Introduction
Key Teaching Points.
-
•
Atrial arrhythmias are common in patients with Brugada syndrome.
-
•
Many medications commonly used to treat atrial arrhythmias are contraindicated in patients with Brugada syndrome.
-
•
Quinidine has been shown to be an effective antiarrhythmic drug for some patients with Brugada syndrome who suffer from ventricular arrhythmias and may be beneficial to patients with Brugada syndrome who suffer from atrial arrhythmias as well.
Brugada syndrome (BrS) was first identified in 1992 as an electrocardiographic (ECG) pattern associated with sudden cardiac death in predominantly young male subjects with structurally normal hearts.1 Although initially considered a primary inherited channelopathy, BrS appears to encompass a spectrum of clinical and pathological diseases that include structural heart disease.2 Although ventricular fibrillation is the most feared consequence of BrS, patients with BrS commonly experience atrial arrhythmias, with a prevalence of up to 40% in some studies.3 Antiarrhythmic drug (AAD) therapy and rate control can be difficult in patients with BrS, as many agents can provoke ventricular arrhythmias. Class IC AADs as well as calcium channel blockers and amiodarone are contraindicated, while β-blockers have been associated with an increased risk of ventricular arrhythmias in some patients with BrS.3 Furthermore, some patients deemed high risk with BrS have implantable cardioverter-defibrillators (ICDs) and are at risk of inappropriate shocks secondary to rapidly conducted atrial arrhythmias.
Hydroquinidine has been identified as the AAD of choice for preventing recurrent ventricular arrhythmias in patients with BrS.4, 5 Hydroquinidine has also been used safely in a limited number of patients with concomitant ventricular and atrial arrhythmias.3 The recent QUIDAM study randomized 50 high-risk patients to hydroquinidine or placebo to determine the impact on life-threatening arrhythmias.6 Hydroquinidine proved to be an effective preventive therapy in preventing ventricular arrhythmias; however, the effect on atrial arrhythmia treatment or prevention was not addressed and has not yet been evaluated. We report the use of quinidine, an isomer of quinine that contains up to 20% hydroquinidine, as an effective treatment of both atrial and ventricular arrhythmias in patients with BrS.
Case reports
Case 1
A 28-year-old man was diagnosed with BrS after developing a type 1 pattern in response to an ajmaline challenge performed as a part of family screening. One year after diagnosis in 2013 while living in the United Kingdom, he presented to the emergency department with chest pain, presyncope, and wide complex monomorphic tachycardia. At this time, he had a negative electrophysiology study with ventricular stimulation and a normal coronary angiogram. After these studies, he was determined to be high risk and received an ICD.
In the summer of 2015, after moving to Canada, he received 4 appropriate ICD shocks for ventricular fibrillation over the course of 2 months (Figure 1). Each of these shocks occurred at night while sleeping after episodes of excessive alcohol intake. The patient had also recently been prescribed β-blockers for symptomatic atrial tachycardia (AT) in 2015. The patient presented for his first device check in Canada, symptomatic with palpitations in 2016. The ECG at that time demonstrated a wide complex tachycardia that was diagnosed as AT with left bundle branch block aberrancy. Device interrogation revealed frequent episodes of AT that were quite symptomatic. After spontaneous cessation of AT, the patient's ECG demonstrated a spontaneous type 1 Brugada pattern (Figure 2). The patient was offered quinidine and an option to proceed to an electrophysiology study and AT ablation. The patient declined another invasive procedure and was initiated on quinidine at a dose of 200 mg 4 times daily. After 17 months of follow-up, the patient has had no further ventricular or atrial arrhythmias.
Figure 1.
Device interrogation of an episode of ventricular fibrillation in case 1, demonstrating the initiation of ventricular fibrillation during an episode of ambient bradycardia (sleeping) and late-coupled premature ventricular complexes.
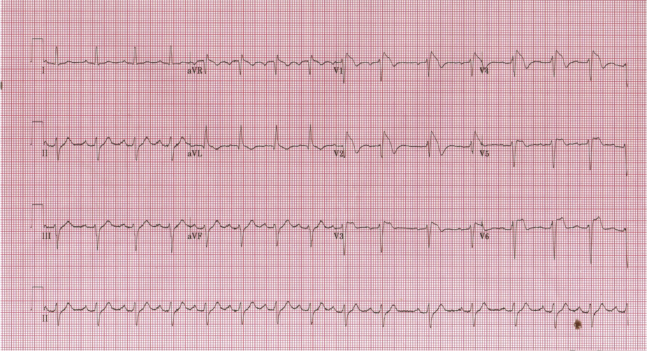
Figure 2.
Modified “high lead” electrocardiogram from case 1, demonstrating first-degree atrioventricular block, left anterior fascicular block, and type 1 Brugada pattern.
Case 2
The second patient is a 37-year-old man who presented to the emergency department with atrial fibrillation (AF). After receiving propafenone for chemical conversion to sinus rhythm, the patient developed a type 1 ECG pattern and had an episode of nonsustained polymorphic ventricular tachycardia (Figure 3). Quinidine was initiated shortly thereafter, which successfully suppressed atrial arrhythmias for the next 2 years without documented recurrence. The patient went on to undergo AF ablation, after which quinidine was weaned off.
Figure 3.
Polymorphic ventricular tachycardia recorded by telemetry after the initiation of propafenone for atrial fibrillation in case 2.
Both patients underwent genetic testing that did not show an SCN5A mutation.
Discussion
Quinidine is the first-line AAD for patients with BrS who have had appropriate ICD shocks. Quinidine is moderately effective in unselected patients with atrial arrhythmias, but is associated with a significant increase in mortality.4, 5, 7 For this reason, quinidine has fallen out of favor in the treatment of AF in patients without associated BrS. However, in the cases described, quinidine effectively suppressed atrial and ventricular arrhythmias in 2 patients with BrS without mutations in SCN5A.
While the pathophysiology of BrS remains disputed, a certain proportion of patients with BrS appear to have an isolated electrical substrate that involves an imbalance of depolarizing sodium current and repolarizing potassium current. The transient outward potassium current (Ito) contributes to early repolarization and is more prominent in the epicardium than in the endocardium, creating a transmural voltage gradient that is believed to underlie the J-wave and J-point elevation on the ECG.8 Either a decrease in the density or an increase in the acceleration of inactivation of the sodium current would lead to unopposed Ito in the early phase of the action potential.9, 10 The regional variability in this voltage gradient is highest between the right ventricular epicardium and pericardium, leading to the typical findings of BrS on the ECG. An imbalance between the sodium current and Ito might also be seen in the atria in patients with BrS. The voltage gradient observed creates dispersion of repolarization, which can facilitate local reexcitation and lead to reentrant arrhythmias in the atria and ventricles. Quinidine increases the effective refractory period, decreases automaticity through the blockade of sodium and potassium channels, and is felt to be a potent blocker of Ito. This agent may play an even more important role in the treatment of patients with BrS suffering from atrial arrhythmias since Ito is more prominent in the atria than in the ventricles.11 This blockade of Ito may then decrease the repolarization gradient and therefore decrease the propensity for reentrant arrhythmias in patients with BrS. Interestingly, in the aforementioned QUIDAM study, hydroquinidine did not change the BrS ECG pattern suggesting a broader or more complex effect on arrhythmia suppression than did Ito blockade alone. While quinidine has been used as monotherapy in a large number of patients with BrS at risk of atrial arrhythmias without evidence of clinical harm, caution must be exercised when treating lone AF because of the theoretical risk of rapidly conducting rates with atrial flutter.
Conclusion
The 2 patients described in this case series experienced a dramatic reduction in atrial dysrhythmia burden when treated with quinidine. Clinicians may consider quinidine, an agent that has generally fallen out of favor in the treatment of atrial arrhythmias, the likely drug of choice in patients with BrS suffering from AT or AF.
References
- 1.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Gray B., Semsarian C., Sy R.W. Brugada syndrome: a heterogeneous disease with a common ECG phenotype? J Cardiovasc Electrophysiol. 2014;25:450–456. doi: 10.1111/jce.12366. [DOI] [PubMed] [Google Scholar]
- 3.Giustetto C., Cerrato N., Gribaudo E., Scrocco C., Castagno D., Richiardi E., Giachino D., Bianchi F., Barbonaglia L., Ferraro A. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm. 2014;11:259–265. doi: 10.1016/j.hrthm.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Belhassen B., Glick A., Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 5.Anguera I., Garcia-Alberola A., Dallaglio P., Toquero J., Pérez L., Martínez J.G., Peinado R., Rubín J.M., Brugada J., Cequier A. Shock reduction with long-term quinidine in patients with Brugada syndrome and malignant ventricular arrhythmia episodes. J Am Coll Cardiol. 2016;67:1653–1654. doi: 10.1016/j.jacc.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Andorin A., Gourraud J.B., Mansourati J. The QUIDAM study: hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm. 2017;14:1147–1154. doi: 10.1016/j.hrthm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Lafuente-Lafuente C., Valembois L., Bergmann J.F., Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 8.Yan G.X., Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 9.Meregalli P.G., Wilde A.A., Tan H.L. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367–378. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T., Nakajima T., Hazama H., Hamada E., Murakawa Y., Sawada K., Omata M. Regional differences in transient outward current density and inhomogeneities of repolarization in rabbit right atrium. Circulation. 1995;92:3061–3069. doi: 10.1161/01.cir.92.10.3061. [DOI] [PubMed] [Google Scholar]