Abstract
Adipose tissue depots can exist in close association with other organs, where they assume diverse, often ‘non-traditional’ functions. In stem cell-rich skin, bone marrow and mammary glands, adipocytes signal to and modulate organ regeneration and remodeling. Skin adipocytes and their progenitors signal to hair follicles, promoting epithelial stem cell quiescence and activation, respectively. Hair follicles signal back to adipocyte progenitors, inducing their expansion and regeneration, as in skin scars. In mammary glands and heart, adipocytes supply lipids to neighboring cells for nutritional and metabolic functions, respectively. Adipose depots adjacent to skeletal structures function to absorb mechanical shock. Adipose tissue near the surface of skin and intestine senses and responds to bacterial invasion, contributing to the body’s innate immune barrier. As the recognition for diverse adipose depot functions increase, novel therapeutic approaches centered on tissue-specific adipocytes are likely to emerge for a range of cancers and regenerative, infectious, and autoimmune disorders.
Introduction
The storage of energy as lipids is a highly conserved mechanism shared by unicellular and multicellular organisms across evolutionary phylogeny. While prokaryotes and single-celled eukaryotes store lipids in intracellular organelles known as lipid droplets or lipid bodies, multicellular organisms developed specialized cells to house them (Driskell et al., 2014; Ottaviani et al., 2011). Lipid-storing cells exist both in invertebrates and vertebrates, although rather than being homologous, they may have evolved convergently to sequester lipid from the extracellular environment (Ottaviani et al., 2011). In vertebrates, adipocyte-like cells have been noted already in lamprey, a group of jawless fish (Muller, 1968).
In mammals, two principal types of adipose tissue exist, white (WAT) and brown (BAT) (Frontini and Cinti, 2010; Rosen and Spiegelman, 2014a). BAT develops embryonically, derived from Myf5- and Pax7- expressing precursor cells in the mesoderm that also give rise to skeletal muscle cells and a portion of white adipocytes (Lepper and Fan, 2010; Sanchez-Gurmaches et al., 2012; Seale et al., 2008; Wang and Seale, 2016). Brown adipocytes, which in rodents are predominantly contained in the interscapular region, contain multilocular lipid droplets and high numbers of mitochondria, and primarily function to dissipate stored energy in the form of heat. Although in humans BAT was thought to be restricted to an interscapular depot in infants and adults chronically exposed to extreme cold, more recent evidence has suggested that brown adipocytes, and/or adipocytes possessing characteristics of both brown and white adipocytes (known as ‘beige’ or ‘brite’ adipocytes), may be more common in adults than had been previously appreciated (Wang and Seale, 2016). That said, the majority of adipose tissue in mammals, including adult humans, and the focus of this review, is WAT, which is primarily comprised of large adipocytes that harbor a single lipid droplet and markedly fewer mitochondria than brown adipocytes. We will also discuss bone marrow adipose tissue (BMAT), which is currently thought to be distinct from either WAT or BAT (Horowitz et al., 2017).
Historically, the study of WAT has centered around its principle function in controlling energy homeostasis via the storage and release of lipids in response to systemic nutritional and metabolic needs. WAT is distributed throughout the body in several distinct depots. These include visceral depots (vWAT), that in humans include omental, mesenteric, retroperitoneal, gonadal, and pericardial WAT (Wajchenberg, 2000), and are commonly associated with metabolic disorders, such as diabetes and cardiovascular disease (Shuster et al., 2012). Another highly studied depot is subcutaneous WAT (sWAT). It is located in several locations under the skin and, in humans, clusters of sWAT exist in upper (deep and superficial abdomen) and lower (gluteofemoral) body regions (Kwok et al., 2016). Clinically, sWAT has been found to confer some beneficial effects on metabolism (Tran et al., 2008). The differing metabolic functions of the major vWAT and sWAT depots have been the subject of numerous excellent reviews including those by Tchkonia et al. (2013) and Rosen and Spiegelman (2014b).
In addition to major WAT depots, discrete tissue-associated adipose depots are broadly distributed across the body (Figure 1). These depots are often small in size, intricate in microanatomy, closely associated with other anatomic structures, and perform novel tissue- and organ-specific functions (Kruglikov and Scherer, 2016). Recognition of their importance is rapidly growing; yet, with few exceptions, their biology remains incompletely understood. Below we review the prominent tissue-associated adipose depots by anatomic location and highlight some of their most distinctive features.
Figure 1: Anatomy of adipose depots.
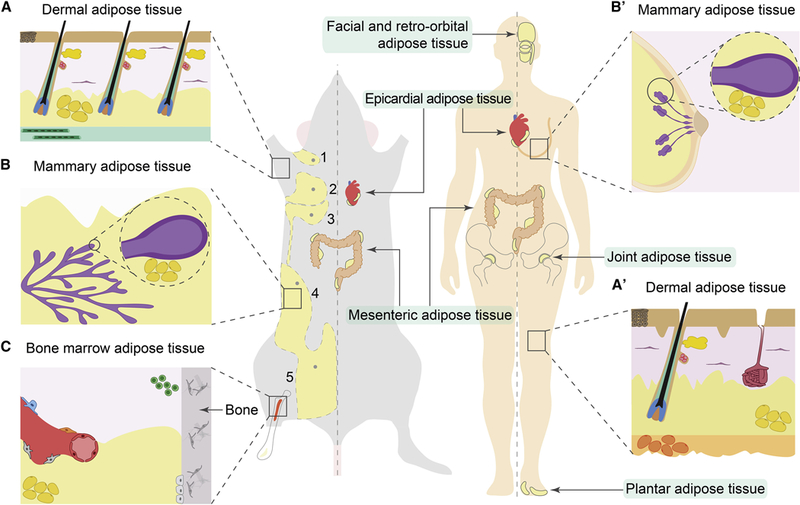
(A, A’) In mouse skin, dermal WAT (dWAT) forms a continuous layer (shown in yellow) separated from subcutaneous WAT (sWAT) by the panniculus carnosus muscle (shown in green). This separation is not prominent in human skin, where dWAT is continuous with underlying sWAT (orange) (A’). Dermal WAT closely associates with HFs and prominently remodels during hair growth cycles. Lower portion of actively growing HFs (shown in red, blue and green) resides within dermal WAT, in close contact with adipocytes. The upper portion of the HF, housing the hair shaft, and containing the HF stem cell compartment and sebaceous gland (shown in red and yellow, respectively) and in humans, a sweat gland (maroon) are also shown. (B, B’) In the mammary gland, adipose tissue (yellow) closely associates with the gland’s epithelium (purple) and undergoes cyclic remodeling during pregnancy, lactation and involution. As shown on D, female mice have five mammary glands, three thoracic (numbers 1, 2 and 3) and two inguinal (numbers 4 and 5), each with its own adipose pads. (C) Specialized adipose tissue is associated with the bone marrow. Micro-anatomically and functionally, it is subdivided into constitutive (yellow) and regulated (red) depots. Adipocytes are shown in yellow, hematopoietic progenitors in green and other mesenchymal bone marrow cell types in grey. Anatomic location of the following additional adipose depots in mouse and human are shown: facial and retro-orbital, epicardial, joint, mesenteric, and plantar adipose tissues.
Anatomy of adipose depots
Dermal adipose tissue.
The skin consists of consecutive layers of stratified epidermis, fibroblast-rich dermis, and dermal WAT (dWAT), containing mature, unilocular white adipocytes. Skin appendages, principally hair follicles (HFs), traverse through multiple layers, and in many species, such as humans and mice, come in close contact with dWAT (Figure 1A, 1A’) (Driskell et al., 2014). Although most mammals have a dedicated dWAT depot, its anatomy can vary substantially across species. In mice, dWAT forms a continuous layer, separated from sWAT by a striated muscle layer, aka the panniculous carnosus (Driskell et al., 2014). Humans largely lack this muscle layer, but plastic surgeons have long reported that fascia superficialis demarcates two distinct skin adipose compartments: namely the superficial areolar and deep lamellar layers (Alexander and Dugdale, 1992; Gasperoni and Salgarello, 1995). These layers of skin-associated WAT differ in cellular distribution and metabolic activity (Miyazaki et al., 2000; Sbarbati et al., 2010; Smith et al., 2001; Walker et al., 2007). Pigs contain three layers of skin-associated WAT, each separated by layers of fascia, with adipocytes in the outer and middle layers having distinct cellular, enzymatic and lipidomic profiles (Anderson et al., 1972; Hilditch and Stainsby, 1935; Monziols et al., 2007).
A signature feature of dWAT is its striking cyclic size fluctuations, which occur in parallel with HF cycling (Chase et al., 1953; Wojciechowicz et al., 2013; Wojciechowicz et al., 2008). HFs repetitively make new hairs via a three-phase regeneration cycle involving: (1) growth (aka anagen), during which HFs elongate via epithelial cell proliferation and dWAT layer prominently expands in thickness; (2) regression (aka catagen), when HFs involute largely via apoptosis and dWAT recedes; and (3) quiescence (aka telogen) when HFs are small and inactive and dWAT is at its thinnest (Figure 2) (Stenn and Paus, 2001). Cyclic dWAT expansion results both from hypertrophy of pre-existing adipocytes and hyperplasia, which involves proliferation of adipocyte precursors (APs) that subsequently differentiate into lipid-laden, unilocular adipocytes (Festa et al., 2011b; Rivera-Gonzalez et al., 2016b; Zhang et al., 2016a). In mouse dWAT, several populations of APs are recognized. Similar to other WAT depots, dermal APs are defined by the expression of the cellular surface markers CD34, CD29, and Sca1 (Festa et al., 2011b; Rodeheffer et al., 2008b). Among these APs, CD24+ cells represent an adipocyte stem cell (ASC) population, while CD24− cells are committed pre-adipocytes (Berry et al., 2013; Rivera-Gonzalez et al., 2016b). Although both CD24+ and CD24− subsets of APs proliferate prior to anagen, the CD24+ ASC population preferentially expands, resulting in the generation of ~20% new adipocytes (Festa et al., 2011b; Rivera-Gonzalez et al., 2016b). Proliferation of both ASCs and pre-adipocytes is Pdgfa-dependent, and dWAT expansion at this stage is abrogated in mice with a mesenchymal cell-specific deletion of Pdgfa (Rivera-Gonzalez et al., 2016b).
Figure 2: Cellular and signaling basis for dermal WAT remodeling.
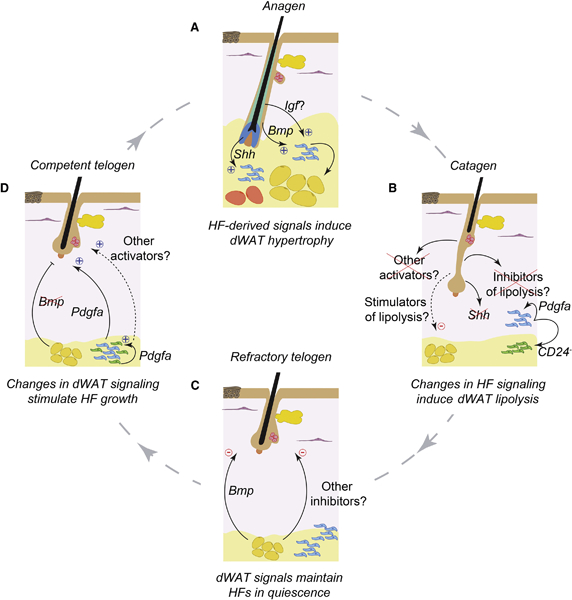
(A) Dermal WAT remodels in parallel with the hair growth cycle. dWAT peaks in thickness during the anagen phase, when HFs are actively growing and their proximal segments closely contact with adipocytes. dWAT contains several generations of adipocytes, earlier formed cells (yellow), that remain from the previous cycle, and later formed cells (orange), that differentiate from progenitors (blue) during new cycle. In each cycle, ~20% of adipocytes form anew from the progenitors. Anagen HFs stimulate dWAT hypertrophy and hyperplasia via several paracrine ligands for Hedgehog, BMP and, possibly IGF pathways. (B) dWAT quickly reduces in size during catagen phase, primarily via lipolysis. Signaling mechanism of catagen-associated lipolysis remains poorly understood. It likely involves loss of pro-adipogenic signaling factors, such as Shh, that are abundant during anagen, and/or production of new anti-adipogenic factors. Associated with catagen is also proliferative expansion of adipose progenitors. Early stage CD24+ ASCs (blue) proliferate to give rise to CD24− late stage progenitors, pre-adipocytes (green). Driving progenitor expansion is autocrine Pdgfa signaling. (C) dWAT reaches is smallest size during telogen phase. During early, aka refractory telogen, dermal adipocytes express BMP ligands that signal to maintain quiescent state of resting HFs. Other adipose-derived hair cycle inhibitors likely exist during this phase. (D) During late, aka competent telogen dermal adipocytes cease to express BMP ligands and telogen HFs become “alert”, able to quickly enter new anagen upon stimulation. Pdgfa, produced by adipose progenitors, can directly signal to stimulate new hair cycle entry by telogen HFs. Other adipose-derived hair cycle activators likely exist during this phase.
HF elongation and dWAT expansion during anagen are not only concomitant, but functionally linked, providing a prime example of cross-regulation between epithelial and adipose cell lineages (Figure 2A). Defects in dermal adipogenesis affect HF regeneration—hair cycle progression becomes disrupted upon pharmacological inhibition of the adipocyte differentiation regulatory factor Pparγ, and after transplantation of normal skin onto Ebf1 mutant mice, in which dermal APs are reduced (Festa et al., 2011b). Conversely, grafting purified APs into normal telogen skin is sufficient to activate a new hair growth cycle (Festa et al., 2011b). Unlike in Ebf1 mutants, hair cycle progresses normally in A-Zip mice which lack mature adipocytes, but feature elevated AP numbers (Festa et al., 2011b; Rodeheffer et al., 2008b). These data show that dermal APs, rather than mature adipocytes, promote new hair cycle entry by HFs.
By contrast, mature dermal adipocytes supply telogen HFs with long-range inhibitory signals that simultaneously stall hair regeneration across large stretches of skin (Plikus et al., 2008). This role for dermal adipocytes was identified in studies on natural hair cycle in mice—certain groups of telogen HFs are “competent” to quickly reenter anagen when challenged with physiological or exogenous growth-activating signals, such as hair plucking (Chen et al., 2015), while other groups of telogen HFs are “refractory” to the very same stimuli and fail to regenerate. Further observations showed that during early telogen, HFs are refractory and acquire competent state only after one month, and that this is associated with prominent downregulation of Bone morphogenetic protein 2 (Bmp2) expression by adjacent dermal adipocytes (Figure 2C, 2D). BMP signaling maintains quiescence of HF stem cells and adipose-derived Bmp2 contributes to highly quiescent, refractory telogen state. Skin-specific overexpression of the soluble BMP antagonist Noggin is sufficient to largely abolish telogen refractivity, leading to precocious hair cycle reentry by HFs (Plikus and Chuong, 2014; Plikus et al., 2008).
Hair cycle-coupled dWAT expansion is also modulated by epithelial Wnt activity (Donati et al., 2014) and Shh signals (Zhang et al., 2016b) (Figure 2A). During HF morphogenesis and regeneration, inhibition of canonical Wnt signaling in epithelial skin cells suppresses adipogenesis, while its ectopic activation increases dWAT expansion, even in the absence of HFs. Responding to Wnt activation in vitro, epithelial cells secrete pro-adipogenic factors, suggesting a potential mechanism by which epithelial cells may control dWAT expansion in parallel with anagen entry. Donati et al. (2014) identified BMP and IGF ligands as the putative Wnt-controlled epithelial pro-adipogenic factors. Another recent study identified pro-adipogenic effect of HF-derived Shh ligands (Zhang et al., 2016b), adding Hedgehog pathway to the list of signals shared between HFs and dWAT.
In addition to local triggers, dWAT can expand in response to systemic cold stress (Kasza et al., 2014). Compared to normal animals, mice lacking syndecan-1, a heparin sulfate proteoglycan, display dWAT size reduction and compromised cold stress responses at room temperature (21˚C), that induces moderate thermal shock, but not at thermo-neutral conditions (31˚C). Boosting adipogenesis with the Pparγ agonist rosiglitazone rescues both the dWAT thickness and cold stress response phenotypes in syndecan-1 null mice. While the role of skin in systemic thermoregulation is not fully understood (Romanovsky, 2014), the syndecan-1 study suggests that dWAT is important for this homeostatic process. The mechanism by which dWAT responds to cold stress is an exciting future direction for the field.
dWAT also plays a major role in several aspects of skin wound healing. Wound repair typically occurs in three sequential stages—inflammation, proliferation, and remodeling (Gurtner et al., 2008). This process can be modeled in mice, with different injury sizes resulting in distinct repair outcomes. Regeneration of hair follicles and lipid-filled adipocytes occur during repair of large but not small wounds (Ito et al., 2007; Plikus et al., 2017).
Lineage studies using Sm22-Cre and Sma-CreER mouse models showed that the majority of newly formed adipocytes that are generated in large healing wounds derive from contractile fibroblasts, aka myofibroblasts (Plikus et al., 2017). De novo adipogenesis in this case is driven by BMP signals generated by new anagen-stage HFs and depends on activation of Zfp423, the key transcriptional regulator of adipose lineage commitment (Gupta et al., 2010; Shao et al., 2017). New adipocyte formation in response to signals from HFs during wound healing thus provides further support for the notion of bidirectional signaling between epithelial and adipocyte lineages (Donati et al., 2014). Anagen HFs derived from human scalp and BMP can also induce human keloid scar fibroblasts to undergo adipogenesis in vitro (Plikus et al., 2017), suggesting at least partial conservation of this phenomenon between mice and humans.
Several studies suggest that dWAT is an active participant in wound repair, though our understanding of the specific roles played by adipocyte lineage cells in the process is not yet fully understood. Transplantation of heterogeneous populations of adipose-derived mesenchymal cells into dermal wounds accelerates healing (Kim et al., 2007), though further studies are needed to clarify whether this function could be attributed to a pure population of adipocyte precursor cells. When adipogenesis is inhibited via pharmacological and genetic means, extracellular matrix deposition and fibroblast recruitment into the wound bed are impaired (Schmidt and Horsley, 2013), suggesting that adipocyte lineage cells enhance normal healing, likely via paracrine signaling. Indeed, several adipocyte-derived factors, or adipokines, have been implicated in controlling wound healing. One such factor, adiponectin, influences wound re-epithelialization, and mice deficient in adiponectin demonstrate a pronounced delay in wound healing (Jin et al., 2015; Salathia et al., 2013; Shibata et al., 2012). Leptin, another adipokine, also enhances wound re-epithelialization as well as re-vascularization (Frank et al., 2000; Ring et al., 2000; Sierra-Honigmann et al., 1998). Systemic and local application of leptin to Ob null mice, which lack endogenous leptin, rescues wound re-epithelialization and contraction defects, but it has no effect in Db null mice, which lack functional leptin receptor (Frank et al., 2000; Ring et al., 2000), suggesting that leptin acts through its receptor to facilitate proper wound healing.
Among other skin-specific roles of dWAT, a recent study identified its importance in local innate immune response to bacterial infection. Responding to skin infection by Staphylococcus aureus, dWAT prominently and rapidly expands in size (Zhang et al., 2015). This bacteria-induced dWAT expansion occurs in telogen skin, does not require new hair cycle entry, and mechanistically involves hypertrophy of preexisting adipocytes and adipogenesis of Pref1+,Zfp423+ pre-adipocytes. Concomitantly with hypertrophy, dermal adipocytes start to express and secrete large amounts of cathelicidin, a membrane-disrupting antimicrobial peptide which directly kills bacteria (Kosciuczuk et al., 2012). A normal adipogenesis program is critical for proper cathelicidin production and skin innate immune response becomes severely compromised in Zfp423 null mice deficient for adipogenic progenitors, and in mice treated with Pparγ inhibitors. For instance, skin infection that would normally be locally contained progresses to septicemia (Zhang et al., 2015).
Facial adipose tissue.
Craniofacial structures closely associate with multiple adipose pads and the effect that WAT has on facial features in humans significantly affects facial recognition and age perception (Figure 1). Developmentally, in mice, many facial adipocytes develop from neural crest (Billon et al., 2007; Lemos et al., 2012), similar to adjacent skeletal structures. However, with age their numbers decrease concomitantly with an increase in non-neural crest mesenchyme-derived adipocytes (Lemos et al., 2012). Existence of neural crest-derived adipocytes in the face can explain why some types of human congenital WAT dystrophies, specifically congenital infiltrating lipomatosis (Couto et al., 2017; Kamal et al., 2010) are restricted to the face. Temporally, facial adipose pads, including these in the cheeks, chin and periocular regions, begin to specify prenatally in the second trimester with cheek WAT forming first and periocular WAT forming last (Poissonnet et al., 1983).
Facial WAT consists of deep and superficial depots (Rohrich and Pessa, 2007). Deep depots include sub-orbital, retro-orbital and buccal adipose pads. Being important for facial reconstruction, they are detailed in plastic surgery literature (Aiache and Ramirez, 1995; Hwang et al., 2007; May et al., 1990; Stuzin et al., 1990). Superficial depots are numerous and distribute broadly under the facial skin. Nasolabial adipose runs along the identically named skin fold next to the nose. Cheek adipose is the largest depot and sub-divides into three distinct compartments—medial, middle, and lateral temporal pads. Of these, middle and lateral temporal pads provide cheek skin with its volume. Cranium contains two large adipose pads—temporal and forehead adipose. Orbital adipose consists of three distinct pads that extend around the eye and into the cheek. Lastly, jowl adipose is found in the inferior part of the face.
In normal adults, facial WAT dramatically reduces with age and this strongly contributes to facial aging—loss of adipose volume results in wrinkling of the skin. Susceptibility of individual facial WAT depots to age-related atrophy differs. For instance, retro-orbital WAT prominently atrophies (Gesta et al., 2007) and this contributes to aged, sunken eye appearance, while other depots, such as nasolabial and jowl WAT, are more resistant to atrophy (Rohrich and Pessa, 2007). Age-related changes that do occur in jowl WAT, however, contribute to a ‘sagging jawline’ appearance.
Clinically, retro-orbital WAT is particularly remarkable. It prominently expands within the eye socket in Grave’s disease, a type of inflammatory autoimmune disorder (Bahn, 2010), and presents as exophthalmos, bulging of the eyes, also known as Graves’ ophthalmopathy. At least in part, exophthalmos is caused by excessive adipogenesis in the retro-orbital space (Forbes et al., 1986) and if left untreated it can result in sight-threatening complications, including optic nerve compression (Wiersinga and Bartalena, 2002). Pathogenesis of Graves’ ophthalmopathy appears to be complex (Gortz et al., 2016), but includes stimulation of thyrotropin receptor, expressed on a subset of retro-orbital pre-adipocytes, by activating autoantibodies (Starkey et al., 2003). Autoantibody-mediated activation of thyrotropin signaling can directly promote adipogenesis, similar to the effect of native thyrotropin ligand (Kumar et al., 2011; Lu and Lin, 2008; Zhang et al., 2009). Excessive retro-orbital adipogenesis can be additionally stimulated by pro-adipogenic prostaglandins secreted by activated T-cells present in large numbers in the affected orbit (Feldon et al., 2006).
Mammary adipose tissue.
In the mammary gland, epithelial ducts, the milk-producing structures, are surrounded by mammary adipose tissue (mgWAT) (Figure 1B, 1B’). It is primarily composed of white adipocytes, although short-lived brown adipocytes, and a brown adipocyte-associated gene expression signature that is cold-inducible, have also been identified in young mice (Gouon-Evans and Pollard, 2002; Master et al., 2002). Mice contain five pairs of mammary glands, numbered one through five according to their anterior-posterior positioning along the mammary line axis (Figure 1). Among these, the fourth gland, located inguinally in sWAT, is the gland of choice in the majority of mouse mammary and breast cancer studies (Richert et al., 2000). Compared to mice, adipocyte abundance represents a lower percentage of the human breast in favor of more fibrous connective tissue (Parmar and Cunha, 2004), and varies widely between individuals (reviewed in (Hovey and Aimo, 2010; Neville et al., 1998)).
Somewhat analogous to HFs, the mammary gland epithelium undergoes cyclic remodeling consisting of pregnancy, lactation, and involution stages, and mgWAT remodels along with it (Anderson et al., 2007; Hovey and Aimo, 2010; Watson, 2006). During pregnancy, mammary adipocytes expand in size and nearly double in lipogenic capacity (Bandyopadhyay et al., 1995; Bartley et al., 1981; Elias et al., 1973; Pujol et al., 2006). However, when lactation initiates, lipogenesis rates within mgWAT plummet (Bandyopadhyay et al., 1995; Bartley et al., 1981) and its area dramatically decreases. mgWAT size reduction is associated with the appearance of small, multilocular adipocytes (Elias et al., 1973; McCready et al., 2014; Rudolph et al., 2003). Work from Cinti and colleagues has suggested that mature mgWAT adipocytes exhibit an unusual degree of cellular plasticity as the mammary gland transitions to lactation, converting into secretory mammary epithelial cells via a process called ‘adipoepithelial transdifferentiation’ (De Matteis et al., 2009; Morroni et al., 2004; Prokesch et al., 2014). However, the relative contribution of adipoepithelial transdifferentiation vs. cell size reduction vs. cell apoptosis vs. another cellular event(s) is not yet clear and warrants further investigation.
An intriguing aspect of mgWAT biology is its ability to shuttle lipids to the adjacent epithelial cells for milk synthesis during lactation. This ‘feeding’ phenomenon likely explains why mammary adipocytes initially expand during pregnancy (to store extra lipids) and then reduce in size during lactation (as the result of lipid shuttling to epithelial cells) with the reduction being most prominent in cells positioned most proximal to the epithelium (Bandyopadhyay et al., 1995; Bartley et al., 1981; Elias et al., 1973). Mammary epithelium likely initiate this ‘feeding’ (Bandyopadhyay et al., 1995; Bartley et al., 1981), by sending as-of-yet unknown ‘nutrient request’ signals. The exact mechanism of lipid ‘feeding’ remains to be investigated.
During the involution phase that follows lactation, mgWAT expands and large unilocular adipocytes quickly re-establish (Rudolph et al., 2003). Although the mechanism of rapid post-lactation mgWAT expansion remains poorly understood, reverse ‘adipoepithelial transdifferentiation’ was proposed to account for it (Morroni et al., 2004; Prokesch et al., 2014). Expansion of mgWAT also depends on active remodeling of the mammary gland microenvironment, and involves activities of matrix-degrading proteases (Alexander et al., 2001; Lilla et al., 2009; Lund et al., 2000; Lund et al., 1996; Selvarajan et al., 2001) and tissue-resident macrophages (O’Brien et al., 2012). Further, mgWAT reemergence is concomitant with epithelial programmed cell death, and delayed epithelial involution in several genetic mouse models is associated with impaired adipocyte expansion, suggesting that epithelial cell death may also provide stimulus for mgWAT dynamics or vice versa. However, our understanding of both the cellular basis of mgWAT remodeling during lactation cycles and the mechanisms controlling this activity remain incomplete and will be an interesting area for further investigation.
What are the effects of mgWAT on mammary epithelium? Studies suggest that adipose tissue strongly promotes epithelial cell growth by providing structural support and regulating signals (reviewed in (Hovey and Aimo, 2010; Wang et al., 2010). In vitro, co-culture of mammary epithelial cells with either the 3T3-L1 adipocyte precursors or mammary adipose explants demonstrate that adipocyte lineage cells promote epithelial proliferation and branching, alveolar and ductal morphogenesis, and their functional differentiation into milk protein-producing cells (Hovey et al., 1998; Levine and Stockdale, 1984, 1985; Pavlovich et al., 2010; Wang et al., 2009; Wiens et al., 1987; Zangani et al., 1999). In vivo, mammary glands in A-Zip mice, which lack mature adipocytes, have rudimentary epithelial ducts with reduced branching and severe distention (Couldrey et al., 2002). Furthermore, induction of generalized loss of adipocytes in the FAT-ATTAC mouse model during pubertal development disrupts epithelial branching and terminal end bud formation, mediated by changes in epithelial proliferation and apoptosis (Landskroner-Eiger et al., 2010). However, adipocyte loss at a later time, 7 weeks of age, results in precocious differentiation of milk-producing alveolar structures and expression of a milk protein gene (Landskroner-Eiger et al., 2010). Collectively, these data suggest that mgWAT adipocytes are required at different points in the mammary gland life cycle, including the initial growth and branching of the ducts, maintenance of the ducts, and functional epithelial differentiation ahead of pregnancy.
The precise molecular mechanisms by which mgWAT and mammary epithelial cells interact are not clearly understood. Many molecules released from mgWAT and other depots influence epithelial cells—including prolactin, estrogen, insulin-like growth factor-I, hepatocyte growth factor, leptin, adiponectin and others (reviewed in (Brisken and Ataca, 2015; Hovey and Aimo, 2010; Wang et al., 2008). A frequently studied example is prolactin, which plays a major role in promoting milk production, and is expressed and released by mgWAT (Ben-Jonathan and Hugo, 2015; Hugo et al., 2008; Zinger et al., 2003). While prolactin receptor isoforms are expressed differentially by epithelial cells at various stages of development, the receptor is also expressed by various stromal cells, including adipocyte lineage cells (Hovey and Aimo, 2010). As another example, stroma-derived Wnt5a was shown recently to enhance mammary epithelial stem cell expansion and inhibit ductal branching in vivo, while Wnt5b represses stem cell expansion (Kessenbrock et al., 2017), implicating non-canonical WNT signaling. Whether adipocyte lineage cells participate in providing these extracellular signals however, remains unknown. Cell specific deletion of ligands and receptors will be needed to fully untangle the signaling crosstalk between adipocytes and epithelial cells in the mammary gland.
mgWAT also influences breast cancer promotion and invasion. Adipocytes in human breast tumors, and those cultured with murine and human breast tumor cells, display decreased adipocyte cellularity, lipid content, white adipocyte-associated gene expression, and in a cohort of Chinese women—increased expression of brown adipocyte-associated genes (DeFilippis et al., 2012; Dirat et al., 2011; Wang et al., 2014). Adipocytes and other stromal cells surrounding several types of breast tumors additionally show repressed expression of the lipid uptake receptor CD36, and depressed adipogenic capacity in vitro (DeFilippis et al., 2012). These findings suggest that epithelial cell malignancy in the breast may influence the phenotype of surrounding adipocytes.
Emerging evidence suggests that cancer associated adipocytes (CAAs) can, in turn, regulate tumor cells by providing lipids and cytokines (Tan et al., 2011). Interestingly, metastasis-initiating epithelial breast cancer cells upregulate CD36 (Pascual et al., 2017), and CAAs contribute lipid to tumor cells (Wang et al., 2017), illustrating another example of ‘lipid feeding’ in the breast, this time promoting pathological behaviors. Lipids are released by CAAs in the mammary tumor microenvironment via the action of adipose triglyceride lipase (ATGL), and are transferred to tumor cells in lipid droplets, where they activate metabolic pathways that stimulate invasiveness (Wang et al., 2017). In addition to lipids, increased expression of pro-inflammatory cytokines and secreted molecules also alter tumor cell behavior (Dirat et al., 2011; Iyengar et al., 2003). In vitro studies have implicated IGF-1, leptin, inflammatory cytokines including interleukin-6 (IL-6), autotaxin, and the β-hydroxybutyrate, an inhibitor of histone deacetylases, in promoting breast cancer growth and invasion (Benesch et al., 2015; D’Esposito et al., 2012; Dirat et al., 2011; Huang et al., 2017; Santander et al., 2015; Surmacz, 2007; Volden et al., 2016). While these studies suggest that a bidirectional interplay between adipocytes and cancer cells exists in the mammary gland, the molecular signals that underlie this interaction are not fully understood in vivo.
The dynamic behavior displayed by mgWAT during lactation cycles may also have implications for breast cancer. Pregnancy-associated breast cancers, or breast cancers diagnosed during pregnancy, lactation, and involution until 5 years postpartum, are more aggressive and have poorer prognosis than other breast cancers (Callihan et al., 2013). Several changes in the mammary stroma during lactation-cycles have been shown to promote tumorigenesis and metastasis (Bemis and Schedin, 2000; McDaniel et al., 2006; Schedin et al., 2007). These include changes to extracellular matrix components and immune cells during involution (Lyons et al., 2011; Martinson et al., 2014), and adipocytes during lactation (McCready et al., 2014). As many studies have shown the interconnected nature of these stromal cell types in the breast, further research into the potential tumor promotional role of mgWAT during lactation cycles holds great clinical promise.
Joint adipose tissue.
Distinct WAT depots reside in and around the joints (Figure 1). Examples of joint adipose include that in the acetabular fossa of the hip joint and infrapatellar adipose in the knee joint. In the hip joint, adipose occupies a large portion of the acetabular fossa and it prominently atrophies, undergoes fibrosis, necrosis and calcification upon hip degeneration (Sampatchalit et al., 2009). The infrapatellar adipose pad, aka Hoffa’s adipose pad, is located within the knee joint capsule (Gallagher et al., 2005). Similar to acetabular fossa adipose, the function of Hoffa’s adipose pad is likely to cushion and reduce friction between adjacent skeletal structures (Draghi et al., 2016). Intriguingly, infrapatellar adipose preserves even upon extreme starvation, suggesting that its responsiveness to systemic stimuli is distinct from other WAT depots, and that its primary role is biomechanical rather than energy storage (Smillie, 1980). Evidence is also emerging for the signaling role of infrapatellar adipose in knee joint pathologies, such as in osteoarthritis (Ioan-Facsinay and Kloppenburg, 2013). In diseased joints, adipocytes secrete elevated levels of IL-6 cytokine and adipokines, including adiponectin and leptin (Distel et al., 2009; Gandhi et al., 2011; Hui et al., 2012), and these are thought to act as paracrine drivers of joint inflammation.
Bone marrow adipose tissue.
Bone marrow is the site of active adult hematopoiesis (Crane et al., 2017; Mikkola and Orkin, 2006; Morrison and Scadden, 2014), and also harbors specialized bone marrow adipose tissue (BMAT) that displays characteristics of brown and white adipose tissue (reviewed in (Horowitz et al., 2017)). Within bone, BMAT exists in two distinct compositions—constitutive and regulated (Scheller et al., 2015; Tavassoli and Crosby, 1970). Constitutive or “yellow” BMAT forms early in postnatal development in the distal skeletal elements, such as distal tibia or tail vertebrae in mice and contains larger adipocytes, while regulated or “red” BMAT forms later in development than constitutive BMAT, is primarily found in more proximal skeletal regions and contains primarily hematopoietic cells (Scheller et al., 2015).
Progenitor sources for BMAT and lineage relationships between adipocytes and other mesenchymal bone marrow cells is a subject of ongoing investigation. Bone marrow harbors diverse mesenchymal progenitors, many of which can be identified on the basis of Osterix expression (Mizoguchi et al., 2014). Lineage tracing studies using Osterix-CreER demonstrated that Osterix-expressing progenitors contribute to neonatal bone as well as bone marrow stroma. Following radiation injury, these progenitors can also contribute to adipocytes. BMAT progenitors can also be marked using Lepr-Cre (Zhou et al., 2014), however, their lineage relationship with Osterix+ mesenchymal progenitors remains unclear. Independent of lineage studies, Ambrosi et al. (2017) identified multipotent bone marrow mesenchymal stem cells (mMSCs), characterized by the cell surface marker profile CD45−,CD31−,Sca1+,CD24+. Intriguingly, this marker profile has been employed previously to isolate ASCs from non-bone marrow adipose depots, including sWAT and dWAT (Berry and Rodeheffer, 2013; Festa et al., 2011a; Rivera-Gonzalez et al., 2016a; Rodeheffer et al., 2008a). These bone marrow mMSCs, however, have broader lineage potential than has so far been observed in non-bone marrow ASCs. They display both adipogenic and osteo-chondrogenic differential potential, depending on experimental conditions in vitro and in vivo (Ambrosi et al., 2017). Isolated mMSCs also express high levels of Cxcl12 and Lepr. The later marker suggests that mMSCs likely overlap and/or give rise to Lepr-Cre+ adipocyte progenitors. Cxcl12 expression indicates that mMSCs and likely Lepr-Cre+ progenitors overlap with CAR cells, another distinct mesenchymal lineage of the bone marrow (Mizoguchi et al., 2014; Omatsu et al., 2010; Sugiyama et al., 2006; Zhou et al., 2014). Taken together, the lineage relationship between adipogenic and non-adipogenic bone marrow progenitors is complex, and future studies will be necessary to fully deconvolute it.
Multiple signaling factors likely regulate alternative fate selection by mMSCs, yet they remain poorly understood. MicroRNA miR-188 is one such lineage regulator, whose activity shuttles mMSCs toward adipogenesis. Mice with deletion of miR-188 display reduced bone marrow adiposity, while miR-188 overexpression in Osterix+ cells results in greater adipose accumulation in the bone marrow. At the signaling level, miR-188 likely promotes adipogenesis by directly targeting and decreasing expression levels of histone deacetylase Hdac9 and Rictor, important regulators of bone metabolism (Li et al., 2015).
What is the functional significance of BMAT? In analogy with other WAT depots, BMAT has been shown to function as an endocrine organ. Paradoxically, BMAT is expanded following chronic calorie restriction in young women with anorexia nervosa in comparison to healthy control patients and individuals who have recovered from anorexia (as reviewed by (Fazeli et al., 2013)). Upon caloric restriction in mice, BMAT expands by approximately 40% and this is accompanied by increased serum levels of adiponectin (Cawthorn et al., 2014). When caloric restriction is performed in mice overexpressing a soluble negative regulator of adipogenesis, Wnt10b, in bone specific Osteocalcin+ cells, BMAT fails to expand and adiponectin serum levels fail to rise. This data suggests that adiponectin secretion by BMAT is an important depot-specific response to caloric restriction. It functions to systemically regulate skeletal muscle adaptation to energy restriction (Cawthorn et al., 2014). In addition to caloric restriction challenge, BMAT also expands in response to aging, obesity, and growth hormone deficiency (Ambrosi et al., 2017; Menagh et al., 2010). Generally, adipose progenitors can proliferate in response to high-fat diet and this response becomes pronounced in aged animals. Consistent with this, BMAT expands in volume during aging and obesity, and high-fat diet challenge shifts mMSCs lineage output in the bone marrow toward adipocytes, although the response of BMAT to high fat diet is age-dependent and variable (Horowitz et al., 2017). On the other hand, BMAT can decrease in size in response to cold challenge. As compared to thermo-neutral conditions, exposure of mice to 4ºC leads to approximately 70% loss of BMAT in the proximal tibia, the region characterized as red BMAT. At least in part, this BMAT volume loss is mediated via lipolysis (Scheller et al., 2015).
BMAT function is also modulated by hormonal cues, particularly via parathyroid hormone (PTH). Genetic ablation of parathyroid signaling via Pht1r deletion in Prx1-Cre mice, which targets mMSCs, including adipocyte progenitors, increases marrow adiposity in the distal tibia (Fan et al., 2017). This is accompanied by a decrease in bone mass. Mechanistically, loss of PTH signaling in mMSCs results in their preferential differentiation toward adipocytes. Furthermore, acting indirectly, signaling crosstalk between marrow adipocytes and osteoclasts likely stimulates bone resorption. PTH-mediated regulation of BMAT is also conserved in humans. Treatment with PTH results in reduced BMAT in patients with idiopathic osteoporosis, suggesting a possible therapeutic role for PTH signaling in reducing marrow adiposity.
In addition, BMAT controls hematopoiesis and its effect appears to be pleotropic and site-dependent. In tail vertebrae of mice, which are rich in BMAT, hematopoetic stem cells (HSCs) and short-term hematopoietic progenitors are reduced and display lower cycling behavior as compared to other bone marrow regions with lower BMAT content (Naveiras et al., 2009). Supporting the negative role of BMAT are competitive repopulation assay experiments in A-Zip mice and mice treated with a Pparγ inhibitor, which lead to accelerated marrow engraftment following irradiation (Naveiras et al., 2009). Further, transplantation of adipose progenitors into the tibia of irradiated mice leads to increased BMAT and results in decreased hematopoetic cell frequency (Ambrosi et al., 2017). Negative regulation of hematopoiesis was found in this study to be confined to cells committed to the adipocyte lineage, whereas their multipotent mMSC precursors increased hematopoietic recovery following irradiation. In contrast, study by Zhou et al. (2017b) suggests that the effect of adipocytes on hematopoiesis varies across the skeleton, and that unlike in tail vertebrae, adipocytes in long bones, in fact, promote HSC maintenance and hematopoietic regeneration following irradiation. This positive effect of adipocytes on hematopoiesis is likely mediated via stem cell factor, SCF (Zhou et al., 2017a). Given the complexity of marrow mesenchymal cells and BMAT, additional molecular mechanisms that control marrow adiposity and its function will be an exciting area of further exploration.
Plantar and palmar adipose tissue.
Another example of WAT with a primarily biomechanical role is located in feet and palms. Plantar adipose consists of several pads and they are important in body weight distribution and pressure attenuation (Fontanella et al., 2016). To better withstand high pressures, adipocytes in plantar adipose distribute into distinct globules encapsulated by collagen- and elastin-rich fibrous septa (Buschmann et al., 1995). In normal individuals, adipocytes within globules are large and uniform in size, but become smaller concurrent with thickening of septa upon fat atrophy, such as with age, in response to trauma or upon diabetes (Dalal et al., 2015). Unlike plantar WAT, which is implicated in various foot pathologies, palmar fat has been seldom studied. It has been reported, however, that the inter-metacarpal palmar adipose pad is important for efficient hand grasping (Clavert et al., 2006).
Cardio-vascular adipose tissue.
Surrounding the heart are two prominent adipose depots, epicardial and pericardial WAT (Figure 1) (Iacobellis, 2009). Of these, epicardial adipose is particularly important in normal heart physiology and in pathogenesis of cardio-vascular diseases (Iacobellis, 2015). Anatomically, it is located between the myocardium and the visceral layer of the pericardium and covers approximately 80% of heart’s surface area (Rabkin, 2007). At the cellular level, epicardial adipocytes are similar to white adipocytes in other WAT depots, albeit they are generally small in size. Importantly, they express high levels of the UCP1 uncoupling protein (Sacks et al., 2009), indicating that they actively generate heat and likely provide heart with thermal protection, in addition to mechanical cushioning. Epicardial adipose also produces high levels of free fatty acids, that likely directly diffuse to the adjacent myocardium and serve as an additional local energy source (Iacobellis, 2015). This feature of epicardial adipose parallels ‘feeding’ role of mgWAT in lactating mammary glands. Furthermore, epicardial adipose has been shown to secrete multiple pro- and anti-inflammatory cytokines that can signal to and significantly affect the function of the nearby heart muscle and coronary arteries (Mazurek et al., 2003; Yudkin et al., 2005). Changes in the epicardial WAT, particularly its hypertrophy during obesity, have detrimental effects of heart function. Excess epicardial adipose mass increases work demand on heart contraction and contributes to cardiac hypertrophy (Iacobellis et al., 2004). It also leads to adipose infiltration of the myocardium and an increase in adipose-derived pro-inflammatory signaling, both of which can have further detrimental effects on heart function (Iacobellis, 2015).
Mesenteric adipose tissue.
Mesenteric WAT (mWAT) is a distinct vWAT depot located in the double fold of peritoneum (Figure 1). Normally, it comes in contact with intestines at the site of mesentery attachment (reviewed by (Tchkonia et al., 2013)), and it also plays an important role in the pathogenesis of inflammatory bowel diseases, including Crohn’s Disease (CD) (Paeschke et al., 2017). In particular, a prominent expansion of mWAT around the circumference of intestines, so-called ‘creeping fat’, is a pathological hallmark of CD (Crohn et al., 1952; Desreumaux et al., 1999). mWAT expansion in CD appears to primarily occur via hyperplasia, as it becomes enriched for many small adipocytes. Also, in contrast to other visceral adipose depots, mWAT expansion in CD is independent of patient’s BMI, indicating local rather than systemic drivers (Kredel and Siegmund, 2014). While the pathogenesis of ‘creeping fat’ in CD remains only partially understood, it is thought to be induced by the increased penetration of bacteria from the intestinal lumen. Responding to bacterial products, such as lipopolysaccharides, mWAT progenitors activate, undergo differentiation and increase production of pro-inflammatory adipokines including IL-6, IL-1β, IL-17 and leptin (Batra et al., 2012; Gewirtz, 2015; Peyrin-Biroulet et al., 2012). The latter contribute to the pro-inflammatory signaling driving CD progression. Supporting a pro-inflammatory role of ‘creeping fat’, cultured mWAT pre-adipocytes derived from CD and ulcerative colitis patients expressed higher levels of pro-inflammatory cytokine mRNA in response to stimulus than those from healthy patients (Sideri et al., 2015). ‘Creeping fat’ likely represents an outcome of exaggerated innate immune response by mWAT to an abnormal increase in bacterial invasion in the diseased gut with compromised epithelial barrier. Normally, however, mWAT likely plays an important defense function, aiding in detecting bacterial translocation, producing anti-inflammatory cytokines and eliminating bacteria, both via macrophage recruitment and via direct phagocytosis (Batra et al., 2012; Paeschke et al., 2017; Peyrin-Biroulet et al., 2012). Importantly, this antibacterial defense function of mWAT adipocytes in intestines closely parallels that of dWAT adipocytes in the skin.
Diversity of adipose depots in the natural world
Highly specialized WAT depots can be found in other mammalian species. Below, we briefly highlight some of the most unique-looking WAT depots with the intention to emphasize the diversity in adipose anatomy and function.
Facial adipose tissue in orangutans.
Adult male orangutans feature the so-called facial flanges, shield-like folds of skin around their faces. Anatomical studies show that flanges largely consist of expanded facial WAT that closely associates with facial muscles (Figure 3A) (Winkler, 1989). Facial flanges in male orangutans develop during adolescence and, although variable in size and shape, their prominence correlates with systemic hormonal levels, natural habitat, and ranking with their established societal hierarchy. Flanges are underdeveloped in immature males and in females. Although the exact function of facial flanges remains unclear, behavioral evidence suggests that their prominence signifies male’s reproductive fitness. Indeed, orangutan females are less likely to mate with younger males lacking prominent facial flanges.
Figure 3: Examples of specialized adipose depots in mammals.
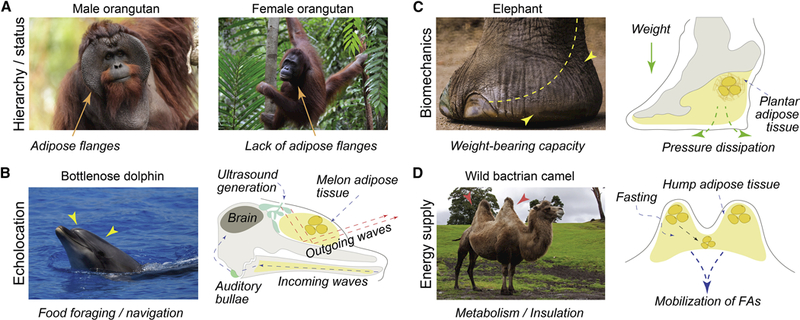
(A) Male orangutans display prominent fat-filled facial flanges (credit: Eric Kilby). Flanges develop in adulthood and are largely absent in adult females (credit: Victor Ulijn) and pubescent males. They are thought to determine hierarchical status, dominance and successful reproductive fitness with estrus females. (B) Cetaceans, including the bottlenose dolphin (shown here) utilize melon, specialized adipose-containing tissue for echolocation (credit: William Warby). Ultrasound is generated in the so-called phonic lips (green), and sound waves travel and collimate in the melon located above maxilla (yellow). Upon echoing, incoming sound waves pass through the adipose-filled mandible and are perceived by the auditory bullae (light green). (C) Elephants evolved prominent plantar adipose (yellow) that aids in dissipating pressure (credit: Aotaro). Similar to other species, plantar adipose protects adjacent skeletal structures from mechanical shock during gating and walking. (D) Hump in camels contain adipose tissue, that has adaptive role in the desert environment (credit: Tony Hisgett). Upon periods of fasting, camels mobilize fatty acids from hump stores. Unique positioning of the adipose tissue in the humps, away from the body core, prevents camels from overheating.
Acoustic adipose tissue in cetaceans.
Multiple cetacean species, including oceanic dolphins, utilize a highly sophisticated sonar system to navigate and locate food in the depths of the ocean. The melon, a highly specialized WAT and muscle-rich organ, constitutes the key component of their sonar system. As the name suggests, the melon is roughly oval in shape. It is located underneath the skin on top of the animal’s face and rests on a prominent skeletal structure. Anatomically, it is placed in front of the blowhole, where ultrasound is generated by the so-called phonic lips and surrounding chambers (Figure 3B). The melon has complex microanatomy; it is rich in adipocytes and is intimately connected to multiple facial muscles, whose contractile activity allows cetaceans to voluntarily control the melon’s shape and size (Harper et al., 2008). Lipid analysis of the melon shows that it has distinct molecular composition compared to traditional WAT depots, and it is enriched for short- and medium-chain triglycerides and wax esters (Scano et al., 2006; Scano et al., 2005; Varansi et al., 1975). Functionally, the melon is thought to act as an acoustic lens by collimating ultrasound waves generated in the blowhole region and coupling acoustic energy to the seawater for minimal energy loss during echolocation. It is also thought that the distribution and composition of the melon’s lipids contributed to its acoustic properties but this speculation is yet to be fully addressed.
Plantar adipose tissue in elephants.
Elephants, the largest extant terrestrial animal species, have column-like legs that feature large plantar adipose pads at their base. Their foot skeleton almost entirely rests on these adipose cushions that act to absorb shock during walking (Michilsens et al., 2009; Weissengruber et al., 2006). Anatomy of plantar adipose in elephants is intricate—it occupies the space both underneath and in between bones, muscles, and tendons, and consists of metatarsal and digital compartments (Figure 3C). Histologically, both compartments differ from one another. Digital WAT is fibrous and contains mostly individually scattered unilocular adipocytes, while metatarsal WAT also contains adipocyte clusters. Curiously, in addition to its shock absorbing function, plantar adipose has been hypothesized to participate in the so-called “seismic” signal perception (O’Connell-Rodwell, 2007). Similar to some other terrestrial species, elephants are able to generate and sense low frequency acoustic waves that preferentially spread through the ground over long distances (O’Connell-Rodwell et al., 2000). Although the elephants’ ability to detect such low-frequency “seismic” signals is obvious from behavioral studies (O’Connell-Rodwell et al., 2006), the perception mechanism remains unclear. It has been proposed that one way for elephants to receive ground-based sound perception is through foot adipose and bone conduction (O’Connell-Rodwell, 2007). Indeed, as mentioned above, aquatic mammals prominently use modified WAT depots for sound conduction, and convergent evolution of this function in elephants’ plantar adipose is likely.
Hump adipose tissue in camels.
Camels are well adapted to the desert environment and able to withstand prolonged heat of up to 40°C, cope with significant water loss (Schmidtnielsen, 1959), and undergo prolonged fasting (Bengoumi et al., 2005). Camels evolved prominent, and signature-looking WAT depots on their back, commonly known as humps (Figure 3D). The Arabian camel (Camelus dromedarius) has one hump, while Bactrian camel (Camelus bactrianus) and wild Bactrian camel (Camelus ferus) have two distinct, prominent humps. WAT depots in the hump are primarily used as energy stores (Mirgani, 1977) and their anatomy is thought to be an adaptation against overheating in the desert environment—that is, by concentrating adipose in the humps, camels minimize thermal insulation on the rest of the body and increase their ability to dissipate heat.
Perspective
New studies on skin, mammary gland and bone marrow point toward several common features of adipose depots that reside in close anatomical association with stem cell-rich tissues (Figure 4). Owing to their prominent secretory profile, APs and mature adipocytes naturally become important signaling centers within stem cell niches. Studies on HF regeneration show how growth factors secreted by APs and adipocytes can either stimulate or inhibit the activity of epithelial stem cells and the pace of hair cycle progression, respectively. Similar roles have emerged for BMAT in regulating hematopoietic progenitors, where the latter respond to adipose-derived growth factors and inflammatory cytokines. Analogous signaling functions are expected for mgWAT in the mammary gland. Indeed, mgWAT is essential for proper growth and differentiation of mammary epithelial cells, albeit signaling regulators are not well understood. The ‘non-traditional’ role for adipose depots is also fast-emerging in the disease context, where adipose-derived inflammatory cytokines become essential drivers of the pathological tissue behaviors. For example, mgWAT adipocytes mount tumor-promoting inflammatory signaling in breast cancer. In analogy, inflammatory signaling by mesenteric adipocytes drives progression of CD in the intestine.
Figure 4: Diverse functions of tissue-associated adipose depots.
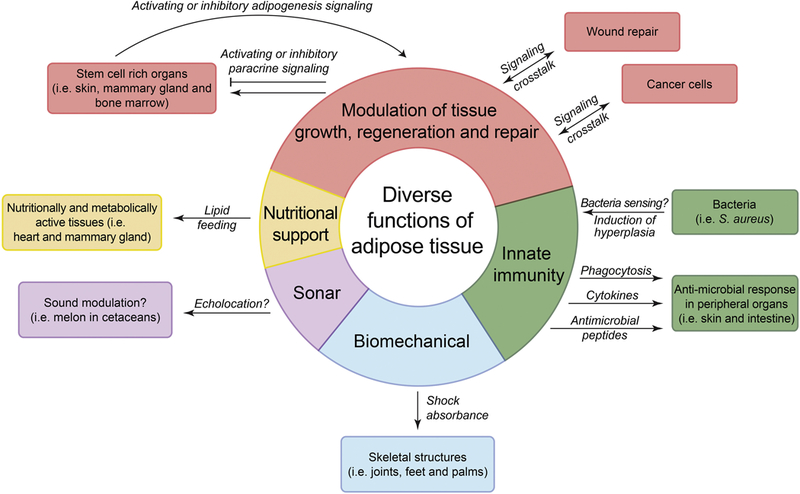
Distinct functions of adipose tissue are represented as color-coded segments on the diagram. Paracrine signaling function of adipose in skin, mammary gland and bone marrow is in red, innate immune function of adipose in skin and intestine is in green, biomechanical function of skeleton-associated adipose is in blue, sound modulating function of melon adipose in cetaceans is in purple, and lipid ‘feeding’ function of epicardial and mammary gland adipose is in yellow. Mechanisms that require further confirmation are designated with the question mark.
Signaling interactions between adipocyte lineage cells and adjacent tissues are reciprocal. In normal skin, cycling HFs act as essential drivers of dWAT hypertrophy cycles, while in skin wounds, HFs signal to induce formation of new adipocytes, leading to permanent dWAT regeneration within scars. In the mammary gland, cyclic remodeling of mgWAT—adipocyte hypertrophy during pregnancy and their emptying during lactation—is expected to be driven by epithelial cell-derived signals, yet their molecular identity remains to be determined. Analogous reciprocal signaling is expected in the bone marrow, where BMAT adipocytes likely respond to the multitude of factors produced both by the hematopoietic cells as well as other diverse mesenchymal cell types with the niche.
Importantly, in addition to signaling inputs, tissue-associated adipose depots also supply their associated tissues with nutrients (Figure 4). This adipose function is prominent in the mammary gland, where mgWAT ‘feeds’ secretory apparatus of the gland during lactation, and in the heart, where epicardial WAT ‘feeds’ adjacent myocardium. It will be intriguing to learn if a similar ‘feeding’ behavior also evolved in other organs, such as in skin, between dWAT adipocytes and cycling HFs.
When adipocytes come in close anatomical association with the skeleton, they commonly assume biomechanical, shock-absorbent roles (Figure 4). As such, adipose tissue cushions the eye globe within the socket, moving skeletal elements within the joints, and multiple skeletal components in the feet. The latter function becomes prominent in animals of the megafauna, such as elephants. Craniofacial adipose depots can also contribute to sculpting facial features, which become essential for socialization via facial expressions in primates, including humans.
Yet another important function of secondary WAT depots is in mounting innate antimicrobial responses in organs that border the outside environment, skin and gastrointestinal tract (Figure 4). In both organs, dWAT and mWAT respectively, are able to directly sense invading bacteria and respond to their presence by phagocytosing them as well as secreting antimicrobial peptides and inflammatory cytokines, that further recruit immune cells. Antimicrobial responses by dWAT and mWAT depend on local depot hyperplasia. Importantly, the ability to expand locally, either via hyperplasia or hypertrophy, out of synchrony with major metabolic WAT depots, appears to be a general shared feature of multiple secondary adipose depots. Indeed, dWAT expands locally in response to activated HFs, while mgWAT expands locally in response to remodeled mammary gland epithelium. Furthermore, many adipose depots, at least partially, uncouple from the systemic metabolic demands, such as nutrient shortage. This feature enables them to perform their respective tissue-specific functions in spite of natural and pathological fluctuations in systemic metabolism.
Taken together, the picture emerges of bodily adipose consisting of multiple anatomically discrete depots with diverse and, commonly, highly specialized functions. Recent studies revealing the complexity of adipocyte biology, particularly in the skin, mammary glands and bone marrow, herald a new chapter in adipose research with the focus shifting toward recognizing adipose tissue as the major signaling, metabolic, biomechanical and innate immune player both in normal organ physiology and in disease. Naturally, expanding knowledge on tissue-specific roles for adipose tissue will lead to better understanding of the impact it has in tissue aging and diverse diseases, including cancer, and will likely direct the discovery of novel cellular and signaling therapeutic targets centered on adipocyte lineage.
Acknowledgements
We thank Drs. M. Rodeheffer and M. Horowitz for critical reading and feedback on the text. R.K.Z. is supported by the NIH (T32 GM007499 and F31 HD082956 from the NICHD) and by the Yale Stem Cell Center as a recipient of the Lo Graduate Fellowship. C.F.G.J. is supported by NSF-GRFP (DGE-1321846) and MBRS-IMSD training grant (GM055246) and Howard A. Schneiderman Graduate Fellowship Award. V.H. is supported by the NIAMS (R01AR060296 and R01AR069550) and the State of Connecticut (12-SCB-YALE-01). M.V.P. is supported by the NIAMS (R01AR067273 and R01AR069653) and Pew Charitable Trust grant.
References
- Aiache AE, and Ramirez OH (1995). The suborbicularis oculi fat pads: an anatomic and clinical study. Plast Reconstr Surg 95, 37–42. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Selvarajan S, Mudgett J, and Werb Z (2001). Stromelysin-1 regulates adipogenesis during mammary gland involution. The Journal of cell biology 152, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander HG, and Dugdale AE (1992). Fascial planes within subcutaneous fat in humans. European journal of clinical nutrition 46, 903–906. [PubMed] [Google Scholar]
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, et al. (2017). Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 20, 771–784 e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DB, Kauffman RG, and Kastenschmidt LL (1972). Lipogenic enzyme activities and cellularity of porcine adipose tissue from various anatomical locations. The Journal of Lipid Research 13, 593–599. [PubMed] [Google Scholar]
- Anderson SM, Rudolph MC, McManaman JL, and Neville MC (2007). Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis! Breast Cancer Research 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn RS (2010). Graves’ ophthalmopathy. N Engl J Med 362, 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Lee LY, Guzman RC, and Nandi S (1995). Effect of reproductive states on lipid mobilization and linoleic acid metabolism in mammary glands. Lipids 30, 155–162. [DOI] [PubMed] [Google Scholar]
- Bartley JC, Emerman JT, and Bissell MJ (1981). Metabolic cooperativity between epithelial cells and adipocytes of mice. American Journal of Physiology - Cell Physiology 10, 204–208. [DOI] [PubMed] [Google Scholar]
- Batra A, Heimesaat MM, Bereswill S, Fischer A, Glauben R, Kunkel D, Scheffold A, Erben U, Kuhl A, Loddenkemper C, et al. (2012). Mesenteric fat - control site for bacterial translocation in colitis? Mucosal Immunol 5, 580–591. [DOI] [PubMed] [Google Scholar]
- Bemis LT, and Schedin P (2000). Reproductive State of Rat Mammary Gland Stroma Modulates Human Breast Cancer Cell Migration and Invasion. Cancer Research 60, 3414–3418. [PubMed] [Google Scholar]
- Ben-Jonathan N, and Hugo E (2015). Prolactin (PRL) in Adipose Tissue: Regulation and Functions (Cham: Springer, Cham; ), pp. 1–35. [DOI] [PubMed] [Google Scholar]
- Benesch MGK, Tang X, Dewald J, Dong W-F, Mackey JR, Hemmings DG, McMullen TPW, and Brindley DN (2015). Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. Faseb Journal 29, 3990–4000. [DOI] [PubMed] [Google Scholar]
- Bengoumi M, Faulconnier Y, Tabarani A, Sghiri A, Faye B, and Chilliard Y (2005). Effects of feeding level on body weight, hump size, lipid content and adipocyte volume in the dromedary camel. Anim Res 54, 383–393. [Google Scholar]
- Berry DC, Stenesen D, Zeve D, and Graff JM (2013). The developmental origins of adipose tissue. Development (Cambridge, England) 140, 3939–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, and Rodeheffer MS (2013). Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, and Dupin E (2007). The generation of adipocytes by the neural crest. Development 134, 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, and Ataca D (2015). Endocrine hormones and local signals during the development of the mouse mammary gland. Wiley Interdisciplinary Reviews: Developmental Biology 4, 181–195. [DOI] [PubMed] [Google Scholar]
- Buschmann WR, Jahss MH, Kummer F, Desai P, Gee RO, and Ricci JL (1995). Histology and histomorphometric analysis of the normal and atrophic heel fat pad. Foot Ankle Int 16, 254–258. [DOI] [PubMed] [Google Scholar]
- Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, and Borges VF (2013). Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Research and Treatment 138, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, et al. (2014). Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HB, Montagna W, and Malone JD (1953). Changes in the skin in relation to the hair growth cycle. Anat Rec 116, 75–81. [DOI] [PubMed] [Google Scholar]
- Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. (2015). Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavert P, Dosch JC, Wolfram-Gabel R, and Kahn JL (2006). New findings on intermetacarpal fat pads: anatomy and imaging. Surg Radiol Anat 28, 351–354. [DOI] [PubMed] [Google Scholar]
- Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, and Green J (2002). Adipose tissue: A vital in vivo role in mammary gland development but not differentiation. Developmental dynamics : an official publication of the American Association of Anatomists 223, 459–468. [DOI] [PubMed] [Google Scholar]
- Couto JA, Konczyk DJ, Vivero MP, Kozakewich HPW, Upton J, Fu X, Padwa BL, Mulliken JB, Warman ML, and Greene AK (2017). Somatic PIK3CA mutations are present in multiple tissues of facial infiltrating lipomatosis. Pediatr Res [DOI] [PMC free article] [PubMed]
- Crane GM, Jeffery E, and Morrison SJ (2017). Adult haematopoietic stem cell niches. Nat Rev Immunol [DOI] [PubMed]
- Crohn BB, Ginzburg L, and Oppenheimer GD (1952). Regional ileitis. The American Journal of Medicine 13, 583–590. [DOI] [PubMed] [Google Scholar]
- D’Esposito V, Passaretti F, Hammarstedt A, Liguoro D, Terracciano D, Molea G, Canta L, Miele C, Smith U, Beguinot F, et al. (2012). Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 55, 2811–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Widgerow AD, and Evans GR (2015). The plantar fat pad and the diabetic foot--a review. Int Wound J 12, 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis R, Zingaretti MC, Murano I, Vitali A, Frontini A, Giannulis I, Barbatelli G, Marcucci F, Bordicchia M, Sarzani R, et al. (2009). In Vivo Physiological Transdifferentiation of Adult Adipose Cells. Stem cells (Dayton, Ohio) 27, 2761–2768. [DOI] [PubMed] [Google Scholar]
- DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen Y-Y, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu D, et al. (2012). CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer discovery 2, 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, Hafraoui S, Emilie D, Ectors N, Peuchmaur M, et al. (1999). Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 117, 73–81. [DOI] [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al. (2011). Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Research 71, 2455–2465. [DOI] [PubMed] [Google Scholar]
- Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, and Benelli C (2009). The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum 60, 3374–3377. [DOI] [PubMed] [Google Scholar]
- Donati G, Proserpio V, Lichtenberger BM, Natsuga K, Sinclair R, Fujiwara H, and Watt FM (2014). Epidermal Wnt/beta-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proc Natl Acad Sci U S A 111, E1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghi F, Ferrozzi G, Urciuoli L, Bortolotto C, and Bianchi S (2016). Hoffa’s fat pad abnormalities, knee pain and magnetic resonance imaging in daily practice. Insights Imaging 7, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Jahoda CA, Chuong CM, Watt FM, and Horsley V (2014). Defining dermal adipose tissue. Exp Dermatol 23, 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JJ, Pitelka DR, and Armstrong RC (1973). Changes in fat cell morphology during lactation in the mouse. The Anatomical record 177, 533–547. [DOI] [PubMed] [Google Scholar]
- Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, et al. (2017). Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab 25, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, and Klibanski A (2013). Marrow fat and bone--new perspectives. J Clin Endocrinol Metab 98, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldon SE, O’Loughlin C W, Ray DM, Landskroner-Eiger S, Seweryniak KE, and Phipps RP (2006). Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol 169, 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, and Horsley V (2011a). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, and Horsley V (2011b). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanella CG, Nalesso F, Carniel EL, and Natali AN (2016). Biomechanical behavior of plantar fat pad in healthy and degenerative foot conditions. Med Biol Eng Comput 54, 653–661. [DOI] [PubMed] [Google Scholar]
- Forbes G, Gorman CA, Brennan MD, Gehring DG, Ilstrup DM, and Earnest F.t. (1986). Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol 7, 651–656. [PMC free article] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, and Pfeilschifter J (2000). Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. Journal of Clinical Investigation 106, 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A, and Cinti S (2010). Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 11, 253–256. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Tierney P, Murray P, and O’Brien M (2005). The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc 13, 268–272. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Takahashi M, Virtanen C, Syed K, Davey JR, and Mahomed NN (2011). Microarray analysis of the infrapatellar fat pad in knee osteoarthritis: relationship with joint inflammation. J Rheumatol 38, 1966–1972. [DOI] [PubMed] [Google Scholar]
- Gasperoni C, and Salgarello M (1995). Rationale of subdermal superficial liposuction related to the anatomy of subcutaneous fat and the superficial fascial system. Aesthetic plastic surgery 19, 13–20. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, and Kahn CR (2007). Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT (2015). Deciphering the Role of Mesenteric Fat in Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol 1, 352–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortz GE, Moshkelgosha S, Jesenek C, Edelmann B, Horstmann M, Banga JP, Eckstein A, and Berchner-Pfannschmidt U (2016). Pathogenic Phenotype of Adipogenesis and Hyaluronan in Orbital Fibroblasts From Female Graves’ Orbitopathy Mouse Model. Endocrinology 157, 3771–3778. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, and Pollard JW (2002). Unexpected deposition of brown fat in mammary gland during postnatal development. Molecular endocrinology (Baltimore, Md.) 16, 2618–2627. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, and Spiegelman BM (2010). Transcriptional control of preadipocyte determination by Zfp423. Nature 464, 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, and Longaker MT (2008). Wound repair and regeneration. Nature 453, 314–321. [DOI] [PubMed] [Google Scholar]
- Harper CJ, McLellan WA, Rommel SA, Gay DM, Dillaman RM, and Pabst DA (2008). Morphology of the melon and its tendinous connections to the facial muscles in bottlenose dolphins (Tursiops truncatus). J Morphol 269, 820–839. [DOI] [PubMed] [Google Scholar]
- Hilditch TP, and Stainsby WJ (1935). The body fats of the pig: Progressive hydrogenation as an aid in the study of glyceride structure. The Biochemical journal 29, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA, Lindskog D, Kaplan JL, Ables G, Rodeheffer MS, et al. (2017). Bone marrow adipocytes. Adipocyte 6, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey RC, and Aimo L (2010). Diverse and active roles for adipocytes during mammary gland growth and function. Journal of mammary gland biology and neoplasia 15, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey RC, MacKenzie DD, and Mcfadden TB (1998). The proliferation of mouse mammary epithelial cells in response to specific mitogens is modulated by the mammary fat pad in vitro. In vitro cellular & developmental biology. Animal 34, 385–392. [DOI] [PubMed] [Google Scholar]
- Huang C-K, Chang P-H, Kuo W-H, Chen C-L, Jeng Y-M, Chang K-J, Shew J-Y, Hu C-M, and Lee W-H (2017). Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via β-hydroxybutyrate. Nature Communications 8, 14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo ER, Borcherding DC, Gersin KS, Loftus J, and Ben-Jonathan N (2008). Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. The Journal of clinical endocrinology and metabolism 93, 4006–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, and Young DA (2012). Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis 71, 455–462. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Hwang K, Jin S, and Kim DJ (2007). Location and nature of retro-orbicularis oculus fat and suborbicularis oculi fat. J Craniofac Surg 18, 387–390. [DOI] [PubMed] [Google Scholar]
- Iacobellis G (2009). Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 17, 625; author reply 626–627. [DOI] [PubMed] [Google Scholar]
- Iacobellis G (2015). Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11, 363–371. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, and Leonetti F (2004). Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol 94, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Ioan-Facsinay A, and Kloppenburg M (2013). An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther 15, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, and Cotsarelis G (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP, et al. (2003). Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene 22, 6408–6423. [DOI] [PubMed] [Google Scholar]
- Jin CE, Xiao L, Ge ZH, Zhan XB, and Zhou HX (2015). Role of adiponectin in adipose tissue wound healing. Genet Mol Res 14, 8883–8891. [DOI] [PubMed] [Google Scholar]
- Kamal D, Breton P, and Bouletreau P (2010). Congenital infiltrating lipomatosis of the face: report of three cases and review of the literature. J Craniomaxillofac Surg 38, 610–614. [DOI] [PubMed] [Google Scholar]
- Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, et al. (2014). Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet 10, e1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Smith P, Steenbeek SC, Pervolarakis N, Kumar R, Minami Y, Goga A, Hinck L, and Werb Z (2017). Diverse regulation of mammary epithelial growth and branching morphogenesis through noncanonical Wnt signaling. PNAS 114, 3121–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, and Park JS (2007). Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48, 15–24. [DOI] [PubMed] [Google Scholar]
- Kosciuczuk EM, Lisowski P, Jarczak J, Strzalkowska N, Jozwik A, Horbanczuk J, Krzyzewski J, Zwierzchowski L, and Bagnicka E (2012). Cathelicidins: family of antimicrobial peptides. A review. Mol Biol Rep 39, 10957–10970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredel LI, and Siegmund B (2014). Adipose-Tissue and Intestinal Inflammation — Visceral Obesity and Creeping Fat. Frontiers in Immunology 5, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, and Scherer PE (2016). Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends Endocrinol Metab 27, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nadeem S, Stan MN, Coenen M, and Bahn RS (2011). A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves’ ophthalmopathy. J Mol Endocrinol 46, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok KHM, Lam KSL, and Xu A (2016). Heterogeneity of white adipose tissue: molecular basis and clinical implications. Experimental & Molecular Medicine 48, e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskroner-Eiger S, Park J, Israel D, Pollard JW, and Scherer PE (2010). Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Developmental biology 344, 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos DR, Paylor B, Chang C, Sampaio A, Underhill TM, and Rossi FM (2012). Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells 30, 1152–1162. [DOI] [PubMed] [Google Scholar]
- Lepper C, and Fan C-M (2010). Inducible Lineage Tracing of Pax7-Descendant Cells Reveals Embryonic Origin of Adult Satellite Cells. Genesis (New York, N.Y. : 2000) 48, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JF, and Stockdale FE (1984). 3T3-L1 adipocytes promote the growth of mammary epithelium. Experimental Cell Research 151, 112–122. [DOI] [PubMed] [Google Scholar]
- Levine JF, and Stockdale FE (1985). Cell-cell interactions promote mammary epithelial cell differentiation. The Journal of cell biology 100, 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, et al. (2015). MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest 125, 1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla JN, Joshi RV, Craik CS, and Werb Z (2009). Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. The Journal of biological chemistry 284, 13792–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, and Lin RY (2008). TSH stimulates adipogenesis in mouse embryonic stem cells. J Endocrinol 196, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Bjorn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, Osterby R, Christensen IJ, Stephens RW, Bugge TH, et al. (2000). Lactational competence and involution of the mouse mammary gland require plasminogen. Development (Cambridge, England: ) 127, 4481–4492. [DOI] [PubMed] [Google Scholar]
- Lund LR, Rømer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Danø K, and Werb Z (1996). Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development (Cambridge, England: ) 122, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan A-C, and Schedin P (2011). Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nature medicine 17, 1109–U1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson HA, Jindal S, Durand-Rougely C, Borges VF, and Schedin P (2014). Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. International Journal of Cancer 136, 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master SR, Hartman JL, D’Cruz CM, Moody SE, Keiper EA, Ha SI, Cox JD, Belka GK, and Chodosh LA (2002). Functional Microarray Analysis of Mammary Organogenesis Reveals a Developmental Role in Adaptive Thermogenesis. Molecular endocrinology (Baltimore, Md.) 16, 1185–1203. [DOI] [PubMed] [Google Scholar]
- May JW Jr., Fearon J, and Zingarelli P (1990). Retro-orbicularis oculus fat (ROOF) resection in aesthetic blepharoplasty: a 6-year study in 63 patients. Plast Reconstr Surg 86, 682–689. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. (2003). Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466. [DOI] [PubMed] [Google Scholar]
- McCready J, Arendt LM, Glover E, and Iyer V (2014). Pregnancy-associated breast cancers are driven by differences in adipose stromal cells present during lactation. Breast Cancer Research 16, R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, and Schedin P (2006). Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. The American journal of pathology 168, 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, and Iwaniec UT (2010). Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res 25, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michilsens F, Aerts P, Van Damme R, and D’Aout K (2009). Scaling of plantar pressures in mammals. J Zool 279, 236–242. [Google Scholar]
- Mikkola HK, and Orkin SH (2006). The journey of developing hematopoietic stem cells. Development 133, 3733–3744. [DOI] [PubMed] [Google Scholar]
- Mirgani T (1977). Fatty-Acid Composition of Hump Triglycerides of Camel Camelus-Dromedarius. Comp Biochem Phys B 58, 211–213. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, and Ntambi JM (2000). The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. The Journal of biological chemistry 275, 30132–30138. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, and Frenette PS (2014). Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell 29, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monziols M, Bonneau M, Davenel A, and Kouba M (2007). Comparison of the lipid content and fatty acid composition of intermuscular and subcutaneous adipose tissues in pig carcasses. Meat science 76, 54–60. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, and Scadden DT (2014). The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni M, Giordano A, Zingaretti MC, Boiani R, De Matteis R, Kahn BB, Nisoli E, Tonello C, Pisoschi C, Luchetti MM, et al. (2004). Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proceedings of the National Academy of Sciences of the United States of America 101, 16801–16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H (1968). [Fine structure and lipid formation in fat cells of the perimeningeal tissue of lampreys under normal and experimental conditions]. Z Zellforsch Mikrosk Anat 84, 585–608. [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, and Daley GQ (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M, Medina D, Monks J, and Hovey R (1998). The Mammary Fat Pad. J Mammary Gland Biol Neoplasia 3, 109–116. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Martinson H, Durand-Rougely C, and Schedin P (2012). Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development (Cambridge, England) 139, 269–275. [DOI] [PubMed] [Google Scholar]
- O’Connell-Rodwell CE (2007). Keeping an “ear” to the ground: seismic communication in elephants. Physiology (Bethesda) 22, 287–294. [DOI] [PubMed] [Google Scholar]
- O’Connell-Rodwell CE, Arnason BT, and Hart LA (2000). Seismic properties of Asian elephant (Elephas maximus) vocalizations and locomotion. J Acoust Soc Am 108, 3066–3072. [DOI] [PubMed] [Google Scholar]
- O’Connell-Rodwell CE, Wood JD, Rodwell TC, Puria S, Partan SR, Keefe R, Shriver D, Arnason BT, and Hart LA (2006). Wild elephant (Loxodonta africana) breeding herds respond to artificially transmitted seismic stimuli. Behavioral Ecology and Sociobiology 59, 842–850. [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, and Nagasawa T (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399. [DOI] [PubMed] [Google Scholar]
- Ottaviani E, Malagoli D, and Franceschi C (2011). The evolution of the adipose tissue: a neglected enigma. Gen Comp Endocrinol 174, 1–4. [DOI] [PubMed] [Google Scholar]
- Paeschke A, Erben U, Kredel LI, Kuhl AA, and Siegmund B (2017). Role of visceral fat in colonic inflammation: from Crohn’s disease to diverticulitis. Curr Opin Gastroenterol 33, 53–58. [DOI] [PubMed] [Google Scholar]
- Parmar H, and Cunha GR (2004). Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocrine-related cancer 11, 437–458. [DOI] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, Berenguer A, Prats N, Toll A, Hueto JA, et al. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45. [DOI] [PubMed] [Google Scholar]
- Pavlovich AL, Manivannan S, and Nelson CM (2010). Adipose Stroma Induces Branching Morphogenesis of Engineered Epithelial Tubules. Tissue Engineering Part A 16, 3719–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Beclin E, Odou MF, et al. (2012). Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 61, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, and Chuong CM (2014). Macroenvironmental regulation of hair cycling and collective regenerative behavior. Cold Spring Harb Perspect Med 4, a015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, et al. (2017). Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, and Chuong CM (2008). Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissonnet CM, Burdi AR, and Bookstein FL (1983). Growth and development of human adipose tissue during early gestation. Early Hum Dev 8, 1–11. [DOI] [PubMed] [Google Scholar]
- Prokesch A, Smorlesi A, Perugini J, Manieri M, Ciarmela P, Mondini E, Trajanoski Z, Kristiansen K, Giordano A, Bogner-Strauss JG, et al. (2014). Molecular Aspects of Adipoepithelial Transdifferentiation in Mouse Mammary Gland. Stem cells (Dayton, Ohio) 32, 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol E, Proenza AM, Roca P, and Llado I (2006). Changes in mammary fat pad composition and lipolytic capacity throughout pregnancy. Cell and Tissue Research 323, 505–511. [DOI] [PubMed] [Google Scholar]
- Rabkin SW (2007). Epicardial fat: properties, function and relationship to obesity. Obes Rev 8, 253–261. [DOI] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, and Anderson SM (2000). An atlas of mouse mammary gland development. Journal of mammary gland biology and neoplasia 5, 227–241. [DOI] [PubMed] [Google Scholar]
- Ring BD, Scully S, Davis CR, Baker MB, Cullen MJ, Pelleymounter MA, and Danilenko DM (2000). Systemically and Topically Administered Leptin Both Accelerate Wound Healing in Diabetic ob/ob Mice. Endocrinology 141, 446–449. [DOI] [PubMed] [Google Scholar]
- Rivera-Gonzalez GC, Shook BA, Andrae J, Holtrup B, Bollag K, Betsholtz C, Rodeheffer MS, and Horsley V (2016a). Skin Adipocyte Stem Cell Self-Renewal Is Regulated by a PDGFA/AKT-Signaling Axis. Cell Stem Cell 19, 738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gonzalez GC, Shook BA, Andrae J, Holtrup B, Bollag K, Betsholtz C, Rodeheffer MS, and Horsley V (2016b). Skin Adipocyte Stem Cell Self-Renewal Is Regulated by a PDGFA/AKT-Signaling Axis. Stem Cell 19, 738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, and Friedman JM (2008a). Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, and Friedman JM (2008b). Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249. [DOI] [PubMed] [Google Scholar]
- Rohrich RJ, and Pessa JE (2007). The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg 119, 2219–2227; discussion 2228–2231. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA (2014). Skin temperature: its role in thermoregulation. Acta Physiologica 210, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, and Spiegelman BM (2014a). What we talk about when we talk about fat. Cell 156, 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, and Spiegelman BM (2014b). What we talk about when we talk about fat. Cell 156, 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MC, McManaman JL, Hunter L, Phang T, and Neville MC (2003). Functional Development of the Mammary Gland: Use of Expression Profiling and Trajectory Clustering to Reveal Changes in Gene Expression During Pregnancy, Lactation, and Involution. Journal of mammary gland biology and neoplasia 8, 287–307. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, et al. (2009). Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 94, 3611–3615. [DOI] [PubMed] [Google Scholar]
- Salathia NS, Shi J, Zhang J, and Glynne RJ (2013). An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. J Invest Dermatol 133, 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampatchalit S, Chen L, Haghighi P, Trudell D, and Resnick DL (2009). Changes in the acetabular fossa of the hip: MR arthrographic findings correlated with anatomic and histologic analysis using cadaveric specimens. AJR Am J Roentgenol 193, W127–133. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung C-M, Sparks CA, Tang Y, Li H, and Guertin DA (2012). PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metabolism 16, 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santander A, Lopez-Ocejo O, Casas O, Agostini T, Sanchez L, Lamas-Basulto E, Carrio R, Cleary M, Gonzalez-Perez R, and Torroella-Kouri M (2015). Paracrine Interactions between Adipocytes and Tumor Cells Recruit and Modify Macrophages to the Mammary Tumor Microenvironment: The Role of Obesity and Inflammation in Breast Adipose Tissue. Cancers 7, 143–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarbati A, Accorsi D, Benati D, Marchetti L, Orsini G, Rigotti G, and Panettiere P (2010). Subcutaneous adipose tissue classification. European Journal of Histochemistry 54, 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scano P, Marincola FC, Locci E, and Lai A (2006). 1H and 13C NMR studies of melon and head blubber of the striped dolphin (Stenella coeruleoalba). Lipids 41, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Scano P, Maxia C, Maggiani F, Crnjar R, Lai A, and Sirigu P (2005). A histological and NMR study of the melon of the striped dolphin (Stenella coeruleoalba). Chem Phys Lipids 134, 21–28. [DOI] [PubMed] [Google Scholar]
- Schedin P, O’Brien J, Rudolph M, Stein T, and Borges V (2007). Microenvironment of the involuting mammary gland mediates mammary cancer progression. Journal of mammary gland biology and neoplasia 12, 71–82. [DOI] [PubMed] [Google Scholar]
- Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, et al. (2015). Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6, 7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BA, and Horsley V (2013). Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development (Cambridge, England: ) 140, 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtnielsen K (1959). The Physiology of the Camel. Sci Am 201, 140–&. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan S, Lund LR, Takeuchi T, Craik CS, and Werb Z (2001). A plasma kallikrein-dependent plasminogen cascade required for adipocyte differentiation. Nature Cell Biology 3, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Hepler C, Vishvanath L, MacPherson KA, Busbuso NC, and Gupta RK (2017). Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol Metab 6, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Tada Y, Asano Y, Hau CS, Kato T, Saeki H, Yamauchi T, Kubota N, Kadowaki T, and Sato S (2012). Adiponectin Regulates Cutaneous Wound Healing by Promoting Keratinocyte Proliferation and Migration via the ERK Signaling Pathway. Journal of immunology (Baltimore, Md. : 1950) 189, 3231–3241. [DOI] [PubMed] [Google Scholar]
- Shuster A, Patlas M, Pinthus JH, and Mourtzakis M (2012). The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. The British Journal of Radiology 85, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideri A, Bakirtzi K, Shih DQ, Koon HW, Fleshner P, Arsenescu R, Arsenescu V, Turner JR, Karagiannides I, and Pothoulakis C (2015). Substance P Mediates Proinflammatory Cytokine Release From Mesenteric Adipocytes in Inflammatory Bowel Disease Patients. Cellular and Molecular Gastroenterology and Hepatology 1, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Honigmann M.R.o., Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, et al. (1998). Biological Action of Leptin as an Angiogenic Factor. Science (New York, N.Y.) 281, 1683–1686. [DOI] [PubMed] [Google Scholar]
- Smillie IS (1980). Diseases of the knee joint (Churchill Livingstone; ). [Google Scholar]
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, and Bray GA (2001). Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 50, 425–435. [DOI] [PubMed] [Google Scholar]
- Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, and Ludgate M (2003). Adipose thyrotrophin receptor expression is elevated in Graves’ and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol 30, 369–380. [DOI] [PubMed] [Google Scholar]
- Stenn KS, and Paus R (2001). Controls of hair follicle cycling. Physiological reviews 81, 449–494. [DOI] [PubMed] [Google Scholar]
- Stuzin JM, Wagstrom L, Kawamoto HK, Baker TJ, and Wolfe SA (1990). The anatomy and clinical applications of the buccal fat pad. Plast Reconstr Surg 85, 29–37. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, and Nagasawa T (2006). Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988. [DOI] [PubMed] [Google Scholar]
- Surmacz E (2007). Obesity hormone leptin: a new target in breast cancer? Breast Cancer Research 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Buache E, and Chenard MP (2011). Adipocyte is a non-trivial, dynamic partner of breast cancer cells. International Journal of Developmental Biology 55, 851–859. [DOI] [PubMed] [Google Scholar]
- Tavassoli M, and Crosby WH (1970). Bone marrow histogenesis: a comparison of fatty and red marrow. Science 169, 291–293. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, and Kirkland JL (2013). Mechanisms and Metabolic Implications of Regional Differences among Fat Depots. Cell Metabolism 17, 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, and Kahn CR (2008). Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metabolism 7, 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varansi U, Feldman HR, and Malins DC (1975). Molecular basis for formation of lipid sound lens in echolocating cetaceans. Nature 255, 340–343. [DOI] [PubMed] [Google Scholar]
- Volden PA, Skor MN, Johnson MB, Singh P, Patel FN, McClintock MK, Brady MJ, and Conzen SD (2016). Mammary Adipose Tissue-Derived Lysophospholipids Promote Estrogen Receptor–Negative Mammary Epithelial Cell Proliferation. Cancer Prevention Research 9, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajchenberg B.L.o. (2000). Subcutaneous and Visceral Adipose Tissue: Their Relation to the Metabolic Syndrome. Endocrine Reviews 21, 697–738. [DOI] [PubMed] [Google Scholar]
- Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, Liuzzi A, and Di Blasio AM (2007). Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity 15, 1933–1943. [DOI] [PubMed] [Google Scholar]
- Wang F, Gao S, Chen F, Fu Z, Yin H, Lu X, Yu J, and Lu C (2014). Mammary Fat of Breast Cancer: Gene Expression Profiling and Functional Characterization. PloS one 9, e109742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Mariman E, Renes J, and Keijer J (2008). The secretory function of adipocytes in the physiology of white adipose tissue. Journal of Cellular Physiology 216, 3–13. [DOI] [PubMed] [Google Scholar]
- Wang W, and Seale P (2016). Control of brown and beige fat development. Nature Reviews Molecular Cell Biology 17, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Reagan MR, and Kaplan DL (2010). Synthetic adipose tissue models for studying mammary gland development and breast tissue engineering. Journal of mammary gland biology and neoplasia 15, 365–376. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Sun L, Subramanian B, Maffini MV, Soto A, Sonnenschein C, and Kaplan DL (2009). Preadipocytes Stimulate Ductal Morphogenesis and Functional Differentiation of Human Mammary Epithelial Cells on 3D Silk Scaffolds. Tissue Engineering Part A 15, 3087–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Attané C, Milhas D, Dirat B, Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, et al. (2017). Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. Journal of Clinical Investigation 2, e87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ (2006). Key stages in mammary gland development - Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Research 8, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissengruber GE, Egger GF, Hutchinson JR, Groenewald HB, Elsasser L, Famini D, and Forstenpointner G (2006). The structure of the cushions in the feet of African elephants (Loxodonta africana). J Anat 209, 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens D, Park CS, and Stockdale FE (1987). Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Developmental biology 120, 245–258. [DOI] [PubMed] [Google Scholar]
- Wiersinga WM, and Bartalena L (2002). Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid 12, 855–860. [DOI] [PubMed] [Google Scholar]
- Winkler LA (1989). Morphology and Relationships of the Orangutan Fatty Cheek Pads. Am J Primatol 17, 305–319. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, and Jahoda CA (2013). Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS One 8, e59811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowicz K, Markiewicz E, and Jahoda CA (2008). C/EBPalpha identifies differentiating preadipocytes around hair follicles in foetal and neonatal rat and mouse skin. Exp Dermatol 17, 675–680. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, and Stehouwer CD (2005). “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365, 1817–1820. [DOI] [PubMed] [Google Scholar]
- Zangani D, Darcy KM, Shoemaker S, and Ip MM (1999). Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Experimental Cell Research 247, 399–409. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tsai P-C, Gonzalez-Celeiro M, Chung O, Boumard B, Perdigoto CN, Ezhkova E, and Hsu Y-C (2016a). Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes & Development 30, 2325–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tsai PC, Gonzalez-Celeiro M, Chung O, Boumard B, Perdigoto CN, Ezhkova E, and Hsu YC (2016b). Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes Dev 30, 2325–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bowen T, Grennan-Jones F, Paddon C, Giles P, Webber J, Steadman R, and Ludgate M (2009). Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem 284, 26447–26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, and Gallo RL (2015). Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, and Morrison SJ (2017a). Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nature Cell Biology 19, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, and Morrison SJ (2017b). Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol 19, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, and Morrison SJ (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger M, McFarland M, and Ben-Jonathan N (2003). Prolactin expression and secretion by human breast glandular and adipose tissue explants. The Journal of clinical endocrinology and metabolism 88, 689–696. [DOI] [PubMed] [Google Scholar]


