Abstract
A body of evidence has revealed positive effects of physical exercise on behavioral, cognitive and physical outcomes in patients with schizophrenia. Notably, the effect of exercise at the neural level may be particularly relevant as well as it is hypothesized that exercise may stimulate the brain in a way that might normalize neural alterations related to the disorder. The aim of the current systematic review was to provide an up to date overview of studies investigating the neural effects of exercise in individuals with a schizophrenia spectrum disorder and healthy individuals. The majority of included studies focused on hippocampal effects, reporting beneficial effects of exercise. In addition, in schizophrenia increased extrastriate body area (EBA) activation and increased white matter fiber integrity in tracts relevant to the disorder were found and in healthy individuals decreased connectivity of the dorsolateral prefrontal cortex (DLPFC) indicating greater cognitive efficiency was reported. Comparing individuals with a schizophrenia spectrum disorder and healthy individuals within a similar age range, most studies found similar effects on hippocampal volume and white matter tracts for both groups, although the effect in schizophrenia spectrum disorders may be attenuated which is in line with previous literature on brain plasticity. The current review indicates a lack of studies investigating neural correlates other than the hippocampus. Although those studies that did focus on other neural correlates revealed promising results, these have not been replicated in other studies and call for replication. Furthermore, future studies should expand their focus, by investigating neural mechanisms underlying positive effects of physical exercise on positive symptoms, negative symptoms and symptoms such as depression, social withdrawal and social cognition.
Keywords: Exercise, Hippocampus, Magnetic resonance imaging (MRI), Neuroimaging, Physical activity, Schizophrenia spectrum disorder
Highlights
-
•
MRI studies on exercise found positive effects on the hippocampus post training in schizophrenia spectrum disorders and controls.
-
•
Positive effects were also found in the EBA and in white matter tracts in schizophrenia spectrum disorders.
-
•
Positive effects were also found in DLPFC connectivity in healthy individuals.
-
•
Effects in schizophrenia may be attenuated.
-
•
There seems to be a dose-response relationship between exercise and neural effects.
1. Introduction
Schizophrenia is a mental disorder that has a considerable impact on patients and their environment (Rössler et al., 2005), because of the disruption of thought, emotion and behavior. The clinical presentation of schizophrenia is characterized by hallucinations and delusions, negative symptoms (e.g. anhedonia, avolition and alogia) and cognitive impairments (van Os and Kapur, 2009). These symptoms have been associated with neural alterations such as changes in volume and functioning of various brain areas (Ross et al., 2006). Studies have consistently demonstrated significant declines in whole brain volume, whole brain gray matter, and frontal lobe volume over time, as well as increases in lateral ventricular volume compared with healthy control subjects (Olabi et al., 2011)With regard to brain function, abnormalities in the prefrontal (both dorsolateral and ventrolateral) cortex and anterior cingulate cortex, the basal ganglia, hippocampus, thalamus, striatum, amygdala and cerebellum have been shown (Fusar-Poli et al., 2007).
Despite comprehensive and evidence-based treatment guidelines, not all patients benefit from standard care (Andrews et al., 2003; Chakos et al., 2001; Dixon and Perkins, 2010) and standard care (e.g. medication, cognitive behavioral therapy and psycho-education) may be insufficient to address all symptoms associated with a schizophrenia spectrum disorder. While anti-psychotic medication is effective for the treatment of psychotic symptoms (Leucht et al., 2014), it is less effective for the treatment of negative symptoms and cognitive deficits (Fusar-poli et al., 2015; Nielsen et al., 2015). Unfortunately, these deficits constitute major determinants of poor functioning and disability (Barch and Keefe, 2010). Adjunctive psychosocial interventions are recommended to complement medication. However, in general, psychological and psychosocial treatments show small to moderate effects on negative symptoms (Lutgens et al., 2017). The most robust findings have been found for social skills training, which might be explained by the activating element in such interventions (Elis et al., 2013). Although cognitive behavioral therapy may have a moderate effect on negative symptoms, findings differ across studies and studies are too heterogeneous to allow for definite conclusions (Aleman et al., 2016). With regard to cognitive deficits, cognitive remediation is a recommended intervention (Wykes et al., 2011). Another difficulty in the treatment of schizophrenia spectrum disorders concerns the fact that the use of anti-psychotic medication, but also the disorder in itself, are associated with metabolic symptoms (Mitchell et al., 2013), such as type two diabetes (Stubbs et al., 2015) and cardiovascular disease (Gardner-Sood et al., 2015). To address the issues outlined above (e.g. negative symptoms, cognitive deficits and physical problems) physical exercise is likely to be a suitable complementary intervention in the treatment of schizophrenia spectrum disorders (Vancampfort et al., 2012; Vera-garcia et al., 2015).
Earlier systematic reviews indicated that moderate to vigorous exercise reduces both positive and negative symptoms in schizophrenia (Dauwan et al., 2016; Firth et al., 2015). Furthermore, it has been shown that exercise interventions may improve cardiometabolic risk factors such as low physical fitness (Vancampfort et al., 2017); they may ameliorate symptoms such as depression and social withdrawal; and exercise interventions significantly improve functioning and low self-esteem (Firth et al., 2015). In addition, there is a growing body of literature that demonstrates the effect of exercise on neurocognition, including social cognition, working memory and attention (Firth et al., 2017).
Although studies have consistently revealed positive effects of physical exercise on behavioral, cognitive and physical outcomes in schizophrenia spectrum disorders, the effect at the neural level remains to be more thoroughly elucidated. Unraveling the effect of physical exercise at the neural level may be particularly relevant as it is hypothesized that physical exercise may stimulate the brain in a way that might normalize neural alterations related to the disorder (Kandola et al., 2016; Ross et al., 2006; Scheewe et al., 2013. Along with other factors, physical inactivity may explain brain alterations associated with schizophrenia spectrum disorders (Lindamer et al., 2009). Indeed, people with schizophrenia often have a sedentary life style, engaging in less moderate and vigorous physical activity compared to the general population (Stubbs et al., 2016). Changing this sedentary life style by exercise interventions may ameliorate brain abnormalities in schizophrenia (Kandola et al., 2016; Scheewe et al., 2013). A meta-analysis on the effect of exercise on hippocampal volume in healthy individuals showed that aerobic exercise interventions indeed may be useful for preventing age-related hippocampal deterioration in the general population (Firth et al., 2018). However, the effect of exercise interventions on hippocampal activation and connectivity, and neural correlates other than the hippocampus in healthy individuals has not been comprehensively reviewed yet. A recent review on schizophrenia samples expanded their view to the whole brain, however, specifically focusing on neurobiological mechanisms through which exercise may reduce cognitive deficits. Most importantly, a direct comparison of the neural effect in individuals with schizophrenia spectrum disorders and healthy individuals is currently lacking.
This systematic review investigates the neural effect of physical exercise interventions at a whole brain level in both patients with a schizophrenia spectrum disorder and healthy individuals. By comparing patients with healthy individuals within a similar age range we aim to distinguish neural effects specific to individuals with a schizophrenia spectrum disorder. Expanding the focus to the whole brain allows to reveal differential pathways in which the brain might be ameliorated. In addition, by means of a broad overview we aim to reveal gaps in the literature to guide future research.
2. Methods
To ensure high quality reporting, PRISMA guidelines for systematic reviews were followed (Shamseer et al., 2015).
2.1. Systematic search
Two systematic database searches in Pubmed, Embase, Psychinfo and Web of Science were conducted in June 2016 (EvdS and ANH) and updated in October 2017 (EvdS). The first search string included suitable indexing terms (i.e. MeSH terms and keywords) regarding schizophrenia spectrum disorders (e.g. psychosis, psychotic disordger) and the second search string included terms regarding healthy individuals (e.g. volunteers, nonclinical). In addition, both search strings included specific indexing terms regarding: i) exercise (e.g. physical activity, endurance training, aerobic training) and ii) neuroimaging (e.g. brain, MRI). (See Appendix for search strings). We checked the reference lists of all included studies for additional publications.
2.2. Selection of studies
Two reviewers (EvdS and ANH) independently screened the title and abstract of all citations identified by the searches. Subsequently, the full texts of all potentially eligible articles were assessed for final selection by the same two reviewers independently. Disagreements were resolved by means of discussion.
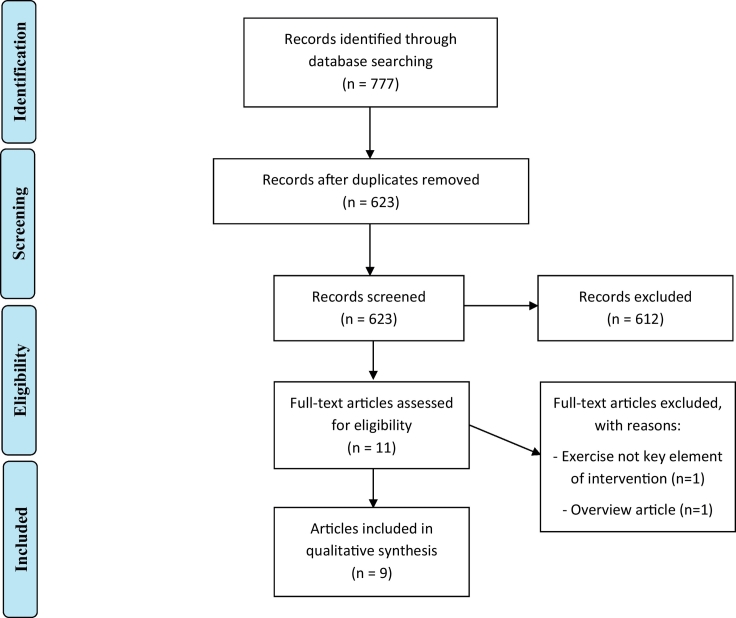
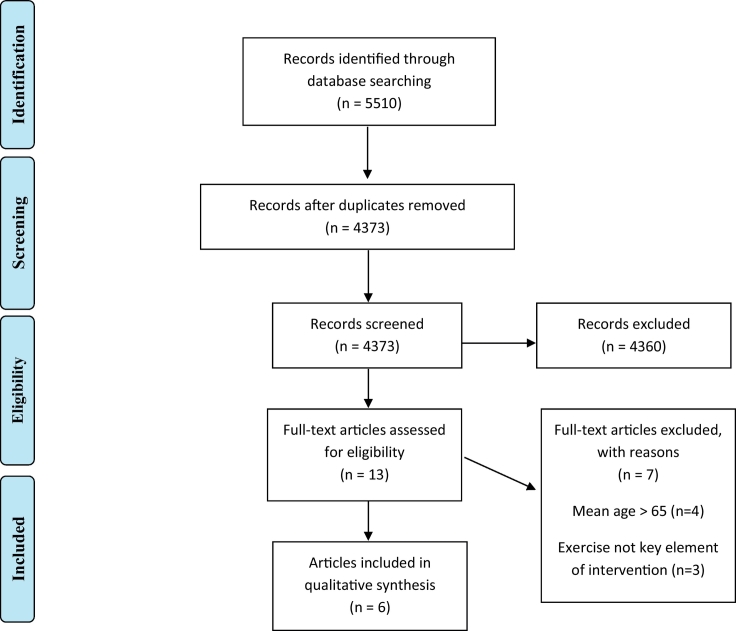
Studies were included if (a) conducted in a sample of individuals with a schizophrenia spectrum disorder and/or healthy individuals (b) the sample of participants had a mean age <65, (c) exercise was the key element of the investigated intervention (e.g. walking, cycling), (d) the study had a longitudinal design, (in case of randomized controlled trials, any control intervention with a similar duration or standard care was allowed as control interventions) and (e) a neuroimaging method (such as MRI, PET, CT or SPECT) was used. Furthermore, only articles published in English in a peer-reviewed journal were included. Reasons for exclusion were recorded, and duplicate articles were identified. Details regarding the selection procedure were recorded to complete a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Liberati et al., 2009).
2.3. Data extraction
Two reviewers (EvdS and ANH) independently extracted data using a predetermined form. The following data were extracted:
(a) Participant characteristics (number of participants, mean age, gender, and for the patient samples: diagnosis, PANSS scores)
(b) Study characteristics (design, control condition)
(c) Exercise protocol characteristics (type of exercise, frequency, duration)
(d) Neuroimaging characteristics (technique, scanner, field of view, outcome)
(e) Functional outcomes.
Separate studies using the same data were included if other neural correlates were investigated or if other neuroimaging techniques were used.
2.4. Visualization
To visualize the results (Fig. 1) of included studies we entered MNI coordinates in BrainNet Viewer (Xia et al., 2013, http://www.nitric.org/projects/bnv/). In studies that reported Talairach coordinates, these were converted to MNI space. For studies that performed a ROI analysis based on the AAL atlas, we used the center coordinates of the ROI according to the atlas. Of note, unweighted results were used. Structural MRI as well as task and resting state fMRI studies, and DTI studies are included in this figure. With regard to functional connectivity studies, MNI coordinates of seed regions and of connected brain areas were used.
Fig. 1.
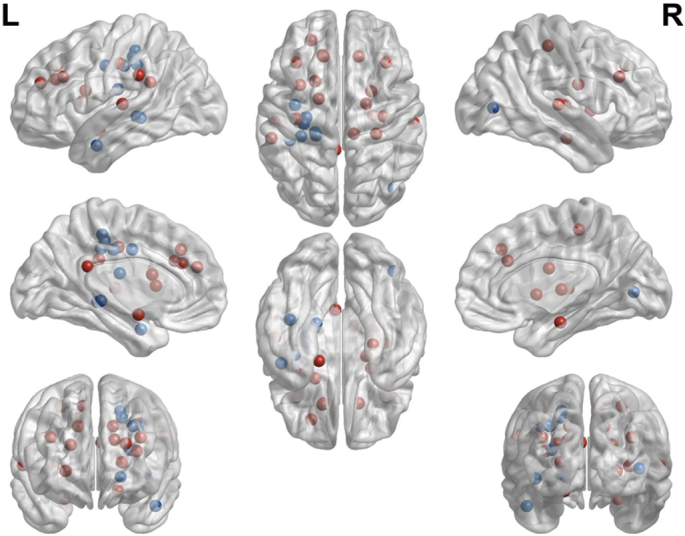
Visual representation of implicated neural correlates of exercise as a summary of empirical findings.
Blue – Schizophrenia spectrum disorders, Red – Healthy.
3. Results
3.1. Search results
The results of the search strategies are presented in two PRISMA flow charts (Fig. 2, Fig. 3).
Fig. 2.
PRISMA flow diagram of the literature search regarding schizophrenia.
Fig. 3.
PRISMA flow diagram of the literature search regarding healthy individuals.
3.2. Participants in the included studies
3.2.1. Schizophrenia
In the studies including patients with a schizophrenia spectrum disorder, the number of participants ranged from 5 to 50 in each study, providing an overall number of 168 unique patients, of whom 81 were women. One study included female participants only (Lin et al., 2015), whereas two other studies only included men (Falkai et al., 2013; Pajonk et al., 2010; Rosenbaum et al., 2015). All participants had a DSM-IV or ICD-10 diagnosis of schizophrenia, schizoaffective disorder or schizophreniform disorder. The mean total PANSS score ranged between 31.8 and 68.1 across studies. Although most studies included relatively young participants, only one study used a first episode psychosis (FEP) sample with illness duration less than three years.
3.2.2. Healthy individuals
In the four studies that were selected during the systematic search focused on healthy individuals, 145 participants were included. Another 56 healthy participants were included in three of the selected studies reporting on individuals with a schizophrenia spectrum disorder. This resulted in a total of 201 healthy individuals (91 female), participating in the studies included in the review. One study only included male participants (Wagner et al., 2015). For more details of participants' demographic characteristics, see Table 1.
Table 1.
Demographic characteristics of participants in included studies.
| Study | Diagnosis | N (gender) | Mean age | Ilness duration | Mean PANSS score | In/out patients |
|---|---|---|---|---|---|---|
| Schizophrenia studies | ||||||
|
Falkai et al., 2013 |
ICD-10 and a DSM-IV diagnosis of schizophrenia |
Exercise group N = 8 (0 female) |
32.9 ± 10.6 | 9.8 ± 1.4 | 68.1 ± 17.6 | Out |
| Control group N = 8 (0 female) |
37.4 ± 8.1 | 65.9 ± 13.9 | ||||
| Healthy control group N = 8 (0 female) |
34,8 ± 10.2 | |||||
|
Lin et al., 2015a |
DSM-IV diagnosis of schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorders, psychosis or NOS |
Exercise group N = 17 (17 female) |
24.6 ± 7.9 | 2.4 ± 2.0 | 46.4 ± 15.9 | Out |
| Yoga group N = 20 (20 female) |
23.8 ± 6.8 | 2.5 ± 2.1 | 47.5 ± 15.4 | |||
| Control group N = 13 (13 female) |
25.3 ± 8.1 | 2.0 ± 2.0 | 45.9 ± 15.3 | |||
|
Lin et al., 2017a |
DSM-IV diagnosis of schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorders, psychosis or NOS |
Exercise group N = 23 (23 female) |
24.6 ± 7.9 | 2.4 ± 2.0 | 46.4 ± 15.9 | |
| Yoga group N = 23 (23 female) |
23.8 ± 6.8 | 2.5 ± 2.1 | 47.5 ± 15.4 | |||
| Control group N = 12 (12 female) |
25.3 ± 8.1 | 2.0 ± 2.0 | 45.9 ± 15.3 | |||
|
Malchow et al., 2015 |
ICD-10 diagnosis of schizophrenia |
Schizophrenia exercise group N = 20 (6 female) |
36.3 | 9.3 ± 7.9 | 31.8 ± 18.8 | In/out |
| Healthy exercise group N = 21 (6 female) |
37.4 | |||||
| Schizophrenia table soccer group N = 19 (7 female) |
35.3 | 11.1 ± 10.8 | 40.4 ± 14.6 | |||
|
Pajonk et al., 2010 |
ICD-10 and a DSM-IV diagnosis of schizophrenia |
Exercise group N = 8 (0 female) |
32.9 ± 10.6 | 9.8 ± 1.4 | 68.1 ± 17.6 | Out |
| Control group N = 8 (0 female) |
37.4 ± 8.1 | 12.5 ± 4.5 | 65.9 ± 13.9 | |||
| Healthy control group N = 8 (0 female) |
34,8 ± 10.2 | |||||
|
Scheewe et al., 2013 |
DSM-IV diagnosis of schizophrenia |
Schizophrenia exercise group N = 18 (4 female) |
28.5 ± 7.3 | 6.0 ± 5.7 | 61.4 ± 11.2 | |
| Healthy exercise group N = 25 (7 female) |
29.5 ± 8.3 | |||||
| Schizophrenia control group N = 14 (2 female) |
31.1 ± 8.0 | 7.9 ± 5.0 | 59.0 ± 10.2 | |||
| Healthy control group N = 27 (9 female) |
28.4 ± 7.0 | In/out | ||||
|
Svatkova et al., 2015 |
DSM-IV diagnosis of schizophrenia |
Schizophrenia exercise group N = 16 (3 female) |
28.8 ± 7.4 | 6.4 ± 5.9 | 62.3 ± 13.2 | In/out |
| Healthy exercise group N = 24 (7 female) |
28.8 ± 7.9 | |||||
| Schizophrenia control group N = 17 (3 female) |
31.3 ± 8.2 | 8.5 ± 6.0 | 60.3 ± 10.1 | |||
| Healthy control group N = 24 (8 female) |
27.7 ± 6.4 | |||||
|
Takahashi et al., 2012 |
DSM-IV diagnosis of schizophrenia |
Exercise group N = 13 (6 female) |
43.5 ± 11.8 | 22.1 (± 15.0) | 37.4 (± 6.7) | In |
| Control group N = 10 (5 female) |
39.9 ± 13.6 | 14.8 (±11.7) | 35.4 (±3.1) | |||
| Rosenbaum et al., 2015 | DSM-IV diagnosis of schizophrenia, schizoaffective disorder or schizophreniform disorder |
Exercise group N = 5 (0 female) |
20.2 ± 4.2 | < 3 year | No PANSS reported | Out |
| Healthy individual studies | ||||||
| Pereira et al., 2007 | Experimental group N = 11 (9 female) |
33 (ranging from 21 to 45) | ||||
|
Tao et al., 2016 |
Tai Chi Chuan group N = 21 (13 female) |
62.38 ± 4.55 | ||||
| Baduanjin group N = 16 (10 female) |
62.18 ± 3.79 | |||||
| Control group N = 25 (19 female) |
59.76 ± 4.83 | |||||
|
Tao et al., 2017 |
Tai Chi Chuan group N = 21 (13 female) |
62.38 ± 4.55 | ||||
| Baduanjin group N = 15 (9 female) |
62.33 ± 3.88 | |||||
| Control group N = 25 (19 female) |
59.76 ± 4.83 | |||||
|
Tozzi et al., 2016 |
Experimental group N = 19 (11 female) |
45.11 ± 13.38 | ||||
| Control group N = 19 (14 female) |
42.89 ± 13.82 | |||||
|
Wagner et al., 2015 |
Experimental group N = 17 (0 female) |
25.0 ± 3.3 | ||||
| Control group N = 17 (0 female) |
23.7 ± 1.7 | |||||
| Wagner et al., 2017 | Experimental group N = 17 (0 female) |
25.0 ± 3.3 | ||||
| Control group N = 17 (0 female) |
23.7 ± 1.7 | |||||
Lin et al. (2015) and Lin et al. (2017) reported Mean age, Ilness duration and Mean PANSS score of the whole group of participants, not for the specific sample which could be included per MRI-analysis.
3.3. Interventions in the included studies
3.3.1. Schizophrenia
All interventions in studies focussing on schizophrenia spectrum disorders consisted of aerobic exercise such as cycling, jogging or rowing; in addition, two interventions also included muscle stretching (Takahashi et al., 2012) and muscle strength exercises (Scheewe et al., 2013; Svatkova et al., 2015).
3.3.2. Healthy individuals
Three of the studies investigating the effect of interventions for this group included aerobic exercise such as cycling or jogging and minor muscle stretching. One study used two kinds of martial arts interventions, respectively Tai Chi Chuan exercises and Badunjin exercises (Tao et al., 2016). Details of the interventions such as duration and frequency are presented in Table 2.
Table 2.
Methodological characteristics & neuroimaging findings.
| Study | Design | Intervention | Neuroimaging technique | Field strength | Included regions | Statistical threshold | Functional outcomes | Neuroimaging outcomes |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia | ||||||||
| Falkai et al., 2013 | RCT | Schizophrenia exercise group 3 times a week, 12 weeks, 30 min, stationary cycling at an individually defined intensity gradually increased over intervention Healthy exercise group 3 times a week, 12 weeks, 30 min cycling Table football control group 3 times a week, 12 weeks, 30 min |
sMRI | 1.5T | Whole brain | Uncorrected | -Patients improved in short-term memory and PANSS total symptoms after exercise. -No time x group effects on fitness outcomes. |
No exercise-related changes in cortical regions. |
| Lin et al., 2015 | RCT | Exercise group 3 times a week, 12 weeks, 60 min, treadmill walking and stationary cycling at 50-60% VO2 max. Yoga group 3 times a week, 12 weeks, 60 min Waitlist control group |
sMRI | 3T | ROI | Uncorrected | -Both types of exercise improved working memory and overall and depressive symptoms (all P ⩽0.01). -A trend towards increased fitness in the aerobic exercise group (7.7%). |
- Aerobic exercise was associated with increased hippocampal gray matter volume (F = 7.52, P = 0.01), mainly related to increases in the left hippocampus (F = 5.13, P = 0.03). - Yoga did not show significant changes in total hippocampal volume. |
| Lin et al., 2017 | RCT | Exercise group 3 times a week, 12 weeks, 60 min, treadmill walking and stationary cycling at 50-60% VO2 max. Yoga group 3 times a week, 12 weeks, 60 min Waitlist control group |
fMRI | 3T | Whole brain | FWE | In yoga group, the ALFF changes in the precuneus were significantly correlated with the changes of PANSS negative scores (r=0.5906, p=0.003), especially with blunted affect subscores (r= 0.551, p = 0.012). | -Yoga was associated with decreased ALFF in precuneus compared to control (P<0.001) and aerobic exercise groups (P<0.001) and -Control group showed increased ALFF in visual cortex (P = 0.0018). |
| Malchow et al., 2015 | RCT | Schizophrenia exercise group 3 times a week, 12 weeks, 30 min, stationary cycling at gradually increasing intensity Healthy exercise group 3 times a week, 12 weeks, 30 min Schizophrenia table soccer group 3 times a week, 12 weeks, 30 min |
VBM | 3T | Whole brain/ROI | FWE | After 3 months of endurance training, both the schizophrenia patient cohort (p= 0.009) and the healthy control cohort (p= 0.003) showed a significant improvement in Physical Working Capacity 130 | - No exercise-related changes in bilateral hippocampal volumes. - Exercise participants showed increased volume of the left anterior temporal lobe (P < 0.05). - Participants from the table soccer control group showed increased motor cortex volumes and anterior cingulate cortex (ACC) volumes (P < 0.05). |
| Pajonk et al., 2010 | RCT | Schizophrenia exercise group 3 times a week, 12 weeks, 30 min, stationary cycling at an individually defined intensity gradually increased over intervention Healthy exercise group 3 times a week, 12 weeks, 30 min cycling Table football control group 3 times a week, 12 weeks, 30 min table football |
sMRI | 1.5T | ROI | Uncorrected | -Patients improved in short-term memory (F1,14 = 4.95; P = .04) and PANSS total symptoms (F1,14 = 6.76; P = .02) after exercise. -No relationship between changes in the PANSS total score and hippocampal volumes. -Change in hippocampal volume correlated with the change in STM r14 = 0.51; P < 0.05) - Changes in hippocampal volume in the exercise group were correlated with improvements in aerobic fitness (r = 0.71; P = .003). -No time x group effects on fitness outcomes (F1,19 = 0.9; P = .35). |
- Hippocampal volume increased with 12% in the schizophrenia exercise group, significantly more than in the control group (F = 13.8, P = 0.002). - 1% volume decrease in the control group (F = 13.8, P = 0.002). - Hippocampal volume increased with 16% in the healthy individuals exercise group. |
| Rosenbaum et al., 2015 | Pilot Longitudinal |
Exercise group 2 times a week, 12 weeks, 45 min, stationary cycling at 65% heart rate of their VO2 max |
sMRI | 1.5T | ROI | Uncorrected | -At follow-up a statistically and clinically significant 20.1% mean increase in VO2 peak. -No statistically significant change was observed in short-term verbal or spatial memory or symptoms. | No significant effect of exercise training on hippocampal volume |
| Scheewe et al., 2013 | RCT | Exercise group 2 times a week, 6 months, 40 min cycling/treadmill/elliptical at up to 75% of max heart rate, then 20 min resistance training Control group 2 times a week, 6 months, occupational therapy |
sMRI | 3T | Whole brain/ROI | FDR | CRF improvement was significantly related to cerebral matter volume increase(0.164ml/W; p=0.045),lateral ventricle (_0.018 ml/W; p=0.035)and third ventricle volume decrease (_0.0018 ml/W; p=0.013)in patients but not in healthy controls | - Exercise therapy did not increase global brain volume, hippocampal volume or cortical thickness in schizophrenia. - Cardio-respiratory fitness improvement was significantly related to cerebral matter volume increase (P = 0.045), lateral (P = 0.035) and third ventricle (P = 0.013) volume decrease and at trend level for cerebral gray matter (P = 0.059). |
| Svatkova et al., 2015 | RCT | Exercise group 2 times a week, 6 months, 40 min cycling/treadmill/elliptical at up to 75% of max heart rate, then 20 min resistance training Schizophrenia control group Occupational therapy Healthy control group Life as usual |
DTI | 3T | Whole brain | FWE | -Significant differences in the exercise and nonexercise group from the first to second measurement were found in the cardiorespiratory fitness parameters (Wpeak, VO2peak), -In patients, a decrease in positive symptoms over time (dPANSSpos) significantly correlated with FA improvement over time (dFApicked) (r = −0.455, P = .008). |
- Exercise improved white matter integrity in fiber tracts in the left corticospinal tract (LCST, P = .032), the left superior longitudinal fascicle (LSLF, P = .032), the left inferior longitudinal fascicle (LILF, P = 0.032), the left inferior fronto-occipital fascicle (LIFOF, P = 0.032), left anterior thalamic radiation (ATR, P = 0.048) and in the body and splenium of the corpus callosum (CC, P = 0.032). |
| Takahashi et al., 2012 | RCT | Exercise group 2 times a day, 5 days/week, 3 months, 30-60 min, walking and jogging, muscle-stretching and sport group (basketball). Control group Care as usual |
fMRI | 1.5T | Whole brain | Uncorrected | -BMI and general psychopathology scale of PANSS were significantly reduced in the program group but not in the control group. -Increase in EBA activation was associated with improvement in the general psychopathology scale of PANSS | Exercise training was associated with activation of the body-selective extrastriate body area (EBA, x=42, y=72, z=4, Z score = 4.12) in the posterior temporal-occipital cortex during observation of sports-related actions. |
| Healthy individuals | ||||||||
| Pereira et al., 2007 | Longitudinal | Exercise group 4 times a week, 12 weeks, 40 min cycling/treadmill/climbing/ellitical, 20 min stretching Aerobic training (treadmill, cycling) and stretching |
CBV | 1.5T | ROI | Uncorrected | - VO2max values significantly increased over time (F=11.6, P= 0.007) -The CBV changes were found to correlate with cardiorespiratory and cognitive function. |
Exercise was found to have a primary effect on dentate gyrus CBV (F = 12, P = 0.006) . |
| Tao et al., 2016 | RCT | Exercise groups 5 days a week, 12 weeks, 30 min exercises, 30 min breathing/relaxation Tai Chi Chuan Baduanjin Control group One time basic health education, physical activity habits as usual |
rsMRI | 3.0T | ROI | FWE | -The memory quotient measured by the Wechsler Memory Scale-Chinese Revision significantly increased after Tai Chi Chuan and Baduanjin practice as compared with the control group, and no significant difference was observed in MQ between the Tai Chi Chuan and Baduanjin groups | -RsFC between the bilateral hippocampus and mPFC significantly increased (peak Z = 3.34) in the Tai Chi Chuan group compared to the control group (also in the Baduanjin group compared to the control group, but at a lower threshold) - No significant difference between the Tai Chi Chuan and Baduanjin groups. |
| Tao et al., 2017 | RCT | Exercise groups 5 days a week, 12 weeks, 30 min exercises, 30 min breathing/relaxation Tai Chi Chuan Baduanjin Control group One time basic health education, physical activity habits as usual |
rsMRI | 3.0T | ROI | FWE | -Both Tai Chi Chuan and Baduanjin groups demonstrated significant improvements in mental control function. -Mental control improvement was negatively associated with rsFC DLPFC-putamen changes across all subjects |
-the Tai Chi Chuan group showed a significant decrease in rsFC between the DLPFC and the left superior frontal gyrus (SFG, peak Z = 4.28) and anterior cingulate cortex (peak Z = 3.89) - the Baduanjin group showed a significant decrease in rsFC between the DLPFC and the left putamen (peak Z = 3.75) and insula (peak Z = 3.61) |
| Tozzi et al., 2016 | RCT | Exercise group 2 times a week, 16 weeks, 5 min warm-up/5 min cool-down aerobic exercises <40% heart rate reserve, 21 min aerobic exercises at 40-59% HRR, 42 min 55-75% HRR Control group Physical activity as usual |
rsMRI | 3.0T | Whole brain/ROI | FDR | -Changes in mood disturbance following exercise were correlated with those in connectivity between parahippocampal gyrus and superior temporal gyrus as well as with the amount of training. -No changes in mood disturbance were detected in the control group. |
- Exercise induced a decrease in local efficiency in the parahippocampal lobe (P = 0.03). - Network-based analysis (NBA) revealed an increased functional connectivity between the right parahippocampal gyrus and the left supramarginal gyrus (P = 0.01), the left precentral area (P = 0.005), the left superior temporal gyrus (P = 0.007) and the right superior temporal pole (P = 0.007) . |
| Wagner et al., 2015 | RCT | Exercise group 3 times a week, 6 weeks, 5 min warm-up/5 min cool-down, 50 min stationary cycling at 77% ±9% VO2max Control group Physical activity as usual |
VBM | 3.0T | ROI | Uncorrected | The exercise group showed physical fitness improvements. Hippocampal volume decrease was negatively correlated with fitness improvement and increased BDNF levels. |
Results revealed an average volume decrease of about 2%, which was restricted to right hippocampal subfields CA2/3 (P = 0.01), subiculum (P = 0.01), and dentate gyrus (P = 0.005). |
| Wagner et al., 2017 | RCT | Exercise group 3 times a week, 6 weeks, 5 min warm-up/5 min cool-down, 50 min stationary cycling at 77% ±9% VO2max Control group Physical activity as usual |
fMRI | 3.0T | ROI | FWE | The exercise group showed physical fitness improvements. Both groups showed cognitive improvements. |
A significantly increased (P < 0.05) activation in the exercise group was observed in the left anterior hippocampus for one of the two task conditions. Changes in exercise-induced BDNF were correlated with left anterior hippocampal activation. Additionally, the motor network showed stronger activation after the exercise intervention in both task conditions. |
3.4. Neural correlates and functional outcomes of exercise
3.4.1. Schizophrenia spectrum disorders
3.4.1.1. Structural MRI/voxel-based morphometry
Six articles reported on the effect of exercise on brain structure in schizophrenia spectrum disorders (Falkai et al., 2013; Lin et al., 2015; Malchow et al., 2015; Pajonk et al., 2010; Rosenbaum et al., 2015; Scheewe et al., 2013), of which five included the hippocampus as a specific region of interest (ROI) in the analysis. Two of these reported a significant effect of exercise on hippocampal gray matter volume (Lin et al., 2015; Pajonk et al., 2010). Pajonk et al. (2010) showed mean hippocampal volume increases of 12% in the exercise group compared to a volume decrease of 1% in the table football control condition. Short-term memory (F1,14 = 4.95; P = 0.04) and PANSS total symptoms (F1,14 = 6.76; P = 0.02) improved after exercise. The change in hippocampal volume in individuals with schizophrenia correlated with changes in short term memory but not with total PANSS score. For the exercise group as a whole, hippocampal volume was correlated with improvements in aerobic fitness measured by change in maximum oxygen consumption. Results showed an increased hippocampal volume of 16% following exercise in healthy individuals. The study did not include a healthy control group to compare to the exercise group. Lin et al. (2015) revealed hippocampal volume increases in the aerobic exercise group compared with the waitlist control group. The yoga group, however, did not show significant changes in hippocampal volume. Both yoga and exercise improved working memory and overall and depressive symptoms and a trend towards improved fitness in terms of VO2 max/kg.
In contrast, three other studies that included the hippocampus as a ROI reported no significant effect of exercise training (Malchow et al., 2015; Rosenbaum et al., 2015; Scheewe et al., 2013). The first mentioned study was a very small pilot study with a sample of five young FEP patients (Rosenbaum et al., 2015). The hippocampus was not affected by exercise. Also, no changes were observed in symptoms and in short-term verbal or spatial memory. However, a significant 20.1% mean increase in VO2 peak was found. Secondly, Scheewe et al. (2013) looked at the whole brain in addition to the hippocampus and revealed no effect of exercise compared to occupational therapy and life as usual on global brain volume, hippocampal volume and cortical thickness in both patients and healthy controls. Nevertheless, overall improvement in cardiorespiratory fitness in only participants with schizophrenia was associated with an increase in total cerebral matter volume and attenuated increase in lateral and third ventricle volumes. In addition, interaction effects between CRF improvement and group were found and this improvement in fitness was associated with cortical thickening in the left hemisphere in patients with schizophrenia as well as in healthy controls. Finally, Malchow et al. (2015) also found no increases in hippocampal structures in both participants with schizophrenia and healthy controls. However, the table soccer control group showed decreased hippocampal volume over time, which implies a potential protective effect of exercise. Additional VBM analyses comparing the effect of exercise with table soccer did reveal an increased volume of the left superior, middle and inferior anterior temporal gyri in patients. Participants from the table soccer control group showed increased motor cortex volumes and anterior cingulate cortex (ACC) volumes. After three months of endurance training, both the schizophrenia group and healthy control group showed a significant improvement in Physical Working Capacity 130 (PWC130) which is a measure of endurance.
One study investigating the effect on brain structure investigated whole brain gray matter density and brain surface expansion (Falkai et al., 2013) as a follow-up on the article of Pajonk et al. (2010). MRI-based cortical pattern matching methods were used on that same dataset to investigate whether exercise training also affects more cortical regions in addition to the subcortical hippocampus found by Pajonk et al. (2010). In contrast to their hypothesis, no significant changes in gray matter density and brain surface expansion were found in participants with schizophrenia. Also no time x group effects were found for fitness measures. However, patients improved in short-term memory and PANSS total symptoms after exercise. Despite equivalent sample size, healthy controls did show a significant increase in gray matter density in the right frontal and occipital pole regions following exercise training.
3.4.1.2. Diffusion tensor imaging
In one article diffusion tensor imaging (DTI) analyses were performed, which is used to investigate white matter tractography within the whole brain. Using data drawn from the same study (‘The outcome of psychosis and fitness therapy’, TOPFIT study) as Scheewe et al. (2013), Svatkova et al. (2015) reported increases of white matter fiber tracts that are related to motor functioning in both participants with schizophrenia and healthy controls. More specifically, exercise improved white matter integrity in fiber tracts in the left corticospinal tract (LCST), the left superior longitudinal fascicle (LSLF), the left inferior longitudinal fascicle (LILF), the left inferior fronto-occipital fascicle (LIFOF), left anterior thalamic radiation (ATR) and in the body and splenium of the corpus callosum (CC). Significant differences in cardiorespiratory fitness measures (Wpeak and VO2peak) were found for the exercise group compared to the non-exercise group. The maximal oxygen uptake (VO2peak) was correlated with changes in overall fiber density. In patients, a decrease in positive symptoms over time significantly correlated with fractional anisotropy (FA) improvement over time.
3.4.1.3. Functional MRI
One study used task (t)fMRI to investigate changes in brain response to sports observation after a three-month sports program (Takahashi et al., 2012). Whole brain analyses revealed that in the exercise group, activation of the body-selective extrastriate body area (EBA) in the posterior temporal-occipital cortex increased during observation of sports-related video-clips. Furthermore, BMI and the general psychopathology scale of PANSS were significantly reduced in the exercise group, but not in the control group. The increase in EBA activation was associated with improvement in the general psychopathology scale of PANSS.
Finally, as a follow-up of Lin et al. (2015), in Lin et al. (2017) resting state (rs) fMRI data was used to investigate local spontaneous neuronal fluctuations at rest, measured by the amplitude of low-frequency fluctuations (ALFF) (Zou et al., 2008). Analyses showed decreased.
ALFF in the precuneus in the yoga group compared to the wait-list control group (p < 0.001) and aerobic exercise groups (p < 0.001) and increased ALFF in the visual cortex (p = 0.0018) in the wait-list control group. In the yoga group, the ALFF changes in the precuneus were significantly correlated with the changes of PANSS negative scores (r = 0.5906, p = 0.003), especially with blunted affect subscores (r = 0.551, p = 0.012).
3.4.2. Healthy individuals
3.4.2.1. Functional MRI
Three studies used rsfMRI to investigate the effect of exercise on brain connectivity (Tao et al., 2016; Tao et al., 2017; Tozzi et al., 2016). Tozzi et al. (2016) performed graph metrics analysis using a network of 90 ROIs, which revealed a decrease in local efficiency in the right parahippocampal gyrus in the exercise group (Tozzi et al., 2016). Next, this structure was used as a specific seed in network-based analysis (NBA). Results revealed an increased functional connectivity between the right parahippocampal gyrus and the left supramarginal gyrus, the left precentral area, the left superior temporal gyrus and the right superior temporal pole. The connectivity between the parahippocampal gyrus and superior temporal gyrus were correlated with changes in mood disturbance following exercise. No changes in mood disturbance were found in the control group.
Comparing a Tai Chi Chuan exercise group to a control group, Tao et al. (2016) found increased functional connectivity between the bilateral hippocampus and the right mPFC and left mPFC by means of a seed-based analysis with the hippocampus as a seed. Similar results were found in the Baduanjin exercise group compared to the control group, although at a lower threshold. With regard to cognitive outcomes, the memory quotient measured by the Wechsler Memory Scale significantly increased after Tai Chi Chuan and Baduanjin practice compared with the control group. No significant difference was observed between the Tai Chi Chuan and Baduanjin groups.
Using the same data-set, Tao et al. (2017) additionally investigated whether the martial arts interventions can modulate mental control function and the resting state functional connectivity (rsFC) of the cognitive control network. Compared to the control group, both Tai Chi Chuan and Baduanjin groups showed significant improvements in mental control function. Seed-based (DLPFC as a seed) rsFC analyses revealed that in the Tai Chi Chuan group rsFC between the DLPFC and the left superior frontal gyrus (SFG) and anterior cingulate cortex was decreased. The Baduanjin group showed a significant decrease in rsFC between the DLPFC and the left putamen and insula.
In one article, task (t)fMRI was used: Wagner et al. (2017) performed ROI (bilateral hippocampus) and whole brain fMRI analyses on the data-set of Wagner et al. (2015) by means of a recognition memory task. The task consisted of the presentation of paired pictures and words, of which specific pairs had to be learned before scanning. Although there were no differences in the amount of correct answers between both groups, the exercise group showed increased activation in the left anterior hippocampus in the familiarity condition. Changes in exercise-induced BDNF were correlated with left anterior hippocampal activation. At the whole brain level, the exercise group showed increased activation in motor areas, supplementary motor areas and in the right superior temporal gyrus in both task conditions.
3.4.2.2. Cerebral blood volume
Pereira et al. (2007) created cerebral blood volume (CBV) maps in the hippocampal formation of healthy individuals. A comparison of pre- and post-neuroimaging data after a three month exercise program showed that exercise had an effect on dendate gyrus CBV, a hippocampal subregion that supports adult neurogenesis. These CBV changes were found to selectively correlate with cardiorespiratory (VO2max) and cognitive function (modified Rey Auditory Verbal Learning Test).
3.4.2.3. Structural MRI/voxel-based morphometry
One of the included studies, which focused on the hippocampus as a ROI, unexpectedly found a volume decrease (2%) of the right hippocampal subfields CA2/3, the subiculum, and the dentate gyrus (Wagner et al., 2015). Additional VBM analysis narrowed the specific location to the anterior part of the right hippocampus. Hippocampal volume decrease was negatively correlated with fitness improvements and increased BDNF levels. This indicates that participants that benefited from the exercise training showed less hippocampal volume decrease and increased BDNF levels.
4. Discussion
4.1. Hippocampal effects
In the studies on both people with a schizophrenia spectrum disorder and healthy individuals the hippocampus was the most studied region in relationship to physical exercise. Three studies from the schizophrenia spectrum disorder search (Lin et al., 2015; Malchow et al., 2015; Pajonk et al., 2010) and four studies resulting from the healthy individuals search (Pereira et al., 2007; Tao et al., 2016; Tozzi et al., 2016; Wagner et al., 2017) reported beneficial effects of exercise on the brain. Pajonk et al. (2010) and Lin et al. (2015) revealed hippocampal volume increase in the exercise group. In Pajonk's study et al. (2010) these effects were correlated with short-term memory and with improvements in aerobic fitness. Lin et al. (2015) also found improved working memory, improved overall and depressive symptoms and a trend towards improved fitness in terms of VO2 max/kg but did not test for correlations. Although Malchow et al. (2015) found no hippocampal volume changes in participants with schizophrenia and healthy individuals following an exercise program, participants with schizophrenia in the table soccer control group showed decreased left hippocampus volume. This indicates that physical exercise could have a protective effect on hippocampal volume decline over time which is in line with research which revealed that aerobic exercise successfully counteracts deteriorating hippocampal function caused by aging (Firth et al., 2017) and Alzheimer's disease (Intlekofer and Cotman, 2013). The positive effect of exercise on neurotrophic factors such as BDNF and insulin-like growth factor-1 (IGF-1) could mediate, in part, beneficial effects on brain structure (McMorris, 2016). With regard to healthy individuals, Pereira et al. (2007) found a primary effect on dentate gyrus CBV, Wagner et al. (2017) showed increased activation in the left anterior hippocampus in response to a recognition memory task, Tao et al. (2016) reported increased functional connectivity between the hippocampus and mPFC and Tozzi et al. (2016) showed increased functional connectivity between the right parahippocampal gyrus and the left supramarginal gyrus, the left precentral area, the left superior temporal gyrus and the right superior temporal pole. Similar findings of beneficial effects of exercise on the hippocampus have been reported previously in animal studies (Van Praag, 2008), in patients with Alzheimer's disease (Erickson et al., 2012) and in elderly (Erickson, 2013).
However, regardless of the robust evidence from studies in animals and other participant groups, results of the included studies that focused on the hippocampus were inconsistent. Two studies from the schizophrenia spectrum disorder search (Rosenbaum et al., 2015; Scheewe et al., 2013) and one study from the healthy individuals search (Wagner et al., 2015) reported no effect on hippocampal structure. The inconsistencies of results across studies investigating the hippocampus might be explained by different segmentation techniques, sample characteristics, and frequency, intensity and duration of exercise protocols. Scheewe et al. (2013) and Rosenbaum et al. (2015) used an automated method of measuring hippocampal volume as opposed to manual segmentation which may have a lower reliability compared to manual segmentation of hippocampal volumes (Morey et al., 2008), as used by Pajonk et al. (2010). With regard to sample characteristics, Rosenbaum et al. (2015) included five relative young (mean age: 20.2) participants who were experiencing a first episode of psychosis (FEP) and the duration of illness (<3 years) may have played a role in the results. Similarly, Wagner et al. (2015) used a male university student sample with a mean age of 25. Several studies have demonstrated that the associations between fitness, physical activity and gray matter volume are mediated by age (Bugg and Head, 2012; Colcombe et al., 2003). The effects of fitness and physical activity on prefrontal and hippocampal volume are larger at older ages (Erickson et al., 2012). Erickson et al. (2014) suggested that there might be certain age periods, characterized by losses in gray matter volume, during which the brain is more susceptible to physical activity-induced changes. In turn, young adulthood is characterized by a relatively stable brain structure, which may be less responsive to physical exercise. However, during the first years of illness onset, age acceleration of the brain is more predominant than in later stages and brain plasticity might be the largest (Schnack et al., 2016), so the null finding in the study of Rosenbaum et al. (2015) might mostly be accounted for by the low power (n = 5). Differences in findings may also have resulted from differences in the frequency and intensity of exercise and the duration of interventions. Rosenbaum et al. (2015) and Scheewe et al. (2013) used an exercise frequency of roughly two times a week, while studies that implemented a frequency of at least three times a week showed more or less positive effects (Lin et al., 2015; Pajonk et al., 2010; Takahashi et al., 2012). In addition, while the studies that found a positive effect implemented an intervention period of twelve (Pereira et al., 2007; Tao et al., 2016) to sixteen weeks (Tozzi et al., 2016), Wagner et al. (2015) used an intervention of six weeks. These findings suggest a dose-response relationship between physical activity and neural changes in both individuals with a schizophrenia spectrum disorder and healthy individuals. Accordingly, Tozzi et al. (2016) have demonstrated that their neuroimaging findings were correlated with the number of exercise sessions that each individual participant attended.
4.2. Other outcomes
Three studies reported on the structure of brain areas other than the hippocampus. First of all, VBM analyses revealed increased volume of the left superior, middle and inferior anterior temporal gyri following exercise in participants with schizophrenia (Malchow et al., 2015). The left temporal lobe is considered an important area for the pathophysiology of schizophrenia and gray matter volume in this region is decreased already in early stages of the disorder and in people at risk (Cooper et al., 2014; Honea et al., 2000). In addition, Scheewe et al. (2013) found that regardless of exercise group, improvement in cardiorespiratory fitness was associated with an increase in total cerebral matter volume and attenuated increase in lateral and third ventricle volumes in patients with schizophrenia. Moreover, cardiorespiratory fitness was associated with cortical thickening in the left hemisphere in both patients and healthy controls. In contrast, Falkai et al. (2013) revealed no exercise-induced changes in the cortical areas in individuals with schizophrenia. This inconsistency might be explained by a small sample size (n = 8 per group) compared to the sample sizes of Malchow et al. (2015) (nmean = 20 per group) and Scheewe et al. (2013) (nmean = 21 per group). However, despite an equivalent sample size, the healthy control group showed significantly increased gray matter densities in the right frontal and occipital cortex in response to exercise suggesting that exercise effects on cortical gray matter may be attenuated in chronic schizophrenia (Falkai et al., 2013).
In line with articles that demonstrated positive effects of exercise on hippocampal volume, activation and connectivity, and brain structure in other areas, Svatkova et al. (2015) showed that exercise also affects white matter integrity in fiber tracts. This effect was found especially in those tracts that have been shown to be altered in schizophrenia (Kubicki et al., 2009) which again implies that exercise can ameliorate abnormalities in schizophrenia. Another study found positive effects of exercise on brain activation by means of task fMRI. Takahashi et al. (2012) demonstrated that exercise has a positive effect on activation of the body-selective extrastriate body area (EBA) during observation of sports-related video-clips. Previous studies have shown that the EBA is involved in planning, execution, rehearsal and imagination of actions as part of the mirror neuron system (MNS) which is implicated in understanding actions and inferring the intention of others (Amoruso, 2011; Jackson et al., 2008). In addition, the EBA is implicated in the motivation and preparation of actions (Kühn et al., 2011) which may have important clinical implications for schizophrenia. A resting state fMRI study reported positive effects of a yoga intervention which consisted mostly of body postures (40–45 min per session) that were designed to cover all body parts to give the body overall strength and flexibility and thus can be seen as a muscle strength- or resilience training rather than aerobic exercise. Results revealed yoga-related ALFF changes in the precuneus, which was positively correlated with the changes of PANSS blunted affect subscores. As the precuneus is involved in intrinsic activity and self-consciousness during rest (Fransson and Marrelec, 2008), these findings may indicate a potential benefit of yoga for attention and self-disturbance of individuals with psychosis. Although no ALFF changes related to aerobic exercise were found, the study did provide support for a yoga intervention focused on strength and flexibility of the body. Lastly, Tao et al. (2017) revealed a decrease in rsFC between the DLPFC and the left superior frontal gyrus (SFG) and anterior cingulate cortex and the DLPFC and the left putamen and insula following exercise in healthy individuals. As increased activity within the CCN might be related to compensatory mechanisms rather than indexing a healthy state, findings of decreased connectivity of the DLPFC within the CCN can be interpreted as a marker for greater cognitive efficiency. Thus, findings demonstrate the potential of Tai Chi Chuan and Baduanjin exercises in preventing cognitive decline.
4.3. Comparison of effect in schizophrenia spectrum disorder and in healthy individuals
Most studies that included individuals with a schizophrenia spectrum disorder as well as healthy individuals found effects in the same direction for both groups on the primary imaging outcome (Malchow et al., 2015; Pajonk et al., 2010; Scheewe et al., 2013; Svatkova et al., 2015). However, Pajonk et al. (2010) found similar results of hippocampal volume increase for both groups but in schizophrenia to a lesser extent (12% compared to 16%). Accordingly, Falkai et al. (2013) found an effect on gray matter density in healthy individuals but no changes in individuals with schizophrenia indicating that neuroplasticity may be attenuated in schizophrenia. This possible attenuated neuroplastic response to exercise is in line with findings of reduced neurogenesis (Reif et al., 2006) and disturbances in synaptic plasticity (Schmitt et al., 2015) in schizophrenia. Five of six studies investigating the effect of exercise in only healthy individuals showed effects of exercise. In contrast, only five of eight articles reporting on individuals with schizophrenia found beneficial effects. However, these studies cannot be compared directly because in general studies on healthy individuals have included older participants and have used more intense exercise programs. Moreover, studies also suggested other variables to be of importance. In addition to age of the participants, frequency and duration of exercise programs and medication, interneuron architecture and genetic factors play an important role in brain plasticity. Papiol et al. (2017) suggest that polygenic risk has an impact on the structural plasticity of hippocampal subfields following aerobic exercise in schizophrenia.
4.4. Limitations and suggestions for future research
A number of important limitations need to be considered. First, as already emphasized, the included studies are heterogeneous with regard to intervention characteristics (frequency, intensity, duration), participant characteristics (in/out-patients, illness duration, symptom severity, age), control groups, neuroimaging techniques and outcomes, which may limit the interpretation of the results. This variety precluded a formal meta-analysis. Consensus on optimal intervention characteristics and close monitoring of adherence and intensity is needed in future physical activity research in order to compare results across studies. An average weekly exercise frequency of at least 2 times a week and a duration of at least 12 weeks might be the minimum (Scheewe et al., 2013; Vancampfort et al., 2014). With regard to control groups, it is recommended to use a control condition in which participants receive at least the same amount of attention and face-to-face interaction to exclude any Hawthorne effect. Secondly, in addition to inconsistencies across study characteristics, genetic factors may play a role in inconsistencies across findings thus far. Future studies should investigate whether hippocampal changes following exercise are mediated by polygenic risk scores (Papiol et al., 2017). Thirdly, as an association between cardiorespiratory fitness and neural effects have been demonstrated in many of the included studies (Lin et al., 2015; Pajonk et al., 2010; Pereira et al., 2007; Scheewe et al., 2013) indicating that cardiorespiratory fitness improvement may be a requirement for positive neural effects, implementation of a standardized measure of aerobic fitness is recommended in future studies. If aerobic fitness is a marker for beneficial neural effects, this may have important clinical implications. In addition, a standardized measure could provide valuable feedback to patients in case of achieving success in changes in cardiorespiratory fitness, as many of them gain weight following treatment (Vancampfort et al., 2015). Finally, some of the included studies used small samples, future studies should include at least twenty participants per group to allow for enough power (Thirion et al., 2007).
Most importantly, nearly all of the studies focused on hippocampal volume. There seems to be a lack of studies investigating other neural correlates. However, those studies that did focus on other brain areas or white matter integrity, did reveal interesting and potentially important positive results of exercise on the brain. For example, Svatkova et al. (2015) revealed improved white matter integrity in motor areas and Takahashi et al. (2012) found increased brain activation in the EBA in individuals with a psychotic disorder. Studies with healthy individuals found decreased connectivity between the DLPFC and other brain areas and increased activation in motor areas and the superior temporal gyrus. As these respective studies report promising results, these have not been replicated in other studies and call for replication.
Furthermore, as previous research has revealed positive effects of physical exercise on positive symptoms, negative symptoms and symptoms such as depression, social withdrawal and social cognition (Firth et al., 2015; Kimhy et al., 2016) it might be interesting to investigate neural mechanisms underlying these behavioral improvements in addition to focusing on the hippocampus as a brain area underlying cognitive improvements. So far, only Takahashi et al. (2012) investigated the effect of exercise on neural mechanisms underlying for example inferring intentions of others and motivation to take action. With regard to negative symptoms such as apathy, future research should investigate the effect on the fronto-subcortical circuitry and the anterior cingulate cortex (ACC) and the inferior parietal cortex as regions of interest (Kos et al., 2016). Regarding the decrease of depressive symptoms or mood improvement associated with exercise, activation and connectivity between the amygdala, the hippocampus and the medial prefrontal cortex may be relevant targets in analyses (Drevets et al., 2008). Furthermore, a focus on activation and connectivity of brain areas involved in social cognition such as the amygdala, the fusiform gyrus, the superior frontal sulcus, the medial prefrontal cortex and the ventrolateral prefrontal cortex could be fruitful in order to determine neural mechanisms underlying effects of exercise on social functioning (Pinkham, 2014).
Finally, in addition to investigating gray matter volumes or activation and connectivity between gray matter brain areas, it might be interesting to look for the effect of exercise on improved white matter integrity in fiber tracts. So far, only Svatkova et al. (2015) studied the effect of exercise on white matter in individuals with schizophrenia.
4.5. Conclusions
The aim of the present article was to provide an up to date overview of studies investigating the neural effects of exercise in individuals with a schizophrenia spectrum disorder and healthy individuals. The majority of included studies focused on hippocampal effects, reporting beneficial effects of exercise. However, results seem inconsistent across studies due to differences in frequency, intensity and duration of exercise protocols, mean age of samples, and segmentation technique used. In addition, in schizophrenia increased EBA activation and increased white matter fiber integrity in tracts relevant to the disorder were found and in healthy individuals decreased connectivity of the DLPFC indicating greater cognitive efficiency was reported. Comparing individuals with a schizophrenia spectrum disorder and healthy individuals, most studies found similar effects for both groups although the effect in schizophrenia spectrum disorders may be attenuated which is in line with previous literature on brain plasticity.
Consensus on optimal intervention characteristics is needed as there seems to be a clear dose-response relationship between exercise and neural effects in both individuals with a schizophrenia spectrum disorder and healthy individuals. As results for both groups indicate a possible relationship between cardiorespiratory fitness and neural effects, implementation of a standardized measure of aerobic fitness is recommended in future trials. Future studies should expand their focus, by investigating neural mechanisms underlying positive effects of physical exercise on positive symptoms, negative symptoms and symptoms such as depression, social withdrawal and social cognition. Finally, future studies should utilize other neuroimaging techniques in addition to structural magnetic resonance imaging more frequently to build upon the promising results of the scarce fMRI and DTI studies and further elucidate the functional neurobiology of exercise-related effects.
Acknowledgments
Acknowledgments
The authors would like to thank Anne Neumann Holbeck, a master student who acted as second reviewer throughout the search process.
Funding
This work was supported by the Netherlands Organization for Scientific Research (NWO) as part of the research programme Violence against psychiatric patients (‘Geweld tegen psychatrisch patienten’), grant number 432-12-807.
Contributor Information
E.C.D. van der Stouwe, Email: e.c.d.van.der.stouwe@rug.nl.
J.T. van Busschbach, Email: j.t.van.busschbach@umcg.nl.
B. de Vries, Email: b.de.vries@rug.nl.
W. Cahn, Email: w.cahn@umcutrecht.nl.
A. Aleman, Email: a.aleman@umcg.nl.
G.H.M. Pijnenborg, Email: g.h.m.pijnenborg@umcg.nl.
Appendix A. Search strings
Search strings which were used in Pubmed:
-
I.
Schizophrenia:
(“Schizophrenia”[Mesh] OR Schizophren*[tiab] OR psychosis[tiab] OR psychot*[tiab])
AND
(“Motor Activity”[Mesh] OR “Sports”[Mesh] OR “Exercise Movement Techniques”[Mesh] OR “Exercise Therapy”[Mesh] OR physical activit*[tiab] OR exercis*[tiab] OR sport*[tiab] OR fitness[tiab] OR endurance training[tiab] OR physical training[tiab] OR aerobic training[tiab] OR resistance training[tiab])
AND
(“Brain”[Mesh] OR “Cognition”[Mesh] OR “Learning”[Mesh] OR “Neuroimaging”[Mesh] OR “Tomography”[Mesh] OR “Neuroimaging”[Mesh] OR “Imaging, Three-Dimensional”[Mesh] OR hippocamp*[tiab] OR cortex[tiab] OR brain[tiab] OR cognit*[tiab] OR memory[tiab] OR neuroimag*[tiab] OR imaging[tiab] OR MRI[tiab] OR fMRI[tiab] OR magnetic resonance[tiab] OR tomograph*[tiab] OR scan*[tiab])
AND
(“Controlled Clinical Trial” [Publication Type] OR “Controlled Clinical Trials as Topic”[Mesh] OR random*[tiab] OR trial[ti])
NOT
((“Animals”[Mesh] NOT “Humans”[Mesh]) OR animal[ti] OR rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti])
-
II.
Healthy individuals
(“Volunteers”[Mesh] OR healthy[tiab] OR normal[tiab] OR volunteer*[tiab] OR nondiseas*[tiab] OR non-diseas*[tiab] OR nonclinical[tiab] OR non-clinical[tiab] OR nonmedical*[tiab] OR non-medical*[tiab])
AND
(“Motor Activity”[Mesh] OR “Sports”[Mesh] OR “Exercise Movement Techniques”[Mesh] OR “Exercise Therapy”[Mesh] OR physical activit*[tiab] OR exercis*[tiab] OR sport*[tiab] OR fitness[tiab] OR endurance training[tiab] OR physical training[tiab] OR aerobic training[tiab] OR resistance training[tiab])
AND
(“Brain”[Mesh] OR “Cognition”[Mesh] OR “Learning”[Mesh] OR “Neuroimaging”[Mesh] OR “Tomography”[Mesh] OR “Neuroimaging”[Mesh] OR “Imaging, Three-Dimensional”[Mesh] OR hippocamp*[tiab] OR cortex[tiab] OR brain[tiab] OR cognit*[tiab] OR memory[tiab] OR neuroimag*[tiab] OR imaging[tiab] OR MRI[tiab] OR fMRI[tiab] OR magnetic resonance[tiab] OR tomograph*[tiab] OR scan*[tiab])
AND
(“Controlled Clinical Trial” [Publication Type] OR “Controlled Clinical Trials as Topic”[Mesh] OR random*[tiab] OR trial[ti])
NOT
((“Animals”[Mesh] NOT “Humans”[Mesh]) OR animal[ti] OR rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti])
Search strings which were used in Psychinfo:
-
I.
Schizophrenia
((DE “Schizophrenia”) OR TI (Schizophren* OR psychosis OR psychot*) OR AB (Schizophren* OR psychosis OR psychot*)) AND ((DE “Physical Activity”) OR (DE “Sports”) OR (DE “Movement Therapy”) OR (DE “Exercise”) OR TI (physical activit* OR exercis* OR sport* OR fitness OR endurance training OR physical training OR aerobic training OR resistance training) OR AB (physical activit* OR exercis* OR sport* OR fitness OR endurance training OR physical training OR aerobic training OR resistance training)) AND ((DE “Brain”) OR (DE “Cognition”) OR (DE “Learning”) OR (DE “Neuroimaging”) OR (DE “Tomography”) OR (DE “Magnetic Resonance Imaging”) OR TI (hippocamp* OR cortex OR brain OR cognit* OR memory OR neuroimag* OR imaging OR MRI OR fMRI OR magnetic resonance OR tomograph* OR scan*) OR AB (hippocamp* OR cortex OR brain OR cognit* OR memory OR neuroimag* OR imaging OR MRI OR fMRI OR magnetic resonance OR tomograph* OR scan*)) AND ((DE “Clinical Trials”) OR TI (random* OR trial) OR AB (random*))
-
II.
Healthy individuals
((DE “Volunteers”) OR (DE “Experimental Subjects”) OR TI (healthy OR normal OR volunteer* OR nondiseas* OR non-diseas* OR nonclinical OR non-clinical OR nonmedical* OR non-medical*) OR AB (healthy OR normal OR volunteer* OR nondiseas* OR non-diseas* OR nonclinical OR non-clinical OR nonmedical* OR non-medical*)) AND ((DE “Physical Activity”) OR (DE “Sports”) OR (DE “Movement Therapy”) OR (DE “Exercise”) OR TI (physical activit* OR exercis* OR sport* OR fitness OR endurance training OR physical training OR aerobic training OR resistance training) OR AB (physical activit* OR exercis* OR sport* OR fitness OR endurance training OR physical training OR aerobic training OR resistance training)) AND ((DE “Brain”) OR (DE “Cognition”) OR (DE “Learning”) OR (DE “Neuroimaging”) OR (DE “Tomography”) OR (DE “Magnetic Resonance Imaging”) OR TI (hippocamp* OR cortex OR brain OR cognit* OR memory OR neuroimag* OR imaging OR MRI OR fMRI OR magnetic resonance OR tomograph* OR scan*) OR AB (hippocamp* OR cortex OR brain OR cognit* OR memory OR neuroimag* OR imaging OR MRI OR fMRI OR magnetic resonance OR tomograph* OR scan*)) AND ((DE “Clinical Trials”) OR TI (random* OR trial) OR AB (random*))
Search strings which were used in Web of Science
-
I.
Schizophrenia
Schizophrenia OR Schizophren* OR psychosis OR psychot*
AND
“Motor Activity” OR “Exercise Movement Techniques” OR “Exercise Therapy” OR “physical activit*” OR exercis* OR sport* OR fitness OR “endurance training” OR “physical training” OR “aerobic training” OR “resistance training”
AND
Brain OR Learning OR Neuroimaging OR “Imaging, Three-Dimensional” OR hippocamp* OR cortex OR cognit* OR memory OR neuroimag* OR imaging OR MRI OR fMRI OR “magnetic resonance” OR tomograph* OR scan*
AND
“Controlled Clinical Trial” OR “Controlled Clinical Trials as Topic” OR random* OR trial.
-
II.
Healthy individuals
Volunteers OR healthy OR normal OR volunteer* OR nondiseas* OR non-diseas* OR nonclinical OR non-clinical OR nonmedical* OR non-medical*
AND
“Motor Activity” OR “Exercise Movement Techniques” OR “Exercise Therapy” OR “physical activit*” OR exercis* OR sport* OR fitness OR “endurance training” OR “physical training” OR “aerobic training” OR “resistance training”
AND
Brain OR Learning OR Neuroimaging OR “Imaging, Three-Dimensional” OR hippocamp* OR cortex OR cognit* OR memory OR neuroimag* OR imaging OR MRI OR fMRI OR “magnetic resonance” OR tomograph* OR scan*
AND
“Controlled Clinical Trial” OR “Controlled Clinical Trials as Topic” OR random* OR trial.
References
- Aleman A., Lincoln T.M., Bruggeman R., Melle I., Arends J., Arango C., Knegtering H. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr. Res. 2016 doi: 10.1016/j.schres.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Amoruso L. Beyond extrastriate body area (EBA) and fusiform body area (FBA): context integration in the meaning of actions. Front. Hum. Neurosci. 2011;5:1–3. doi: 10.3389/fnhum.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G., Sanderson K., Corry J., Issakidis C., Lapsley H. Cost-effectiveness of current and optimal treatment for schizophrenia (Structured abstract) Br. J. Psychiatry. 2003;183:427–435. doi: 10.1192/bjp.183.5.427. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Keefe R.S.E. Anticipating DSM-V: opportunities and challenges for cognition and psychosis. Schizophr. Bull. 2010;36:43–47. doi: 10.1093/schbul/sbp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg J.M., Head D. Lobe. 2012;32(3):506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakos M., Lieberman J., Hoffman E., Bradford D., Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am. J. Psychiatry. 2001;158:518–526. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- Colcombe S.J., Erickson K.I., Raz N., Webb A.G., Cohen N.J., McAuley E., Kramer A.F. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. J. Gerontol. Ser. A Biol. Med. Sci. 2003;58(2):M176–M180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Cooper D., Barker V., Radua J., Fusar-poli P., Lawrie S.M. Psychiatry Research : Neuroimaging Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res. Neuroimaging. 2014;221(1):69–77. doi: 10.1016/j.pscychresns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Dauwan M., Begemann M.J.H., Heringa S.M., Sommer I.E. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 2016;42:588–599. doi: 10.1093/schbul/sbv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L.B., Perkins D.O. Treatment of patients with schizophrenia second edition. Am. Psychiatr. Assoc. 2010;1–184 [PubMed] [Google Scholar]
- Drevets W.C., Price Æ.J.L., Furey M.L. 2008. Brain Structural and Functional Abnormalities in Mood Disorders: Implications for Neurocircuitry Models of Depression; pp. 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elis O., Caponigro J.M., Kring A.M. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin. Psychol. Rev. 2013;33:914–928. doi: 10.1016/j.cpr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I. The ageing Hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2013;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K., Weinstein A., Lopez O. Physical activity, brain plasticity, and Alzheimer's disease. Arch. Med. Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Leckie R.L., Weinstein A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging. 2014;35:S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P., Malchow B., Wobrock T., Gruber O., Schmitt A., Honer W.G., Pajonk F.G., Sun F., Cannon T.D. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:469–473. doi: 10.1007/s00406-012-0383-y. [DOI] [PubMed] [Google Scholar]
- Firth J., Cotter J., Elliott R., French P., Yung A.R. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol. Med. 2015;45:1343–1361. doi: 10.1017/S0033291714003110. [DOI] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Rosenbaum S., Vancampfort D., Malchow B. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. 2017;43:546–556. doi: 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Vancampfort D., Schuch F., Lagopoulos J., Rosenbaum S., Ward P.B. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. NeuroImage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Perez J., Broome M., Borgwardt S., Placentino A., Caverzasi E.…McGuire P. Neurofunctional correlates of vulnerability to psychosis: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2007;31(4):465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Fusar-poli P., Papanastasiou E., Stahl D., Rocchetti M., Carpenter W., Shergill S., Mcguire P. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. 2015;41:892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Sood P., Lally J., Smith S., Atakan Z., Ismail K., Greenwood K.E., Keen A., O'Brien C., Onagbesan O., Fung C., Papanastasiou E., Eberhard J., Eberherd J., Patel A., Ohlsen R., Stahl D., David A., Hopkins D., Murray R.M., Gaughran F., team IMPaCT. Cardiovascular risk factors and metabolic syndrome in people with established psychotic illnesses: baseline data from the IMPaCT randomized controlled trial. Psychol. Med. 2015;45:2619–2629. doi: 10.1017/S0033291715000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R., Sc B., Crow T.J., Ph D., Passingham D., Ph D.…Ph D. 2000. Reviews and Overviews Regional Deficits in Brain Volume in Schizophrenia : A Meta-Analysis of Voxel-Based Morphometry Studies, i; pp. 2233–2245. [DOI] [PubMed] [Google Scholar]
- Intlekofer K.A., Cotman C.W. Neurobiology of disease exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol. Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Meltzoff A.N., Decety J. NIH public access. Growth. 2008;23:1–7. [Google Scholar]
- Kandola A., Hendrikse J., Lucassen P.J., Yücel M. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: practical implications for mental health treatment. Front. Hum. Neurosci. 2016;10(373) doi: 10.3389/fnhum.2016.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D., Lauriola V., Bartels M.N., Armstrong H.F., Vakhrusheva J., Ballon J.S., Sloan R.P. Aerobic exercise for cognitive deficits in schizophrenia - the impact of frequency, duration, and fidelity with target training intensity. Schizophr. Res. 2016;172:213–215. doi: 10.1016/j.schres.2016.01.055. [DOI] [PubMed] [Google Scholar]
- Kos C., van Tol M.J., Marsman J.B.C., Knegtering H.… Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci. Biobehav. Rev. 2016;69:381–401. doi: 10.1016/j.neubiorev.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Kubicki M., McCarleya R., Westin C.-F., Parka H.-J., Maier S., Kikinis R., Jolesz F.A., Shenton M.E. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2009;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Keizer A., Rombouts S., Hommel B. The functional and neural mechanism of action preparation: roles of EBA and FFA in voluntary action control. J. Cogn. Neurosci. 2011;23:214–220. doi: 10.1162/jocn.2010.21418. [DOI] [PubMed] [Google Scholar]
- Leucht S., Cipriani A., Spineli L., Mavridis D., Örey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lässig B., Salanti G., Davis J.M. 2014. Comparative Efficacy and Tolerability of 15 Antipsychotic Drugs in Schizophrenia: A Multiple-Treatments Meta-analysis; pp. 951–962. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lin J., Chan S.K., Lee E.H., Chang W.C., Tse M., Su W.W., Sham P., Hui C.L., Joe G., Chan C.L., Khong P.L., So K.F., Honer W.G., Chen E.Y. Aerobic exercise and yoga improve neurocognitive function in women with early psychosis. NPJ Schizophr. 2015;1(15047) doi: 10.1038/npjschz.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Geng X., Lee E.H., Chan S.K., Chang W.C., Hui C.L.…Chen E.Y. Yoga reduces the brain’s amplitude of low-frequency fluctuations in patients with early psychosis results of a randomized controlled trial. Schizophr. Res. 2017;184:141–142. doi: 10.1016/j.schres.2016.11.040. [DOI] [PubMed] [Google Scholar]
- Lindamer L.A., McKibbin C., Norman G.J., Jordan L., H. K., Abeyesinhe S., Patrick K. Assessment of Physical Activity in Middle-aged and Older Adults with Schizophrenia. 2009;104:294–301. doi: 10.1016/j.schres.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens D., Gariepy G., Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta-analysis. Br. J. Psychiatry. 2017;210:324–332. doi: 10.1192/bjp.bp.116.197103. [DOI] [PubMed] [Google Scholar]
- Malchow B., Keeser D., Keller K., Hasan A., Rauchmann B.-S., Kimura H., Schneider-Axmann T., Dechent P., Gruber O., Ertl-Wagner B., Honer W.G., Hillmer-Vogel U., Schmitt A., Wobrock T., Niklas A., Falkai P. Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophr. Res. 2015:1–10. doi: 10.1016/j.schres.2015.01.005. [DOI] [PubMed] [Google Scholar]
- McMorris T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016;165:291–299. doi: 10.1016/j.physbeh.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Mitchell A.J., Vancampfort D., Sweers K., Van Winkel R., Yu W., De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-a systematic review and meta-analysis. Schizophr. Bull. 2013;39:306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Pettya C.M., Xua Y., Hayesa J.P., Wagner R., Lewisa D.V., LaBara K.S., Stynerg M., McCarthya G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2008;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Levander S., Kjaersdam T. 2015. Second-generation Antipsychotic Effect on Cognition in Patients With Schizophrenia — A Meta-analysis of Randomized Clinical Trials; pp. 185–196. [DOI] [PubMed] [Google Scholar]
- Olabi B., Ellison-wright I., Mcintosh A.M., Wood S.J., Bullmore E., Lawrie S.M. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- van Os J., Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Pajonk F.G., Wobrock T., Gruber O., Scherk H., Berner D., Kaizl I., Kierer A., Muller S., Oest M., Meyer T., Backens M., Schneider-Axmann T., Thornton A.E., Honer W.G., Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Papiol S., Popovic D., Keeser D., Hasan A., Degenhardt F., Rossner M.J.…Schmitt A. Polygenic risk has an impact on the structural plasticity of hippocampal sub fields during aerobic exercise combined with cognitive remediation in multi-episode schizophrenia. Nat. Publ. Group. 2017;7(6) doi: 10.1038/tp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A.C., Huddleston D.E., Brickman A.M., Sosunov A.A., Hen R., McKhann G.M., Sloan R., Gage F.H., Small S.A., Brown T.R. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E. Social cognition in schizophrenia. J. Clin. Psychiatry. 2014;75:14–19. doi: 10.4088/JCP.13065su1.04. [DOI] [PubMed] [Google Scholar]
- Reif A., Fritzen S., Finger M., Strobel A., Lauer M., Schmitt A., Lesch K.-P. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S., Lagopoulos J., Curtis J., Taylor L., Watkins A., Barry B.K., Ward P.B. Aerobic exercise intervention in young people with schizophrenia spectrum disorders; improved fitness with no change in hippocampal volume. Psychiatry Res. Neuroimaging. 2015;232:200–201. doi: 10.1016/j.pscychresns.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Ross C.A., Margolis R.L., Reading S.A.J., Pletnikov M., Coyle J.T. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Rössler W., Joachim Salize H., Van Os J., Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005;15:399–409. doi: 10.1016/j.euroneuro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Scheewe T.W., van Haren N.E.M., Sarkisyan G., Schnack H.G., Brouwer R.M., de Glint M., Hulshoff Pol H.E., Backx F.J.G., Kahn R.S., Cahn W. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur. Neuropsychopharmacol. 2013;23:675–685. doi: 10.1016/j.euroneuro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Schmitt A., Hasan A., Gruber O., Falkai P. Schizophrenia as a disorder of molecular pathways. Biol. Psychiatry. 2015;77:22–28. doi: 10.1016/j.biopsych.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack H.G., Van Haren N.E.M., Nieuwenhuis M., Pol H.E.H., Cahn W., Kahn R.S. Accelerated brain aging in schizophrenia: a longitudinal pattern recognition study. Am. J. Psychiatry. 2016;173:607–616. doi: 10.1176/appi.ajp.2015.15070922. [DOI] [PubMed] [Google Scholar]
- Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349 doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Vancampfort D., De Hert M., Mitchell A.J. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr. Scand. 2015;132:144–157. doi: 10.1111/acps.12439. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Williams J., Gaughran F., Craig T. How sedentary are people with psychosis? A systematic review and meta-analysis. Schizophr. Res. 2016;171:103–109. doi: 10.1016/j.schres.2016.01.034. [DOI] [PubMed] [Google Scholar]
- Svatkova A., Mandl R.C.W., Scheewe T.W., Cahn W., Kahn R.S., Hulshoff Pol H.E. Physical exercise keeps the brain connected: biking increases white matter integrity in patients with schizophrenia and healthy controls. Schizophr. Bull. 2015;41:869–878. doi: 10.1093/schbul/sbv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Sassa T., Shibuya T., Kato M., Koeda M., Murai T., Matsuura M., Asai K., Suhara T., Okubo Y. Effects of sports participation on psychiatric symptoms and brain activations during sports observation in schizophrenia. Transl. Psychiatry. 2012;2 doi: 10.1038/tp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Egorova N., Chen X., Sun S., Xue X., Huang J., Zheng G., Wang Q., Chen L., Kong J. Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after tai chi Chuan practice in elder adults. Front. Aging Neurosci. 2016;8:1–9. doi: 10.3389/fnagi.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Chen X., Egorova N., Liu J., Xue X., Wang Q., Zheng G., Li M., Hong W., Sun S., Chen L., Kong J. Tai chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion B., Pinel P., Mériaux S., Roche A., Dehaene S., Poline J.B. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. NeuroImage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Tozzi L., Carballedo A., Lavelle G., Doolin K., Doyle M., Amico F., Mccarthy H., Gormley J., Lord A., O'Keane V., Frodl T. Longitudinal functional connectivity changes correlate with mood improvement after regular exercise in a dose-dependent fashion. Eur. J. Neurosci. 2016;43:1089–1096. doi: 10.1111/ejn.13222. [DOI] [PubMed] [Google Scholar]
- Van Praag H. 2008. Neurogenesis and Exercise: Past and Future Directions; pp. 128–140. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Probst M., Helvik Skjaerven L., Catalán-Matamoros D., Lundvik-Gyllensten A., Gómez-Conesa A., Ijntema R., De Hert M. Systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia. Phys. Ther. 2012;92:11–23. doi: 10.2522/ptj.20110218. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Probst M., De Hert M., Soundy A., Stubbs B., Stroobants M., De Herdt A. Neurobiological effects of physical exercise in schizophrenia: a systematic review. Disabil. Rehabil. 2014;36:1–6. doi: 10.3109/09638288.2013.874505. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Rosenbaum S., Ward P.B., Stubbs B. Exercise improves cardiorespiratory fitness in people with schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2015;169:453–457. doi: 10.1016/j.schres.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Rosenbaum S., Schuch F., Ward P.B., Richards J., Mugisha J., Probst M., Stubbs B. Cardiorespiratory fitness in severe mental illness: a systematic review and meta-analysis. Sports Med. 2017;47:343–352. doi: 10.1007/s40279-016-0574-1. [DOI] [PubMed] [Google Scholar]
- Vera-garcia E., Mayoral-cleries F., Vancampfort D., Stubbs B., Cuesta-vargas A.I. A systematic review of the bene fi ts of physical therapy within a multidisciplinary care approach for people with schizophrenia: an. Psychiatry Res. 2015;229:828–839. doi: 10.1016/j.psychres.2015.07.083. [DOI] [PubMed] [Google Scholar]
- Wagner G., Herbsleb M., La Cruz F.D., Schumann A., Brünner F., Schachtzabel C., Gussew A., Puta C., Smesny S., Gabriel H.W., Reichenbach J.R., Bär K.-J. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J. Cereb. Blood Flow Metab. 2015:1–9. doi: 10.1038/jcbfm.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., Herbsleb M., De F., Schumann A., Köhler S., Puta C., Gabriel H.W., Reichenbach J.R., Bär K. Changes in fMRI activation in anterior hippocampus and motor cortex during memory retrieval after an intense exercise intervention. Biol. Psychol. 2017;124:65–78. doi: 10.1016/j.biopsycho.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Wykes T., Huddy V., Cellard C., McGurk S.R., Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool forhuman brain connectomics. PLoS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.H., Zhu C.Z., Yang Y., Zuo X.N., Long X.Y., Cao Q.J., Wang Y.F., Zang Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]