Abstract
This study aimed to investigate possible differences in spatio-temporal gait parameters of people with Parkinson’s Disease (pwPD) when they are tested either in laboratory using 3D Gait Analysis or in a clinical setting using wearable accelerometers. The main spatio-temporal gait parameters (speed, cadence, stride length, stance, swing and double support duration) of 31 pwPD were acquired: i) using a wearable accelerometer in a clinical setting while wearing shoes (ISS); ii) same as condition 1, but barefoot (ISB); iii) using an optoelectronic system (OES) undressed and barefoot. While no significant differences were found for cadence, stance, swing and double support duration, the experimental setting affected speed and stride length that decreased (by 17% and 12% respectively, P<0.005) when passing from the clinical (ISS) to the laboratory (OES) setting. These results suggest that gait assessment should be always performed in the same conditions to avoid errors, which may lead to inaccurate patient’s evaluations.
Key words: gait, Parkinson’s Disease, inertial sensor, spatio-temporal parameters
Introduction
Parkinson’s disease (PD) is the most frequent neurodegenerative movement disorder characterized by a gradual loss of motor and non-motor functions. Of motor symptoms, bradykinesia, rigidity, tremor, postural instability, and gait disturbance (usually associated with an increased probability of falls) are the most commonly present.1 The inability to walk properly is responsible for severe limitations in terms of functional independence and tends to reduce the overall quality of life.2 Within the wide range of gait alterations exhibited by individuals with PD, the most evident and easy to assess (even by simple visual observation) include decreased speed, reduced stride length and increased double support phase. Nevertheless, there are more subtle changes involving left-right asymmetry and stride-to-stride variability, which become detectable only when quantitative movement analysis techniques are used.
Early and accurate gait assessment is significant for PD diagnosis and is an important prerequisite to properly treat patients and reduce both disability burden and health care costs. Objective and noninvasive assessment strategies are urgent need in order to achieve this goal.
Indeed, in the routine clinical setting, but also in many studies present in the scientific literature, gait assessment is often performed with qualitative and subjective methods (e.g. the Unified Parkinson Disease Rating Scale, UPDRS) which are sometimes integrated with the results of timed tests such as the 10-meter and 6- minute walking test, the Timed Up and Go (TUG) etc. Unfortunately, such methods do not allow a detailed and precise knowledge of all the spatio-temporal and kinematic variables associated with the gait cycle, so that more refined analyses cannot be performed without the instrumental support of devices specifically designed for human movement analysis.
The gold standard for gait analysis is currently represented by optoelectronic stereophotogrammetry.3 In this technique, spherical passive reflective markers are placed on the lower extremities, pelvis, and trunk of the subject according to standardized protocols4 and, during walking, the three-dimensional trajectories of the markers are captured by a certain number of high-frequency cameras (typically six or more with frequencies ranging from 50 to 240 Hz) which also provide stroboscopic infrared illumination. Such data are then processed by a workstation to provide: i) spatio-temporal parameters (stance, swing, double support phase duration, stride time, cadence, step/stride length, step width and gait speed); ii) variation of kinematic parameters (usually pelvic tilt, rotation and obliquity, hip flexion-extension, adductionabduction and rotation, knee flexion-extension, ankle dorsiflexion, and foot progression) within the gait cycle.
Nevertheless, this technique is expensive, it requires a dedicated laboratory (i.e. the whole system is not easily portable), and data acquisition and processing is time-consuming and can be performed only by specialized personnel. Moreover, the final report of a gait analysis is complex and not easy for the clinician to interpret.
To overcome such limitations, research has been directed towards the development of lightweight, portable and wearable sensors suitable for quick and reliable clinical assessment of gait, which allow the testing of patients in more ecological conditions. Nowadays, micro electro-mechanical systems (MEMS) technology makes it possible to use miniaturized sensors (including accelerometers and gyroscopes) at a reasonable cost, and this possibility has been exploited by researchers involved in the study of PD.
Wearable accelerometric sensors (alone or in combination with gyroscopes thus composing an inertial measurement unit, IMU) have been regularly used for a decade in PD research to evaluate, among other aspects, gait alterations including freezing,5 even though they are still not fully part of routine clinical assessment.
On the basis of these considerations, this study proposes the application of inertial sensors to assess spatio-temporal gait parameters in individuals with PD in a typical clinical environment. The results will be compared with those obtained (for the same cohort of patients) in the laboratory using the gold standard (i.e. the optoelectronic system). The main purpose of the investigation is not to supply validation of the inertial sensor (that should now be taken for granted) but rather to verify what parameters are possibly influenced by the different external conditions that unavoidably characterize the clinical and the laboratory settings (e.g. wearing shoes, being undressed). Our aim is to assess if the cogency of gait parameters may be affected by the different clinical/laboratory environmental conditions and, in particular, if they may be less reliable when testing conditions become less ecological.
Materials and Methods
Participants
In the period October 2016-March 2017, a convenience sample involved 50 outpatients with PD followed at the Neurology Department, AOB G. Brotzu General Hospital (Cagliari, Italy). They were informed about the study by the hospital neurologists and assessed. All screened patients met the PD UK Brain Bank criteria.6 None of them had significant cognitive impairment (e.g. Mini-Mental Status Examination MMSE <24; Frontal Assessment Battery FAB <13). We also excluded patients who had a history of psychiatric or severe systemic illnesses, such as chronic infectious diseases, diabetes, or other metabolic, endocrine, or autoimmune illnesses; cancer; chronic alcohol consumption; toxic exposure, and any family history of myopathy or neuropathy. Levodopa equivalent daily dose (LEDD, including Levodopa and all antiparkinsonian therapies, such as dopamine agonists, rasagiline, I-COMT)7 was calculated for each patient.
All patients included presented mild to moderate disability assessed by means of the modified Hoehn and Yahr (H&Y) staging scale (1≤H&Y≤3). Evaluation was performed in ON state 60’-90’ after intake of the usual morning Levodopa dose). Assessment using the motor subscale from the Unified Parkinson’s Disease Rating Scale (UPDRS III) and a complete neurological examination were carried out in all patients. Screening for eligibility criteria and H&Y score attribution, as well as clinical motor assessment was performed by a neurologist experienced in PD (GC).
The present study was conducted in accordance with the guidelines on human experimentation (Declaration of Helsinki). The local ethics committee approved the study and all participants signed an informed consent agreeing to participate in the study.
Measurement of spatio-temporal gait parameters
The acquisition of the spatial-temporal gait parameters was performed separately using two independent techniques, namely optoelectronic stereophotogrammetry and inertial sensors. In the first case, an optoelectronic system composed of eight infrared Smart-D cameras (BTS Bioengineering, Italy) set at a frequency of 120 Hz was used. After anthropometric data collection, 22 spherical retroreflective passive markers (14 mm in diameter) were placed on the skin of the individual’s lower limbs and trunk at specific landmarks, following the protocol described by Davis et al.8 Participants were then asked to walk barefoot at a self-selected speed in the most natural manner possible on a 10 m walkway at least six times, allowing suitable rest times between the trials. The raw data were then processed with the Smart Analyzer (BTS Bioengineering, Italy) dedicated software to calculate speed, cadence, stride length, stance, swing and double support phase duration (calculated as a percentage of the gait cycle). The mean value of the six trials was considered representative of each patient. The same parameters were also acquired using a validated9 wireless inertial sensing device (G-Sensor, BTS Bioengineering, Italy), previously used in studies involving individuals with PD.10 In this case, participants were instructed to walk along an 15-m hallway adjacent to the neurologist’s office at a self-selected speed and in the most natural manner possible. The traveled distance roughly corresponds to 10-20 strides, depending on the participant’s height and stride length. Using a semi-elastic belt, the inertial sensor was attached at the lower lumbar level (centered on the L4-L5 intervertebral disc), and provided accelerations along three orthogonal axes: anteroposterior (AP), mediolateral (ML), and superoinferior (SI). Acceleration data, acquired at 100 Hz frequency, were transmitted via Bluetooth to a PC and processed using dedicated software (GWalk, BTS Bioengineering, Italy) that calculated the spatial-temporal parameters previously mentioned. The software automatically removes the first and last two strides from the computation to consider only steady velocity conditions (i.e. acceleration and deceleration phases are discarded).
To simulate the most realistic clinical condition, participants were tested fully dressed and with their shoes. Then, they were asked to remove their shoes, and a second trial (more similar to the laboratory conditions in terms of foot-ground contact) was performed. To sum up, all patients were tested under three conditions: i) using the inertial sensors while wearing their own shoes (ISS condition from now on) to simulate clinical conditions; ii) using inertial sensors while they were barefoot (ISB condition from now on) to partly simulate the laboratory conditions; iii) using the optoelectronic system (OES condition from now on) in the typical laboratory situation, that is, undressed, with markers and barefoot. The sequence of trials was fully randomized.
Statistical analysis
Data analyses were conducted using SPSS software (v.20, IBM, Armonk, NY, USA). A set of one-way repeated measure ANOVAs were run to examine the effect of the different measurement conditions on the following gait parameters: speed, cadence, stride length, stance, swing and double support phase duration. A set of paired-sample t-tests was used for planned contrasts. The level of significance was set at p = 0.05 and was adjusted with the Bonferroni correction to avoid type I errors associated with multiple testing. As a consequence, we adjusted the levels of significance both for ANOVAs and t-tests, resulting equal to P=0.0083 (0.05/6 tests) and P=0.017 (0.05/3 tests), respectively. Data were preliminarily checked for normality and equal variance using the Shapiro-Wilk and Levene tests.
Results
Of the 50 patients assessed for eligibility to enter the study, 19 declined to participate, leaving 31 patients (26 males, 5 females) who completed the three gait assessments in laboratory and clinic. Their main anthropometric and clinical features are given in Table 1.
Table 1.
Main features of the participants. Values are expressed as mean ± SD.
| Parameter | Value |
|---|---|
| Age (years) | 61.1±11.3 |
| PD Duration (years) | 7.5±5.4 |
| Hoehn & Yahr | 2.4±0.5 |
| Unified Parkinson's Disease Rating Scale (UPDRS III) | 27.3±9.5 |
| Mini-Mental Status Examination (MMSE) | 28.7±1.9 |
| Frontal Assessment Battery (FAB) | 16.9±1.4 |
| LEDD (Levodopa Equivalent Daily dose) | 542.1±212.4 |
As preliminary analyses, we ran a set of t-tests to compare the data of right and left limbs (for the parameters that are supplied separately for each limb) and we found no significant differences. For this reason, data were summarized across the right and left sides, and the values reported here refer to the mean value between them.
The spatio-temporal parameters calculated for the three experimental conditions analyzed are shown in Table 2.
Table 2.
Spatio-temporal parameters of gait assessed in the three experimental conditions.
| Spatio-temporal Gait Parameters | ISS | ISB | OES | P-value |
|---|---|---|---|---|
| Gait speed (m s-1) | 1.24±0.20 | 1.20±0.26 | 1.06±0.28 | 0.001* |
| Cadence (steps min-1) | 114.32±21.31 | 119.48±17.16 | 114.68±13.27 | 0.253 |
| Stride Length (m) | 1.27±0.19 | 1.19±0.19 | 1.13±0.20 | 0.003* |
| Stance phase (% of the gait cycle) | 61.90±2.90 | 62.52±3.81 | 60.88±2.92 | 0.053 |
| Swing phase (% of the gait cycle) | 38.12±2.92 | 37.46±3.80 | 38.78±2.59 | 0.138 |
| Double support (% of the gait cycle) | 11.79±2.81 | 12.35±3.55 | 11.86±2.83 | 0.625 |
ISS = inertial sensor wearing shoes, ISB = inertial sensor barefoot, OES = optoelectronic system barefoot. Values are expressed as mean ± SD. The symbol * denotes a main effect of the condition after Bonferroni correction.
While no significant effects related to the type of test were found for cadence, stance, swing or double support phase duration, the experimental condition appears to affect gait speed and stride length. In particular, with regard to speed, we found a significant main effect, F(2, 60)=8.49; P<0.001; η2=0.22, and a significant linear trend, F(1, 30)=11.39; P<0.005; η2=0.28 (Figure 1). In line with our expectations, a set of paired-sample t-tests revealed higher speeds for patients when they were in the ISS condition compared to when they were in the OES condition, t(30)=3.38; P<0.005; d=0.74, and higher speeds in the ISB condition compared to the OES condition, t(30)=2.56; P<0.01; d=0.50. Moreover, the speed in the ISS condition is lower than in the ISB condition, t(30)=1.89; P<0.05; d=0.19, although this comparison did not reach the adjusted alpha value.
Figure 1.
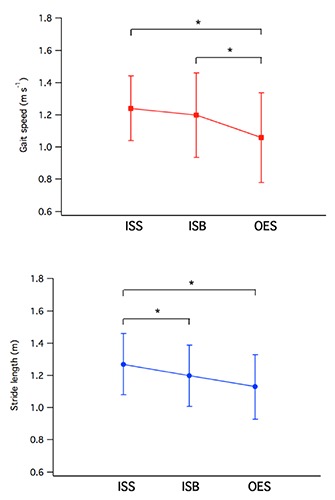
Trend of gait speed (top) and stride length (bottom) for the three experimental conditions tested. Values are expressed as mean ± SD. The symbol * denotes statistical significance after Bonferroni correction.
Similar to the results obtained for speed, we also found a significant main effect for stride length, F(2, 60)=6.56; P<0.005; η2=0.18, and a significant linear trend, F(1, 30)=8.41; P<0.01; η2=0.22 (Figure 1). In line with our expectations, a set of paired-sample t-tests revealed a longer average stride length in the ISS condition than in that of the ISB, t(30)=6.26; P<0.001; d=0.38, and OES, t(30)=2.90; P<0.005; d=0.69, conditions. However, the stride length in the ISB condition was not significantly higher than that in the OES condition, t(30)=1.46; P=0.077; d=0.33.
Discussion
The aim of the present study was to compare the assessment of patients in different conditions to see whether gait parameters may be affected by the different external factors typical of the clinical and laboratory settings. In particular, we compared the data obtained through inertial sensors, both when patients wore shoes (ISS) and when they were barefoot (ISB), with the data obtained through an optoelectronic system (OES), the gold standard for laboratory gait analysis.
The results partly confirm our expectations, showing that speed and stride length are influenced by the situations in which patients are assessed. In particular, we found that the more ecological the setting is, the higher the speed is and the longer the strides are. The data revealed the highest speed and longest strides when patients were assessed in the most ecological situation (dressed and wearing shoes, ISS), while both parameters linearly worsened when conditions become less ecological, that is, when patients were dressed and barefoot (ISB), and when they were undressed and barefoot (OES), in the typical laboratory condition.
From this point of view, our findings are consistent with previous studies that assessed the effect of the presence/absence of footwear on gait parameters of individuals of an age range close to those tested here, both in healthy conditions and affected by neurologic and orthopedic pathologies. Arnadottir and Mercer11 examined 35 healthy women aged between 65 and 93 years and found that they performed significantly better (i.e. exhibited a higher walking speed) in the 10-meter walking test when they wore shoes than when they were barefoot. Ng et al.12 observed higher gait speed and step length when footwear was worn in a cohort of thirty individuals with stroke who had undergone a gait rehabilitation program. It is interesting to observe that the step length changes were of the same order of magnitude as those detected in the present study (+10% footwear vs. barefoot condition, +12% in the study by Ng et al.12). A 7% significant reduction in stride length, but no changes in gait speed, were also observed by Shakoor and Block13 in 75 adult subjects affected by knee osteoarthritis when they were tested barefoot.
Such different performances can be explained by considering that the subjects were exposed to foot-ground contacts in which socks+footwear, socks only, or nothing at all were interposed between feet and ground. Each of these conditions is associated with different friction levels and also originates decreasing perceptions of safety (i.e. correct balance) when the individual walks on the floor. In previous studies it was found that older individuals are more at risk of unintentional falls when they walk barefoot or are wearing socks only.14 This appears to be characterized by better postural control15 and thus it is reasonable to hypothesize that when tested in the laboratory using optoelectronic-based gait analysis, participants felt more uncomfortable owing to both the barefoot condition and the presence of markers, and thus unconsciously adjusted their gait pattern (reducing speed and shortening stride) to achieve better control and reduce the risk of falls.
Some limitations of this study are to be acknowledged: firstly we did not formulate any special request of participants regarding their shoes. They arrived at the experimental test sessions with footwear and socks of their own choice, so there it was no standardization in terms of materials and thus of coefficient of friction. Similarly, there was no standardization in terms of walking surface, which was wood for the optoelectronic- based gait analysis and ceramic tiles for the inertial sensor tests. Finally, it is to be recalled that differences also exist in the overall length of the path that participants were requested to walk: in fact, even though in both experimental condition care was taken to acquire the gait parameters in steady conditions (e.g. constant speed) the walkway located inside the room in which the optoelectronic system is installed is 10 m long, while the available walking distance for the inertial sensor tests was 18 m long.
Conclusions
The results of the present study have strong implications in the clinical assessment of individuals with PD, in particular as regards the homogeneity of situations in which they are assessed. It is demonstrated that even when a quantitative analysis of gait is used, improvement/worsening of gait parameters may be due to the different conditions in which tests are performed (e.g. presence of footwear, external environment), rather than reflecting real fluctuations in the severity of symptoms. Moreover, our results suggest that clinicians should avoid making absolute comparisons of spatio-temporal gait parameters calculated using inertial sensors with those obtained, for the same individual, through optoelectronic systems. This is not owing to reliability/accuracy problems of inertial sensors (the validity of which is not questioned here), but to the different strategies that people with PD may adopt when tested in different settings. In sum, we recommend that clinicians test the same individuals always in the same conditions to minimize measurement errors, which may lead to erroneous (or at least imprecise) diagnosis and evaluations and, consequently, to potentially mistaken medical decisions. The meticulous description of how tests are performed is extremely important, especially in those situations in which patients are independently assessed by different specialists.
Acknowledgments
The authors wish to express their gratitude to the physical therapists of the AOB “G. Brotzu” general hospital Marilena Fara, Giovanna Ghiani, Alessandra Pani, Elsa Sau, Gino Sedda, and Mauro Usala for their valuable support.
Funding Statement
Funding: This study was partly supported by the Autonomous Region of Sardinia (grant CRP-78543 L.R. 7/2007) and the author Mauro Murgia was supported by Autonomous Region of Sardinia, Master&Back Programme 2013 (PRR-MAB-A2013-19330).
References
- 1.Davie CA. A review of Parkinson's disease. Brit Med Bull 2008;86:109-27. [DOI] [PubMed] [Google Scholar]
- 2.Hausdorff JM. Gait dynamics in Parkinson's disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 2009;19:26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappozzo A, Della Croce U, Leardini A, Chiari L. Human movement analysis using stereophotogrammetry. Part 1: Theoretical background. Gait Posture 2005;21:186-96. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari A, Benedetti MG, Pavan E, et al. Quantitative comparison of five current protocols in gait analysis. Gait Posture 2008;28:207-16. [DOI] [PubMed] [Google Scholar]
- 5.Maetzler W, Domingos J, Srulijes K, et al. Quantitative wearable sensors for objective assessment of Parkinson's disease. Mov Disord 2013;28:1628-37. [DOI] [PubMed] [Google Scholar]
- 6.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Ps 1998;51:745-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649-53. [DOI] [PubMed] [Google Scholar]
- 8.Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci 1991;10:575-87. [Google Scholar]
- 9.Bugané F, Benedetti MG, Casadio G, et al. Estimation of spatial-temporal gait parameters in level walking based on a single accelerometer: validation on normal subjects by standard gait analysis. Comput Meth Prog Bio 2012;108:129-37. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner A, Galli M, Gaglione M, et al. The Parkinsonian Gait Spatiotemporal Parameters Quantified by a Single Inertial Sensor before and after Automated Mechanical Peripheral Stimulation Treatment. Parkinsons Dis 2015;390512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnadottir SA, Mercer VS. Effects of footwear on measurements of balance and gait in women between the ages of 65 and 93 years. Phys Ther 2000;80:17-27. [PubMed] [Google Scholar]
- 12.Ng H, McGinley JL, Jolley D, et al. Effects of footwear on gait and balance in people recovering from stroke. Age Ageing 2010;39:507-10. [DOI] [PubMed] [Google Scholar]
- 13.Shakoor N, Block JA. Walking barefoot decreases loading on the lower extremity joints in knee osteoarthritis. Arthritis Rheum 2006;54:2923-7. [DOI] [PubMed] [Google Scholar]
- 14.Menant JC, Steele JR, Menz HB, et al. Optimizing footwear for older people at risk of falls. J Rehabil Res Dev 2008;45:1167-81. [PubMed] [Google Scholar]
- 15.Horgan NF, Crehan F, Bartlett E, et al. The effects of usual footwear on balance amongst elderly women attending a day hospital. Age Ageing 2009;38:62-7. [DOI] [PubMed] [Google Scholar]


