Abstract
Purpose
Radiation may have significant immuno-modulatory effects that impact tumor response and could potentiate immunotherapeutic approaches. The purpose of this study was to prospectively investigate circulating lymphoid cell population (CLP) fractions during hypofractionated proton therapy (HPT) in blood samples of liver cancer patients, and to explore their association with survival.
Methods and Materials
We collected serial blood samples pre-treatment and at days 8 and 15 of HPT from 43 liver cancer—22 hepatocellular carcinoma (HCC) and 21 intrahepatic cholangiocarcinoma (ICC) —patients enrolled in a phase II clinical trial. All patients received 15 fractions of proton therapy to a median dose of 58 Gy (RBE). We used flow cytometry to measure the changes in the fractions of total CD3+, CD4+ and CD8+ T cells, CD4+CD25+ T cells, CD4+CD127+ T cells, CD3+CD8+CD25+ activated cytotoxic T lymphocytes (CTLs) and CD3–CD56+ natural killer (NK) cells.
Results
With a median follow-up of 42 months, median overall survival (OS) in the study cohort was 30.6 months for HCC and 14.5 months for ICC patients. Longer OS was significantly correlated with greater CD4+CD25+ T cell (p=0.003) and CD4+CD127+ T cell (p=0.01) fractions at baseline only in ICC patients. In HCC patients, the fraction of activated CTLs mid-treatment (at day 8) was significantly associated with OS (p=0.007). These findings suggest a differential relevance of immuno-modulation by HPT in these liver cancers.
Conclusion
Anti-tumor immunity may depend on maintenance of a sufficiently high number of activated CTLs during HPT in HCC patients, and CD4+CD25+ T cells and CD4+CD127+ T cells prior to treatment in ICC patients. These results could guide the design of future studies to determine the optimal treatment schedules when combining radiation with specific immunotherapy approaches.
Keywords: Immune response, proton therapy, liver cancer
Introduction
There is emerging data on the immunomodulatory effects of radiation associated with anti-tumor immunity, and potential synergistic effects with certain immunotherapeutic approaches (1). Radiation, particularly when delivered over protracted courses, can also cause rapid depletion of circulating lymphoid cell populations (CLPs) (2). Moreover, these responses may significantly vary between individuals and CLP type (3). Despite the recognition of a differential impact of treatment fractionation and prescription dose on immune cells (4), little is known about the effects of proton therapy in various tumors and their impact on treatment outcomes. The goal of this study was to evaluate key effector and suppressor CLPs (5) during hypofractionated proton therapy (HPT) in hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) patients, and to investigate their association and survival.
Materials and Methods
The patient cohort includes the participants enrolled at one institution in a multi-site phase II clinical trial (NCTXXXXXXXX) that investigated the safety and efficacy of high-dose HPT in HCC and ICC patients. The protocol was reviewed and approved by local institutional IRBs, for patient details see Ref. (6) and Supplementary Material. Patients received 15 fractions to a maximum total dose of 67.5Gy-equivalent, with the relative biological effectiveness set at 1.1 per institutional guidelines (6). Dose de-escalation was permitted to maintain the normal liver mean dose below 24Gy-equivalent. We analyzed CLP fractions in patients with biopsy-confirmed HCC (n=22) and ICC (n=21) by flow cytometric analysis after immunostaining peripheral mononuclear cells in freshly drawn samples using an LSR-II cytometer (BD) (7). We evaluated the fractions of total CD3+ CLPs, CD4+ and CD8+ T cells, CD4+CD25+ T cells, CD4+CD127+ T cells, CD3+CD8+CD25+ activated cytotoxic T lymphocytes (CTLs) and CD3–CD56+ natural killer (NK) cells in serial fresh blood samples collected at days 1 (baseline), 8 and 15 of HPT (see gating strategy in Supplementary Figure S1). We examined the changes in CLP fractions during treatment, their differences between HCC and ICC patients, and their associations with OS. Wilcoxon rank-sum test were used for comparisons, Wilcoxon signed-rank for changes over time, and log-rank tests for association with OS.
Results and Discussion
Table 1 lists the patient characteristics for the HCC and ICC cohorts. The only significant difference between the HCC and ICC patients was the higher proportion of males in the HCC group, consistent with the known predominance of this disease in males. Data were analyzed with a median follow-up of 42 months (43 months for the HCC patients and 36 months for the ICC cohort), when 9 patients were still alive. Figure 1 shows the OS distributions in the two cohorts: median OS was 30.6 months for HCC and 14.5 months for ICC patients. These results compare favorably to historical data (8).
Table 1. Patient Characteristics.
Data were collected from all HCC and ICC patients enrolled at XXX in NCTXXXXXX. p-values are from log-rank p statistics for OS and from Mann-Whitney-U test for the rest. Values in parentheses designate range.
| Characteristics | Hepatocellular Carcinoma | Intrahepatic Cholangiocarcinoma | P value |
|---|---|---|---|
| Patients (n) | 22 | 21 | |
| Age median (years) | 69.5 [54–88] | 66.4 [36–82] | 0.063 |
| Male/Female | 19/3 | 10/11 | 0.003 |
| No cirrhosis/CTP1 Class A/B | 3/16/3 | 1/17/3 | 0.27 |
| CLIP2 score 0-1/2-3 | 16/6 | 19/2 | 0.13 |
| TVT3 yes/no | 9/13 | 9/12 | 0.45 |
| Mean GTV4 volume (cm3) | 151.5 [17–501] | 117.7 [4–310] | 0.34 |
| Mean Liver Dose (Gray) | 16.7 [6.2–24.3] | 17.2 [3.2–27.3] | 0.35 |
| Median Dose to Tumor (Gray) | 58 [45–67.7] | 58 [45–67.7] | 0.24 |
| Median OS (months) | 30.6 | 14.5 | 0.09 |
CTP = Child-Turcotte-Pugh
CLIP = Cancer of the liver Italian program
TVT = Tumor vascular thrombosis
GTV = Gross Tumor Volume
Figure 1. Kaplan-Meier overall survival (OS) distributions by disease type.
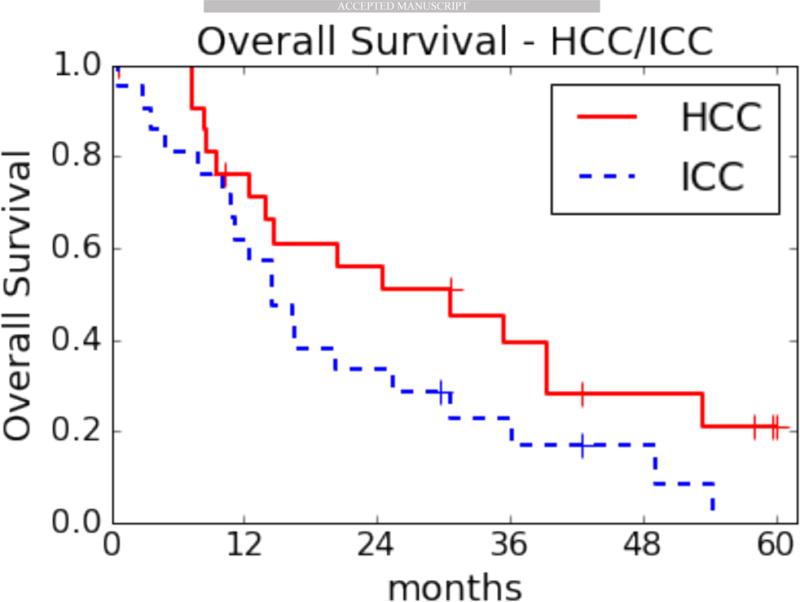
HCC, hepatocellular carcinoma, median OS: 30.6 months (range 0.7–60.1). ICC, intrahepatic cholangiocarcinoma, median OS: 14.5 months (range 0.7–54.3).
To examine whether the fractions of the CLP subsets correlate with OS, we divided each cohort at their median CLP fraction. At baseline, improved OS strongly correlated with greater CD4+CD25+ T cell and CD4+CD127+ (naïve and central memory) T cell fractions in the ICC patients (p=0.003 and p=0.01, Figure 2D,E). This association was not seen in HCC patients (Figure 2A,B). In contrast, we detected a significant association between longer OS and greater activated CTL fraction at day 8 during HPT, only in the HCC patients (p=0.007, Figure 2C) but not in the ICC cohort (p=0.65, Figure 2E). We found no association with OS for any of the CLP fractions at day 15 of HPT. These correlations were confirmed in multivariate analyses that included other relevant parameters such as tumor volume, target dose and the Child-Turcotte-Pugh (CTP) score, with the exception of CD4+CD25+ T cells that only showed a trend for correlation at baseline with OS in ICC patients in multivariate analyses (p=0.08, see Supplementary Material and Tables S1-S3).
Figure 2. Associations between circulating lymphocyte population (CLP) fractions and overall survival (OS) in liver cancer patients treated with hypofractionated proton therapy (HPT).
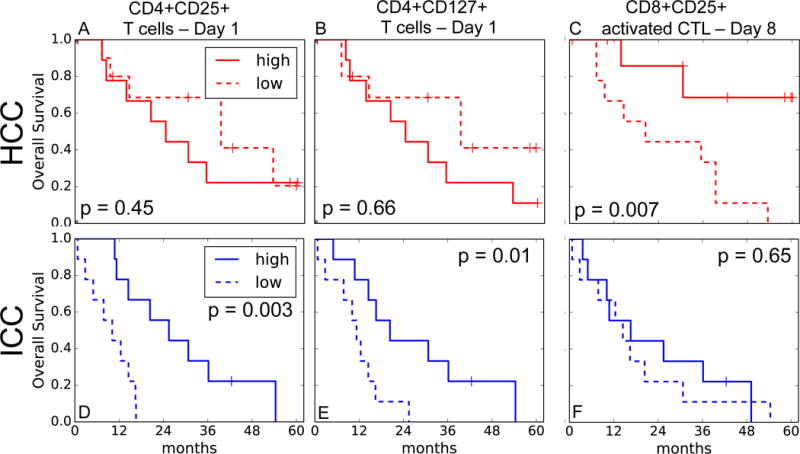
Association between CLP fractions (higher versus lower than median) during HPT and OS in hepatocellular carcinoma (HCC) (red, A-C) and intrahepatic cholangiocarcinoma (ICC) (blue, D-F) patients. A,D, CD4+CD25+ T cells at day 1. B,E, CD4+CD127+ T cells at day 1. C,F, activated cytotoxic T lymphocytes (CTLs) at day 8. p-values from log-rank test.
All CLPs showed a significant drop during HPT (see Supplementary Material and Figure S2). These effects were transient, as all CLP fractions recovered when measured at the first follow-up except for activated CTLs, which remained low in HCC patients but increased in ICC patients (see Supplementary Material).
Survival analyses of patients after liver-directed radiotherapy are often affected by the competing risk of death by underlying liver disease. In the current analysis, only 9 patients (6 HCC, 3 ICC) died without progression – 5 died of cirrhosis, and the rest of unknown causes. These data indicate that for this cohort, OS is an appropriate measure of disease-specific outcome.
Taken together, these results indicate that cellular immune responses might occur in liver cancer patients early during HPT. They also suggest that they may depend on maintenance of sufficiently high fractions of activated CTLs in the blood circulation of HCC patients during HPT, and CD4+CD25+ T cells and CD4+CD127+ (naïve and central memory T cells) in ICC patients before HPT. If these associations are confirmed in larger studies, they may suggest differential avenues to improve outcomes of HPT for HCC and ICC. For HCC, these data would suggest a potential benefit for increasing hypo-fractionation, especially if used in combination with immunotherapeutic approaches, with the goal of increasing lymphocyte-sparing (2, 9).
The CD4+CD25+ T cell population includes T regulatory cells (Tregs) and activated CD4+ T cells. Previous studies reported that increased Tregs in peripheral blood correlate with tumor burden (10), and a high number of infiltrating Tregs are thought to be an unfavorable prognostic factor (11). However, and importantly, we found no significant association between circulating CD4+CD25+CD127–Treg fraction and OS in this study.
Our observations suggest a different immuno-modulation during HPT in the HCC and ICC cohorts. As the changes occur early in therapy, adaptation of the treatment for high/low-risk populations could increase the therapeutic ratio. The immune escape pathways of HCC and ICC are complex, with PD-1 and PD-L1 playing an important role (12). PD-1 inhibition has been recently approved and is currently being investigated in advanced HCC as monotherapy, but response rates are less than 20% in unselected patients (13). Radiation has the potential to enhance the effect of immunotherapeutic approaches (14), though the optimal treatment schedules still have to be determined to render immunotherapy-radiotherapy approaches effective in these diseases (15). These hypothesis-generating findings warrant further investigations of these cellular biomarkers, and are important for the pursuit of combinations of radiation with specific immunotherapy approaches as well as for their optimal scheduling in future studies.
Supplementary Material
Summary.
This study investigated key effector and suppressor lymphocytes during radiotherapy in liver cancer patients. Results indicate that cellular immune responses may occur early during radiotherapy, associate with survival and significantly differ between histological subtypes. These data provide new insights for the pursuit of rational combinations of radiation with immunotherapy in liver cancer.
Acknowledgments
We thank Mrs. Anna Khachatryan (Steele Laboratories, Department of Radiation Oncology, Massachusetts General Hospital) for technical support.
Financial Support: This work was supported by grants from the XXX. The work of XXX and XXX is supported by NIH grant XXXXXXXXX. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The authors declare no potential conflicts of interest.
Author Contributions: Dr. Duda had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Grassberger and Hong contributed equally to this work. Concept and design: Hong, Zhu and Duda. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Grassberger, Hong and Duda. Critical revision of the manuscript for important intellectual content: All Authors. Statistical analysis: Grassberger and Yeap. Study supervision: Hong, Zhu and Duda.
References
- 1.Chi K-H, Liu S-J, Li C-P, et al. Combination of Conformal Radiotherapy and Intratumoral Injection of Adoptive Dendritic Cell Immunotherapy in Refractory Hepatoma. Journal of Immunotherapy. 2005;28:129. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 2.Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–579. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz A, Bayer J, Dechamps N, et al. Heritability of susceptibility to ionizing radiation-induced apoptosis of human lymphocyte subpopulations. Radiation Oncology Biology. 2007;68:1169–1177. doi: 10.1016/j.ijrobp.2007.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:1–15. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. JCO. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolaney SM, Ziehr DR, Guo H, et al. Phase II and Biomarker Study of Cabozantinib in Metastatic Triple-Negative Breast Cancer Patients. The Oncologist. 2017;22:25–32. doi: 10.1634/theoncologist.2016-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 9.Popp I, Grosu A-L, Niedermann G, et al. Immune modulation by hypofractionated stereotactic radiation therapy: Therapeutic implications. Radiotherapy and Oncology. 2016;120:185–194. doi: 10.1016/j.radonc.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Cao M, Cabrera R, Xu Y, et al. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Invest. 2007;87:582–590. doi: 10.1038/labinvest.3700540. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 12.Long J, Lin J, Wang A, et al. PD-1/PD-L blockade in gastrointestinal cancers: lessons learned and the road toward precision immunotherapy. J Hematol Oncol. 2017;10:146. doi: 10.1186/s13045-017-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1:1325–8. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 15.Young KH, Baird JR, Savage T, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE. 2016;11:e0157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


