Abstract
Muscle disuse results in the loss of muscular strength and size, due to an imbalance between protein synthesis (MPS) and breakdown (MPB). Protein ingestion stimulates MPS, although it is not established if protein is able to attenuate muscle loss with immobilization (IM) or influence the recovery consisting of ambulatory movement followed by resistance training (RT). Thirty men (49.9 ± 0.6 yr) underwent 14 days of unilateral leg IM, 14 days of ambulatory recovery (AR), and a further six RT sessions over 14 days. Participants were randomized to consume an additional 20 g of dairy protein or placebo with a meal during the intervention. Isometric knee extension strength was reduced following IM (−24.7 ± 2.7%), partially recovered with AR (−8.6 ± 2.6%), and fully recovered after RT (−0.6 ± 3.4%), with no effect of supplementation. Thigh muscle cross-sectional area decreased with IM (−4.1 ± 0.5%), partially recovered with AR (−2.1 ± 0.5%), and increased above baseline with RT (+2.2 ± 0.5%), with no treatment effect. Myofibrillar MPS, measured using deuterated water, was unaltered by IM, with no effect of protein. During AR, MPS was increased only with protein supplementation. Protein supplementation did not attenuate the loss of muscle size and function with disuse or potentiate recovery but enhanced myofibrillar MPS during AR.
NEW & NOTEWORTHY Twenty grams of daily protein supplementation does not attenuate the loss of muscle size and function induced by 2 wk of muscle disuse or potentiate recovery in middle-age men. Average mitochondrial but not myofibrillar muscle protein synthesis was attenuated during immobilization with no effect of supplementation. Protein supplementation increased myofibrillar protein synthesis during a 2-wk period of ambulatory recovery following disuse but without group differences in phenotype recovery.
Keywords: atrophy, deuterium, disuse, milk protein, resistance training
INTRODUCTION
Periods of muscle disuse are a common consequence of illness, disability, and injury, including bone fractures. These episodes of extreme inactivity result in rapid muscle atrophy and loss of contractile function (7, 19, 34). Thus, immobility is a risk for falls (66), loss of independence (60), prolonged health care requiring hospitalization (71) and may further lead to premature mortality (44). There are currently limited therapeutic options for the management of disuse atrophy (20, 56), with significantly delayed or incomplete recovery a frequent consequence of even short periods of muscle disuse in the elderly (42, 74). Thus, repeated periods of disuse may accelerate the age-related loss of muscle mass (26), which begins in middle age (79).
While only a few high-intensity muscle contractions have been shown to effectively attenuate disuse atrophy, ongoing health needs and complications may limit the introduction of adequate mobilization and exercise (18, 20, 56). Nutritional interventions therefore may present a practical countermeasure to limit muscle loss during muscular disuse and immobility and further aid recovery (17, 25, 29, 57, 81). Limited analysis in animal models has shown protein supplementation can be effective in attenuating muscle loss during extreme models of muscle disuse (47, 63). However, clinically the evidence is not well established. A recent study demonstrated that protein supplementation failed to attenuate muscle loss during a 5-day period of muscle disuse in older individuals (19). Conversely, high-dose leucine supplementation may attenuate the loss of muscle size and strength following longer periods of bedrest (25); however, a lower dose of leucine contained within a high-quality protein might have a similar effect (13). The potential ability of supplemental protein to enhance recovery following a period of muscle disuse has not been investigated. Therefore, it needs to be established whether protein supplementation during a follow-up period of habitual activity or structured exercise may accelerate recovery.
Fundamentally, muscle mass is regulated by the balance between synthesis and degradation of contractile (myofibrillar) proteins. Disuse atrophy is likely the result of both an increase in myofibrillar protein breakdown (MPB) and a suppression of myofibrillar protein synthesis (MPS) (67). However, the relative importance of these two processes has not yet been defined (59, 61). It is well established that a period of muscle disuse results in “anabolic resistance” to protein feeding such that the MPS response to protein consumption is blunted after immobilization or inactivity compared with when the same dose of protein is ingested after normal muscle activity (7, 19, 33). This is in part responsible for the observed atrophy following muscle disuse (59). A similar anabolic resistance to feeding is often observed in older adults and can be partially overcome through the consumption of larger protein doses (16, 53). In the resting fasted state, anabolically resistant older adults are not able to further stimulate MPS with doses of protein beyond 20 g of dairy protein (85) or the equivalent dose of essential amino acids (16). However, some authors have suggested that ~32 g of protein would be required to maximally stimulate MPS in older men (53). In anabolically resistant muscle that is sensitized to feeding by prior resistance exercise, larger protein doses (~40 g) may further stimulate MPS (85). This quantity of protein is not frequently achieved at morning or midday meals (11) but would be achieved when a standard meal is supplemented with 20 g of diary protein. Therefore, it is hypothesized that the consumption of additional high-quality protein, with morning meals, during a period of limb immobilization might partially ameliorate anabolic resistance and attenuate the degree of muscle atrophy induced by disuse.
The purpose of the present study is to determine whether daily supplementation with 20 g of high-quality dairy protein, consumed with morning meals, is able to attenuate the reduction in muscle size and function induced by unilateral lower limb immobilization in middle-aged men. This model of disuse has previously been shown to result in significant but reversible muscle atrophy in 14 days (33). The secondary aim was to determine if dairy protein supplementation would increase the rate of muscle recovery during both ambulatory recovery (AR) and resistance training (RT) follow ups. This study also analyzed the integrated MPS response to immobilization and recovery using deuterium labeling to determine the regulation of MPS in disuse atrophy and recovery.
METHODS
Participants.
Thirty healthy middle-aged men between the ages of 45 and 60 yr were recruited from Auckland, New Zealand through local newspaper advertisements. The participants were free from metabolic conditions such as diabetes, musculoskeletal impairments, and a history of heart disease or cancer, or a family history of blood clots. Smokers were excluded from the study. Participants taking medications such as statins, steroids, or nonsteroidal anti-inflammatory drugs, which may affect muscle biology, were excluded. Plasma biochemistry was measured at the familiarization visit using a Roche C311 autoanalyzer, (Roche, Mannheim, Germany) by enzymatic colorimetric assay. Plasma insulin concentrations were analyzed using electrochemiluminescence immunoassay on a Cobas e11 (Roche) and are shown along with participant’s demographic information in Table 1. All 30 participants randomized into the study completed the intervention. All participants who received the intervention or control supplements completed the trial. One participant in the protein group had an abdominal injury during the RT phase but was able to complete the full protocol. Participants provided informed oral and written consent before the commencement of the study. This study was approved by the Northern Health and Disability Ethics Committee (New Zealand) and is in compliance with the most recent declaration of Helsinki. The trial was registered with the Australia New Zealand Clinical Trial Registry No. ACTRN12615000454572 on May 11, 2015.
Table 1.
Participant characteristics
| Dairy Protein (n = 15) | Placebo (n = 15) | |
|---|---|---|
| Age, yr | 51.5 ± 3.8 | 48.5 ± 2.4 |
| Height, cm | 177.5 ± 7.4 | 176.3 ± 7.5 |
| Weight, kg | 87.0 ± 14.2 | 87.9 ± 11.5 |
| BMI, kg/m2 | 27.5 ± 3.2 | 28.3 ± 3.2 |
| HDL cholesterol, mmol/l | 1.23 ± 0.40 | 1.18 ± 0.48 |
| LDL cholesterol, mmol/l | 3.50 ± 0.85 | 3.23 ± 0.77 |
| Triglycerides, mmol/l | 1.89 ± 1.62 | 1.61 ± 1.41 |
| Total cholesterol, mmol/l | 5.80 ± 0.86 | 5.36 ± 0.77 |
| Insulin, pmol/l | 60.42 ± 40.14 | 79.1 ± 42.9 |
| Glucose, mmol/l | 5.59 ± 0.49 | 5.53 ± 0.55 |
| HOMA-IR | 1.81 ± 0.98 | 1.80 ± 0.90 |
| Body fat, % | 25.1 ± 7.4 | 25 ± 6.1 |
Values are means ± SD. BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance.
Experimental design.
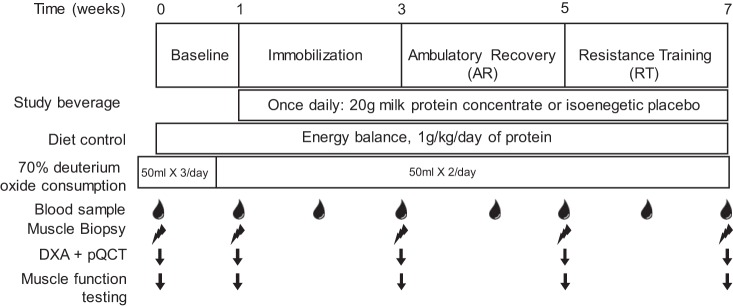
The study consisted of a total of four phases over the course of 7 wk (Fig. 1) during which time all participants underwent a 2-wk period of unilateral lower limb immobilization. Participants were randomized to either the placebo or dairy protein supplement groups in a parallel design; the leg to be immobilized was counterbalanced based on dominance. Random sequences were generated with https://www.random.org/, and the dairy protein supplement was dispensed in a double-blind manner. Allocation was conducted with a one to one ratio between groups by an investigator (M. Coronet) and concealed using a locked spreadsheet. All testing took place at the Liggins Institute Clinical Research Unit. The a priori primary outcomes of the study were changes in knee extensor isometric strength and thigh muscle cross sectional area (CSA) at 50% of femur length. Before the beginning of the baseline phase, a biopsy was obtained from the vastus lateralis muscle with a Bergström needle modified for manual suction under local anesthetic (1% lidocaine). Participants were then familiarized with the muscle function testing procedure (described below). They were then asked to maintain their normal lifestyle and physical activity patterns for 1 wk while wearing a wristband accelerometer (Fitbit Charge, San Francisco, CA) to count daily steps. After 1 wk participants returned to the laboratory and underwent a dual-energy X-ray absorptiometry (DXA) scan, peripheral quantitative computed tomography (pQCT) scan of the lower leg and thigh, and a second muscle biopsy from the same leg followed by muscle function testing. Afterwards a rigid knee brace (DonJoy; IROM, Vista, CA) was worn for 14 days on the contralateral leg from the first two biopsies. The brace was locked at 60°C during the immobilization (IM) phase (33). Participants were provided with a set of crutches and asked to avoid any weight bearing on the immobilized leg. The brace was removed and readjusted every 2 days by an investigator who sealed the brace with tape and then signed the seal to ensure compliance (33). After 2 wk of immobilization the brace was removed and the scans were repeated before a muscle biopsy was obtained from the immobilized leg and muscle function measurements were repeated. Participants were then asked to resume their normal lifestyle [ambulatory recovery (AR)] for 2 wk while physical activity, and daily steps were recorded. Following the AR, the biopsy, scans, and muscle function testing were repeated, and a one repetition maximum (1-RM) for leg extension and leg press was estimated independently for each leg using the Brzycki equation (8). The final 2-wk stage of the study consisted of three weekly RT sessions. Each RT session included four sets at 80% of 1-RM of both leg press and extension (Sygeum; Gym80, Gelsenkirchen, Germany). Each exercise was performed unilaterally with the previously immobilized leg always training first. Ten repetitions were performed for the first three sets of each exercise while the last set of each exercise was performed until the point of failure; this protocol has previously been shown to acutely increase MPS (14). The final scans, muscle biopsy, and muscle function testing were performed 48 h after the last RT session to minimize the effects of fatigue on functional tests and edema on the imaging measures, while still allowing six RT sessions to be completed in 14 days with at least 1 day of rest between sessions.
Fig. 1.
Study timeline. The study consisted of 4 phases over 7 wk. The timing of each phase, diet control and deuterium oxide ingestion protocol are shown. The timing of sample collection, muscle function testing and scanning is also shown. Muscle function testing consisted of single legged vertical jump, isometric strength testing, and a single leg incremental cycling test. DXA, dual-energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography.
Dairy protein supplement and dietary intake.
Beginning at the start of the baseline phase, participants were prescribed a partially controlled diet with the aim of controlling protein intake at 1 g·kg−1·day−1, excluding the supplements. Dietary control was intended to reduce potential effects of variability in habitual diet on muscle mass (41) and MPS changes (85). Protein intake of 1 g·kg−1·day−1 was chosen because it has been recommended as the minimum to maintain lean mass and function in older adults (4), although the participants in the present study were middle aged. Previous work has suggested the adults within the study age range require more than the current recommended daily allowance of protein to maintain muscle mass (10). Energy requirements were estimated using the Harris-Benedict equation (65) to predict basal metabolic rate and an activity factor of 1.3 based on the requirements of men with low activity levels (1.4–1.6) and then adjusted slightly for under reporting of dietary intake (30). Breakfasts (25% of energy and protein requirements, ~20 g of protein) and evening meals (50% of energy and protein requirements, ~42 g of protein) were provided to participants, which accounted for ~75% of protein and energy requirements, and individual dietary advice was provided for lunch meals to ensure participants met their protein and energy intake targets. The unequal balance of protein intake throughout the day is not optimal for maximizing MPS (55) but is reflective of normal eating habits (11). Once daily during the IM, AR, and RT phases, participants consumed either 20 g of milk protein concentrate (MPC 485 (Fonterra Cooperative Group, Auckland, New Zealand; containing 2.1 g of leucine) (52) or an isoenergetic carbohydrate placebo (maltodextrin); both supplements were vanilla flavored and artificially sweetened. Sachets containing the powdered supplements were provided in identical packaging, and participants were instructed to add the powder to 250 ml of water. The milk protein concentrate used in the study consisted of 80% casein and 20% whey protein and has previously been demonstrated to induce a similar MPS response to whey protein in middle-aged men (52). Supplements (protein or placebo) were consumed with a meal immediately after RT to stimulate postexercise MPS (15) or with breakfast on nontraining days because morning meals often contain less protein than midday or evening meals (11). Twenty-four-hour urine collections were completed at the end of each phase of the study, and protein intake was estimated based on urine urea nitrogen excretion as described by Maroni et al. (48). The average total protein intake for the three study phases when the dairy supplement was consumed was averaged for analysis. Dietary intake was analyzed using Foodworks software (Version 8, Xyris). To confirm that participants were in energy balance over the 7-wk period of diet control, changes in whole body DXA derived fat and lean mass were used to calculate average daily energy deficit or surplus (24). Chemical energy equivalents for changes in fat mass of 39.5 MJ/kg and lean mass of 7.6 MJ/kg were used (38).
Imaging.
Whole body DXA (Lunar Prodigy; GE, Waltham, MA) scans were performed at the beginning and end of each study phase to assess body composition. The whole body scan was automatically segmented by the software, and segmentation of tissue regions was defined by lines positioned on the image. These lines were adjusted where necessary to include all of the tissue within the appropriate regions. Segmentation of all scans were completed by a single investigator who was blinded to treatment allocation.
Muscle CSA of the immobilized leg was assessed using a Stratec XCT 3000 pQCT with software version 6.20C (Stratec Medizintechnik, Pforzheim, Germany). Participants were positioned supine with the immobilized leg centered within the machine’s gantry and anchored by a foot rest with straps to limit movement during each scan. Muscle CSA was measured at 20 and 50% of femur length and at 66% of tibia length. Femur length was measured from the lateral knee joint space to the greater trochanter and tibia length from the lateral malleolus to the lateral knee joint space. For the upper leg scans, a scout view scan was used to position the anatomical reference line at the distal femur joint surface. For the lower leg scan, the computed tomography position was defined manually. The following measurements were obtained: total area with subcutaneous fat removed (mm2) and cortical area (mm2). Muscle CSA was determined as the difference in these measures. For analysis of the total area, threshold was set at 40 mg/cm3 (contour mode 1/peel mode 1). For cortical area, the threshold was set at 280 mg/cm3 (contour mode 1/peel mode 1). All pQCT scans were analyzed by the same operator.
Muscle function.
Single leg vertical jump height was used as a measure of muscle power production. Participants performed four maximal single leg vertical jumps on a force platform (Leonardo Mechanograph GRFP; Novatech Medical, Pforzheim, Germany) with a 5-s rest between jumps, and the average of four jumps was reported. Isometric muscle strength of the knee flexors and extensors was tested using a Biodex dynamometer (Shirley, NY) with the knee angle set to 90° of flexion. Five maximal contractions were performed for each movement with 30 s of rest in between and the highest values were used for analysis. Muscle aerobic capacity was measured using an incremental single leg cycling test. Participants were seated on a cycle ergometer (Velotron; Racemate, Seattle, WA), with one foot attached to the pedal with tape, while the other pedal was removed and in its place was an 8.5-kg counterweight. Participants cycled at 50 W for 1 min, and then, the resistance was increased 2 W every 12 s until their cadence dropped below 50 rpm; motivation and encouragement were provided throughout the test.
Real-time polymerase chain reaction.
Gene expression of atrogenes (MuRF1/TRIM63 and Atrogin-1/FBXO32) and myostatin (MSTN) was analyzed by real-time polymerase chain reaction (RT-PCR). RNA was extracted from ~15 mg of muscle tissue using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The High-Capacity RNA-to-cDNA Kit (Life Technologies, Carlsbad, CA) was used to synthesize cDNA. RT-PCR was performed using SYBR Green I Master Mix (Roche Applied Science, Penzberg, Germany) on a LightCycler 480 II (Roche Applied Science). Primers used in this study are listed in Table 2. For normalization purposes, several proposed stable reference genes, (C1orf43, CHMP2A, EMC7, and VCP) (23) and genes commonly used as reference genes in the muscle literature (TBP, PPIA, and HPRT) were analyzed. The geometric mean of the three most stable genes across the whole population set for all time points (78) was used for normalization. The levels of TBP, HPRT, and CHMP2A mRNAs were identified as the three least variable and were therefore used as reference genes. Standard and melting curves were performed for every target to confirm primer efficiency and single product amplification. The 2−ΔCT method was used to compare difference in raw gene expression (45).
Table 2.
RT-PCR primer sequences
| Target | Primer Sequence |
|---|---|
| Atrogin-1 | |
| Forward | AATAAGGAGAATCTTTTCAACAGCC |
| Reverse | TCCATGGCGCTCTTTAGTACTTC |
| MuRF1 | |
| Forward | GGGACAAAAGACTGAACTGAATAAC |
| Reverse | GGCTCAGCTCTTCCTTTACCT |
| MSTN | |
| Forward | CTACAACGGAAACAATCATTACCA |
| Reverse | GTTTCAGAGATCGGATTCCAGTAT |
| TBP | |
| Forward | TGTGCTCACCCACCAACAAT |
| Reverse | TCTGCTCTGACTTTAGCACCTG |
| CHMP2A | |
| Forward | CGCTATGTGCGCAAGTTTGT |
| Reverse | GGGGCAACTTCAGCTGTCTG |
| HPRT | |
| Forward | CCTGGCGTCGTGATTAGTGAT |
| Reverse | TCGAGCAAGACGTTCAGTCC |
Muscle protein synthesis.
For 2 days before the first study visit, participants consumed three 50-ml aliquots of 70% deuterium oxide (D2O; Isowater, Collingwood, ON, Canada) each day, and they continued to consume thrice daily aliquots (morning, midday, and evening) of deuterium oxide for the first 5 days of the study. For the remainder of the study participants consumed 2 daily (morning and evening) 50-ml aliquots of 70% D2O. This protocol has previously been demonstrated to rapidly achieve a precursor pool enrichment of 1–2% (64, 69). Plasma samples were collected weekly to measure isotopic enrichment of total body water.
Skeletal muscle tissue was fractionated according to previously published procedures (21, 22, 49, 50, 64). Muscle (30–50 mg) was homogenized 1:10 with a bead homogenizer (Next Advance, Averill Park, NY) in isolation buffer (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris base, 5 mM MgCl2, 1 mM EDTA, and 1 mM ATP, pH 7.5) with phosphatase and protease inhibitors (HALT; Thermo Scientific, Rockford, IL). After homogenization, subcellular fractions of myofibrillar (containing primarily contractile elements and nuclei) and mitochondrial proteins were isolated via differential centrifugation as previously described (21, 22, 49, 50, 64, 69). After muscle fractions were isolated and purified, 250 μl of 1 M NaOH were added, and pellets were incubated for 15 min at 50°C and centrifuged at 900 rpm. Protein was hydrolyzed by incubation for 24 h at 120°C in 6 N HCl. The hydrolysates were ion exchanged, dried under vacuum, and resuspended in 1 ml molecular biology grade H2O. Five-hundred microliters of suspended samples were derivatized [500 μl acetonitrile, 50 μl 1 M K2HPO4 (pH 11), and 20 μl of pentafluorobenzyl bromide (Pierce Scientific, Rockford, IL)], sealed, and incubated at 100°C for 1 h. Derivatives were extracted into ethyl acetate. The organic layer was removed and dried by N2 followed by vacuum centrifugation. Samples were reconstituted in 1 ml ethyl acetate and then analyzed. The pentafluorobenzyl-N,N-di(pentafluorobenzyl) derivative of alanine was analyzed on an Agilent 7890B GC coupled to an Agilent 5977A MS as previously described (21, 22, 49, 50, 64, 69). To determine body water enrichment, 125 μl of plasma were placed into the inner well of an O-ring screw cap and inverted on a heating block overnight. Two microliters of 10 M NaOH and 20 μl of acetone were added to all samples and to 100 μl 0–20% D2O standards and then capped immediately. Samples were vortexed at low speed and left at room temperature overnight. Extraction was performed by the addition of 200 μl hexane. The organic layer was transferred through anhydrous Na2SO4 into GC vials and analyzed via EI mode with a DB-17MS column.
The newly synthesized fraction of proteins was calculated from the true precursor enrichment with plasma analyzed for D2O enrichment and adjusted by mass isotopomer distribution analysis (MIDA) (9). Protein synthesis was calculated as the ratio of deuterium-labeled to unlabelled alanine (9) in proteins over the entire labeling period and then converted to fractional synthesis rate by dividing by time (day) and multiplying by 100.
Citrate synthase activity.
Citrate synthase (CS) activity was measured from a separate piece of tissue. Briefly, tissue was weighed and homogenized in ice-cold homogenization buffer (25 mM Tris·HCl pH7.8, 1 mM EDTA, 2 mM MgCl2, 50 mM KC, and 0.50% Triton X-100) using a TissueLyser II (Qiagen, Dusseldorf, Germany). Homogenates were centrifuged at 14,000 g for 10 min at 4°C, and the supernatant was frozen at −80°C until further use. All assays were performed using the Molecular Devices Spectramax-340 96-well microplate reading spectrophotometer at 25°C. CS activity was determined by measuring absorbance at 412 nm in 50 mM Tris·HCl (pH 8.0) with 0.2 mM DTNB, 0.1 mM acetyl-coA, and 0.25 mM oxaloacetate. The rate of change of absorbance and path length of each well were determined using SoftMax pro version 3.1.1 (Molecular Devices, Sunnyvale, CA), and CS activity was calculated using an extinction coefficient of 13.6 mM/cm.
Statistical analysis.
Sample size was based on a 20% decrease in knee extension torque following immobilization and was powered to detect a 50% attenuation in torque loss with 80% power, resulting in 15 participants per group. Estimated variability in torque loss was based on Dirks et al. (19) and Oates et al. (56). Differences in muscle size, function, and fractional synthetic rate were assessed using a mixed models approach to repeated-measures ANOVA (SAS, SAS Institutes, Cary, NC, version 9.4, [proc mixed function]). Baseline values were used as a covariate, time was used as a within subjects fixed factor, and supplement was used as a between subject factor. The subject was added to the model as a random factor. Where appropriate pairwise comparisons were examined using Tukey's post hoc test. PCR data were analyzed using two-way ANOVA as a fold change from baseline. Two-way random effects model interclass correlation coefficient (ICC)(2,1) was used to test the reliability of measurements of muscle function and size between the familiarization visit (week 0) and the baseline visit (week 1) (82). Least square means are shown as ± SE in the figures and text and ± SD in the tables. α Was set at P < 0.05.
RESULTS
Muscle strength and function.
The reliability of all physical function measurements was excellent (knee extension ICC = 0.92; keen flexion ICC = 0.87; single leg cycling peak power ICC = 0.96) with the exception of isometric plantar flexion (ICC = 0.64) and single leg jump height (ICC = 0.60), which were classified as good. Baseline knee extension torque was 231 ± 14 and 212 ± 19 Nm in the placebo and protein groups, respectively; there were no group or group-by-time effects (P = 0.839), but there was a main effect of time (P < 0.0001). Knee extension torque decreased with IM, was partially recovered after AR, and fully recovered after RT (Fig. 2A). Baseline knee flexion torque was 89 ± 4 Nm in the placebo group and 90 ± 6 Nm in the protein group. There were no group or group-by-time effects (P = 0.542), but there was a main effect of time (P < 0.0001). Knee flexion torque was also reduced with IM, returned to baseline levels with AR (Fig. 2B), and increased above baseline following RT. Baseline plantar flexion strength was 59 ± 7 and 69 ± 8 Nm in the placebo and protein groups, respectively. There were no group or group-by-time effects (P = 0.257), but there was a main effect of time (P = 0.019). Plantar flexion strength decreased with IM, increased above baseline with AR, and increased further following RT (Fig. 2C).
Fig. 2.
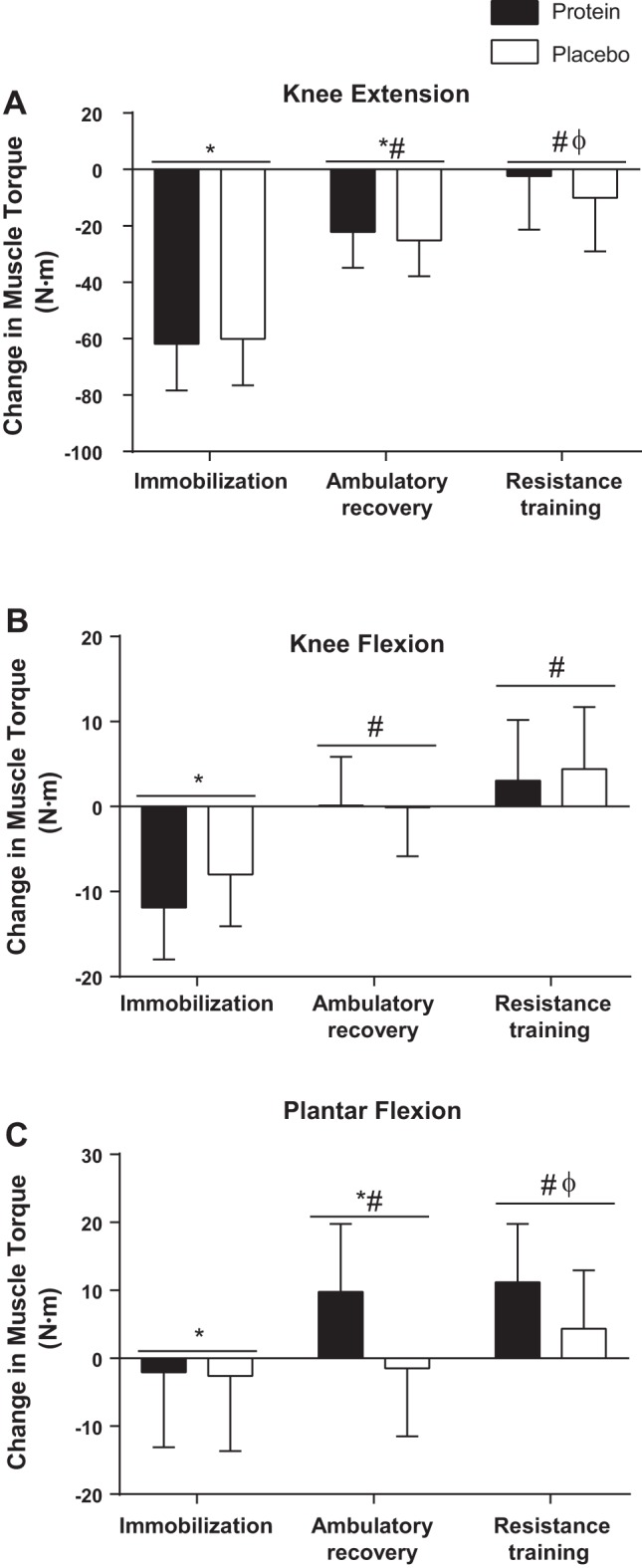
Muscle torque. Changes from baseline in maximal voluntary muscle torque for knee extension (A), knee flexion (B), and plantar flexion (C). Bars are means ± SE. Horizontal line represents a main effect for time. *P < 0.05, significantly different than baseline. #P < 0.05, significantly different from immobilization. ΦP < 0.05, significantly different from ambulatory recovery.
Single leg jump height was used as a measure of muscular power and at baseline was 25.7 ± 0.1 cm in the placebo group and 23.6 ± 0.1 cm in the protein group. There were no group or group-by-time effects (P = 0.479), but there was a main effect of time (P < 0.0001). Jump height decreased with IM, was partially recovered following AR, and fully recovered following RT (Fig. 3A). Peak power production during the incremental cycling test was 149 ± 9 and 148 ± 9 W in the placebo and protein groups, respectively, at baseline. Peak power decreased with IM, was partially recovered following AR, and fully recovered following RT (Fig. 3B). There were no group or group-by-time effects (P = 0.314), but there was a main effect of time (P < 0.0001).
Fig. 3.
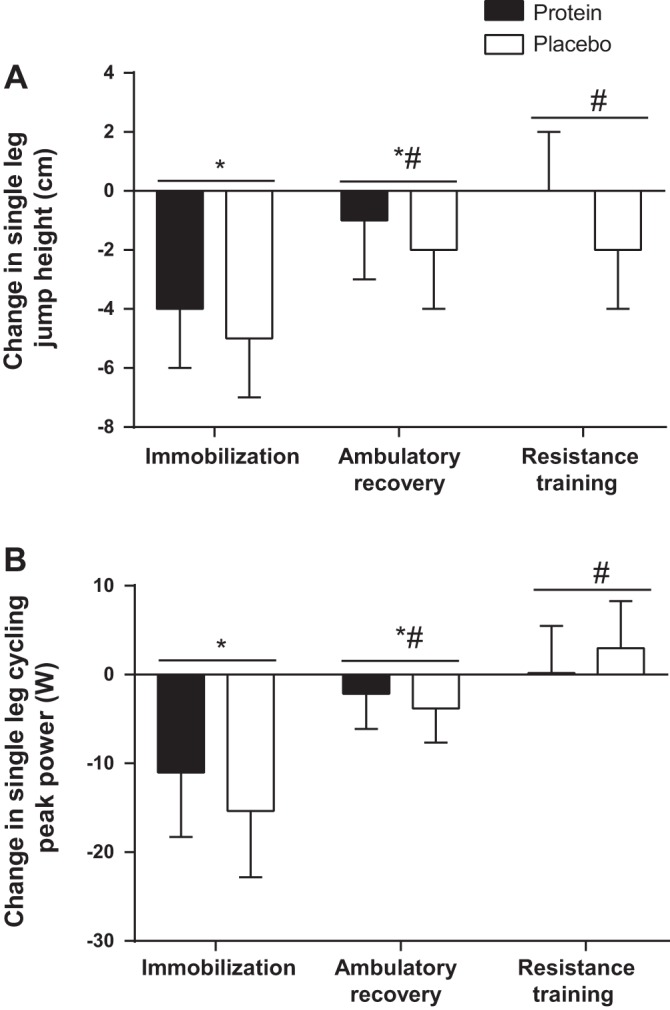
Muscle function. Changes in single leg jump height (A) and peak power during an incremental single leg cycling test performed until exhaustion (B). Bars are means ± SE. Horizontal line represents a main effect for time. *P < 0.05, significantly different than baseline. #P < 0.05, significantly different from immobilization.
Muscle size.
Reliability of all imaging measures was excellent (ICC = 0.99). Muscle CSA was measured using pQCT. Thigh muscle CSA at 50% femur length was 16,469 ± 637 and 16,033 ± 831 mm2 at baseline in the placebo and protein groups, respectively. There were no group or group-by-time effects (P = 0.582), but there was a main effect of time (P < 0.0001). Muscle CSA at 50% femur length was reduced with IM, partially recovered with AR, and increased above baseline with RT (Fig. 4A). Muscle CSA at 20% femur length was 8,687 ± 426 and 8,726 ± 512 mm2 at baseline in the placebo and protein group, respectively. There were no group or group-by-time effects (P = 0.943), but there was a main effect of time (P < 0.0001). Thigh muscle CSA at 20% femur length reduced with IM, recovered with AR, and increased above baseline with RT (Fig. 4B). Calf muscle CSA at 66% tibia length was 9,330 ± 345 mm2 in the placebo group at baseline and 8,867 ± 388 mm2 in the protein group at baseline. Calf muscle CSA reduced with IM, recovered with AR, and increased above baseline with RT (Fig. 4C). There were no group or group-by-time effects (P = 0.648), but there was a main effect of time (P < 0.0001).
Fig. 4.
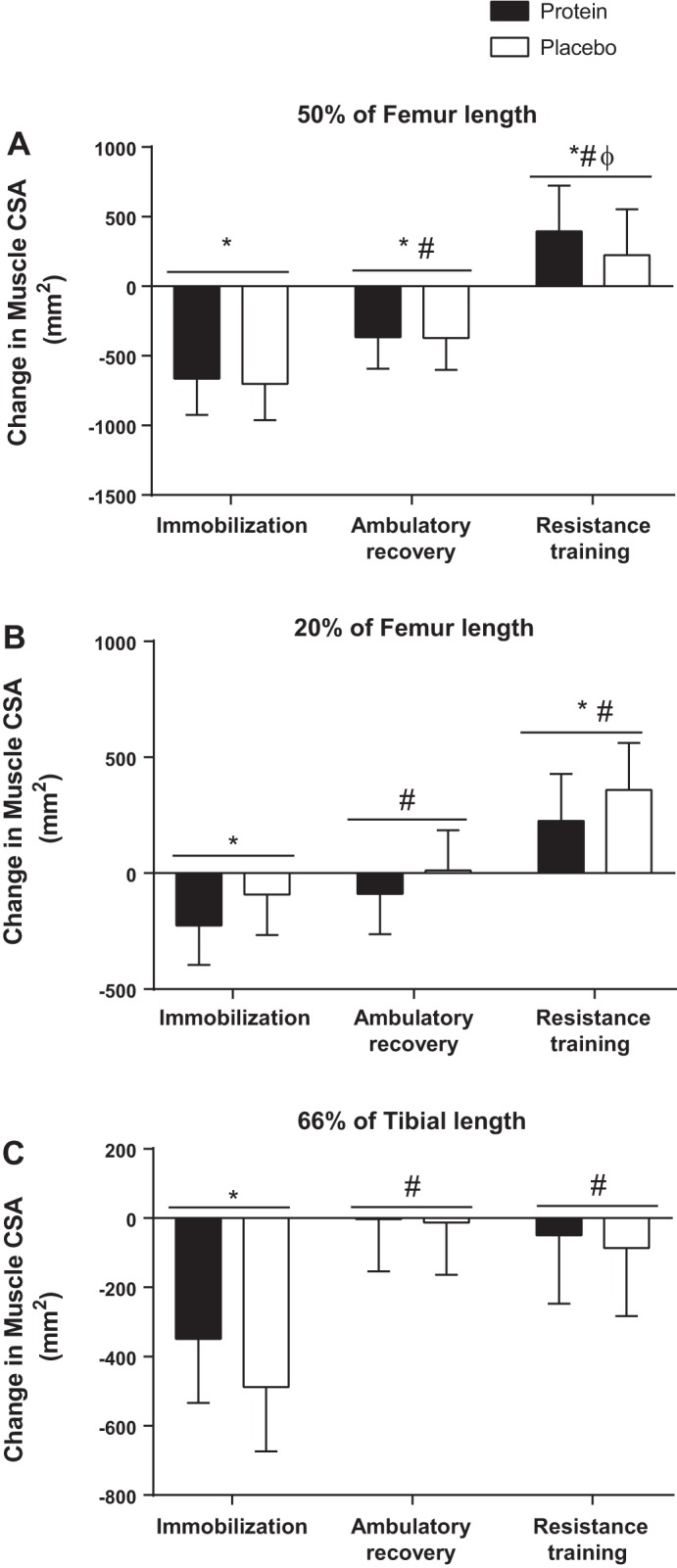
Muscle cross-sectional area (CSA). Changes from baseline in thigh muscle CSA at 50% (A) and 20% of femur length (B) as well as calf CSA at 66% of tibia length (C). Bars are means ± SE. Horizontal line represents a main effect for time. *P < 0.05, significantly different than baseline. #P < 0.05, significantly different from immobilization. ΦP < 0.05, significantly different from ambulatory recovery.
DXA was also used to estimate changes in muscle mass throughout the study. There were no changes in fat or lean mass of the control (nonimmobilized) leg throughout the study with the exception of a main effect for an increase in lean mass from the post-IM time point to the post-RT time point (P = 0.043) (data not shown). Fat mass of the immobilized leg did not change during the study. The immobilized leg decreased in lean mass from 10,510 ± 339 and 10,412 ± 528 g in the placebo and protein group, respectively, by 127 ± 256 g in the protein group and 199 ± 256 g in the placebo group; however, this change did not reach significance (P = 0.076). Lean leg mass increased above baseline after RT 116 ± 177 and 123 ± 177 g in the protein and placebo groups, respectively, although significance was not reached (P = 0.060). However, there was a significant difference between the post-IM and post-RT time points (P = 0.002).
Protein intake and activity level.
Urea values from 24-h urine collections were used to estimate different protein intakes of 67.6 ± 6.3 g/day in the placebo group and 91.1 ± 7.4 g/day in the supplement group (P = 0.023). When expressed relative to body weight, estimated protein intakes were 0.79 ± 0.09 g·kg−1·day−1 in the control group and 1.08 ± 0.11 g·kg−1·day−1 in the protein group (P = 0.06). Dietary intake (Table 3) was unchanged in fat or carbohydrate content across the study phases. Protein consumption increased in the protein supplementation group after the onset of supplementation (IM phase) and was maintained for the AR and RT phases. The pattern was apparent when consumption was expressed as total protein (P = 0.004), as relative to body weight (P = 0.005), and as a percentage of energy intake (P = 0.002). Participants in both groups self-reported to slightly exceeding their lunch protein targets resulting in an average daily protein intake of ~1.1 g·kg−1·day−1 in the diets of both groups exclusive of the supplements provided. Dietary protein intake at the midday meal was prescribed as ~20 g; however, participants in both groups consumed ~30 g of protein at the midday meal. Daily steps were tracked with a wrist worn accelerometer that counted baseline steps per day of 10,060 ± 1,042 and 11,427 ± 1,389 in the placebo and protein groups, respectively. Daily steps were not recorded during the IM phase because participants were ambulating using crutches. Steps per day during the AR phase were 11,825 ± 726 and 11,677 ± 901 in the placebo and protein groups, respectively. During the RT phase, steps per day were 11,778 ± 722 and 12,300 ± 1,130 in the placebo and protein groups, respectively. There were no differences in daily steps between diet groups or study phases. The average energy balance over the 7-wk diet control period was a deficit of 42.3 ± 81.0 and 38.5 ± 90.6 kcal/day in the placebo and protein groups, respectively. These values were not different than zero (P = 0.501) and were not different between groups (P = 0.971).
Table 3.
Dietary intake during the 4 phases of the intervention
| Baseline |
Immobilization |
Ambulatory Recovery |
Resistance Training |
|||||
|---|---|---|---|---|---|---|---|---|
| Protein | Placebo | Protein | Placebo | Protein | Placebo | Protein | Placebo | |
| Protein, g·kg−1·day−1 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.3 ± 0.3* | 1.1 ± 0.1 | 1.2 ± 0.2* | 1.1 ± 0.2 | 1.3 ± 0.3* | 1.1 ± 0.1 |
| Protein, g/day | 93.6 ± 10.3 | 92.0 ± 15.5 | 115.0 ± 21.4* | 97.5 ± 17.6 | 109.1 ± 13.4* | 92.0 ± 12.0 | 113.9 ± 14.4* | 92.7 ± 13.9 |
| Protein, %energy | 15.5 ± 2.0 | 16.2 ± 1.8 | 18.4 ± 1.7* | 15.3 ± 1.9 | 17.7 ± 1.4* | 14.9 ± 1.3 | 17.8 ± 1.5* | 15.4 ± 1.6 |
| Carbohydrate, g/day | 281.9 ± 27.7 | 280.0 ± 55.3 | 279.6 ± 52.3 | 319.7 ± 61.4 | 282.2 ± 37.8 | 313.8 ± 67.4 | 288.4 ± 32.2 | 303.0 ± 43.0 |
| Carbohydrate, %energy | 46.5 ± 3.0 | 49.0 ± 3.7 | 44.6 ± 2.6 | 50.0 ± 2.7 | 45.8 ± 2.2 | 50.2 ± 2.8 | 45.2 ± 3.1 | 50.2 ± 3.6 |
| Fat, g/day | 95.8 ± 19.5 | 80.1 ± 11.1 | 98.4 ± 31.9 | 93.0 ± 20.6 | 94.6 ± 24.9 | 85.3 ± 21.0 | 100.2 ± 25.0 | 87.4 ± 17.0 |
| Fat, %energy | 35.2 ± 3.3 | 31.8 ± 2.5 | 34.7 ± 3.6 | 32.6 ± 2.8 | 34.0 ± 2.9 | 30.9 ± 5.2 | 34.8 ± 3.6 | 32.4 ± 3.7 |
| Total energy, kcal | 2,434 ± 291 | 2,280 ± 368 | 2,523 ± 555 | 2,559 ± 461 | 2,476 ± 406 | 2,493 ± 468 | 2,568 ± 374 | 2,420 ± 329 |
Values are means are ± SD.
P < 0.05, different from baseline within the same group. All values include both intake of meals and supplements (protein or placebo).
Muscle protein synthesis.
The mitochondrial protein synthesis rate was measured using D2O as a tracer and was 1.45 ± 47%/day and 1.03 ± 0.13%/day in the placebo and protein groups, respectively; there were no group effects or group by time interactions (P = 0.879). However, there was a main effect of time such that the fractional synthetic rate of mitochondrial proteins decreased during the IM phase compared with baseline (P = 0.030) (Fig. 5A). The myofibrillar protein synthesis rate at baseline was 1.18 ± 0.34 and 1.22 ± 0.39%/day in the placebo and protein groups, respectively. There were effects of both supplement group (P = 0.010) and phase of the study (P = 0.040), but the group-by-time interaction did not reach significance (P = 0.150). IM had no effect on myofibrillar protein synthesis whereas myofibrillar protein synthesis was increased above baseline during the AR phase only in the protein group (P = 0.049) (Fig. 5B). Total body water deuterium enrichment was not different between groups and increased from 1.02 ± 0.06% during the baseline phase to 1.36 ± 0.08% during the IM phase (P < 0.001). Enrichment was then maintained at 1.53 ± 0.11 and 1.58 ± 0.10% in the AR and RT phases, respectively (P = 0.524).
Fig. 5.
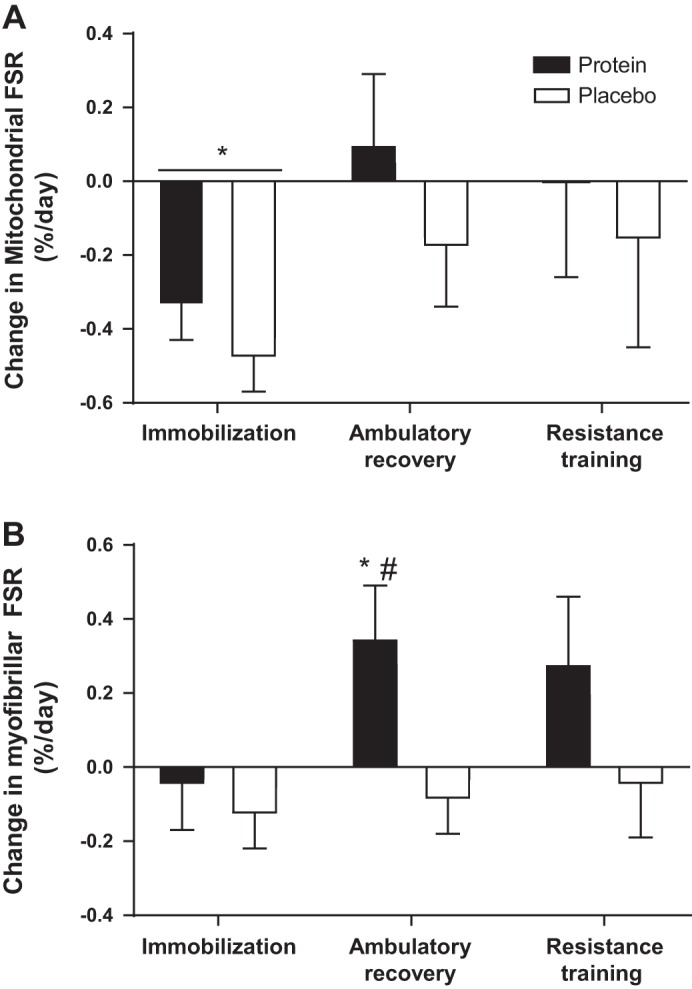
Muscle protein synthesis. The changes from baseline in fractional synthetic rate of mitochondrial (A) and myofibrillar (B) protein fractions. Bars are means ± SE. Horizontal line represents a main effect for time. *P < 0.05, significantly different than baseline. #P < 0.05, significantly different from immobilization.
Gene expression and citrate synthase activity.
Atrogin gene expression increased following IM (P < 0.001) with no difference between groups. Expression returned to baseline after AR and remained at baseline levels following RT (Fig. 6A). MuRF-1 gene expression also increased following IM (P = 0.003) with no difference between groups and returned to baseline following AR and remained at baseline (Fig. 6B) after RT. mRNA expression of myostatin was not increased above baseline after IM or AR with no difference between groups. Myostatin mRNA was greater after IM when compared with after RT (P = 0.001) (Fig. 6C). CS activity was 13.15 ± 1.3 and 11.56 ± 1.21 nM·min−1·µg−1 protein in the protein and control group, respectively, which did not change over the course of the study (P = 0.124) and was not different between groups.
Fig. 6.
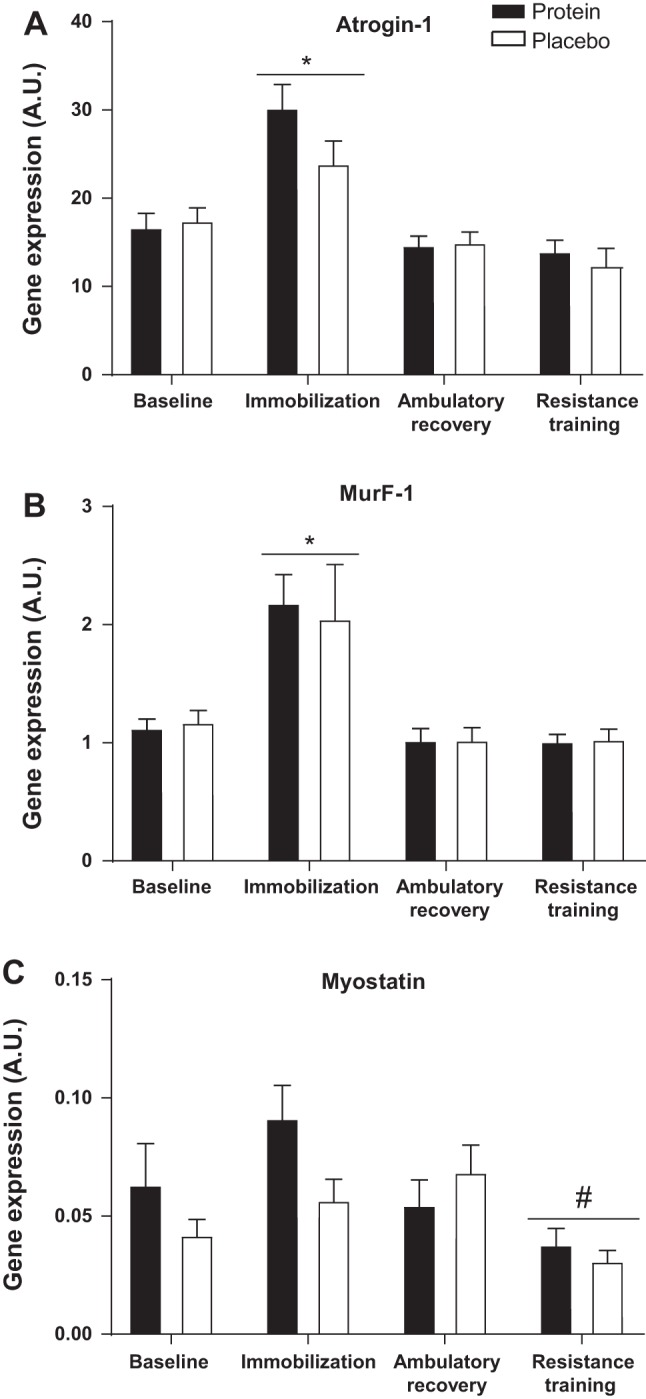
Expression of genes regulating muscle catabolism. Changes in mRNA expression of Atrogin1 (A), MuRF-1 (B), and Myostatin (C). Data are expressed as fold change from baseline for each subject. AU, arbitrary units. Bars are means ± SE. Horizontal line represents a main effect for time. *P < 0.05, significantly different than baseline. #P < 0.05, significantly different from immobilization.
DISCUSSION
The consumption of 20 g of daily supplemental dairy protein, consumed along with a morning meal, did not attenuate the decline of any measures of muscle size or function in this group of middle-aged men consuming 1.1 g total protein·kg body wt−1·day−1 during a 2-wk period of single leg immobilization. Despite supplemental protein increasing the rate of synthesis of contractile proteins during recovery from immobilization, the recovery of muscle size or function were not improved. Restoration of muscle function and size was not fully achieved with 2 wk of normal activity, but full recovery was attained with an additional 2 wk of RT.
The ingestion of 20 g of milk protein is known is stimulate MPS in middle-aged men, and this dose of protein can increase RT-mediated gains in muscle mass (12). However, this finding is not consistent across all studies (62). Given the actions of 20 g of high-quality protein on MPS, it was hypothesized that this dose of supplemental dairy protein could potentially attenuate muscle loss during immobilization. Muscle disuse or immobilization is known to decrease the sensitivity of muscle to protein feeding in both younger and older adults (33, 80). No dose-response studies have been conducted to identify the optimal dose of protein to overcome disuse-induced anabolic resistance. However, it has been suggested 40 g of protein are sufficient to maximally simulate MPS in anabolically resistant older adults (53). Similarly, 40 g of protein ingested after exercise were more effective at simulating MPS in anabolically resistant older adults than the 20 g required to maximize MPS in those without anabolic resistance (54, 85). During disuse, the present study provided 20 g of dairy protein along with a breakfast containing 20 g of protein. The consumption of 40 g of protein was likely sufficient to result in a maximal but transient stimulation of MPS following in the morning meal. The provided evening meals also contained ~42 g of protein, which again was likely sufficient to maximally simulate MPS; however, it is possible that inclusion of some lower quality plant-based proteins reduced the total anabolic potential of the provided mixed meals (77). It is also possible that an attenuation of the muscle loss during immobilization might have been achieved by providing high-quality supplemental protein with the lunch meal and before bed (76) to maximally simulate MPS four times daily rather than the twice daily that was likely achieved in the current study (46).
Recently, Dirks et al. (19) reported a lack of efficacy for 20 g of dairy-based protein ingested twice daily to attenuate muscle loss during just 5 days of immobilization in men aged over 60 yr. Our study agrees with these findings over a 2-wk period of muscle disuse. A number of studies have examined the ability of protein or amino acid supplementation to attenuate atrophy during longer periods of bed rest (reviewed in Ref. 72). The findings of these studies are variable but suggest that a of benefit of supplemental protein is evident more frequently when compared with control participants consuming at or below the recommended daily allowance (RDA) (0.8 g·kg−1·day−1) for dietary protein (72). In the present study, participants consumed 1.1 g·kg−1·day−1 of protein daily, exclusive of the supplement. This habitual protein ingestion exceeding the RDA may partially explain the lack of efficacy of the supplement. A lower habitual protein intake might have increased the potential of the dairy protein to attenuate disuse atrophy. Yet relevance would be limited, as less than 10% of men in the study age range consume protein at or below the RDA (31).
In the present study, protein supplementation did not alter the rate at which muscle size and function recovered following immobilization, yet supplementation increased the aggregate myofibrillar MPS rate, during the first 2 wk of recovery. Given the lack of effect of protein supplementation on muscle size during this period, the observed increase in MPS might represent a higher turnover with protein supplementation compared with the placebo group. This higher turnover could suggest a greater rate of remodelling (39). Yet, in the absence of a measurable phenotype adaptation definitive conclusions cannot be made. Speculatively, either the changes in MPS could have been unrelated to the gains in mass and strength, or that the changes were below the limits of detection. It is then possible that a more sensitive measure of muscle size such as magnetic resonance imaging-derived muscle volume or a longer AR period may have resulted in a measurable difference in muscle size recovery with protein supplementation.
The relative contributions of blunted MPS and increased MPB on muscle mass during human disuse atrophy are unknown. Animal studies suggest that MBP is likely a major contributor to disuse atrophy (5). However, it is has been argued that because the magnitude of atrophy observed in animal models is much larger than is observed even with severe human disuse models such as bed rest, findings from animal studies might not directly translate to human physiology (59). In humans, multiple studies have shown that disuse lasting longer than 5 days decreases both postprandial and postabsorptive MPS (27, 32, 58, 80), while in vivo human measurements of muscle MPB are rarely performed. Of the available data, fasted MPB in six men did not to change after 14 days of bedrest (27). Furthermore, MPB has also been inferred not to change with muscle disuse, based on declining MPS and changes in muscle size (32). Thus the consensus of existing literature suggests that accelerated MPB is unlikely to exert a predominant role in the loss of muscle mass with immobilization (2). Yet, in the present study, baseline myofibrillar MPS rates were ~1.2%/day, and the mean change in muscle CSA was −4.5% following immobilization. Thus a ~27% daily increase in MPB, decrease in MPS, or combination of the two would be required to explain the observed muscle loss. It is likely that this imbalance, at least in part, was due to increased MPB. To date the only available measures of muscle protein turnover during disuse have been over the course of several hours in the postprandial and postabsorptive periods, measured following weeks of prior disuse (58, 80). Long-term protein turnover measurements have not previously been reported, so no direct comparisons can be made. Animal studies (35, 68) along with measures of human catabolic gene expression (19, 37, 73) and breakdown products (75) suggest MPB may transiently increase at the onset of disuse. In the present study, catabolic gene expression (MurF-1, Atrogin-1, and Myostatin) was also elevated after 2 wk of disuse supporting at least some elevation of MPB. Given that MPB in humans has not been measured at the beginning of disuse, it is possible an increase in MPB during the first several days of immobilization is in part contributing to the ~27% imbalance between MPS and MPB measured over the 2-wk intervention. However, it also possible that true suppression of MPS was simply not detected.
Loss of mitochondrial mass and loss of function are common consequences of muscle disuse (1, 36). We developed an incremental single leg cycling protocol to evaluate muscular aerobic function (6). Along with the commonly observed decreases in strength and power, the present study shows a decline in aerobic power that was not fully recovered after 2 wk of AR. This finding was accompanied by a decrease in mitochondrial MPS but no change in CS activity, a marker of mitochondrial content (43). The depressed mitochondrial MPS is the result of a slower turnover of mitochondrial proteins, and this may result in a decline in mitochondrial function resulting from a slower clearance of damaged mitochondrial proteins (36).
The present study was conducted in middle-aged males, an inadequately defined life stage (51,) which marks a transition between the ability of young adults to rapidly recover from muscle disuse to the impaired recovery observed in older adults (74). Suetta et al. (74) have shown that even when structured RT is commenced immediately after immobilization and continued for 4 wk, older men do not fully recover muscle lost after 2 wk of immobilization. Numerous animal studies have also shown an impaired ability to recover from disuse in older animals (3, 84). Due to the design of the current study, it is unclear if the middle-aged participants would have recovered their preimmobilization muscle strength and size without the RT phase or how long this recovery would take. Based on the findings, it seems prudent to recommend resistance exercise following periods of disuse such as those induced by casting and hospital stays in middle-aged and older adults to ensure the recovery of muscle function and morphology.
The study design that was employed enabled analysis of the actions of protein supplementation during muscle disuse and recovery on muscle size, function, and protein synthesis. Because of the complexity of the design, it is important to acknowledge a number of limitations. First, it is not possible to separate the effects of RT from the effects of an additional 2 wk of active recovery, so it is not known if the recovery observed after RT would have also occurred with an additional 2 wk of free-living recovery. It is also unknown if a longer period of immobilization or RT would have been able to differentiate any actions of the protein supplementation on muscle mass and function. Second, it was not technically possible to measure MPB or protein net balance at the same time as MPS, so definitive quantification of MPB is not possible. Further research using a tracer dilution technique will be required to draw firm conclusions regarding the extent of MPB during muscle disuse (40). The present study only recruited men, which limits the generalizability of the findings. Premenopausal young women display similar resting and postexercise MPS responses to men of the same age (83), whereas older postmenopausal women have very different muscle protein metabolism compared with men of the same age (70). Middle-aged women may respond differently to both disuse and protein intake compared with men of the same age and thus should be the subject of further research.
Short-term muscle disuse resulted in a rapid decline in muscle function and size, which was not recovered after 2 wk of normal activity; however, an additional 2 wk of RT fully normalized muscle size and function. The addition of 20 g of high-quality protein to the controlled diets of middle-aged men did not attenuate the loss of muscle function or size during immobilization, possibly due in part to the participants consuming adequate protein. During disuse, MPS of contractile proteins was not altered. This maintenance of MPS during immobilization suggests that MPB may be important for the loss of muscle mass during disuse. Interestingly during recovery, dairy protein supplementation increased myofibrillar MPS in the absence of measurable differences in size and function, making the physiological significance unclear. Importantly, for those in middle-age, even a brief period of muscle disuse results in skeletal muscle atrophy and functional decline. This is not modified with 20 g of daily supplemental protein and is not fully normalized by 2 wk of ambulatory recovery.
The age-related loss of muscle mass and strength begins in middle age and may be exacerbated by incomplete recovery from periods of muscle disuse. RT should be prescribed after periods of casting, immobilization, bedrest, and reduced activity to support the full normalization of muscle function. Protein supplementation does not appear to be beneficial in attenuating the loss of muscle mass or strength during immobilization in middle-aged men consuming adequate protein. Further nutritional research should focus on the potential use of protein to enhance recovery from disuse and the use of higher dose more frequent protein supplementation during disuse.
DISCLOSURES
C. J. Mitchell, R. F. D’Souza, and D. Cameron-Smith received financial support from the New Zealand Primary Growth Partnership (PGP) post-farm gate program, funded by Fonterra Cooperative Group and the NZ Ministry for Primary Industries (MPI), to conduct this study. SDP is the Fonterra Chair in Human Nutrition, University of Auckland; A. C. Fanning is a current employee of Fonterra Cooperative Group. The other authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
C.J.M., B.F.M., K.L.H., A.C.F., S.D.P., and D.C.-S. conceived and designed research; C.J.M., R.F.D., S.M.M., V.C.F., F.F.P., M.C., C.A.P., and B.D. performed experiments; C.J.M., R.F.D., S.M.M., V.C.F., B.F.M., K.L.H., F.F.P., and C.A.P. analyzed data; C.J.M., B.F.M., and K.L.H. interpreted results of experiments; C.J.M. prepared figures; C.J.M. drafted manuscript; C.J.M., R.F.D., S.M.M., V.C.F., K.L.H., F.F.P., M.C., C.A.P., B.D., A.C.F., S.D.P., and D.C.-S. approved final version of manuscript; R.F.D., S.M.M., V.C.F., B.F.M., K.L.H., F.F.P., C.A.P., A.C.F., S.D.P., and D.C.-S. edited and revised manuscript.
ACKNOWLEDGMENTS
We acknowledge William C. Castor, Sarah E. Ehrlicher, and Justin Reid for help with preparation of samples for the gas chromatography-mass spectrometry.
REFERENCES
- 1.Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, Phillips SM, Tarnopolsky M. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One 4: e6518, 2009. doi: 10.1371/journal.pone.0006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 311: E594–E604, 2016. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E, Boirie Y. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14: 542–559, 2013. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boushel R, Saltin B. Ex vivo measures of muscle mitochondrial capacity reveal quantitative limits of oxygen delivery by the circulation during exercise. Int J Biochem Cell Biol 45: 68–75, 2013. doi: 10.1016/j.biocel.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98: 2604–2612, 2013. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 8.Brzycki M. A Practical Approach to Strength Training. Indianapolis, IN: Blue River, 2012. [Google Scholar]
- 9.Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760: 730–744, 2006. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 56: M373–M380, 2001. doi: 10.1093/gerona/56.6.M373. [DOI] [PubMed] [Google Scholar]
- 11.Cardon-Thomas DK, Riviere T, Tieges Z, Greig CA. Dietary protein in older adults: adequate daily intake but potential for improved distribution. Nutrients 9: 9, 2017. doi: 10.3390/nu9030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96: 1454–1464, 2012. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 13.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99: 276–286, 2014. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- 14.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 590: 2751–2765, 2012. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab (Lond) 9: 40, 2012. doi: 10.1186/1743-7075-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 32: 704–712, 2013. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Devries MC, Breen L, Von Allmen M, MacDonald MJ, Moore DR, Offord EA, Horcajada MN, Breuillé D, Phillips SM. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep 3: 3, 2015. doi: 10.14814/phy2.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr 144: 1196–1203, 2014. doi: 10.3945/jn.114.194217. [DOI] [PubMed] [Google Scholar]
- 20.Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf) 210: 628–641, 2014. doi: 10.1111/apha.12200. [DOI] [PubMed] [Google Scholar]
- 21.Drake JC, Bruns DR, Peelor FF 3rd, Biela LM, Miller RA, Hamilton KL, Miller BF. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol Endocrinol Metab 307: E813–E821, 2014. doi: 10.1152/ajpendo.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake JC, Peelor FF 3rd, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci 68: 1493–1501, 2013. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenberg E, Levanon EY. Human housekeeping genes, revisited. Trends Genet 29: 569–574, 2013. doi: 10.1016/j.tig.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Elia M, Livesey G. Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev Nutr Diet 70: 68–131, 1992. doi: 10.1159/000421672. [DOI] [PubMed] [Google Scholar]
- 25.English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr 103: 465–473, 2016. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 29: 18–23, 2010. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RL, Schatzkin A, Spiegelman D, Subar AF, Tinker LF, Willett W. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 180: 172–188, 2014. doi: 10.1093/aje/kwu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 87: 1554S–1557S, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 72: 503–509, 1987. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- 33.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol 264: 267–282, 1977. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gram M, Vigelsø A, Yokota T, Helge JW, Dela F, Hey-Mogensen M. Skeletal muscle mitochondrial H2 O2 emission increases with immobilization and decreases after aerobic training in young and older men. J Physiol 593: 4011–4027, 2015. doi: 10.1113/JP270211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustafsson T, Osterlund T, Flanagan JN, von Waldén F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol (1985) 109: 721–727, 2010. doi: 10.1152/japplphysiol.00110.2009. [DOI] [PubMed] [Google Scholar]
- 38.Hall KD. What is the required energy deficit per unit weight loss? Int J Obes 32: 573–576, 2008. doi: 10.1038/sj.ijo.0803720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hesselink MK, Minnaard R, Schrauwen P. Eat the meat or feed the meat: protein turnover in remodeling muscle. Curr Opin Clin Nutr Metab Care 9: 672–676, 2006. doi: 10.1097/01.mco.0000247471.64532.7d. [DOI] [PubMed] [Google Scholar]
- 40.Holm L, Kjaer M. Measuring protein breakdown rate in individual proteins in vivo. Curr Opin Clin Nutr Metab Care 13: 526–531, 2010. doi: 10.1097/MCO.0b013e32833c3c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB; Health ABC Study . Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 87: 150–155, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Hvid LG, Suetta C, Nielsen JH, Jensen MM, Frandsen U, Ørtenblad N, Kjaer M, Aagaard P. Aging impairs the recovery in mechanical muscle function following 4 days of disuse. Exp Gerontol 52: 1–8, 2014. doi: 10.1016/j.exger.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Legrand D, Vaes B, Matheï C, Adriaensen W, Van Pottelbergh G, Degryse JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc 62: 1030–1038, 2014. doi: 10.1111/jgs.12840. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr 35: 1506–1511, 2016. doi: 10.1016/j.clnu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Maki T, Yamamoto D, Nakanishi S, Iida K, Iguchi G, Takahashi Y, Kaji H, Chihara K, Okimura Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr Res 32: 676–683, 2012. doi: 10.1016/j.nutres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 49.Miller BF, Ehrlicher SE, Drake JC, Peelor FF 3rd, Biela LM, Pratt-Phillips S, Davis M, Hamilton KL. Assessment of protein synthesis in highly aerobic canine species at the onset and during exercise training. J Appl Physiol (1985) 118: 811–817, 2015. doi: 10.1152/japplphysiol.00982.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11: 150–161, 2012. doi: 10.1111/j.1474-9726.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell CJ, D’Souza RF, Zeng N, McGregor RA, Fanning AC, Poppitt SD, Cameron-Smith D. Understanding the sensitivity of muscle protein synthesis to dairy protein in middle-aged men. Int Dairy J 63: 35–41, 2016. doi: 10.1016/j.idairyj.2016.07.008. [DOI] [Google Scholar]
- 52.Mitchell CJ, McGregor RA, D’Souza RF, Thorstensen EB, Markworth JF, Fanning AC, Poppitt SD, Cameron-Smith D. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients 7: 8685–8699, 2015. doi: 10.3390/nu7105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 54.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 55.Murphy CH, Oikawa SY, Phillips SM. Dietary protein to maintain muscle mass in aging: a case for per-meal protein recommendations. J Frailty Aging 5: 49–58, 2016. [DOI] [PubMed] [Google Scholar]
- 56.Oates BR, Glover EI, West DW, Fry JL, Tarnopolsky MA, Phillips SM. Low-volume resistance exercise attenuates the decline in strength and muscle mass associated with immobilization. Muscle Nerve 42: 539–546, 2010. doi: 10.1002/mus.21721. [DOI] [PubMed] [Google Scholar]
- 57.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 89: 4351–4358, 2004. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 58.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985) 107: 645–654, 2009. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- 59.Phillips SM, McGlory C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 592: 5341–5343, 2014. doi: 10.1113/jphysiol.2014.273615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rantanen T, Avlund K, Suominen H, Schroll M, Frändin K, Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res 14, Suppl: 10–15, 2002. [PubMed] [Google Scholar]
- 61.Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: The dominant mechanism causing disuse muscle atrophy is proteolysis. J Physiol 592: 5345–5347, 2014. doi: 10.1113/jphysiol.2014.279406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reidy PT, Borack MS, Markofski MM, Dickinson JM, Deer RR, Husaini SH, Walker DK, Igbinigie S, Robertson SM, Cope MB, Mukherjea R, Hall-Porter JM, Jennings K, Volpi E, Rasmussen BB. Protein supplementation has minimal effects on muscle adaptations during resistance exercise training in young men: a double-blind randomized clinical trial. J Nutr 146: 1660–1669, 2016. doi: 10.3945/jn.116.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribeiro CB, Christofoletti DC, Pezolato VA, de Cássia Marqueti Durigan R, Prestes J, Tibana RA, Pereira EC, de Sousa Neto IV, Durigan JL, da Silva CA. Leucine minimizes denervation-induced skeletal muscle atrophy of rats through akt/mtor signaling pathways. Front Physiol 6: 73, 2015. doi: 10.3389/fphys.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 40: 168–182, 1984. [DOI] [PubMed] [Google Scholar]
- 66.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 35, Suppl 2: ii37–ii41, 2006. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 67.Rudrappa SS, Wilkinson DJ, Greenhaff PL, Smith K, Idris I, Atherton PJ. Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance-a qualitative review. Front Physiol 7: 361, 2016. doi: 10.3389/fphys.2016.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21: 140–155, 2007. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 69.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HL, Szallar SE, Wood LM, Peelor FF 3rd, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. doi: 10.1096/fj.13-246595. [DOI] [PubMed] [Google Scholar]
- 70.Smith GI, Villareal DT, Sinacore DR, Shah K, Mittendorfer B. Muscle protein synthesis response to exercise training in obese, older men and women. Med Sci Sports Exerc 44: 1259–1266, 2012. doi: 10.1249/MSS.0b013e3182496a41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia and length of hospital stay. Eur J Clin Nutr 70: 595–601, 2016. doi: 10.1038/ejcn.2015.207. [DOI] [PubMed] [Google Scholar]
- 72.Stein TP, Blanc S. Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit Rev Food Sci Nutr 51: 828–834, 2011. doi: 10.1080/10408398.2010.482679. [DOI] [PubMed] [Google Scholar]
- 73.Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Bayer M, Petersson SJ, Schrøder HD, Andersen JL, Heinemeier KM, Aagaard P, Schjerling P, Kjaer M. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One 7: e51238, 2012. doi: 10.1371/journal.pone.0051238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107: 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 75.Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol (1985) 105: 902–906, 2008. doi: 10.1152/japplphysiol.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trommelen J, van Loon LJ. Pre-sleep protein ingestion to improve the skeletal muscle adaptive response to exercise training. Nutrients 8: 8, 2016. doi: 10.3390/nu8120763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr 145: 1981–1991, 2015. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 78.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: H0034, 2002. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 1: 129–133, 2010. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wall BT, Snijders T, Senden JM, Ottenbros CL, Gijsen AP, Verdijk LB, van Loon LJ. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab 98: 4872–4881, 2013. doi: 10.1210/jc.2013-2098. [DOI] [PubMed] [Google Scholar]
- 81.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 71: 195–208, 2013. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 82.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19: 231–240, 2005. [DOI] [PubMed] [Google Scholar]
- 83.West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, Hawley JA, Coffey VG, Phillips SM. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol (1985) 112: 1805–1813, 2012. doi: 10.1152/japplphysiol.00170.2012. [DOI] [PubMed] [Google Scholar]
- 84.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol 64: 17–32, 2015. doi: 10.1016/j.exger.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108: 1780–1788, 2012. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]