Abstract
Lung cancer is the leading cause of cancer deaths throughout the world. The majority of patients are diagnosed with locally advanced or metastatic disease when surgery, the best curative option, is no longer feasible. Thus, the prognosis of lung cancer remains poor and heterogeneous and new biomarkers are needed. As the immune system plays a pivotal role in cancer, the study of tumor microenvironment, with regard to the immune component, may provide valuable information for a better comprehension of the pathogenesis and progression of the disease. Through a detailed and critical evaluation of the most recent publications on this topic, we provide evidences of the prognostic and predictive significance of immune markers in tumor and in peripheral blood of lung cancer patients: from the landscape of immune cells (macrophages, neutrophils, lymphocytes and natural killer) and their cytokines, to the analysis of immune-checkpoints (PD-L1 and CTLA4), up to the genetic and epigenetic regulation of the immune response (immune gene signatures and miRNA). We also argue about the lights and shadows related to immune marker use in clinical practice, emphasizing on one hand the importance of their assessment in the choice of therapeutic treatment, on the other, the difficulty in their determination and reproducibility of literature data. The following review gives a foundation and a suggestion for future studies investigating tumor immunology in lung cancer.
Introduction
Immunosurveillance in Lung Cancer: The Prognostic Role of Tertiary Lymphoid Structures (TLSs)
Almost 50 years passed since Burnet first introduced the concept of immunosurveillance [1], refined later in immuno-editing by Dunn and colleagues [2]. According to the immunosurveillance theory, the host can control tumor growth through the activation of adaptive and innate immune mechanisms, during the early stage of cancer (elimination phase). Under the constant immune pressure (continued deletion of cancer cells recognized by the immune system), some tumor cells undergo genetic and epigenetic changes (immune-editing), enabling them to avoid immune attack. Tumor escape occurs when neoplastic cells evade immunosurveillance and the tumor microenvironment (TME) provides a survival advantage for neoplastic cells. As for other cancers, the concept of the immune-editing can be applied to the lung cancer [3]; thus, the immunosurveillance of lung cancer can be effective in early oncogenesis but it is inhibited in cancer progression, developing a clinically detectable tumor. Evidence for immunosurveillance in lung cancer lies firstly in the proper histology of lung; secondly in the large body of scientific literature demonstrating an immune infiltrate of adaptive and innate immune cell populations [4]. The lung is a mucosal surface of the body, exposed constantly to inhaled particles including pathogens, as well as other potential toxins [5]. Lung protects itself using local tissue structures such as the mucus layer, ciliary ladder, and smooth muscles. Moreover, the respiratory epithelium is also able to directly sense pathogens and respond by releasing antimicrobial molecules able to opsonise bacteria. These innate processes are usually able to maintain sterility of the lung without the intervention of immune system cells. The latter are the next line of defense in the lung. Indeed, pulmonary immune homeostasis is maintained by a network of tissue-resident immune cells that continually monitor the external environment [5]. In health conditions, they contribute to tolerance to innocuous inhaled particles, while ensure an efficient and rapid immune response against invading pathogens. Immune cells of lung tissue are heterogeneous and involve alveolar macrophages, dendritic cells (DCs), and lymphocytes. CD8+ T cells and CD4+ T cells are the most prevalent subtypes of lymphocytes in lung tissue, although natural killer (NK) cells and NK T cells are also present. Very few B cells were found in the lung. The major part of CD4+ subset in the lung are T helper 1 (Th1), while T helper 2 (Th2) and regulatory T cells (Tregs) were detected at low levels [6]. Lung mononuclear phagocytes have been shown to adapt specifically to the lung environment, and contribute to lung homeostasis, scavenging, and immunosurveillance [7]. In lung cancers these immune cells are highly organized in ectopic lymph node-like structures, called TLSs, not present under normal conditions [8]. TLSs resemble and function like secondary lymph-nodes, and antigen presentation take place in them. TLSs are considered a gateway for the entrance of immune cells from the blood to the tumor, through specialized blood vessels, named endothelial venules, which surrounded TLSs [8]. The role of TLSs in the immunosurveillance is supported by a positive correlation between high density TLSs, containing CD8+ T cells, and improved survival of patients, also suggesting a good prognostic value of infiltrating CD8+ T cells in lung cancer [9]. Interestingly, other authors found that patients with few TLSs, but high number of infiltrating CD8+ T cells, had poor survival, underlying the importance of TLS structures themselves; in these structures CD8 + T cells alone were not capable to satisfactorily fulfill their antitumor role without mature DCs [10]. Moreover measurable IgG and/or IgA versus tumor antigens, have been isolated from TLSB cells [8]. In all cases, in lung cancer, the density of TLSs correlates with a favorable prognosis.
Prognostic Immune Cells in Lung Cancer
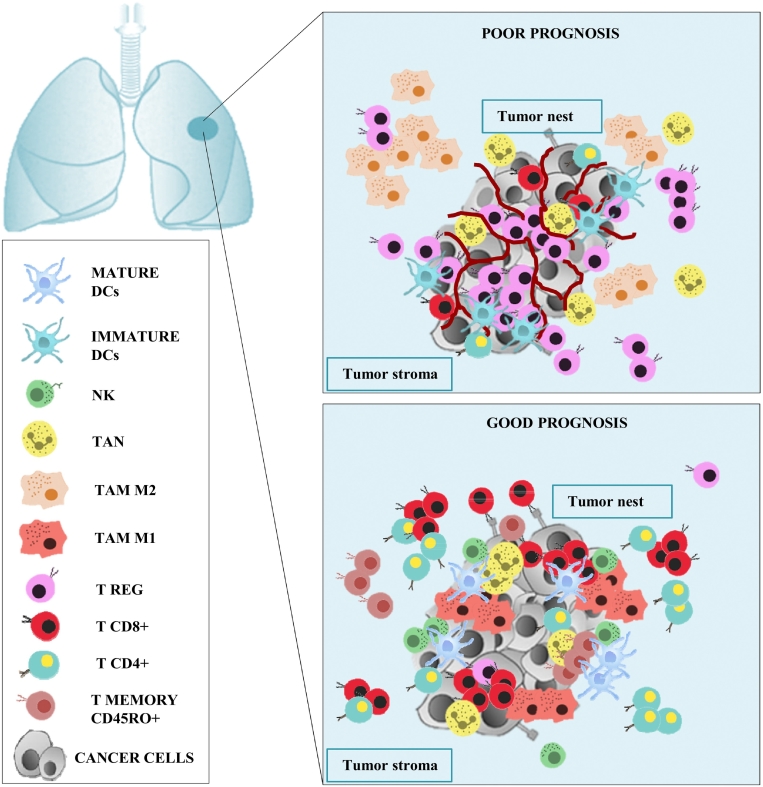
Tumor infiltrating immune cells as macrophages, neutrophils, lymphocytes, have a pivotal contribution in cancer progression and critically influences the clinical outcome of patients depending on density and localization. In Figure 1 we can see a representation of the positive and negative prognostic significance of immune cells in lung cancer microenvironment.
Figure 1.
Prognostic significance of lung cancer infiltrating immune cells. Top panel: poor prognosis lung cancer. Prevalence of Treg compared to T CD8+ and T CD4+ lymphocytes in both tumor nest and stroma. Presence of M2 macrophages in tumor stroma and immature DCs. In patients with adenocarcinoma and lymph node metastases a high neutrophil intratumoral density was correlated with a poor prognosis too. Bottom panel: good prognosis lung cancer. Prevalence of T CD8+ compared to Treg lymphocytes in both tumor nest and stroma. Presence of M1 macrophages, mature DCs and NK cells in tumor nest. High density of T CD4+ lymphocytes in tumor stroma rather than in tumor nest. In patients with SCC, T memory CD45RO+ lymphocytes, in both tumor nest and stroma, and high neutrophil intratumoral density were correlated with a better prognosis too. Abbreviations: DCs = dendritic cells, NK = natural killer; TAN = tumor associated neutrophil; tumor associate macrophage M1/M2; TREG = T regulatory.
Tumor Associated Macrophages (TAMs)
Given the prevalence of macrophages in the lung, our knowledge of TAMs and the spectrum of macrophage phenotypes (tumor suppressing, M1; tumor-promoting, M2) has progressed over the past decades [11], [12]. This can be seen in the evolution of studies investigating macrophage infiltration as a prognostic indicator of lung cancer [13]. Some papers demonstrated that high numbers of macrophages in tumor islets were positively correlated with favorable clinical outcomes and longer survival, in both surgically resected and advanced-stage lung cancers, whereas high numbers of macrophages in the tumor stroma were negatively correlated with patient outcome [14], [15]. However, the prognostic significance of tumor islet or stromal TAMs, lacked consensus of another study reporting no association with survival [16]. Of note, these studies differed in the methodology used (tissue macroarrays or whole sections, score, antibodies used to mark macrophages as anti-CD163+ instead of anti-CD68+). To more accurately define macrophage phenotypes, recently Jacute et al. used multiple stains (CD68+, CD163+, HLA-DR, inducible nitric oxide synthase iNOS) providing a more extensive panel of putative M1 and M2 markers [17]. Despite these contradictory findings, the majority of data suggest that high macrophage densities in tumor islets favor better prognosis. Precisely, M1 macrophages, generally located in tumor cell islets, have been associated with better prognosis, whereas M2 macrophages, more abundant in the tumor stroma, have been associated with poorer prognosis [18].
Tumor-Associated Neutrophils (TANs)
TANs represent a significant portion of tumor-infiltrating cells and accumulate in many types of cancers including lung cancer [19]. It has been hypothesized that TANs polarize into either an N1 antitumoral or N2 protumoral phenotype, in response to cancer epithelial- and stromal-derived signals [20]. CD66b + is an established marker of TANs, stored in neutrophil granules and constitutively expressed by human neutrophils [21]. The prognostic role of CD66b + TANs has been associated with unfavorable outcome for a number of malignancies [22], [23]. In non–small cell lung cancer (NSCLC), two previous studies failed to reveal significant association between TANs and patient outcome [24], [25] but none of these evaluated cancer histological subtypes. Recently Rakaee et al. conducted a study on 536 NSCLC patients of which 172 harbored lymph node metastases [26]. The authors demonstrated that high intratumoral CD66b + TAN density in squamous cell carcinoma (SCC) subgroup, was an independent positive prognostic factor for disease-free survival; by contrast, in adenocarcinoma subgroup, high intratumoral TAN density was an independent negative prognostic factor [26]. Likewise, in patients with lymph node metastases, high level of intratumoral TANs was associated with poor prognosis. Differently, stromal CD66b + TANs were not associated with outcome of NSCLC patients [26]. Eruslanov et al. demonstrated that in early stages of lung cancer, the cross talk between TANs and distant activated T cells led to the up-regulation of CD54, CD86, OX40L and 4-1BBL, costimulatory molecules on the neutrophil surface, which activated T cell proliferation in a positive-feedback loop [27], [28]. Considering the results of these studies, we think that in the earliest stage of lung cancer TANs are not immunosuppressive, but stimulate T cell response, while in advanced lung cancer their phenotype changes supporting the tumor.
Tumor Infiltrating DCs (TIDCs)
DCs represent a heterogeneous and highly plastic immune cell system with a central role in controlling immune responses. In cancer, DCs are able to take up and process apoptotic and necrotic tumor fragments and present tumor antigens to antigen-specific helper and cytotoxic T cells. In this interaction, the mature DCs crucially need to display T-co-stimulatory molecules (CD40, CD86) that will favor cytotoxic T-cell responses. Accordingly, the intratumoral infiltration and activation status of DCs are emerging as clinically relevant parameters in lung cancer, having a substantial prognostic impact. Got et al. in 458 NSCLC lesions found that a high density of mature DC (DC-lamp+) in the TLSs correlated with infiltration of the lesions by T cells and expression of immune-related genes indicating T-cell activation, T helper 1 phenotype and cytotoxic differentiation [9]. A high density of TLS-associated DCs was also associated with improved survival [9]. However, the majority of TIDCs, in resected lung cancer specimen, was shown to reside in an immature state, to strongly overexpress the T-cell inhibitory molecule PD-L1 [29], [30] and PD-L2 [30], and to acquire classical surface markers and functions commonly ascribed to TAMs and myeloid-derived suppressor cells. These TIDCs are capable of actively suppress T-cell function through the secretion of Arginase-1 or indoleamine 2,3-dioxygenase. Thus, compare to peritumoral lung tissue, lung tumors are heavily infiltrated by cells sharing prototypical markers of CD11b + DCs and M2-polarized/tumor-supporting macrophages, with high cell surface levels of PD-L129. Furthermore, low expression of IL-12 and of the co-stimulatory molecules CD80/CD80 in lymph nodes draining lung adenocarcinoma predict a poorer outcome [29]. Finally, Pyfferoen et al. demonstrated that TIDCs-associated miRNA signatures have a negative prognostic impact in NSCLC [29].
Tumor infiltrating lymphocytes (TILs)
TILs are a heterogeneous population of tumor microenvironment comprising mainly T lymphocytes and to a lesser degree B lymphocytes and NK cells. According to the cell surface markers, T lymphocytes include CD8+ cytotoxic T lymphocytes (CTL), CD4+ T helper lymphocytes (Th), CD45RO+ memory T cells (Tm) and FOXP3 regulatory (Tregs) cells. The correlation between TILs and clinical outcome of patients has been extensively studied in lung cancer. Many studies acted to demonstrate the prognostic role of TILs in lung cancer have been published from 2003 to 2014 [31], [32], [33], [34], [35]. However, these studies reported contradictory results with limited statistical power, due to multiple factors examined, or to small number of patients, and they were non homogeneous with regard to stage, histological types of lung tumor and distribution site of lymphocytes (stromal lymphocytes, sTILs and intratumoral lymphocytes, iTILs). Fortunately in 2015 Geng et al. made clarity, publishing an excellent meta-analysis of studies investigating the prognostic impact of TILs in lung cancer patients. This study included 29 reports involving 8600 patients with NSCLC [36].The statistical results confirmed that high density of TILs was associated with favorable progression free survival (PFS), rather than OS. Subgroup analysis was performed according to TIL subsets including CD8+, CD4+ and FOXP3 T cells and reported a better OS in patients with high level of CD8+ T cell infiltration in tumor stroma (TS) and tumor nest (TN), and in both TS and TN. Compared with CD8+ T cells in TN, the prognostic effect of CD8+ T cells in TS appeared more significant. High density of CD4+ T cell infiltration in TS, rather than in TN, was associated with better prognosis in lung cancer. By contrast high density of FOXP3 T cell infiltration in TS could be recognized as a negative prognostic factor for NSCLC [36]. More recently Zeng et al. published another meta-analysis study, on the prognostic value of TILs in NSCLC, included sixteen reports of the Geng's study plus 8 additional studies [37]. The authors showed results overlapping those of Geng's study underlying TILs have a prognostic significance for both OS and recurrence [37]. Among the meta-analysis studies reported by Zeng, very interesting, for what concern the quantification of TILs, was that of Schalper et al. who used multiplex quantitative immunofluorescence to measure the level of CD3+, CD8+ and CD20+ in 552 NSCLC patients [38]. The level of TILs was obtained in different tumor compartments by using cytokeratin stain to define tumor cells, and 4′,6-Diamidino-2-Phelylindolo. The authors found that increased levels of CD3+ and CD8+ TILs were associated with better outcome in NCLSC [38]. In the majority of published papers, T cell subsets were assessed by immunohistochemistry (IHC) with a manual semi-quantitative approach. Different cut off were used to define “low” and “high” for each marker and for the epithelial/tumor nest compartment and the stromal compartment, according to the staining distribution [32]. Of note, the study of Donnem et al., who recently demonstrated that the stromal CD8+ T cell density, scored on a manual semi-quantitative 4-point scale, had an independent prognostic value and could stratify patients within each tumor/lymph node/metastasis (TNM) stage [39]. This paper was followed by a second study and proposal to introduce an IHC-based “TNM immune” staging system into clinical use for NSCLC [40]. After these two big meta-analysis studies, all researchers have oriented their studies in order to propose a reproducible method for TIL quantification in lung tumor microenvironment, that could clarify their prognostic and predictive role. One of these, is the study of Brambilla et al [41]. For these authors the discrimination between stromal and epithelial infiltration may add more confusion than precision, due to the lack of inter-observer reproducibility, and from a pathology point of view adds little, because by definition, the tumor environment includes stroma [41]. Finally the last relevant publication concerning this topic is by Obeid and colleagues [42]. The authors assess 9 sampling strategies of 23 primary NSCLCs with the purpose to evaluate which of these 9 methods had the closest correlation with CD8+ TIL density measures of a whole tumor section, as well as with survival outcomes. The strategy showed the greater concordance with whole tumor, was that used multiple random samples of 20% of the tumor or a random core biopsy measuring 10x1mm [42]. The authors found that patients who had higher CD8+ counts in the center of the tumor experienced longer OS than those with low CD8+ counts [42].
Anticancer immune response is to date especially investigated for NSCLC, without regard to histological types. Recently it was reported a significant lower proportion of CD8+ cells and higher FOXP3+/CD8+ ratio in metastatic versus free lymph nodes only in adenocarcinoma, indicating a particular biology of this type of NSCLC [43]. Other authors demonstrated that CD8+ T cell infiltration strongly contributed to a better prognosis in adenocarcinoma when tumor cells retained the expression of classical HLA class I and did not express HLA-E [44]. Therefore, analysis of HLA-A, -B/C and HLA-E expression should be included as biomarkers together with CD8+ analyses, to predict the response to immunotherapy. In relation to small cell carcinoma (SCC), it has demonstrated the prognostic role of T memory CD45RO+ cells. These cells alone and in combination with CD8+ TILs, in tumor and stromal compartments and within each pathological stage (from stage I to IIIA), were a significant prognostic indicator of improved survival time [45]. The putative contribution of NK cells to immunosurveillance in lung cancer has been an ongoing topic. Some studies reported that NK infiltrating the tumor tissue were associated with better prognosis in several tumors, included lung cancer [46]. Other studies refer to the prognostic role of peripheral blood rather than tumor tissue NKcells; thus we reserve to discuss it in the next paragraph.
Prognostic Immune Circulating Markers in Lung Cancer
Immune Circulating Cells
For lung cancer <30% of the tumors are resectable and available for a complete microscopic examination. In other cases, the material for the study of inflammatory infiltration may be a tumor biopsy. Histological biopsies or cytological samples are too small and not representative for evaluating inflammatory infiltration. The immune response may be evaluated easier by peripheral blood examination, although it reflects systemic changes, different from local ones.
Neutrophils account for the most peripheral white blood cells [47]. A series of studies have explored the correlation between peripheral neutrophils and lymphocytes ratio (NLR) and the prognosis of lung cancer. A meta-analysis, including 14 studies and 2735 lung cancer cases, showed that high NLR yielded a worse OS in NSCLC and SCLC [48]. These data agree with those recently published by Deng et al [49]. The latter reported that elevated pretreatment values of NLR were an independent factor for poor prognosis in SCLC patients. Moreover high platelet and lymphocytes ratio were associated with poor prognosis too [49]. More recently Akinci Ozyurek et al. demonstrated that NLR was more significant in determining the prognosis in NSCLC than in SCLC cases [50]. Wang et al. studied, by flow cytometry, T lymphocyte subgroups in peripheral blood of NSCLC patients and healthy adults, evaluating their clinical significance in diagnosis, treatment and prognosis [51]. CD3+, CD4+, CD4+/CD8+ ratio and NK cells in NSCLC were decreased significantly in comparison with the control group, and their levels inversely correlated with the clinical stage of NSCLC, decreasing with the increase of clinical stage; CD8+ cells demonstrated no significant change and Treg cells were significantly more frequent than in the control group, and increased with the clinical stage of NSCLC [51].
NK activity was also related to lung cancer prognosis [46] and peripheral NK cell cytotoxicity was reduced in lung cancer patients [52]. Xu et al. demonstrated that the overexpression of T cell immunoglobulin and mucin-domain-containing molecule-3 (Tim3), on CD3+ CD56+ NK cells and CD3+ CD56dimNK subset, was associated with lymph node metastasis, and a shorter OS of patients with lung adenocarcinoma [53]. Other authors demonstrated an aberration of NK cell function in NSCLC. This aberration consisted in a constitutively low expression at the mRNA level of the three NK isoform receptors (NCR1/NKp46; NCR3/NKp30; NKp30), which correlated with poor OS and PFS [54].
For deeper and specific characterization of local immune response, the analysis of bronchoalveolar lavage (BAL) fluid may be used [55]. In the BAL obtained from a lung afflicted by cancer, the following changes may be observed: increased number of neutrophils, predominance of T-cells and cytotoxic CD8+ T cells, prominent percentage of Tregs, polarization of macrophages to the M2 population, significantly increased concentration of TGF-β [56]. These alterations were significantly different than peripheral blood and BAL material obtained from the healthy lung, symmetrically to tumor localization in the lung afflicted by cancer.
Interleukines and chemokines
Cancer cells communicate with the microenvironment via a complex network of many growth factors, chemokines, interleukines and their own receptors. In the last decades several studies focused on the association between the expression level of interleukines, measured in serum, BAL and in lung tumor tissue, and patient survival or progression. Interleukin-20 (IL-20) and interleukin-22 (IL-22) have modulatory and opposing effects on cancer cells: IL-20 is an inhibitor of angiogenesis, while IL-22 stimulates tumor growth [57]. Recently, Naumnik et al. found that IL-20 and IL-22 in the serum and BAL of NSCLC patients are prognostic factors of cancer progression [58]. The authors reported high serum levels of IL-20 were negatively associated with cancer progression, while they failed to find an association between survival and serum levels of IL-22. Moreover the found that lower levels of IL-22 in the BAL of NSCLC patients, compared to healthy control, were associated to worse survival. A possible explanation of this founding could lie in the distribution of IL-22 receptors. IL-22 receptor 1 (IL-22R1) is expressed exclusively on epithelial and tissue cells of lung cancer. The BAL concentration of IL-22 in NSCLC patients could be reduced due to its binding to the receptor [58]. Increased levels of IL-17 have been found in advanced NSCLC. Lin et al. demonstrated that IL-17 levels were significantly elevated in the serum of SCLC patients and correlated with tumor metastasis, stage, and shorter OS [59]. Thus IL-17 may be a novel prognostic biomarker in SCLC [59]. Other authors emphasized the importance to identify a “combined cytokine prognostic classifier” to detect patients at high risk of recurrence of lung cancer, thus requiring more aggressive treatment regimens at the time of diagnosis [60], [61]. These authors firstly found that the high combined expression of IL-8 with IL-6 [60], and secondly of IL-6 with IL-17 [61], was negative prognostic factors for stage I lung cancer. In addition to interleukins, also chemokines and/or their receptors expression have been correlated with patient survival or progression in lung cancer. Moreover, most of the studies evaluated chemokine expression in tumor or stromal compartment than in patient serum. A very recent and exhaustive review on the role of chemokines in NSCLC was recently published [62]. In summary, in these patients, high levels of C-C motif chemokine ligand 2 (CCL2), CCL19, CXCL16, and low levels of CCL5 were associated to a better survival; high levels of C-X-C motif chemokine ligand 8, CXCL8, and C-X-C motif chemokine receptor 4, CXCR4, were associated to a worse survival [62].
Immunocheckpoints as Prognostic and Predictive Immune Markers in Lung Cancer
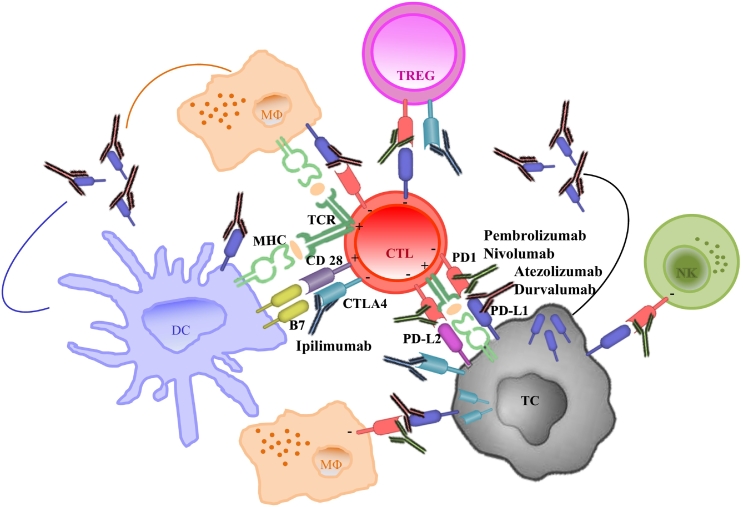
Immunocheckpoint are pathways that induce costimulatory and inhibitory signals, crucial for regulating the physiologic T cell immune response, maintaining self-tolerance and inducing tumor escape from immunosurveillance [63]. Programmed death protein 1 (PD1) and Cytotoxic T-lymphocyte-associate protein 4 (CTLA4) pathways, are considered the main checkpoints for effective immunotherapy in solid tumor and also in lung cancer (Figure 2).
Figure 2.
PD-L1/PD1 and CTLA4 immune regulatory pathways. Schematic representation of immunochekpoints in lung cancer immune contexture. Cytotoxic T lymphocyte activation (CTL) require two signals: peptide-major histocompatibility complex (MHC) binding, that is recognized by the T cell receptor (TCR), and the B7 costimulatory molecule-CD28 complex. The immunocheckpoint protein CTLA4 competes with CD28 to bind the B7 ligand on antigen presenting cells, leading to the inhibition of T cell activation. CTLA4 is expressed on T cells and was also found on the membrane and in the cytoplasm of NSCLC cells. The monoclonal antibody Ipilimumab blocks CTLA4 binding to B7. Similarly to CTLA4 function, the binding of PD-L1 immunocheckpoint protein to its receptor PD1, keeps T cells from killing tumor cells. PD-L1 is expressed by lung cancer cells on cell membrane and in the cytoplasm and can be up-regulated on DCs, macrophages, NK and activated T cells. PD1 is expressed by activated T cells, NK and myeloid cells. PD-L1 soluble form, deriving by tumor and immune infiltrating cells, compete for PD1 ligation with anti-PD1 monoclonal antibodies. In green we represented anti-PD1 antibodies (Pembrolizumab and Nivolumab); in brown anti PD-L1 antibodies (Atezolizumab and Durvalumab).Abbreviations: TC = tumor cell; MΦ = macrophage; DC = dendritic cell; NK = natural killer; TREG = T regulatory; CTL = cytotoxic T lymphocyte.
PD1/PD-L1 axis
PD1 is expressed by activated T cells, B cells, NK T cells, and myeloid cells and is often highly expressed by TILs [62]. PD1 ligand (PD-L1) is expressed by cancer cells, and can be up-regulated on TAM, DCs, fibroblasts, and activated T cells [64]. The expression of PD-L1 on tumor cells was demonstrated on cell membrane, in the cytoplasm, or both, in focal or scattered pattern. Ligation of PD-L1 with PD1 mediates suppression of T cell function, differentiation and survival [64]. In NSCLC, it has been reported that 20–60% of tumors were positive for PD-L1 and/or for PD-L2, at lower frequency [3]. PD-L1 is not only membrane bound, but also secreted as a soluble form (sPD-L1). In lung cancer, the source of sPD-L1 could be the tumor cell, or the tumor-infiltrating immune cells [64]. Soluble PD-L1 may compete for PD1 ligation with anti-PD1 monoclonal antibodies. Although some studies reported high PD-L1 expression in lung tumor cells or TILs, are predictive of the response to PD1 pathway inhibitors, PD-L1 has not proved adequately reliable as a single biomarker [64]. One explanation may be the current use of non-standardized IHC techniques for measuring PD-L1 levels in tissue. Different commercial anti-PD-L1 antibodies (Dako 28–8, Dako 22C3, VentanaSP142, Ventana SP263) have been developed and validated in clinical trials. The American Association for Cancer Research (AACR) and the International Association for the Study of Lung Cancer (IASLC), together with four pharmaceutical companies (Brystol-Meyers, Merck, Genentech/Roche and AstraZeneca) and two diagnostic companies (Dako and Ventana), in the Bluprint Project, compared four PD-L1 assays on the same set of lung cancers. The results were similar for the 22C3, 28-8 and SP263 PD-L1 antibodies, while PD-L1 expression, tested by SP142 antibody, was generally low [65]. The difference may be explained by the binding of PD-L1 extracellular domain for the three antibodies and PD-L1 cytoplasmic domain for SP142. Moreover, when the results of the different assays were translated into “positive” or “negative”, based on the cut-offs, only the 50% of specimen showed the same results for all tests.
Recently Brody et al. published a meta-analyses study to clarify the prognostic role of PD-L1 in advanced NSCLC. A total of 35 eligible studies were selected for analysis. Among these, three large meta-analysis studies concluded that high tumor PD-L1 expression was associated with shorter survival [66]. A possible link between PD-L1 expression and poor prognosis in advanced NSCLC fits with the role of PD1/PD-L1 in suppressing anti-tumor response. Paulsen et al. showed that a low density of PD-L1+ stromal immune cells and PD1+ intraepithelial TILs+ predicted for unfavorable survival outcome, especially for patients with SSC [67]. By contrast, Velcheti and colleagues in a multivariate analysis of 544 patients (Stage I-IV NSCLC) reported a significant association between PD-L1 expression with increased TILs, and longer OS [68]. Similar results were found by Cooper et al. who demonstrated PD-L1 expression in ≥50% of NSCLC (Stage I-III) tumor cells was associated with longer OS, with the exception of adenocarcinoma patients [69].
The value of PD-L1 as a predictive biomarker for the therapy with anti-PD1/PD-L1 agents (nivolumab, pembrolizumab/atezolizumab, durvalumab), was evaluated in 16 studies of Brody's meta-analyses [66]. Among nivolumab monotherapy studies, 3 reported greater treatment benefit in patients with high versus low tumor PD-L1 expression. Conversely, a randomized controlled trial in squamous NSCLC patients concluded that the treatment benefit of nivolumab was independent of tumor PD-L1 expression. For pembrolizumab, high PD-L1 expression was correlated with improved treatment effect in 2 studies. Three of 4 atezolizumab studies, reported that higher PD-L1 expression levels were associated with greater treatment effects and longer survival. For durvalumab monotherapy, a Phase 1/2 study demonstrated that the overall response rate was higher in patients with high versus low tumor PD-L1 expression [66]. The Food and Drug Administration (FDA) approved nivolumab, pembrolizumab and atezolizumab for the treatment of NSCLC patients with disease progression or after platinum-based chemotherapy. Furthermore, in October 2016 pembrolizumab was approved by the FDA as a 1st line treatment for advanced NSCLC patients, based on the results of the clinical trial KEYNOTE-024 that showed significantly improved response rate, PFS and OS when advanced NSCLC patients, whose tumors harbored PD-L1 expression by IHC in 50% or greater of tumor cells, were treated with this drug compared to platinum-based chemotherapy in the 1st line setting [70].
CTLA4
CTLA4 is an inhibitory molecule expressed on T cells involved in the negative regulation of T cell interaction with antigen-presenting cells (APC). It inhibits binding of CD28 on T cells, to B7 proteins on APCs, thus weakening the costimulation on T cells [71]. CTLA4 is also constitutively expressed on Treg and promotes their regulatory function [72]. CTLA4 was found on the cell surface and in the cytoplasm of tumor cells in about 50% of NSCLC patients. High CTLA4expression, but not PD1, predicted worse survival in NSCLC and other malignancies, like nasopharyngealor esophageal carcinoma [73]. Contrarily, other authors found CTLA4 overexpression correlated with good survival and reduced death rate in radically resected NSCLC [74]. Recently, Paulsen et al. evaluated by IHC CTLA4 expression in 536 patients with primary resected stage I-IIIA NSCLC [75]. CTLA4 expression in neither tumor epithelial cells, nor stromal cells, was significantly associated with disease specific survival. However, high stromal CTLA4 expression predicted improved disease specific survival in SCC subgroup. By contrast, CTLA4 expression in metastatic lymph nodes, was an independent negative prognostic factor [75]. Basing on these studies, we think that CTLA4 expression has diverging prognostic impact with regard to the histological lung tumor and stage of disease. The anti CTLA4 IgG1 humanized antibody, ipilimumab, binds to CTLA4 and prevents the inhibition of CD-28/B7 signaling. It leads to the reactivation of the antitumor immune response mediated by specific T cells and depletion of Tregs [76]. A phase 2 study with ipilimumab, in combination with chemotherapy, in advanced NSCLC patients, showed promising results with a significant improvement in PFS versus the group treated with chemotherapy alone. However, actually there is no validated biomarker considered able to predict response to anti-CTLA4 therapy [76].
Exosomes as Modulators of Lung Cancer Immune Response
Exosomes are nanovesicles of 50-100 nm of diameter that are released from most viable cells and play an important role in intercellular communication. They are exocytosed in a constitutive manner in both physiologic and pathological conditions and can be found in several body fluids: urine, saliva, blood, BAL. Exosomes contain messenger RNA (mRNA), microRNA (miRNA), double-stranded DNA (dsDNA) and proteins that could serve as diagnostic, prognostic and predictive biomarkers for different tumors [77]. Many correlations were found between tissue and exosomal biomarkers in different cancers, included lung cancer [77]. Exosomes are reported to mediate lung cancer invasion, metastasis and drug resistance and to transport proteins (Epidermal growth factor receptor, EGFR, claudins, KRAS) and miRNA (tumor suppressor miRNA, non-coding RNA) related to poor OS of adenocarcinoma and NSCLC patients [77]. In lung cancer, tumor-derived exosomes disable anti-tumor immune effector cells and promote tumor escape from immune control. The EGFR carried by lung cancer cell exosomes, can induce immune-resistance of DCs and CD8+ T cells, by tumor-specific Treg cells, with immune escape of cancer cells [78]. Liu et al. demonstrated that tumor exosomal RNAs promoted lung pre-metastatic niche formation by the up-regulation of alveolar epithelial talk like receptor 3, TLR3, with neutrophils recruitment and increasing cytokines production [79]. By contrast, the exosome derived miR-302b suppressed lung cancer cell proliferation and migration through its interaction with the transforming growth factor β receptor II, TGFβRII, mRNA [80]. Beside tumor cell derived exosomes, these vesicles are also released by normal immune cells. Since 2003, it was demonstrated that lung cell-derived exosomes, present in healthy human BAL, express MHC class I/II and costimulatory molecules, suggesting their exocytosis from antigen presenting cells and activity as immunomodulatory agents [81]. DC-derived exosomes prime specific cytotoxic T lymphocytes and activate anti-tumor immune response [81]. The presence of MHC-I/MHC-II molecules, on the surface of dendritic cell-derived exosomes, facilitates the direct stimulation of CTLs and CD4 + T cells [82]. Therefore while exosomes deriving from lung cancer cells have a pro-tumor effect, exosomes from immune cells of lung tumor environment are useful tool for tumor antigen-specific immunity and may exhibit utility in lung cancer immunotherapy.
Epigenetic Regulation of Lung Cancer Immune Response
Advances in the field of lung cancer epigenetics, provide a very promising step towards the direction of novel biomarker development. Epigenetics consists of heritable modifications in the chromatin that influence gene expression without directly altering the DNA coding sequence [83]. Epigenetics mechanism can be grouped into DNA methylation, DNA acetylation (histone/nucleosome remodeling) and micro RNA (miRNA) [84].
DNA Methylation in Lung Cancer and Immune Response
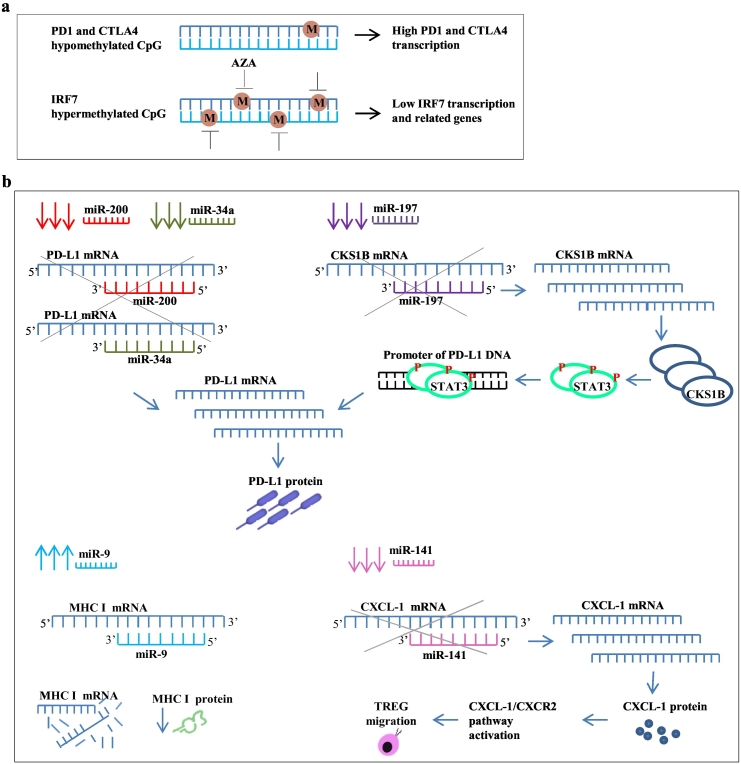
Global hypomethylation is frequent in NSCLC and is associated with genome instability [84]. CpG islands methylation is completed by different DNA methyltransferase whose expression is implicated in the pathogenesis of lung cancer. A large number of aberrantly methylated genes have also been identified in lung cancer [84], among which, the immune-checkpoint genes. Marwitz et al. analyzed the epigenetic modification of PDC1 (PD1), CD274 (PD-L1) and CTLA4 in NSCLC tissue from 39 patients [85]. Results were correlated with transcriptome data. Significant differences in the CpG-methylation patterns between tumor tissues and controls were observed for CTLA4 and PD1: NSCLC tumors exhibited a decreased level of CpG methylation in these loci compared to tumor-free tissues, while no differences for PD-L1 could be observed [85]. Hypermethylation may also have significance in the pathogenesis of lung cancer. Recent data suggest that the modulation of DNA methylation via methyl transferase inhibitors might triggers anti-tumor immune responses. Wrangle et al. studied the expression signatures of immune genes and pathways activated in NSCLC by Azacytidine (AZA), an hypomethylating agent [86]. They found that the interferon regulating factor 7 (IRF7), an upstream activator of genes involved in type 1 interferon signaling, hypermethylated in lung cancer, was up-regulated by AZA [86]. These data suggest that IRF7 silencing by DNA methylation could result in suppression of immune-regulatory genes important for the immunosurveillance involved in cytotoxic immune mechanisms against cancer (Figure 3A).
Figure 3.
Epigenetic regulation of lung cancer immune response. (a) Aberrant DNA methylation of immune genes in NSCLC: hypomethylation in PD1 and CTLA4 and hypermethylation in IRF7 loci leaded respectively to their decreased and increased transcription. The hypomethylating agent AZA, reverted IRF7 silencing, promoting the transcription of IRF7 target genes involved in type 1 interferon signaling. (b) MicroRNA regulation of immune genes in lung cancer. The expression of three different miRNA (miR-200, miR-34a and miR-197), affecting PD-L1 expression, was reduced in NSCLC cells. Low levels of miR-200 and miR-34a were directly associated to the increase of PD-L1 expression, because PD-L1 mRNA has complementary sequences to these miRNAs. By contrast miR-197 indirectly affected PD-L1 expression, because PD-L1 mRNA has no complementary sequence to this miRNA. Low levels of miR-197 correlated with the expression of CD28 kinase regulatory subunit 1 B. The latter phosphorylated STAT3 which bound PD-L1 promoter inducing its transcription. High levels of miR-9 in lung cancer cells correlated with the down regulation of MHC I, favoring tumor escape. Low levels of miR-141 in mice model of malignant pleural effusion, resulted in increased production of CXCL1 and Treg recruitment and migration. Abbreviations: PD1 = programmed death protein 1; CTLA4 = Cytotoxic T-lymphocyte-associate protein 4; IRF7 = interferon regulating factor 7; PD-L1 = programmed death protein ligand 1; CKS1B = CD28 protein kinase regulatory subunit 1 B; STAT3 = signal transducer and activator of transcription; CXCL1 = C-X-C motif chemokine ligand 1; CXCR2 = C-X-C motif chemokine receptor ligand 2.
Histone Modification in Lung Cancer and Immune Response
Histone epigenetic modifications play a crucial role in lung carcinogenesis. Histone deacetylases1 and 3 (HDAC1, HDAC3) gene expression appears to correlate with lung cancer progression and poor prognosis in adenocarcinoma patients [83]. Histone deacetylase inhibitors (HDACIs), in addition to their direct anti-cancer effects, strengthen the immune system, by up-regulating the expression of MHC class I/II proteins, and co-stimulatory/adhesion molecules such as CD80, CD86, human leukocyte antigen (HLA-DR, HLA-ABC) and intracellular adhesion molecule I (ICAM-1) [83]. HDACIs may also enhance the immune response by altering the activities of immune cells, directly or indirectly through the modulation of cytokine secretion [87]. HDACI-treated NSCLC cells down-regulated tumor necrosis factor receptor 1 (TNFR-1) mRNA and surface protein expression, and responded to TNF-treatment with attenuated NF-KB nuclear translocation and DNA binding [88].
MicroRNAs (miRNA) as Modulators of Lung Cancer Immune Response
MicroRNAs are a class of small noncoding RNA of 18 to 25 nucleotides that post-transcriptionally regulate gene expression. miRNA play important roles in the regulation of immune responses in cancer cells [89]. Many different miRNA have been reported to be abnormally expressed or deregulated in lung carcinoma and to have diagnostic and prognostic value as biomarkers [90], [91]. Some articles demonstrated a strictly association between PD-L1 expression and three different miRNAs: miR-200, miR-34 and miR-197 [92], [93], [94]. Chen et al. found that high PD-L1 expression in primary tumor cells was strongly associated with high epithelial-mesenchymal transition (EMT) score and could be determined by miR-200 expression [92]. These authors indicated that PD-L1tumor expression in NSCLC was regulated by miR-200/ZEB1 axis: miR-200 acted as a cell-autonomous suppressor of EMT and metastasis and directly targeted and inhibited PD-L1 [92]. Low expression of miR-200 was associated with increased PD-L1 expression and suppression of T CD8+ infiltration [92]. Thus the assessment of miR-200 expression may be useful for the therapy with anti-PD-1 or anti PD-L1 antibodies. Recently Cortez et al. found that miR-34 regulated PD-L1 expression binding the 3’UTRof PD-L1 mRNA [93]. They demonstrated that mutated TP53 NSCLC cell lines had significantly higher expression of PD-L1 and lower expression of mi-R34a, but no differences in the expression of miR34b and miR34ccompared to cancer cells wild type for TP53 [93]. Fujita et al. showed a negative correlation between miR-197 and PD-L1 expression: lower miR-197 expression was associated with shorter OS of NSCLC patients [94]. It was suggested that miR-197 is involved in chemo-resistance, tumorigenesis and metastasis. Indeed, miR-197 knockdown led to cisplatin and paclitaxel resistance in four lung cancer cell lines, while miR-197 overexpression induced cell sensitivity to these compounds [94]. Hence, miR-197 may be considered an important predictive immune marker in lung cancer. Interestingly, PD-L1 mRNA has no complementary sequence to miR-197 region. This miRNA regulates the CD28 protein kinase regulatory subunit 1B (CKS1B), and the authors hypothesized that CKSB modulated PD-L1 expression through the interaction with the signal transducer and activator of transcription (STAT3), which binds the promoter of PD-L1 gene [94](Figure 3B). However the possibility to use miRNA as predictive factors for immune checkpoint inhibitor based therapy, appears to be a distant future.
Another miRNA involved in the modulation of the immune response against lung cancer is miR-9 [95]. It is overexpressed in several malignancies, and down-regulates MHC class I, preventing the detection of cancer cells by the immune system [96].
Finally miR-141-CXCL1-CXCR2 signaling in malignant pleural effusion may be a potent factor implicated in NSCLC patient survival. It was demonstrated that CXCL1 is a miR-141target and that CXCL1-CXCR2 pathway regulate Treg cell migration into MPE. Furthermore miR-141 significantly inhibited tumor growth and metastasis in immune-competent mice model. This suppressive function was mediated by blocking CXCL1-CXCR2 pathway and Treg recruitment [97]. Hence, the decreased expression of miR-141 resulted in the increased production of CXCL1 and recruitment of Treg to promote tumor escape [97].
Immune Gene Signatures as Lung Cancer Immune Markers
Immune-related gene signatures have been seen having prognostic value of clinical outcome across multiple cancer types [98], [99], [100]. Showe et al. showed that peripheral blood mononuclear cell associated gene signatures can predict outcome in NSCLC patients, independent of demographic data or TNM staging [101]. The authors identified 23 survival-related genes specific to T cells. Eight of these, associated with better survival, included T-cell transcriptional activators and CTL response genes. The remaining 15 genes, among which genes for chemokine (CCNA2, CCNB2, CDK1, CDCA5 and UEBE2C), indicated poor survival and were significantly associated with adenocarcinoma [101]. Other authors studied the expression levels of similar genes for chemokines, chemokine receptors and interleukines (CXCL1, CXCL5, CXCL8, CXCL9, CXCL10, CXCL11, CXCR3, IL8, IL6, CXCL2, CXCL3, CXCL12 CXCR4, TNF, CHKA, AGFG1, CTC1) differentially expressed between tumor and non-tumor tissue and suggested to be relevant for early staged NSCLC patients' postoperative outcome [102]. Changes in gene expression were more pronounced in squamous carcinomas as compared to tumors of nonsquamous histology. However, only CXCL5 showed a significant prognostic effect: CXCL5upregulation in lung tumor was found to be a favorable independent prognostic factor for both OS and DFS [102].
Recently Chifman et al. identified 9 immune gene signatures able to predict and quantify the different immune infiltrates in a range of solid tumor types included lung cancer [103]. The first 3 signatures, classified as T/NK, included genes with conserved roles in T-cell receptor signaling such as TRAC, TRBC1, CD3D, CD3G, TRAT1, CD2, CD7, CD28, LCK and CD247, as well as genes activating CTLs, including CD8A, PRF1, CCL5, CXCL9, GZMB, GZMA, GZMH, GZMK, CTSW, IL2RB and CRTAM. One signature, termed B/P/T/NK included B cell signaling genes such as CD19, CD79A and CD180, and genes involved in lymphocyte differentiating and trafficking including IKZF1, CXCR3, IL16 and ITGB7. One signature, termed B/P was composed of immunoglobulin encoding genes such as IGKC, IGHD, IGLC1, IGLJ3, IGHA1, IGHM, IGJ and IGK. One signature, termed B/M/D was predominated by genes belonged to the MHC class II family (HLA-DRA, HLA-DRB1, HLA-DPA1, HLA-DPB1, HLADQB1, CD74) consistent with roles in professional antigen presentation. Two gene signatures, termed M/D/N, comprised genes involved in the activation and recruitment of effector lymphocytes (CD84, CD86, CCR1), regulation of immune responses (LILRB2, LILRB4, CD300A), macrophage differentiation and function (CSF1R, CCL2, CD14, CD163, CYBB, CLEC4A, CLEC7A) and myeloid IgG receptor signaling (FCER1G, FCGR1A, FCGR1B, FCGR2A, FCGR2B, FCGR3A, FCGR3B). Finally, one gene signature, termed D (Lipopolysaccharides, LPS), showed greatest enrichment in LPS-stimulated DCs and was composed of MHC class I family genes (HLA-B, HLA-C, HLA-G, HLA-J) and a large number of genes with direct roles in interferon signaling (IRF7, IRF9, STAT1, ISG15, OAS1, OAS2, OAS3, IFI35, IFI44, IFI6, IFIH1, IFIT3, IFIT5, HERC5, HERC6, DDX58, DDX60). These markers of immune involvement were significantly associated with patient prognosis.
Conclusions
The microenvironment of lung cancer has a strong prognostic value. The analysis of the immune contexture of this tumor revealed a set of cellular and molecular immune markers which could effectively and reproducibly classify patients according to their survival. Immune markers may be combined with the standard pathological TNM classification to form a TNM-Immunoscore for lung cancer. Further studies investigating larger cohorts of patients, uniform in histology, stage, methodologies, and assessing tumor-immune system interactions are warranted to fully assess the prognostic/predictive power of these markers.
Acknowledgments
Acknowledgements
The authors thank Pauline Maselli Campagna for language revision.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Remark R, Becker C, Gomez JE, Damotte D, Dieu-Nosjean MC, Sautès-Fridman C. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 5.Rodero MP, Poupel L, Loyher PL, Hamon P, Licata F, Pessel C. Immune surveillance of the lung by migrating tissue monocytes. eLife. 2015;4 doi: 10.7554/eLife.07847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenhals JE, Seyedin SN, Anderson C, Brooks ED, Li YR, Younes AI. Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation. Transl Lung Cancer Res. 2017;6:148–158. doi: 10.21037/tlcr.2017.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 8.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 9.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 10.Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A. Immune contexture and histological response after neoadjuvant chemotherapy. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 12.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 15.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T. Predominant infiltration of macrophages and CD8 (+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55:301–312. doi: 10.1111/j.1365-2559.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 17.Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Vaitkiene S. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018;19:3. doi: 10.1186/s12865-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193:116–130. doi: 10.1164/rccm.201508-1545CI. [DOI] [PubMed] [Google Scholar]
- 19.Singhal S, Bhojnagarwala PS, O'Brien S, Moon EK, Garfall AL, Rao AS. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12:7. doi: 10.1186/1479-5876-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coëlle C, Mouroux J. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectablenon small cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 25.Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81:130–137. doi: 10.1016/j.lungcan.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Rakaee M, Busund LT, Paulsen EE, Richardsen E, Al-Saad S, Andersen S. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget. 2016;7:72184–72196. doi: 10.18632/oncotarget.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eruslanov EB. Phenotype and function of tumor-associated neutrophils and their subsets in early-stage human lung cancer. Cancer Immunol Immunother. 2017;66:997–1006. doi: 10.1007/s00262-017-1976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyfferoen L, Brabants E, Everaert C, De Cabooter N, Heyns K, Deswarte K. The transcriptome of lung tumor-infiltrating dendritic cells reveals a tumor-supporting phenotype and a microRNA signature with negative impact on clinical outcome. Oncoimmunology. 2016;6 doi: 10.1080/2162402X.2016.1253655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pachéco Y. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 33.Kayser G, Schulte-Uentrop L, Sienel W, Werner M, Fisch P, Passlick B. Stromal CD4/CD25 positive T-cells are a strong and independent prognostic factor in non-small cell lung cancer patients, especially with adenocarcinomas. Lung Cancer. 2012;76:445–451. doi: 10.1016/j.lungcan.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita T, Ishii G, Hiraoka N, Hirayama S, Yamauchi C, Aokage K. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci. 2013;104:409–415. doi: 10.1111/cas.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: a meta-analysis. Cell Physiol Biochem. 2015;37:1560–1571. doi: 10.1159/000438523. [DOI] [PubMed] [Google Scholar]
- 37.Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ, Xie CM. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget. 2016;7:13765–13781. doi: 10.18632/oncotarget.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3) doi: 10.1093/jnci/dju435. [pii:dju435] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non–small cell lung cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 40.Donnem T, Kilvaer TK, Andersen S, Richardsen E, Paulsen EE, Hald SM. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol. 2016;27:225–232. doi: 10.1093/annonc/mdv560. [DOI] [PubMed] [Google Scholar]
- 41.Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol. 2016;34:1223–1230. doi: 10.1200/JCO.2015.63.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obeid JM, Wages NA, Hu Y, Deacon DH, Slingluff CL., Jr. Heterogeneity of CD8+ tumor-infiltrating lymphocytes in non-small-cell lung cancer: impact on patient prognostic assessments and comparison of quantification by different sampling strategies. Cancer ImmunolImmunother. 2017;66:33–43. doi: 10.1007/s00262-016-1908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domagala-Kulawik J, Kwiecien I, Pankowski J, Pasieka-Lis M, Wolosz D, Zielinski M. Elevated Foxp3/CD8 ratio in lung adenocarcinoma metastatic lymph nodes resected by transcervical extended mediastinal lymphadenectomy. Biomed Res Int. 2017;2017:5185034. doi: 10.1155/2017/5185034. [Epub 2017 Aug 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.TalebianYazdi M, van Riet S, van Schadewijk A, Fiocco M, van Hall T, Taube C. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget. 2016;7:3477–3488. doi: 10.18632/oncotarget.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulsen EE, Kilvaer T, Khanehkenari MR, Maurseth RJ, Al-Saad S, Hald SM. CD45RO(+) Memory T lymphocytes—a candidate marker for TNM-immunoscore in squamous non-small cell lung cancer. Neoplasia. 2015;17:839–848. doi: 10.1016/j.neo.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 47.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 48.Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai S. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524–530. doi: 10.6061/clinics/2015(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer? Oncotarget. 2017;8:37200–37207. doi: 10.18632/oncotarget.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.AkinciOzyurek B, SahinOzdemirel T, BuyukyaylaciOzden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18:1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang WJ, Tao Z, Gu W, Sun LH. Variation of blood T lymphocyte subgroups in patients with non- small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:4671–4673. doi: 10.7314/apjcp.2013.14.8.4671. [DOI] [PubMed] [Google Scholar]
- 52.Al Omar SY, Marshall E, Middleton D, Christmas SE. Increased killer immunoglobulin-like receptor expression and functional defects in natural killer cells in lung cancer. Immunology. 2011;133:94–104. doi: 10.1111/j.1365-2567.2011.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Fend L, Rusakiewicz S, Adam J, Bastien B, Caignard A, Messaoudene M. Prognostic impact of the expression of NCR1 and NCR3 NK cell receptors and PD-L1 on advanced non-small cell lung cancer. Oncoimmunology. 2016;6 doi: 10.1080/2162402X.2016.1163456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osińska I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, Dziedzic D, Domagała-Kulawik J. CD4+/CD25(high)/FoxP3+/CD127- regulatory T cells in bronchoalveolar lavage fluid of lung cancer patients. Hum Immunol. 2016;77:912–915. doi: 10.1016/j.humimm.2016.07.235. [DOI] [PubMed] [Google Scholar]
- 56.Domagala-Kulawik J, Raniszewska A. How to evaluate the immune status of lung cancer patients before immunotherapy. Breathe (Sheff) 2017;13:291–296. doi: 10.1183/20734735.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim C, Savan R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014;25:257–271. doi: 10.1016/j.cytogfr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Naumnik W, Naumnik B, Niklińska W, Ossolińska M, Chyczewska E. Clinical implications of hepatocyte growth factor, interleukin-20, and interleukin-22 in serum and bronchoalveolar fluid of patients with non-small cell lung cancer. Adv Exp Med Biol. 2016;952:41–49. doi: 10.1007/5584_2016_66. [DOI] [PubMed] [Google Scholar]
- 59.Lin Q, Xue L, Tian T, Zhang B, Guo L, Lin G. Prognostic value of serum IL-17 and VEGF levels in small cell lung cancer. Int J Biol Markers. 2015;30:e359–363. doi: 10.5301/jbm.5000148. [DOI] [PubMed] [Google Scholar]
- 60.Ryan BM, Pine SR, Chaturvedi AK, Caporaso N, Harris CC. A combined prognostic serum interleukin-8 and interleukin-6 classifier for stage 1 lung cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J Thorac Oncol. 2014;9:1494–1503. doi: 10.1097/JTO.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meaney CL, Zingone A, Brown D, Yu Y, Cao L, Ryan BM. Identification of serum inflammatory markers as classifiers of lung cancer mortality for stage I adenocarcinoma. Oncotarget. 2017;8:40946–40957. doi: 10.18632/oncotarget.16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rivas-Fuentes S, Salgado-Aguayo A, PertuzBelloso S, GorocicaRosete P, Alvarado-Vásquez N, Aquino-Jarquin G. Role of chemokines in non-small cell lung cancer: angiogenesis and inflammation. J Cancer. 2015;6:938–952. doi: 10.7150/jca.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soria JC, Marabelle A, Brahmer JR, Gettinger S. Immune checkpoint modulation for non-small cell lung cancer. Clin Cancer Res. 2015;21:2256–2262. doi: 10.1158/1078-0432.CCR-14-2959. [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11:964–975. doi: 10.1016/j.jtho.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brody R, Zhang Y, Ballas M, Siddiqui MK, Gupta P, Barker C. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. 2017;112:200–215. doi: 10.1016/j.lungcan.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Paulsen EE, Kilvaer TK, Khanehkenari MR, Al-Saad S, Hald SM, Andersen S. Assessing PDL-1 and PD-1 in non-small cell lung cancer: a novel immunoscore approach. Clin Lung Cancer. 2017;18:220–233.e8. doi: 10.1016/j.cllc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Mino-Kenudson M. Immunohistochemistry for predictive biomarkers in non-small cell lung cancer. Transl Lung Cancer Res. 2017;6:570–587. doi: 10.21037/tlcr.2017.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shtivelman E, Hensing T, Simon GR, Dennis PA, Otterson GA, Bueno R. Oncotarget. 2014;5:1392–1433. doi: 10.18632/oncotarget.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res. 2015;4:177–190. doi: 10.3978/j.issn.2218-6751.2015.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Usó M, Jantus-Lewintre E, Calabuig-Fariñas S, Blasco A, García Del Olmo E, Guijarro R. Analysis of the prognostic role of an immune checkpoint score in resected non-small cell lung cancer patients. Oncoimmunology. 2016;6 doi: 10.1080/2162402X.2016.1260214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1463–1472. doi: 10.1007/s00262-012-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paulsen EE, Kilvaer TK, Rakaee M, Richardsen E, Hald SM, Andersen S. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol Immunother. 2017;66:1449–1461. doi: 10.1007/s00262-017-2039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villalobs P, Ignacio I, Wistuba Lung Cancer Biomarkers. Hematol Oncol Clin N Am. 2017;31:13–29. doi: 10.1016/j.hoc.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reclusa P, Taverna S, Pucci M, Durendez E, Calabuig S, Manca P. Exosomes as diagnostic and predictive biomarkers in lung cancer. J Thorac Dis. 2017;9:S1373–S1382. doi: 10.21037/jtd.2017.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest. 2013;31:330–335. doi: 10.3109/07357907.2013.789905. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Li J, Yu J, Zhang H, Wang B, Guo H, Bai J. Exosomes-derived MiR-302b suppresses lung cancer cell proliferation and migration via TGFβRII inhibition. Cell Physiol Biochem. 2016;38:1715–1726. doi: 10.1159/000443111. [DOI] [PubMed] [Google Scholar]
- 81.Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Extracellular vesicles: new players in lung immunity. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2017-0293TR. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 82.Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ansari J, Shackelford RE, El-Osta H. Epigenetics in non-small cell lung cancer: from basics to therapeutics. Transl Lung Cancer Res. 2016;5:155–171. doi: 10.21037/tlcr.2016.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balgkouranidou I, Liloglou T, Lianidou ES. Lung cancer epigenetics: emerging biomarkers. Biomark Med. 2013;7:49–58. doi: 10.2217/bmm.12.111. [DOI] [PubMed] [Google Scholar]
- 85.Marwitz S, Scheufele S, Perner S, Reck M, Ammerpohl O, Goldmann T. Epigenetic modifications of the immune-checkpoint genes CTLA4 and PDCD1 in non-small cell lung cancer results in increased expression. Clin Epigenetics. 2017;9:51. doi: 10.1186/s13148-017-0354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyanaga A, Gemma A, Noro R, Kataoka K, Matsuda K, Nara M. Antitumor activity of histone deacetylase inhibitors in non-small cell lung cancer cells: development of a molecular predictive model. Mol Cancer Ther. 2008;7:1923–1930. doi: 10.1158/1535-7163.MCT-07-2140. [DOI] [PubMed] [Google Scholar]
- 88.Imre G, Gekeler V, Leja A, Beckers T, Boehm M. Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappaB by tumor necrosis factor-alpha receptor-1 down-regulation. Cancer Res. 2006;66:5409–5418. doi: 10.1158/0008-5472.CAN-05-4225. [DOI] [PubMed] [Google Scholar]
- 89.Inamura K. Diagnostic and therapeutic potential of MicroRNAs in lung cancer. Cancers (Basel) 2017;9(5) doi: 10.3390/cancers9050049. [pii: E49] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 91.Fan F, Zhu Z, Gao C, Liu Y, Wang B, Wang Z. Prognostic value of lncRNAs in lung carcinoma: a meta-analysis. Oncotarget. 2017;8:83292–83305. doi: 10.18632/oncotarget.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv303. [pii: djv303] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujita Y, Yagishita S, Hagiwara K, Yoshioka Y, Kosaka N, Takeshita F. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther. 2015;23:717–727. doi: 10.1038/mt.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu G, Shao G, Pan Q, Sun L, Zheng D, Li M. MicroRNA-9 regulates non-small cell lung cancer cell invasion and migration by targeting eukaryotic translation initiation factor 5A2. Am J Transl Res. 2017;9:478–488. [eCollection 2017] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao F, Zhao ZL, Zhao WT, Fan QR, Wang SC, Li J. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;431:610–616. doi: 10.1016/j.bbrc.2012.12.097. [DOI] [PubMed] [Google Scholar]
- 97.Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y. miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther. 2014;13:3152–3162. doi: 10.1158/1535-7163.MCT-14-0448. [DOI] [PubMed] [Google Scholar]
- 98.Bedognetti D, Hendrickx W, Marincola FM, Miller LD. Prognostic and predictive immune gene signatures in breast cancer. Curr Opin Oncol. 2015;27:433–444. doi: 10.1097/CCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Lu Z, Che Y, Wang J, Sun S, Huang J. Immune signature profiling identified predictive and prognostic factors for esophageal squamous cell carcinoma. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1356147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.An N, Shi X, Zhang Y, Lv N, Feng L, Di X. Discovery of a novel immune gene signature with profound prognostic value in colorectal cancer: a model of cooperativity disorientation created in the process from development to cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Showe MK, Kossenkov AV, Showe LC. The peripheral immune response and lung cancer prognosis. Oncoimmunology. 2012;1:1414–1416. doi: 10.4161/onci.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kowalczuk O, Burzykowski T, Niklinska WE, Kozlowski M, Chyczewski L, Niklinski J. CXCL5 as a potential novel prognostic factor in early stage non-small cell lung cancer: results of a study of expression levels of 23 genes. Tumour Biol. 2014;35:4619–4628. doi: 10.1007/s13277-014-1605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chifman J, Pullikuth A, Chou JW, Bedognetti D, Miller LD. Conservation of immune gene signatures in solid tumors and prognostic implications. BMC Cancer. 2016;16:911. doi: 10.1186/s12885-016-2948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]