Abstract
Background
Bicycle and treadmill exercise tests are used in sports medicine and occupational medicine to detect latent disease, to monitor treatment, and to measure patients’ physical performance ability and reserve. In this review, we describe the indications, contraindications, and manner of performance of these tests, along with the variables tested, criteria for evaluation, (sub)maximal stress, and the factors that affect these tests, including age, sex, and medications.
Methods
This review is based on pertinent articles retrieved by a selective literature search and on the ergometry guidelines of four medical specialty societies.
Results
The proper performance of ergometric stress tests calls for preparation and monitoring by qualified staff as well as standardized testing conditions. Ergometric studies are indispensable as a clinical diagnostic method for the early recognition of disease, for follow-up over time, and for individual counseling. The patient’s maximal achievable performance is a predictor of morbidity and mortality. Among the variables that can be measured in the submaximal performance range, the respiratory rate, heart rate, and lactate performance curves are more accurate prognostic predictors than the so-called threshold values (physical working capacity, anaerobic-aerobic threshold). Ergometric stress tests can be used to detect (among other conditions) latent hypertension, pulmonary diseases (e.g., exertional asthma), pabnormal ECG changes, and cardiovascular disorders (e.g., ischemia, arrhythmia, congestive heart failure). The ergometric findings are influenced by the choice of stress-inducing protocol. They provide important information for the planning and monitoring of exercise training and for the treatment of persons suffering from diverse physical conditions, as well as for leisure-time athletes. They are less suitable for use in the design of training programs for high-performance athletes.
Conclusion
Ergometric stress tests provide important data in clinical and preventive medicine. The findings are often difficult to interpret because of the wide range of normal findings, the use of different stress-inducing protocols, and the lack of generally accepted reference values. The establishment of a nationwide fitness and health registry for ergometric data would be very helpful for the individualized interpretation of test findings and for the monitoring of exercise training and therapy.
Ergometric stress testing is indicated to answer relevant questions for athletes, asymptomatic persons, and patients with a wide range of diseases (box 1). Its most important use is to detect latent disease (health-related indication for testing). It can also be used to measure physical performance ability and reserve and to assess the prognosis of patients with various diseases, as well as to monitor and guide exercise training and the training of athletes. When used in ill patients, it is helpful for evaluating treatment, monitoring interventions, and and assessing the prognosis (1 [p. 51], 2 [p. 60], 3, 4 [p. 268]). Very different groups of persons can benefit from ergometric stress testing, ranging from professional athletes and ambitious leisure-time athletes to untrained persons and ill patients (1 [p. 51], 2 [p. 60], 5, 6, 7 [p. 10]). Further diagnostic objectives are listed in Box 1.
BOX 1. Indications for ergometry in sports medicine*1 .
-
Asymptomatic persons
Diagnosis of latent disease and of possible risks in sport
Assessment of physical performance ability and counseling before the start of training; monitoring and guidance of training
Assessment of performance capacity and physical performance ability in occupational medicine
-
Patients
Diagnosis of cardiovascular and pulmonary diseases, before the start of training if applicable
Evaluation of symptoms: dyspnea, chest pain, palpitations, dizziness (syncope)
-
Follow-up assessment during training (for patients as well)
Recommendations on the extent and intensity of training (FITT rules*2)
Diagnostic objectives
In accordance with the above, the diagnostic objectives are the assessment of performance, development, suitability, and structure. Stress is measured by external parameters and effort by “internal” ones as a response of the subject’s bodily organs to the task.
*1 from (1 [pp.173, 355, 401], 2, 4, 9, 20)
*2 FITT: frequency of the training stage, intensity of training, type of training, time of the training unit; from Pescatello et al. (2)
The most common standard methods of ergometric testing are bicycle ergometry (in Europe) and treadmill ergometry (in the USA) (1 [p. 21], 2 [p. 162], 4 [p. 238], 8–11). Other testing procedures, such as rowing ergometry, the field step test, rotational ergometry, supine bicycle ergometry, step-climbing, a six-minute walking test, and strength tests are used to measure performance in sport-specific testing and to answer specific clinical questions (4 [p. 238], 12 [p. 519], 13–17). These will not be described in any greater detail in this review.
Method
In this review, we present the indications and contraindications of ergometric stress testing, the manner in which these tests are performed, the variables they measure and their informative value, the criteria for evaluating submaximal and maximal performance, and factors that influence the outcome of the tests (e.g., age, medications, illnesses). This review is based on the current ergometry guidelines of the relevant medical specialty societies (9, 11– 13, 18– 20), the literature cited in these guidelines, and a selective literature search in the PubMed/MEDLINE, Cochrane Library, Embase, Scopus, and Web of Science databases (search terms: “ergometry,” “exercise testing,” “functional capacity,” “fitness,” “stress testing”).
Indications
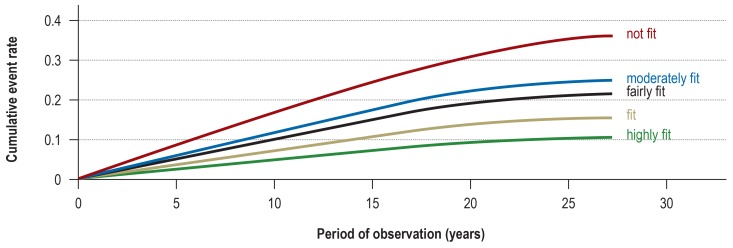
In sports medicine, ergometry is used primarily to measure fitness (“physical capacity”) and endurance, in order to monitor the effects of exercise training in healthy individuals or the effects of treatment (rehabilitation, other measures) in persons with various diseases (box 1). Further indications include the differential diagnostic evaluation of diseases (heart, lungs) and symptoms, e.g., chest pain or shortness of breath (1 p. 173], 7, 13 [p. 329], 21, 22). The measurement of physical performance for prognostic purposes has become much more common in recent years. Physical fitness (figure 1) is an indicator of future cardiovascular, metabolic, or even psychiatric events, as well as a means of lowering risk (1 [p. 355, 401], 2, 3, 5, 10, 15, 23, e1–e5). This is true not only for the standard tests (bicycle and treadmill ergometry) but also for grasp strength (e6– e9). In a longitudinal study published in 2015, with more than 140 000 participants, grasp strength was a better predictor of morbidity and mortality than systolic blood pressure (e6).
Figure 1.
The probability of a serious cardiovascular event as a function of physical fitness (fitness categories based on the maximal performance attained on the ergometric treadmill; N = 20 590 subjects; figure modified from Kokkinos et al. 2017 [23])
The performance of ergometry
History-taking, physical examination, and rest electrocardiography (ECG) are mandatory prerequisites before the patient is put under any physical stress to detect risks due to latent or overt disease, such as coronary heart disease or hypertension. Standardized conditions must be maintained in the testing room (ambient temperature, humidity, etc.) (ebox 1) (1 [p. 40], 4 [p. 250], 11, 13 [p. 14], 15). Continuous heart rate, blood pressure, and ECG monitoring and observation of the patient are mandatory so that the test can be terminated at once in case any complications arise. The response to emergency situations should be practiced at regular intervals with the nonphysician members of the testing team (ebox 1).
eBOX 1. Requirements for ergometric testing*.
Preliminary examination
history incl. current medications, physical examination incl. blood pressure at rest, ECG at rest
Test conditions
room temperature 16–24°C, humidity 30–60%
calibrated ergometer (certificate of conformity), control monitor, continuous digital ECG recording and storage
60–80 rpm, may be higher at maximal performance
blood pressure measurement every 1–2 minutes
telephone number of emergency response team in view
Subject
normal body temperature
at least 2 hours since last meal
at least 12 hours since last alcohol consumption or tobacco use
medications: adequate pause since last intake, if indicated
Examiners
presence of an experienced physician (up-to-date knowledge of resuscitation techniques)
appropriately trained personnel (trained in ECG monitoring, symptoms that may arise, first-aid measures, and cardiopulmonary resuscitation in case of an emergency)
Emergency equipment is mandatory, a defibrillator must be on hand, the staff must be trained in what to do should an emergency arise, and a physician should be with the subject or patient at all times (permissibly in the next room if the subject is healthy, but in any case able to be at their side within one minute).
* after (1 [p. 40], 4 [p. 259], 12, 13 [p. 14])
Remarks on testing methods
Bicycle ergometry is used in Europe in preference to treadmill ergometry. Its main advantage is that it less commonly causes artifacts in the stress ECG; treadmill ergometry can also make patients feel insecure or anxious because of the moving surface (4, 7). On the other hand, bicycle ergometry activates a smaller amount of muscle mass than treadmill ergometry and thus generates a maximal performance level that is ca. 10% lower than what is achievable on a treadmill (12 [p. 619], 24 [p. 273]). In supine bicycle ergometry, exhaustion in reached earlier, and the maximal achievable performance is therefore ca. 20% lower.
Standardized stress protocols should be used in order to ensure the validity of inter- and intra-individual comparisons. Different stress protocols are used depending on the category of patient tested and the questions that are to be answered (eTable 1); these protocols vary widely with respect to the initial performance level, the rate increase of performance demanded over time, and the duration of the entire test (1 [p. 47], 9, 25 [p. 155], 26–31). Ramped performance increases and increases in small steps at brief intervals have the advantage of enabling a relatively rapid increase of cardiocirculatory performance. Stress protocols of this type (e.g., an increase by 25 W every 2 minutes, eFigure 1) are suitable, for example, for use in adolescents, or to answer questions of a cardiological nature. The oxygen uptake and the serum lactate concentration respond more slowly to the level of physical performance; thus, measurements of these variables cannot always be reliably attributed to the current performance level (32).
eTable 1. Recommendations for bicycle ergometric testing protocols*1.
| Application | Initial performance level | Increment / step duration |
| Routine ergometry in sports medicine (Lactate) performance evaluation, stress ECG |
40 W | 40 W/4 min |
| As above in routine ergometry in sports medicine; also German Olympic Sports Confederation, Olympic Training Centers |
50 W*2 | 50 W/3 min (25 W steps in near-peak performance range) |
| Routine in the inpatient setting, stress ECG, right-heart catheterization, nuclear medicine |
50 W | 25 W/2 min |
| Pulmonology (including blood gases, spiroergometry, right-heart catheterization) |
(25) 50 W | 25 W/4 or 5 min |
| Pediatrics | 1 W/kg body weight | 0.5 W/kg/3 min |
|
5-minute break before the start of the test (“resting” values from the last 3 min); 6-to-10-minute recovery phase after the end of the test (“recovery” values) | ||
*1 after (1 [pp. 8, 21ff.],18, 33 [p.117])
*2 In high-performmance athletes, the initial performance level can also be set at = 200 W (e.g., bicycle racers, rowers).
eFigure 2.
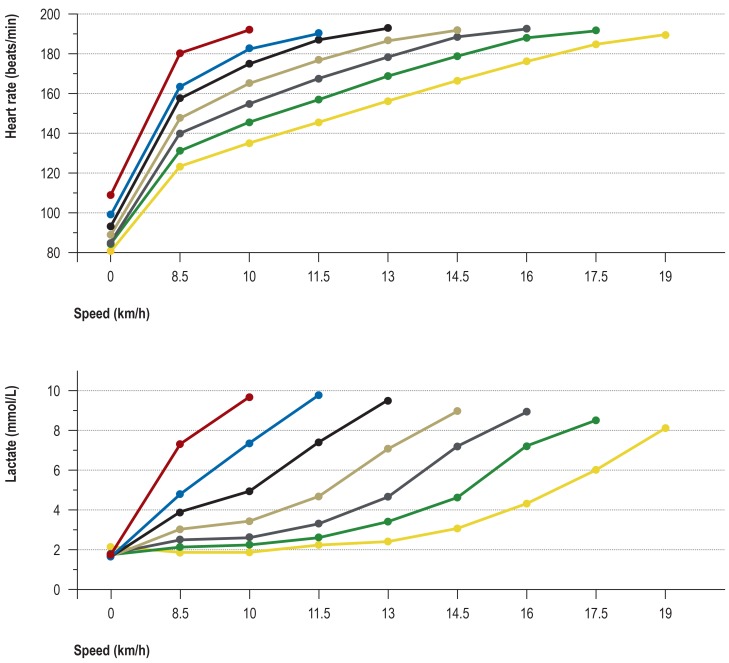
The course of heart rate and lactate concentration in healthy 18- tos 35-year-olds undergoing treadmill ergometry (performance steps of 4 minutes’ duration, with 30-second breaks in between). The figure shows the means obtained in different performance groups whose maximal treadmill speed ranged from 10 to 19 km/h (n = 385). The data were acquired in the setting of diagnostic studies of physical performance carried out in the Institute for Preventive Medicine of the German Federal Armed Forces.
Performance increases by 40 W or 1.5 km/h every 3 to 5 minutes are often used for the assessment of both the cardiocirculatory and the metabolic component of performance (8, 29, 33) (Figure 2, eFigure 2, eTable 1). If the subject is a high-performance athlete (e.g., a professional cyclist or rower), the test can begin at a higher performance level, e.g., 200–300 W, and maximal performance levels above 500 W are not unusual. At such high performance intensities, blood pressure values of up to 300 mmHg can be regarded as normal (4 [p. 248], 22, 24 [p. 31, 479], 34).
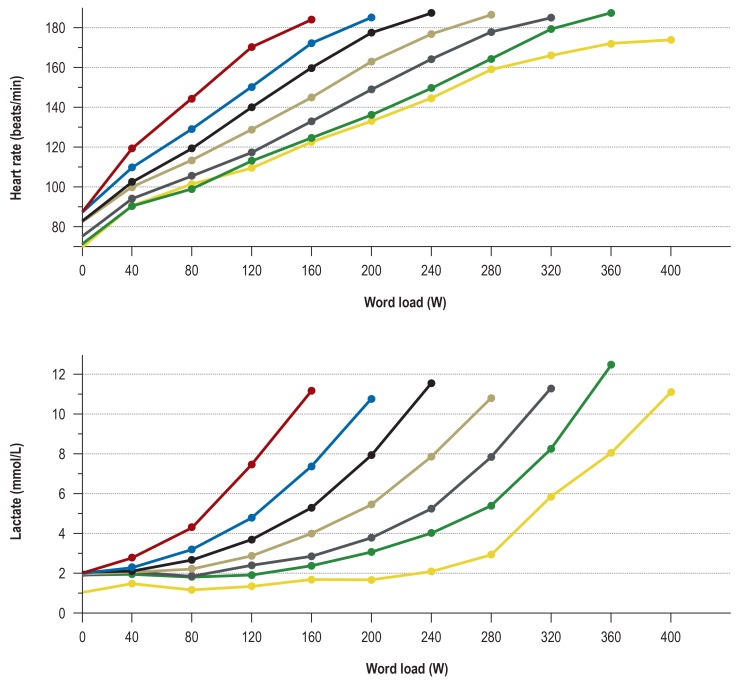
Figure 2.
The course of heart rate and lactate concentration in healthy 18- to 35-year-olds undergoing bicycle ergometry (with 4-minute performance steps). The mean values of different performance groups with maximal performance ranging from 160 to 400 W are shown (n = 334). These data were acquired in the setting of diagnostic studies of physical performance carried out at the Institute for Preventive Medicine of the German Federal Armed Forces. The minimum and maximum values and the 5th, 25th, 50th, and 75th percentiles for each level of performance are listed in eTable 3a (heart rate) and eTable 4a (lactate concentration).
As soon as the stress test is over, all subjects should be observed for at least a further 6 minutes, as complications such as arrhythmias or a drop in blood pressure tend to occur during this time (4, 20). Staying in motion at a low intensity (0–50 W) helps prevent excessively rapid blood pressure drops (1 [p. 51], 4).
Ergometric stress testing is contraindicated in persons suffering from
any acute or severe chronic cardiorespiratory disease causing marked functional impairment (e.g., severe congestive heart failure, high-grade congenital heart defects, cardiomyopathy, severe arrhythmias, thromboses, malignant hypertension, or pulmonary hypertension), or
any acute or severe disease of other organ systems, e.g., nephritis, poorly controlled diabetes mellitus, or electrolyte disturbances (1 [p. 51], 11, 26).
The criteria for premature termination of ergometric stress testing (box 2) are based, among other things, on the experience of the physician conducting the test, as well as on the availability of appropriate emergency measures, such as hemodynamic stabilization, treatment of arrhythmias, cardiopulmonary resuscitation, and defibrillation, in case a severe complication should arise. It follows that ergometric testing in a physician’s outpatient office may be contraindicated for a patient who can, in fact, undergo it in an appropriately equipped testing facility, even in the presence of severe disease, e.g., aortic valvular stenosis. The risk of a life-threatening event or death in a healthy person or athlete undergoing ergometric stress testing has fallen markedly in recent decades to its current level of 0–0.5 cases per 10 000 tests (1 [p. 56], 4 [p. 248], 13). In an older study, 17 fatalities (mainly with supine bicycle testing) and 96 nonfatal complications were reported among 712 285 tested patients with coronary heart disease (e10).
BOX 2. Criteria for the termination of ergometric testing*.
Symptoms:
dizziness, incoordination, progressive chest pain or shortness of breath
Signs:
-
ECG changes
-
progressively severe arrhythmias
couplets, salvoes, ventricular tachycardia, increasing supraventricular extrasystoles, atrial tachycardias, atrial flutter, new-onset atrial fibrillation
-
progressive intracardiac conduction disturbance
increasing QRS widening
appearance of a left bundle branch block
-
progressive repolarization disorder
ST depression:
horizontal depression greater than 0.2 mV
progressive ST elevation iin the electrocardiogram (ECG) (>0.1 mV)
monophasic deformation in the ECG as ST elevation (obligatory termination of test)
-
-
- hemodynamic changes
progressive drop in blood pressure
insufficient rise in blood pressure (less than 10 mmHg for each level of performance)
excessive rise in blood pressure (ca. 250 mmHg at low or intermediate performance intensity)
Note: A number of current guidelines and standard texts no longer distinguish between relative and absolute contraindications (see above) or criteria for test termination, but refer to progressive changes instead.
* after (1 [p. 56], 4 [p. 248],18, e30)
Variables measured and the interpretation of findings
The main objective of ergometric stress testing, aside from the diagnostic evaluation of clinical manifestations (dyspnea, dizziness, exhaustion, pain, etc.), is the measurement of parameters relating to exercise physiology for the assessment of stress and performance capacity. The main parameters to be measured and monitored include the performance achieved (in watts or km/h), heart rate (HR), ECG, arterial blood pressure (BP), lactate concentration (Lac), and the spiro-ergometric parameters oxygen uptake (VO2), carbon dioxide release (VCO2), respiratory quotient (RQ = VO2/VCO2), respiratory rate (RR), respiratory minute volume (V?E), and respiratory equivalent (RE = V?E/VO2).
The subjective perception of exertion is assessed on the Borg scale (1 [p. 71], 35), ranging from 6 (very, very easy) to 20 (maximal stress). Borg chose this range of values to reflect the heart rate of healthy individuals doing physical work: the Borg score multiplied by 10 can be used to compare the subjective perception of exertion with the heart rate during the work in question. Substantial differences, if present, may reflect the subject’s over- or underestimation of his or her own reserves; they may also be seen, for example, in patients taking beta-blockers. For special questions there are also shorter Borg scales, the so-called CR10 scales (35, e11).
The interpretation of findings: general considerations
The interpretation of the findings requires specialized knowledge and experience, particularly in view of the wide range of normal physiologic values of the measured variables (both within and across individuals). For example, the literature indicates individual ranges of 7–15% for heart rate and arterial blood pressure under stress (22 [p. 161], 36, 37). There are no generally accepted reference values: many studies of highly variable methodological quality are available for each of the measured variables (1 [p. 62], 10, 22 [p. 161], 28, 34).
This variability in the quality of studies is one of the reasons why multiple meta-analyses have not yielded any definitive reference values with corresponding evidence levels (10, 26, 28). Moreover, the results of measurement are heavily influenced by the particular stress-inducing protocol used. For example, the maximal performance attainable in a protocol with stress increments at 2-minute intervals is, understandably, higher than that attainable in an otherwise identical protocol with 3-minute intervals (4 [p. 240], 7, 33). In the submaximal range as well, different settings in different stress protocols (e.g., with respect to HR, VO2, and Lac) can lead to significant differences in the values obtained (4 [p. 270], 32, 33).
Criteria for peak performance
The main criteria for peak performance are the maximal achieved performance, maximal heart rate (HRmax), maximal lactate concentration (Lacmax), maximal oxygen uptake (VO2max), and other spiroergometric parameters (1 [p. 147], 11, 13) (table). The peak performance criteria are age-dependent and must be set lower for persons over 65 (38). The values given in the Table are reference values that are to be exceeded when the individual achieves peak performance; in other words, only when at least one of the measured values exceeds the corresponding value in the Table can peak performance be said to have been reached. Healthy subjects who have attained one of these reference values can and should stress themselves further up to the point of exhaustion.
Table. Criteria for peak word load—minimal values for adults*1.
| Performance (W) | >75% of norm*2 |
| Heart rate | >208 − 0.7 × age (years), bicycle ergometry >209.3 − 0.72 × age 8 (years), treadmill ergometry |
| Perceived effort | >17 (Borg scale) |
| Blood gases and lactate | |
| pH | <7.25 |
| Base excess | <9 mmol/l (normal subjects), <5 mmol/l (patients) |
| Lactate | >9 mmol/L (normal subjects), >5 mmol/L (patients) |
| Spiroergometry | |
| VO2max | >35 mL/kg/min (men), > 30 mL/kg/min (women) |
| Respiratory quotient | >1.15 |
| Respiratory equivalent | >35 |
Peak peformance is said to have been achieved only when at least one of these values has been exceeded.Ergometric stress testing should not be terminated when one of these values has been reached; the person undergoing testing should continue working up to the individual point of exhaustion. Ergometric testing should, however, be terminated if pathological changes arise (Box 2).
*1 after: (4, [pp. 268–9], 22 [pp. 168–80], 33 [p. 133], 38, e18 [pp. 123–7]); Values for bicycle ergometry from (e15), for treadmill ergometry from Arena et al. 2016 (28)
*2 See eTable 2 norms for maximal performance (W) with increasing performance levels.
The maximal achieved performance is more than just an important indicative value for diagnostic evaluation during exercise training (etable 2). It is now considered to be a good predictor of the severity of illness and a prognostic criterion for patients with cardiopulmonary disease, and it is used in preventive interventions (figure 1) (1 [p. 335, 401], 3, 15, 23, 39). In the context of medical evaluations of physical performance, a distinction must be made between maximal performance and performance capacity. The latter is the highest performance that can be achieved without any pathological changes such as ST elevation or cardiac arrhythmia (1 [p. 243], 4 [p. 279]).
eTable 2. Norms for maximal work load (W) with increasing performance level, by age, sex, and body weight (after [e31]).
| Men | Age (years) | ||||||||
| 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | |
| Weight (kg) | W | ||||||||
| 60–65 | 220 | 210 | 200 | 185 | 175 | 170 | 155 | 150 | 135 |
| 66–69 | 225 | 215 | 205 | 195 | 180 | 175 | 160 | 155 | 140 |
| 70–73 | 230 | 220 | 210 | 200 | 190 | 180 | 165 | 160 | 145 |
| 74–77 | 235 | 225 | 215 | 205 | 195 | 185 | 170 | 165 | 150 |
| 78–81 | 240 | 230 | 220 | 210 | 200 | 190 | 180 | 170 | 155 |
| 82–85 | 245 | 235 | 225 | 215 | 205 | 195 | 185 | 175 | 160 |
| 86–89 | 250 | 240 | 230 | 220 | 210 | 200 | 190 | 180 | 170 |
| 90–93 | 255 | 245 | 235 | 225 | 215 | 205 | 195 | 185 | 175 |
| 94–97 | 260 | 250 | 240 | 230 | 220 | 210 | 200 | 190 | 180 |
| 98–101 | 265 | 255 | 245 | 235 | 225 | 215 | 205 | 195 | 185 |
| 102–105 | 270 | 260 | 250 | 240 | 230 | 220 | 210 | 200 | 190 |
| 106–109 | 280 | 270 | 260 | 250 | 235 | 225 | 215 | 205 | 195 |
| Women | Age (years) | ||||||||
| 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | |
| Weight (kg) | W | ||||||||
| 40–45 | 110 | 105 | 100 | 95 | 90 | 90 | 85 | 75 | 75 |
| 46–49 | 115 | 110 | 105 | 100 | 100 | 95 | 90 | 85 | 80 |
| 50–53 | 120 | 115 | 110 | 105 | 100 | 100 | 95 | 90 | 85 |
| 54–57 | 125 | 120 | 120 | 115 | 110 | 105 | 100 | 100 | 95 |
| 58–61 | 130 | 125 | 125 | 120 | 115 | 110 | 105 | 100 | 100 |
| 62–65 | 135 | 135 | 130 | 125 | 120 | 120 | 115 | 110 | 105 |
| 66–69 | 140 | 140 | 135 | 130 | 130 | 125 | 120 | 115 | 110 |
| 70–73 | 150 | 145 | 140 | 135 | 130 | 130 | 125 | 120 | 115 |
| 74–77 | 155 | 150 | 145 | 140 | 135 | 135 | 130 | 125 | 120 |
| 78–81 | 160 | 155 | 150 | 150 | 145 | 140 | 135 | 130 | 130 |
| 82–85 | 165 | 160 | 155 | 150 | 150 | 145 | 140 | 140 | 135 |
| 86–89 | 170 | 165 | 160 | 160 | 155 | 150 | 145 | 140 | 140 |
Maximal oxygen uptake is considered to be the most important gross spiro-ergometric criterion of peak performance ability. Healthy persons without special training attain VO2max values of 30–45 mL O2/kg/min; world-class rowers, cross-country skiers and marathon runners attain values around 80 mL O2/kg/min. The VO2max can be roughly estimated without direct measurement of gas exchange by way of the maximal achieved performance (e.g., in bicycle ergometry, VO2max = 360 + 11 × performance [W]; in treadmill ergometry, VO2max = 4.25 + 2.98 × speed [km/h]), as the curve of oxygen uptake vs. physical performance is nearly a straight line when the usual ergometric test settings are used (15, 16, 24, e12, e13). The VO2max can also be approximated from the subject’s body weight, HR, and maximal performance with the aid of the classic Astrand rhyming nomogram (2, 24 [p. 273]). Some authors recommend the indirect approximation of oxygen uptake from the heart rate alone, but the values obtained by this method are markedly less reliable than those obtained by direct measurement, because HR values in the maximal range are highly variable (standard deviation ca. 10%) (1, 4, 9, 18, e14).
The maximal heart rate of healthy persons under stress depends mainly on age, rather than the degree of exercise training (28, 33, e15). A further important factor is the mass of working muscle: a higher heart rate can be achieved on a treadmill than on an exercise bicycle. The currently recommended formulae for assessment of the HRmax are, for bicycle ergometry, 208 - 0.7 × age in years (e15) and, for treadmill ergometry, 209.3 - 0.72 × age in years (28). These formulae, based on measured values in large sample groups, are equally valid for men and women.
Interpretation of measured values in the submaximal performance range
The maximal values obtained at peak performance are often the main focus of the diagnostic study, but values that are obtained in the submaximal performance range also have their advantages:
The effects of training and therapy can be recognized earlier and more clearly (e16).
Motivation plays a smaller role than in peak-performance testing (often a problem in tests done for medicolegal purposes).
The risk of a medical event (complication) is lower.
Incipient pathological changes already become visible at moderate intensities of stress.
Latent hypertension, for example, can become evident under stress in persons who have a normal blood pressure at rest (e17). On the other hand, an inadequate rise in blood pressure may indicate cardiac dysfunction (e.g., systolic heart failure or arterial hypertension). The response of the heart rate to stress can also reveal pathological changes: a low or slow rise in HR may indicate inadequate chronotropic function (e.g., in coronary heart disease or congestive heart failure), which the clinical history can distinguish from a bradycardic reaction due to training. An excessive rise in HR may be due to lack of training, hyperthyroidism, heart failure, or atrial fibrillation (1 [p. 287], 7, 13 [p. 387]). Pathological ECG changes (e.g., ST elevation or depression, arrhythmias) can arise even at low intensities of stress. Thus, blood pressure measurement and monitoring of the HR and ECG in stress testing in the submaximal performance range are important and, indeed, mandatory safety checks that can reveal ischemia, arrhythmias, and heart failure (2 [p. 130], 9, 11, 13 [p. 351], 23, e18).
Further very important criteria for evaluation in the submaximal performance range are the respiratory rate, heart rate, and lactate concentration curves over time (Figure 2, eFigure 2, eTables 3a, b, eTables 4a, b). In the past, various “threshold” concepts for these values were developed as an aid to the diagnostic evaluation of physical performance and the guidance and monitoring of exercise training (29, 33, e19). For example, the physical working capacity was defined in reference to a given heart rate (PWC 150 = the performance achieved at a heart rate of 150/min, and so forth). This method, however, underestimates performance in persons with a high maximal heart rate and overestimates it in persons with a low maximal heart rate. Moreover, PWC values depend on the subject’s age and sex, among other factors (1 [p. 76], e14, e20, e21). The uncertainties involved in the use of PWC have led to their no longer being used in sports medicine or in clinical medicine (33).
eTable 3a. Heart rate values in healthy 18- to 35-year-olds with different levels of maximal work load in bicycle ergometry (achieved maximal performance 160–320 W, 4-minute performance levels, initial level 40 W, increments of 40 W). This table contains the minimal and maximal values and the 5th, 25th, 50th, 75th, and 95th percentile values (p) for each performance level.*.
| 18- to 35-year-olds | 160 W group | 200 W group | 240 W group | 280 W group | 320 W group | |
| Performance groups | n | 13 | 24 | 25 | 29 | 13 |
| Heart rate at 40 W | min | 81.2 | 86.8 | 79.3 | 79.3 | 81.1 |
| p05 | 81.2 | 88.0 | 82.5 | 85.8 | 81.1 | |
| p25 | 103.8 | 95.8 | 97.0 | 93.2 | 85.7 | |
| p50 | 121.8 | 106.4 | 100.4 | 98.6 | 94.6 | |
| p75 | 126.4 | 119.5 | 109.4 | 111.0 | 104.0 | |
| p95 | 144.0 | 128.4 | 119.4 | 127.6 | 112.3 | |
| max | 144.0 | 132.7 | 121.5 | 132.0 | 112.3 | |
| Heart rate at 80 W | min | 115.0 | 100.3 | 85.1 | 89.8 | 92.0 |
| p05 | 115.0 | 101.8 | 104.6 | 96.3 | 92.0 | |
| p25 | 126.0 | 115.3 | 108.3 | 100.9 | 98.0 | |
| p50 | 142.0 | 125.3 | 116.6 | 110.3 | 101.1 | |
| p75 | 155.0 | 133.4 | 120.0 | 120.0 | 113.8 | |
| p95 | 161.6 | 144.8 | 130.7 | 140.5 | 123.0 | |
| max | 161.6 | 148.2 | 137.7 | 141.4 | 123.0 | |
| Heart rate at 120 W | min | 147.1 | 117.4 | 96.2 | 101.5 | 101.0 |
| p05 | 147.1 | 124.4 | 119.2 | 106.0 | 101.0 | |
| p25 | 152.2 | 132.6 | 128.6 | 118.4 | 106.0 | |
| p50 | 163.0 | 144.6 | 133.6 | 124.0 | 113.0 | |
| p75 | 175.0 | 156.9 | 144.0 | 134.0 | 123.4 | |
| p95 | 185.1 | 166.6 | 152.8 | 154.0 | 136.4 | |
| max | 185.1 | 178.5 | 158.7 | 158.4 | 136.4 | |
| Heart rate at 160 W | min | 166.0 | 143.0 | 113.3 | 113.2 | 112.1 |
| p05 | 166.0 | 147.1 | 137.6 | 120.6 | 112.1 | |
| p25 | 172.1 | 160.6 | 147.7 | 134.1 | 120.7 | |
| p50 | 180.0 | 164.7 | 155.3 | 140.0 | 127.1 | |
| p75 | 189.2 | 175.6 | 164.6 | 150.0 | 139.4 | |
| p95 | 199.1 | 185.8 | 172.3 | 165.9 | 149.1 | |
| max | 199.1 | 198.4 | 175.0 | 174.0 | 149.1 | |
| Heart rate at 200 W | min | 160.0 | 128.9 | 126.5 | 124.8 | |
| p05 | 163.0 | 154.2 | 133.7 | 124.8 | ||
| p25 | 175.9 | 167.8 | 150.0 | 138.6 | ||
| p50 | 183.6 | 174.0 | 158.0 | 147.1 | ||
| p75 | 190.7 | 182.0 | 168.4 | 151.7 | ||
| p95 | 203.9 | 188.5 | 178.9 | 160.2 | ||
| max | 209.3 | 191.0 | 182.0 | 160.2 | ||
| Heart rate at 240 W | min | 144.0 | 144.0 | 138.3 | ||
| p05 | 170.2 | 146.0 | 138.3 | |||
| p25 | 179.1 | 168.8 | 152.1 | |||
| p50 | 187.0 | 174.4 | 163.1 | |||
| p75 | 192.1 | 180.7 | 168.2 | |||
| p95 | 198.0 | 191.0 | 170.5 | |||
| max | 198.4 | 195.5 | 170.5 | |||
| Heart rate at 280 W | min | 161.0 | 150.8 | |||
| p05 | 165.0 | 150.8 | ||||
| p25 | 179.1 | 168.8 | ||||
| p50 | 186.0 | 175.0 | ||||
| p75 | 190.0 | 178.0 | ||||
| p95 | 202.3 | 184.8 | ||||
| max | 203.6 | 184.8 | ||||
| Heart rate at 320 W | min | 162.7 | ||||
| p05 | 162.7 | |||||
| p25 | 176.0 | |||||
| p50 | 183.1 | |||||
| p75 | 186.0 | |||||
| p95 | 194.0 | |||||
| max | 194.0 | |||||
* The data were acquired in the framework of diagnostic studies of physical performance at the Institute for Preventive Medicine of the German Federal Armed Forces.
eTable 3b. Heart rate values of healthy 18- to 35-year-olds with different levels of maximal work load in treadmill ergometry (achieved maximal ?performance 10–19 km/h, 4-minute performance steps, initial speed 8.5 km/h, speed increment 1.5 km/h).This table contains the minimal and maximal values and the 5th, 25th, 50th, 75th, and 95th percentile values (p) for each performance level.*.
| 18- to 35-year-olds |
10 km/h group |
11.5 km/h group |
13 km/h group |
14.5 km/h group |
16 km/h group |
17.5 km/h group |
19 km/h group |
|
| Performance groups | n | 10 | 46 | 83 | 110 | 95 | 27 | 9 |
|
Heart rate at 8.5 km/h |
min | 163.1 | 133.0 | 128.4 | 118.2 | 97.1 | 106.0 | 107.0 |
| p05 | 163.1 | 139.0 | 132.8 | 122.9 | 116.0 | 114.4 | 107.0 | |
| p25 | 167.0 | 151.9 | 145.1 | 134.8 | 129.0 | 117.8 | 113.0 | |
| p50 | 172.6 | 158.8 | 153.0 | 143.6 | 136.0 | 126.6 | 123.6 | |
| p75 | 178.1 | 166.0 | 159.0 | 151.2 | 144.0 | 136.8 | 130.8 | |
| p95 | 180.5 | 170.8 | 168.0 | 166.0 | 158.0 | 151.9 | 132.0 | |
| max | 180.5 | 173.1 | 172.1 | 176.0 | 162.0 | 158.4 | 132.0 | |
|
Heart rate at 10 km/h |
min | 175.6 | 155.1 | 146.4 | 132.0 | 116.7 | 116.8 | 120.6 |
| p05 | 175.6 | 163.1 | 151.0 | 138.0 | 128.6 | 128.0 | 120.6 | |
| p25 | 183.8 | 171.0 | 164.0 | 153.4 | 145.2 | 133.5 | 123.6 | |
| p50 | 187.0 | 176.7 | 171.6 | 160.9 | 151.0 | 139.0 | 129.8 | |
| p75 | 194.0 | 183.1 | 179.1 | 169.1 | 158.0 | 150.0 | 139.0 | |
| p95 | 205.0 | 188.0 | 186.1 | 180.0 | 171.0 | 171.0 | 146.0 | |
| max | 205.0 | 190.1 | 190.9 | 187.1 | 178.0 | 173.0 | 146.0 | |
|
Heart rate at 11.5 km/h |
min | 172.1 | 157.0 | 147.0 | 123.9 | 132.3 | 133.9 | |
| p05 | 175.9 | 162.1 | 155.1 | 143.4 | 138.8 | 133.9 | ||
| p25 | 181.0 | 178.3 | 166.6 | 158.0 | 147.0 | 137.0 | ||
| p50 | 186.1 | 184.4 | 173.5 | 165.0 | 151.1 | 143.0 | ||
| p75 | 192.0 | 190.1 | 181.1 | 174.0 | 160.8 | 146.2 | ||
| p95 | 195.1 | 200.0 | 191.8 | 183.0 | 177.0 | 155.0 | ||
| max | 197.8 | 207.4 | 198.0 | 192.6 | 181.0 | 155.0 | ||
|
Heart rate at 13 km/h |
min | 163.0 | 161.2 | 142.0 | 148.4 | 147.0 | ||
| p05 | 174.0 | 168.0 | 156.0 | 150.2 | 147.0 | |||
| p25 | 181.6 | 177.6 | 169.0 | 159.0 | 148.2 | |||
| p50 | 190.0 | 182.7 | 175.2 | 164.0 | 155.0 | |||
| p75 | 195.8 | 191.6 | 183.0 | 172.2 | 156.0 | |||
| p95 | 206.6 | 199.1 | 191.2 | 184.4 | 164.0 | |||
| max | 216.0 | 208.0 | 199.9 | 192.0 | 164.0 | |||
|
Heart rate at 14.5 km/h |
min | 161.2 | 154.7 | 160.2 | 156.0 | |||
| p05 | 173.3 | 169.1 | 161.0 | 156.0 | ||||
| p25 | 182.0 | 178.2 | 169.1 | 161.7 | ||||
| p50 | 188.0 | 184.2 | 177.0 | 163.0 | ||||
| p75 | 194.9 | 193.1 | 184.0 | 167.0 | ||||
| p95 | 206.0 | 199.6 | 191.0 | 171.0 | ||||
| max | 212.0 | 207.2 | 199.0 | 171.0 | ||||
|
Heart rate at 16 km/h |
min | 154.5 | 170.1 | 164.4 | ||||
| p05 | 170.4 | 173.0 | 164.4 | |||||
| p25 | 183.1 | 178.5 | 172.0 | |||||
| p50 | 189.0 | 185.0 | 174.0 | |||||
| p75 | 195.6 | 192.0 | 175.2 | |||||
| p95 | 205.0 | 201.0 | 179.0 | |||||
| max | 209.2 | 207.0 | 179.0 | |||||
|
Heart rate at 17.5 km/h |
min | 174.6 | 172.0 | |||||
| p05 | 175.2 | 172.0 | ||||||
| p25 | 179.0 | 180.4 | ||||||
| p50 | 188.0 | 183.1 | ||||||
| p75 | 196.8 | 185.0 | ||||||
| p95 | 200.1 | 186.1 | ||||||
| max | 205.2 | 186.1 | ||||||
|
Heart rate at 19 km/h |
min | 172.0 | ||||||
| p05 | 172.0 | |||||||
| p25 | 183.0 | |||||||
| p50 | 186.6 | |||||||
| p75 | 189.6 | |||||||
| p95 | 195.1 | |||||||
| max | 195.1 | |||||||
* The data were acquired in the framework of diagnostic studies of physical performance at the Institute for Preventive Medicine of the German Federal Armed Forces.
eTable 4a. Lactate values in healthy 18- to 35-year-olds with different levels of maximal work load in bicycle ergometry (achieved maximal performance 160–320 W, 4-minute performance levels, initial level 40 W, increments of 40 W).This table contains the minimal and maximal values and the 5th, 25th, 50th, 75th, and 95th percentile values (p) for each performance level.*.
| 18- to 35-year-olds | 160 W group | 200 W group | 240 W group | 280 W group | 320 W group | |
| Performance groups | n | 28 | 88 | 103 | 67 | 30 |
|
Lactate concentration at 40 W |
min | 1.2 | 0.8 | 0.8 | 0.8 | 0.8 |
| p05 | 1.2 | 1.1 | 1.1 | 1.1 | 1.0 | |
| p25 | 2.2 | 1.7 | 1.7 | 1.4 | 1.3 | |
| p50 | 2.8 | 2.2 | 2.1 | 1.8 | 1.6 | |
| p75 | 3.0 | 2.8 | 2.4 | 2.3 | 2.2 | |
| p95 | 4.4 | 3.5 | 3.1 | 2.8 | 3.0 | |
| max | 4.5 | 5.8 | 3.9 | 4.5 | 3.5 | |
|
Lactate concentration at 80 W |
min | 1.6 | 1.2 | 0.8 | 0.9 | 0.8 |
| p05 | 2.2 | 1.6 | 1.4 | 1.0 | 0.9 | |
| p25 | 3.5 | 2.6 | 2.1 | 1.7 | 1.5 | |
| p50 | 4.3 | 3.1 | 2.6 | 2.2 | 1.9 | |
| p75 | 5.1 | 3.9 | 3.3 | 2.7 | 2.3 | |
| p95 | 6.4 | 4.8 | 4.1 | 3.7 | 2.7 | |
| max | 6.8 | 8.7 | 6.3 | 5.1 | 3.0 | |
|
Lactate concentration at 120 W |
min | 4.3 | 2.4 | 1.6 | 0.9 | 1.0 |
| p05 | 5.0 | 2.8 | 2.1 | 1.1 | 1.3 | |
| p25 | 5.7 | 3.8 | 2.9 | 2.4 | 2.0 | |
| p50 | 6.9 | 4.8 | 3.7 | 3.0 | 2.4 | |
| p75 | 8.9 | 5.6 | 4.4 | 3.5 | 2.7 | |
| p95 | 11.7 | 7.0 | 5.3 | 4.3 | 3.5 | |
| max | 11.8 | 8.6 | 6.6 | 5.4 | 3.8 | |
|
Lactate concentration at 160 W |
min | 6.2 | 3.4 | 2.6 | 1.2 | 1.3 |
| p05 | 8.4 | 4.4 | 3.2 | 2.2 | 1.4 | |
| p25 | 9.2 | 5.8 | 4.3 | 3.4 | 2.2 | |
| p50 | 11.2 | 7.2 | 5.0 | 3.9 | 2.9 | |
| p75 | 13.2 | 8.8 | 6.3 | 4.6 | 3.3 | |
| p95 | 14.2 | 10.6 | 7.8 | 5.7 | 4.1 | |
| max | 16.3 | 14.8 | 9.1 | 8.2 | 5.3 | |
|
Lactate concentration at 200 W |
min | 5.7 | 3.7 | 2.3 | 1.6 | |
| p05 | 6.3 | 4.6 | 3.3 | 2.0 | ||
| p25 | 8.8 | 6.1 | 4.0 | 3.0 | ||
| p50 | 10.5 | 7.8 | 5.2 | 3.9 | ||
| p75 | 12.7 | 9.4 | 6.7 | 4.3 | ||
| p95 | 15.8 | 11.3 | 8.3 | 5.2 | ||
| max | 18.2 | 16.1 | 11.4 | 5.4 | ||
|
Lactate concentration at 240 W |
min | 4.9 | 1.5 | 3.3 | ||
| p05 | 7.1 | 4.1 | 3.8 | |||
| p25 | 9.2 | 6.1 | 4.5 | |||
| p50 | 11.5 | 8.2 | 5.1 | |||
| p75 | 13.2 | 9.8 | 5.9 | |||
| p95 | 15.8 | 12.1 | 7.1 | |||
| max | 25.0 | 14.8 | 8.3 | |||
|
Lactate concentration at 280 W |
min | 4.5 | 4.3 | |||
| p05 | 5.7 | 4.4 | ||||
| p25 | 8.3 | 6.0 | ||||
| p50 | 11.2 | 8.1 | ||||
| p75 | 13.4 | 9.1 | ||||
| p95 | 15.3 | 11.7 | ||||
| max | 16.4 | 12.3 | ||||
|
Lactate concentration at 320 W |
min | 6.3 | ||||
| p05 | 6.8 | |||||
| p25 | 9.8 | |||||
| p50 | 11.3 | |||||
| p75 | 13.0 | |||||
| p95 | 14.5 | |||||
| max | 15.4 | |||||
* The data were acquired in the framework of diagnostic studies of physical performance at the Institute for Preventive Medicine of the German Federal Armed Forces.
eTable 4b. Lactate values values of healthy 18- to 35-year-olds with different levels of maximal work load in treadmill ergometry (achieved maximal performance 10–19 km/h, 4-minute performance steps, initial speed 8.5 km/h, speed increment 1.5 km/h).This table contains the minimal and maximal values and the 5th, 25th, 50th, 75th, and 95th percentile values (p) for each performance level.*.
| 18- to 35-year-olds | 10 km/hgroup | 11.5 km/hgroup | 13 km/hgroup | 14.5 km/hgroup | 16 km/hgroup | 17.5 km/hgroup | 19 km/hgroup | |
| Performance groups | n | 10 | 46 | 83 | 110 | 95 | 27 | 9 |
|
Lactate concentration at 8.5 km/h |
min | 4.9 | 2.4 | 1.5 | 1,1 | 0,8 | 0,8 | 1,1 |
| p05 | 4.9 | 3.1 | 1.9 | 1,6 | 1,4 | 0,9 | 1,1 | |
| p25 | 5.8 | 4.1 | 3.2 | 2,5 | 2,1 | 1,7 | 1,5 | |
| p50 | 6.9 | 4.3 | 4.0 | 3.0 | 2.5 | 2.0 | 1.9 | |
| p75 | 8.7 | 5.6 | 4.7 | 3.7 | 2.8 | 2.8 | 2.1 | |
| p95 | 10.7 | 6.7 | 5.4 | 4.2 | 3.9 | 3.3 | 2.4 | |
| max | 10.7 | 7.9 | 9.4 | 5.4 | 4.5 | 3.3 | 2.4 | |
|
Lactate concentration at 10 km/h |
min | 7.4 | 3.2 | 2.6 | 1.2 | 0.8 | 0.9 | 1.2 |
| p05 | 7.4 | 4.1 | 3.0 | 1.9 | 1.4 | 0.9 | 1.2 | |
| p25 | 8.2 | 5.2 | 3.8 | 2.7 | 2.2 | 1.9 | 1.4 | |
| p50 | 9.6 | 7.1 | 4.6 | 3.3 | 2.5 | 2.2 | 1.7 | |
| p75 | 11.4 | 9.3 | 5.7 | 4.0 | 3.1 | 2.9 | 2.0 | |
| p95 | 12.7 | 11.5 | 8.2 | 5.2 | 3.7 | 3.4 | 2.7 | |
| max | 12.7 | 13.3 | 12.4 | 6.9 | 4.6 | 3.7 | 2.7 | |
|
Lactate concentration at 11.5 km/h |
min | 4.6 | 3.0 | 1.4 | 1.3 | 1.0 | 1.6 | |
| p05 | 5.9 | 4.0 | 2.9 | 1.9 | 1.5 | 1.6 | ||
| p25 | 7.6 | 5.6 | 3.7 | 2.8 | 2.0 | 1.7 | ||
| p50 | 9.7 | 7.2 | 4.4 | 3.3 | 2.8 | 2.0 | ||
| p75 | 11.2 | 8.8 | 5.4 | 3.9 | 3.1 | 2.4 | ||
| p95 | 14.5 | 11.7 | 7.5 | 4.8 | 3.6 | 3.6 | ||
| max | 14.8 | 15.2 | 8.7 | 5.7 | 3.9 | 3.6 | ||
|
Lactate concentration at 13 km/h |
min | 4.3 | 2.6 | 1.8 | 2.0 | 1.5 | ||
| p05 | 5.3 | 4.3 | 3.0 | 2.2 | 1.5 | |||
| p25 | 7.9 | 5.6 | 3.9 | 2.8 | 2.2 | |||
| p50 | 9.3 | 6.5 | 4.5 | 3.6 | 2.4 | |||
| p75 | 10.9 | 8.7 | 5.2 | 3.9 | 2.4 | |||
| p95 | 15.0 | 11.2 | 7.1 | 4.7 | 3.0 | |||
| max | 18.0 | 13.6 | 8.3 | 5.2 | 3.0 | |||
|
Lactate concentration at 14.5 km/h |
min | 4.6 | 3.5 | 3.0 | 2.3 | |||
| p05 | 5.8 | 4.5 | 3.0 | 2.3 | ||||
| p25 | 7.4 | 5.5 | 3.7 | 2.5 | ||||
| p50 | 8.8 | 6.8 | 4.6 | 3.0 | ||||
| p75 | 10.2 | 8.7 | 5.4 | 3.4 | ||||
| p95 | 12.9 | 11.6 | 6.3 | 3.9 | ||||
| max | 14.9 | 14.1 | 7.9 | 3.9 | ||||
|
Lactate concentration at 16 km/h |
min | 5.0 | 4.1 | 2.6 | ||||
| p05 | 5.2 | 4.5 | 2.6 | |||||
| p25 | 7.2 | 5.5 | 3.7 | |||||
| p50 | 8.7 | 7.2 | 4.1 | |||||
| p75 | 10.7 | 8.3 | 5.0 | |||||
| p95 | 14.7 | 10.7 | 6.0 | |||||
| max | 15.5 | 11.4 | 6.0 | |||||
|
Lactate concentration at 17.5 km/h |
min | 3.3 | 3.8 | |||||
| p05 | 3.8 | 3.8 | ||||||
| p25 | 7.1 | 4.5 | ||||||
| p50 | 8.7 | 5.9 | ||||||
| p75 | 10.3 | 7.3 | ||||||
| p95 | 12.2 | 8.4 | ||||||
| max | 12.4 | 8.4 | ||||||
|
Lactate concentration at 19 km/h |
min | 5.3 | ||||||
| p05 | 5.3 | |||||||
| p25 | 6.5 | |||||||
| p50 | 8.2 | |||||||
| p75 | 9.4 | |||||||
| p95 | 10.4 | |||||||
| max | 10.4 | |||||||
* The data were acquired in the framework of diagnostic studies of physical performance at the Institute for Preventive Medicine of the German Federal Armed Forces.
Rightward and leftward shifts of the lactate-performance curve permit assessment of performance changes and training effects and their monitoring over time (33, e18, e19, e22). Many lactate “threshold” concepts were previously used to detect the highest level of performance (in km/h or watts) at which lactate was still at equilibrium (33) and then derive recommendations for training from this information (1 [S. 213]). It turned out, however, that this transfer was only possible to a limited degree, even in endurance sports (29, 31– 33).
Interpretation of the post-exercise phase
In the early recovery phase after ergometric stress testing, complications such as arrhythmia and circulatory collapse can arise, and therefore the blood pressure, heart rate, and ECG should still be monitored. The HR response in this phase also provides further diagnostic information: the HR should fall by at least 12 beats/min in the first minute of the recovery period (e23, 22), indicating a good autonomic balance between sympathetic and parasympathetic function (e24). Delayed HR renormalization may indicate disturbed neurohumoral function due to stress, hypertension, lack of training, or other factors (4 [p. 287, 291], e24). On the other hand, slow HR recovery kinetics and high HR in the recovery period are normal if the subject has performed physical work above the limit of sustainable performance (e23).
Factors affecting the ergometrically measured variables
Norms for maximal performance (in watts) are listed in eTable 2 as a function of the main physiological factors affecting them—age, sex, and body weight. The subject’s food intake and physical activity right before testing can also markedly affect performance and lactate values, which depend on glycogen reserves in muscle (1 [p. 216], 13, 24 [p. 31, 71], 32).
Drug intake can also markedly affect ergometric values (ebox 2). Drugs can alter the response to stress, giving rise to either false-positive or false-negative findings on the stress ECG (e.g., ST depression in the absence of disease or lack of ST elevation in the presence of disease). The drug(s) now being taken must therefore be asked about and thoroughly documented, along with the patient’s past medical history and current illnesses, before ergometric testing is performed. Various commonly consumed substances such as nicotine, caffeine, and energy drinks can also affect the ergometric findings, possibly giving rise to tachycardia or an arrhythmia (1 [p. 335], 2 [p. 153], 9, e25).
eBOX 2. Medications that can influence ergometric stress testing*.
Beta-blockers lower the heart rate, cardiac output, and blood pressure at any given level of physical performance. They also raise the performance capacity of patients with coronary heart disease (the ischemia threshold rises).
Nitrates increase performance capacity and lessen the ischemic response to exercise at any given level of physical performance.
Calcium-channel blockers of the diltiazem and verapamil types lower the heart rate, blood pressure, and cardiac output and increase the threshold for ischemia; calcium-channel blockers of the nifedipine type have similar effects, except that they raise the heart rate.
Digitalis can give rise to a false-positive finding resembling ischemia in the stress ECG of 25–40% of all persons tested (including healthy subjects), and may lower the heart rate during stress testing in persons with atrial fibrillation.
Diuretics have no direct effect on the ergometric findings but may cause hypokalemia, leading, in turn, to arrhythmias and a falsely positive stress ECG; when taken over the long term, they can cause hypovolemia and an increased heart rate under stress.
Vasodilators lower the blood pressure and may increase the heart rate at any given level of physical performance.
Antiarrhythmic drugs can prolong the QRS duration and the QT interval. Quinidine can cause a falsely positive stress ECG, and all antiarrhythmic drugs can cause ventricular arrhythmias (proarrhythmia, torsade des pointes).
Bronchodilators (ß-agonists, theophylline preparations) elevate the heart rate at rest and under stress and can cause supraventricular arrhythmias under stress.
Psychoactive drugs: tranquilizers and antidepressants can cause a falsely positive stress ECG (prolonged QT duration).
Antihistamines (terfenadine) can cause arrhythmias (torsade des pointes or atypical atrial tachycardia).
Thyroid-hormone supplementation can elevate the heart rate, cardiac output, and blood pressure and worsen ischemic reactions.
* after (1 [p. 355], 2 [p. 383], 9, 26 [p. 155])
Overview and future perspective
Ergometry is one of the main forms of preventive testing in sports medicine (5, e26– e28). Its prerequisites are a clinical history, a physical examination, and a digital ECG at rest with computer-assisted interpretation. If there are any abnormal findings, ergometric stress testing is the next step in the diagnostic evaluation; cardiac ultrasonography and other tests may also be indicated, but only after stress testing (39, e26, e29). It is a matter of concern that older persons who are just beginning to exercise regularly, as well as persons who only engage in sports in their leisure time, much less commonly undergo sports-related medical evaluations than high-performance athletes do. A survey of over 10 000 long-distance runners revealed that 59.9% of the ambitious athletes, but only 46.8% of the free-time exercisers and 42.0% of the beginning participants, had ever had such a check-up. The corresponding figure for respondents over age 50 was only 40% (1, 5, e26, e29).
Ergometric stress testing yields important information for the planning and monitoring of exercise and treatment. This is true, in particular, with regard to the increasing use of so-called fitness trackers that register fitness– and health-related parameters (e.g., heart rate, distance, speed, sleep duration) through sensors in a bracelet or chest strap. In high-performance sports, the transfer of ergometric laboratory data into detailed training plans is more difficult, as the sport-specific stress pattern is usually very different from the stress protocol used in testing. Further considerations regarding training recommendations can be found on the website of the European Federation of Sports Medicine (www.efsma-scientific.eu).
The creation of a nationwide fitness and health registry would be of great help for the individualized assessment of test findings and of the course of training and treatment effects over time (5, 25, e28). Such a registry would also enable comprehensive statistical and epidemiological studies and would be helpful for the development and evaluation of health initiatives (e28).
Key Messages.
Ergometric stress testing is an important means of diagnosing latent cardiopulmonary and metabolic disease and of monitoring medical treatment, both in the hospital and in the outpatient setting.
It is contraindicated in the presence of acute and severe diseases of the heart, lungs, or other internal organs. The criteria for terminating ergometric stress testing include both objective findings (e.g., abnormal ECG or blood pressure changes) and subjective symptoms (dizziness, incoordination, progressive chest pain or shortness of breath).
Physical capacity, also called physical fitness, is considered to be of prognostic significance for morbidity and mortality in a wide range of diseases.
Pathological changes may already be evident in an ergometric stress test with physical performance in the submaximal range; the same is true of the effects of physical training and medical treatment. The time curves of respiratory rate, heart rate, and lactose concentration are more informative than the threshold values that were previously used (physical working capacity, lactate thresholds).
Ergometric measurements can be useful to both leisure-time athletes and ill patients by serving as a guide for the planning of physical training and medical treatment.
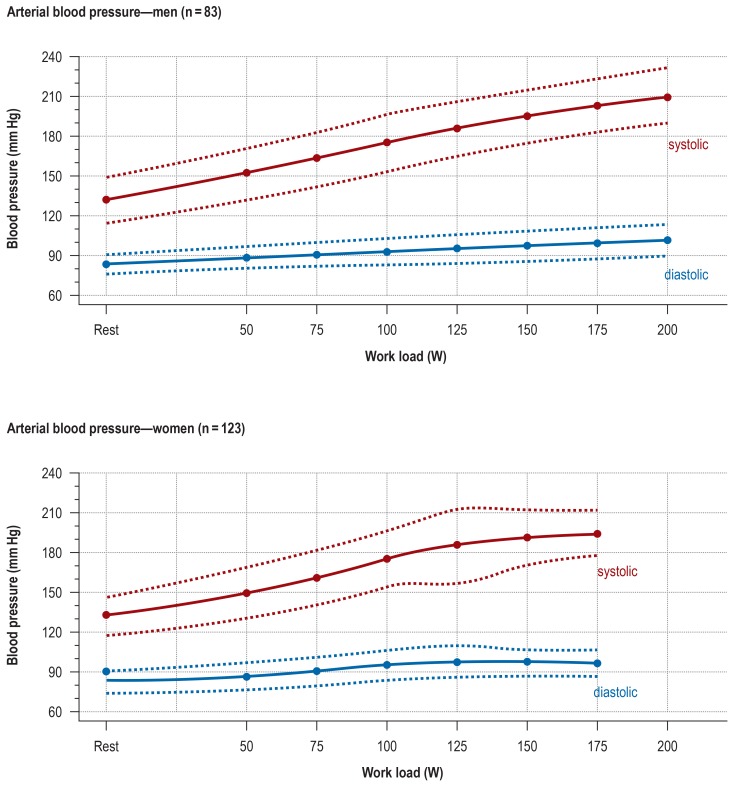
eFigure 1.
The course of arterial blood pressure during ergometric testing while seated on an exercise bicycle, with performance increments every 2 minutes (mean ± standard deviation) (1, 4)
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Acknowledgement
The authors would like to thank Matthias Krapick (Institute for Preventive Medicine of the German Federal Armed Forces) for his collaboration.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Löllgen H, Erdmann E, Gitt AK, editors. Springer. Berlin, Heidelberg: 2010. Ergometrie. Belastungsuntersuchungen in Klinik und Praxis. [Google Scholar]
- 2.Pescatello LS. ACSM‘s guidelines for exercise testing and prescription. Baltimore: Lippincott Williams & Wilkins. 2014 [Google Scholar]
- 3.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice A case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 4.Löllgen H. Kardiopulmonale Funktionsdiagnostik. Nürnberg: Novartis Pharma. 2005 [Google Scholar]
- 5.Leyk D, Rüther T, Wunderlich M, Sievert A, Erley OM, Löllgen H. Utilization and implementation of sports medical screening examinations Survey of more than 10 000 long-distance runners. Dtsch Arztebl Int. 2008;105:609–614. doi: 10.3238/arztebl.2008.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löllgen H, Leyk D, Löllgen D. Verordnen Sie Bewegung auf Rezept! Evidenzbasierte Empfehlungen für die Trainingsberatung im Breitensport. MMW-Fortschritte der Medizin. 2011;153:29–32. [PubMed] [Google Scholar]
- 7.Rost R, Lagerstrom D, Völker K. Die Fahrradergometrie und körperliches Training bei Herz-Kreislauf-Patienten. Köln: Echo-Verlag. 1996 [Google Scholar]
- 8.Meyer FJ, Borst MM, Buschmann HC, et al. Belastungsuntersuchungen in der Pneumologie. Pneumologie. 2013;67:16–34. doi: 10.1055/s-0032-1325901. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training A scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 10.Forman DE, Arena R, Boxer R, et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2016;133:1–18. doi: 10.1093/eurheartj/ehw180. [DOI] [PubMed] [Google Scholar]
- 12.McArdle WD, Katch FI, Katch VL. Wolters Kluwer. Philadelphia: 2016. Essentials of exercise physiology. [Google Scholar]
- 13.Froelicher VF, Myers J. Saunders Elsevier. Philadelphia: 2006. Exercise and the heart 5th ed. [Google Scholar]
- 14.Zhang Y, Zhang J, Zhou J, et al. Nonexercise estimated cardiorespiratory fitness and mortality due to all causes and cardiovascular disease Mayo Clinic Proceedings. Innovations, Quality & Outcomes. 2017;1:16–25. doi: 10.1016/j.mayocpiqo.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers J, Nead KT, Chang P, Abella J, Kokkinos P, Leeper NJ. Improved reclassification of mortality risk by assessment of physical activity in patients referred for exercise testing. Am J Med. 2015;128:396–402. doi: 10.1016/j.amjmed.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Nes BM, Vatten LJ, Nauman J, Janszky I, Wisløff U. A simple nonexercise model of cardiorespiratory fitness predicts long-term mortality. Med Sci Sports Exerc. 2014;46:1159–1165. doi: 10.1249/MSS.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 17.Dal Monte A. Belastungsmessverfahren und Ergometer Olympia-Buch der Sportmedizin. Eine Veröffentlichung des IOC in Zusammenarbeit mit der FIMS. In: Dirix A, Knuttgen HA, Tittel K, editors. Deutscher Ärzteverlag. Köln: 1989. pp. 113–134. [Google Scholar]
- 18.Deutsche Gesellschaft für Kardiologie - Herz- und Kreislaufforschung (DGK) Leitlinien zur Ergometrie. Z Kardiol. 2000;89:136–143. [Google Scholar]
- 19.Löllgen H, Hansel J, Boldt F, et al. DGSP Leitlinie Vorsorgeuntersuchung im Sport. www.bayerischersportaerzteverband.de/fileadmin/user_upload/html/Download/S1_Leitlinie_DGSP.pdf (last accessed on 12 March. 2018) [Google Scholar]
- 20.Wonisch M, Berent R, Klicpera M, et al. Praxisleitlinien Ergometrie. Austr J Cardiolog. 2008;15:3–17. [Google Scholar]
- 21.Löllgen H. Bedeutung und Evidenz der körperlichen Aktivität zur Prävention und Therapie von Erkrankungen. Dtsch Med Wochenschr. 2013;138:2253–2259. doi: 10.1055/s-0033-1349606. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman K, Hansen JE, Sue DY, Stringer W, Whipp BJ. Lippincott Williams & Wilkins. Philadelphia: 2012. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. [Google Scholar]
- 23.Kokkinos PF, Faselis C, Myers J, et al. Cardiorespiratory fitness and incidence of major adverse cardiovascular events in US Veterans: a cohort study. Mayo Clin Proc. 2017;92:39–48. doi: 10.1016/j.mayocp.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Åstrand PO. Textbook of work physiology Physiological bases of exercise. Champaign: Human Kinetics. (4) 2003 [Google Scholar]
- 25.Finger JD, Gösswald A, Härtel S, et al. Messung der kardiorespiratorischen Fitness in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsblatt. 2013;56:885–893. doi: 10.1007/s00103-013-1694-5. [DOI] [PubMed] [Google Scholar]
- 26.Lilly LS. Elsevier. Philadelphia: 2016. Braunwald’s heart disease Review and assessment. [Google Scholar]
- 27.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin Proc. 2015;90:1515–1523. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arena R, Myers J, Kaminsky LA. Revisiting age-predicted maximal heart rate: Can it be used as a valid measure of effort? Am Heart J. 2016;173:49–56. doi: 10.1016/j.ahj.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leyk D, Baum K, Wamser P, Selle K, Hoffman U, Essfeld D. Sport und Buch Strauss. Köln: 1998. Grenzen der Standard-Ausdauerverfahren in den Sportspielen In: Jeschke D, Lorenz R (eds.): Sportartspezifische Leistungsdiagnostik. Energetische Aspekte, pp. 243–250. [Google Scholar]
- 30.Baum K, Leyk D, Hoy S. Metabolische und kardiovaskuläre Beanspruchungen bei Badminton, Tennis und Squash im Überblick. Sportorthopädie und Sporttraumatolgie. 1997;13:1–4. [Google Scholar]
- 31.Leyk D, Schirrmacher L, Hoffmann U, Baum K. Leistungsdiagnostik in den Sportspielen: Kurze Richtungsänderungen Sprints mit Vergleich zwischen Handballspieler, Sprinter, und Ausdauertrainierten. Leistungssport. 2000;30:31–35. [Google Scholar]
- 32.Wackerhage H, Leyk D. Sport und Buch Strauss. Köln: 2000. Muskulärer Energiestoffwechsel und Sport. [Google Scholar]
- 33.Heck H. Energiestoffwechsel und medizinische Leistungsdiagnostik. Schorndorf: Hofmann. 1990 [Google Scholar]
- 34.Wilson MG, Drezner J, Sharma S. IOC manual of sports cardiology. Chichester: Wiley Blackwell. 2017 [Google Scholar]
- 35.Borg G. Borg’s perceived exertion and pain scales. Champaign: Human Kinetics. 1998 [Google Scholar]
- 36.Löllgen H, Haninger B, Just H. Kindermann W, Hort W, editors. Langzeitvariabilität ergometrischer Messgrössen Sportmedizin für Breiten- und Leistungssport: Berichtsband Deutscher Sportärztekongress Saarbrücken 16.10. 1910.1980. Gräfelfing: Demeter-Verlag. 1980:S. 73–S 78. [Google Scholar]
- 37.von Nieding G, Krekeler H, Löllgen H, Ripplinger E. Intraindividuelle Variabilität von Lungenfunktionsgrössen im Längsschnitt und ihre Bedeutung für die arbeitsmedizinische Untersuchung. Prax Klin Pneumol. 1977;31:858–871. [PubMed] [Google Scholar]
- 38.Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age. A cross-sectional study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085276. e85276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. J Cardiopulm Rehabil. 2005;25:386–387. [Google Scholar]
- E1.Liu R, Sui X, Laditka JN, et al. Cardiorespiratory fitness as a predictor of dementia mortality in men and woman. Med Sci Sports Exerc. 2012;44:253–259. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Can Med Ass J. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk Their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- E4.Brill PA, Kohl HW, Blair SN. Anxiety, depression, physical fitness, and all-cause mortality in men. J Psychosom Res. 1992;36:267–273. doi: 10.1016/0022-3999(92)90091-f. [DOI] [PubMed] [Google Scholar]
- E5.Thomson D, Turner A, Lauder S, et al. A brief review of exercise, bipolar disorder, and mechanistic pathways. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- E7.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31:3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- E8.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- E9.Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death. Cohort study of one million participants. BMJ. 2012;345 doi: 10.1136/bmj.e7279. e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Scherer D, Kaltenbach M. Häufigkeit lebensbedrohlicher Komplikationen bei ergometrischen Belastungsuntersuchungen. Dtsch Med Wochenschr. 1979;104:1161–1165. doi: 10.1055/s-0028-1129062. [DOI] [PubMed] [Google Scholar]
- E11.Borg G. Anstrengungsempfinden und körperliche Aktivität. Dtsch Ärztebl. 2004;101:A1016–A1021. [Google Scholar]
- E12.Jackson AS, Blair SN, Mahar MT, Wier LT, Ross RM, Stuteville JE. Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc. 1990;22:863–870. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- E13.Artero EG, Jackson AS, Sui X, et al. Longitudinal algorithms to estimate cardiorespiratory fitness: associations with nonfatal cardiovascular disease and disease-specific mortality. J Am Coll Cardiol. 2014;63:2289–2296. doi: 10.1016/j.jacc.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Ulmer HV, Hufnagel B. Mellerowicz H, editor. Zur Vergleichbarkeit von W170-Testergebnissen in Abhängigkeit von Alter, Geschlecht und Ausdauertrainingszustand Standardisierung, Kalibrierung und Methodik in der Ergometrie. Ausgewählte Beiträge vom 4. Internationalen Seminar für Ergometrie. Erlangen: Perimed. 1981:122–127. [Google Scholar]
- E15.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- E16.Lannoy de L, Sui X, Lavie CJ, Blair SN, Ross R. Change in submaximal cardiorespiratory fitness and all-cause mortality. Mayo Clin Proc. 2018;93:184–190. doi: 10.1016/j.mayocp.2017.11.020. [DOI] [PubMed] [Google Scholar]
- E17.Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am J Hypertens. 2013;26:357–366. doi: 10.1093/ajh/hps053. [DOI] [PubMed] [Google Scholar]
- E18.Hollmann W, Strüder HK, Predel HG, Tagarakis CVM. Schattauer. Stuttgart: 2006. Spiroergometrie Kardiopulmonale Leistungsdiagnostik des Gesunden und Kranken. [Google Scholar]
- E19.Dickhuth HH, Yin L, Niess A, et al. Ventilatory, lactate-derived and catecholamine thresholds during incremental treadmill running: relationship and reproducibility. Int J Sports Med. 1999;20:122–127. doi: 10.1055/s-2007-971105. [DOI] [PubMed] [Google Scholar]
- E20.Liesen H, Stein N, Heinsberg KE, Völker K, Hollmann W. Mellerowicz H, editor. Über Wertigkeit und Bedeutung der PWC zur Leistungsbeurteilung im Alter und von Trainingsanpassungen Standardisierung, Kalibrierung und Methodik in der Ergometrie. Ausgewählte Beiträge vom 4. Internationalen Seminar für Ergometrie. Erlangen: Perimed. 1981:128–134. [Google Scholar]
- E21.Rösler JA. Ergometrie in der Arbeitsmedizin Ergometrie. Belastungsuntersuchungen in Klinik und Praxis. 3rd ed., In: Löllgen H, Erdmann E, Gitt AK, editors. Springer Verlag. Berlin, Heidelberg: 2010. pp. 375–382. [Google Scholar]
- E22.Steinacker JM. Energieliefernde Systeme und Laktat in der Ergometrie Ergometrie. In: Löllgen H, Erdmann E, Gitt AK, editors. Springer Verlag. Berlin, Heidelberg: 2010. pp. 213–227. [Google Scholar]
- E23.Borresen J, Lambert MI. Autonomic control of heart rate during and after exercise. Sport Med. 2008;38:633–646. doi: 10.2165/00007256-200838080-00002. [DOI] [PubMed] [Google Scholar]
- E24.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- E25.Busuttil M, Willoughby S. A survey of energy drink consumption among young patients presenting to the emergency department with the symptom of palpitations. Int J Cardiol. 2016;204:55–56. doi: 10.1016/j.ijcard.2015.11.118. [DOI] [PubMed] [Google Scholar]
- E26.Löllgen H, Leyk D, Hansel J. The pre-participation examination for leisure time physical activity: general medical and cardiological issues. Dtsch Arztebl Int. 2010;107:742–749. doi: 10.3238/arztebl.2010.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Löllgen H, Leyk D. Prävention durch Bewegung. Bedeutung der körperlichen Leistungsfähigkeit. Internist. 2012;53:663–670. doi: 10.1007/s00108-011-2932-2. [DOI] [PubMed] [Google Scholar]
- E28.Leyk D, Franke E, Hofmann M, et al. Gesundheits- und Fitnessförderung in der Bundeswehr Von ressourcenorientierter Präventionsforschung zur Umsetzung in die Fläche. Wehrmed Mschr. 2013;57:162–166. [Google Scholar]
- E29.Löllgen H, Börjesson M, Cummiskey J, Bachl N, Debruyne A. The pre-participation examination in sports: EFSMA statement on ECG for pre-participation examination. Dtsch Z Sportmed. 2015;15:151–155. [Google Scholar]
- E30.Steinacker JM, Liu Y, Reißnecker Standards der Sportmedizin: Abbruchkriterien bei der Ergometrie. Dtsch Z Sportmed. 2002;53:228–229. [Google Scholar]
- E31.Reiterer W. Methodik eines rektangulären triangulären Belastungstests. Herz Kreislauf. 1975;7:457–462. [Google Scholar]