Abstract
We aimed to examine the effects of a sodium glucose co-transporter 2 (SGLT2) inhibitor on systemic and intrarenal renin–angiotensin system (RAS) in subtotally nephrectomized non-diabetic rats, a model of chronic kidney disease (CKD). Oral administration of the selective SGLT2 inhibitor, TA-1887 (10 mg/kg/ day), for 10 weeks induced glycosuria. However, plasma renin activity, plasma angiotensinogen levels, kidney angiotensin II contents and renal injury were not significantly affected by TA-1887. These data indicate that chronic treatment with an SGLT2 inhibitor does not activate the systemic and intrarenal RAS in subjects with non-diabetic CKD.
Keywords: SGLT2 inhibitor, Renin–angiotensin system (RAS), Chronic kidney disease (CKD)
Sodium glucose co-transporter 2 (SGLT2) in the proximal tubular S1 segment reabsorbs approximately 90% of the filtered glucose in urine,1 and SGLT2 inhibitors have been used as novel hypoglycemic agents for patients with type 2 diabetes.1 More recently, the EMPA-REG OUTCOME trial has shown that treatment with the SGLT2 inhibitor, empagliflozin, improves cardiovascular event and all-cause mortality in patients with type 2 diabetes and established cardiovascular disease.2 Additionally, sub-analyses of the EMPA-REG OUTCOME study have identified a potential reduction of rates of hospitalization for heart failure in patients treated with empagliflozin.3 Interestingly, these beneficial effects of the SGLT2 inhibitor were independent of changes in glycemic control, plasma lipid levels and blood pressure.2, 3
However, side effects of SGLT2 inhibitors, particularly polyuria and polydipsia, have been reported during the early stages of treatment.4 These clinical symptoms indicate that SGLT2 inhibitors promote diuresis and negative water balance. We recently demonstrated that treatment with SGLT2 inhibitors caused temporary natriuresis and body fluid loss in obese rats.5, 6 Similarly, in patients with type 2 diabetes, urinary excretion of sodium significantly increased for only a few days and then returned to pre-treatment levels during chronic treatment with an SGLT2 inhibitor.7 These data indicate that the diuretic effect of a SGLT2 inhibitor is soon compensated for, possibly by an adaptive physiological mechanism. In this regard, Cherny et al8 reported that urinary angiotensinogen (AGT) excretion, which reflects intrarenal renin–angiotensin system (RAS) activity,9, 10 was significantly increased by treatment with a SGLT2 inhibitor in patients with type 1 diabetes mellitus. These data suggest that intrarenal RAS is activated to compensate for acute loss of sodium and body fluid. In contrast, a recent study by Shin et al11 showed that during treatment with a SGLT2 inhibitor, type 2 diabetic rats exhibited a significant decrease in urinary AGT excretion and an associated reduction in blood glucose levels. Thus, the effects of SGLT2 inhibitors on intrarenal RAS activity are still controversial because of a lack of data from direct measurements of intrarenal angiotensin II levels.
In the present study, we directly measured the angiotensin II content of renal tissues to determine whether a SGLT2 inhibitor alters intrarenal RAS activity. Blood glucose level is a critical factor determining intrarenal AGT expression and associated RAS activity.10 Furthermore, side effects of SGLT2 inhibitors, particularly polyuria and polydipsia, have been clinically reported during the early stages of treatment,4 leading to restricted use of SGLT2 inhibitors, particularly concerning in patients with chronic kidney disease (CKD). Therefore, we examined the effect of an SGLT2 inhibitor in non-diabetic rats subjected to subtotal nephrectomy, to avoid possible effects of blood glucose changes on intrarenal RAS activity.
Thirty male 4-week-old Sprague Dawley (SD) rats were purchased from Japan SLC Inc. (Shizuoka, Japan). The rats were acclimatized for 1 week, then the remnant kidney model was induced by surgical renal reduction, as previously described.12 All experimental protocols and procedures were carried out according to the guidelines for care and use of animals established by Kagawa University and Osaka City General Hospital. One week after the surgery, we excluded nephrectomized rats with blood urea nitrogen (BUN) levels less than 50 mg/dL or greater than 100 mg/dL. A total of 20 nephrectomized rats were then randomly assigned to receive the SGLT2 inhibitor TA-1887 (10 mg/kg/day, per os, n = 10)13 or vehicle (carboxymethylcellulose, n = 10) at the age of 17 weeks. TA-1887 is a potent and selective SGLT2 inhibitor. The IC50 values of TA-1887 for human SGLT1 and SGLT2 were 230 nM and 1.1 nM, respectively.13 Treatment was continued for 10 weeks.
Systolic blood pressure was measured every four weeks in conscious rats using a tail-cuff plethysmograph (BP-98A; Softron Co, Tokyo, Japan). Urine produced over a 1-day period was obtained every 4 weeks using metabolic cages. At 17 weeks of age, the rats were decapitated and trunk blood was collected. Half of the left kidney was homogenized in cold methanol and processed for measurement of angiotensin II content,14 and the remaining renal tissues were fixed with 10% formalin (pH 7.4), embedded in paraffin, sectioned into 3-μm-thick slices and subjected to Azan and periodic acid-Schiff (PAS) staining, as described previously.6 Plasma BUN, creatinine (both Wako Pure Chemical Industries Ltd., Osaka, Japan), urinary albumin (Shibayagi Co. Ltd., Shibukawa, Japan), and plasma total and intact AGT (IBL Co. Ltd., Fujioka, Japan) were determined using commercially available ELISA kits. Plasma renin activity (PRA) and angiotensin II content of renal tissue were measured by a radioimmunoassay, as previously described.14
Data are presented as mean ± SEM. Data from vehicle- and TA-1887-treated rats were compared using an unpaired t-test. P < 0.05 was considered statistically significant.
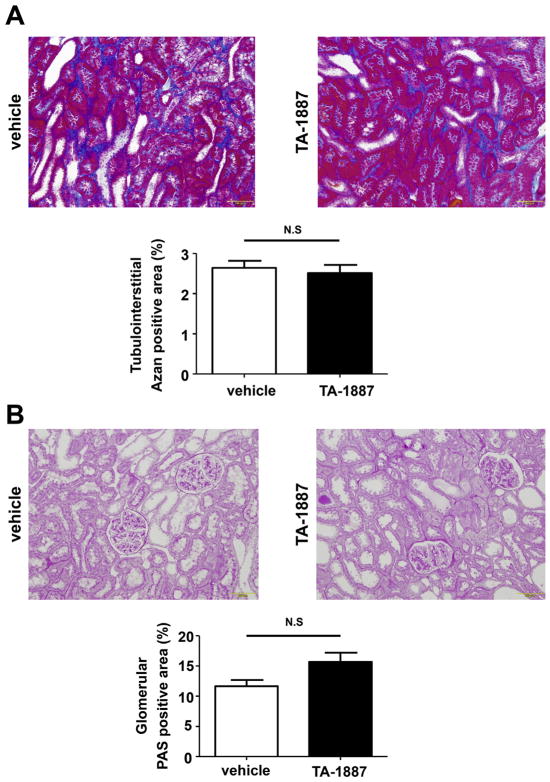
During the experimental period, 3 vehicle- and 2 TA-1887-treated 5/6 nephrectomized rats died. All analyses were performed in the remaining animals. As shown in Table 1, TA-1887 significantly increased urinary volume and urinary glucose excretion in 5/6 nephrectomized rats, which suggests that TA-1887 effectively blocked renal tubule SGLT2. However, administration of TA-1887 did not significantly change the plasma glucose level (147 ± 5 mg/dL) as compared with vehicle treatment (150 ± 2 mg/ dL) in non-diabetic 5/6 nephrectomized rats. Consistent with previous studies that used other SGLT2 inhibitors in rats,6 TA-1887 administration for 10 weeks tended to decrease body weight and blood pressure, but these changes were not significant. TA-1887 significantly increased kidney weight, kidney weight/body weight ratio, and urinary volume in 5/6 nephrectomized rats. However, TA-1887 did not significantly change plasma BUN or creatinine levels, creatinine clearance, or urinary albumin excretion (Table 1). Similarly, TA-1887 did not change the tubulointerstitial Azan-positive area (Fig. 1A and B) or the glomerular PAS-positive area (Fig. 1C and D) in 5/6 nephrectomized rats. These data are consistent with a recent study12 demonstrating that SGLT2 inhibitor treatment did not affect the development of hypertension, albuminuria or renal tissue injury in 5/6 nephrectomized rats.
Table 1.
Systemic and renal parameters in non-diabetic 5/6 nephrectomized rats.
| Vehicle (n = 7) | TA-1887 (n = 8) | |
|---|---|---|
| Urine volume (ml/24 h) | 52 ± 1 | 89 ± 4* |
| Urinary glucose excretion (mg/day) | 3 ± 1 | 1933 ± 339* |
| Blood pressure (mmHg) | 150 ± 3 | 147 ± 2 |
| Body weight (g) | 483 ± 7 | 456 ± 13 |
| Kidney weight (g) | 2.2 ± 0.2 | 3.0 ± 0.2* |
| plasma BUN (mg/dl) | 45 ± 3 | 55 ± 6 |
| Plasma creatinine (mg/dl) | 0.9 ± 0.1 | 0.8 ± 0.1 |
| Creatinine clearance (ml/min) | 3.0 ± 0.5 | 2.1 ± 0.2 |
| Urinary albumin excretion (mg/day) | 151 ± 4 | 159 ± 5 |
All data are mean ± SEM.
P < 0.05 vs. vehicle.
Fig. 1.
Effects of the sodium glucose co-transporter 2 (SGLT2), TA-1887, on glomerular and tubulointerstitial injuries in non-diabetic 5/6 nephrectomized rats. A, Tubulointerstitium stained with Azan reagents (200× magnification). B, Glomeruli stained with periodic acid-Schiff (PAS) reagents (200× magnification). Neither tubulointerstitial Azan-positive area nor glomerular PAS-positive area differs between vehicle- and TA-1887-treated non-diabetic 5/6 nephrectomized rats.
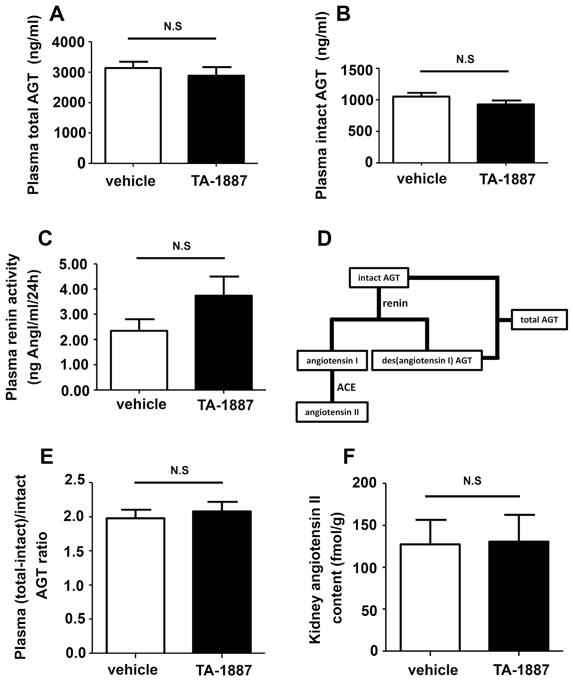
TA-1887 administration for 10 weeks tended to increase PRA in 5/6 nephrectomized rats; however, these changes were not significant (Fig. 2A, P = 0.087).These data are consistent with a recent study15 showing that chronic treatment with an SGLT2 inhibitor, empagliflozin, did not significantly change PRA in type 2 diabetic db/db mice (P = 0.059). The present study also showed that TA-1887 did not change either total or intact AGT levels in plasma (Fig. 2B and C). These data indicate that AGT production by the liver is not influenced by long-term administration of a SGLT2 inhibitor. We also determined the amount of des (angiotensin I) AGT (calculated as total AGT – intact AGT). As shown in Fig. 2D, the ratio of des (angiotensin I) AGT to intact AGT in the plasma may reflect how much intact AGT is cleaved by renin.10 The results showed that chronic TA-1887 treatment did not significantly change the ratio of des (angiotensin I) AGT to intact AGT in plasma (Fig. 2E). These data suggest that chronic administration of the SGLT2 inhibitor does not influence the amount of cleaved AGT.
Fig. 2.
Effects of TA-1887 on the systemic and intrarenal renin–angiotensin system in non-diabetic 5/6 nephrectomized rats. Chronic treatment with TA-1887 does not change plasma renin activity (PRA; A), total angiotensinogen (AGT) (B) and intact AGT levels (C). The ratio of des (angiotensin I) AGT (calculated as total AGT – intact AGT) to intact AGT indicates the activity of renin (D). TA-1887 does not change the ratio of (des-angiotensin I) AGT to intact AGT (E) in plasma, and kidney angiotensin II contents (F) in non-diabetic 5/6 nephrectomized CKD rats.
In the present study, we directly measured kidney angiotensin II contents to determine whether a SGLT2 inhibitor activates the intrarenal RAS. We found that chronic treatment with TA-1887 did not significantly change the angiotensin II content of renal tissues (Fig. 2F). These data indicate that the intrarenal RAS is not activated by chronic treatment with a SGLT2 inhibitor in 5/6 nephrectomized rats.
In summary, the present study showed that chronic administration of TA-1887 did not further impair renal function in 5/6 nephrectomized rats. In these animals, neither the systemic nor the intrarenal RAS was activated by treatment with TA-1887. These data indicate that chronic treatment with a SGLT2 inhibitor may not cause deterioration of renal function by activation of the RAS in subjects with non-diabetic CKD.
Acknowledgments
TA-1887 was provided by Tanabe Mitsubishi Pharma Co. Ltd. (Tokyo, Japan).
Footnotes
Peer review under responsibility of Japanese Pharmacological Society.
Conflict of interest statement
This work received partial financial support from the Mitsubishi Tanabe Pharma Corporation (to Akira Nishiyama). The other authors declare no conflict of interest.
References
- 1.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 4.Haneda M, Seino Y, Inagaki N, et al. Influence of renal function on the 52-week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2016;38(1):66–88.e20. doi: 10.1016/j.clinthera.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Rahman A, Kittikulsuth W, Fujisawa Y, et al. Effects of diuretics on sodium-dependent glucose cotransporter 2 inhibitor-induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J Hypertens. 2016;34(5):893–906. doi: 10.1097/HJH.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 6.Takeshige Y, Fujisawa Y, Rahman A, et al. A sodium-glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens Res. 2016;39(6):415–422. doi: 10.1038/hr.2016.2. [DOI] [PubMed] [Google Scholar]
- 7.Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86(5):1057–1058. doi: 10.1038/ki.2014.246. [DOI] [PubMed] [Google Scholar]
- 9.Mizushige T, Kobori H, Hitomi H, et al. Urinary angiotensinogen could Be a prognostic marker of the renoprotection of olmesartan in metabolic syndrome patients. Int J Mol Sci. 2016;17(11) doi: 10.3390/ijms17111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 11.Shin SJ, Chung S, Kim SJ, et al. Effect of sodium-glucose Co-Transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One. 2016;11(11):e0165703. doi: 10.1371/journal.pone.0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Thai K, Kepecs DM, Gilbert RE. Sodium-glucose linked Cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS One. 2016;11(1):e0144640. doi: 10.1371/journal.pone.0144640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oguma T, Kuriyama C, Nakayama K, et al. Changes in glucose-induced plasma active glucagon-like peptide-1 levels by co-administration of sodium-glucose cotransporter inhibitors with dipeptidyl peptidase-4 inhibitors in rodents. J Pharmacol Sci. 2016;132(4):255–261. doi: 10.1016/j.jphs.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi K, Murase M, Nakano D, et al. Angiotensin-converting enzyme inhibitor does not suppress renal angiotensin II levels in angiotensin I-infused rats. J Pharmacol Sci. 2013;122(2):103–108. doi: 10.1254/jphs.13045fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallo LA, Ward MS, Fotheringham AK, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6:26428. doi: 10.1038/srep26428. [DOI] [PMC free article] [PubMed] [Google Scholar]