Abstract
We aimed to explore the interaction among lncRNA MALAT1, miR‐129 and SOX2. Besides, we would investigate the effect of MALAT1 on the proliferation of glioma stem cells and glioma tumorigenesis. Differentially expressed lncRNAs in glioma cells and glioma stem cells were screened out with microarray analysis. The targeting relationship between miR‐129 and MALAT1 or SOX2 was validated by dual‐luciferase reporter assay. The expressions of MALAT1, miR‐129 and SOX2 mRNA in both glioma non‐stem cells and glioma stem cells were examined by qRT‐PCR assay. The impact of MALAT1 and miR‐129 on glioma stem cell proliferation was observed by CCK‐8 assay, EdU assay and sphere formation assay. The protein expression of SOX2 was determined by western blot. The effects of MALAT1 and miR‐129 on glioma tumour growth were further confirmed using xenograft mouse model. The mRNA expression of MALAT1 was significantly up‐regulated in glioma stem cells compared with non‐stem cells, while miR‐129 was significantly down‐regulated in glioma stem cells. MALAT1 knockdown inhibited glioma stem cell proliferation via miR‐129 enhancement. Meanwhile, miR‐129 directly targeted at SOX2 and suppressed cell viability and proliferation of glioma stem cells by suppressing SOX2 expression. The down‐regulation of MALAT1 and miR‐129 overexpression both suppressed glioma tumour growth via SOX2 expression promotion in vivo. MALAT1 enhanced glioma stem cell viability and proliferation abilities and promoted glioma tumorigenesis through suppressing miR‐129 and facilitating SOX2 expressions.
Keywords: glioma, lncRNA MALAT1, miR‐129, proliferation, SOX2
1. INTRODUCTION
Glioma is one of the most prevalent and aggressive malignant tumours in human central nervous system with high mortality rate around the world.1 Nowadays, although many advances have been achieved in treatment strategies, such as immunotherapy, stereotactic radiotherapy and new chemotherapy drugs, the recurrence rate and mortality rate were still not reduced because of metastasis.2, 3 Moreover, due to our poor understanding of glioma pathogenesis, therapeutic strategies for glioma are limited.4 Glioma stem cells (GSCs) are a subgroup of glioma cells with the ability of self‐renewal,5, 6 and are remarkably resistant to chemotherapy and radiotherapy in malignant gliomas.7 Thus, it is imperative to focus on the mechanisms of glioma tumorigenesis by elucidating GSCs.
Long non‐coding RNAs (lncRNAs), a group of non‐protein coding transcripts longer than 200 nucleotides, play a key role in many types of malignant tumours, including glioma.8 LncRNAs may function as competing endogenous RNAs (ceRNAs) to modulate miRNA expressions.9 Metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) is an lncRNA with a length of approximately 8000‐nt.8 Wang et al revealed that MALAT1 was aberrantly expressed in carcinoma cells.10 Ma et al11 found that MALAT1 was up‐regulated in glioma tissues and correlated with the progression of glioma. However, the molecular mechanism of MALAT1 in glioma cells remains unknown.
MicroRNAs (miRNAs) are short single‐stranded RNA molecules which function as a critical modulator in gene expression by wholly or partially binding to corresponding mRNAs.12 MiRNAs play a crucial role in the progression of various cancers, such as osteosarcoma 12 and glioma.13 MiR‐129 is a miRNA family mainly composed of miR‐129‐3p, miR‐129‐2‐3p and miR‐129‐5p.14 Previous researches have already demonstrated that miR‐129‐5p acted as a tumour inhibitor in multiple cancers such as ovarian cancer,15 breast cancer 16 and also glioma.17 Chen et al18 found that Notch‐1/ E2F7/Beclin‐1 axis was regulated and further the viability of malignant glioma cells could be impaired by up‐regulation of miR‐129. However, the role of miR‐129 in glioma stem cells has not been elucidated so far.
SRY (sex determining region Y)‐box 2 (SOX2) is revealed as a stemness marker in glioma 19 and a transcription factor highly associated with pluripotency.20 Current study investigated the relationship between SOX2 and miR‐129 to further elucidate the molecule network of miR‐129 in glioma stem cells progression. Activated SOX2 expression maintained stemness and self‐renewal of GSCs.21 SOX2 is associated with GBM stem‐like phenotype which is more resistant to γ‐radiation.22 Therefore, SOX2 expression changes are significant for GSC stemness maintainment.
This study hypothesized the promoter role of lnc MALAT1 in glioma and validated its effect in both in vivo and in vitro experiments. MALAT1 was suspected to bind to miR‐129 which target at SOX2, an oncogene, and their interaction was depicted in this study. By illustrating the underlying mechanism that facilitated glioma progression, this study may contribute to the application of glioma target therapy.
2. MATERIALS AND METHODS
2.1. Clinical specimens
Fourteen histologically verified glioma tissue specimens based on the WHO‐2007 classification from 2015‐2017 were obtained from patients treated with surgery at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Inclusion criteria were the following: (i) WHO graded gliomas confirmed by histopathology. Exclusion criteria were as follows: (i) an unknown IDH1‐mutation status, (ii) patients with a previous history of brain tumours, (iii) patients younger than 18 years of age. All glioma tissues were first preserved in liquid nitrogen and stored at −80°C for the subsequent experiment. This project was ratified by the Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and written informed consents were collected from all patients.
2.2. Glioma cell culture and stem cell isolation
Glioma cells were cultured in Dulbecco's modified Eagle medium (DMEM)/high glucose with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and were maintained in a humidified incubator at 37°C with 5% CO2. GSCs were separated as described previously from tissue cells.23, 24 Primary cells were detached with trypsin, washed once in FACs buffer (PBS containing 1%‐2% BSA and 5 mM EDTA), then stained with anti‐CD24‐FITC (Invitrogen, Carlsbad, CA, USA) and anti‐CD133‐PE (Invitrogen) using 10 μl of antibody per 106 cells, and incubated at 4°C for 15 minutes. Following incubation, cells were washed once with FACs buffer. The CD44+CD24− cells were considered to be GSCs.
2.3. Microarray analysis
GSE23806 microarray data obtained from Gene Expression Omnibus database was applied to the filtration of aberrantly expressed lncRNAs. “Match matrix” file from GEO website (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23806) were downloaded and processed for samples and corresponding probes. Corresponding platform information of GPL570 ([HG‐U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array) was downloaded and processed as well for matching probes and corresponding gene symbols. Expressions of lncRNAs in 14 conventional glioma cell lines (G121, G84, G120, G61, G112, G130, U118MG, SF268, G124, G118, G142, G44, SW1783 and G22) and 5 glioma stem‐like cell lines (GS01, GS02, GS03, GS04 and GS05) were analysed using unpaired T‐test method in the LIMMA package and the obtained P values were adjusted with Benjamini‐Hochberg method. A volcano plot filtering (fold change > 4, adjusted P‐value < .01) was drawn and all the differentially expressed lncRNAs were listed in the heat map.
2.4. QRT‐PCR
The extraction of total RNA was implemented by Trizol reagent (Invitrogen, USA), and the purity and concentration of RNA were detected by NanoDrop 1000 (Nanodrop Technologies Inc., USA). First Strand cDNA Synthesis Kit (Biochemical Industry Inc., USA) was used for the reverse transcription according to the instructions. Quantitative PCR was conducted through Bio‐Rad MiniOpticon real‐time PCR system, using Maxima SYBR Green qPCR Master Mix (2X) kit (Fermentas, Burlington, Canada) for amplified detection according to the manual. GAPDH was deemed as a reference gene of SOX2 and MALAT1, while U6 was an internal control of miR‐129. The relative expression levels of MALAT1, miR‐129 and SOX2 were determined by using the 2−ΔΔCT method. The primers were synthesized by Sangon Biotech (Shanghai, China). The detailed primers were exhibited in Table 1.
Table 1.
Primer sequences of qRT‐PCR
| Primer sequence (5′‐3′) | ||
|---|---|---|
| MALAT1 | Sense | ATGCGAGTTGTTCTCCGTCT |
| Anti‐Sense | TATCTGCGGTTTCCTCAAGC | |
| miR‐129 | Sense | CGGCGGTTTTTTGCGGTCTGGGCT |
| Anti‐Sense | CAACCTGGAGGACTCCATGCTG | |
| SOX2 | Sense | GGAGTTGTCAAGGCAGAGAAG |
| Anti‐Sense | CGCCGCCGATGATTGTTAT | |
| GAPDH | Sense | ACCCCGCCGCCTGTGGArGG |
| Anti‐Sense | TTCTGACGGCAGGTCAGGT | |
| U6 | Sense | TCGAACAGGAGGAGCAGAGAGCGA |
| Anti‐Sense | TCGAACAGGAGGAGCAGAGAGCGA | |
2.5. Western blot
The isolation of total protein from tissues and protein concentration measurement were performed by using RIPA lysis buffer and BCA protein concentration kit (Beyotime, Shanghai, China). Proteins were segregated by SDS‐PAGE and then transferred onto PVDF membranes (Invitrogen) in accordance with the instructions, followed by blocking in 5% nonfat milk for 60 minutes and incubation with primary antibodies against SOX2 (ab137385, 1:1000, Abcam corporation) at 4°C overnight. After that, the membranes were washed with Tris Buffered Saline Tween (TBST) every 5 minutes for four times and then incubated in HRP‐conjugated goat anti‐rabbit IgG secondary antibody (1:2000) for 2 hours. After being rinsed twice in TBST, the immunoreactive bands were developed using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Buckinghamshire, England).
2.6. Cell transfection
Glioma stem cells in logarithmic growth were first seeded in the culture dish, and then placed onto the 6‐well plate when cell growth reached 80%‐90% confluence. MALAT1 siRNA, miR‐129 mimics and miR‐129 inhibitor were synthesized by Genepharma Company (Shanghai, China). 293T cells were respectively transfected with lncRNA MALAT1 siRNA, miR‐129 mimics, miR‐129 inhibitor and negative control lncRNA MALAT1 or negative control mimics using Lipofectamine™ 2000 reagent (Life Technologies Inc., USA) following the instructions. After transfection for 48 hours, the sample was collected and transfection efficiency was detected. The experimental groups were generally divided into five groups as follows: Blank group (without transfection), Negative Control (NC) group (transfected with negative control lncRNA MALAT1 or mimics), si‐MALAT1 group (transfected with lncRNA MALAT1 siRNA), miR‐129 group (transfected with miR‐129 mimics) and miR‐129 inhibitor group (transfected with miR‐129 inhibitor).
2.7. Dual‐luciferase reporter gene assay
Plasmid pmirGLO vectors were bought from Promega Corporation (Madison, USA). The recombinant reporter gene pmir‐GLO‐MALAT1 and pmir‐GLO‐SOX2 was constructed. Glioma stem cells were seeded into the 24‐well culture plate until 90% confluency. Then Recombinant vector (wild‐type or mutated type) was transfected into the cells together with miR‐129 mimics or mimics control by using Lipofectamine 2000 reagent, followed by incubation for 48 hours. After that, the fluorescence intensity of transfected cells was examined by luciferase reporter assay kit (Promega, Madison, WI, USA).
2.8. CCK‐8 assay
Cell proliferation was assessed by Cell Counting Kit‐8 (CCK‐8; Beyotime, Shanghai, China). Transfected glioma stem cells were seeded into 96‐well culture plate at a density of 2 × 103 cells per well containing 10 μL CCK‐8 solutions and cultured overnight. The optical density (OD) value of each well was assessed at 24, 48, 72 and 96 hours using a microplate reader. Absorbance was recorded at 450 nm. The assay was repeated three times.
2.9. EdU assay
Transfected glioma stem cells were cultured in 96‐well plates. Briefly, glioma stem cells were incubated with EdU labelling medium at moderate concentration for 2 hours. The cells were then fixed with 0.5% TritonX‐100 in PBS (100 μL) for 25 minutes, and stained with 100 μL Apollo dye solution (Ribobio) for 30 minutes at room temperature. The cells were subsequently stained using DAPI (Invitrogen) and incubated for half an hour. The percentage of EdU positive cells was calculated using ImageJ software.
2.10. Sphere formation assay
Glioma stem cells were seeded into 6‐well plate at 5 × 103 cells/mL in the culture medium containing 20 ng/mL basic Fibroblast Growth Factor (bFGF) and Epidermal Growth Factor (EGF), 5 L g/mL insulin (Sigma‐Aldrich,St. Louis, MO, USA), 0.4% BSA (Invitrogen, USA) medium and 0.02% B27 (Invitrogen). After incubation for 7 days, cells were fixed using 10% formalin and photographed under a conventional microscope. Sphere Formation Efficiency (SFE) representing the ability of sphere formation (diameter > 75 μm) was calculated. The formula of SFE was: the numbers of cell sphere in each well / the total number of cells originally seeded in each well.
2.11. Xenograft mouse model
Nine male nude mice (5 weeks old) were purchased from Shanghai Experimental Animal Centre (Shanghai, China). Animal experiments were strictly conducted in accordance with the protocols of Animal Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. MALAT1 shRNA sequence was sub‐cloned into the pGLV2‐U6‐Puro vector (Genepharma), the lentivirus was packaged in 293T cells according to the protocol and then infected glioma cells at the multiplicity of infection (MOI) of 20. Stable MALAT1 knockout cells were selected using 1 μg/mL puromycin. AgomiR‐129 (Rio‐bio, Guangzhou, China) was then transfected into glioma stem cells using Lipofectamine 2000 (Invitrogen). After transfection, cells were collected and washed thrice with ice‐cold PBS and then suspended in PBS. 2 × 106 transfected glioma stem cells were injected into the left flanks of nude mice. The mice were sacrificed executed 28 days after injection. Mice injected with glioma stem cells without transfection was diagnosed as control group. Tumour volumes were detected every week. Tumour volume was monitored by measuring the length and width and calculated using the formula: V = (L × W 2) × 0.5. At 28th day after implantation, mice were sacrificed and isolated tumours were weighed. In addition, total proteins were extracted from the tumour samples and detected by Western blot.
2.12. Statistical analysis
Statistical analyses were performed through GraphPad Prism 6.0 (GraphPad Software, San Diego, California, USA). All quantitative data are presented as means ± standard deviation (SD). Differences between groups were compared using Student's t‐test while comparison in multiple groups was practiced with one‐way ANOVA. Benjamini‐Hochberg was used to adjust multiple testing for selection of differentially expressed lncRNAs. Statistical significance was based on P value < .05.
3. RESULTS
3.1. MALAT1 is highly expressed in glioma stem cells
The differentially expressed lncRNAs, including 43 up‐regulated lncRNAs and 16 down‐regulated lncRNAs, were screened out in glioblastoma stem‐like cells and conventional glioma cells based on the filtration criteria of fold change > 4 and adjusted P < .01 (Table 2, Figure 1A). Microarray analysis results revealed that MALAT1 was significantly up‐regulated in glioma stem‐like cells. To validate the prediction, we used 14 patient tissue samples to isolate GBCs. Those CD133+CD24− cells were regarded as GSCs as reported previously.25, 26 Characteristics of patients were listed in Table 3. After we obtained glioma stem cells from glioma cells, qRT‐PCR was utilized to measure the expression level of MALAT1. As shown in Figure 1B,C, MALAT1 expression in glioma stem cells was remarkably higher than compared with non‐stem cells (P < .0001).
Table 2.
The results of fold‐change and P value of differential genes
| Gene symbol | logFC | P.Value | Adj. P.Value |
|---|---|---|---|
| TRDV3 | 8.575920353 | 1.69E‐18 | 1.36E‐15 |
| TRDC | 8.182903102 | 8.45E‐21 | 1.02E‐17 |
| SNORD3A | 7.003737202 | 2.01E‐23 | 4.85E‐20 |
| LINC01158 | 6.679955378 | 1.45E‐07 | 2.99E‐06 |
| LINC00461 | 4.680745748 | 0.001802104 | 0.007229372 |
| LINC01003 | 4.616437767 | 4.15E‐08 | 1.13E‐06 |
| FGF14‐AS2 | 4.250067783 | 2.40E‐11 | 2.52E‐09 |
| CAHM | 4.035562004 | 2.63E‐13 | 5.77E‐11 |
| FGF14‐AS2 | 3.778449578 | 4.34E‐10 | 2.56E‐08 |
| LINC00936 | 3.764411621 | 1.24E‐09 | 6.25E‐08 |
| PEG3‐AS1 | 3.719100724 | 3.95E‐13 | 7.95E‐11 |
| ARHGAP5‐AS1 | 3.53093737 | 9.48E‐10 | 4.98E‐08 |
| PAXIP1‐AS1 | 3.412324551 | 3.62E‐07 | 6.43E‐06 |
| CCEPR | 3.056910193 | 1.11E‐15 | 5.34E‐13 |
| ILF3‐AS1 | 3.054249994 | 1.97E‐11 | 2.38E‐09 |
| ZNF793‐AS1 | 2.966212306 | 1.21E‐08 | 4.29E‐07 |
| YTHDF3‐AS1 | 2.876594005 | 4.92E‐11 | 4.40E‐09 |
| GS1‐124K5.4 | 2.859079983 | 5.22E‐11 | 4.51E‐09 |
| MINCR | 2.856789138 | 1.16E‐07 | 2.58E‐06 |
| UG0898H09 | 2.840202417 | 0.000173758 | 0.001067752 |
| URB1‐AS1 | 2.724625797 | 3.22E‐12 | 4.58E‐10 |
| LINC01315 | 2.591253926 | 1.04E‐08 | 3.86E‐07 |
| TRAF3IP2‐AS1 | 2.546802754 | 4.06E‐15 | 1.64E‐12 |
| BAALC‐AS2 | 2.54080024 | 3.42E‐11 | 3.31E‐09 |
| MALAT1 | 2.523271109 | 3.14E‐05 | 0.000261728 |
| IDH1‐AS1 | 2.491701012 | 2.70E‐12 | 4.35E‐10 |
| DLEU2 | 2.488769448 | 1.57E‐09 | 7.57E‐08 |
| TOLLIP‐AS1 | 2.479583102 | 3.21E‐17 | 1.94E‐14 |
| LINC00665 | 2.464888348 | 2.14E‐11 | 2.43E‐09 |
| BOLA3‐AS1 | 2.457662529 | 4.87E‐14 | 1.47E‐11 |
| PWAR6 | 2.443116861 | 7.73E‐05 | 0.000546506 |
| SEPT7‐AS1 | 2.422505772 | 1.00E‐13 | 2.69E‐11 |
| ATP13A4‐AS1 | 2.395337515 | 1.58E‐10 | 1.09E‐08 |
| PRKAG2‐AS1 | 2.367825007 | 1.32E‐07 | 2.85E‐06 |
| TBX2‐AS1 | 2.316519621 | 0.000103617 | 0.000697036 |
| ELOVL2‐AS1 | 2.243879181 | 7.32E‐15 | 2.52E‐12 |
| GBAT2 | 2.216352939 | 1.43E‐06 | 2.00E‐05 |
| ENO1‐AS1 | 2.175228792 | 1.62E‐10 | 1.09E‐08 |
| LINC01465 | 2.161541462 | 1.28E‐10 | 9.40E‐09 |
| LINC00526 | 2.158895263 | 4.93E‐10 | 2.77E‐08 |
| MALAT1 | 2.135200437 | 0.000172118 | 0.001061614 |
| SNHG9 | 2.087679222 | 9.08E‐05 | 0.000621076 |
| LINC00938 | 2.029574413 | 2.45E‐06 | 3.19E‐05 |
| SNHG15 | 2.00342226 | 0.000455262 | 0.002349268 |
| BRWD1‐IT2 | 2.000447174 | 1.33E‐13 | 3.21E‐11 |
| CEBPZOS | −2.030429459 | 4.17E‐05 | 0.000332317 |
| MMP24‐AS1 | −2.08914189 | 1.63E‐05 | 0.000152843 |
| DLEU2 | −2.098699898 | 0.000268268 | 0.001504038 |
| EBLN3 | −2.114406503 | 1.09E‐08 | 4.00E‐07 |
| SNHG16 | −2.286897084 | 2.15E‐07 | 4.05E‐06 |
| NEAT1 | −2.33227962 | 0.002655131 | 0.009759727 |
| GAS5 | −2.431689548 | 2.76E‐07 | 5.06E‐06 |
| PSMD5‐AS1 | −2.451857035 | 0.000929539 | 0.004203816 |
| AGAP2‐AS1 | −2.673571961 | 1.16E‐07 | 2.58E‐06 |
| SERTAD4‐AS1 | −2.758723401 | 1.42E‐05 | 0.000137251 |
| LINC00152 | −3.068202392 | 2.36E‐09 | 1.09E‐07 |
| STX17‐AS1 | −3.299781124 | 0.000265796 | 0.001503275 |
| MSC‐AS1 | −3.711235086 | 0.002113499 | 0.008140511 |
| IGHG1 | −3.984993575 | 2.70E‐06 | 3.47E‐05 |
| LINC01116 | −4.329934135 | 8.08E‐07 | 1.25E‐05 |
| LOXL1‐AS1 | −5.600952763 | 7.29E‐06 | 7.79E‐05 |
| LINC01296 | −5.95432695 | 1.69E‐06 | 2.33E‐05 |
Negative values (−) denote low expression and positive denote high expression. LogFC represents the gene expression fold change of stem cells compared with cancer cells. P.Value and adj. P.Value refer to statistical values, P < .01 indicates statistical significance.
Figure 1.
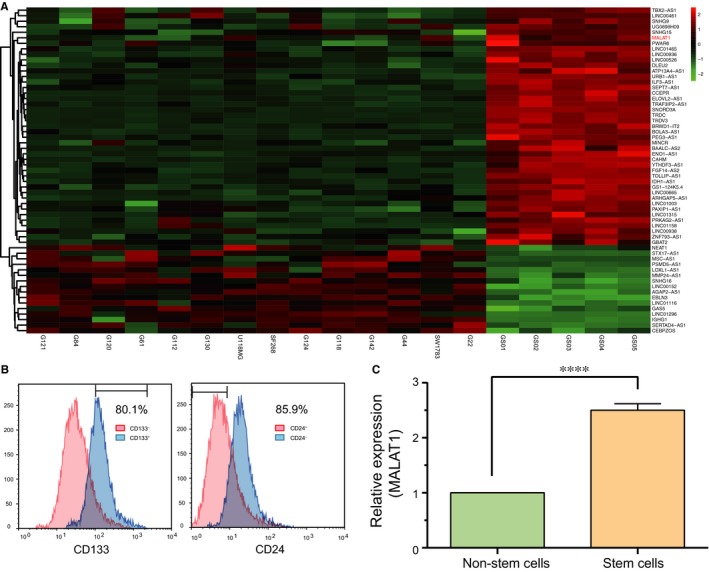
MALAT1 is highly expressed in glioma stem cells. A, The differentially expressed lncRNAs in glioma stem cells (including GS01, GS02, GS03, GS04 and GS05) and glioma tissue cells (including G121, G84, G120, G61, G112, G130, U118MG, SF268, G124, G118, G142, G44, SW1783 and G22) were screened through microarray analysis. MALAT1 was found to be significantly up‐regulated in GSCs. B, Glioma stem cells were isolated from glioma cancer cells with flow cytometry and those with CD144+ CD24− markers were considered as GSCs. C, The expression level of MALAT1 in glioma stem cells was remarkably higher than that in glioma tissue cells examined by qRT‐PCR. ****P < .0001, compared with cancer cells (glioma tissue cells)
Table 3.
Patient characteristics
| Factor | Number |
|---|---|
| Patients | 14 |
| Gender (m, f) | 10/4 |
| Age, y (median, range) | 47.6 (18.1‐84.0) |
| Surgical procedure | |
| Biopsy | 238 |
| Surgery | 62 |
| WHO grade | |
| II | 2 |
| III | 6 |
| IV | 6 |
| Tumour location | |
| Frontal | 3 |
| Temporal | 6 |
| Parietal | 2 |
| Occipital | 1 |
| Midline/basal ganglia/corpus callosum | 2 |
| IDH1 R132H status | |
| Wild‐type | 8 |
| Mutated | 6 |
3.2. MALAT1 promotes the growth of glioma stem cells
After transfection with si‐MALAT1 (si‐MALAT1#1 or si‐MALAT1#2), the expression of MALAT1 in glioma stem cells significantly decreased (Figure 2A, P < .01). The result of CCK‐8 assay suggested that cell viability in two si‐MALAT1 groups significantly reduced in comparison with NC group, indicating that cell growth of GBCs was significantly suppressed by si‐MALAT1 (Figure 2B, both P < .01). Meanwhile, the percentage of EdU positive cells in si‐MALAT1 group also dramatically declined, suggesting that knockdown of MALAT1 could significantly repress the proliferation of glioma stem cells (Figure 2C,D, both P < .01). In addition, sphere formation assay revealed that the sphere formation efficiency in si‐MALAT1 groups significantly decreased in comparison with NC group (Figure 2E,F, both P < .01). Therefore, we concluded that MALAT1 promoted GSCs proliferation.
Figure 2.
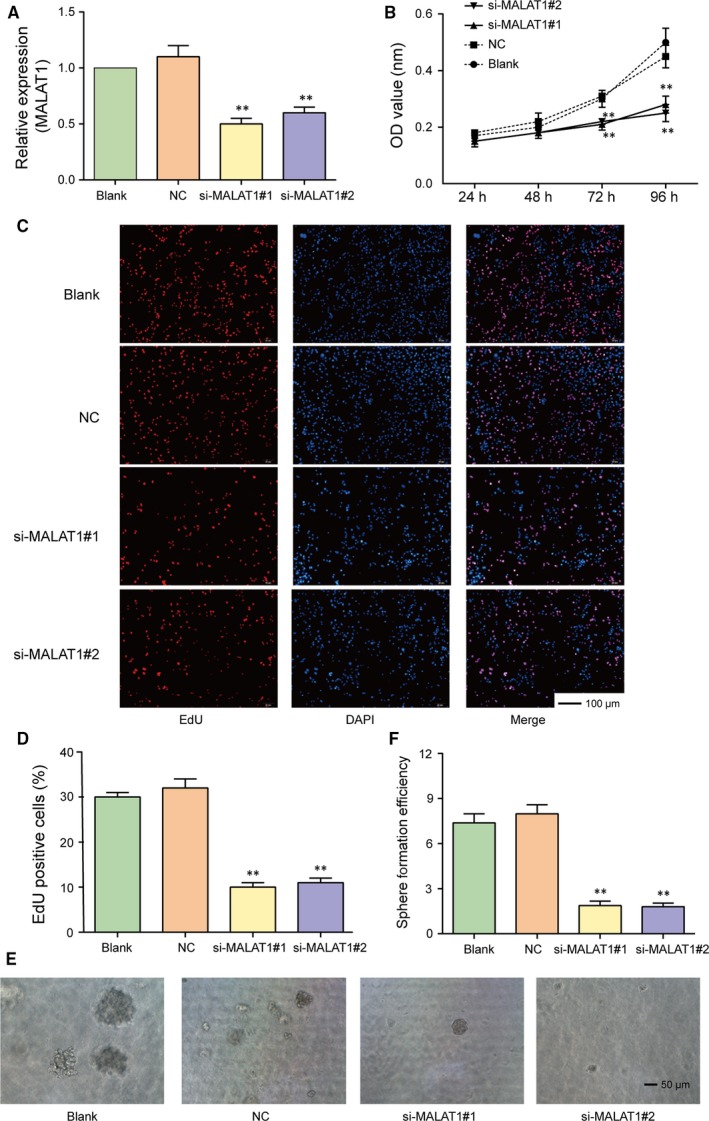
MALAT1 promotes the growth of glioma stem cells. A, The expression of MALAT1 in glioma stem cells significantly decreased after transfection with si‐MALAT1 (si‐MALAT1#1 or si‐MALAT1#2). B, The cell growth of glioma stem cells transfected with si‐MALAT1 was suppressed significantly confirmed by CCK‐8 assay. C‐D, The percentage of EdU positive cells transfected with si‐MALAT1 dramatically declined, suggesting that knockdown of MALAT1 could significantly repress the proliferation of glioma stem cells. E‐F, The sphere formation efficiency of glioma stem cells in si‐MALAT1 groups significantly decreased in comparison with NC group detected by sphere formation assay. Scale bar, 50 μm. **P < .01, compared with NC group
3.3. MiR‐129 is a potential target of MALAT1
Predicted by miRcode database (http://www.mircode.org/), potential targeting relationships between MALAT1 and miR‐129, miR‐205, miR‐155, miR‐194, as well as the binding sites were presented in Figure 3A,B. Dual‐luciferase reporter assay results indicated that only miR‐129 mimics restrained the luciferase activity of the reporting vector containing MALAT1 sequence significantly (Figure 3C, P < .01). We also constructed a MALAT1‐WT 3′ UTR and a MALAT1‐MUT 3′ UTR luciferase reporter vectors to validate the direct targeting relationship between miR‐129 and MALAT1. The luciferase activity of the cells co‐transfected with MALAT1‐WT 3′ UTR and miR‐129 mimics group was considerably weaker (Figure 3D,E, P < .01). Additionally, we also examined the expression of miR‐129 using qRT‐PCR after si‐MALAT1 transfection. As shown in Figure 3F, the expression level of miR‐129 was significantly up‐regulated in si‐MALAT1#1 and si‐MALAT1#2 groups in comparison with NC group (both P < .01).
Figure 3.
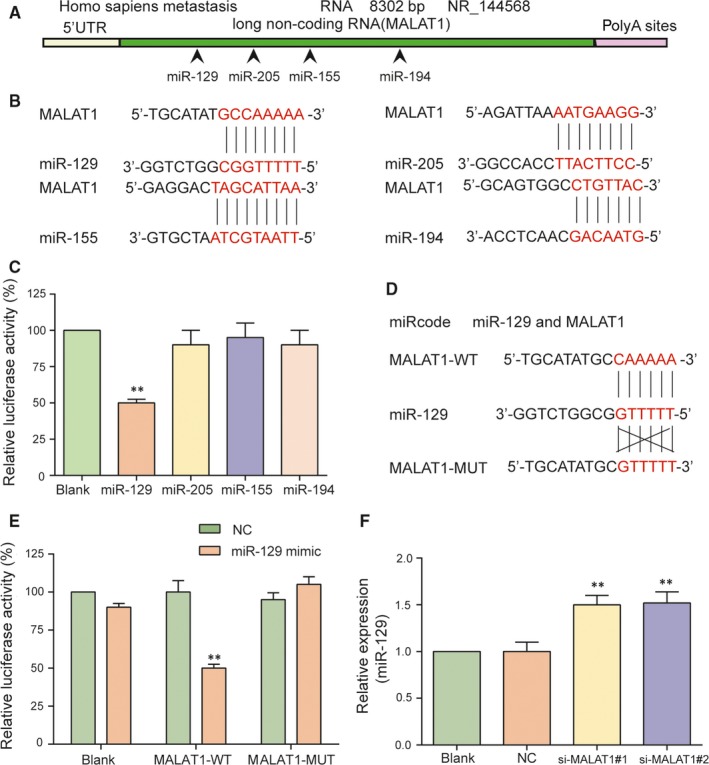
MiR‐129 is a potential target of MALAT1. A‐B, The binding sites of MALAT1 and miR‐129, miR‐205, miR‐155 as well as miR‐194 were predicted by miRcode. C, Compared with blank group, the luciferase activity in miR‐129 group significantly decreased, while there was no significant difference between miR‐205, miR‐155, miR‐194 and blank group. D‐E, Verified by dual luciferase reporter gene assay, MALAT1 could directly target miR‐129. F, The expression level of miR‐129 was significantly up‐regulated in si‐MALAT1#1 and si‐MALAT1#2 groups in comparison with NC group examined by qRT‐PCR. **P < .01, compared with blank group
3.4. MiR‐129 restrains the propagation of glioma stem cells
The results of qRT‐PCR indicated that miR‐129 expression in glioma stem cells was remarkably lower than that in non‐stem cells (Figure 4A, P < .001). Moreover, after being transfected with miR‐129 mimics, the expression of miR‐129 in glioma stem cells dramatically increased, while the expression level of miR‐129 in cells transfected with miR‐129 inhibitor significantly decreased (Figure 4B, both P < .01). The results from CCK‐8 assay and EdU assay suggested that the glioma stem cell viability was significantly attenuated in miR‐129 overexpression group, whereas the proliferation ability of glioma stem cells in miR‐129 inhibitor group was drastically enhanced compared with NC group (Figure 4C‐E, all P < .01). Meanwhile, the results of sphere formation assay indicated that the sphere formation efficiency of glioma stem cells in miR‐129 mimics group was notably lower (P < .0001), while the cells transfected with miR‐129 inhibitor presented a higher sphere formation efficiency in comparison with NC group (Figure 4F,G, P < .01). Therefore, overexpression of miR‐129 could significantly suppress the proliferation ability and stemness of glioma stem cells.
Figure 4.
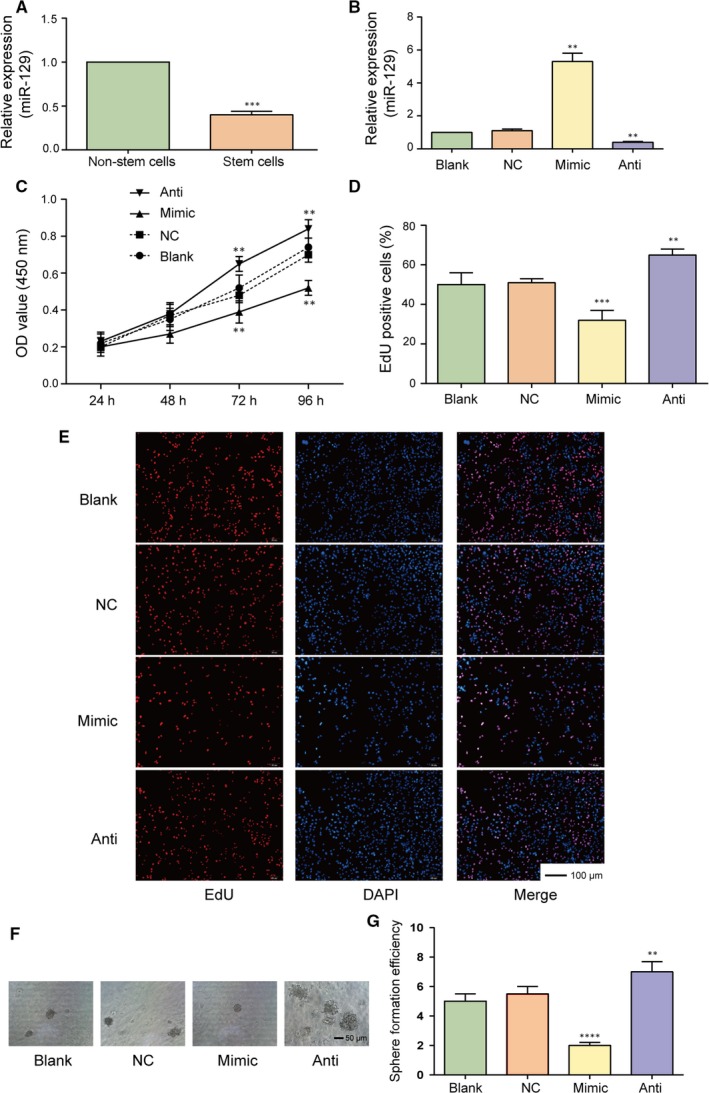
MiR‐129 suppresses the proliferation of glioma stem cells. A, The expression of miR‐129 in glioma stem cells was significantly lower than that in the glioma tissue cells detected by qRT‐PCR. B, The expression of miR‐129 in miR‐129 mimics group dramatically increased, while that in miR‐129 inhibitor group significantly decreased observed by qRT‐PCR. C‐E, CCK‐8 assay and EdU assay showed that cell proliferation of glioma stem cells were significantly attenuated in miR‐129 overexpression group but remarkably enhanced in miR‐129 inhibitor group compared with NC group. F‐G, The sphere formation assay results also revealed that the sphere formation efficiency of glioma stem cells in miR‐129 mimics group was significantly lower than that in NC group, while the cells transfected with miR‐129 inhibitor presented higher sphere formation efficiency in comparison with NC group determined by sphere formation assay. Scale bar, 100 μm. **P < .01. ***P < .001, ****P < .0001, compared with NC group
3.5. SOX2 is a direct target of miR‐129
Previous studies ever identified that SOX2 was required for cancer cell line progression in lung and esophageal squamous carcinoma, highlighting the importance of SOX2 as a lineage‐survival oncogene.27 High SOX2 expression has been associated with several human solid tumours, including glioma.28 The present study evaluated that expression of SOX2 mRNA in glioma stem cells was significantly higher than that in glioma cells detected by qRT‐PCR (Figure 5A, P < .001). TargetScan predicted the direct targeting relationship between miR‐129 and SOX2 and the dual‐luciferase reporter assay validated their direct targeting relationship since the luciferase activity in SOX2‐WT + miR‐129 mimics group was remarkably weaker compared with that in NC group (Figure 5B,C, P < .001). Hence, SOX2 was a direct target of miR‐129. Furthermore, miR‐129 mimic suppressed SOX2 expression, while miR‐129 inhibitor enhanced SOX2 expression (Figure 5D, P < .01). In addition, after being transfected with MALAT1 siRNA (si‐MALAT1#1 or si‐MALAT1#2), the mRNA and protein expressions of SOX2 were significantly down‐regulated (Figure 5E, P < .01). We thus confirmed negative regulation between miR‐129 and SOX2 and positive regulation between MALAT1 and SOX2.
Figure 5.
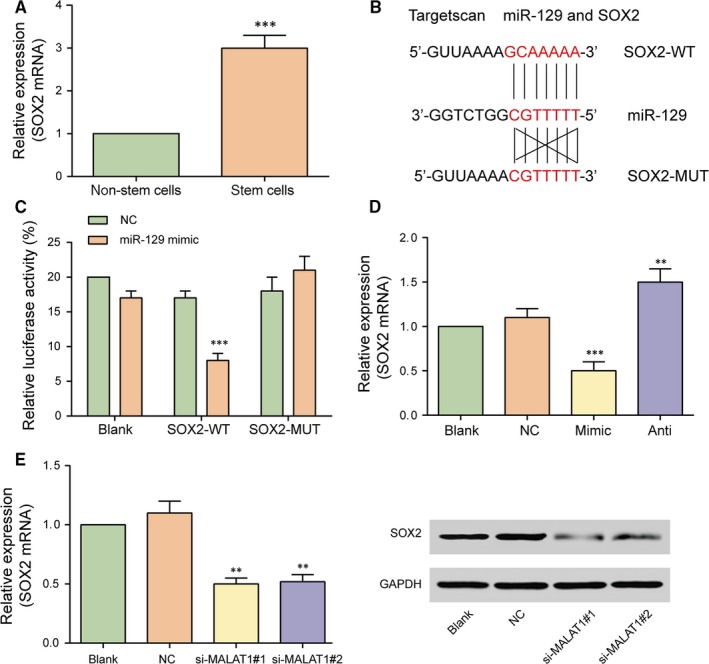
SOX2 is a direct target of miR‐129. A, The expression of SOX2 mRNA in glioma stem cells was significantly higher than that in glioma tissue cells detected by qRT‐PCR. ***P < .001, compared with cancer cells (glioma tissue cells). B, SOX2 was a candidate target of miR‐129 predicted by TargetScan. C, The targeted relationship between miR‐129 and SOX2 was validated by dual luciferase reporter gene assay. D, The expression of SOX2 in glioma stem cells transfected with miR‐129 mimics remarkably decreased, while glioma stem cells transfected with miR‐129 inhibitor presented a significant increase in the expression of SOX2. E, Compared with NC group, SOX2 mRNA and protein expression drastically decreased after transfected with si‐MALAT1#1 and si‐MALAT1#2 detected by qRT‐PCR and Western blot respectively. **P < .01, ***P < .001, all compared with NC group
3.6. The down‐regulation of MALAT1 expression and miR‐129 overexpression suppresses glioma tumour growth in vivo
Xenograft mice models were established by subcutaneously injecting glioma stem cells with different expression of MALAT1 and miR‐129. The tumour size was measured every 7 days. At the 28th day, mice were sacrificed and tumours were obtained and weighed. The results disclosed that the tumour volume and weight in sh‐MALAT1 and AgomiR‐129 groups significantly reduced in comparison with control group (Figure 6A‐C, all P < .01). Meanwhile, western blot was used to examine the relative protein expression of SOX2 in mice. Consistent with the previous experiment results, the expression of SOX2 protein in mice tumour was significantly down‐regulated after stable transfection with sh‐MALAT1 or AgomiR‐129 mimics (Figure 6D). Overall, down‐regulation of MALAT1 or overexpression of miR‐129 suppressed glioma tumour growth in vivo.
Figure 6.
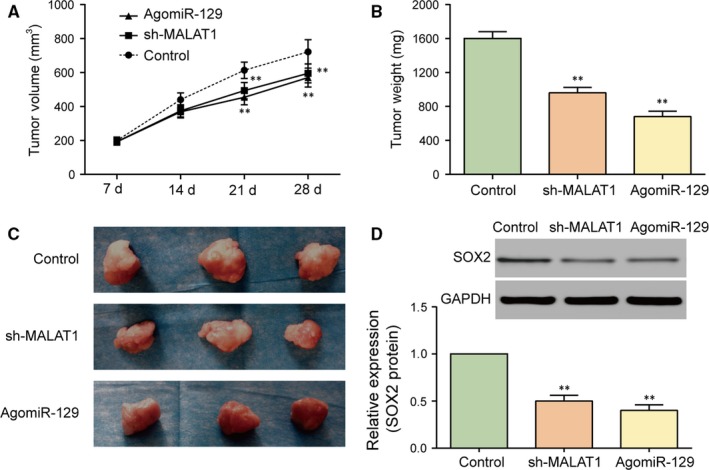
Either MALAT1 down‐regulation or miR‐129 overexpression suppresses glioma tumour growth in vivo. A‐C, The tumour volume and weight of mice transfected with sh‐MALAT1 and AgomiR‐129 significantly reduced compared with control group. D, The expression of SOX2 protein in mice transfected with sh‐MALAT1 or AgomiR‐129 was significantly down‐regulated. **P < .01, compared with control group
4. DISCUSSION
Glioma has been regarded as a kind of aggressive malignancy of the central nervous system worldwide. Glioma stem cells play essential role in glioma tumorigenesis. Previous studies have revealed that MALAT1 functions as oncogenic lncRNA in multiple cancers.29 Chen et al30 reported that the expression of MALAT1 was down‐regulated in preeclampsia, suppressing proliferation and cell cycle of JEG‐3 cells. Ma et al11 revealed that MALAT1 was correlated with the malignant status in glioma. Li et al9 found that MALAT1 expression was up‐regulated in glioma, promoting cell propagation and inhibiting cell apoptosis. Consistent with previous research results, our data demonstrated that MALAT1 was involved in glioma tumorigenesis as a tumour promoter. Through CCK‐8 proliferation assay, EdU assay and sphere formation assay, we also found that MALAT1 could promote the proliferation of glioma stem cells.
Emerging evidence has indicated that lncRNA could sponge miRNA and regulate the functions of miRNAs.31 Previous studies also indicated that MALAT1 presented various functions as miRNA sponges in different cancers. For example, MALAT1 promoted malignant melanoma growth and metastasis by sponging miR‐22.32 In our research, MALAT1 bound to miR‐129 directly to suppress its expression, which was firstly studied.
Earlier studies have reported that miR‐129‐2 suppressed glioma by targeting HMGB1 in glioma in an DNA methylation way.33 MiR‐129 also impair human malignant glioma progression via autophagic flux enhancement by regulating a novel Notch‐1/ E2F7/Beclin‐1 axis.18 Kang et al34 revealed that miR‐129‐2 suppressed proliferation of esophageal carcinoma cells through down‐regulation of SOX4 expression. Elevated SOX2 enforces glioblastoma stem cell identity.35 High miR‐21/low SOX2 axis was capable of classifying patients with longer survival.36 We also diagnosed miR‐129 as a tumour suppressor via SOX2 modulation firstly in glioma tumorigenesis. Besides, the MALAT1/miR‐129/SOX4 was of great significance in present molecular system investigation.
As an important pluripotent marker of stem cells, SOX2 has been recognized as serving a crucial role in maintaining the properties of cancer stem cells.37 According to the study of Wang et al38 SOX2 was considered as a predictor of survival in gastric cancer to inhibit cell proliferation and metastasis. Chen et al also revealed that silencing of SOX2 could regulate the apoptosis rate of human lung cancer cells.39 In our research, we speculated that the interaction between miR‐129 and SOX2 was associated with the suppression of glioma tumorigenesis and the inhibition of glioma stem cell activities. The results of experiments indicated that miR‐129 inhibited the expression of SOX2, suppressing GSCs proliferation. On the contrary, MALAT1 promoted the expression of SOX2, thus boosting the viability and proliferation of GSCs. Additionally, the effects of MALAT1 and miR‐129 on the glioma tumour were confirmed in a xenograft mouse model, indicating that MALAT1 promoted glioma tumour growth by regulating miR‐129 and SOX2. However, some concerns still existed in the current study. For example, MALAT1 could bind to other miRNAs in glioma stem cells. The downstream pathway of SOX2 MALAT1/miR‐129/SOX4 axis could further been investigated as well.
5. CONCLUSION
In summary, our research showed that MALAT1 was up‐regulated in glioma stem cells and acted as a tumour promoter in glioma progression. Silencing of MALAT1 suppressed the proliferation and stemness of GSCs and in vivo tumour growth via up‐regulating miR‐129 and further inhibiting SOX2, providing a promising therapy target for the treatment of glioma.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Zhiyong Xiong and Luyang Wang contributed to research conception and design as well as manuscript drafting; Ye Yuan analysed and interpreted data; Luyang Wang made statistical analysis; Qiangping Wang revised the manuscript and took the role of funding collectors. In addition, all authors approved final manuscript.
Xiong Z, Wang L, Wang Q, Yuan Y. LncRNA MALAT1/miR‐129 axis promotes glioma tumorigenesis by targeting SOX2. J Cell Mol Med. 2018;22:3929–3940. 10.1111/jcmm.13667
Funding information
The study was supported by the Funds for Creative Research of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (02.03.2017–65).
Zhiyong Xiong and Luyang Wang are first co‐authors.
[Correction added on 14 June 2018, after first online publication: The title has been amended to reflect the correct information discussed throughout the article]
REFERENCES
- 1. Quan J, Qu J, Zhou L. Microrna‐539 inhibits glioma cell proliferation and invasion by targeting dixdc1. Biomed Pharmacother. 2017;93:746‐753. [DOI] [PubMed] [Google Scholar]
- 2. Popescu ID, Codrici E, Albulescu L, et al. Potential serum biomarkers for glioblastoma diagnostic assessed by proteomic approaches. Proteome Sci. 2014;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruceru ML, Enciu AM, Popa AC, et al. Signal transduction molecule patterns indicating potential glioblastoma therapy approaches. OncoTargets Ther. 2013;6:1737‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhi T, Jiang K, Zhang C, et al. Microrna‐1301 inhibits proliferation of human glioma cells by directly targeting n‐ras. Am J Cancer Res. 2017;7:982‐998. [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Xiao Z, Du X, Huang L, Du G. Silencing of the long non‐coding rna neat1 suppresses glioma stem‐like properties through modulation of the mir‐107/cdk6 pathway. Oncol Rep. 2017;37:555‐562. [DOI] [PubMed] [Google Scholar]
- 6. Codrici E, Enciu AM, Popescu ID, Mihai S, Tanase C. Glioma stem cells and their microenvironments: providers of challenging therapeutic targets. Stem Cells Int. 2016;2016:5728438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bazzoli E, Pulvirenti T, Oberstadt MC, et al. Mef promotes stemness in the pathogenesis of gliomas. Cell Stem Cell. 2012;11:836‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Yuan J, Li L, Yang Y, Xu X, Wang Y. Long non‐coding rna xist exerts oncogenic functions in human glioma by targeting mir‐137. Am J Transl Res. 2017;9:1845‐1855. [PMC free article] [PubMed] [Google Scholar]
- 9. Li Z, Xu C, Ding B, Gao M, Wei X, Ji N. Long non‐coding rna malat1 promotes proliferation and suppresses apoptosis of glioma cells through derepressing rap1b by sponging mir‐101. J Neurooncol. 2017;134:19‐28. [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Li M, Wang Z, et al. Silencing of long noncoding rna malat1 by mir‐101 and mir‐217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J Biol Chem. 2015;290:3925‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma KX, Wang HJ, Li XR, et al. Long noncoding rna malat1 associates with the malignant status and poor prognosis in glioma. Tumour Biol. 2015;36:3355‐3359. [DOI] [PubMed] [Google Scholar]
- 12. Jiang K, Zhi T, Xu W, et al. Microrna‐1468‐5p inhibits glioma cell proliferation and induces cell cycle arrest by targeting rrm1. Am J Cancer Res. 2017;7:784‐800. [PMC free article] [PubMed] [Google Scholar]
- 13. Labak CM, Wang PY, Arora R, et al. Glucose transport: meeting the metabolic demands of cancer, and applications in glioblastoma treatment. Am J Cancer Res. 2016;6:1599‐1608. [PMC free article] [PubMed] [Google Scholar]
- 14. Xu H, Hu Y, Qiu W. Potential mechanisms of microrna‐129‐5p in inhibiting cell processes including viability, proliferation, migration and invasiveness of glioblastoma cells u87 through targeting fndc3b. Biomed Pharmacother. 2017;87:405‐411. [DOI] [PubMed] [Google Scholar]
- 15. Tan G, Cao X, Dai Q, et al. A novel role for microrna‐129‐5p in inhibiting ovarian cancer cell proliferation and survival via direct suppression of transcriptional co‐activators yap and taz. Oncotarget. 2015;6:8676‐8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang QY, Tang J, Zhou CX, Zhao Q. The down‐regulation of mir‐129 in breast cancer and its effect on breast cancer migration and motility. Sheng Li Xue Bao. 2012;64:403‐411. [PubMed] [Google Scholar]
- 17. Yang Y, Huang JQ, Zhang X, Shen LF. Mir‐129‐2 functions as a tumor suppressor in glioma cells by targeting hmgb1 and is down‐regulated by DNA methylation. Mol Cell Biochem. 2015;404:229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Zhang Y, Shi Y, et al. Mir‐129 triggers autophagic flux by regulating a novel notch‐1/ e2f7/beclin‐1 axis to impair the viability of human malignant glioma cells. Oncotarget. 2016;7:9222‐9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soroceanu L, Matlaf L, Khan S, et al. Cytomegalovirus immediate‐early proteins promote stemness properties in glioblastoma. Cancer Res. 2015;75:3065‐3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hubert CG, Rivera M, Spangler LC, et al. A three‐dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Can Res. 2016;76:2465‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin J, Park G, Kim TH, et al. Pigment epithelium‐derived factor (pedf) expression induced by egfrviii promotes self‐renewal and tumor progression of glioma stem cells. PLoS Biol. 2015;13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez‐Bertoni H, Lal B, Li A, et al. Dnmt‐dependent suppression of microrna regulates the induction of gbm tumor‐propagating phenotype by oct4 and sox2. Oncogene. 2015;34:3994‐4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu M, Xue Y, Zheng J, et al. Linc00152 promotes malignant progression of glioma stem cells by regulating mir‐103a‐3p/fezf1/cdc25a pathway. Mol Cancer. 2017;16:110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. Cd44 + cd24(‐) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem‐like cells give rise to tumour endothelium. Nature. 2010;468:829‐833. [DOI] [PubMed] [Google Scholar]
- 26. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755‐768. [DOI] [PubMed] [Google Scholar]
- 27. Bass AJ, Watanabe H, Mermel CH, et al. Sox2 is an amplified lineage‐survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Annovazzi L, Mellai M, Caldera V, et al. Sox2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics. 2011;8:139‐147. [PubMed] [Google Scholar]
- 29. Li J, Wang J, Chen Y, et al. Lncrna malat1 exerts oncogenic functions in lung adenocarcinoma by targeting mir‐204. Am J Cancer Res. 2016;6:1099‐1107. [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Meng T, Liu X, et al. Long non‐coding rna malat‐1 is downregulated in preeclampsia and regulates proliferation, apoptosis, migration and invasion of jeg‐3 trophoblast cells. Int J Clin Exp Pathol. 2015;8:12718‐12727. [PMC free article] [PubMed] [Google Scholar]
- 31. Cui B, Li B, Liu Q, Cui Y. Lncrna ccat1 promotes glioma tumorigenesis by sponging mir‐181b. J Cell Biochem. 2017. Dec;118(12):4548‐4557. [DOI] [PubMed] [Google Scholar]
- 32. Luan W, Li L, Shi Y, et al. Long non‐coding rna malat1 acts as a competing endogenous rna to promote malignant melanoma growth and metastasis by sponging mir‐22. Oncotarget. 2016;7:63901‐63912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhai J, Qu S, Li X, et al. Mir‐129 suppresses tumor cell growth and invasion by targeting pak5 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;464:161‐167. [DOI] [PubMed] [Google Scholar]
- 34. Kang M, Li Y, Liu W, et al. Mir‐129‐2 suppresses proliferation and migration of esophageal carcinoma cells through downregulation of sox4 expression. Int J Mol Med. 2013;32:51‐58. [DOI] [PubMed] [Google Scholar]
- 35. Bulstrode H, Johnstone E, Marques‐Torrejon MA, et al. Elevated foxg1 and sox2 in glioblastoma enforces neural stem cell identity through transcriptional control of cell cycle and epigenetic regulators. Genes Dev. 2017;31:757‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sathyan P, Zinn PO, Marisetty AL, et al. Mir‐21‐sox2 axis delineates glioblastoma subtypes with prognostic impact. J Neurosci. 2015;35:15097‐15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He J, Shi J, Zhang K, et al. Sox2 inhibits wnt‐beta‐catenin signaling and metastatic potency of cisplatin‐resistant lung adenocarcinoma cells. Mol Med Rep. 2017;15:1693‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang S, Tie J, Wang R, et al. Sox2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating pten. Cancer Lett. 2015;358:210‐219. [DOI] [PubMed] [Google Scholar]
- 39. Chen S, Li X, Lu D, et al. Sox2 regulates apoptosis through map4k4‐survivin signaling pathway in human lung cancer cells. Carcinogenesis. 2014;35:613‐623. [DOI] [PubMed] [Google Scholar]


