Abstract
Objective
To determine if the coding strategies used to identify severe sepsis in administrative datasets could identify cases with comparable case mix, hospitalization characteristics, and outcomes as a cohort of children diagnosed with severe sepsis using strict clinical criteria.
Methods
We performed a retrospective cohort study using data from 2005-2011 from the New York and Florida State Inpatient Databases, available from the U.S. Healthcare Cost and Utilization Project. We compared four coding strategies: the single ICD-9-CM codes for 1) severe sepsis or 2) septic shock, and the algorithms developed by 3) Angus, et al. or 4) Martin, et al. which use a combination of ICD-9-CM codes for infection and organ dysfunction. We compared the cases identified by each strategy with each other and with children enrolled in the RESOLVE trial.
Results
The Angus criteria was nine times larger (n=23,995) than the smallest cohort, identified by the ‘septic shock’ code (n=2,601). Cases identified by the Angus and Martin strategies had low mortality rates, while the cases identified by the ‘severe sepsis’ and ‘septic shock’ codes had much higher mortality at all time points (e.g. 28-day mortality of 4.4% and 7.4% vs. 15.4% and 16.0%, respectively). Mortality in the ‘severe sepsis’ and ‘septic shock’ code cohorts was similar to that presented in the RESOLVE trial.
Conclusions
The ICD-9-CM codes for ‘severe sepsis’ and ‘septic shock’ identify smaller, but higher acuity cohorts of patients that more closely resemble the children enrolled in the largest clinical trial of pediatric severe sepsis to date.
Keywords: Sepsis, Septic Shock, Epidemiology, International Classification of Diseases, Mortality
To date, epidemiologic investigation of pediatric severe sepsis using large, administrative datasets has been hindered by variability in the definition of ‘severe sepsis’, yielding widely varying estimates of incidence, mortality and long-term outcome.1–5 Such disparate results, in turn, have raised additional questions about these studies’ generalizeability and applicability to populations of children diagnosed with severe sepsis via clinical criteria. However, administrative datasets remain an efficient and cost effective means to study large cohorts of severe sepsis patients over an extended period of time, revealing important epidemiologic data that would take years and considerable expense to collect using prospective cohorts of human subjects. For similar reasons, they are also uniquely suited to studying rare conditions and outcomes. Given these advantages, it would be of significant practical and scientific utility to identify a coding strategy that generates an administrative dataset population of children with severe sepsis that has similar illness characteristics and long-term outcomes as clinically identified populations of children with severe sepsis.
Recent epidemiologic studies of children with severe sepsis exemplify this problem. For example, three recent studies of pediatric severe sepsis using three unique datasets and coding strategies identified a prevalence among children hospitalized in the U.S. ranging between 0.45% - 7.7% and an in-hospital mortality rate ranging from 6.8% - 21.2%.3,6,7 Much of this variation appeared to be related to coding strategy, with certain strategies consistently identifying smaller, higher acuity patient populations than others. Only one of these studies assessed outcomes past 28 days, but since no comparison was made to a clinically identified population, it is unclear how their outcomes at one year might have differed from a clinical cohort.6 Weiss et al. compared a severe sepsis cohort developed with administrative data to two clinically identified populations, and found significant discrepancies in the case mix, PICU length of stay, and hospital costs.8 However, the administrative dataset cohort was identified using a pool of strategies and a large combination of codes, making it difficult to know if certain coding strategies would have been more comparable to the clinical cohorts than others.
Therefore, we conducted this study to determine if any of the commonly used coding strategies to identify severe sepsis in administrative datasets could identify a pediatric population with comparable case mix, hospitalization characteristics, short-term and long-term outcomes as a prospective cohort of children diagnosed with severe sepsis using strict clinical criteria. We selected four well-established and frequently used coding strategies to compare, including: the single ICD-9-CM codes for 1) severe sepsis or 2) septic shock, and the algorithms developed by 3) Angus, et al.1 and 4) Martin, et al.9 which use a combination of ICD-9-CM codes for infection and organ dysfunction. We compared the population of children with severe sepsis identified by each strategy with each other and with children diagnosed with severe sepsis in the RESOLVE trial.10 Conducted from November, 2002 - April, 2005 in 18 countries, the RESOLVE trial randomized 477 pediatric severe sepsis patients to treatment with activated drotrecogin alfa (n=240) or placebo (n= 237). Enrollment in the RESOLVE trial required demonstration of clinical criteria for severe sepsis including suspicion or proven infection, cardiovascular and respiratory organ dysfunction within the 12 hours prior to enrollment. For purposes of comparison with our administrative dataset cohorts, we combined the placebo and study drug groups in the RESOLVE trial, and then compared the characteristics and outcomes of this prospective cohort to the children identified by one or more of the four different coding algorithms.
Material and Methods
Data sources
To create our administrative dataset cohorts, we used the New York (NY) and Florida (FL) State Inpatient Databases (SID) from 2005 to 2011 to identify cases of severe sepsis. The SID are part of the collection of databases and software tools maintained by the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ). We specifically chose the NY and FL SID because they contain large, diverse populations and include an encrypted patient identifier that permits researchers to longitudinally follow pediatric patients over time. The SID contain basic demographic information (age, sex, and race), ICD-9-CM discharge diagnoses, type and number of in-hospital procedures, hospital length of stay, and discharge status for each hospitalization. For our comparison clinical cohort, we used the data published in 2007 as part of the RESOLVE trial, including baseline characteristics of the trial participants (study table 1) and clinical outcomes (study table 3). Because we used only publically available, de-identified data, the Washington University Institutional Review Board deemed this study to be exempt from review.
Coding strategies
To be consistent with the RESOLVE trial, we defined the study population as any patient < 18 years of age at the time of the first hospitalization for severe sepsis from 2005-2011. We defined a severe sepsis case in the administrative dataset as any hospitalization coded for one or more of our four coding algorithms: 1) severe sepsis (ICD-9-CM code 995.52), 2) septic shock (ICD-9-CM code 785.52), 3) the criteria of Angus, et al.1 or 4) Martin, et al.9 These latter two methods use a combination of ICD-9-CM codes for infection and acute organ dysfunction that are recognized to be consistent with severe sepsis. Briefly, the method used by Angus and colleagues combines 1,286 distinct infection codes with an additional 13 codes for acute organ dysfunction in the cardiovascular, respiratory, neurologic, hematologic, hepatic or renal systems.1 The method used by Martin et al. uses only six codes for sepsis or infection combined with an additional 32 codes for acute organ dysfunction.9 For the comparison clinical cohort, severe sepsis was defined by the RESOLVE trial’s inclusion criteria: ‘a suspected or proven infection, and systemic inflammation, sepsis-induced cardiovascular, and respiratory organ dysfunction within 12 hours before entering the study’.10
Analysis
To focus on typical sepsis populations, we restricted analyses to hospitalizations in an acute-care hospital with total length of stay of 180 days or less (this excluded only 1.2% of the total population coded for severe sepsis by one of the four algorithms). To eliminate out-of-state visitors who would be unlikely to have follow-up data, we restricted analyses to patients who were residents of Florida or New York.
To understand how the four administrative dataset cohorts compared, we first determined the demographics, case mix, short-term outcomes and one-year mortality of the four overlapping cohorts. We then developed several additional cohorts by 1) creating mutually exclusive groups for each coding strategy, then 2) creating several additional cohorts through combinations of the four coding algorithms. This permitted determination and comparison of the demographics, infection groupings, and outcomes in the populations identified by each strategy in total, uniquely by each strategy, and by combinations of codes.
To understand how the administrative cohorts compared to clinical patients, we then compared the administrative dataset cohorts to the patients in the RESOLVE trial. Because organ failure was identified and defined differently in the administrative datasets than the clinical trial, we chose not to make direct comparisons of organ failure rates and severity. Similarly, because the RESOLVE trial ended at 28 days, it was not possible to make a direct comparison of one-year mortality between RESOLVE study patients and the administrative dataset cohorts.
All categorical variables were compared using the Pearson Chi square test and mortality was compared using Chi square, Kaplan-Meier plots, and log-rank tests. To account for repeated comparisons, we defined statistical significance as a p-value ≤ 0.0125 (0.05/4 different comparisons). All data were analyzed using SAS Enterprise Guide version 7.1 (SAS, Cary, NC) and STATA version 14 (Stata, College Station, TX).
RESULTS
Administrative dataset cohorts: case mix
Of the 3,013,028 pediatric hospitalizations in the New York and Florida SIDs between 2005-2011, we identified 25,389 admissions that met the criteria for severe sepsis by at least one of the four coding strategies (Table 1). This corresponded to an overall prevalence of 0.84% of pediatric severe sepsis among hospitalized children. The coding strategy used by Angus, et al. identified the largest cohort of patients (n=23,995, over three times larger than the cohort identified by the Martin strategy and over seven times larger than the single ICD-9-CM code strategies). All four cohorts had similar distributions of race, sex and age (Table 1).
Table 1.
Demographics and Disease Characteristics of the Four Cohorts
| RESOLVE | Angus Strategy | Angus Strategy only | Martin Strategy | Martin Strategy only | Severe Sepsis code | Severe Sepsis code only | Septic Shock code | Septic Shock code only | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n=477 | n=23,995 | n=17,327 | n=7,388 | n=954 | n=3,155 | n=219 | n=2,601 | n=88 | |
| Male sex, n (%) | 28 (54.1%) | 13,086 (54.5%) | 9,457 (54.6%) | 4,058 (54.9%) | 548 (57.4%) | 1,695 (53.7%) | 119 (54.3%) | 1,399 (53.8%) | 52 (59.1%) |
| Age in years, n (%) | |||||||||
| 0 to < 1 | 151 (31.7%) | 10,095 (42.1%) | 7,632 (44.1%) | 2,588 (35.0%) | 316 (33.1%) | 1046 (33.2%) | 148 (67.6%) | 748 (28.8%) | 79 (89.8%) |
| 1 to < 5 | 149 (31.2%) | 4,328 (18.0%) | 3,126 (18.0%) | 1,499 (20.3%) | 298 (31.2%) | 560 (17.8%) | 19 (8.7%) | 465 (17.9%) | * |
| 5 to < 10 | 101 (21.2%) | 2,735 (11.4%) | 1,942 (11.2%) | 905 (12.3%) | 119 (12.5%) | 407 (12.9%) | 14 (6.4%) | 369 (14.2%) | * |
| 10 to < 18 | 76 (15.9%) | 6,837 (28.5%) | 4,627 (26.7%) | 2,396 (32.4%) | 221 (23.2%) | 1,142 (36.2%) | 38 (17.4%) | 1,019 (39.2%) | * |
| Race | |||||||||
| White | 314 (65.8%) | 10,161 (42.4%) | 7,357 (43.3%) | 3,049 (41.3%) | 342 (35.8%) | 1,292 (41.0%) | 74 (33.8%) | 1,136 (43.7%) | 40 (45.5%) |
| Hispanic | 95 (19.9%) | 4,206 (17.5%) | 3,043 (17.9%) | 1,365 (18.5%) | 222 (23.3%) | 573 (18.2%) | 27 (12.3%) | 471 (18.1%) | 12 (13.6%) |
| African-American | 28 (5.9%) | 5,962 (24.9%) | 4,307 (25.3%) | 1,884 (25.5%) | 286 (30.0%) | 790 (25.0%) | 67 (30.6%) | 592 (22.8%) | 17 (19.3%) |
| Asian or Pacific Islander | 13 (2.7%) | 725 (3.0%) | 510 (3.0%) | 224 (3.0%) | 98 (3.1%) | 12 (5.5%) | 84 (3.2%) | ||
| Other or Missing Race | 27 (5.7%) | 2,941 (12.3%) | 2,100 (12.1%) | 866 (11.7%) | 72 (7.5%) | 402 (12.7%) | 39 (17.8%) | 318 (12.2%) | 15 (17.0%) |
| Disease Severity | |||||||||
| Number of organ failures | |||||||||
| 1 | 9,439 (39.3%) | 7,353 (42.4%) | 2,611 (35.3%) | 585 (61.3%) | 714 (22.6%) | 67 (30.6%) | 570 (21.9%) | 29 (33.0%) | |
| 2 | 5,591 (23.3%) | 3,567 (20.6%) | 2,076 (28.1%) | 149 (15.6%) | 979 (31.0%) | 25 (11.4%) | 807 (31.0%) | 15 (17.1%) | |
| 3 | 1,812 (7.6%) | 697 (4.0%) | 1,074 (14.5%) | * | 732 (23.2%) | * | 681 (26.2%) | * | |
| 4 | 627 (2.6%) | 114 (0.7%) | 499 (6.8%) | 0 | 389 (12.33%) | * | 362 (13.9%) | * | |
| 5 | 209 (0.9%) | 20 (0.1%) | 188 (2.5%) | 0 | 154 (4.9%) | 0 | 164 (6.3%) | 0 | |
| Number of organ failures, median | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | |
| Cardiovascular Failure, n (%) | 3,113 (13.0%) | 430 (2.5%) | 2,460 (33.3%) | 0 | 2,245 (71.2%) | * | 2,601 (100%) | 88 (100%) | |
| Hematologic Failure, n (%) | 5,113 (21.3%) | 3,028 (17.5%) | 2,062 (27.9%) | 16 (1.7%) | 830 (26.3%) | 17 (7.8%) | 695 (26.7%) | * | |
| Hepatic-Metabolic Failure, n (%) | 9,460 (39.4%) | 5,993 (34.6%) | 3,829 (51.8%) | 471 (49.4%) | 1,773 (56.2%) | 72 (32.9%) | 1493 (57.4%) | 33 (37.5%) | |
| Renal Failure, n (%) | 2,520 (10.5%) | 1,441 (8.3%) | 1,022 (13.8%) | 0 | 695 (22.03%) | 38 (17.4%) | 477 (18.3%) | 13 (14.8%) | |
| Respiratory Failure, n (%) | 7,377 (30.7%) | 4,554 (26.3%) | 3,198 (43.3%) | 397 (41.6%) | 1,574 (50.0%) | 16 (7.3%) | 1,186 (45.6%) | 14 (15.9%) | |
| Neurologic Failure, n (%) | 2,141 (8.9%) | 1,694 (9.8%) | 452 (6.1%) | 17 (1.8%) | 185 (5.9%) | * | 145 (5.6) | * | |
| Site of infection | |||||||||
| Bacteremia | 151 (31.7%) | 6,551 (27.3%) | 457 (2.6%) | 7,060 (95.6%) | 928 (97.3%) | 2,743 (86.9%) | 96 (43.8%) | 2,743 (86.9%) | 88 (100%) |
| Respiratory | 1,336 (50.6%) | 10,293 (42.9%) | 8,091 (46.7%) | 2,434 (33.0%) | 301 (31.6%) | 1,133 (35.9%) | 40 (18.3%) | 894 (34.4%) | * |
| Intra-abdominal | 530 (20.1%) | 2,273 (9.5%) | 1,914 (11.1%) | 747 (10.1%) | 112 (11.7%) | 342 (10.8%) | 13 (5.9%) | 284 (10.9%) | 0 |
| Urinary Tract/GU | 268 (10.2%) | 2,692(11.2%) | 2,876 (16.6%) | 657 (8.9%) | 122 (12.8%) | 238 (8.5%) | 14 (6.4%) | 196 (7.5%) | * |
| Other | 479 (18.1%) | 11,857 (49.4%) | 8,965 (51.7%) | 3,195 (43.4%) | 446 (46.75%) | 1,053 (33.4%) | 41 (18.7%) | 864 (33.2%) | * |
| Mortality | |||||||||
| 14 day mortality | 67 (14.1%) | 732 (3.1%) | 308 (1.8%) | 393 (5.3%) | 11 (1.2%) | 364 (11.5%) | 27 (12.3%) | 316 (12.2%) | 28 (31.8%) |
| 28 day mortality | 82 (17.3%) | 1,053 (4.4%) | 456 (2.6%) | 545 (7.4%) | 13 (1.4%) | 486 (15.4%) | 39 (17.8%) | 415 (16.0%) | 33 (37.5%) |
| In-hospital mortality | 82 (17.3%) | 1,572 (6.6%) | 681 (3.9%) | 815 (11.0%) | 20 (2.1%) | 667 (21.1%) | 52 (23.7%) | 533 (20.5%) | 41 (46.6%) |
| One year mortality | N/A | 2,110 (8.8%) | 980 (5.7%) | 1,077 (14.6%) | 44 (4.6%) | 755 (24.0%) | 55 (25.1%) | 611 (23.5%) | 43 (48.9%) |
Overall, there was significant overlap in the cohorts identified by the four coding strategies: over 80% of cases identified by the single ICD-9-CM codes for ‘severe sepsis’ and ‘septic shock’ were also identified by the strategies used by Angus and Martin, and 86.4% of cases identified by the Martin strategy were also identified by the Angus strategy (Figure 1). The majority of cases identified by the Angus strategy (72.2%) were uniquely identified by that method alone (Table 1). In contrast, only 12.9% of the Martin cohort was unique to that strategy (n=954 cases), 6.9% of the ‘severe sepsis’ cohort was uniquely identified by that code (n=219 cases) and only 3.9% of the ‘septic shock’ cohort was uniquely identified by that code alone (n=88 cases). Perhaps due to the significant overlap, the cohort identified uniquely by the Angus strategy had similar gender, age and racial distributions to the larger parent cohort from which it derived (Table 1). However, markers of disease severity in the Angus definition-only cohort indicated less organ dysfunction, less cardiovascular failure, and less renal failure than the larger parent cohort (Table 1). A similar pattern was seen in the cohort identified uniquely by the Martin strategy, with no cases of cardiovascular or renal failure in this group, compared to 33.3% (cardiovascular) and 13.8% (renal) in the larger parent cohort from which they derived (Table 1).
Figure 1. Pediatric Severe Sepsis Cases Identified by Four ICD-9-CM Code Strategies.
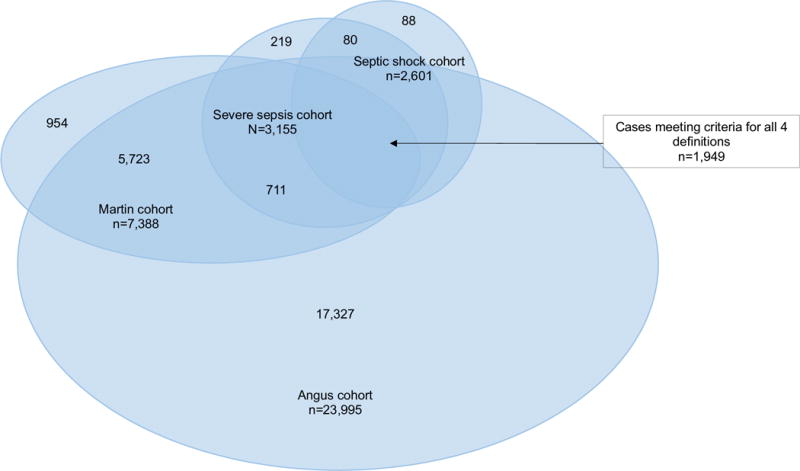
A total of 25,389 admissions included a diagnosis of severe sepsis by at least one of the four coding strategies. The cohort identified by Angus, et al. was the largest (n=23,995, over three times larger than the cohort identified by Martin, et al. and over seven times larger than the cohorts identified by single ICD-9-CM codes). Almost 8% (n=1,949) of cases met criteria for all four coding strategies.
The cohorts uniquely identified by the ‘severe sepsis’ and ‘septic shock’ codes were very small and had different demographics than their parent cohorts, with a significantly higher proportion of cases under one year old and many fewer adolescents (Table 1). In addition, cases identified uniquely by the ‘severe sepsis’ code had much lower proportions of cardiovascular, hepatic and respiratory failure than the parent cohort and a much lower proportion of ‘bacteremia’ as their site of infection. Because cardiovascular failure is in the definition of septic shock, all cases identified by the ‘septic shock’ code had cardiovascular failure. However, the cohort uniquely identified by the ‘septic shock’ code had much smaller proportions of cases with respiratory, hematologic and hepatic failure (Table 1).
Consistent with their parent cohorts, the 3,510 cases identified by both the Martin and Angus strategies had predominantly one or two organs failing, a low proportion of cardiovascular failure, a high proportion of respiratory failure, and a very high proportion of bacteremia as the site of infection (Table 2). Only 80 cases were identified by both of the single code strategies but neither the Angus or Martin strategies (‘severe sepsis’ AND ‘septic shock’, Table 2). This cohort was much younger (mean age 0.6 yrs, ± 2.5 yrs) and much sicker than any cohort identified by any single strategy or combination of strategies, with 48.8% mortality during the index hospitalization. In contrast, the cohort identified by the presence of the ‘severe sepsis’ code OR the ‘septic shock’ code was relatively large (n=3,598) and had severity of illness and distribution of organ failures most similar to the parent single code cohorts and the group identified by all four coding strategies. Almost 8% (n=1,949) of cases met criteria for severe sepsis by all four coding strategies. This group had the highest proportion of adolescents of any strategy, had the highest proportion of multiple organ dysfunction with 3 or more organs failing, and had very high proportions of cardiovascular, hepatic and respiratory failure (Table 2).
Table 2.
Demographic and Disease Characteristics of Cohorts identified by Combinations of Codes
| RESOLVE | Septic Shock OR Severe Sepsis codes | Septic Shock AND Severe Sepsis codes only | Angus AND Martin strategies only | All 4 Strategies | |
|---|---|---|---|---|---|
|
| |||||
| n=477 | n=3,598 | n=80 | n=3,510 | n=1,949 | |
| Male sex, n (%) | 28 (54.1%) | 1,939 (53.9%) | 43 (53.8%) | 1,933 (55.1%) | 1,048 (53.9%) |
| Age in years, n (%) | |||||
| 0 to < 1 | 151 (31.7%) | 1,245 (34.6%) | 75 (93.8%) | 1,541 (43.9%) | 387 (19.9%) |
| 1 to < 5 | 149 (31.2%) | 622 (17.3%) | * | 615 (17.5%) | 394 (20.2%) |
| 5 to < 10 | 101 (21.2%) | 449 (12.5%) | * | 366 (10.4%) | 318 (16.3%) |
| 10 to < 18 | 76 (15.9%) | 1,282 (35.6%) | * | 988 (28.1%) | 850 (43.6%) |
| Race | |||||
| White | 314 (65.8%) | 1,504 (42.3%) | 32 (41.0%) | 1,461 (42.3%) | 838 (42.9%) |
| Hispanic | 95 (19.9%) | 637 (17.9%) | * | 589 (17.1%) | 383 (19.7%) |
| African-American | 28 (5.9%) | 885 (24.9%) | 23 (29.4%) | 894 (25.9%) | 440 (22.6%) |
| Asian or Pacific Islander | 13 (2.7%) | 118 (3.3%) | 0 | 115 (3.3%) | 59 (3.0%) |
| Other or Missing Race | 27 (5.7%) | 454 (12.6%) | 13 (16.6%) | 451 (12.8%) | 229 (11.7%) |
| Disease Severity | |||||
| Number of organ failures | |||||
| 1 | 859 (23.9%) | 38 (47.5%) | 1,403 (40.0%) | 353 (18.1%) | |
| 2 | 1,118 (31.1%) | 26 (32.5%) | 1,002 (28.6%) | 590 (30.3%) | |
| 3 | 826 (23.0%) | 12 (15.0%) | 326 (9.3%) | 545 (28.0%) | |
| 4 | 428 (11.9%) | * | 92 (2.6%) | 308 (15.8%) | |
| 5 | 180 (5.0%) | 0 | * | 137 (7.0%) | |
| Number of organ failures, median | 2 | 2 | 1 | 3 | |
| Cardiovascular Failure, n (%) | 2,688 (74.7%) | 80 (100%) | 167 (4.8%) | 1,949 (100%) | |
| Hematologic Failure, n (%) | 928 (25.8%) | * | 1,188 (33.9%) | 568 (29.14%) | |
| Hepatic-Metabolic Failure, n (%) | 1,993 (55.4%) | 31 (38.8%) | 1,635 (46.6%) | 1,177 (60.4%) | |
| Renal Failure, n (%) | 755 (21.0%) | 19 (23.8%) | 394 (11.2%) | 368 (18.9%) | |
| Respiratory Failure, n (%) | 1,721 (47.8%) | * | 1,1173 (33.4%) | 1,004 (51.5%) | |
| Neurologic Failure, n (%) | 208 (5.8%) | * | 246 (7.0%) | 115 (5.9%) | |
| Site of infection | |||||
| Bacteremia | 151 (31.7%) | 3,005 (83.5%) | * | 3,239 (92.3%) | 1,934 (99%) |
| Respiratory | 1,336 (50.6%) | 1,247 (34.7%) | * | 1,028 (29.3%) | 735 (37.7%) |
| Intra-abdominal | 530 (20.1%) | 528 (14.7%) | 0 | 421 (12.0%) | 324 (16.6%) |
| Urinary Tract/GU | 268 (10.2%) | 425 (11.8%) | * | 557 (15.9%) | 235 (12.1%) |
| Other | 479 (18.1%) | 1,183 (32.8%) | * | 1,780 (50.7%) | 657 (33.7%) |
| Mortality | |||||
| 14 day mortality | 67 (14.1%) | 417 (11.6%) | 24 (30.0%) | 87 (2.5%) | 213 (10.9%) |
| 28 day mortality | 82 (17.3%) | 560 (15.6%) | 30 (37.5%) | 140 (4.0%) | 276 (14.2%) |
| In-hospital mortality | 82 (17.3%) | 763 (21.2%) | 39 (48.8%) | 261 (7.4%) | 352 (18.1%) |
| One year mortality | 867 (24.1%) | 41 (51.3%) | 406 (11.6%) | 1.0%) | |
Indicates number <11, which cannot be presented due to the terms of our data use agreement (to protect patient confidentiality).
Administrative dataset cohorts: short-term and one-year mortality
Among the original (overlapping) four administrative dataset cohorts, 14- and 28- day mortality ranged between 11-16% for the cohorts identified by the ‘severe sepsis’ and ‘septic shock’ codes, but was much lower in the cohorts identified by the Angus and Martin strategies (Table 1). Mortality at 14 and 28 days was even lower (< 3%) in the cohorts identified uniquely by the Angus and Martin strategies. In contrast, cases identified uniquely by the ‘severe sepsis’ and ‘septic shock’ codes had same or higher mortality at all time points compared to their parent cohorts, and nearly a third of patients uniquely identified by the single code for ‘septic shock’ had died by 14 days. Among the original (overlapping) four administrative dataset cohorts, in-hospital mortality ranged from a low of 6.6% in those identified by Angus, et al. to a high of 21.1% in the group identified by the ‘severe sepsis’ code (Table 1). The cases identified by both of the single code strategies but neither the Angus or Martin strategies (‘severe sepsis’ AND ‘septic shock’) had the highest mortality of all the groups at all time points (Table 2).
Among the original (overlapping) four administrative dataset cohorts, total mortality at one year after hospital discharge ranged from a low of 8.8% among the cases identified by Angus, et al. to a high in the single code cohorts of 23.5% (‘severe sepsis’) and 24.0% (‘septic shock’). These differences in mortality were apparent almost immediately after hospital discharge, with the majority of deaths occurring in the first two months after hospital discharge (Figure 2). Only half of all the observed mortality in the cohorts identified by Angus, et al. and Martin, et al. occurred within 28 days of presentation, whereas approximately two-thirds of deaths in the ‘severe sepsis’ and ‘septic shock’ code cohorts occurred in the same time frame (median time to death in these two groups was 16 days and 14 days, respectively). Mortality rates in the cases that met criteria for all four definitions were comparable to those in the group identified by the combination of ‘severe sepsis’ or ‘septic shock’ codes (Table 2).
Figure 2. One-year Mortality for Pediatric Severe Sepsis Patients.
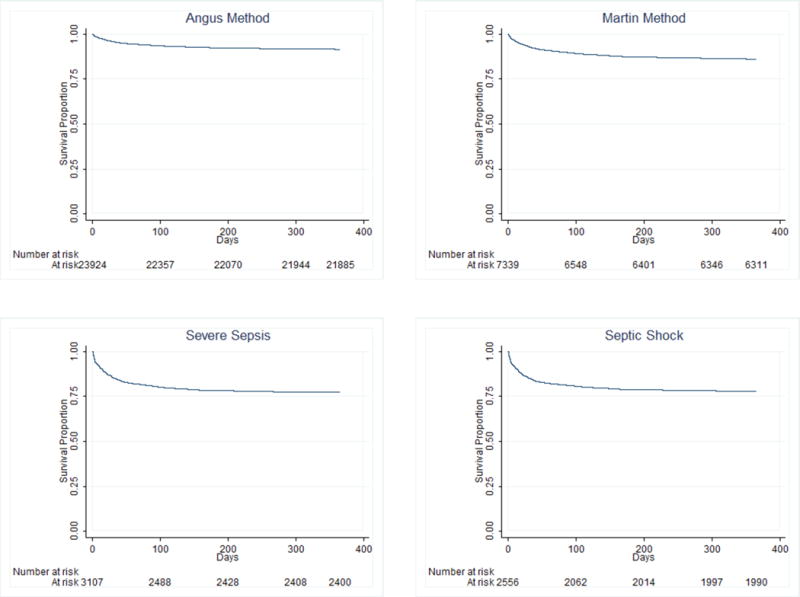
Mortality in the year after hospital discharge ranged from a low of 8.8% among the cases identified by Angus, et al. to a high in the single code cohorts of 23.5% (‘severe sepsis’) and 24.0% (‘septic shock’). The majority of deaths occurred in the first two months after hospital discharge.
Comparison to patients in the RESOLVE trial
Compared to the RESOLVE trial, all four of the original (overlapping) administrative dataset cohorts were older and more racially diverse (Table 1). Site of infection was also different in the RESOLVE trial than in the four coding cohorts, with higher proportions of respiratory and intra-abdominal infections and a concomitantly lower proportion of infection involving ‘other’ sites in the RESOLVE study patients (Table 1). In-hospital, 14- and 28-day mortality in the RESOLVE trial (14.1% and 17.3%, respectively) was similar to that in both cohorts identified by the single ICD-9-CM code strategies, but was higher than the mortality identified by the Angus and Martin coding strategies Table 1).
DISCUSSION
Although ICD-9-CM diagnosis codes have been used extensively to identify patients with severe sepsis in large datasets, we found that depending on the strategy used, the characteristics and long-term outcomes of patients identified can vary quite widely. Over all, we found that strategies that use single ICD-9-CM codes for ‘severe sepsis’ or ‘septic shock’ identify cohorts with organ failure and mortality rates most similar to children enrolled in the largest clinical trial of pediatric severe sepsis to date, the RESOLVE trial. If the patients enrolled in the RESOLVE trial are a true representation of pediatric severe sepsis patients generally, then these single code cohorts appear to be good proxy populations based on the characteristics we used for comparison. If the one-year mortality of clinically identified sepsis patients is similar to that found in our ‘severe sepsis’ and ‘septic shock’ dataset cohorts, then use of these codes in large datasets may be an efficient and cost-effective means to study long-term outcomes for children with severe sepsis.
Unfortunately, we were unable to perform detailed comparison of the patient characteristics of these administrative cohorts and the children in the RESOLVE trial. Therefore, how these administrative cohorts compare on other characteristics, such as the frequency and severity of underlying comorbidities, details of their hospital care, and other important clinical variables remains unknown. In addition, the cohorts of children identified by single codes for ‘severe sepsis’ and ‘septic shock’ are markedly smaller than the groups identified using strategies that combine codes for organ failure and infection, which have been used extensively in the past to determine estimates of incidence and prevalence of severe sepsis in children. A shift to preferential use of single code strategies in administrative data research may result in the creation of cohorts that more closely resemble children included in clinical trials, but simultaneously result in significant changes in the estimates of the epidemiology of severe sepsis in children. From an epidemiologic perspective, the cohort we identified by the combination of ‘severe sepsis’ OR ‘septic shock’ codes might be more useful, because it maintained many of the characteristics of the individual parent cohorts but was larger. Combining these codes, therefore, may provide clinically relevant case cohorts with less risk of underestimating the size of the true septic population. Certainly, more information is needed to understand the ways in which these patient populations compare and how we can best move forward with epidemiologic studies of severe sepsis in children.
We also found that the risk of mortality after a hospitalization for severe sepsis in childhood is substantial, and certainly well above population norms for children in the U.S.11 Due to the inability of our dataset to capture any deaths outside of an inpatient admission, it is possible that we have underestimated the true mortality rate. Work from other researchers suggest that many pediatric deaths occur outside the inpatient setting, particularly in cases of chronic disease with planned outpatient hospice care or severe trauma, where death is declared in an emergency department without hospital admission.12,13 These data suggest the need for additional long-term studies to identify the true rates of post-discharge mortality and the subsets of pediatric severe sepsis patients at risk for death in the year after hospital discharge. Can these patients be identified at the time of their hospital discharge? Are there modifiable factors that could be addressed to improve their long-term outcome? Large datasets may be useful in exploring these questions, once we have determined the ideal coding strategy and patient case-mix to identify these patients.
Although our study has many strengths, there are some limitations to acknowledge. First, we used the RESOLVE trial as our clinical ‘gold standard’ for pediatric severe sepsis, but to be included in the RESOLVE trial, patients had to have signs of systemic infection and require mechanical ventilatory support. This established a mandatory frequency of respiratory failure of 100%, which is not necessarily representative of the entire population of children with severe sepsis. Indeed, respiratory failure was found in only 30-50% of patients in our four original dataset cohorts. In addition, the RESOLVE trial excluded children with a history of bone marrow transplantation, certain organ failures > 24-36 hours’ duration, and significant thrombocytopenia.10 These conditions are likely associated with severe sepsis and risk of mortality, and exclusion of these populations may affect the generalizability of the RESOLVE trial and it’s utility as a gold standard.
Because we only used data from two east coast states, our results may not be generalizable to the entire United States or to cohorts of children treated for life-threatening infections in other countries. While we intentionally selected large, populous states to minimize this concern, it is possible that a nationally representative dataset might have been more generalizable. There are certainly data available from large clinical cohorts other than the RESOLVE trial, such as those presented by Schlabach, et al. describing 11,574 children with severe infections in Australia and New Zealand over a ten year period from 2002-2013.14 More detailed comparison of the clinical and hospitalization characteristics of of our proxy cohorts with the children in this and other studies may yield more information on the generalizability of our study. Lastly, it is not possible to determine the exact cause of death in the SID. Our mortality rates, while accurate, reflect all-cause mortality and not just that attributable to severe sepsis. To our knowledge, however, clinical studies, the RESOLVE trial included, also considered all-cause mortality in making final mortality calculations. This makes our methods consistent with others.
CONCLUSIONS
The ICD-9-CM diagnosis codes for ‘severe sepsis’ and ‘septic shock’ identify smaller, but higher acuity patients that more closely resemble the children enrolled in the largest clinical trial of pediatric severe sepsis to date. Mortality continued to be higher than the general pediatric population in the year after hospital discharge, indicating a need to understand more about the causes and possible treatments for the late effects of sepsis in childhood.
Acknowledgments
Funding Source: Dr. Olsen is the director of the Washington University Center for Administrative Data Research (CADR). Her contributions to this work was funded in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NIH) and by grant number R24 HS19455 (PI: V. Fraser) from the Agency for Healthcare Research and Quality (AHRQ).
Abbreviations
- HCUP
Healthcare Cost and Utilization Project
- NY
New York
- FL
Florida
- SID
State Inpatient Database
Footnotes
Financial Disclosure: None
Conflict of Interest: None
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 3.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. 2014;15(9):798–805. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the Epidemiology of Pediatric Severe Sepsis. Pediatr Crit Care Med. 2013 doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 5.Odetola FO, Gebremariam A, Freed GL, et al. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487–94. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 6.Czaja AS, Zimmerman JJ, Nathens AB, et al. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–57. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 7.Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med. 2014;15(9):828–38. doi: 10.1097/PCC.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SL, Parker B, Bullock ME, et al. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13(4):e219–26. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 9.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 10.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369(9564):836–43. doi: 10.1016/S0140-6736(07)60411-5. [see comment] [DOI] [PubMed] [Google Scholar]
- 11.U.S. CDC. 2015 [cited 2016. Available from: http://www.cdc.gov/nchs/fastats/child-health.htm.
- 12.Feudtner C, Hays RM, Haynes G, et al. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 13.Feudtner C, Silveira MJ, Shabbout M, et al. Distance from home when death occurs: a population-based study of Washington State, 1989-2002. Pediatrics. 2006;117(5):e932–9. doi: 10.1542/peds.2005-2078. [DOI] [PubMed] [Google Scholar]
- 14.Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15(1):46–54. doi: 10.1016/S1473-3099(14)71003-5. [DOI] [PubMed] [Google Scholar]


