance therapy. Three patients had a partial response with a median time to response of 1.5 months. At final follow up, eight of thirteen patients were alive without evidence of progression while the remaining five patients had expired. Causes of death were related to septic shock (n=3) and pulmonary embolism (n=2). Four of 5 deaths occurred months (range 9–141mo) after achieving CR (n=3) or PR (n=1) and were not attributed to AZT/GCV/Rituximab/Dexamethasone therapy or disease progression. Patient 4 was disease free for 145 months, but developed pseudomonal pneumonia after total colectomy for colon cancer and succumbed to septic shock. Patient 5 died in CR after suffering a pulmonary embolism and withdrawing cardiopulmonary support. After developing a PR, patient 6 also suffered a pulmonary embolism, was stabilized in the hospital, and then opted for home hospice given advanced age, multiple comorbidities including severe emphysema, and desire to be home with family. Patient 8 died in CR from septic shock. Only patient 9 was found to have progressive disease leading to endotracheal intubation for airway protection and hospital acquired infection, sepsis and death in the ICU. This patient developed concomitant septic shock after two months of therapy. Patients 4 and 8 did not have documented leukopenias, whereas patient 9 did have leukopenia at the time of death. At two years the estimate of overall survival (OS) and progression free survival (PFS) were both 76.9% (95% CI: 44.2–91.9%) as depicted in the Kaplan-Meier curves seen in Figure 4.
Table 3.
Overall response rates and duration of response in 13 PCNS-PTLD patients treated with GCV, AZT, rituximab, and dexamethasone
| ID | Transplant- PTLD* |
Lesion Location Histology |
Radiologic Response |
Time to Response (mo.) |
Response Duration (mo.) |
Survival (cause of death) |
|---|---|---|---|---|---|---|
| 1 | 156† | FR/O LyG III | CR | 5 | 79 | Alive |
| 2 | 63 | T/P LyG III | CR | 3 | 71 | Alive |
| 3 | 156 | FR DLBCL | CR | 2 | 67.5 | Alive |
| 4 | 252† | FR DLBCL | CR | 2 | 141 | Deceased (sepsis) |
| 5 | 22 | FR DLBCL | CR | 6 | 39 | Deceased (pulmonary embolism) |
| 6 | 72 | Multifocal LyG III | PR | 1 | 18.5 | Deceased (pulmonary embolism) |
| 7 | 170 | P/pons DLBCL | CR | 2 | 59 | Alive |
| 8 | 38 | FR/P DLBCL | CR | 1 | 9 | Deceased (sepsis, DIC) |
| 9 | 162 | Multifocal LyG III | PD | NA | NA | Deceased (sepsis) |
| 10 | 74 | Multifocal LyG III | PR | 1.5 | 26.5 | Alive |
| 11 | 132 | Multifocal DLBCL | CR | 2 | 26 | Alive |
| 12 | 169 | T DLBCL | PR | 4 | 18.5 | Alive |
| 13 | 125 | Multifocal DLBCL | CR | 2 | 20 | Alive |
DLBCL, diffuse large b-cell lymphoma; LyG III, lymphomatoid granulomatosis grade III; FR, frontal lobe; T, Temporal lobe; P, Parietal lobe; O, Occipital lobe; CR, complete remission; PR, partial response; PD, progressive disease; NA, not applicable
Interval between transplant and PTLD diagnosis in months.
The interval for patients 1 and 4 is the interval from the original transplant to PTLD diagnosis
Figure 4.
Kaplan Meier curves representing overall survival (A) and progression free survival (B). At two years the OS and PFS were equal at 76.9% (95% CI: 44.2–91.9%). Patient’s censored are represented by a tick mark.
Tissue specimens
CD20 expression using L26 anti CD20 monoclonal antibody (Dako) and secondary goat-anti-mouse HRP conjugated antibody per Ohio State University pathology protocols was documented on every patient to confirm B-cell lineage. EBV positivity was documented on every patient using EBER-ISH. Evaluation of BXLF1/vTK and BGLF4 was completed in seven patients and found to be positive. Areas of tumor expressing viral kinases did not express latent membrane protein 1 (LMP1) (Figure 2).
In vitro viral kinase expression
Transfection of either BXLF1/vTK or BGLF4 conferred increased sensitivity of 293T cells to GCV and AZT as shown by a decrease in cellular proliferation at all time points tested. After treating 293T cells with AZT and GCV transfected with both BXLF1/vTK and BGLF4 vectors for 72 hours there was greater than 60% reduction in cellular proliferation (Figure 3A&B). Flow cytometry with annexin V/PI double staining was used to analyze whether transfection of lytic phase kinases led to increased apoptosis when exposed to GCV+AZT, Rituximab, dexamethasone, or a combination of all 4 drugs. The rates of apoptosis were increased in cells exposed to a combination of all 4 drugs for 48 and 72 hours compared to any drug alone at any exposure duration (Figure 3C). Akata cells were exposed to our four-drug regimen for distinct time periods which resulted in BZLF1 induction at all time periods tested (Figure 3D) and BXLF1 expression at 24 hours. The expression of BXLF1 correlated with sensitization to combination GCV+AZT, Rituximab and dexamethasone treatment (Figure 3C).
DISCUSSION
PCNS-PTLD is a rare complication of SOT with no standard treatment. The most common therapeutic options after reduction in immune suppression therapy include cytotoxic chemotherapy with high dose methotrexate (HD-MTX), with or without whole brain radiotherapy (WBRT)13. A 2013 multicenter retrospective review by Evens et al. analyzed first line treatment after reduction of immune suppression in 84 PCNS-PTLD patients over a 14 year period12. HD-MTX was given to 48% of the cohort alone or in combination with other agents. High dose cytarabine was administered to 33%, while rituximab was used alone (10%) or in combination with other agents in 45% of patients. CR was seen in 38% and PR in 21% of patients, while 32% of patients had PD. The median OS was 17 months. In another retrospective review by Cavaliere and colleagues11 spanning 25 years, 34 patients with PCNS-PTLD received systemic therapy (temozolamide, HD-MTX alone or in combination with other systemic chemotherapy, or CHOP), intrathecal chemotherapy, immunotherapy (rituximab), and radiation therapy. The authors report a 47-month median survival with all treatment outcomes combined. This exceeded a previously reported median survival of 26 months by Snanoudj et al. in patients with primary brain lymphomas after kidney transplant36. Using an antiviral approach, we observed an ORR of 92% and estimated two-year OS was 76.9%.
Our treatment regimen demonstrates a comparable OS to previously described chemotherapeutic and radiation-based therapies and may have a milder side-effect profile. Importantly, only one of thirteen patients died with PD, with other patients in this study succumbing to sepsis and thrombotic events. Using an antiviral-based regimen we avoid toxicities attributed to standard induction or chemotherapeutic regimens such as HD-MTX. HD-MTX in combination with WBRT can cause delayed leukoencephalopathy37–39, which can be progressive and in extreme cases, fatal38. In addition, HD-MTX is associated with renal injury directly related to methotrexate clearance by the kidney40–42. Aoki et al. reported a 22% reduction in creatinine clearance with HD-MTX alone in PCNSL42. These nephrogenic changes pose an increased risk in a patient population where renal health and performance status are often compromised. The risk of nephrotoxicity is much lower with AZT/GCV and was not a significant dose limiting toxicity in our patients. AZT and GCV do come with their own toxicities and are known to be myelosuppressive. However, the incidence of hematologic toxicities in our study was expected and these toxicities were typically reversible. Non-hematologic side effects were not observed during our study.
In a review by Cavaliere et al.11, eight patients received undisclosed antiviral therapy concomitantly with chemotherapy and/or radiation. Antiviral therapy efficacy could not be determined at that time given the confounding treatment modalities. This review highlights the limitations of antiviral therapy, recognizing that antivirals like GCV require phosphorylation by lytic phase kinases and have no effect on tumor cells in latently infected B-cells. There is ongoing research focused on inducing lytic phase kinases with therapies like histone deacetylase inhibitors (HDACi), steroids, radiotherapy, and other chemotherapeutic agents22,43–45. Other studies have demonstrated that a single fraction of WBRT can induce EBV lytic kinases in implanted PCNS lymphomas in rats. Subsequent treatment with AZT and GCV improved survival time compared to either therapy alone22. Arginine butyrate, an HDACi, was used in conjunction with GCV in 15 patients with refractory EBV+ lymphoid malignancies resulting in 4 CRs and 6 PRs44. In a study looking at EBV+ PCNSL, Roychowdhury and colleagues22 demonstrated that EBV thymidine kinase expression by in situ RT-PCR in one patient eliminated the need for induction therapies. They further report that this 45-year-old man with PCNS-PTLD and kinase expression in tumor tissue was successfully treated with GCV and AZT without WBRT. At the time of paper submission, the patient remained on GCV and AZT and had been disease free for 36 months. Our study expands upon this finding by reporting the expression of lytic protein (BGLF4) and thymidine (BXLF/vTK) kinases in PCNS-PTLD without induction therapies. Identification of predictive and/or prognostic biomarkers is critical to select patients for targeted treatment. Expression of these kinases provides the rationale for using AZT and GCV as primary treatment in these tumors. Constitutive lytic phase kinase activity in PCNS-PTLD suggests that we may be able to replace intensive induction or chemotherapeutic regimens in future trials with antiviral therapy. At the University of Miami, Ramos and colleagues reported dramatic responses in patients with AIDS-related PCNSL treated with AZT-based treatment21. Furthermore, AZT induced EBV lytic gene expression (BZLF1 and BXLF/vTK) followed by apoptosis in primary EBV+ lymphoma cell lines derived from AIDS patients21. Likewise, we show the lytic phase protein BZLF1 and BXLF1 kinase was induced in EBV+ Akata cells using our combination steroid, rituximab and antiviral regimen. Prior work has demonstrated that treatment of EBV+ cell lines with glucocorticoid or nucleoside analogues is capable of triggering activation of BZLF1 and downstream kinases21,31,46. Here we were able to demonstrate in two separate cell line models that combination treatment with nucleoside analogues, rituximab and dexamethasone led to maximal anti-tumor effect and lytic gene expression. This may also provide explanation of the attractive clinical activity seen in patients treated with combination therapy despite only portions of the PCNS PTLD tumors showing expression of BGLF4 and BXLF1. We would expect BGLF4 to be induced, however, we were unable to demonstrate this due to lack of an appropriate antibody. Despite this shortcoming, showing induction of lytic phase genes like BZLF1 is evidence of the effectiveness of our drug regimen despite lack of a specific induction regimen.
Primary treatment in our study included steroids and rituximab, in addition to antivirals. The question remains which adjuncts to antiviral therapy, if any, are necessary for effective treatment of PCNS-PTLD. In the aforementioned case report by Roychowdhury et al., steroids but not rituximab, were used22. The use of rituximab in PCNS-PTLD is controversial given poor CNS penetration across the blood-brain barrier27. However, case reports have shown complete remission of PCNS-PTLD with rituximab monotherapy27. Prospective, randomized controlled trials designed to evaluate the effect of rituximab on PCNS-PTLD with and without antiviral therapy are needed to delineate the role of combined versus single agent therapy.
Special consideration should be given to the patients that were successfully retransplanted after treatment of PCNS-PTLD with this regimen. The role of retransplantation after treatment of PTLD has been controversial due to the reintroduction of increased immunosuppression. In an analysis by Johnson et al.47 69 retransplants were performed after the treatment for PTLD. Conclusions from their study showed patient and graft survival were 85.5% and 73.9%, respectively. Here we include two patients, 1 and 4 from Table 1, who received a second kidney transplant after successful treatment of PCNS-PTLD. Patient 1 was still alive 4 years after transplantation and patient 4 survived another 5 years after the second transplant before dying from complications of colon cancer. Taking these cases into consideration, a prohibitive stance on retransplantation should not be the rule. Several features may predispose a patient to better outcomes following retransplantation including type of organ transplanted, time from PTLD diagnosis to retransplantation, EBV seronegativity, and type of immunosuppressive medication used47. Further study is needed to determine which patients will benefit most from retransplantation after treatment of PCNS-PTLD.
Limitations to our study include the small sample size, variability in tumor subtype, and treatment modifications due to myelosuppression. We saw approximately 20 PCNS PTLD patients over the time period of this report. Patients with poor performance status (i.e. ECOG 3+), unable to consent to treatment, inability to tolerate any drug in this regimen, CNS/systemic infection, or cytopenias on presentations were excluded. While interpretation of our results is limited by the small sample size; the excellent responses observed are notable. Additionally, we illustrate proof of concept by demonstrating the expression of viral kinases in tumor tissue and increased in vitro cellular sensitivity to antiviral therapy after viral kinase cDNA transfection. Further prospective investigation is needed to replicate our findings and justify the utility of viral kinase expression as a biomarker in EBV+ PCNSL. In our study 5/13 patients were identified as having lymphomatoid granulomatosis (LyG) grade III, which is higher than expected based on prior case reports. LyG III and DLBCL can have overlapping pathologic features and in our study tumors of both LyG (N=2) and DLBCL (N=5) expressed the viral kinases in question and both responded to antiviral therapy. Hematologic toxicity was our major limiting factor in maintenance treatment although reversible in most instances. The percentage of patients with grade 3–4 anemia or neutropenia was greater than those previously reported using HD-MTX. We observed a 30% rate of anemia and 38% rate of neutropenia compared to 0% and 20–32%42,48. Four patients required modification of their antiviral therapy and three patients ultimately had AZT discontinued due to toxicity or intolerance. Although three patients died from septic shock patient 4 and 8 had no documented leukopenias. Patient 9 did have documented leukopenias and succumbed to septic shock with progressive PCNS-PTLD. The question remains as to whether an extended duration of maintenance treatment is necessary or whether a shorter duration of treatment could be provided to induce less toxicity while preserving the durability of response.
In summary, this report highlights the efficacy of antiviral therapy as first line treatment for PCNS-PTLD and provides a mechanistic rationale with targetable viral kinase biomarkers. We observed a 92% ORR. Anti-viral therapy potentially offers a milder and reversible side effect profile when compared to other standard therapies. In this small cohort of patients, we demonstrated durable responses without disease recurrence. One patient in our cohort enjoyed disease remission for nearly 12 years. Further study of this regimen is warranted. A multi-center phase II trial is currently in development.
Supplementary Material
Statement of Translational Relevance.
Primary central nervous system post-transplant lymphoproliferative disorder (PCNS-PTLD) is a rare disorder that arises in immunosuppressed patients and is associated with poor outcomes. Treatment options include high dose methotrexate or radiation which are often poorly tolerated in this population. PCNS-PTLD is universally associated with the Epstein Barr virus and prior preclinical work has suggested that this subtype of lymphoma can express viral lytic kinase proteins that may confer sensitivity to nucleoside analogue antiviral therapy. Here we document expression of lytic kinase proteins in PCNS-PTLD tumors and demonstrate robust and sustained clinical activity and tolerability of an anti-viral based regimen delivered over a 2-week period. Supporting mechanistic laboratory data provides rationale for considering this approach in PCNS-PTLD patients. We believe this therapeutic approach to be a reasonable upfront strategy for patients who may not be candidates for high dose methotrexate.
Acknowledgments
This work was supported by the Friends of Jason Gould Foundation (Baiocchi). BMH was supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA165998.
Footnotes
Conflict of interest statement: None declared. The authors confirm that neither the submitted manuscript nor any similar manuscript, in whole or in part, other than an abstract, is under consideration, in press, or published elsewhere.
References
- 1.Ziegler J, Miner R, Rosenbaum E, Lennette E, Shillitoe E, Casavant C, et al. Outbreak of Burkitt's-like lymphoma in homosexual men. The Lancet. 1982;320:631–3. doi: 10.1016/s0140-6736(82)92740-4. [DOI] [PubMed] [Google Scholar]
- 2.Greenspan JS, Greenspan D, Lennette ET, Abrams DI, Conant MA, Petersen V, et al. Replication of Epstein–Barr Virus within the Epithelial Cells of Oral Hairy Leukoplakia, an AIDS-Associated Lesion. New England Journal of Medicine. 1985;313:1564–71. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 3.Ambinder RF. Epstein-barr virus and hodgkin lymphoma. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2007:204–9. doi: 10.1182/asheducation-2007.1.204. [DOI] [PubMed] [Google Scholar]
- 4.Rezk SA, Weiss LM. Epstein-Barr virus-associated lymphoproliferative disorders. Human pathology. 2007;38:1293–304. doi: 10.1016/j.humpath.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Fink SE, Gandhi MK, Nourse JP, Keane C, Jones K, Crooks P, et al. A comprehensive analysis of the cellular and EBV-specific microRNAome in primary CNS PTLD identifies different patterns among EBV-associated tumors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:2577–87. doi: 10.1111/ajt.12858. [DOI] [PubMed] [Google Scholar]
- 6.Penn I, Port G. Central Nervous System Lymphomas In Organ Allograft Recipients. Transplantation. 1995;59:240–4. [PubMed] [Google Scholar]
- 7.Kamel-Reid S, Dick JE. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988;242:1706–9. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- 8.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 9.Baiocchi RA, Caligiuri MA. Low-dose interleukin 2 prevents the development of Epstein-Barr virus (EBV)-associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5577–81. doi: 10.1073/pnas.91.12.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baiocchi RA, Ross ME, Tan JC, Chou CC, Sullivan L, Haldar S, et al. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood. 1995;85:1063–74. [PubMed] [Google Scholar]
- 11.Cavaliere R, Petroni G, Lopes MB, Schiff D. Primary central nervous system post-transplantation lymphoproliferative disorder. Cancer. 2010;116:863–70. doi: 10.1002/cncr.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evens AM, Choquet S, Kroll, Desrosiers AR, Jagadeesh D, Smith SM, et al. Primary CNS Posttransplant Lymphoproliferative Disease (PTLD): An International Report of 84 Cases in the Modern Era. American Journal of Transplantation. 2013;13:1512–22. doi: 10.1111/ajt.12211. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:95–102. doi: 10.1182/asheducation-2013.1.95. [DOI] [PubMed] [Google Scholar]
- 14.Lake WMD, Chang JEMD, Kennedy TMD, Morgan AMD, Salamat SMDP, Baskaya MKMD. A Case Series of Primary Central Nervous System Posttransplantation Lymphoproliferative Disorder: Imaging and Clinical Characteristics. Neurosurgery. 2013;72:960–70. doi: 10.1227/NEU.0b013e31828cf619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JI. Epstein–Barr Virus Infection. New England Journal of Medicine. 2000;343:481–92. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 16.Meng Q, Hagemeier SR, Fingeroth JD, Gershburg E, Pagano JS, Kenney SC. The Epstein-Barr Virus (EBV)-Encoded Protein Kinase, EBV-PK, but Not the Thymidine Kinase (EBV-TK), Is Required for Ganciclovir and Acyclovir Inhibition of Lytic Viral Production. Journal of Virology. 2010;84:4534–42. doi: 10.1128/JVI.02487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murata T, Kondo Y, Sugimoto A, Kawashima D, Saito S, Isomura H, et al. Epigenetic histone modification of Epstein-Barr virus BZLF1 promoter during latency and reactivation in Raji cells. J Virol. 2012;86:4752–61. doi: 10.1128/JVI.06768-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woellmer A, Arteaga-Salas JM, Hammerschmidt W. BZLF1 governs CpG-methylated chromatin of Epstein-Barr Virus reversing epigenetic repression. PLoS pathogens. 2012;8:e1002902. doi: 10.1371/journal.ppat.1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirsch JD, Stratta RJ, Sollinger HW, Hafez GR, D'Allesandro AM, Kalayoglu M, et al. Treatment of severe Epstein-Barr virus-induced lymphoproliferative syndrome with ganciclovir: Two cases of after solid organ transplantation. The American Journal of Medicine. 1989;86:241–4. doi: 10.1016/0002-9343(89)90279-9. [DOI] [PubMed] [Google Scholar]
- 20.Raez L, Cabral L, Cai JP, Landy H, Sfakianakis G, Byrne GE, Jr, et al. Treatment of AIDS-related primary central nervous system lymphoma with zidovudine, ganciclovir, and interleukin 2. AIDS research and human retroviruses. 1999;15:713–9. doi: 10.1089/088922299310809. [DOI] [PubMed] [Google Scholar]
- 21.Bayraktar UD, Diaz LA, Ashlock B, Toomey N, Cabral L, Bayraktar S, et al. Zidovudine-based lytic-inducing chemotherapy for Epstein-Barr virus-related lymphomas. Leukemia & lymphoma. 2014;55:786–94. doi: 10.3109/10428194.2013.818142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roychowdhury S, Peng R, Baiocchi RA, Bhatt D, Vourganti S, Grecula J, et al. Experimental Treatment of Epstein-Barr Virus-associated Primary Central Nervous System Lymphoma. Cancer Research. 2003;63:965–71. [PubMed] [Google Scholar]
- 23.Porcu P, Eisenbeis CF, Pelletier RP, Davies EA, Baiocchi RA, Roychowdhury S, et al. Successful treatment of posttransplantation lymphoproliferative disorder (PTLD) following renal allografting is associated with sustained CD8+ T-cell restoration. Blood. 2002;100:2341–8. doi: 10.1182/blood-2002-01-0210. [DOI] [PubMed] [Google Scholar]
- 24.Aboulafia DM, Ratner L, Miles SA, Harrington WJ, Jr Consortium AAMCT. Antiviral and immunomodulatory treatment for AIDS-related primary central nervous system lymphoma: AIDS Malignancies Consortium pilot study 019. Clinical lymphoma & myeloma. 2006;6:399–402. doi: 10.3816/clm.2006.n.017. [DOI] [PubMed] [Google Scholar]
- 25.Blaes AH, Peterson BA, Bartlett N, Dunn DL, Morrison VA. Rituximab therapy is effective for posttransplant lymphoproliferative disorders after solid organ transplantation. Cancer. 2005;104:1661–7. doi: 10.1002/cncr.21391. [DOI] [PubMed] [Google Scholar]
- 26.Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa A-M, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107:3053–7. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 27.Patrick A, Wee A, Hedderman A, Wilson D, Weiss J, Govani M. High-dose intravenous rituximab for multifocal, monomorphic primary central nervous system posttransplant lymphoproliferative disorder. Journal of Neuro-Oncology. 2011;103:739–43. doi: 10.1007/s11060-010-0425-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised Response Criteria for Malignant Lymphoma. Journal of Clinical Oncology. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow SC, Harris NLE, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. World Health Organization Classification of Tumours of the Haematopoietic and Lmphoid Tissue. IARC Press; 2008. [Google Scholar]
- 30.van Hemel BM, Suurmeijer AJ. Effective application of the methanol-based PreservCyt fixative and the Cellient automated cell block processor to diagnostic cytopathology, immunocytochemistry, and molecular biology. Diagnostic cytopathology. 2013;41:734–41. doi: 10.1002/dc.22963. [DOI] [PubMed] [Google Scholar]
- 31.Daibata M, Bandobashi K, Kuroda M, Imai S, Miyoshi I, Taguchi H. Induction of lytic Epstein-Barr virus (EBV) infection by synergistic action of rituximab and dexamethasone renders EBV-positive lymphoma cells more susceptible to ganciclovir cytotoxicity in vitro and in vivo. J Virol. 2005;79:5875–9. doi: 10.1128/JVI.79.9.5875-5879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, et al. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–903. doi: 10.1182/blood-2011-06-363879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J, Cahir-McFarland E, Zhao B, Kieff E. Virus and cell RNAs expressed during Epstein-Barr virus replication. J Virol. 2006;80:2548–65. doi: 10.1128/JVI.80.5.2548-2565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cytovene-IV (ganciclovir sodium) [package insert] Genentech USA; South San Francisco, CA: 2017. [Google Scholar]
- 35.Retrovir (zidovudine) [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2008. [Google Scholar]
- 36.Snanoudj R, Durrbach A, Leblond V, Caillard S, Hurault De Ligny B, Noel C, et al. Primary brain lymphomas after kidney transplantation: presentation and outcome. Transplantation. 2003;76:930–7. doi: 10.1097/01.TP.0000079253.06061.52. [DOI] [PubMed] [Google Scholar]
- 37.DeAngelis LM, Delattre J-Y, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 38.Schlegel U, Pels H, Oehring R, Blumcke I. Neurologic Sequelae of Treatment of Primary CNS Lymphomas. Journal of Neuro-Oncology. 1999;43:277–86. doi: 10.1023/a:1006214804736. [DOI] [PubMed] [Google Scholar]
- 39.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. International Journal of Radiation Oncology*Biology*Physics. 1980;6:1215–28. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 40.DeAngelis LM. Primary CNS Lymphoma: Treatment with Combined Chemotherapy and Radiotherapy. Journal of Neuro-Oncology. 1999;43:249–57. doi: 10.1023/a:1006258619757. [DOI] [PubMed] [Google Scholar]
- 41.Mutsando H, Fahim M, Gill DS, Hawley CM, Johnson DW, Gandhi MK, et al. High dose methotrexate and extended hours high-flux hemodialysis for the treatment of primary central nervous system lymphoma in a patient with end stage renal disease. American journal of blood research. 2012;2:66–70. [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki H, Ogura R, Tsukamoto Y, Okada M, Natsumeda M, Isogawa M, et al. Advantages of dose-dense methotrexate protocol for primary central nervous system lymphoma: comparison of two different protocols at a single institution. Neurologia medico-chirurgica. 2013;53:797–804. doi: 10.2176/nmc.oa2013-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore SM, Cannon JS, Tanhehco YC, Hamzeh FM, Ambinder RF. Induction of Epstein-Barr Virus Kinases To Sensitize Tumor Cells to Nucleoside Analogues. Antimicrobial Agents and Chemotherapy. 2001;45:2082–91. doi: 10.1128/AAC.45.7.2082-2091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrine SP, Hermine O, Small T, Suarez F, Reilly R, Boulad F, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus–associated lymphoid malignancies. Blood. 2006;109:2571–8. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004;78:1893–902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang EV, Webster Marketon JI, Chen M, Lo KW, Kim SJ, Glaser R. Glucocorticoids activate Epstein Barr virus lytic replication through the upregulation of immediate early BZLF1 gene expression. Brain, behavior, and immunity. 2010;24:1089–96. doi: 10.1016/j.bbi.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson SR, Cherikh WS, Kauffman HM, Pavlakis M, Hanto DW. Retransplantation after post-transplant lymphoproliferative disorders: an OPTN/UNOS database analysis. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:2743–9. doi: 10.1111/j.1600-6143.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 48.Roth P, Martus P, Kiewe P, Mohle R, Klasen H, Rauch M, et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology. 2012;79:890–6. doi: 10.1212/WNL.0b013e318266fcb2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.