Abstract
Transient adenosine signaling has been recently discovered in vivo, where the concentration is on average 180 nM and the duration only 3-4 seconds. In order to rapidly screen different brain regions and mechanisms of formation and regulation, here we develop a rat brain slice model to study adenosine transients. The frequency, concentration, and duration of transient adenosine events were compared in the prefrontal cortex (PFC), hippocampus (CA1), and thalamus. Adenosine transients in the PFC were similar to those in vivo, with a concentration of 160 ± 10 nM, and occurred frequently, averaging one every 50 ± 5 s. In the thalamus, transients were infrequent, occurring every 280 ± 40 s, and lower concentration (110 ± 10 nM), but lasted twice as long as in the PFC. In the hippocampus, adenosine transients were less frequent than in the PFC, occurring every 79 ± 7 s, but the average concentration (240 ± 20 nM) was significantly higher. Adenosine transients are largely maintained after applying 200 nM tetrodotoxin, implying they are not activity dependent. The response to adenosine A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) differed by region; DPCPX had no significant effects in the PFC, but increased the average transient concentration in the thalamus and both the transient frequency and concentration in the hippocampus. Thus, the amount of adenosine available to activate receptors, and the ability to upregulate adenosine signaling with DPCPX, varies by brain region. This is an important consideration for designing treatments that modulate adenosine in order to cause neuroprotective effects.
Keywords: adenosine, electrochemistry, fast-scan cyclic voltammetry, A1 receptor, brain slices
Introduction
Adenosine is a neuromodulator that also protects the brain from adverse events such as hypoxia or traumatic brain injury.1 Adenosine activates inhibitory A1 receptors which, in turn, decrease cAMP concentrations resulting in a decrease in neuronal firing.2 A1 adenosine receptors play an inhibitory role, modulating neurotransmitter release and are found both presynaptically and postsynaptically.3,4 The A1 antagonist DPCPX increases cell excitability while the A1 agonist N6-cyclopentyladenosine decreases excitability.5,6 Adenosine also activates excitatory A2A receptors on blood vessels to cause vasodilation, increasing oxygen delivery to the brain.7 Changes in extracellular adenosine concentrations have been observed on time scales ranging from minutes to hours.8,9,10 However, a faster form of transient adenosine release has recently been characterized, which lasts only a few seconds.11 While adenosine release can be electrically stimulated,12 recent studies have found transient adenosine events that are not stimulated, but spontaneous, and occur randomly.11,13,14 Rapid adenosine can modulate electrically-stimulated dopamine release.15 There is also a correlation between transient adenosine and transient oxygen events, supporting the hypothesis that rapid adenosine serves a neuroprotective role.16 However, there are many open questions about mechanisms of spontaneous adenosine release and the source of the adenosine that are not easy to decipher in vivo and different in situ or in vitro models are needed.
In anesthetized rats, the average concentration of a spontaneous adenosine transient is 170 nM in the caudate-putamen and 190 nM in the prefrontal cortex (PFC).11 Each event lasts approximately 3 s and the average time between two consecutive events ranges from 1-3 min depending on the brain region. Adenosine transients are cleared in part by extracellular metabolic enzymes such as adenosine deaminase and adenosine kinase as well as equilibrative nucleoside transporters.13 While the previous work has elucidated some of the functions and mechanisms of transient adenosine, it is unclear if transient adenosine behaves in a similar manner throughout the brain. So far, differences in concentration and frequency have been discovered in the caudate and PFC but other brain regions are largely unexplored. Adenosine is neuroprotective and adenosine transients may play a role in stressful circumstances such as hypoxia or ischemia.17 As such, it is important to understand adenosine transients in regions typically studied in stroke, including the thalamus and the hippocampus.18,19 Defining differences between regions could help determine the extent to which adenosine modulation varies throughout the brain.
To characterize neurochemical release, pharmacological experiments are often used and brain slice models are widely employed because they allow rapid screening while bypassing the blood brain barrier. Brain slices have been used to elucidate the basic mechanisms of long-term adenosine release, identifying intracellular and extracellular mechanisms of formation.20,21,22,23 In particular, brain slices are convenient models for studying the effects of hypoxia and ischemia on adenosine pathways, by restricting the flow of oxygenated buffer to the slice.24,25,26,27 Using fast-scan cyclic voltammetry (FSCV), stimulated adenosine release has been measured in brain slices as well.12,28 However, it is not as obvious whether spontaneous transients will occur in brain slices since only the terminals are present. In spinal cord slices of the dorsal horn, the Zylka group did report low frequency transient adenosine changes, occurring every few minutes, that were due to the breakdown of ATP in the extracellular space by prostatic acid phosphatase and ecto-5′-nucleotidase.29,30 However, the extent to which transient adenosine is released in slices from central brain regions is unknown.
In this study, we used FSCV with carbon fiber microelectrodes (CFMEs) to characterize transient adenosine events in PFC, thalamus, and hippocampus brain slices and define differences in how they are regulated by A1 receptors. We also investigated the activity dependence of spontaneous, transient adenosine by treating slices with tetrodotoxin (TTX). Adenosine frequency, as well as the concentration and duration of events, varied by brain region. The effect of the A1 antagonist DPCPX also varied by brain region, showing there are regional differences in both the amount of adenosine neuromodulation and the regulation of adenosine transients by A1 receptors. TTX had little impact on transient adenosine, suggesting an alternative mechanism of release. This work establishes brain slices as a platform for pharmacological experiments to understand the mechanism, formation, and regulation of transient adenosine release, facilitating experiments that are not feasible in vivo due to toxicity or blood-brain barrier permeability. Understanding these differences in how transient adenosine is regulated will lead to a better understanding of how much adenosine is available in different regions to act as a neuromodulator.
Results and Discussion
Adenosine detection in slices with FSCV
The main goal of this study was to compare spontaneous, transient adenosine efflux in multiple brain regions. Adenosine was measured in real-time with FSCV, where it undergoes two oxidation processes that are visualized as two oxidation peaks.31 Using a voltage waveform from −0.4 V to 1.45 V and back at 400 V/s, the primary oxidation process yields a peak at 1.4 V on the cathodic scan. On subsequent scans, a secondary oxidation process occurs, giving a peak at 1.2 V on the anodic scan which has less current than the primary peak. These peaks are seen in Fig. 1, a 3D color plot of an adenosine transient in the PFC. The applied voltage is displayed on the y-axis, time on the x-axis, and the current in false color. There are two green/purple peaks in the center of the plot that are due to adenosine oxidation, and the secondary peak at 1.2 V always comes after the primary peak at 1.4 V. The inset is the cyclic voltammogram, which plots the current at each voltage and is a fingerprint for adenosine detection. There is also an artifact at the onset of the adenosine transients in situ, seen as a vertical colored line in the color plot (Fig. 1). This artifact is largest around 0.5 V, but the duration is much shorter than adenosine release and we hypothesize it is due to ionic changes. Additionally, there is a small peak at −0.2 V. However, this negative peak is at the start of the anodic scan so it’s more likely to be a background shift due to adsorption than a reduction process. To examine how adenosine changes over time, the current at the peak potential of the primary oxidation peak (i vs t) is shown above the color plot. The duration of a transient is calculated as the time it takes to rise from and decline to 10% of the maximum current; this transient lasts about 2.8 s. The peak concentration of this transient is 500 nM, converted from the current using a post-calibration factor, and this is an example of one of the larger transients that are observed.
Figure 1.
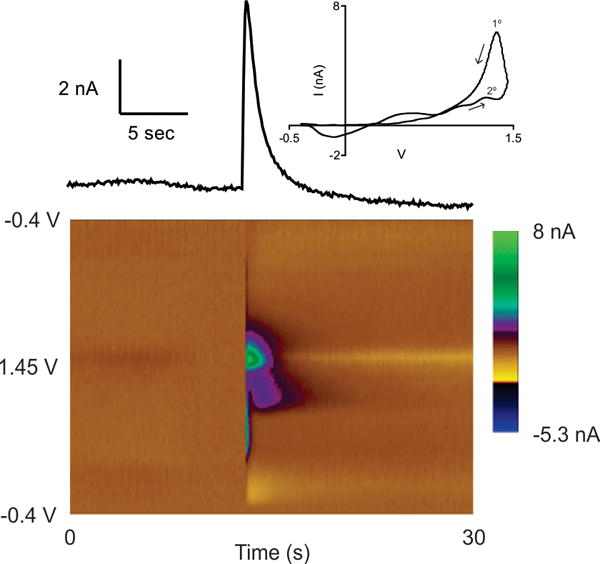
Adenosine transient in the hippocampus. The color plot shows all data, with scanned voltage on the y-axis, time on the x-axis, and current depicted in color. A horizontal slice in the 3D color plot results in the i vs t plot (above) and a vertical slice gives the cyclic voltammogram at a given time (inset).
Regional variation of spontaneous adenosine
From previous work studying stimulated adenosine release and long-term adenosine changes, we hypothesized that there would be regional differences in spontaneous adenosine transients in brain slices. Three brain regions were chosen: the PFC, where frequent transients had been reported in vivo,11 and two new regions where spontaneous adenosine had not been explored in vivo: the hippocampus CA1 and the thalamus. All of these regions express intermediate to high levels of adenosine A1 receptors and are important in memory formation and recollection.32 The PFC is, in part, responsible for cognitive control, connecting working memory and personality.33,34 The hippocampus is associated with declarative and spatial memory in both humans and rats,35 and the CA1 plays an important role in maintaining an individual’s autobiographical memory and ability to mentally place themselves in time frames other than the present.36 The thalamus is involved with memory, learning, speech, personality and relaying sensory information, all of which can be compromised due to a stroke.37
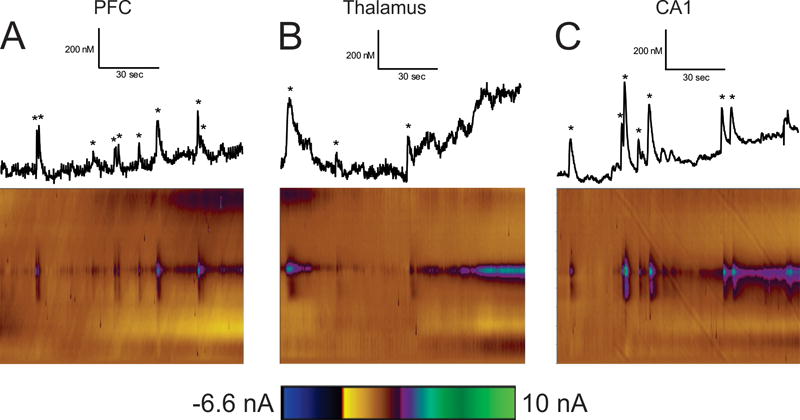
Fig. 2 shows example color plots and data traces in each region. In the PFC, transients occur frequently, with 9 transients happening within this 2 min window (Fig. 2A). In the thalamus, transient adenosine events are less frequent with only 3 events in 2 min (Fig. 2B). Although the events in the thalamus were relatively infrequent, some do still occur in rapid succession which results in a broad range of inter-event times for this region. In the hippocampus CA1, transient adenosine events are less frequent than in the PFC but more frequent than in the thalamus. Seven transient adenosine events are identified in this 2-minute sample (Fig. 2C). Other regions of the hippocampus, including the CA2, CA3, and dentate gyrus were also tried, but robust transients were rarely seen in those locations.
Figure 2.
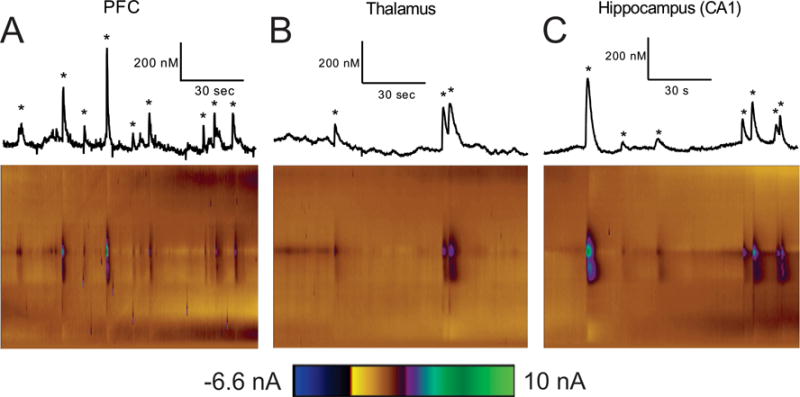
Concentration traces (top) and 3D color plots (bottom) for the (A) PFC, (B) thalamus, and (C) CA1. Adenosine transients are marked with stars in the concentration traces, which are all scaled the same to highlight the variety of concentrations in each region.
In order to evaluate differences in regions statistically, one hour of data was collected from 8 slices per region. The number of transients varied by region, with 65 ± 17 transients per hour in the PFC, 10 ± 2 transients in the thalamus, and 42 ± 7 transients in the CA1. There is an overall significant effect of brain region on number of transients (one-way ANOVA, p = 0.005) and a significant difference between the PFC and the thalamus (Bonferroni post-test, p = 0.004), but there is no significant difference between the CA1 and the PFC (p = 0.20) or the thalamus (p = 0.28). In all brain regions, the frequency of adenosine events is higher in the initial part of the hour (Fig. S1A). However, this drop in frequency was consistent between slices and the time measured was the same for all slices. The drop in frequency may be due to the tissue being unable to synthesize adenosine, and a previous study found improvement by adding ribose and adenine in the perfusion buffer.26 However, adenine is electroactive and adding large amounts of it to the slice interferes with adenosine detection by FSCV. Alternatively, the increased frequency at the beginning could be due to electrode implantation disturbing tissue and causing more release.38 Sample colors plots in Fig. 2 were taken from the first 10 minutes in their respective experiments when the transient frequencies were highest, and trends in concentration, duration, and relative frequency were also compared for both 1 hour and the first 10 min, and no differences were observed.
To examine frequency, we compared the distribution of inter-event times, which is the time between two consecutive transients. The inter-event times for each region were binned into 20 second bins and plotted as a cumulative distribution due to the Poisson nature of the relative frequency distribution (Fig. 3A). The distribution of inter-event times in the PFC (blue) rises rapidly, indicating a high frequency of events, while the rise for the CA1 (green) is slower. In the thalamus (red), the distribution rises very slowly, not even reaching 50% until the 120-140 s bin, indicating that half of all transients were more than 2 min apart. A Kruskal-Wallis (K-W) test indicates a significant difference in the cumulative inter-event frequency distributions (p < 0.0001, n = 510 transients in the PFC, 75 in the thalamus, and 328 in the CA1). Dunn’s post-tests indicate a significant difference between inter-event time distributions for the CA1 and the PFC (p = 0.0007), the CA1 and the thalamus (p < 0.0001), and the PFC and the thalamus (p < 0.0001). Table 1 lists the median and mean inter-event times for each region.
Figure 3.
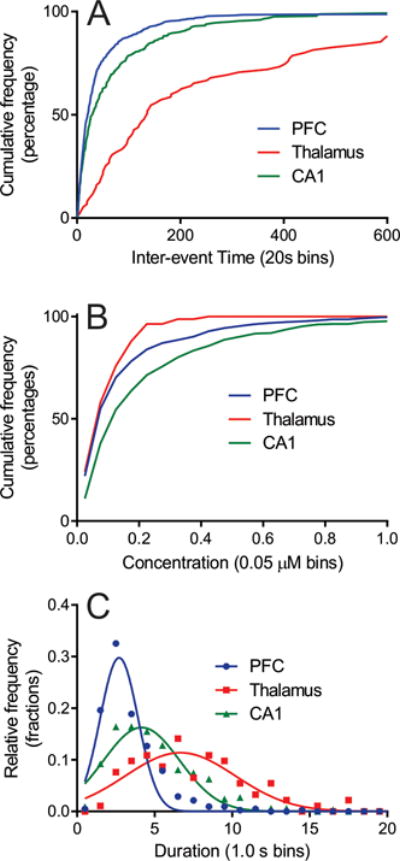
Differences in (A) interevent time (K–W test, p < 0.0001), (B) concentration (K–W test, p < 0.0001), and (C) duration (ANOVA, p < 0.0001) between the prefrontal cortex, thalamus, and CA1 region of the hippocampus. All are n = 8 slices.
Table 1.
Inter-event time, concentration and duration of spontaneous adenosine transients in the pre-frontal cortex, thalamus, and CA1 region of the hippocampus under control conditions and with A1 antagonist, DPCPX (100 nM)
| Brain Region | Inter-event Time (s) | Concentration (nM) | Duration (s) | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | 100nM DPCPX | Median | Mean | 100nM DPCPX | Mean | 100nM DPCPX | |
| Prefrontal cortex | 20 | 50 (±5) |
53 (±4) |
90 | 160 (±10) |
160 (±10) |
3.4 (±0.1) |
3.6 (±0.1) |
| Thalamus | 133 | 280 (±40) |
210 (±30) |
90 | 110 (±10) |
210 (±20)**** |
7.4 (±0.4) |
6.2 (±0.4)* |
| Hippocampus (CA1) | 30 | 79 (±7) |
58 (±7)** |
140 | 240 (±20) |
310 (±10)**** |
4.9 (±0.2) |
5.4 (±0.2) |
Errors are SEM
Significantly different distributions than control for that region (Kolmogorov-Smirnov tests for inter-event times and concentrations; unpaired t-test for duration)
Fig. 2 also demonstrates that there is a large range of concentrations of adenosine transients. Many transients are small, less than 50 nM, and the majority of transients have concentrations around or below 100 nM. Larger transients are also observed; for example, the large event in the CA1 trace is 350 nM and the largest shown in the PFC is 470 nM. In general, larger transients were more likely to be observed in the hippocampus. Concentrations ranged from 10 nM up to 2.7 μM and Fig. 3B shows the cumulative distribution of transients (50 nM bins). The relative frequency distribution of concentration is not Gaussian, so we assume a Poisson distribution, as we did with inter-event time. The thalamus has a larger percentage of transients in the small concentration bins causing it to reach 100% quickly while the PFC has several transients higher than 200 nM so it takes longer to reach 100%. The CA1 has the most large concentration transients, so it takes longer for its cumulative frequency curve to rise. A K-W test reveals a main effect of brain region for the concentration distribution (p < 0.0001). Dunn’s post-test indicates the distribution in the CA1 is significantly different from that in the PFC (p < 0.0001, n = 336 and 518, respectively) as well as the thalamus (p < 0.0001, n = 336 and 83, respectively). However, there was no significant difference between the PFC and thalamus (p > 0.99, n = 518 and 83, respectively). Table 1 gives mean and median event concentrations in each region. The mean concentration of each adenosine transient was 160 ± 10 nM in the PFC, 110 ± 10 nM in the thalamus, and 240 ± 20 nM in the CA1.
Another parameter to compare between brain regions is the event duration, defined here as the time it takes for a transient to rise and decline to 10% of its maximum concentration. Table 1 compares the mean duration of transients which were 3.4 ± 0.1 s in the PFC, 7.4 ± 0.4 s in the thalamus, and 4.9 ±0.1 s in the CA1. The frequency distribution in Fig. 3C shows that the distribution of durations is Gaussian in each brain region, allowing for a one-way ANOVA for comparison. Overall, there was a main effect of brain region on event duration (ANOVA, p < 0.0001). Bonferroni post-tests indicate a significant difference between the PFC and thalamus (p < 0.0001), and a significant difference for the CA1 with both the PFC (p = 0.01) and the thalamus (p = 0.002).All three regions have transients that last less than 2 s. However, the distribution is very narrow, in the PFC, while the distribution is broader in the CA1 and even more so in the thalamus.
Because of the drop off of adenosine transients with time, we also repeated this analysis with only the first 10 minutes of data (Figs. S1). Frequency of release was higher in the first 10 min, as expected, (Figs. S1B and C) but the trends were the same, with the PFC producing the most frequent events and the thalamus the least frequent events.11 The 10 min. data have similar trends to the 1 hour data, with no changes in the mean concentration or durations of transients compared to the 1 hour analysis (Fig. S1D-G). Because the number of transients is low in only 10 min, particularly in the thalamus, it would take many more slices to have enough transients to fully define the distribution, so the full hour of data from each slice was analyzed for the subsequent drug experiments.
Biological Implications of Regional Variations in Adenosine
One of the main findings of this work is that the frequency of adenosine transients varies dramatically between regions. Past pharmacological experiments in vivo have pointed to the frequency of transients as the primary way that transient adenosine is regulated, and this work extends that to show frequency has major differences between brain regions.11,39 The median inter-event time in the thalamus (132 s) is 6 times larger than the PFC (20 s) and 4 times larger than the hippocampus (30 s). In the thalamus, the median inter-event time is similar to the frequency observed by Zylka’s group in spinal cord slices, where transients were not as frequent.29 These differences in frequency show that the number of transient adenosine release events that could modulate neurotransmission or blood flow is substantially higher in the PFC. In particular, more frequent transients would increase the chance that receptors were activated, and the highest frequency transients occur in the PFC which has a lower expression of A1 receptors than the thalamus or CA1, so more transients might be needed to activate receptors that are more diffuse.32
The concentration of adenosine transients also varied by brain region. The hippocampus CA1 had more large events than the other two regions, the largest of which was 2.7 μM. In contrast, the highest concentration in the PFC was just over 1 μM and only 400 nM in the thalamus. Most events in both the thalamus and the PFC were less than 90 nM, which is about the affinity of adenosine for A1 receptors.40 However, these transients are occurring on top of the basal concentration of adenosine, which is about 33 nM in the thalamus and cortex, and 200 nM in the hippocampus.41,42 Thus, most transients would be large enough to active A1 receptors.41 Since excitatory A2A receptors require a higher concentration of adenosine to become activated (Kd~150 nM), the wide distribution of transient concentrations in the CA1 and PFC may be to differentiate the need for A1 activation versus A2A activation.40 In addition, the concentrations are highest in the hippocampus, which has lower vascular density, and where adenosine might have to diffuse further to provide blood flow modulation.16,43
Duration is important because it, along with concentration, would control how long adenosine receptors could be activated. Transient adenosine is cleared fastest in the PFC and much slower in the thalamus. Equilibrative nucleoside transporter 1 (ENT1), adenosine kinase, and adenosine deaminase are all clearance mechanisms for transient adenosine.13 Interestingly, ENT1 is expressed at higher levels in the thalamus and cortex, where clearance is slower, than in the hippocampus.44 Likewise, the thalamus also exhibits higher activity of adenosine deaminase than either of the other two regions.45 Thus, the slow clearance of adenosine in the thalamus suggests that ENT1 and adenosine deaminase are not the primary mechanisms of transient adenosine clearance. Previously, even when ENT1, adenosine kinase, and adenosine deaminase were blocked simultaneously, there was still fast clearance, so other mechanisms of clearance may be responsible for the rapid clearance rates in the PFC.13 There is an inverse relationship between frequency/concentration and duration, with the thalamus having lower frequencies and concentrations, but longer durations. The longer duration may therefore compensate for the lower frequency and allow more receptor activation. Alternatively, the need for adenosine may vary among regions, particularly since the differences between transients in each region would likely result in different downstream effects such as glutamate modulation.
Adenosine transients are similar in vivo and in situ
In the first report of transient adenosine measurements in vivo, the mean inter-event time in the PFC was 108 s, whereas it is 48 s in brain slices.11 The established detection limit in vivo was 40 nM, but lower background noise in slices allows a lower limit of detection, 10 nM. This increased sensitivity means more small events are detected and counted, driving both the average concentration and the average inter-event time down. Excluding all events in PFC slices below 40 nM increases the mean inter-event time from 48 s to 63 s, which is still more frequent than in vivo. Excluding these low concentration events also increases the mean concentration to 190 nM, the same as the 190 nM average found in vivo. Transient adenosine release lasts longer in brain slices than in vivo. The mean duration in vivo in the PFC was 2.8 (± 0.1) s whereas in situ, it was 3.6 (± 0.1) s. This may indicate that transporters or metabolic processes are impaired in slices compared to in vivo measurements. However, the concentrations, frequencies, and durations are still very similar between the two models which indicates that slices are a viable method to study transient adenosine release and will be useful for understanding mechanisms of release or pharmacological studies in the future.
Effect of tetrodotoxin on transient adenosine release
While the mechanism of spontaneous, transient adenosine release is unknown, previous research has demonstrated that adenosine can be released as adenosine through nucleoside transporters or be formed from extracellular metabolism of ATP that is released via exocytosis.9,46,47 Dale’s group has shown that rapid, stimulated adenosine in the cerebellum is mainly activity dependent.48 To test if spontaneous adenosine release is activity dependent, 200 nM tetrodotoxin (TTX) was used to block Na+ channels, inhibiting activity dependent exocytotic release. TTX activity was confirmed in the slice by first measuring stimulated dopamine release in the caudate putamen, then perfusing with 200 nM TTX in aCSF for 20 minutes. This concentration of TTX eliminated the stimulated dopamine signal (Fig. 4A). The electrode was then moved to the CA1 in the same slice and transient adenosine detected. Adenosine transients were still observed in the CA1 with no significant difference in the mean inter-event time (t-test, 101 ± 9 s TTX vs 79 ± 7 s control p = 0.11) or concentration (t-test,190 ± 10 nM TTX vs 240 ± 20 nM control, p =0.38). When plotting distributions, there is a significant difference in the cumulative distribution of inter-event times with TTX (KS test, p < 0.0001) (Fig 4B) but not in the cumulative distribution of transient event concentrations (KS test, p = 0.65). The slightly lower frequency distribution of transients may be due to downstream effects of other neurotransmitters, such as glutamate, that can modulate transient adenosine. Thus, it appears that adenosine release events are not primarily due to exocytosis as the frequency and concentration of release is largely maintained with doses of TTX that eliminate exocytotic release of dopamine. We postulate that the release mechanism of transient adenosine is not activity dependent, but more experiments are need to thoroughly characterize alternative routes such as pannexin channels, connexin channels, or nucleoside transporters.13,47 Brain slice models were particularly helpful to this experiment as TTX cannot be given to an intact animal.
Figure 4.
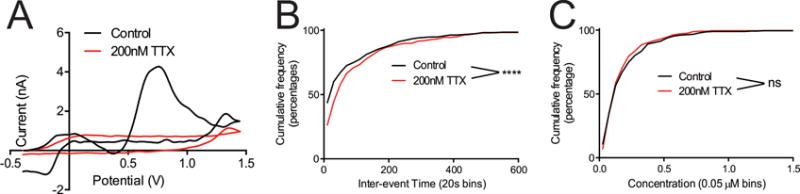
Effect of TTX. (A) 200 μM TTX eliminated stimulated dopamine release in caudate of a sagittal slice. (B) In the same slices, but in the hippocampus, there was no effect of TTX on the mean interevent time or concentration, but there was a significant effect of TTX on the interevent time distribution. K–S test, **** p <0.0001. (C) There was no significant effect of TTX on the concentration of adenosine transients in the CA1 (K–S test).
Effect of A1 antagonism on transients in each of the brain regions
Spontaneous adenosine release is mediated by A1 receptors but the extent to which this occurs in different brain regions is not known.11 A1 receptors are inhibitory G protein coupled receptors, lowering cAMP levels and subsequently decreasing cell excitability.2 Adenosine modulates glutamate and GABA release through activation of presynaptic A1 autoreceptors.49 DPCPX is a high affinity inhibitor of adenosine A1 receptors (Ki = 0.5 nM).50 Brain slices were bathed in aCSF containing 100 nM DPCPX50 before and during measurements of adenosine. Fig. 5 shows sample color plots and concentration traces. The number of transients in each region is about the same as control (Fig. 2), but the concentrations do change, particularly in the thalamus and hippocampus where larger transients are more commonly observed after DPCPX. The average number of transients per hour in the PFC (65 ± 8) after DPCPX was exactly the same as control slices (65 ± 17). The average number of transients were also not significantly different in other regions, going up only slightly after DPCPX in the thalamus from 10 ± 2 to 13 ± 3, (t-test, p = 0.49, n = 8) and in the CA1 from 42 ± 7 to 51 ± 12 (t-test, p = 0.31, n = 8). Fig. 6 shows cumulative frequency distributions for each brain region with and without DPCPX. There is no change in cumulative distribution of inter-event times in PFC slices treated with DPCPX (KS-test, p = 0.15) or in the thalamus (KS-test, p = 0.14). However, there was a significant increase in the inter-event time distribution in the CA1 (KS-test, p = 0.003), as the transients became more frequent (Table 1). This significant change in frequency is interpreted with some caution because the number of transients did not also significantly change.
Figure 5.
Concentration changes (top) and 3D color plots (bottom) for the (A) PFC, (B) thalamus, and (C) CA1 when treated with 100 nM DPCPX. Verified adenosine transients are marked with a star in the concentration traces.
Figure 6.
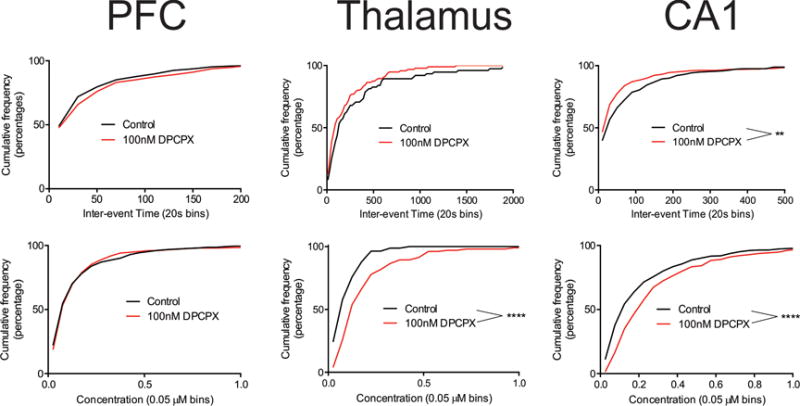
Cumulative distributions of the interevent times (top) and concentrations (bottom) of adenosine transients in the PFC (left), thalamus (middle), and CA1 (right). Control is black, and treated with 100 nM DPCPX is red. A K–S test was performed for all graphs, and significant differences are marked by asterisks, **p < 0.01, ****p < 0.0001.
The average concentration of all transient adenosine events in the PFC remained unchanged with DPCPX, but the distribution of concentrations increased significantly in the CA1 (KS, p < 0.0001) and the thalamus (KS, p < 0.0001). This means that there were more large transients after A1 inhibition. The change was particularly large in the thalamus, where the event concentration doubled with DPCPX. Thus, DPCPX had more of an effect on concentration than frequency, with large changes in both the CA1 and thalamus. However, DPCPX had little effect in the PFC on either frequency or concentration. No significant difference was anticipated of DPCPX on duration since A1 receptors are not responsible for clearance. The only significant effect was in the thalamus, where duration decreased with DPCPX, but the sample size was small.
Biological implications of regional differences in response to A1 antagonist
DPCPX blocks the inhibitory effects of adenosine A1 receptors and previous work in vivo shows that adenosine transients increased in frequency in both the PFC and caudate-putamen after DPCPX.11 In contrast, in PFC slices, there were no significant differences in the inter-event time, concentration, or duration of adenosine transients when perfused with 100 nM DPCPX. As described above, adenosine transients in situ occur at a higher frequency than in vivo and the PFC has the highest rate of adenosine transients, so it is possible that A1 receptors may not be able to increase the rate in this region. DPCPX did have effects in both the CA1 and thalamus. It is especially interesting that the concentration shifts in the hippocampus, where it was already large without drug. The average concentration in the CA1 after DPCPX indicates that a higher proportion of transients here are large enough to activate A2a receptors, and thus may better link adenosine signaling to blood flow.40 There was also a significant increase in the concentration of transients in the thalamus with DPCPX which would also shift more transients to values that are able to activate A2a receptors.
These results are interesting because they show that A1 receptors may not regulate adenosine transients to the same magnitude in all brain regions. A1 receptors are highly expressed in both the thalamus and the hippocampus while moderately expressed in the cortex.32 Thus, the effects may be correlated to A1 expression levels. A1 receptors may have less effect in the PFC, leading to the higher frequency without drug and no change in frequency when they are blocked with A1 antagonist DPCPX. A1 receptors self-regulate adenosine release and these results are important because A1 receptor antagonists might be used to increase the amount of adenosine available to act as a neuroprotective agent, particularly during events that stress the tissues such as ischemia or physical damage.5,11 However, the regional differences suggest that the drug might not be equally effective in all regions, showing that regulation of adenosine neuromodulation via A1 activation states may differ regionally.
Conclusions
Here, we demonstrate regional differences in spontaneous, transient adenosine release in brain slices. The release frequency is highest in the PFC, while the hippocampus has the largest concentration, and the thalamus has the longest duration. Because frequency, concentration, and duration control the amount of adenosine available to act at receptors, the profile of rapid adenosine release varies between regions. Adenosine release was still maintained after TTX, showing that the mechanism is not likely activity-dependent. More studies are needed to determine the mechanism of release. The TTX studies do demonstrate that slices are useful for pharmacological studies where the drug might be lethal if given to the intact animal. A1 inhibition with DPCPX increased the concentration of adenosine transients in the hippocampus and the thalamus but not in the PFC. The differences in regulation by A1 inhibition show that drugs that regulate adenosine release also have different effects in different regions. Overall, this study is important because if shows the amount of adenosine available to provide neuromodulatory and neuroprotective effects varies by brain region, and that regional difference may be an important consideration when designing experiments to promote the neuroprotective effects of adenosine.
Experimental
Slice preparation
Protocols for animal experiments were approved by the Animal Care and Use Committee at the University of Virginia. Male Sprague-Dawley rats weighing between 250-350 grams were housed in a university vivarium and were provided food and water ad libitum prior to experimentation. Rats were anesthetized with isoflurane (approximately 1mL/100g) in a desiccator and promptly beheaded. The brain was quickly removed and placed in a beaker of cold (0-5°C) oxygenated aCSF to recover for 2 minutes. 400 μm thick coronal slices were collected from the PFC and thalamus. 400 μm thick sagittal slices were collected from the hippocampus. Slices were made with a Leica vibratome (LeicaVT1000S, Bannockburn, IL), and kept in a beaker of oxygenated aCSF at 37°C in a water bath for 1 hour of recovery. During experiments, 37°C oxygenated aCSF was perfused over the slices at a rate of 2 mL/min. Approximate coordinates are +4.6 mm anterior-posterior (AP), +2.0 mm mediolateral (ML), and −2.0 mm dorsoventral (DV) for the PFC; −3.1 mm AP, +3.0 mm ML, and −6.0 mm DV for the thalamus, and −4.5 mm AP, +3.8 mm ML, and −3.0 mm DV for the CA1 region of the hippocampus (Fig. S2). After allowing the slice and the electrode to equilibrate in the slice chamber, electrodes were implanted 75 μm into the tissue. Once the electrode was implanted and the background stabilized (approximately 5 min), FSCV data was collected for 1 hour. After experimentation, each electrode was calibrated with 1 μM adenosine. Depending on the length and taper of the electrode, the calibration factor varied between 6 and 37 nA/μM with most electrodes around 13 nA/μM.
Chemicals
Slices were kept in oxygenated artificial cerebral spinal fluid (aCSF), as described previously.51 aCSF was comprised of 126 mM NaCl, 2.5 mMKCl, 1.2 mM NaH2PO4 monohydrate, 2.4 mM CaCl2 dihydrate, 1.2 mM MgCl2 hexahydrate, 25 mM NaHCO3, 11 mM glucose, and 15 mM tris(hydroxymethyl)aminomethane and was adjusted to pH 7.4 immediately prior to experimentation. A 10 mM stock solution of adenosine was prepared in 0.1 mM HClO4 and this was diluted daily in aCSF to 1 μM for post-calibration of the CFMEs. One mM stock solutions of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, Sigma-Aldrich) were prepared in dimethyl sulfoxide (DMSO) and kept frozen until used. Stock DPCPX was added to perfusion aCSF to make a 100 nM solution and slices were perfused for 15 min before the electrode was implanted and adenosine measurements made while DPCPX was perfused. Tetrodotoxin (TTX, Tocris) was reconstituted to 3 mM in 0.2 M citrate buffer, diluted to 50 μM aliquots, and kept frozen until used. 50 μM aliquots were added to perfusion aCSF to make a 200 nM working solution and perfused over slices in the same manner as DPCPX. After each experiment all solutions and surfaces that came into contact with TTX were treated with 10% bleach to deactivate residual TTX.
Electrochemistry
Adenosine transients were measured using FSCV with CFMEs as described previously.31 Briefly, electrodes were fabricated by aspirating a 7 μm diameter T-650 carbon fiber (Cytec Engineering Materials, West Patterson, NJ, USA) into a glass capillary. The capillaries were pulled on a vertical puller and the exposed fiber subsequently cut to ~50 μm. Traditionally, the tips of electrodes are sealed with epoxy to prevent buffer from leaking into the capillary. However, we have found that this decreases our electrodes’ sensitivity to adenosine transients (Fig. S3). If there is a long taper to the glass to ensure a watertight seal, the epoxy step is unnecessary to electrode fabrication. Waveform generation and cyclic voltammogram collection was performed through HDCV (from UNC Chemistry, Chapel Hill, NC) with a Dagan ChemClamp potentiostat (Dagan Corporation; Minneapolis, MN, USA). The waveform used for adenosine detection scans from a holding potential of −0.4V to a switching potential of +1.45V at a rate of 400V/s and a frequency of 10Hz.
Data Analysis and Statistics
Adenosine transients were analyzed using the principal components analysis (PCA) in the HDCV Analysis software as describe previously.11 Briefly, the 5 largest transients from each slice were used to create a training set to which other transients would be compared. The principal components were extracted and the raw data was then transformed into a concentration vs time trace to identify and quantify transients while excluding any signal that generated excessive residual current. Transients collected for TTX experiments were analyzed using a new automated algorithm and compared with control slices analyzed in the same manner.52 All statistics were performed using Graphpad Prism 6. Mean values are given ± standard error of the mean (SEM). The times between consecutive transients, or inter-event time, were pooled and binned in 20 second bins for cumulative frequency graphs. The inter-event times of the three brain regions were compared with each other using the Kruskal-Wallis test (non-parametric, unpaired, one-way ANOVA). Comparison of the impact of TTX and DPCPX on inter-event time was analyzed with the Kolmogorov-Smirnov test (non-parametric, unpaired t-test). 8 brain slices were used for each brain region with no more than 3 slices coming from the same animal (each group of 8 slices was from at least 4 different animals). 8 additional slices were used for each brain region for TTX experiments as well as DPCPX experiments. There was no noticeable effect of different coordinates within each region.
Supplementary Material
Acknowledgments
This research was supported by a grant from NIH (R01NS076875) to BJV.
Footnotes
Supporting Information
Two supplemental figures: S1: Adenosine transient frequency over time; 10 minute analysis vs. 1 hour analysis S2: Electrode implantation sites in the three brain regions S3: Sensitivity of epoxied vs non-epoxied electrodes (PDF)
The authors declare no competing financial interests.
Orcid
B. Jill Venton: 0000-0002-5096-9309
Author Contributions
B.J.V. oversaw the experiments. S.T.L. performed the experiments and analyzed the data. Both S.T.L. and B.J.V. wrote the manuscript.
References
- 1.Tominaga K, Shibata S, Watanabe S. A neuroprotective effect of adenosine A1-receptor agonists on ischemia-induced decrease in 2-deoxyglucose uptake in rat hippocampal slices. Neurosci Lett. 1992;145:67–70. doi: 10.1016/0304-3940(92)90205-l. [DOI] [PubMed] [Google Scholar]
- 2.Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- 4.Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules. 2017;22:676. doi: 10.3390/molecules22040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cechova S, Elsobky AM, Venton BJ. A1 receptors self-regulate adenosine release in the striatum: evidence of autoreceptor characteristics. Neuroscience. 2010;171:1006–1015. doi: 10.1016/j.neuroscience.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda MF, Hamani C, de Almeida ACG, Amorim BO, Macedo CE, Fernandes MJS, Nobrega JN, Aarão MC, Madureira AP, Rodrigues AM, Andersen ML, Tufik S, Mello LE, Covolan L. Role of adenosine in the antiepileptic effects of deep brain stimulation. Front Cell Neurosci. 2014;8:312. doi: 10.3389/fncel.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther. 1998;284:1066–1073. [PubMed] [Google Scholar]
- 8.Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int. 1995;26:387–95. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- 9.Pazzagli M, Pedata F, Pepeu G. Effect of K+ depolarization, tetrodotoxin, and NMDA receptor inhibition on extracellular adenosine levels in rat striatum. Eur J Pharmacol. 1993;234:61–5. doi: 10.1016/0014-2999(93)90706-n. [DOI] [PubMed] [Google Scholar]
- 10.Cui M, Bai X, Li T, Chen F, Dong Q, Zhao Y, Liu X. Decreased extracellular adenosine levels lead to loss of hypoxia-induced neuroprotection after repeated episodes of exposure to hypoxia. PLoS One. 2013;8:e57065. doi: 10.1371/journal.pone.0057065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of Spontaneous, Transient Adenosine Release in the Caudate-Putamen and Prefrontal Cortex. PLoS One. 2014;9:e87165. doi: 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pajski ML, Venton BJ. Adenosine Release Evoked by Short Electrical Stimulations in Striatal Brain Slices is Primarily Activity Dependent. ACS Chem Neurosci. 2010;1:775–787. doi: 10.1021/cn100037d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen MD, Ross AE, Ryals M, Lee ST, Venton BJ. Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism. Pharmacol Res Perspect. 2015;3:e00189. doi: 10.1002/prp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamah-Biassi EB, Almonte AG, Blagovechtchenski E, Grinevich VP, Weiner JL, Bonin KD, Budygin EA. Real time adenosine fluctuations detected with fast-scan cyclic voltammetry in the rat striatum and motor cortex. J Neurosci Methods. 2015;256:56–62. doi: 10.1016/j.jneumeth.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross AE, Venton BJ. Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors. J Neurochem. 2015;132:51–60. doi: 10.1111/jnc.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Venton BJ. Correlation of transient adenosine release and oxygen changes in the caudate-putamen. J Neurochem. 2017;140:13–23. doi: 10.1111/jnc.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526(Pt 1):143–55. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo HG, Kim DY, Park HW, Lee SU, Park SH. Early motor balance and coordination training increased synaptophysin in subcortical regions of the ischemic rat brain. J Korean Med Sci. 2010;25:1638–45. doi: 10.3346/jkms.2010.25.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha RA, Vizi ES, Ribeiro JA, Sebastião AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–7. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JB, Lupica CR, Dunwiddie TV. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J Neurosci. 1993;13:3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wall M, Dale N. Activity-dependent release of adenosine: a critical re-evaluation of mechanism. Curr Neuropharmacol. 2008;6:329–337. doi: 10.2174/157015908787386087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diógenes MJ, Neves-Tomé R, Fucile S, Martinello K, Scianni M, Theofilas P, Lopatář J, Ribeiro JA, Maggi L, Frenguelli BG, Limatola C, Boison D, Sebastião AM. Homeostatic control of synaptic activity by endogenous adenosine is mediated by adenosine kinase. Cereb Cortex. 2014;24:67–80. doi: 10.1093/cercor/bhs284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan NK, Young AMJ, Gibson CL, Davidson C. Inhibition of pre-ischeamic conditioning in the mouse caudate brain slice by NMDA- or adenosine A1 receptor antagonists. Eur J Pharmacol. 2013;698:322–329. doi: 10.1016/j.ejphar.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J Neurochem. 2003;86:1506–1515. doi: 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- 26.Nedden S Zur, Doney AS, Frenguelli BG. Modulation of intracellular ATP determines adenosine release and functional outcome in response to metabolic stress in rat hippocampal slices and cerebellar granule cells. J Neurochem. 2014;128:111–124. doi: 10.1111/jnc.12397. [DOI] [PubMed] [Google Scholar]
- 27.Duarte JM, Cunha RA, Carvalho RA. Adenosine A1 receptors control the metabolic recovery after hypoxia in rat hippocampal slices. J Neurochem. 2016;136:947–57. doi: 10.1111/jnc.13512. [DOI] [PubMed] [Google Scholar]
- 28.Pajski ML, Venton BJ. The mechanism of electrically stimulated adenosine release varies by brain region. Purinergic Signal. 2013;9:167–174. doi: 10.1007/s11302-012-9343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, Vihko P, Wightman RM, Zylka MJ. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol Pain. 2011;7:80. doi: 10.1186/1744-8069-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Street SE, Kramer NJ, Walsh PL, Taylor-Blake B, Yadav MC, King IF, Vihko P, Wightman RM, Millan JL, Zylka MJ. Tissue-Nonspecific Alkaline Phosphatase Acts Redundantly with PAP and NT5E to Generate Adenosine in the Dorsal Spinal Cord. J Neurosci. 2013;33:11314–11322. doi: 10.1523/JNEUROSCI.0133-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swamy BEK, Venton BJ. Subsecond Detection of Physiological Adenosine Concentrations Using Fast-Scan Cyclic Voltammetry. Anal Chem. 2007;79:744–750. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- 32.Goodman RR, Snyder SH. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982;2:1230–41. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 34.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced Prefrontal Gray Matter Volume and Reduced Autonomic Activity in Antisocial Personality Disorder. Arch Gen Psychiatry. 2000;57:119. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- 35.Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 36.Bartsch T, Dohring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci. 2011;108:17562–17567. doi: 10.1073/pnas.1110266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 38.Ross AE, Nguyen MD, Privman E, Venton BJ. Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. J Neurochem. 2014;130:50–60. doi: 10.1111/jnc.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen MD, Wang Y, Ganesana M, Venton BJ. Transient Adenosine Release Is Modulated by NMDA and GABAB Receptors. ACS Chem Neurosci. 2017;8:376–385. doi: 10.1021/acschemneuro.6b00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: An in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 42.Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 43.Coney AM, Marshall JM. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J Physiol. 1998;509(Pt 2):507–18. doi: 10.1111/j.1469-7793.1998.507bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology. 2001;40:722–731. doi: 10.1016/s0028-3908(00)00207-0. [DOI] [PubMed] [Google Scholar]
- 45.Geiger JD, Nagy JI. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci. 1986;6:2707–2714. doi: 10.1523/JNEUROSCI.06-09-02707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu S, Xiong W, Zhang DL, Soylu H, Sun C, Albensi BC, Parkinson FE. Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol Sin. 2013;34:60–66. doi: 10.1038/aps.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinrich A, Andõ RD, Túri G, Rõzsa B, Sperlágh B. K+ depolarization evokes ATP, adenosine and glutamate release from glia in rat hippocampus: A microelectrode biosensor study. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klyuch BP, Dale N, Wall MJ. Deletion of ecto-5′-nucleotidase (CD73) reveals direct action potential-dependent adenosine release. J Neurosci. 2012;32:3842–7. doi: 10.1523/JNEUROSCI.6052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Fan S, Yan J, Li B, Chen F, Xia J, Yu Z, Hu Z. Adenosine modulates the excitability of layer II stellate neurons in entorhinal cortex through A1 receptors. Hippocampus. 2011;21:265–280. doi: 10.1002/hipo.20745. [DOI] [PubMed] [Google Scholar]
- 50.Sebastião AM, Stone TW, Ribeiro JA. The inhibitory adenosine receptor at the neuromuscular junction and hippocampus of the rat: antagonism by 1,3,8-substituted xanthines. Br J Pharmacol. 1990;101:453–9. doi: 10.1111/j.1476-5381.1990.tb12729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross AE, Nguyen MD, Privman E, Venton BJ. Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. J Neurochem. 2014;130:50–60. doi: 10.1111/jnc.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borman RP, Wang Y, Nguyen MD, Ganesana M, Lee ST, Venton BJ. Automated Algorithm for Detection of Transient Adenosine Release. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00262. acschemneuro.6b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


