Abstract
Accumulating studies supported that lncRNAs played important roles in tumorigenesis. LncRNA HOXA11‐AS was a novel lncRNA that has been proved to involved in several tumours. However, the role of HOXA11‐AS in the development of hepatocellular carcinoma (HCC) remains to be explained. In our study, we showed that HOXA11‐AS expression was up‐regulated in the HCC tissues, and the higher expression of HOXA11‐AS was associated with the advanced stage in the HCC samples. In addition, we indicated that the expression of HOXA11‐AS was up‐regulated in HCC cell lines (Hep3B, SMMC‐7721, MHCC97‐H and BEL‐7402) compared with normal liver cell lines (HL‐7702). Overexpression of HOXA11‐AS promoted HCC proliferation and invasion and induced the epithelial‐mesenchymal transition (EMT) and knockdown of HOXA11‐AS suppressed the HCC cell proliferation and invasion. However, we showed that miR‐214‐3p expression was down‐regulated in the HCC tissues and cell lines. Ectopic expression of miR‐214‐3p suppressed HCC cell proliferation and invasion. Furthermore, we indicated that overexpression of HOXA11‐AS decreased the miR‐214‐3p expression and the expression of miR‐214‐3p was negatively related with the HOXA11‐AS expression in HCC samples. Ectopic expression of HOXA11‐AS increased HCC proliferation and invasion and induced EMT through inhibiting miR‐214‐3p expression. These data suggested that HOXA11‐AS/miR‐214‐3p axis was responsible for development of HCC.
Keywords: hepatocellular carcinoma, HOXA11‐AS, LncRNA, miR‐214‐3p
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is one of most common tumour worldwide and is the 2nd leading cause of cancer‐related death.1, 2, 3, 4, 5 The high mortality rate of this disease is due to lack of impactful treatments.6, 7, 8 Despite interventional therapy, liver transplantation, chemotherapy and surgery could cure HCC cases successfully; the 5‐year survival rate was still dissatisfied.9, 10, 11 Hepatocarcinogenesis was one complicated procedure and that several factors were involved in the initiation, development and progression of this disease.12, 13, 14 However, the detail molecular mechanism of HCC remains largely unknown.15 Therefore, it is crucial to unravel the molecular mechanism and find the diagnostic markers for HCC.
Long non‐coding RNAs (lncRNAs) are one type of non‐coding RNA that modulates gene expression at post‐transcriptional or transcriptional level with the length more than 200 nucleotides.16, 17, 18 Emerging studies indicated that lncRNA acts critical roles in a wide range of cell processes such as cell cycle, differentiation, proliferation, migration, metabolism and apoptosis.19, 20 LncRNAs can serve as oncogenes or tumour suppressor genes via crosstalk with other RNA or by several chromatin‐based mechanisms.21, 22, 23 The expression of lncRNAs was found to be deregulated in a large number of tumours including lung cancer, osteosarcoma, ovarian cancer, breast cancer, gastric cancer, colorectal cancer and HCC.24, 25, 26, 27, 28, 29, 30 LncRNA HOXA11‐AS was a novel lncRNA that has been proved to involved in development of some tumours such as gastric cancer, lung cancer and osteosarcoma.31, 32, 33 However, the role of lncRNA HOXA11‐AS in the HCC remains largely unknown.
In this study, we investigated the expression and the functional role of HOXA11‐AS in HCC. We demonstrated that the expression of HOXA11‐AS was down‐regulated in HCC cell lines and tissues and overexpression of HOXA11‐AS promoted HCC growth, invasion and epithelial‐mesenchymal transition (EMT).
2. MATERIALS AND METHODS
2.1. Samples
A total of 40 pairs of HCC samples and the adjacent noncancerous samples were selected from cases undergone surgery at the Guangdong Second Provincial General Hospital. All tissues were obtained with written informed consent of each patient, and our study was approved by Review Board of Guangdong Second Provincial General Hospital. Tissues were snapping frozen in the liquid nitrogen until protein or RNA extraction. Both non‐tumour and tumour samples were histologically confirmed.
2.2. Cell lines and culture and transfection
Four human HCC cell lines (MHCC‐97H, Bel‐7404, SMMC7721 and QGY‐7703) and one normal liver cell line (HL‐7702) were purchased from the Cell Biology of Chinese Academy of Science from Shanghai. These cells were maintained in the 1640 medium (Invitrogen, CA, USA) supplemented with FBS and penicillin/treptomycin. pcDNA‐HOXA11‐AS and pcDNA‐control, siRNA‐control, siRNA‐HOXA11‐AS, miR‐214‐3p miminc and scramble were purchased from RiboBio (Guangzhou, China) and transfected to the cells using Lipofectamine 2000 kit (Invitrogen) according to the protocol.
2.3. RNA extraction and quantitative RT‐PCR
Total sample and cell RNA were extracted with TRIzol kit (Invitrogen), and then cDNA was synthesized following to manufacturer's information. Quantitative real‐time PCR (qRT‐PCR) was conducted to determine the mRNA and lncRNA using SYBR Green Master Mix (MBI Fermentas) on the IQ5 PCR machine. The relative expression level of gene was calculated by the 2−(DDCt) method. The primers in our manuscript were used as following: HOXA11‐AS‐F 5′‐GAGTGTTGGCCTGTCCTCAA‐3′, HOXA11‐AS‐R 5′‐TTGTGCCCAGTTGCCTGTAT‐3′; miR‐214‐3p‐F 5′‐GAGTGTTGGCCTGTCCTCAA‐3′, miR‐214‐3p‐R 5′‐TTGTGCCCAGTTGCCTGTAT‐3′. The mRNA and miRNA expression levels were normalized to GAPDH and RNU6B expression levels, respectively.
2.4. Cell proliferation assay and invasion assay
3‑(4,5‑dimethylthiazol‑2‑yl)‑2,5‑diphenyltetrazolium (MTT; Sigma) was used to determine the cell proliferation. Cells were cultured in the 96‑well plate for 0, 24, 48 and 72 hours. The absorbance at the 540 nm was determined at the spectrophotometer. Cell invasion was conducted using Transwell chamber coated with Matrigel (BD Biosciences, USA). Cells were cultured on the upper chamber in serum‐free 1640 medium. As chemo‐attractant, 1640 medium containing 20% FBS was put to the lower chamber. Invaded cell was stained with crystal violet and photographed (Olympus, Japan).
2.5. Western blot analysis
Protein from HCC cells or samples was extracted using RIPA buffer with the proteinase inhibitor. The concentration of protein was evaluated by the Bradford kit (Bio‐Rad, CA). Twenty micrograms protein lysate was resolved by 10% SDS‐PAGE and electrophoretically transferred to polyvinylidene difluoride (PVDF). The membrane was blotted with the primary antibodies (anti‐MELK, anti‐E‐cadherin, N‐cadherin, vimentin and Snail antibodies, Abcam). The secondary antibody was IgG‐HRP, and immunoreactive band was visualized by enhanced chemiluminescence detection system.
2.6. Statistical analysis
The data were expressed as the mean ± standard deviation (SD). Statistical analysis was conducted by SPSS 17.0 (IBM, NY, USA). The Student's t test was performed to assess significance of differences between 2 groups. ANOVA was used assess significance of differences between more than 2 groups. P < .05 was determined as significant difference.
3. RESULT
3.1. HOXA11‐AS was up‐regulated in HCC samples
The expression of HOXA11‐AS was examined using qRT‐PCR in tumorous samples and compared normal samples of 40 patients. As shown in the Figure 1A, HOXA11‐AS level was higher in tumorous samples than that of compared normal tissues. In addition, expression of HOXA11‐AS was lower in initial clinical stage (I‐II phase) cases than that advanced clinical stage (III‐IV phase) cases (Figure 1B). Of 40 HCC samples, HOXA11‐AS was up‐regulated in 20 patients (20/30, 67%) compared with adjacent normal tissues (Figure 1C). Moreover, we found that the expression of HOXA11‐AS was up‐regulated in the HCC tissues compared with in normal tissues using RT‐PCR (Figure 1D).
Figure 1.

HOXA11‐AS was up‐regulated in HCC samples. A, The expression of HOXA11‐AS in tumorous samples and compared normal samples of 40 patients was determined by qRT‐PCR. U6 was used as the internal control. B, The expression of HOXA11‐AS was lower in initial clinical stage (I‐II phase) cases than that advanced clinical stage (III‐IV phase) cases. C, Of 40 HCC samples, HOXA11‐AS was up‐regulated in 20 patients (20/30, 67%) compared with adjacent normal tissues. D, The expression of HOXA11‐AS in tumorous samples and compared normal samples of 3 HCC patients was detected by RT‐PCR. ***P < .001
3.2. miR‐214‐3p was down‐regulated in HCC samples
The expression of miR‐214‐3p was measured using qRT‐PCR in tumorous samples and compared normal samples of 40 patients. As shown in the Figure 2A, miR‐214‐3p level was lower in tumorous samples than that of compared normal tissues. In addition, expression of miR‐214‐3p was higher in initial clinical stage (I‐II phase) cases than that advanced clinical stage (III‐IV phase) cases (Figure 2B). Of 40 HCC samples, miR‐214‐3p was down‐regulated in 20 patients (20/30, 67%) compared with adjacent normal tissues (Figure 2C). Moreover, we indicated that the expression of miR‐214‐3p was negatively related with the HOXA11‐AS expression in HCC samples (Figure 1D).
Figure 2.

miR‐214‐3p was down‐regulated in HCC samples. A, The expression of miR‐214‐3p in tumorous samples and compared normal samples of 40 patients was determined by qRT‐PCR. U6 was used as the internal control. B, The expression of miR‐214‐3p was higher in initial clinical stage (I‐II phase) cases than that advanced clinical stage (III‐IV phase) cases. C, Of 40 HCC samples, miR‐214‐3p was down‐regulated in 20 patients (20/30, 67%) compared with adjacent normal tissues. D, The expression of miR‐214‐3p was negatively related with the HOXA11‐AS expression in HCC samples. ***P < .001
3.3. Overexpression of HOXA11‐AS promoted HCC proliferation and invasion
In the HCC cell lines (Hep3B, SMMC‐7721, MHCC97‐H and BEL‐7402), HOXA11‐AS expression was up‐regulated compared with normal liver cell lines (HL‐7702) (Figure 3A). Then, in SMMC‐7721 transfected with pcDNA3.1‐HOXA11‐AS, HOXA11‐AS expression was increased compared with control group (Figure 2B). Ectopic expression of HOXA11‐AS promoted the SMMC‐7721 cell proliferation (Figure 3C). Overexpression of HOXA11‐AS increased the cell invasion in the SMMC‐7721 cell (Figure 3D), and the relative invasive cells are shown in Figure 3E.
Figure 3.

Overexpression of HOXA11‐AS promoted HCC proliferation and invasion. A, The expression of HOXA11‐AS in the HCC cell lines (Hep3B, SMMC‐7721, MHCC97‐H and BEL‐7402) and normal liver cell lines (HL‐7702) was measured by qRT‐PCR. B, HOXA11‐AS expression in the SMMC‐7721 cell was detected by qRT‐PCR. C, Ectopic expression of HOXA11‐AS promoted the SMMC‐7721 cell proliferation. D, Overexpression of HOXA11‐AS increased the cell invasion in the SMMC‐7721 cell. E, The relative invasive cell numbers were shown. *P < .05, **P < .01 and ***P < .001
3.4. Knockdown of HOXA11‐AS suppressed HCC proliferation and invasion
Then, in SMMC‐7721 transfected with siRNA‐HOXA11‐AS, HOXA11‐AS expression was down‐regulated compared with control group (Figure 4A). Knockdown of HOXA11‐AS suppressed the SMMC‐7721 cell growth (Figure 4B). Inhibition of HOXA11‐AS decreased the cell invasion in the SMMC‐7721 cell (Figure 4C), and the relative invasive cells are shown in Figure 4D.
Figure 4.

Knockdown of HOXA11‐AS suppressed HCC proliferation and invasion. A, HOXA11‐AS expression in the SMMC‐7721 cell was detected by qRT‐PCR. B, Knockdown of HOXA11‐AS suppressed the SMMC‐7721 cell growth. C, Inhibition of HOXA11‐AS decreased the cell invasion in the SMMC‐7721 cell. D, The relative invasive cells were shown. *P < .05, **P < .01 and ***P < .001
3.5. Ectopic expression of HOXA11‐AS induced epithelial‐mesenchymal transition (EMT)
Overexpression of HOXA11‐AS suppressed E‐cadherin expression in the SMMC‐7721 cell (Figure 5A). Ectopic expression of HOXA11‐AS increased the N‐cadherin expression in the SMMC‐7721 cell (Figure 5B). HOXA11‐AS overexpression promoted the vimentin expression in the SMMC‐7721 cell (Figure 5C). Ectopic expression of HOXA11‐AS induced the Snail expression in the SMMC‐7721 cell (Figure 5D). In addition, we confirmed that ectopic expression of HOXA11‐AS decreased the protein expression of E‐cadherin and increased the N‐cadherin, vimentin and Snail expression in the SMMC‐7721 cell (Figure 5E).
Figure 5.

Ectopic expression of HOXA11‐AS induced epithelial‐mesenchymal transition (EMT). A, The mRNA expression of E‐cadherin was determined by qRT‐PCR. B, Ectopic expression of HOXA11‐AS increased the N‐cadherin expression in the SMMC‐7721 cell. C, The mRNA expression of vimentin was measured by qRT‐PCR. D, Overexpression of HOXA11‐AS induced the Snail expression in the SMMC‐7721 cell. E, The protein expression of E‐cadherin, N‐cadherin, vimentin and Snail was determined by Western blot. GAPDH was used as the control
3.6. Ectopic expression of miR‐214‐3p suppressed HCC proliferation and invasion
In the HCC cell lines (Hep3B, SMMC‐7721, MHCC97‐H and BEL‐7402), miR‐214‐3p expression was down‐regulated compared with normal liver cell lines (HL‐7702) (Figure 6A). Then, in SMMC‐7721 transfected with miR‐124‐3p mimics, miR‐124‐3p expression was increased compared with control group (Figure 6B). Ectopic expression of miR‐124‐3p decreased the SMMC‐7721 cell proliferation (Figure 6C). Overexpression of miR‐124‐3p suppressed the cell invasion in the SMMC‐7721 cell (Figure 6D), and the relative invasive cells are shown in Figure 6E.
Figure 6.

Ectopic expression of miR‐214‐3p suppressed HCC proliferation and invasion. A, The expression of miR‐214‐3p in the HCC cell lines (Hep3B, SMMC‐7721, MHCC97‐H and BEL‐7402) and normal liver cell lines (HL‐7702) was measured by qRT‐PCR. B, The expression of miR‐124‐3p in the SMMC‐7721 cell was measured by qRT‐PCR. C, Ectopic expression of miR‐124‐3p suppressed the SMMC‐7721 cell proliferation. D, The relative invasive cell numbers were shown. *P < .05, **P < .01 and ***P < .001
3.7. HOXA11‐AS overexpression suppressed the miR‐214‐3p expression in HCC cell
Ectopic expression of HOXA11‐AS inhibited the miR‐214‐3p expression in SMMC‐7721 cell (Figure 7A). HOXA11‐AS overexpression enhanced the MELK expression in the SMMC‐7721 cell (Figure 7B). Overexpression of miR‐214‐3p decreased the MELK expression in the SMMC‐7721 cell (Figure 7C).
Figure 7.
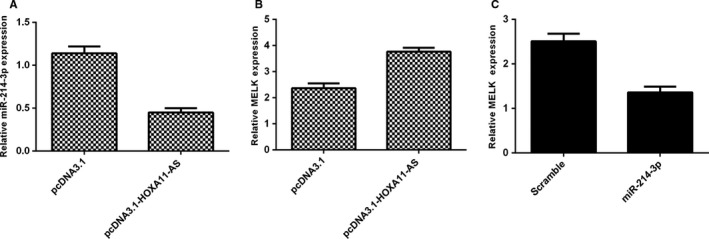
HOXA11‐AS overexpression suppressed the miR‐214‐3p expression in HCC cell. A, The miR‐214‐3p expression was measured by qRT‐PCR in the SMMC‐7721 cell. B, HOXA11‐AS overexpression enhanced the MELK expression in the SMMC‐7721 cell. C, Overexpression of miR‐214‐3p decreased the MELK expression in the SMMC‐7721 cell
3.8. HOXA11‐AS overexpression promoted HCC proliferation and invasion and induced EMT through inhibiting miR‐214‐3p expression
To determine the role of miR‐214‐3p in the HOXA11‐AS‐promoting HCC progression, we transfected miR‐214‐3p mimic in HOXA11‐AS overexpressing SMMC‐7721 cells. We found that ectopic expression of miR‐214‐3p attenuated the proliferation effect of HOXA11‐AS in the SMMC‐7721 cells (Figure 8A). Furthermore, we indicated that overexpression of miR‐214‐3p decreased the invasion ability of HOXA11‐AS overexpressing SMMC‐7721 cells (Figure 8B) and the relative invasive cells were shown (Figure 8C). Overexpression of miR‐214‐3p enhanced the E‐cadherin expression in the HOXA11‐AS overexpressing SMMC‐7721 cell (Figure 8D). Ectopic expression of miR‐214‐3p decreased the N‐cadherin expression in the HOXA11‐AS overexpressing SMMC‐7721 cell (Figure 8E). Overexpression of miR‐214‐3p suppressed the vimentin (Figure 8F) and Snail (Figure 8G) in the HOXA11‐AS overexpressing SMMC‐7721 cell.
Figure 8.
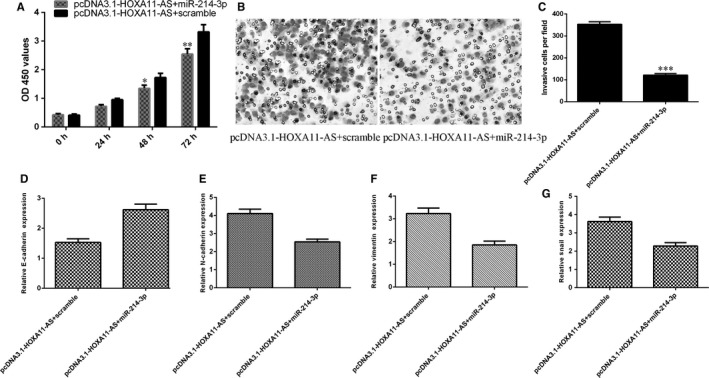
HOXA11‐AS overexpression promoted HCC proliferation and invasion and induced EMT through inhibiting miR‐214‐3p expression. A, Ectopic expression of miR‐214‐3p attenuated the proliferation effect of HOXA11‐AS in the SMMC‐7721 cells. B, Overexpression of miR‐214‐3p decreased the invasion ability of HOXA11‐AS overexpressing SMMC‐7721 cells. C, The relative invasive cell numbers were shown. D, The expression of E‐cadherin was detected by qRT‐PCR. E, Ectopic expression of miR‐214‐3p decreased the N‐cadherin expression in the HOXA11‐AS overexpressing SMMC‐7721 cell. F, The mRNA expression of vimentin was detected by qRT‐PCR. G, The mRNA expression of Snail was detected by qRT‐PCR. *P < .05, **P < .01 and ***P < .001
4. DISCUSSION
In this study, we demonstrated that the expression of HOXA11‐AS was up‐regulated in the HCC samples and the higher expression of HOXA11‐AS was associated with the advanced stage in the HCC samples. However, we showed that miR‐214‐3p expression was down‐regulated in the HCC tissues and the lower expression of miR‐214‐3p was correlated with advanced stage in the HCC tissues. Overexpression of HOXA11‐AS promoted HCC proliferation and invasion and induced the epithelial‐mesenchymal transition (EMT). HOXA11‐AS overexpression suppressed the miR‐214‐3p expression and enhanced the MELK expression. HOXA11‐AS overexpression promoted HCC proliferation and invasion and induced EMT through inhibiting miR‐214‐3p expression. These data suggested that HOXA11‐AS/miR‐214‐3p axis was responsible for development of HCC.
Previous evidences have proved that lncRNA HOXA11‐AS expression was found to be overexpressed and played an oncogene in several tumours.34, 35, 36, 37, 38 Xu et al39 showed that HOXA11‐AS expression was up‐regulated in glioma cell lines and samples. Knockdown expression of HOXA11‐AS suppressed glioma cell invasion, migration and growth partly through promoting miR‐214‐3p expression. Chen et al34 demonstrated that HOXA11‐AS increased EMT through regulating miR‐200b expression in the non‐small‐cell lung cancer (NSCLL). Wang et al40 indicated that ectopic expression of HOXA11‐AS induced the glioma cell growth and knockdown of HOXA11‐AS decreased the cell growth. Liu et al32 showed that HOXA11‐AS expression was up‐regulated in gastric cancer tissues and HOXA11‐AS knockdown decreased gastric cancer cell cycle and inhibited gastric cancer cell invasion, metastasis and migration through regulating β‐catenin and KLF2. In this study, the HOXA11‐AS expression in 40 paired primary HCC tissues and HCC cell lines was up‐regulated compared with adjacent normal samples and normal liver cell lines (HL‐7702). Moreover, ectopic expression of HOXA11‐AS promoted the HCC cell growth, invasion and EMT and knockdown of HOXA11‐AS suppressed the HCC cell proliferation and invasion. These data suggested that HOXA11‐AS might act as oncogene in HCC.
The underlying mechanism by how HOXA11‐AS involved in HCC tumorigenesis still remains to be researched. Growing studies indicated that lncRNAs acted crucial roles in several biological processes by playing as ceRNAs (competing endogenous RNAs) or molecular sponges to modulate‐specific miRNAs.34, 37, 41, 42, 43 Previous study showed that HOXA11‐AS functions as an oncogenic gene which enhanced glioma cell metastasis and growth by inhibiting miR‐214‐3p/EZH2 expression.39 In this regard, we also found that ectopic expression of HOXA11‐AS suppressing the miR‐214‐3p expression in the HCC cell. miR‐214 was demonstrated to be one tumour suppressor miRNA in a lot of tumours including HCC.44, 45, 46, 47 Yang et al48 indicated that miR‐214 inhibited the HCC cell proliferation through targeting E2F3 expression. Wang et al49 also confirmed that the expression of miR‐214 was down‐regulated in HCC specimens and cells and overexpression of miR‐214 suppressed HCC cell proliferation by regulating β‐catenin expression. In addition, Li et al50 showed that the expression of miR‐214‐3p was down‐regulated in HCC tissues and miR‐214‐3p overexpression decreased HCC cell cycle, cell proliferation and induced cell apoptosis partly by regulating embryonic leucine zipper kinase (MELK) expression. Therefore, we focused on the miR‐214‐3p as a ceRNA of HOXA11‐AS. We demonstrated that miR‐214‐3p expression was down‐regulated in the HCC tissues and the expression of miR‐214‐3p was negatively correlated with the expression of HOXA11‐AS. HOXA11‐AS overexpression promoted HCC proliferation and invasion and induced EMT through inhibiting miR‐214‐3p expression. These results suggested that lncRNA HOXA11‐AS played an oncogene role in HCC through acting as ceRNA for miR‐214‐3p.
From this project, we indicated that the expression of HOXA11‐AS was up‐regulated in the HCC samples and cell lines. Overexpression of HOXA11‐AS promoted HCC cell proliferation, invasion and EMT partly through inhibiting miR‐214‐3p expression. These data suggested that HOXA11‐AS may be a potential target for HCC treatment.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest statement.
Zhan M, He K, Xiao J, et al. LncRNA HOXA11‐AS promotes hepatocellular carcinoma progression by repressing miR‐214‐3p. J Cell Mol Med. 2018;22:3758–3767. 10.1111/jcmm.13633
Funding information
This work was supported by the National Natural Science Foundation of China (No. 81641110, 81571785, 81771957), Science and Technology Foundation of Guangdong Province, China (No. 2013B021800180), Natural Science Foundation of Guangdong Province, China(No. 2015A030313725, 2016A030311055, 2016A030313770, Science and Technology Program of Guangzhou Province, China (No.201707010305) and Youth Science Fund of Guangdong Second Provincial General Hospital (No.YQ2006‐001).
Zhan, He and Xiao are co‐first authors.
Contributor Information
Guoan Xiang, Email: guoan_66@163.com.
Ligong Lu, Email: luligong1969@126.com.
REFERENCES
- 1. Yu L, Ding GF, He C, Sun L, Jiang Y, Zhu L. MicroRNA‐424 is down‐regulated in hepatocellular carcinoma and suppresses cell migration and invasion through c‐Myb. PLoS ONE. 2014;9:e91661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Utsunomiya T, Ishikawa D, Asanoma M, et al. Specific miRNA expression profiles of non‐tumor liver tissue predict a risk for recurrence of hepatocellular carcinoma. Hepatol Res. 2014;44:631‐638. [DOI] [PubMed] [Google Scholar]
- 3. Damania P, Sen B, Dar SB, et al. Hepatitis B virus induces cell proliferation via HBx‐induced microRNA‐21 in hepatocellular carcinoma by targeting programmed cell death protein4 (PDCD4) and phosphatase and tensin homologue (PTEN). PLoS ONE. 2014;9:e91745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu T, Zhang X, Sha K, Liu X, Zhang L, Wang B. miR‐709 up‐regulated in hepatocellular carcinoma, promotes proliferation and invasion by targeting GPC5. Cell Prolif. 2015;48:330‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu L, Zhou L, Cheng Y, et al. MicroRNA‐543 acts as an oncogene by targeting PAQR3 in hepatocellular carcinoma. Am J Cancer Res. 2014;4:897‐906. [PMC free article] [PubMed] [Google Scholar]
- 6. Li T, Yin J, Yuan L, et al. Downregulation of microRNA‐139 is associated with hepatocellular carcinoma risk and short‐term survival. Oncol Rep. 2014;31:1699‐1706. [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Wang X, Ruan A, et al. miR‐141 is a key regulator of renal cell carcinoma proliferation and metastasis by controlling EphA2 expression. Clin Cancer Res. 2014;20:2617‐2630. [DOI] [PubMed] [Google Scholar]
- 8. Hung TM, Ho CM, Liu YC, et al. Up‐regulation of microRNA‐190b plays a role for decreased IGF‐1 that induces insulin resistance in human hepatocellular carcinoma. PLoS ONE. 2014;9:e89446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen Q, Bae HJ, Eun JW, et al. MiR‐101 functions as a tumor suppressor by directly targeting nemo‐like kinase in liver cancer. Cancer Lett. 2014;344:204‐211. [DOI] [PubMed] [Google Scholar]
- 10. Wang L, Zhang X, Jia LT, et al. c‐Myc‐mediated epigenetic silencing of MicroRNA‐101 contributes to dysregulation of multiple pathways in hepatocellular carcinoma. Hepatology. 2014;59:1850‐1863. [DOI] [PubMed] [Google Scholar]
- 11. Yang XW, Zhang LJ, Huang XH, et al. miR‐145 suppresses cell invasion in hepatocellular carcinoma cells: miR‐145 targets ADAM17. Hepatol Res. 2014;44:551‐559. [DOI] [PubMed] [Google Scholar]
- 12. Zha Y, Gan P, Yao Q, Ran FM, Tan J. Downregulation of Rap1 promotes 5‐fluorouracil‐induced apoptosis in hepatocellular carcinoma cell line HepG2. Oncol Rep. 2014;31:1691‐1698. [DOI] [PubMed] [Google Scholar]
- 13. Govaere O, Komuta M, Berkers J, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63:674‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA‐7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3‐kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852‐1862. [DOI] [PubMed] [Google Scholar]
- 15. Yu L, Gong X, Sun L, Yao H, Lu B, Zhu L. miR‐454 functions as an oncogene by inhibiting CHD5 in hepatocellular carcinoma. Oncotarget. 2015;6:39225‐39234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang YY, Yang R, Lian JC, Xu HY. LncRNA Sox2ot overexpression serves as a poor prognostic biomarker in gastric cancer. Am J Transl Res. 2016;8:5035‐5043. [PMC free article] [PubMed] [Google Scholar]
- 17. Yu QY, Zhou XF, Xia Q, et al. Long non‐coding RNA CCAT1 that can be activated by c‐Myc promotes pancreatic cancer cell proliferation and migration. Am J Transl Res. 2016;8:5444‐5454. [PMC free article] [PubMed] [Google Scholar]
- 18. Wang ZW, Jin YY, Ren HT, Ma XL, Wang BF, Wang YL. Downregulation of the long non‐coding RNA TUSC7 promotes NSCLC cell proliferation and correlates with poor prognosis. Am J Transl Res. 2016;8:680‐U1079. [PMC free article] [PubMed] [Google Scholar]
- 19. Ma GX, Tang MQ, Wu YQ, Xu XM, Pan F, Xu RA. LncRNAs and miRNAs: potential biomarkers and therapeutic targets for prostate cancer. Am J Transl Res. 2016;8:5141‐5150. [PMC free article] [PubMed] [Google Scholar]
- 20. Chen C, Cheng GQ, Yang XN, Li CS, Shi R, Zhao NN. Tanshinol suppresses endothelial cells apoptosis in mice with atherosclerosis via lncRNA TUG1 up‐regulating the expression of miR‐26a. Am J Transl Res. 2016;8:2981‐2991. [PMC free article] [PubMed] [Google Scholar]
- 21. Chen ZH, Hu HK, Zhang CR, et al. Down‐regulation of long non‐coding RNA FOXD3 antisense RNA 1 (FOXD3‐AS1) inhibits cell proliferation, migration, and invasion in malignant glioma cells. Am J Transl Res. 2016;8:4106‐4119. [PMC free article] [PubMed] [Google Scholar]
- 22. Sun L, Sun P, Zhou QY, Gao XC, Han Q. Long noncoding RNA MALAT1 promotes uveal melanoma cell growth and invasion by silencing of miR‐140. Am J Transl Res. 2016;8:3939‐3946. [PMC free article] [PubMed] [Google Scholar]
- 23. Yu X, Li Z. Long non‐coding RNA HOTAIR: a novel oncogene (Review). Mol Med Rep. 2015;12:5611‐5618. [DOI] [PubMed] [Google Scholar]
- 24. Zhu H, Zhou X, Chang H, et al. CCAT1 promotes hepatocellular carcinoma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8:5427‐5434. [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Ma M, Liu W, Ding W, Yu H. Enhanced expression of long noncoding RNA CARLo‐5 is associated with the development of gastric cancer. Int J Clin Exp Pathol. 2014;7:8471‐8479. [PMC free article] [PubMed] [Google Scholar]
- 26. Kam Y, Rubinstein A, Naik S, et al. Detection of a long non‐coding RNA (CCAT1) in living cells and human adenocarcinoma of colon tissues using FIT‐PNA molecular beacons. Cancer Lett. 2014;352:90‐96. [DOI] [PubMed] [Google Scholar]
- 27. Liu SP, Yang JX, Cao DY, Shen K. Identification of differentially expressed long non‐coding RNAs in human ovarian cancer cells with different metastatic potentials. Cancer Biol Med. 2013;10:138‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Wan L, Lu K, et al. The long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinoma. PLoS ONE. 2015;10:e0114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun L, Li Y, Yang B. Downregulated long non‐coding RNA MEG3 in breast cancer regulates proliferation, migration and invasion by depending on p53′s transcriptional activity. Biochem Biophys Res Comm. 2016;478:323‐329. [DOI] [PubMed] [Google Scholar]
- 30. Tian ZZ, Guo XJ, Zhao YM, Fang Y. Decreased expression of long non‐coding RNA MEG3 acts as a potential predictor biomarker in progression and poor prognosis of osteosarcoma. Int J Clin Exp Pathol. 2015;8:15138‐15142. [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, He RQ, Dang YW, et al. Comprehensive analysis of the long noncoding RNA HOXA11‐AS gene interaction regulatory network in NSCLC cells. Cancer Cell Int. 2016;16:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu ZL, Chen ZY, Fan RH, et al. Over‐expressed long noncoding RNA HOXA11‐AS promotes cell cycle progression and metastasis in gastric cancer. Mol Cancer. 2017;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cui MX, Wang JY, Li QJ, Zhang JL, Jia JL, Zhan XL. Long non‐coding RNA HOXA11‐AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR‐124‐3p in osteosarcoma. Biomed Pharmacother. 2017;92:437‐444. [DOI] [PubMed] [Google Scholar]
- 34. Chen JH, Zhou LY, Xu S, Zheng YL, Wan YF, Hu CP. Overexpression of lncRNA HOXA11‐AS promotes cell epithelial‐mesenchymal transition by repressing miR‐200b in non‐small cell lung cancer. Cancer Cell Int. 2017;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li N, Yang ML, Shi K, Li W. Long non‐coding RNA HOXA11‐AS in human cancer: a meta‐analysis. Clin Chim Acta. 2017;474:165‐170. [DOI] [PubMed] [Google Scholar]
- 36. Cui Y, Yi L, Zhao JZ, Jiang YG. Long noncoding RNA HOXA11‐AS functions as miRNA sponge to promote the glioma tumorigenesis through targeting miR‐140‐5p. DNA Cell Biol. 2017;36:822‐828. [DOI] [PubMed] [Google Scholar]
- 37. Lu QK, Zhao N, Zha GP, Wang HY, Tong QH, Xin SH. LncRNA HOXA11‐AS exerts oncogenic functions by repressing p21 and miR‐124 in uveal melanoma. DNA Cell Biol. 2017;36:837‐844. [DOI] [PubMed] [Google Scholar]
- 38. Su JC, Hu XF. Long non‐coding RNA HOXA11‐AS promotes cell proliferation and metastasis in human breast cancer. Mol Med Rep. 2017;16:4887‐4894. [DOI] [PubMed] [Google Scholar]
- 39. Xu C, He T, Li Z, Liu H, Ding B. Regulation of HOXA11‐AS/miR‐214‐3p/EZH2 axis on the growth, migration and invasion of glioma cells. Biomed Pharmacother. 2017;95:1504‐1513. [DOI] [PubMed] [Google Scholar]
- 40. Wang QX, Zhang JX, Liu YW, et al. A novel cell cycle‐associated IncRNA, HOXA11‐AS, is transcribed from the 5‐prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251‐259. [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR‐21. Cancer Biol Ther. 2016;17:104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang XN, Zhang LH, Cui XD, Wang MX, Zhang GY, Yu PL. lncRNA HOXA11‐AS is involved in fracture healing through regulating mir‐124‐3p. Eur Rev Med Pharmacol Sci. 2017;21:4771‐4776. [PubMed] [Google Scholar]
- 43. Chen D, Sun Q, Zhang LF, et al. The lncRNA HOXA11‐AS functions as a competing endogenous RNA to regulate PADI2 expression by sponging miR‐125a‐5p in liver metastasis of colorectal cancer. Oncotarget. 2017;8:70642‐70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orso F, Quirico L, Virga F, et al. miR‐214 and miR‐148b Targeting Inhibits Dissemination of Melanoma and Breast Cancer. Can Res. 2016;76:5151‐5162. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Q, Zhang SX. miR‐214 promotes radioresistance in human ovarian cancer cells by targeting PETN. Biosci Rep 2017;37:BSR20170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ecke TH, Stier K, Weickmann S, et al. miR‐199a‐3p and miR‐214‐3p improve the overall survival prediction of muscle‐invasive bladder cancer patients after radical cystectomy. Cancer Med. 2017;6:2252‐2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J, Zhao XZ, Guo ZJ, Ma XL, Song YQ, Guo Y. Regulation of NEAT1/miR‐214‐3p on the growth, migration and invasion of endometrial carcinoma cells. Arch Gynecol Obstet. 2017;295:1469‐1475. [DOI] [PubMed] [Google Scholar]
- 48. Yang Y, Chang S, Zhao Z, et al. MicroRNA‐214 suppresses the proliferation of human hepatocellular carcinoma cells by targeting E2F3. Oncol Lett. 2015;10:3779‐3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X, Chen J, Li F, et al. MiR‐214 inhibits cell growth in hepatocellular carcinoma through suppression of β‐catenin. Biochem Biophys Res Commun. 2012;428:525‐531. [DOI] [PubMed] [Google Scholar]
- 50. Li Y, Chen Y, Xie Q, et al. MicroRNA‐214‐3p inhibits proliferation and cell cycle progression by targeting MELK in hepatocellular carcinoma and correlates cancer prognosis. Cancer Cell Int. 2017;17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]


