Abstract
On average, memory capacity is significantly higher in populations of 50-60 year olds than in populations of 80 year olds. We define SuperAgers as individuals 80 or older whose episodic memory capacity is at least as good as that of cognitively average individuals in their 50s and 60s. SuperAgers therefore have memory capacity that is superior for age. Previous work showed that SuperAgers have greater cortical volumes and greater resistance to age-related cortical atrophy than ‘cognitively average’ individuals of the same age. Here we report on the cognitive, personality, and neuropathologic characteristics of the first 10 autopsy cases in the Northwestern SuperAging Program. During the follow-up period, seven SuperAgers maintained episodic memory performance within or above the average range for 50–65 year-old norms and all 10 SuperAgers maintained episodic memory scores within normal limits for their own age. Extraversion scores tended to be high on the NEO-PI-R measure of personality. The 10 autopsy specimens showed variable findings within the spectrum of Alzheimer pathology. The hippocampus and entorhinal cortex contained neurofibrillary degeneration mostly in the Braak II-III stages. However, even these limbic areas contained many healthy appearing neurons and the neocortex was generally free of neurofibrillary degeneration. In contrast, neocortical areas in at least 5 of the cases contained moderate to high densities of neuritic plaques. These findings need to be placed in context by comparing them to the neuropathology of cognitively average individuals of the same age. Future research on SuperAgers is likely to offer insights into factors that either prevent the emergence of involutional changes in the brain or that makes cognitive function more resistant to their consequences.
Keywords: Aging, Memory, Successful Aging, Alzheimer’s disease, dementia
INTRODUCTION
Complaints of declining episodic memory are nearly universal as people age. Are they inevitable? This is a central question addressed by the Northwestern SuperAging research program. The goal of the program is to explore biologic, psychosocial, genetic, and neuropathologic factors that may promote the preservation of episodic memory in advanced old age. In contrast to ‘successful aging,’ an ambiguous term with multiple definitions (Depp & Jeste, 2006), we have qualified the term ‘SuperAging’ with a strict operational definition that facilitates systematic investigations. SuperAgers are defined (Rogalski et al., 2013) as those aged 80 and above whose episodic memory performance is at least at the level of cognitively average individuals in their 50s and 60s.
Cross-sectional and longitudinal MR studies showed that SuperAgers have better cortical integrity (Harrison, Weintraub, Mesulam, & Rogalski, 2012; Rogalski et al., 2013), a thicker anterior cingulate cortex (Harrison et al., 2012; Rogalski et al., 2013), and slower rates of in vivo atrophy than their cognitively average peers (Cook et al., 2017) along with maintenance of episodic memory performance over 18-months (Gefen et al., 2014). Moreover, the brains of SuperAgers studied at post-mortem showed a greater number of von Economo neurons (VENs), unique spindle-shaped neurons implicated in higher-order functioning, and less Alzheimer’s pathology in the anterior cingulate compared to their age-matched cognitively normal peers (Gefen et al., 2014; Rogalski et al., 2013). This report provides a detailed account of the cognitive trajectories, personality profiles, and neuropathologic features of the first 10 SuperAging participants who died and donated their brains for post-mortem autopsy.
METHODS
Participants
Participants were enrolled into the SuperAging research program and, contemporaneously, into the Northwestern Alzheimer’s Disease Center (ADC) Clinical Core. Individuals who met SuperAging criteria at enrollment (described below) and who came to autopsy were included in this report. Recruitment into the SuperAging research program occurred through Northwestern’s ADC Clinical Core, community lectures, and word of mouth. Northwestern University’s Institutional Review Board approved this study. Informed consent was obtained from each participant.
To be designated a SuperAger, individuals were required to be age 80 or above and to perform at or above average normative values for 50–65 year-olds (scaled score ≥ 10) on an episodic memory test, the delayed recall score of the Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, 2004) and within one standard deviation of the average range for their age on non-memory measures according to published normative values (Heaton, 2004; Randolph, 1998; Saxton et al., 2000). The non-memory measures were a 30-item version of the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983), the Trail Making Test Part B (Randolph, 1998), and the Category Fluency Test (Morris et al., 1989). These measures are particularly vulnerable to change in aging and dementia (see Weintraub et al., 2009). Full scale IQ was measured with the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III). Personality profiles of SuperAgers were assessed with the 240-item NEO-PI-R (Costa & McCrae, 1992), a self-report questionnaire measuring the interpersonal, motivational, emotional, and attitudinal styles of adults. Items on the NEO-PI-R consist of self-statements that load onto five different personality factors: Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness. Raw factor scores from the NEO-PI-R were standardized as T-scores (M = 50, SD = 10) using gender-specific adult norms and five descriptive characterizations: Very Low (T = 20–34), Low (T = 35–44), Average (T = 45–54), High (T = 55–64), and Very High (T = 65–80). ApoE genotype was assessed from DNA extracted from blood samples provided by each participant in the course of the procedures for enrollment into the Clinical Core of the Northwestern ADC.
Post-mortem Specimens
Brain autopsies of SuperAging participants were performed by the Neuropathology Core of the Northwestern ADC. All brains were evaluated grossly for cortical, caudate, hippocampal, cerebellar, and brainstem atrophy, vascular pathology, and microscopically for Alzheimer’s disease (AD), Lewy body, and vascular pathologies, and FTLD-tau, FTLD-TDP, FTLD-FUS, and Lewy and non-Lewy alpha-synucleinopathies. Two board-certified neuropathologists with special expertise in neurodegenerative disorders (E.H.B. and Q.M.) rated the gross hippocampal atrophy subjectively and semi-quantitatively, as none, mild, moderate, or severe for each case.
Tissue Processing and Histopathology
Post-mortem intervals ranged from 4 to 58 hours, and brains were fixed in formalin or paraformaldehyde. Blocks of tissue from regions of interest were embedded in paraffin, cut at 5μm thickness, and mounted on charged slides. For immunohistochemical stains, sections were deparaffinized in xylene and stained using the avidin-biotin-peroxidase (ABC) method. The Vecstastain Elite Kit (Vector Laboratories, Cat #PK 6100, Burlingame, CA, USA) was utilized with streptavidin- or avidin- peroxidase, and amino ethylcarbazole (Biogenex, San Ramon, CA, USA) or diaminobenzidine (Cat # 34065, Pierce-Endogen, Rockford, IL, USA) as chromogen.
To assess AD pathology, specific antibodies to phosphorylated tau (AT8, Pierce-Endogen, Rockford, IL, USA) and beta-amyloid (4G8, Signet, Dedham, MA, USA) were used to visualize neurofibrillary tangles (NFTs) and beta-amyloid plaques, respectively. For evaluation of TDP-43 pathology, an antibody against TDP-43 phosphorylated at Ser409/410 (pS409–410–2, Cosmo Bio, Tokyo, Japan) was used. All brains were also evaluated for Lewy body pathology with phosphorylated alpha-synuclein antibody (phospho S129, abcam Cambridge MA), and for non-Alzheimer- and non-Lewy-type deposits of tau, alpha-synuclein, and ubiquitin (DAKO polyclonal, Carpinteria, CA, USA) or p62 (p62Lck ligand, BD Biosciences, San Jose, California).
Sections from each cortical area were also stained with 1.0% Thioflavin-S (Sigma-Aldrich), which recognizes β-pleated sheet protein conformations to visualize neurofibrillary tangles and neuritic amyloid plaques. The Gallyas Silver Stain was used to confirm the presence of neurofibrillary tangles, and H & E was used to note obvious neuronal loss and astrocytosis, superficial microvacuolation and gliosis, and/or depopulation of pigmented nuclei. For this project, sections from Cases 4 and 10 were stained for Nissl substance with .5–1.0% cresyl violet to visualize neurons in entorhinal cortex and the CA1 region of the hippocampus.
RESULTS
Demographic information is provided in Table 1. All ten participants were right-handed women. SuperAgers were required to be at least 80 years; however, age at enrollment ranged from 80–95 years. Each participant was followed for at least 3 years (Table 1). Length of follow-up differed by participant due to rolling admission into the study and variable age at death. Education ranged from 12 to 19 years. WAIS-III Full-scale IQ (FSIQ) was in the High Average to Superior range for all participants (Table 1). Six SuperAgers showed a significant split between verbal (VIQ) and nonverbal (PIQ) abilities, with relative strengths demonstrated in verbal abilities for five of the participants (Wechsler, 1997). There was no significant correlation between RAVLT-delay performance and WAIS-III FSIQ, PIQ, VIQ in this small sample (all p’s > 0.05).
Table 1.
Demographics and IQ Scores
| Case # | Age at Enrollment | Age at Death | Education | VIQ | PIQ | FSIQ |
|---|---|---|---|---|---|---|
| Case 1 | 87 | 90 | 14 | 111a | 108a | 111a |
| Case 2 | 84 | 87 | 18 | 122b | 113 | 119 |
| Case 3 | 89 | 95 | 18 | 131b | 113 | 127 |
| Case 4 | 80 | 87 | 12 | 134 | 134 | 133 |
| Case 5 | 87 | 90 | 18 | 140b | 119 | 135 |
| Case 6 | 84 | 87 | 19 | 133b | 116 | 135 |
| Case 7 | 95 | 99 | 16 | 124b | 95 | 112 |
| Case 8 | 81 | 91 | 14 | 125 | 142b | 143 |
| Case 9 | 85 | 94 | 14 | 116 | 114 | 115 |
| Case 10 | 83 | 92 | 12 | 119b | 107 | 112 |
Age and education are provided in years.
The WAIS-III (Wechsler, 1997) was used to measure current level of intellectual abilities (i.e., Full-Scale IQ or FSIQ) for all participants except Case 1, which was estimated based on the Barona formula (Barona, Reynolds, & Chastain, 1984). WAIS-III IQ scores were based on norms for 80+ year olds. VIQ: Verbal Intelligence Quotient, PIQ: Performance Intelligence Quotient;
Indicates significant split between WAIS-III PIQ and VIQ. A difference of 8.63 between scores is considered significant (p<0.05) for those age 80–84 while difference of 9.49 is considered significant for those age 85–89 (Wechsler, 1997). Cases 1, 2 and 5 were included in a previous report (Rogalski et al. 2013).
Cognitive trajectories
Seven of the 10 participants (Cases 1–7) were able to maintain episodic memory performance within the average range or above for 50–65 year-old norms (Figure 1). Three participants (Case 8, 9, 10) declined below the average range for 50–65 year-olds norms during the follow-up period (scaled score ≤ 7; Figure 1). However, according to age-appropriate norms, scores for each of the 10 participants remained within the average range or above for their age on the RAVLT-delayed memory test (scaled score ≥ 8) across all visits.
Figure 1. Episodic memory performance over time for each SuperAger.
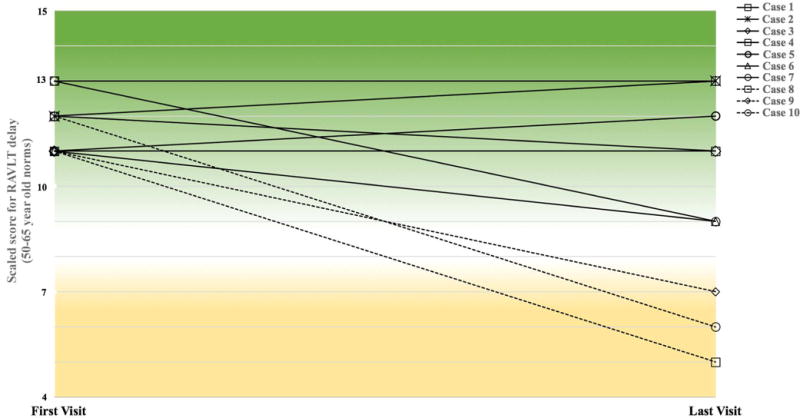
The delayed recalled score on the RAVLT test was used to assess episodic memory performance at enrollment and over time. Participants were required to score at least average compared to 50–65 year-old norms (scaled score ≥10) at their initial visit. Each case is represented by a separate line. Cases 1–7, represented by solid lines, maintained performance in the average range for 50–65 year-old norms (scaled score ≥ 8) over time. Cases 8, 9 and 10, represented by dashed lines, performed below the average range compared to 50–65 year-old norms at the last visit prior to death. Scaled scores for the initial and final visits are provided for each case. The mean number of years between the first and last visits was 4.9 +/−2.8. Green shading indicates psychometric average to superior range (scaled score ≥ 8) while yellow shading signifies below average range (scaled score ≤7).
Across all visits, performance for non-memory domains generally fell within average to superior ranges for age including executive function, attention, and language, and remained stable for Cases 1–8. Case 9 showed evidence of decline in non-memory domains over time with scores falling in average or below average ranges. In non-memory cognitive domains scores, Case 10 generally fell within average or above average ranges for age with some evidence of decline just prior to death.
ApoE
The Apolipoprotein (ApoE)-ε4 allele is one of the strongest and most extensively reported risk factors for dementia due to Alzheimer neuropathology. ApoE genotypes were available for all 10 SuperAging participants. Of the 10 SuperAging cases, two (20%) had an ε4 allele, one had ε2,ε3 and the rest had ε3,ε3, which is the most common genotype in the general population. The ε4 frequency is slightly lower than that of a sample of nondemented older control participants previously reported from the Northwestern ADC Clinical Core, which showed 26% had at least one ε4 allele (Rogalski et al., 2013).
Personality Factors
Age-related changes in personality factors such as neuroticism, extraversion, and openness, have been studied (Roberts, Walton, & Viechtbauer, 2006) and linked to changes in cognitive acuity, physical health, mood, and daily living activities (Boyle et al., 2010; Donnellan & Lucas, 2008; Steunenberg, Twisk, Beekman, Deeg, & Kerkhof, 2005). For example, aging has been associated with a decrease in Extraversion and Openness (Donnellan & Lucas, 2008). Increases in Neuroticism have also been reported above age 70 or 80 and at times associated with cognitive decline and/or dementia (Steunenberg et al., 2005; Terracciano, McCrae, Brant, & Costa, 2005). It remains unclear whether the reported personality changes seen in elderly adults are more closely related to the typical decline in cognitive abilities associated with ‘normal’ aging, or related to the passage of time. Examination of personality profiles of SuperAgers provides a unique opportunity to inspect personality traits in older adults in a setting where episodic memory performance is better than expected for age.
Nine of the 10 participants completed the NEO-PI-R personality inventory. As a group, there were no appreciable deviations from the average range in Neuroticism (Mean T = 53.5), Openness (Mean T = 50.1), Agreeableness (Mean T = 44.3), or Conscientiousness (Mean T = 51.5). Extraversion scores tended to be high (Mean T = 59), with all but one participant characterized as having a High (n=5) or Very High (n= 3) disposition towards extraversion.
Case Vignettes
Brief vignettes, qualitatively juxtaposing the demographic, medical, cognitive, lifestyle and personality data are provided for Case 4, who maintained stable performance until death, and Case 10, who showed decline over time (Figure 2). These two participants also participated in an optional Life Story Interview, a guided interview designed to examine the prosocial aspects of adult development (http://www.sesp.northwestern.edu/foley/research/stories/).
Figure 2. Episodic memory performance by visit and MR imaging for Case 4 and Case 10.
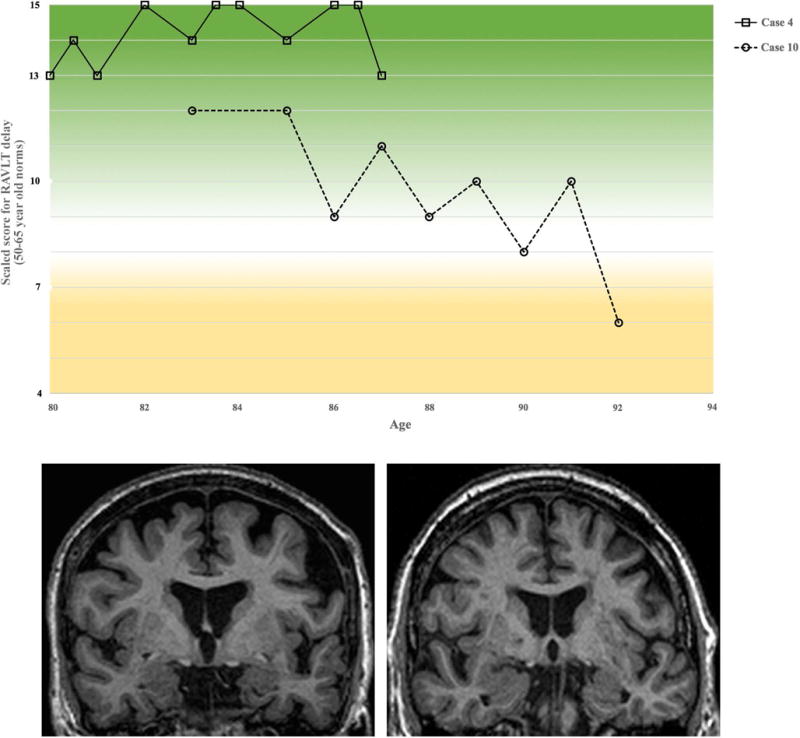
(Top) RAVLT delayed recall scaled scores are shown for both cases from entry into study until last visit prior to death. Case 4 is represented by the solid line, while Case 10 is represented by the dashed line. Coronal MR images from Case 4 (bottom left) and Case 10 (bottom right) are provided.
Case 4 was a right-handed Caucasian woman with 12 years of education who enrolled into the SuperAging research program at age 80. At that time, her level of general intellectual functioning fell within the superior range (WAIS-III FSIQ = 133). Over the course of 7 years, she performed within the high average to superior range on the RAVLT test of episodic memory (raw score RAVLT-delay range: 13–15 of 15 total possible), compared to norms for individuals in their 50s and 60s (Figure 2). Performance on tests of language, attention, and executive functioning were within average to superior ranges according to 80+-year-old norms and remained stable across all visits. The Mini Mental State Examination (MMSE) score remained in the range of 29–30 and the Clinical Dementia Rating (CDR) scale score was 0 (i.e., normal) over the 7 years. Neurological examinations did not reveal diagnostic abnormalities referable to central nervous system (CNS) damage. She did not complain of mood disorder. Medical history noted cardiac and thyroid problems. She denied substance abuse. There was no reported family history of neurodegenerative disorders. She reported normal birth and development, and described growing up “extremely poor,” helping to raise a younger family member from an early age. She was divorced with two children. She worked as a secretary until retirement at age 66 but remained highly active, participating in volunteer work and in various leadership positions in her 80s. She enjoyed traveling and was an avid reader. She described herself as “independent”, “giving”, and “optimistic”, with a strong belief in God. She recalled that her first memories were of the love her parents showed: “No money, just love. That worked.” She was found dead at home at age 87 of unknown cause.
Case 10 was a right-handed Caucasian woman with 13 years of education who was 85-years-old at enrollment. At age 85, her level of general intellectual functioning fell within the high average range (WAIS-III FSIQ = 112), with a relative strength demonstrated in verbal abilities (WAIS-III VIQ =119 and PIQ =107). Though her initial episodic memory performance at age 85 was within the high average range for 50–65 year olds, she showed fluctuating scores over the 7–8 years of follow-up, within a range of >2 standard deviations. Nonetheless, episodic memory performance remained within the average range for 50–65 year old norms until the month prior to death (Figure 2) and within average-for-age range for her own age throughout her life (RAVLT-delay 80+ year old norms ss = 9, age 92). In non-memory cognitive domains, scores were generally within average or above average ranges for her age. Across all visits the MMSE was ≥26; CDR was 0 until the months prior to death, where it declined to 0.5; she was independent in activities of daily living. Medical history was remarkable for hypertension, dyslipidemia, atrial fibrillation, hypercholesterolemia, hysterectomy (with estrogen replacement therapy), and right pontine infarct (age 87). Following the stroke, she experienced serious depression; however, her RAVLT-delay score remained in the average range for 50–65 year old norms until her last visit one month prior to death. She described emotional and physical trauma in childhood and a long history of heavy alcohol use. There was no family history of neurodegenerative disorders. She outlived her children. Over her life, she held several management level occupations and retired at age 78. On the NEO-PI-R measure of personality, her scores reflected a high tendency for openness. She described herself as “fiercely independent”, “rebellious”, and “liberal”. She questioned religion at a young age and considered herself agnostic. She loved traveling, reading, and poetry. She died at age 92 in part from malnutrition/dehydration.
Neuropathologic Features
Neuropathologic features for each of the 10 participants are provided in Tables 2 and 3. Seven of 10 participants were over age 90 at death (Mean: 91.2 +/− 3.9; Range: 87–99). Gross cortical atrophy was not present or mild for nearly all cases, with rare indications (n=2) of asymmetric atrophy (left greater than right hemisphere) in the temporal lobe. Gross hippocampal atrophy was absent in 3 cases and mild in 7 (Table 3).
Table 2.
Alzheimer neuropathology by case
| Case # | Age at Death | ApoE | Brain weight in grams |
Gross Hippocampal Atrophy |
Braak Stage | Neuritic Plaques | Neurofibrillary Tangles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hippocampo-entorhinal Complex | Neocortex | Hippocampal Complex | Neocortex | ||||||||||||
| H | EC | Frontal | Temporal | Parietal | H | EC | Frontal | Temporal | Parietal | ||||||
| Case 1 | 90 | 2,3 | 1100 | none | II-III | 0 | 0 | 0 | 0 | 0 | ++ | ++ | 0 | 0 | 0 |
| Case 2 | 87 | 3,3 | 1090 | none | II-III | + | + | 0 | ++ | ++ | + | + | 0 | 0 | 0 |
| Case 3 | 95 | 3,4 | 1280 | mild | IV | 0 | 0 | 0 | 0 | 0 | +++ | +++ | 0 | ++ | 0 |
| Case 4 | 87 | 3,3 | 1240 | mild | III | 0 | 0 | ++ | 0 | ++ | +++ | +++ | 0 | 0 | 0 |
| Case 5 | 90 | 3,3 | 990 | none | II-III | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | 0 |
| Case 6 | 87 | 3,4 | 1230 | moderate L, mild R | III | + | ++ | ++ | +++ | +++ | ++ | ++ | 0 | 0 | 0 |
| Case 7 | 99 | 3,3 | 1020 | mild | III | 0 | + | +++ | ++ | ++ | +++ | +++ | 0 | 0 | 0 |
| Case 8 | 91 | 3,3 | 1080 | mild | III | + | + | +++ | + | + | ++ | +++ | 0 | 0 | 0 |
| Case 9 | 94 | 3,3 | 1198 | mild | III | ++ | ++ | +++ | ++ | ++ | ++ | +++ | 0 | 0 | 0 |
| Case 10 | 92 | 3,3 | 1040 | mild | III | 0 | 0 | 0 | 0 | 0 | +++ | ++ | 0 | 0 | 0 |
H: hippocampus; EC: Entorhinal Cortex; 0: None to rare; +: sparse, ++: moderate; +++: frequent. The highest of the two hemisphere ratings was provided in the event of rare asymmetry.
Table 3.
Non-Alzheimer neuropathology by case
| Case # | Cerebrovascular disease (CVD) | FTLD-TDP | HS | PART | Lewy bodies | ARTAG | AGD |
|---|---|---|---|---|---|---|---|
| Case 1 | No infarcts/hemorrhages | N | N | Definite | DMN vagus (Incidental) | Y | N |
| Case 2 | No infarcts/hemorrhages | N | N | N | N | Y | N |
| Case 3 | 5 remote microinfarcts, left caudate | N | N | Definite | N | Y | N |
| Case 4 | No infarcts/hemorrhages | N | N | N | N | Y | Y |
| Case 5 | 1 remote lacunar infarct, left globus pallidus | N | N | N | N | N | N |
| Case 6 | No infarcts/hemorrhages | Y | Y | N | N | Y | Y |
| Case 7 | Multiple cortical microinfarcts (likely non-significant); One remote lacunar infarct, left putamen | N | N | N | N | Y | Y |
| Case 8 | Remote lacunar infarct, left putamen | N | N | N | N | Y | N |
| Case 9 | No infarcts/hemorrhages | N | N | N | N | Y | N |
| Case 10 | Incomplete white matter infarction & remote lacunar infarct, left thalamus | N | N | Possible | Amygdala (Incidental) | Y | Y |
FTLD-TDP: frontotemporal lobar degeneration with TDP-43 positive inclusions; HS: hippocampal sclerosis; PART: primary age-related tauopathy; ARTAG: Aging-related tau astrogliopathy; AGD: Argyrophilic grain disease DMN: dorsal motor nucleus
Cognitively average older individuals commonly show the neurofibrillary tangles (NFT) and amyloid plaques pathognomonic of AD, albeit at low densities and restricted distributions. The reasons for this are incompletely understood but important for disentangling the complex relationship between aging and AD.
Neurofibrillary tangle densities were qualitatively ranked according to the widely used Braak staging system where Stage 0 or I indicate the lowest density of NFT and Stage VI is the highest (Braak & Braak, 1991, 1995). Nine of 10 SuperAging cases showed tangle densities consistent with Braak Stage II or III. Case 3 was the only case that showed Braak Stage IV but without neuritic plaques (NP), a combination that is also known as primary age-related tauopathy (PART) (Crary et al., 2014). Case 1 (Braak II-III) and Case 10 (Braak III) also had NFT without additional NP and therefore qualified for the PART designation (Table 3).
The semiquantitative neuroanatomical distribution of NP and NFT were based on Thioflavin-S- and Gallyas- stained sections (Table 2). Between two and six sections from the hippocampus, frontal, temporal, and parietal cortices cut at 5μm were used for the analyses. Density of lesions was assessed on a scale of 0 (virtually no tangles or plaques in multiple sections) to +++ (frequent) based on a modified version of a published scale (Mirra, Hart, & Terry, 1993; Montine et al., 2012). Tangles ranged from sparse to frequent in the hippocampus but were absent in neocortical regions for nine of 10 cases, demonstrating the focality of tangle deposition. Case 3, with NFT but no amyloid (i.e., PART), was the only case with neocortical tangles, but only in temporal cortex. NP were more variable and present in the neocortex of six cases at moderate to frequent quantities. In contrast to NFT, NP were less frequent in the hippocampus.
The photomicrographs in Figure 3 from Cases 4 and 10 show that, while tangles were present in the entorhinal cortex and CA1 region of the hippocampus, the same areas contained many viable neurons, a feature that may explain why Braak stages of II and III may still be compatible with preserved functionality of the hippocampo-entorhinal complex.
Figure 3. Preserved hippocampo-entorhinal complex neurons in the presence of tangles.
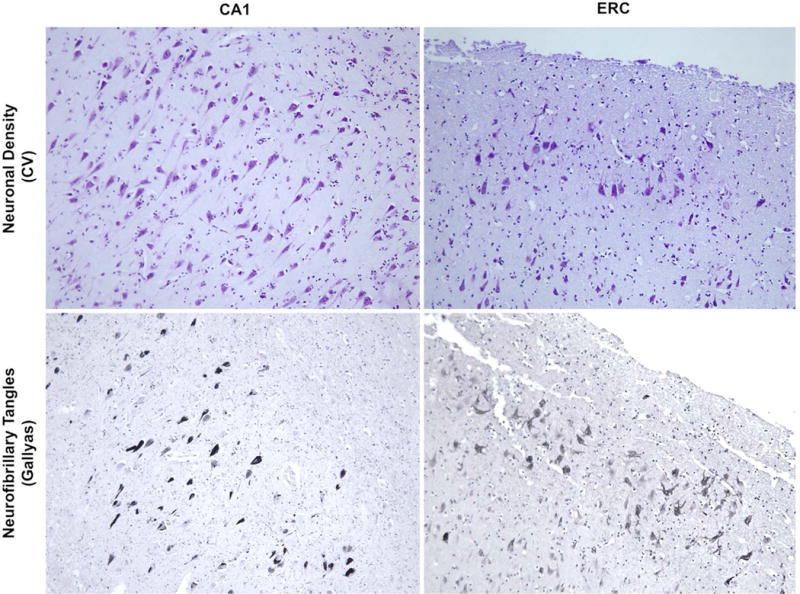
(Top) Nissl-stained sections showed preservation of neuronal integrity in the CA1 subfield of the hippocampus and in the entorhinal cortex (ERC), while (Bottom) adjacent Gallyas stained sections showed tangles consistent with Braak Stage III. All photomicrographs were obtained at 10x. Sections from the left panel are from Case 10, while sections in the right panel are from Case 4.
Three of the cases had one notable infarct (Cases 5, 7, 8); Case 10 had two infarcts and Case 3 had 5 infarcts (Table 3). Hippocampal sclerosis (HS) was seen in a single case and showed comorbid TDP-43 immunoreactive inclusions, which is consistent with the 10% occurrence of HS in older adults and the 90% comorbidity of HS with TDP-43 (Nelson et al., 2011). Aging-related tau astrogliopathy (ARTAG), another common feature in individuals over 60 years old (Kovacs et al., 2016), was present in each of the 10 SuperAging cases. Argyrophilic grain disease (AGD) was seen in four of 10 cases. There were incidental Lewy bodies, one involving the dorsal motor nucleus of the vagus nerve, and one involving the amygdala, in two of the SuperAgers (Table 3).
Integration of Demographic, Cognitive and Neuropathologic Features
The descriptive characterization of these participants did not reveal clear relationships among age at death, amount of pathologic burden, and cognitive trajectory. For example, Case 6 showed significant neuropathology including moderate AD, unilateral HS, moderate cerebrovascular disease with two remote microinfarcts, pTDP-43 positive inclusions, and AGD. Yet, she was among the youngest in our group (age 87 at death) and her cognitive performance, including both memory and non-memory domains, was in the average to superior range for age 10-months prior to death.
Previous studies suggest a relationship between worse memory performance and greater tangle burden in medial temporal memory-related regions even in cognitively normal individuals (Guillozet, Weintraub, Mash, & Mesulam, 2003). It remains to be seen whether participants with Braak stage III or IV tangles were able to maintain superior memory performance because the remaining healthy neurons were compensating for the neurofibrillary degeneration of adjacent neurons or if there were additional factors that made the hippocampo-entorhinal complex resistant to at least some of the toxic effects of neurofibrillary degeneration.
DISCUSSION
The Northwestern SuperAging research program recruits subjects 80 years of age or older whose episodic memory capacity is within the normative range for individuals in their 50s and 60s. Northwestern SuperAgers therefore have memory capacity that is superior for their age. In previous investigations we found that SuperAgers have greater cortical thickness than same-age peers with average memory, that they do not show cortical thinning when compared to cognitively average individuals in their 50s and 60s, that they have a region of anterior cingulate cortex with greater thickness and density of VENs than cognitively average peers, and that this cingulate region is less vulnerable to age-related Alzheimer pathology (Gefen et al., 2015; Gefen et al., 2014; Harrison et al., 2012; Rogalski et al., 2013). We also found that SuperAgers show maintenance of memory capacity and less decline of cortical thickness than cognitively average peers over an interval of 18 months (Cook et al., 2017; Gefen et al., 2014). This last finding suggests that the greater cortical volume of SuperAgers reflects a resistance to age-related atrophy rather than the consequence of thicker cortex at birth. We also reported preliminary significant associations between the SuperAging phenotype and single nucleotide polymorphisms in the MAP2K3 gene (Mitogen-Activated Protein Kinase Kinase 3) (Huentelman et al., in press).
The Northwestern SuperAging program has enrolled 74 participants to date with a greater percentage of women than men (74% female). It is probably premature to comment on gender-specific biases in SuperAging, as there are several factors to consider, including the slightly longer lifespan for women, a tendency for women to volunteer for research more often than men, and suggestions that women tend to have stronger episodic memory abilities than men (de Frias, Nilsson, & Herlitz, 2006; Finkel, Reynolds, McArdle, Gatz, & Pedersen, 2003; McCarrey, An, Kitner-Triolo, Ferrucci, & Resnick, 2016). Yet, the predominance of women SuperAgers is intriguing given the higher predominance of Alzheimer’s disease in women than men (Bachman et al., 1992; “Canadian study of health and aging: study methods and prevalence of dementia,” 1994; Manubens et al., 1995).
This small sample of SuperAgers tended to have higher than average endorsements of Extraversion. Higher than average Extraversion in SuperAgers may not be surprising as their preserved cognitive functioning may enable them to be more independent and active in their community and allow them to be interested in and enthusiastic about engaging in new activities and meeting new people. Confirmation of the consistency of the unique personality profile observations in a larger cohort of SuperAgers is required before conclusions can be drawn. Such an investigation could offer opportunities for disentangling relationships among personality, aging, and cognition and may eventually lead to potential targets for cognitive-behavioral interventions.
The two vignettes highlight optimism, resilience, and perseverance in the face of life challenges as well as active lifestyles. One SuperAger reported: “The negatives in my life certainly influenced me but I think, all-in-all, I always had a positive attitude.” Reading and travel were consistent themes. These themes align well with the suggested importance of participating in cognitively stimulating activities for maintaining cognitive health in older age (Festini, McDonough, & Park, 2016; Hultsch, Hertzog, Small, & Dixon, 1999; Park et al., 2014; Singh-Manoux, Richards, & Marmot, 2003; Wilson et al., 2002; Wilson, Scherr, Schneider, Tang, & Bennett, 2007). The descriptions of the participants are consistent with our recent report examining psychological wellbeing, where 31 SuperAgers endorsed higher ‘positive social relationships with others’ compared to their similarly-aged cognitively average peers (Cook Maher et al., 2017). Further evaluation of the occurrence of other prosocial behaviors including generativity, altruism, life-long learning, and the development of self-understanding through The Life Story Interview or other systematic investigation across the individuals is required before drawing definitive conclusions.
While cognitive reserve remains a powerful concept, there is no single biologically relevant gold standard measurement for its characterization. IQ and education are commonly used as proxy measures. While all SuperAging participants in this report showed IQ scores in the high average to superior range, formal education varied from 12 to 18 years. In our previous SuperAging studies (Cook et al., 2017; Gefen et al., 2014; Harrison et al., 2012; Rogalski et al., 2013) education and/or IQ of the comparison groups (e.g. cognitively average controls, amnestic mild cognitive impairment, aMCI) was similar to that of the SuperAgers, suggesting that, while high IQ may be advantageous, it does not guarantee youthful memory performance in older age.
There was no overrepresentation of the ε2 allele of ApoE, which has been proposed as protective against AD. Identification of potentially protective genetic factors (e.g. our recent MAP2K3 gene report) may elucidate molecular mechanisms that promote superior memory performance in aging, and offer novel insights for memory-related disorders like Alzheimer’s Disease.
All cases had substantial concentrations of neurofibrillary degeneration in the hippocampo-entorhinal complex. There was no obvious relationship between memory performance at the last visit and this type of pathology. The residual healthy neurons in the hippocampo-entorhinal complex and absence of neurofibrillary degeneration in neocortex may turn out to be critical factors. These cases suggest at least two possible pathways for those with superior memory performance in older age. Some SuperAgers with lower than expected neuropathologic burden given their age may be somehow resistant to the emergence of pathology. However, other SuperAgers with substantial neuropathologic burden may be somehow resistant to the negative cognitive consequences associated with neurodegenerative disease.
The SuperAging project has generated novel opportunities for exploring the determinants of superior cognitive aging. Although the reported sample of 10 autopsy cases is too small for definitive conclusions, the results indicate that excellent memory capacity is biologically possible in late life and that it can be maintained for years, even when there is significant neuropathologic burden. The findings also suggest that SuperAgers may have unique personality profiles. Understanding factors associated with superior memory capacity in older age offers an opportunity for preventing or mitigating age-related changes of cognition and understanding the relationships among aging, Alzheimer’s disease, and other neuropathologic mechanisms.
Acknowledgments
We would like to acknowledge our research participants for the time they devoted to this research project, Emmaleigh Loyer, Hannah McKenna, Kristin Whitney, Amanda Rueter, Joseph Boyle, and Marie Saxon for assistance with scheduling and testing participants, and Jaiashre Sridhar for her technical assistance with the figures for this manuscript. We would like to acknowledge Regina Logan and The Foley Center for the Study of Lives for their assistance with the Life Story Interviews.
This work was funded in part by grants from the National Institutes of Health, including R01 AG045571 from the from the National Institute on Aging, P30 AG13854 Alzheimer’s Disease Core Center from the National Institute on Aging, and the Davee Foundation.
Footnotes
The authors do not have any conflicts of interest to report.
References
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42(1):115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Boyle LL, Lyness JM, Duberstein PR, Karuza J, King DA, Messing S, Tu X. Trait neuroticism, depression, and cognitive function in older primary care patients. Am J Geriatr Psychiatry. 2010;18(4):305–312. doi: 10.1097/JGP.0b013e3181c2941b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer’s disease. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278-284. [DOI] [PubMed] [Google Scholar]
- Canadian study of health and aging: study methods and prevalence of dementia. CMAJ. 1994;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
- Cook AH, Sridhar J, Ohm D, Rademaker A, Mesulam MM, Weintraub S, Rogalski E. Rates of Cortical Atrophy in Adults 80 Years and Older With Superior vs Average Episodic Memory. JAMA. 2017;317(13):1373–1375. doi: 10.1001/jama.2017.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook Maher A, Kielb S, Loyer E, Connelley M, Rademaker A, Mesulam MM, Rogalski E. Psychological well-being in elderly adults with extraordinary episodic memory. PLoS One. 2017;12(10):e0186413. doi: 10.1371/journal.pone.0186413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychological assessment. 1992;4(1):5–13. doi: 10.1037/1040-3590.4.1.5. [DOI] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Nilsson LG, Herlitz A. Sex Differences in Cognition are Stable Over a 10-Year Period in Adulthood and Old Age. Aging, Neuropsychology, and Cognition. 2006;13(3-4):574–587. doi: 10.1080/13825580600678418. [DOI] [PubMed] [Google Scholar]
- Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2006;14(1):6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, Lucas RE. Age differences in the Big Five across the life span: evidence from two national samples. Psychol Aging. 2008;23(3):558–566. doi: 10.1037/a0012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festini SB, McDonough IM, Park DC. The Busier the Better: Greater Busyness Is Associated with Better Cognition. Front Aging Neurosci. 2016;8:98. doi: 10.3389/fnagi.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39(3):535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, Geula C. Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity. J Neurosci. 2015;35(4):1781–1791. doi: 10.1523/JNEUROSCI.2998-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Shaw E, Whitney K, Martersteck A, Stratton J, Rademaker A, Rogalski E. Longitudinal neuropsychological performance of cognitive SuperAgers. J Am Geriatr Soc. 2014;62(8):1598–1600. doi: 10.1111/jgs.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Archives of Neurology. 2003;60(5):729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam MM, Rogalski E. Superior memory and higher cortical volumes in unusually successful cognitive aging. Journal of the International Neuropsychological Society : JINS. 2012;18(6):1081–1085. doi: 10.1017/S1355617712000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RM, W S, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults - Professional Manual. Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- Huentelman M, Piras I, Siniard A, De Both M, Richholt R, Bigio EH, Rogalski E. Association of MAP2K3 gene variation and the SuperAging phenotype detected by whole exome sequencing. Alzheimers & Dementia: The Journal of the Alzheimer’s Association in press. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14(2):245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, Dickson DW. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2016;131(1):87–102. doi: 10.1007/s00401-015-1509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manubens JM, Martinez-Lage JM, Lacruz F, Muruzabal J, Larumbe R, Guarch C, Rocca WA. Prevalence of Alzheimer’s disease and other dementing disorders in Pamplona, Spain. Neuroepidemiology. 1995;14(4):155–164. doi: 10.1159/000109791. [DOI] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31(2):166–175. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Archives of Pathology and Laboratory Medicine. 1993;117(2):132–144. [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Alzheimer’s, A. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(Pt 5):1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lodi-Smith J, Drew L, Haber S, Hebrank A, Bischof GN, Aamodt W. The impact of sustained engagement on cognitive function in older adults: the Synapse Project. Psychol Sci. 2014;25(1):103–112. doi: 10.1177/0956797613499592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C. Repeatable battery for the assessment of neuropsychological status (RBANS) San Antonio: The Pyschological Corporation; 1998. [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol Bull. 2006;132(1):1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Mesulam MM. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci. 2013;25(1):29–36. doi: 10.1162/jocn_a_00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J, Ratcliff G, Munro CA, Coffey EC, Becker JT, Fried L, Kuller L. Normative data on the Boston Naming Test and two equivalent 30-item short forms. The Clinical Neuropsychologist. 2000;14(4):526–534. doi: 10.1076/clin.14.4.526.7204. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Western Psychological Services; 2004. [Google Scholar]
- Singh-Manoux A, Richards M, Marmot M. Leisure activities and cognitive function in middle age: evidence from the Whitehall II study. J Epidemiol Community Health. 2003;57(11):907–913. doi: 10.1136/jech.57.11.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steunenberg B, Twisk JW, Beekman AT, Deeg DJ, Kerkhof AJ. Stability and change of neuroticism in aging. J Gerontol B Psychol Sci Soc Sci. 2005;60(1):P27–33. doi: 10.1093/geronb/60.1.p27. [DOI] [PubMed] [Google Scholar]
- Terracciano A, McCrae RR, Brant LJ, Costa PT., Jr Hierarchical linear modeling analyses of the NEO-PI-R scales in the Baltimore Longitudinal Study of Aging. Psychol Aging. 2005;20(3):493–506. doi: 10.1037/0882-7974.20.3.493. doi:2005-13210-012 [pii] 10.1037/0882-7974.20.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Disease and Associated Disorders. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]


