Abstract
Context
No standard advance care planning (ACP) process exists in oncology. We previously developed and validated the values questions for Person-Centered Oncologic Care and Choices (P-COCC), a novel ACP intervention combining a patient values interview with an informational care goals video.
Objectives
To pilot study acceptability, and, using randomization, explore potential utility of P-COCC.
Methods
Eligibility included patients with advanced gastrointestinal cancer cared for at a comprehensive cancer center. Participants were randomized 2:2:1 to P-COCC vs. video alone vs. usual care. Validated assessments of wellbeing and decisional conflict were completed. Participants in the P-COCC arm also completed 3 Likert scales (was the intervention helpful, comfortable, and recommended to others); a positive score on at least 1 of 3 indicated acceptability.
Results
Patients were screened from 9/2014–11/2016; 151 were consented and randomized, 99 whom completed study measures (most common attrition reason: disease progression or death). The primary aim was met: Among 33 participants, P-COCC was acceptable to 32 (97%, 95%CI: 0.84–0.99, p<0.001). Mean distress scores (0–10) increased (0.43) in the P-COCC arm but decreased in the video alone (−0.04) and usual care (−0.21) arms (p=0.03 and 0.04, P-COCC versus video alone and usual care arms, respectively). There were no significant pre-post change scores on other measures of wellbeing (e.g., anxiety, depression, stress), or intergroup differences in decisional conflict.
Conclusions
Our values-based ACP paradigm is acceptable but may increase distress in cancer outpatients. Further studies are investigating the underpinnings of these effects and ways to best support cancer patients in ACP.
Keywords: advance care planning, cancer, communication, goals, patient participation
INTRODUCTION
Advance care planning (ACP) is the process by which adults discuss and consider future medical care in the context of their personal values and preferences.(1) However, in oncology, and despite some recent promising ACP initiatives,(2, 3) there is no gold-standard ACP process, and, ACP discussions have not become more prevalent over the last several years.(4) A lack of these discussions has been linked with worse patient quality of life, worse bereavement adjustment,(5) and greater health care costs in the last week of life.(6) As such, a recent Institute of Medicine report(7) concluded that patient-physician communication at the end of life remains a key area for improving the delivery of patient-centered care.
Our prior work with video decision aids have mostly focused directly on patient preferences for or against specific medical treatment options.(8, 9) Specialized interventions targeting patient values have also been developed, such as in Dignity Therapy(10) and Meaning-Centered Psychotherapy(11) and have utility in end-stage illnesses, including cancer. However, there is a need for scalable and earlier ACP uptake in oncology clinics. There is a disconnect between how much cancer patients,(12) even those in phase I trials,(13) believe ACP should be discussed, and how much it actually occurs. A recent report(14) highlighted the research imperative of defining and evaluating the beneficial elements of ACP in communication occurring with any patient with a serious (life-threatening and/or life-altering(15)) illness, regardless of prognosis.
To address these unmet needs, we created the Person-Centered Oncologic Care and Choices (P-COCC), which combines an informational care goals video with a brief patient values interview.(16) As an ACP intervention, P-COCC addresses key components of the cancer patient experience to help patients consider future medical care in the context of their personal values and preferences. The P-COCC intervention addresses both the internal (e.g., symptoms, psycho/spiritual values, and social relationship values) and external patient factors (e.g., family, the health care team, and medical care options). P-COCC parallels established behavioral science theoretical models, particularly the intrapersonal level Information-Motivation-Behavioral Skills Model,(17) which hinges on a person’s assessment of information, their personal and social motivation, and their ability to effect behavioral change. Through cognitive interviewing (in which semi-structured successive rounds are conducted in order to refine survey items(18)), we have previously validated the appropriateness of the questions used in the values interview.(16) In the pilot study reported herein, we primarily examined the acceptability of P-COCC to participants, and also sought, using a randomized design, hypothesis-generating data on its effects on wellbeing and decisional conflict. We hypothesized that the intervention would be acceptable (primary aim), and, secondarily, might lead to greater benefits in patient wellbeing and decision making compared to participants randomized to a usual care arm, or to an arm of the study (video alone arm) in which they watched a care goals video without being interviewed about their values.
METHODS
Participants
English-speaking patients 21 years of age or older with advanced gastrointestinal (GI) cancers were recruited consecutively from the Memorial Sloan Kettering (MSK) GI medical oncology clinics. We studied advanced GI cancer as this is a large and diverse population of serious illnesses. Participants were ineligible if their medical oncologists believed their patient’s life expectancy to be less than one month, or (when asked the surprise question(19)), greater than 12 months. Also, these timeframes were chosen to test the P-COCC intervention in patients with prognostically serious illness (prognosis <1 year) and to minimize attrition (prognosis of >1 month). Exclusion criteria included (1) a Short Portable Mental Status Questionnaire(20) (SPMSQ) score of “less than intact mental functioning” and (2) any patient condition (e.g., concern for markedly triggering or exacerbating underlying anxiety and/or depression) that the primary MSK medical oncologist deemed would make the study inappropriate for the patient.
Randomization and Study Design
This study was reviewed by the IRB and privacy board at MSK. The research team identified patients from MSK’s GI medical oncology service by review of the electronic medical records. After identification of potentially eligible patients, the treating medical oncologist was asked to confirm suitability for consideration of trial enrolment. During a scheduled visit, the oncologist broached the topic of ACP and introduced the study. If the patient expressed interest, the staff answered any further questions and confirmed their eligibility, including by testing mental function as above with the SPMSQ.(20) Subsequently eligible participants consented to participate in the study.
After consent, participants were randomized 2:2:1 by random permuted block to the P-COCC intervention (values interview with care goals video), video only, or usual care (no values interview or video viewing). Randomization was performed for secondary, hypothesis-generating, data to be generated about intervention effects. Because of the goal to maximize the number of patients receiving the intervention components without losing an adequate reference control sample, even (1:1:1) randomization was not chosen. Given the distinct nature of the processes in the three study arms, participants could not be blinded to their randomization assignment, but, treating oncologists were not informed of the allocation arm of their patients. All testing procedures were carried out by a research study assistant (who had received formal coursework training at MSK in cognitive interviewing and techniques for collection of patient reported data) in private. With the research study assistant, participants in all arms completed 7 validated questionnaires by paper (see online supplement) at baseline and at the next follow-up visit (one month or sooner). The questionnaires on perceived stress, anxiety, peacefulness, depression, distress, treatment satisfaction, and quality of life, were chosen to examine how the different study arms (particularly the P-COCC arm as an advance care planning intervention) would affect wellbeing.
After baseline questionnaires, participants in the P-COCC arm were interviewed about their values by a member of the research team. The eleven P-COCC values questions, previously validated and described in detail,(16) focus on goals (e.g., experiences most important to live well at this time), concerns (e.g., fears or worries about the illness) and sources of support (e.g., in the face of serious challenges), and two of them specifically ask about goals and concerns related to the care goals video. Interviews were audio-recorded and transcribed for later study. Immediately after their values interview, P-COCC arm participants answered 3 Likert scale questions about the values interview (was the process helpful, comfortable, and recommended to others). Given the individual and complex nature of ACP, we posited that a positive score on at least 1 of 3 indicated acceptability for that patient (e.g., a participant’s ratings reflecting that the intervention was uncomfortable, but nonetheless recommended to others and/or helpful, would count as acceptable). These 3 questions have been used in our prior studies, including our most recent one(21) in this patient population at MSK, and, acceptability is a commonly used indicator of intervention feasibility for studies of this pilot nature.(22) Before the next follow-up visit, the interviewer condensed each recorded transcript into a 1 page document that was reviewed with participants at the next follow-up visit. Participants could make corrections to the transcript summary and were encouraged to keep this values document and share with loved ones.
Participants in the video only arm watched the same video (more detail available on acpdecisions.org) as used in the P-COCC arm. The video has also been used in our prior research.(16, 23, 24) The images accompanying the video’s narration were designed to aid the viewer in understanding the different care measures available for patients becoming progressively more ill with advanced cancer, e.g., that 1) CPR and mechanical ventilation are components of life-prolonging care aiming to prolong life, and that they usually take place in an intensive care unit; 2) limited care includes all measures including disease-directed treatments (such as chemotherapy), sometimes in the hospital, but not CPR or mechanical ventilation; and 3) comfort care prioritizes symptom management.
At a follow-up visit approximately 1 month following participation (this timepoint, and all the preceding ones were chosen to collect data longitudinally but minimize attrition due to disease progression), all participants were asked about the degree to which their ACP values changed (assessed on a Likert scale -- and those who expressed change were asked what they felt caused that change; results to be presented separately) and decisional conflict(25) regarding overall cancer treatment decisions.
Statistical Analysis
The sample size was estimated by powering for the primary aim of acceptability of the P-COCC intervention (using the originally planned accrual of 30 participants randomized to the P-COCC arm). Powering utilized an underlying population acceptability of 70% (P-COCC intervention acceptable under the alternative hypothesis) against a null of 40% (P-COCC intervention not acceptable). This yielded an estimated statistical power of 0.92 with a two-sided type-I error of p ≤ 0.05 if 18 out of 30 planned P-COCC arm participants responded favorably to the acceptability items, in a binomial test between the alternative and the null hypotheses. The >70% acceptable boundary would be inclusive of the majority of the population, and is in line with acceptability ranges of 80–90% observed in our most recent video study in this patient population at MSK.(21) The power calculation was based on the P-COCC arm alone because it was the intervention of primary interest. For the secondary analyses, which were hypothesis-generating in nature, independent-sample t-tests were used to compare differences between intervention conditions with respect to pre- and post-test intervention change in the questionnaire scale scores. Decisional conflict scores (obtained post-test only) between intervention conditions were compared using an independent-sample t-test.
RESULTS
Study Participants
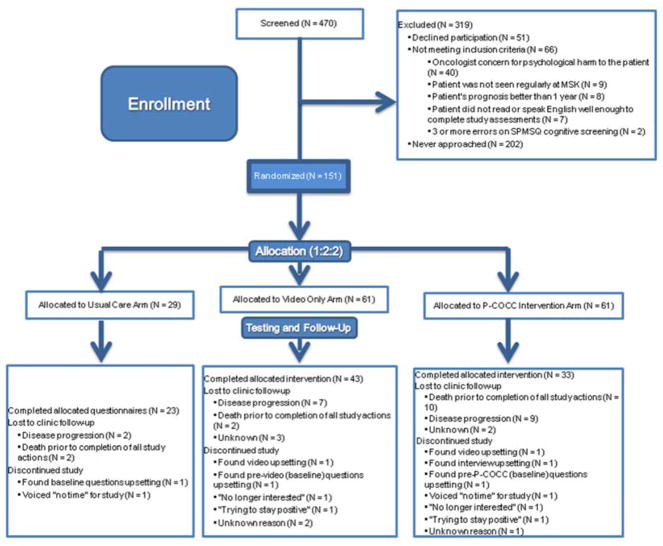
Two hundred and two potentially eligible patients were approached (Figure 1) between September 2014 and November 2016. Fifty-one patients (25%) declined participation. The most common reason for declining to participate in the study, N = 31, was not wanting to discuss the topic. The most common reason for study ineligibility was the primary medical oncologist’s concern for psychological harm through study participation (N = 40). One hundred and fifty-one patients consented and were randomized 2:2:1 to the P-COCC intervention vs. video alone vs. usual care. Ninety-one (66%) completed all study requirements. Interviews in the P-COCC arm averaged 20 minutes which included the 6-minute video duration. The most common reason for study participants not completing the study measures, N = 34, was disease progression (precluding either enough energy to continue study participation, or return visits to clinic at all) or death. Fifteen (7 in the P-COCC arm, of whom 2 completed the combined values interview/informational care goals video) voluntarily withdrew consent from the study. The reasons the 7 participants randomized to the P-COCC arm withdrew are also shown in Figure 1.
FIGURE 1.
CONSORT Diagram
Of the randomized participants, 63% of the participants were male, the mean age was 61 years, and the mean Eastern Cooperative Oncology Group (ECOG) performance status(26) was 1. The most common cancer types were colorectal, gastroesophageal, and pancreatic. Most of the participants were white, and most had a college or higher degree. Table 1 details the study population characteristics. The study arms were well balanced, with no statistically significant differences between arms.
TABLE 1.
Participant Baseline Demographics
| Demographic (available at time of consent) | Video (N = 61) | P-COCC (N = 61) | Usual Care (N = 29) | |
|---|---|---|---|---|
| Age [mean age in years (range)] | 63 (32–86) | 61 (38–86) | 59 (36–87) | |
| Gender | Male | 39 (64%) | 37 (61%) | 19 (66%) |
| Malignancy Type | Pancreatic | 17 (28%) | 20 (33%) | 11 (38%) |
| Colorectal | 19 (31%) | 18 (30%) | 7 (24%) | |
| Gastroesophageal | 15 (25%) | 15 (25%) | 8 (28%) | |
| Cholangiocarcinoma | 6 (10%) | 5 (8%) | 1 (3%) | |
| Hepatocellular Carcinoma | 1 (2%) | 1 (2%) | 2 (7%) | |
| Neuroendocrine | 2 (3%) | 2 (3%) | 0 (0%) | |
| Adrenal | 1 (2%) | 0 (0%) | 0 (0%) | |
| Mean ECOG Performance Status (range) | 1 (0–2) | 1 (0–3) | 1 (0–3) | |
| Education | ||||
|
| ||||
| Grade School | 0 (0%) | 1 (1.6%) | 0 (0%) | |
| Some High School | 2 (3%) | 2 (3%) | 0 (0%) | |
| High School or GED | 7 (11%) | 12 (20%) | 5 (17%) | |
| Some College | 10 (16%) | 10 (16%) | 2 (7%) | |
| College | 13 (21%) | 17 (28%) | 14 (48%) | |
| Post-Graduate | 23 (38%) | 15 (25%) | 4 (14%) | |
| Missing Data | 6 (10%) | 4 (7%) | 4 (14%) | |
| Race | White | 38 (62%) | 33 (54%) | 16 (55%) |
| Asian | 6 (10%) | 7 (11%) | 1 (3%) | |
| Black | 4 (7%) | 10 (16%) | 2 (7%) | |
| More than one race | 2 (3%) | 1 (2%) | 1 (3%) | |
| Missing Data | 6 (10%) | 7 (11%) | 6 (21%) | |
| Other | 5 (8%) | 3 (5%) | 3 (10%) | |
| Hispanic | 4 (7%) | 2 (3%) | 7 (24%) | |
| Religion | Catholic | 18 (30%) | 22 (36%) | 11 (38%) |
| Protestant | 3 (5%) | 6 (10%) | 1 (3%) | |
| Other Christian | 4 (7%) | 9 (15%) | 1 (3%) | |
| Jewish | 11 (18%) | 7 (18%) | 5 (17%) | |
| Muslim | 2 (3%) | 1 (2%) | 0 (0%) | |
| Other | 14 (23%) | 8 (13%) | 5 (17%) | |
| Missing Data | 9 (15%) | 8 (13%) | 6 (21%) | |
| Marital Status | Married or Partnered | 32 (52%) | 41 (67%) | 18 (62%) |
| Widowed | 3 (5%) | 7 (11%) | 2 (7%) | |
| Never Married | 10 (16%) | 4 (7%) | 1 (3%) | |
| Divorced | 9 (15%) | 1 (2%) | 2 (7%) | |
| Missing Data | 7 (11%) | 8 (13%) | 6 (21%) | |
Acceptability of the P-COCC intervention (primary aim)
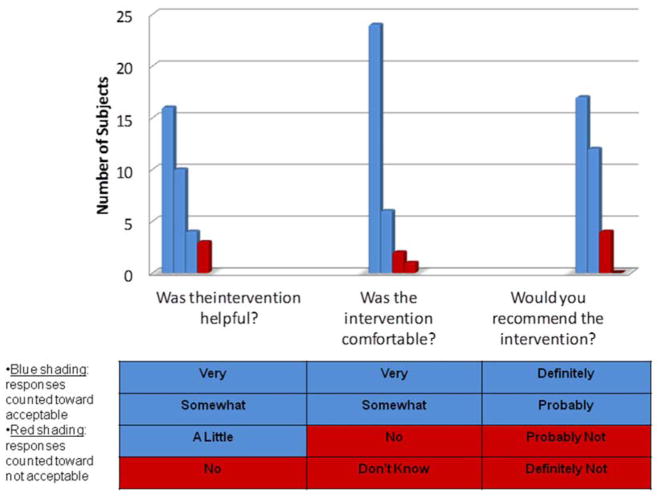
The primary aim was met: of the 33 participants who were randomized to and completed the P-COCC intervention, the intervention was rated acceptable in 32 (97%, 95%CI: 0.84–0.99, p<0.001). If the 7 participants who dropped out of the P-COCC study arm were included in the acceptability calculations (according to intention to treat), the primary aim still would have been met (32/40 = 80%). Table 2 depicts the Likert acceptability scores of each of the participants randomized to the P-COCC intervention arm. Of the 33 participants, the scores of the 1 participant that deemed the intervention to be unacceptable were that the intervention was not helpful, not comfortable to complete, and “probably not” recommended to others. Of the 32 participants whose acceptability scores indicated acceptability of the intervention, there were 4 participants who rated the intervention poorly on one (N=2) or two (N=2) of the acceptability questions.
TABLE 2.
Pre- and Posttest Wellbeing Comparisons, and Decisional Conflict Differences
| preb | P-COCCa | d | pre | Video | d | pre | Usual Care | d | p-valuec | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n=61 | n=61 | n=29 | |||||||||
| post | post | post | 1v2 | 1v3 | |||||||
|
| |||||||||||
| mean (sd) | mean (sd) | mean (sd) | mean (sd) | mean (sd) | mean (sd) | ||||||
|
| |||||||||||
| FACIT-Sp | 3.00 (0.52) | 2.95 (0.51) | −.10 | 3.05 (0.62) | 2.95 (0.68) | −.16 | 2.94 (0.63) | 3.02 (0.56) | .13 | 0.58 | 0.15 |
|
|
|||||||||||
| FACIT-TS-G | 3.50 (0.41) | 3.32 (0.49) | −.44 | 3.35 (0.58) | 3.41 (0.60) | .10 | 3.35 (0.63) | 3.40 (0.52) | .08 | 0.40 | 0.30 |
| Distress Thermometer | 2.96 (2.81) | 4.16 (2.81) | .43 | 3.41 (3.19) | 3.28 (2.99) | −.04 | 3.25 (3.48) | 2.52 (2.31) | −.21 | .029 | .042 |
| Perceived Stress Scaled | 1.33 (0.75) | 1.56 (0.75) | .31 | 1.35 (0.90) | 1.42 (0.91) | .08 | 1.37 (0.72) | 1.17 (0.62) | −.28 | .089 | .515 |
| GAD 7-Iteme | 0.52 (0.64) | 0.62 (0.60) | .15 | 0.69 (0.79) | 0.73 (0.84) | .04 | 0.52 (0.16) | 0.54 (0.51) | .03 | .764 | .713 |
| PHQ-9f | 0.52 (0.43) | 0.58 (0.43) | .14 | 0.63 (0.60) | 0.62 (0.57) | −.02 | 0.62 (0.58) | 0.44 (0.40) | −.31 | 0.641 | 0.160 |
| PEACE Scale | 3.13 (0.60) | 3.13 (0.62) | .00 | 3.19 (0.62) | 3.20 (0.67) | .01 | 3.21 (0.55) | 3.41 (0.44) | .36 | .960 | .310 |
| Peaceful Acceptance of Illness | 3.31 (0.74) | 3.33 (0.65) | .02 | 3.35 (0.68) | 3.42 (0.70) | .10 | 3.51 (0.51) | 3.58 (0.43) | .13 | 0.81 | 0.81 |
| Struggle With Illness | 2.00 (0.69) | 2.02 (0.71) | .03 | 1.92 (0.69) | 1.96 (0.80) | .05 | 2.01 (0.74) | 1.71 (0.51) | −.39 | 0.93 | 0.20 |
| Decisional Conflict Scale | 9.09 (2.35) | 9.19 (2.49) | 9.48 (2.23) | .870 | .540 | ||||||
The P-COCC intervention included both the values interview and the video.
Descriptive statistics reported herein are means and standard deviations of survey scale scores unless otherwise noted.
Between-arm comparisons in the pre-post differences: “1v2” compares the P-COCC arm participants (1) and the video only arm participants (2); “1v3” compares the P-COCC arm participants (1) and the usual care participants (3).
Perceived Stress, higher score represents more stress (0=“Never”, 1=“Almost Never”, 2=“Sometimes”, 3=“Fairly Often”, 4=“Very Often”).
Generalized Anxiety Disorder 7-Item
Patient Health Questionnaire-9
Secondary Aims: Effects on wellbeing and decisional conflict (Table 2)
From baseline to follow-up measurement, mean distress scores (on a 0–10 scale) increased (0.43) in the P-COCC arm but decreased in the video (−0.04) and usual care (−0.21) arms (p=0.03 and 0.04, P-COCC vs video and usual care, respectively). There were no significant pre-post change scores on other measures of wellbeing (anxiety, depression, stress, acceptance, quality of life), or intergroup differences in decisional conflict.
Qualitative Analyses
A forthcoming manuscript will present detailed analyses of the audio-recorded P-COCC values interview transcripts. Two main themes emerged from the interviews that accounted for the statistically increased distress scores in the P-COCC arm. First, some patients felt a fear of poor quality of life and value-discordant care that they associated with some of the images of the life-prolonging interventions in the video (e.g., CPR and mechanical ventilation), eliciting a negative emotional response. Second, patients were concerned about the uncertainty regarding their illness and prognosis, and their desire to have open communication with their providers.
DISCUSSION
The primary aim was met in this randomized trial: Person-Centered Oncologic Care and Choices (P-COCC), an advance care planning (ACP) intervention combining a brief patient values interview and an informational care goals video, was acceptable to the participants randomized to the P-COCC intervention arm. Additionally, compared to the other study arms (usual care and video-only), P-COCC was associated with an increase in distress scores, and no improvement in decisional conflict or other validated measures of wellbeing, such as quality of life, depression or anxiety.
Our study is an important next step in our group’s ACP research in video educational media,(9, 21) specifically by investigating the additional effects of eliciting patient values through a patient interview that we previously validated.(16) Firstly, this study met its primary aim of acceptability and lays groundwork for future work on an intervention that addresses what is important to patients, which our prior work(12) and that of others(27) suggest is a sensitive but critical aspect of ACP. An additional strength is that we previously employed cognitive interviewing methodology(16) to ensure the appropriateness of the patient values questions we asked as part of the P-COCC intervention. Furthermore, the incorporation of the care goals video enabled us to cover topics clinically pertinent to patients with advanced cancer that were not included in the version(28) of the Living Well interview we built our patient values questions upon.
Our findings also answer the call(14) for more communication research, and offer important contributions to the existing body of such work. For instance, our methodology and results are complimentary to other recent ACP initiatives in oncology, such as the CONNECT intervention (Care Management by Oncology Nurses),(3) or the VOICE trial,(29) which combined oncologist communication training and patient assistance. P-COCC also adds to the literature of other ACP interventions like the Serious Illness Care Program(2) as well as the PREPARE website.(30) Finally, P-COCC attempts to bring patient values earlier and to a broader clinical fore than Dignity Therapy,(10) off of which we modeled our process of generating and providing values narratives to patients.
While our trial met its primary endpoint of acceptability to participants, the secondary results were contrary to our hypotheses that P-COCC would improve patient wellbeing and decision making. There are several potential explanations for these findings. Our prior work has highlighted the dynamic of “necessary discomfort”(12) of discussing important but naturally challenging and potentially distressing issues at the intersection of patient values and advanced cancer. Other recent data indicate that other negative psychological outcomes like post-traumatic stress disorder,(31, 32) anxiety,(33) or depressive symptoms(34) may occur in the process of discussing serious illness. Perhaps this post-intervention uptick in distress is therefore necessary for, or at least part of the process of, patients’ informed consent and decision making. Regarding the other measurements of wellbeing, it is possible that other factors related to the patients’ experience, unrelated to the study interventions, influenced the results of these measurements and contributed to the lack of difference between arms.
The statistically significant increase in distress scores seen in the P-COCC intervention relative to the other arms may not represent a clinically meaningful difference. The Distress Thermometer correlates with clinically relevant levels of distress only at 4 or greater on the 0–10 scale(35) and most the participants in all arms of our trial, including P-COCC, were at or less than 4, suggesting the possibility of a floor effect. As we did not target, at baseline, patients with high distress scores, it’s possible the intervention may have had a positive impact in such a select population, as seen in a recent cognitive behavioral intervention in cancer survivors with high fear of recurrence.(36) Our ongoing qualitative analyses of data on participant values (including longitudinal values change) will be reported separately. These analyses will shed light on the intervention’s increase in distress, which our preliminary results suggest is brought on by discussing sensitive but pertinent ACP topics regarding end-of-life medical care. The distress phenomenon we observed may parallel the anxiety that is intentionally elicited in some cognitive behavioral therapies, including in oncology,(37) to help patients habituate to such disease-related emotions.
Our study has limitations. One is that about 25% of those approached chose not to participate, which is not unusual in behavioral research. We could not analyze the sociodemographics of non-participating patients, but the complexity of informed consent and the trepidation these and other patients and physicians may have about such studies may have been at play.(38) Second, it is possible that selection bias influenced the results, as patients referred by their oncologists and amenable to participating in the study may have had opinions about our ACP topics different from those patients who were not referred, or those who chose not to participate. These two limitations have influenced the evolution of our advance care planning efforts at MSK in making values assessments normative and routine from the outset of cancer care for all patients, irrespective of stage or prognosis.(39) Third, due to attrition, a third of consented participants were unable to complete study requirements, primarily due to disease progression and death. Attrition is a phenomenon we have seen before in our behavioral intervention research.(11) We do not believe attrition significantly impacted our study, as we were still able to accrue and complete assessments in a sufficient number of participants to meet the statistical powering of the primary aim, and generate data for the secondary aims, which were designed to be exploratory. Fourth, most questionnaires were only administered at baseline and one follow-up time point. It is possible that wellbeing and other outcomes related to ACP would be different at additional time points. Finally, we did not analyze health outcomes relating to ACP, such as discussions documented in the medical record, or measures of health care utilization, including at the end of life.
This randomized trial demonstrated acceptability and additional outcomes of our novel advance care planning approach, Person-Centered Oncologic Care and Choices (P-COCC). Future efforts in advance care planning research should build upon P-COCC’s use of questions which elicit patient values. We are using the results from this trial in our current research efforts(39) to make these values assessments operational and routinely delivered by oncology nurses from the outset in the care of all cancer patients. This next phase of research also builds on this study with longitudinal tracking of wellbeing and health utilization outcomes, as well as with a communication framework of empathic “ready responses” nurses can use with patients responding to questions about their core values.(39) As appropriate, oncology nurses in this process also involve the oncologists or other specialists, including those in palliative care. In these ways, we hope to normalize and expand the reach of advance care planning endeavors.
Supplementary Material
FIGURE 2.
Ratings of Acceptability of the P-COCC Values Narrative Recording Process (Primary Aim)
Acknowledgments
Research support: National Palliative Care Research Center (2013 Career Development Award to Andrew S. Epstein, MD). Also, partly funded through the NIH/NCI Cancer Center Support Grant P30 CA008748.
We thank the patients and families, as well as the colleagues who referred patients to the trial. Colleagues include Drs. Geoffrey Ku, Yelena Janjigian, Leonard Saltz, Anna Varghese, James Harding, Zsofia Stadler, Diane Reidy, David Ilson, Neil Segal, Armin Shahrokni, Rona Yaeger, Sree Chalasani, Monica Shcherba, and Kenneth Yu.
Footnotes
Prior Presentation of this Work: 2017 Annual Meeting of the American Society of Clinical Oncology and the 2017 Palliative and Supportive Care in Oncology Symposium
Disclosure/Conflict of Interest Statement: Dr. Epstein reports receiving grant funds from the National Palliative Care Research Center in support of this study. None of the other authors have any disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sudore RL, Lum HD, You JJ, et al. Defining Advance Care Planning for Adults: A Consensus Definition From a Multidisciplinary Delphi Panel. J Pain Symptom Manage. 2017;53:821–832. e1. doi: 10.1016/j.jpainsymman.2016.12.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015:5. doi: 10.1136/bmjopen-2015-009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenker Y, White D, Rosenzweig M, et al. Care management by oncology nurses to address palliative care needs: a pilot trial to assess feasibility, acceptability, and perceived effectiveness of the CONNECT intervention. J Palliat Med. 2015;18:232–40. doi: 10.1089/jpm.2014.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narang AK, Wright AA, Nicholas LH. Trends in advance care planning in patients with cancer: Results from a national longitudinal survey. JAMA Oncology. 2015 doi: 10.1001/jamaoncol.2015.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169:480–8. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington DC: National Academies Press; 2014. [PubMed] [Google Scholar]
- 8.Volandes A, El-Jawahri A. Improving CPR Decision-Making for Patients and Families with Video Decision Aids. In: Doyle LJ, Saltsman RA, editors. Cardiopulmonary Resuscitation: Procedures and Challenges. Nova Science Publishers; 2012. [Google Scholar]
- 9.Volandes AE, Paasche-Orlow MK, Mitchell SL, et al. Randomized Controlled Trial of a Video Decision Support Tool for Cardiopulmonary Resuscitation Decision Making in Advanced Cancer. Journal of Clinical Oncology. 2012 doi: 10.1200/JCO.2012.43.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23:5520–5. doi: 10.1200/JCO.2005.08.391. [DOI] [PubMed] [Google Scholar]
- 11.Breitbart W, Rosenfeld B, Pessin H, et al. Meaning-Centered Group Psychotherapy: An Effective Intervention for Improving Psychological Well-Being in Patients With Advanced Cancer. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.57.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein AS, Shuk E, O'Reilly EM, Gary KA, Volandes AE. ‘We have to discuss it’: cancer patients' advance care planning impressions following educational information about cardiopulmonary resuscitation. Psycho-Oncology. 2015 doi: 10.1002/pon.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu S, Barber FD, Naing A, et al. Advance Care Planning in Patients With Cancer Referred to a Phase I Clinical Trials Program: The MD Anderson Cancer Center Experience. Journal of Clinical Oncology. 2012 doi: 10.1200/JCO.2011.38.0758. [DOI] [PubMed] [Google Scholar]
- 14.Tulsky JA, Beach M, Butow PN, et al. A research agenda for communication between health care professionals and patients living with serious illness. JAMA Internal Medicine. 2017 doi: 10.1001/jamainternmed.2017.2005. [DOI] [PubMed] [Google Scholar]
- 15.SKA Defining “Serious Illness”. Journal of Palliative Medicine. 2014;17:985–985. doi: 10.1089/jpm.2014.0164. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AS, O'Reilly EM, Shuk E, et al. Development of an advance care planning paradigm for advanced cancer: person-centered oncologic care and choices (P-COCC) Psycho-Oncology. 2016 doi: 10.1002/pon.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shumaker SA, Ockene JK, Riekert KA. The Handbook of Health Behavior Change. 3. New York: Springer Publishing Company; 2009. [Google Scholar]
- 18.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: SAGE Publications, Inc; 2005. [Google Scholar]
- 19.Moroni M, Zocchi D, Bolognesi D, et al. The 'surprise' question in advanced cancer patients: A prospective study among general practitioners. Palliat Med. 2014;28:959–964. doi: 10.1177/0269216314526273. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AS, Volandes AE, Chen LY, et al. A Randomized Controlled Trial of a Cardiopulmonary Resuscitation Video in Advance Care Planning for Progressive Pancreas and Hepatobiliary Cancer Patients. J Palliat Med. 2013;16:623–631. doi: 10.1089/jpm.2012.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36:452–7. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Jawahri A, Podgurski LM, Eichler AF, et al. Use of Video to Facilitate End-of-Life Discussions With Patients With Cancer: A Randomized Controlled Trial. Journal of Clinical Oncology. 2010;28:305–310. doi: 10.1200/JCO.2009.24.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volandes AE, Levin TT, Slovin S, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: A preintervention-postintervention study. Cancer. 2012;118:4331–8. doi: 10.1002/cncr.27423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 26.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 27.Gawande A. Being Mortal: Medicine and What Matters in the End. Macmillan; 2014. [Google Scholar]
- 28.Schwartz C, Lennes I, Hammes B, et al. Honing an advance care planning intervention using qualitative analysis: the Living Well interview. J Palliat Med. 2003;6:593–603. doi: 10.1089/109662103768253704. [DOI] [PubMed] [Google Scholar]
- 29.Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The voice randomized clinical trial. JAMA Oncology. 2016 doi: 10.1001/jamaoncol.2016.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudore RL, Boscardin J, Feuz MA, et al. Effect of the prepare website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: A randomized clinical trial. JAMA Internal Medicine. 2017;177:1102–1109. doi: 10.1001/jamainternmed.2017.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–94. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 32.Carson SS, Cox CE, Wallenstein S, et al. Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. Jama. 2016;316:51–62. doi: 10.1001/jama.2016.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120:278–85. doi: 10.1002/cncr.28369. [DOI] [PubMed] [Google Scholar]
- 34.Curtis J, Back AL, Ford DW, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: A randomized trial. JAMA. 2013;310:2271–2281. doi: 10.1001/jama.2013.282081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 36.Wal Mvd, Thewes B, Gielissen M, Speckens A, Prins J. Efficacy of Blended Cognitive Behavior Therapy for High Fear of Recurrence in Breast, Prostate, and Colorectal Cancer Survivors: The SWORD Study, a Randomized Controlled Trial. Journal of Clinical Oncology. 2017;35:2173–2183. doi: 10.1200/JCO.2016.70.5301. [DOI] [PubMed] [Google Scholar]
- 37.Traeger L, Greer JA, Fernandez-Robles C, Temel JS, Pirl WF. Evidence-Based Treatment of Anxiety in Patients With Cancer. Journal of Clinical Oncology. 2012;30:1197–1205. doi: 10.1200/JCO.2011.39.5632. [DOI] [PubMed] [Google Scholar]
- 38.Mock V, Hill MN, Dienemann JA, Grimm PM, Shivnan JC. Challenges to behavioral research in oncology. Cancer Pract. 1996;4:267–73. [PubMed] [Google Scholar]
- 39.Epstein AS, Klimek VM, Chow K, et al. Palliative care from cancer diagnosis for all: Memorial Sloan Kettering’s “One-Two-Three” program. Journal of Clinical Oncology. 2017;35:111–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.