Introduction
Key Teaching Points.
-
•
Ehlers-Danlos syndrome is a disease of disordered collagen matrix formation and can cause fragility of blood vessels or organs leading to rupture and premature death.
-
•
Management of atrial and ventricular arrhythmias in patients with Ehlers-Danlos syndrome can be a clinical challenge.
-
•
Invasive cardiac procedures, including implantable cardioverter-defibrillator implantation and catheter ablations, in patients with Ehlers-Danlos syndrome should be undertaken with extreme caution and thorough risk/benefit analysis.
Ehlers-Danlos syndrome (EDS) is a hereditary disease of disordered collagen matrix formation leading to skin laxity, joint hypermobility, and, in the worst case, fragility of blood vessels or organs, leading to rupture and premature death. As a result, invasive procedures in EDS patients are fraught with concern for complications. In this case report, we describe the first reported case of sustained symptomatic ventricular arrhythmias in a patient with EDS requiring placement of an implantable cardioverter-defibrillator (ICD) with the subsequent development of lead perforation necessitating operative repair.
Case report
A 59-year-old man with hypertension, hyperlipidemia, depression, and EDS with unknown subtype presented to our hospital with multiple episodes of syncope and presyncope. His other medical history included thoracic surgery 12 years ago for a mediastinal air-filled cyst that was thought to be due to a subclinical esophageal perforation from an esophagogastroduodenoscopy he had had several years prior. His examination was notable for mildly hyperextensible joints, extremely elastic skin, and multiple atrophic “cigarette paper” scars from various superficial injuries. His children also all demonstrated the same skin laxity and joint hypermobility. There were no premature or unexplained deaths in his family.
His symptoms were concerning for an arrhythmic etiology. A 2-week ambulatory monitor demonstrated frequent PVCs with a 21% burden, 614 episodes of nonsustained ventricular tachycardia, and multiple episodes of sustained ventricular tachycardia of different morphologies, the longest lasting 49 seconds with a rate of 150–200 beats/min (300–400 ms), correlating with his symptoms. Echocardiogram showed no cardiac anomalies and overall normal cardiac function, with normal left ventricular ejection fraction, valvular function, and aortic root. To evaluate for ischemic etiology, coronary angiography was performed, which showed no evidence of coronary artery disease or coronary anomalies. Importantly, immediately post-procedure, the patient developed a femoral access site hematoma despite use of a MYNX vascular closure device. While not an uncommon occurrence, this complication was a first sign of vascular fragility.
Since the presyncope and syncope events were thought to be owing to the ventricular arrhythmias, invasive treatment was preferred. After informed discussion of therapeutic options, the decision was made for transvenous defibrillator implantation; subcutaneous ICD was considered but decided against because of the patient’s cutaneous laxity and concern for device/lead migration. Additionally, given the presence of short atrial arrhythmias on his ambulatory monitor but no pacing indications, as well as the aim to implant a lead on the ventricular septum, a single active fixation VDD lead, rather than a single-chamber ventricular passive fixation lead, was chosen to allow for atrial sensing and better supraventricular and ventricular tachycardia discrimination. During the procedure, the patient was noted to have extremely friable cutaneous tissue with a fragile cobweb-like subcutaneous layer, minimal if any adipose, and no obvious fascial layer. Additionally, tissue layers very easily separated solely with manual dissection. No other obvious vascular fragility or abnormal bleeding was noted. A secondary prevention single-lead VDD ICD was successfully implanted via cephalic cut-down, positioning the lead tip in the mid-low right ventricular (RV) septum, confirmed using multiple orthogonal fluoroscopic views, without incident. Post-procedure portable chest radiograph again verified the location of the RV lead. He was discharged on postoperative day (POD)#1 without complications.
On POD#3, he returned to the emergency department with progressive worsening of pleuritic chest pain over several hours. Electrocardiography demonstrated atrial flutter with 2:1 conduction with heart rate approximately 150 beats/min. Device interrogation showed poor RV capture threshold and RV pacing reproduced sharp chest pain. Given concern for possible RV lead perforation, bedside echocardiogram was performed. Lead migration was noted through the RV apex with a small pericardial effusion (Figures 1 and 2). He was urgently taken to the operating room for lead revision. Arterial line placement failed after multiple attempts owing to repeated vessel rupture. Ultimately, the RV lead was withdrawn with the development of significant hemopericardium, requiring conversion to a full sternotomy. Upon visualization after opening the pericardium, the right coronary artery appeared intact, but very large and patulous. Gentle placement of malleable retractor caused a small laceration at the base of the right ventricle. The area of lead perforation was found on the RV diaphragmatic surface. Owing to concerns regarding anchoring and stability of sutures, Bio-Glue and Nu-Knit were used to successfully patch the perforations. The ICD was explanted and decision was made to not attempt subsequent lead placement owing to profound myocardial fragility. The postoperative course was complicated by a left pneumothorax, which resulted in significant bilateral chest, neck, and face subcutaneous air. The patient also developed a 15-cm rectal prolapse during a bowel movement, requiring gastroenterology consult and manual reduction. He was discharged home in stable condition on antiarrhythmic medications on POD#12.
Figure 1.
Transthoracic echocardiogram showing right ventricular (RV) perforation. ICD = implantable cardioverter-defibrillator.
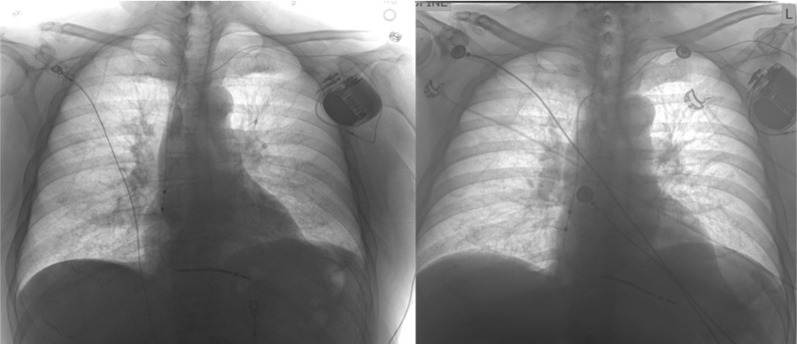
Figure 2.
Chest radiograph (CXR). Left: Post–implantable cardioverter-defibrillator placement. Right: CXR with lead migration and perforation at right ventricular apex.
Since discharge, he has had 2 subsequent admissions in 4 months for atrial flutter with 2:1 atrioventricular block. Transesophageal echocardiogram with cardioversion was attempted; however, the ultrasound probe was unable to be advanced owing to coiling within his pliable esophagus. Because of cardiovascular risk factors with a CHADS-VASC score of 1, he was started on rivaroxaban for anticoagulation. After discussion with multiple other electrophysiologists, the decision was made to medically manage his atrial and ventricular arrhythmias owing to high risk of further perforations of cardiac structures if ablation were to be pursued. Antiarrhythmic therapy with amiodarone and electrical cardioversion have improved symptoms thus far, without the need for further admissions for any sustained arrhythmias. Unfortunately, most recently, he has been admitted for a complicated contained sigmoid colon perforation.
Discussion
EDS encompasses a group of inherited heterogeneous disorders characterized by abnormal collagen synthesis. Systemic involvement can range from isolated skin and joint hyperextensibility to significant vascular and tissue fragility. Importantly, clinical manifestations overlap among different types of EDS, making clinical assessment of EDS type particularly challenging. Genetic testing in conjunction with clinical manifestations aid in the diagnosis of particular EDS subtypes. Also, rare variants of EDS have also been reported, thus underscoring the complexity in diagnosis.1
Cardiovascular involvement, most predominant in type IV (vascular subtype), includes spontaneous arterial dissections, aneurysms, hemorrhages/hematomas, mitral valve prolapse, aortic dilatation, and dysautonomia.2 Although vascular and valvular correlation has been noted, there is no clear link between EDS and cardiac arrhythmias, particularly ventricular tachycardia, that has been documented in current literature. Additionally, no link between myocardial fragility or cardiac rupture and EDS has been reported. Patients with EDS are at particular risk of periprocedural complications owing to vascular friability.3, 4 Serious consideration regarding procedural indications and alternative therapies should be pursued prior to embarking on any interventional or surgical procedure, particularly in the vascular subtype, although as demonstrated in our patient, vascular and myocardial complications can occur despite not having the classic type IV EDS diagnosis. Our patient likely has classic EDS (type I or II) with overlap of vascular (type IV) features. It was surmised that owing to his cardiac tissue fragility, during normal ventricular contractions, the RV lead sliced through the myocardium down the septum and through RV the diaphragmatic surface, leading to perforation. Genetic testing is pending.
To the best of our knowledge, this is the first report describing ventricular arrhythmias requiring ICD implantation in a patient with EDS. This case illustrates the hazards of cardiovascular percutaneous and surgical intervention in EDS patients, highlighting the diffuse nature of the connective tissue disorder. Cardiovascular involvement may not be limited to vascular and valvular structures, but can include the myocardium itself. Patients with EDS pose a unique management challenge in cardiac electrophysiology. Ablations and device implantations should be undertaken with extreme caution, thorough risk/benefit analysis, and comprehensive discussion.
References
- 1.Rombaut L., Malfait F., De Wandele I., Cools A., Thijs Y., De Paepe A., Calders P. Medication, surgery, and physiotherapy among patients with the hypermobility type of Ehlers-Danlos syndrome. Arch Phys Med Rehabil. 2011;92:1106. doi: 10.1016/j.apmr.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Pepin M., Schwarze U., Superti-Furga A., Byers P.H. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 3.Lum Y.W., Brooke B.S., Arnaoutakis G.J., Williams T.K., Black J.H., 3rd Endovascular procedures in patients with Ehlers-Danlos syndrome: a review of clinical outcomes and iatrogenic complications. Ann Vasc Surg. 2012;26:25. doi: 10.1016/j.avsg.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Eagleton M.J. Arterial complications of vascular Ehlers-Danlos syndrome. J Vasc Surg. 2016;64:1869–1880. doi: 10.1016/j.jvs.2016.06.120. [DOI] [PubMed] [Google Scholar]