Abstract
Purpose
To evaluate the outcomes of surgical intervention and active surveillance in patients diagnosed with cystic renal cell carcinoma at our hypothesized radiologic cutoff of >50% cystic.
Materials and Methods
We identified all patients with a pathologically confirmed cystic renal mass that fit our criteria from 2000 to 2015 (n=430). Patients with lack of CT imaging, tumors <50% cystic on imaging, multifocal tumors and prior renal cell carcinoma were excluded (n=292). Patients were stratified into benign or malignant subgroups, and radiologic, clinicopathologic and oncologic features were obtained. Univariate and multivariate associations between clinicoradiologic parameters in each group were analyzed. A separate cohort of patients managed by active surveillance for cystic renal cell carcinoma was similarly reviewed.
Results
Of the 138 cases of cystic renal cell carcinoma cases identified, 102 (73.9%) were renal cell carcinoma and 36 (26.1%) benign masses. Most tumors were Fuhrman grade 1–2 (77.5%), ≤pT2 stage (83.4%) and clear cell histology (65.9%). On univariate analysis, male gender, a solid component and increasing Bosniak classification were significant for malignancy. In a separate cohort, we identified 38 patients on active surveillance. Growth rate was found to be 1.0 mm/year overall and 2.3 mm/year for the solid component. After a median follow-up of >4 years for all cohorts, there was no evidence of recurrence or metastasis from cystic renal cell carcinoma.
Conclusions
Patients with unifocal cystic renal cell carcinoma evaluated using a standardized radiologic threshold of >50% cystic have an excellent prognosis on both active surveillance and following surgical resection.
Keywords: Carcinoma, Renal Cell, Cysts, Nephrectomy, Watchful Waiting
Introduction
Complex-cystic renal masses have long posed a challenge to clinicians, as they represent a heterogenous group of entities, both benign and malignant, that share overlapping clinical and radiologic features. Presently, the CT-based Bosniak classification system remains the gold standard utilized to predict malignancy risk in cystic renal masses and guides management and counseling 1. However, strict adherence to the Bosniak score in the absence of other diagnostic tools may lead to the surgical overtreatment of many cystic lesions2. Emerging evidence has suggested AS as an acceptable initial management strategy in this population 2–5, thus improved imaging criteria are required to help guide treatment algorithms in cystic renal masses and allow for increased implementation of AS protocols.
Historically, cRCC has been used to describe an indolent version of RCC composed predominantly of cysts 6. The threshold of cystic involvement used to define cRCC has traditionally been >75% cystic on pathologic review7, however this classification provides no contribution to preoperative decision-making. Cross-sectional imaging provides the benefit of assessing tumor morphology without surgical manipulation, allowing for an accurate assessment of the solid and cystic components and classification of cRCC 3, 8. Recent studies have evaluated radiologic criteria for cRCC diagnosis, suggesting cystic changes in 5–45% of the total mass on imaging to be associated with favorable survival 3, 8. Given the improved prognostic value that this cystic component yields 8, 9, a consistent and more inclusive classification of cRCC is essential.
Therefore, we aim to advance the preoperative assessment of cystic renal masses by evaluating cRCC as an enhancing renal lesion that is >50% cystic on cross-sectional imaging. We propose that this classification, a simple approach that can be easily be adapted into clinical practice, may yield an improved risk profile allowing for conservative management in the carefully selected patient. The objective of our study is to compare the long-term outcomes of surgery and AS in patients with cRCC using this hypothesized threshold, and to identify any clinicoradiologic parameters that may further predict malignancy risk.
Materials and Methods
Cohorts
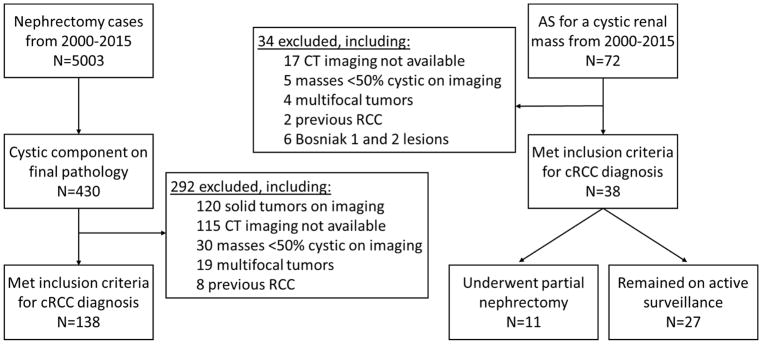
After institutional review board approval, all patients who underwent surgery for a renal mass from January 2000 to December 2015 (n=5003) were queried from a prospectively maintained institutional database. All masses that contained a cystic component on final pathology were identified (n=430). From this cohort we identified 138 cases that met our inclusion criteria (Figure 1).
Figure 1.
Summary of our study cohort and flow chart of exclusion criteria
A separate cohort of 72 complex-cystic masses initially managed by AS from January 2000 to December 2015 was also identified. AS protocols consisted of initial cross-sectional imaging followed by US every 6 months for the first year with annual US studies used thereafter if no growth was present. US studies were included as a part of our follow-up protocol but not for our radiologic assessment. Our final AS cohort consisted of 38 cases (Figure 1). Core biopsies were not utilized given that they are known to have a low diagnostic yield in cystic renal masses 10.
Radiologic review
CT imaging was re-reviewed by one of three genitourinary radiologists (AH, CD, OA), who were blinded to clinical and pathologic characteristics. The solid component of the entire tumor was measured and visually graded into four groups: none, <25%, 25–50% or >50%. Additional imaging characteristics were also recorded, such as the Bosniak classification, maximal diameter (cm), tumor location (>50% exophytic, <50% exophytic, endophytic) and the presence of calcifications (yes/no), septations (yes/no), and other cysts (yes/no).
In our AS cohort, we evaluated the growth rate of the entire lesion calculated by maximal diameter in the first and last CT scan when available. In those patients with a measurable solid component, growth rate of the solid component was also calculated.
Outcomes
Clinical, surgical, radiologic, pathologic 11, and post-operative outcomes were analyzed. Based on final pathology, patients were stratified into either a RCC or benign tumor subgroup. The benign subgroup consisted of mixed epithelial and stromal tumor family, benign multilocular cysts and multilocular cystic renal neoplasm of low malignant potential. In a subset of patients with Bosniak 3 and 4 lesions, associations between the solid component percentage and malignancy risk were similarly assessed. Follow-up was based on last available CT.
Statistical analysis
Univariate analyses of clinical, radiologic and pathologic parameters were evaluated using Fisher’s exact test and student’s t-test. For multivariate analysis, we fitted a logistic model with 5 covariates (sex, Bosniak classification, solid component, solid percentage, calcifications) that we found to be significant in our univariate analysis. Survival analyses were done by constructing Kaplan-Meier curves and log-rank tests. The pre-rejection threshold for all statistical tests was predefined at 0.05. All analyses were performed with the R platform.
Results
Clinicopathologic features
Clinicopathologic and surgical characteristics of our initial cohort of 138 cRCC cases are summarized in Table 1. On pathologic review, 102 (73.9%) were RCC and 36 (26.1%) were benign tumor subtypes. The majority had less aggressive features, presenting with Fuhrman grade 1–2 (77.5%) and ≤pT2 stage (83.4%). Our cohort also had various histopathologic subtypes, with ccRCC (65.9%) being the most common followed by tumors of benign etiology (26.1%). When comparing gender between subgroups, we found that males were more likely to have cystic masses with malignant histology (p=0.006). At a median of follow-up of 5.4 years (IQR 2.8–7.8), there was no evidence of tumor recurrence or metastasis from cRCC. A total of 7 (5.1%) patients died from other causes.
Table 1.
Clinicopathologic and surgical characteristics of cRCC patients in our surgical cohort
| No. gender (%) | |
| Male | 78 (57) |
| Female | 60 (43) |
| No. race (%) | |
| White | 132 (96) |
| Black | 2 (1) |
| Asian | 4 (3) |
| Median age at surgery, years (IQR) | 54.1 (45.5–63.9) |
| No. surgical technique (%) | |
| Partial | 122 (88.4) |
| Radical | 16 (11.6) |
| Open | 113 (81.9) |
| Laparoscopic | 10 (7.2) |
| Robotic | 15 (10.9) |
| No. presenting symptom (%) | |
| Incidental, asymptomatic | 122 (88.4) |
| Local | 16 (11.6) |
| No. histologic subtype (%) | |
| Clear cell renal cell carcinoma | 91 (65.9) |
| Multilocular cystic renal cell neoplasm of low malignant potential | 18 (13) |
| Benign multilocular cyst | 16 (11.6) |
| Papillary renal cell carcinoma | 5 (3.6) |
| Clear cell capillary renal cell carcinoma | 4 (2.9) |
| Unclassified renal cell carcinoma | 2 (1.4) |
| Mixed epithelial and stromal tumor family | 2 (1.4) |
| No. Fuhrman grade (%) | |
| 1 | 22 (15.9) |
| 2 | 85 (61.6) |
| 3 | 9 (6.5) |
| NA | 22 (15.9) |
| No. pathologic stage (%) | |
| pT1a | 97 (70.3) |
| pT1b | 15 (10.9) |
| pT2a | 3 (2.2) |
| pT3a | 4 (2.9) |
| pT3b | 1 (0.7) |
Radiologic and pathologic features
On imaging re-review, the median overall maximal tumor size preoperatively was 3.3 cm (IQR 2.2–4.7). Most cRCC lesions were Bosniak 3 and 4 (93.5%) with a solid component of <25% (83.3%). Figure 2 depicts representative imaging.
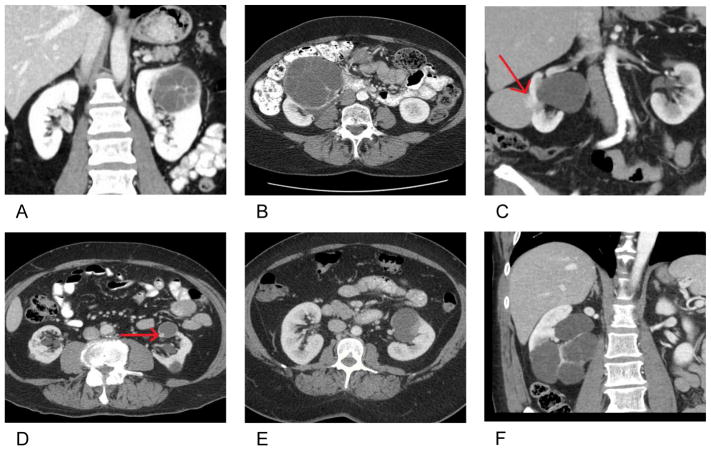
Figure 2. Radiologic representation of cRCC in various histologic subtypes.
A) Clear cell renal cell carcinoma (RCC), B) Multilocular cystic renal cell neoplasm of low malignant potential, C) Papillary RCC, D) Clear cell papillary RCC, E) Unclassified RCC and F) Benign tumor subtype
Table 2 summarizes the associations of radiologic features between RCC and benign tumor subgroups. The presence of any solid component on imaging (p=0.01), solid component percentage (p=0.01), median solid component size (p=0.004) and calcifications (p=0.02) were all found to be significant between the two subgroups.
Table 2.
Comparison of clinical, surgical and radiologic characteristics between pathologically confirmed renal cell carcinoma and benign tumor subtypes in our surgical cohort
| Renal Cell Carcinoma n=102 | Benign Tumors* n=36 | P-value | |
|---|---|---|---|
| No. sex (%) | |||
| Male | 65 (64) | 13 (36) | 0.006 |
| Female | 37 (36) | 23 (64) | |
| No. race (%) | |||
| White | 98 (96) | 34 (94) | 0.77 |
| Black | 1 (1) | 1 (3) | |
| Asian | 3 (3) | 1 (3) | |
| No. presenting symptom (%) | |||
| Incidental, asymptomatic | 88 (86.3) | 34 (94.4) | 0.24 |
| Local | 14 (13.7) | 2 (5.6) | |
| Median age at surgery (IQR) | 53.6 (44.9–63.7) | 55.6 (46.7–64.6) | 0.50 |
| No. surgical technique (%) | |||
| Partial | 91 (89.2) | 31 (86.1) | 0.76 |
| Radical | 11 (10.8) | 5 (13.9) | |
| Open | 84 (82.4) | 29 (80.6) | 0.93 |
| Laparoscopic | 7 (6.9) | 3 (8.3) | |
| Robotic | 11 (10.8) | 4 (11.1) | |
| Median radiologic tumor size, cm (IQR) | 3.3 (2.3–4.7) | 2.9 (2.0–4.9) | 0.39 |
| No. solid component (%) | |||
| Yes | 58 (56.9) | 11 (30.6) | 0.01 |
| No | 44 (43.1) | 25 (69.4) | |
| No. solid component (%) | |||
| 25–50% | 18 (17.6) | 5 (13.9) | 0.01 |
| <25% | 40 (39.2) | 6 (16.7) | |
| No measurable solid component | 44 (43.1) | 25 (69.4) | |
| Median solid component size, cm (IQR) | 0.8 (0.6–1.2) | 0.6 (0.5–0.9) | 0.004 |
| No. Bosniak Classification (%) | |||
| 4 | 49 (48) | 10 (27.8) | <0.0001 |
| 3 | 52 (51) | 18 (50) | |
| 2F | 1 (1) | 8 (22.2) | |
| No. tumor location (%) | |||
| >50% Exophytic | 80 (78.4) | 26 (72.2) | 0.44 |
| <50% Exophytic | 7 (6.9) | 5 (13.9) | |
| Endophytic | 15 (14.7) | 5 (13.9) | |
| No. presence of calcifications (%) | |||
| Yes | 17 (16.7) | 13 (36.1) | 0.02 |
| No | 85 (83.3) | 23 (63.9) | |
| No. presence of septations (%) | |||
| Yes | 92 (90.2) | 31 (86.1) | 0.54 |
| No | 10 (9.8) | 5 (13.9) | |
| No. presence of other cysts (%) | |||
| Yes | 57 (55.9) | 19 (52.8) | 0.85 |
| No | 45 (44.1) | 17 (47.2) | |
Benign tumor subtypes include multilocular cystic renal cell neoplasm of low malignant potential, mixed epithelial and stromal tumor family, and benign multilocular cysts
On multivariate analysis, we found males to have a significantly higher risk of having malignant disease (p=0.007) and that Bosniak 3 lesions were more likely to be malignant (p=0.01). Although Bosniak 4 lesions also showed an increased malignancy risk, this did not reach statistical significance in our multivariate model (p=0.29).
Bosniak 3 and 4
In a subset of patients with Bosniak 3 and 4 lesions (Table 3), we found no significant difference between the RCC and benign tumor subgroups when comparing the solid component percentage (p=0.12). Even when comparing these differences in Bosniak 3 and 4 lesions individually, we still found no significant difference between the 2 groups (p=0.25 and p=0.71, respectively).
Table 3.
Comparison of solid component percentage and malignancy risk in a subset of patients with Bosniak 3 and 4 lesions in our surgical cohort
| Radiologic Solid Component % | Bosniak 3 & 4 | Bosniak 3 | Bosniak 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Renal Cell Carcinoma | Benign Tumors | P-value | Renal Cell Carcinoma | Benign Tumors | P-value | Renal Cell Carcinoma | Benign Tumors | P-value | |
|
|
|
|
|||||||
| Purely cystic | 43 | 18 | 0.12 | 43 | 18 | 0.25 | 0 | 0 | 0.71 |
|
| |||||||||
| <25% | 40 | 6 | 6 | 0 | 34 | 6 | |||
|
| |||||||||
| 25–50% | 18 | 4 | 3 | 0 | 15 | 4 | |||
Active surveillance cohort
Overall, 27 (71.1%) remained on AS and 11 (28.9%) went on to receive surgery, of which all underwent partial nephrectomy. The median overall growth rate was 1.0 mm/year (IQR 0–2.8) over 25.3 months (IQR 16.3–44.8). In 15 (39.5%) lesions with a solid component, the growth rate of the solid portion was 2.3 mm/year (IQR 0.3–4.9). In patients who either remained on AS or ultimately underwent surgery, there was no evidence of recurrence or metastasis from cRCC at a median follow-up of 4.3 years (IQR 2.1–5.7) from first imaging diagnosis or 6.9 years (IQR 4.9–8.5) after surgery, respectively. A total of 8 (21.1%) patients died from other causes.
Of the AS cases that underwent delayed surgical intervention, AS was initially chosen due to favorable tumor characteristics on imaging (63.6%) or significant medical comorbidities (54.5%). A change in tumor character (45.4%) and patient preference to no longer remain on AS (18.2%) were noted indications for surgery. On imaging, most lesions were Bosniak 4 (63.6%) with the presence of a solid component (54.5%). On pathologic review, the majority were ccRCC (63.6%), pT1 stage (54.5%) and Fuhrman grade ≤2 (63.6%).
Surgery vs. active surveillance cohort
Table 4 summarizes the associations of clinicoradiologic features between patients initially managed with surgery and AS. The median age at diagnosis was significantly higher in the AS subgroup (p=<0.0001). Bosniak classification (p=<0.0001), solid component percentage (p=0.01) and median solid component size (p=0.004) were also found to be significantly different between the subgroups, however median overall tumor size was not (p=0.59).
Table 4.
Comparison of clinical and radiologic characteristics between patients initially managed with surgical intervention and active surveillance in our overall cohort
| Surgical Intervention n=138 | Active Surveillance n=38 | P-value | |
|---|---|---|---|
| No. sex (%) | |||
| Male | 78 (57) | 21 (55) | 1.0 |
| Female | 60 (43) | 17 (45) | |
| Median age at diagnosis (IQR) | 54.0 (45.4–64.2) | 67.5 (61.6–73.1) | <0.0001 |
| Median radiologic tumor size, cm (IQR) | 3.3 (2.2–4.7) | 3.1 (2.3–4.5) | 0.59 |
| No. solid component (%) | |||
| Yes | 69 (50.0) | 13 (34.2) | 0.09 |
| No | 69 (50.0) | 25 (65.8) | |
| No. solid component (%) | |||
| 25–50% | 23 (16.7) | 8 (21.1) | 0.01 |
| <25% | 46 (33.3) | 5 (13.2) | |
| No measurable solid component | 69 (50.0) | 25 (65.8) | |
| Median solid component size, cm (IQR) | 0.8 (0.6–1.1) | 1.5 (1.4–3.3) | 0.004 |
| No. Bosniak Classification (%) | |||
| 4 | 59 (42.8) | 9 (23.7) | <0.0001 |
| 3 | 70 (50.7) | 12 (31.6) | |
| 2F | 9 (6.5) | 17 (44.7) | |
| No. tumor location (%) | |||
| >50% Exophytic | 106 (76.8) | 28 (73.7) | 0.01 |
| <50% Exophytic | 12 (8.7) | 9 (23.7) | |
| Endophytic | 20 (14.5) | 1 (2.6) | |
| No. presence of calcifications (%) | |||
| Yes | 30 (21.7) | 7 (18.4) | 0.82 |
| No | 108 (78.3) | 31 (81.6) | |
| No. presence of septations (%) | |||
| Yes | 123 (89.1) | 28 (73.7) | 0.03 |
| No | 15 (10.9) | 10 (26.3) | |
| No. presence of other cysts (%) | |||
| Yes | 76 (55.1) | 26 (68.4) | 0.19 |
| No | 62 (44.9) | 12 (31.6) | |
When comparing patients who initially underwent surgery with those who were initially managed with AS followed by surgery, we found no significant difference between the proportion of tumors with malignant histology (p=0.9). Furthermore, when looking at overall-survival between the same subgroups, we also found no significant difference (p=0.07). Overall, no patients in our entire cohort died of kidney cancer.
Discussion
In this study, we evaluated cRCC as a radiologically distinct subtype of RCC defined by an enhancing renal mass that is >50% cystic on cross-sectional imaging. At this hypothesized threshold, we found that a solitary cRCC lesion in patients with no prior history of RCC has an excellent prognosis on both AS and following surgical resection.
The excellent prognosis of patients with cRCC compared to ccRCC has been recognized in several previous reports, showing that they present with less aggressive pathologic features and have minimal risk of recurrence or metastasis following surgery 3, 9. Our study consisted of 5 (3%) patients with pT3 disease, like a recent study reporting pT3 disease in 4% of their cohort 12. Even at higher stage and grade, patients with cRCC have been shown to have better outcomes compared to ccRCC 12. Given that these lesions are predominantly cystic, the solid portions of these tumors are often significantly smaller than the size of the entire lesion3, 8. The median overall tumor size for cRCC cases in our cohort was 3.3 cm, whereas the median solid component size was 0.8 cm. The solid portion is thought to contain the malignant components of these lesions, thus the tumor burden is often significantly less than other tumors of similar size 3, 8.
Similar to other reports, ccRCC was the most common histology in our cohort followed by tumors of benign etiology 3, 13. Additionally, no patients presented with chromophobe RCC 3, 8; however, two (1.6%) patients were diagnosed with unclassified RCC, which has not been documented previously in the literature. It has been well established that differentiation of benign and malignant complex-cystic masses by current imaging techniques is difficult, thus any radiologic definition for cRCC will also ultimately include benign tumors and other RCC subtypes.
In a recent study investigating the pathologic and radiologic correlates of complex renal cysts, the authors found that the likelihood of malignancy increased with a rising Bosniak score and the presence of mural nodules 14. We found a similar trend in our cohort. In addition, we also found that females with cRCC lesions were more likely to have benign pathology (p=0.006), which remained true in our multivariate model (p=0.007). Female gender is associated with a higher probability of benign renal neoplasms overall 15, thus gender may need to be implemented into future diagnostic protocols for cRCC. However, further analyses need to be conducted.
Although it is well established that Bosniak 4 lesions have an increased malignancy risk compared to Bosniak 3 2, a Bosniak 4 classification did not reach statistical significance in our multivariate model. We attribute this discordant finding to the overlap between the defining criteria of Bosniak 4 lesions2 and covariates which indicate the presence of a solid component on imaging. Since one of the main criteria to categorize a mass as Bosniak 4 is the evidence of a solid component 1, 2, adding this covariate to the multivariate model results in an overlap of cases and a consequent loss of the association between Bosniak score and malignancy.
In a recent study assessing preoperative growth in cystic renal lesions, the authors found that most tumors (73%) did not show a significant increase in size 13. In a similar analysis of our AS cohort, we found the median overall growth rate to be 1.0 mm/year. This compared to a mean growth rate of 2.8 mm/year in a meta-analysis of patients with solid renal tumors on AS 16. Although we also noted the solid portion growth rate to be 2.3 mm/year, this was only in 15 (39.5%) patients and may not be enough to appropriately quantify growth. Given the slow growth rate of cRCC lesions and their more indolent nature, radiologic diagnosis of cRCC allows for AS to be more confidently discussed with appropriately selected patients.
In patients undergoing surgery for a solitary Bosniak 3 or 4 cRCC lesion, partial nephrectomy is recommended when technically feasible4. Similarly, most patients in our study underwent partial nephrectomy (90%). Current post-operative surveillance guidelines for RCC are predicated on pathologic TNM staging 11, with more intensive surveillance recommended for high stage disease 17–19. With the improved outcomes that have been demonstrated in patients with cRCC 3, 9, follow-up care could be optimized and less frequent and aggressive observation could be recommended following surgery with reliance on simple chest imaging and renal US. This could potentially lower health care costs and reduce patient anxiety.
Although most patients with Bosniak 3 and 4 complex-cystic renal masses undergo surgery, AS can be an acceptable initial approach 4. Recent guidelines on the management of localized renal cancer state that AS can be initially implemented in patients with Bosniak 3 and 4 complex-cystic lesions who have a limited life expectancy, are at higher risk for surgical complications, or have favorable tumor characteristics on imaging 4, 20. Our data showed that management initially by AS followed by surgery had no difference in either the proportion of malignancy or overall-survival compared to patients initially managed with surgery. Given that cRCC lesions have a smaller overall size, smaller solid component and lower oncologic risk compared to similar RCC masses, initial AS strategies should be considered 9.
There are several limitations to our study, including its retrospective design, limited follow-up and relatively small cohort. We chose to exclude patients with a history of RCC or other renal lesions to focus exclusively on solitary cystic masses. The cutoff we implemented of >50% cystic on imaging was hypothesis generated and will need to be validated in independent cohorts. We were not able to assess growth rate in our original cRCC cohort given that the majority did not have 2 preoperative CT scans available. In addition, given that patients on AS for cystic renal masses will often be followed with serial US instead of CT, we could only assess growth rate and radiologic characteristics from last available CT. We also could not comment on the incidence of these lesions given that we identified our cohort on retrospective pathology review initially followed by radiologic review, therefore any lesion that did not contain a cystic portion on pathology would not have been included.
Conclusions
Our data suggests that patients with unifocal cRCC evaluated using a standardized radiologic threshold of >50% cystic have an excellent prognosis on both AS and following surgery. In patients with serial imaging, we also noted a similar interval growth rate of cRCC lesions compared to solid renal tumors. We believe that our radiologic definition allows for more inclusive criteria of cRCC and would encourage kidney sparing approaches or implementation of AS protocols when feasible.
Supplementary Material
Acknowledgments
Funding: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and an NIH/NCI Cancer Center Support Grant (P30 CA008748). MG, AS, and BM were supported by the Ruth L. Kirschstein National Research Service Award T32CA082088. JC was sponsored by the German Research Foundation (DFG) Grant CA1403/1-1.
Abbreviations and Acronyms
- AS
active surveillance
- ccRCC
clear cell renal cell carcinoma
- cRCC
cystic renal cell carcinoma
- CT
computerized tomography
- IQR
interquartile range
- RCC
renal cell carcinoma
- US
ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 2.Schoots IG, Zaccai K, Hunink MG, et al. Bosniak Classification for Complex Renal Cysts Reevaluated: A Systematic Review. J Urol. 2017;198:12. doi: 10.1016/j.juro.2016.09.160. [DOI] [PubMed] [Google Scholar]
- 3.Park JJ, Chang Jeong B, Kim CK, et al. Postoperative Outcome of Cystic Renal Cell Carcinoma Defined on Preoperative Imaging: A Retrospective Study. J Urol. 2016 doi: 10.1016/j.juro.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 4.Richard PO, Violette PD, Jewett MA, et al. CUA guideline on the management of cystic renal lesions. Can Urol Assoc J. 2017;11:E66. doi: 10.5489/cuaj.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekar T, Ahmad AE, Fadaak K, et al. Natural History of Complex Renal Cysts: Clinical Evidence Supporting Active Surveillance. J Urol. 2017 doi: 10.1016/j.juro.2017.09.078. [DOI] [PubMed] [Google Scholar]
- 6.Hartman DS, Davis CJ, Jr, Johns T, et al. Cystic renal cell carcinoma. Urology. 1986;28:145. doi: 10.1016/0090-4295(86)90109-3. [DOI] [PubMed] [Google Scholar]
- 7.Corica FA, Iczkowski KA, Cheng L, et al. Cystic renal cell carcinoma is cured by resection: a study of 24 cases with long-term followup. J Urol. 1999;161:408. doi: 10.1016/s0022-5347(01)61903-7. [DOI] [PubMed] [Google Scholar]
- 8.Huber J, Winkler A, Jakobi H, et al. Preoperative decision making for renal cell carcinoma: cystic morphology in cross-sectional imaging might predict lower malignant potential. Urol Oncol. 2014;32:37.e1. doi: 10.1016/j.urolonc.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Winters BR, Gore JL, Holt SK, et al. Cystic renal cell carcinoma carries an excellent prognosis regardless of tumor size. Urol Oncol. 2015;33:505.e9. doi: 10.1016/j.urolonc.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60:578. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt JR, Jewett MA, Richard PO, et al. Multilocular Cystic Renal Cell Carcinoma: Pathological T Staging Makes No Difference to Favorable Outcomes and Should be Reclassified. J Urol. 2016;196:1350. doi: 10.1016/j.juro.2016.05.118. [DOI] [PubMed] [Google Scholar]
- 13.Jhaveri K, Gupta P, Elmi A, et al. Cystic Renal Cell Carcinomas: Do They Grow, Metastasize, or Recur? American Journal of Roentgenology. 2013;201:W292. doi: 10.2214/AJR.12.9414. [DOI] [PubMed] [Google Scholar]
- 14.Reese AC, Johnson PT, Gorin MA, et al. Pathological characteristics and radiographic correlates of complex renal cysts. Urol Oncol. 2014;32:1010. doi: 10.1016/j.urolonc.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violette P, Abourbih S, Szymanski KM, et al. Solitary solid renal mass: can we predict malignancy? BJU Int. 2012;110:E548. doi: 10.1111/j.1464-410X.2012.11245.x. [DOI] [PubMed] [Google Scholar]
- 16.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 17.Donat SM, Diaz M, Bishoff JT, et al. Follow-up for Clinically Localized Renal Neoplasms: AUA Guideline. J Urol. 2013;190:407. doi: 10.1016/j.juro.2013.04.121. [DOI] [PubMed] [Google Scholar]
- 18.Jewett M, Finelli A, Kollmannsberger C, et al. Management of kidney cancer: canadian kidney cancer forum consensus update 2011. Can Urol Assoc J. 2012;6:16. doi: 10.5489/cuaj.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:175. doi: 10.6004/jnccn.2014.0018. [DOI] [PubMed] [Google Scholar]
- 20.Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol. 2017;198:520. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.