Abstract
The entorhinal cortex, a brain area critical for memory, contains neurons that fire when a rodent is in a certain location (eg, grid cells), or when a monkey looks at certain locations. In rodents, these spatial representations align to visual objects in the environment by firing when the animal is in a preferred location defined by relative position of visual environmental features. Recently, our laboratory found that simultaneously recorded entorhinal neurons in monkeys can exhibit different spatial reference frames for gaze position, including a reference frame of visual environmental features. We also discovered that most of the neurons represent gaze position. These results suggest that gaze information in multiple spatial reference frames is a potent signal used in the primate memory system. Here, I describe how these findings support three underappreciated views of the hippocampal memory system.
Keywords: Entorhinal cortex, memory, medial temporal lobe, eye movement, reference frame, hippocampus, primate, gaze
Comment on: Meister MLR, Buffalo EA. Neurons in primate entorhinal cortex represent gaze position in multiple spatial reference frames. J Neurosci. 2018;38:2430-2441. pii: 2432-17. doi:10.1523/JNEUROSCI.2432-17.2018. PubMed PMID: 29386260; PubMed Central PMCID: PMC5858590. https://www.ncbi.nlm.nih.gov/pubmed/29386260
The entorhinal cortex is a brain area important for memory and among the first areas to degrade in Alzheimer’s disease.1 The entorhinal cortex is the primary input and output to the hippocampus, and these two brain areas are collectively referred to here as the hippocampal formation (HF). The HF is critical for forming new memories of life events in humans.2
In a somewhat separate line of research in rodents, entorhinal neurons show spatial representations by firing action potentials when the animal is in a certain location.3,4 A well-studied type of entorhinal spatial activity is that shown by grid cells, which fire at periodic locations across space so that the firing fields resemble a grid.5 Grid cells and other spatial cells in the HF fire selectively for particular locations relative to visible environmental features.4-7 In other words, these spatial representations are anchored to visible environmental features that constitute a spatial reference frame. In the primates, HF neurons exhibit spatial activity by firing selectively for where a monkey is looking.8 Recently, our laboratory discovered that entorhinal neurons fired in a grid-like spatial pattern when a monkey looked at certain screen locations while freely viewing complex images.9 These firing fields for gaze position were stable across a recording session in which the monkey explored different images. However, because images were always presented in the same location on the screen, the spatial reference frame of these representations was unknown. Were spatial representations locked to conspicuous environmental features, such as the bounds of the image display, or instead relative to the monkey’s head position?
To determine the spatial reference frame for monkey entorhinal neurons, we recorded the action potentials of single neurons while monkeys freely viewed large (30° wide), complex images presented in different, but overlapping, locations on a screen. Specifically, on a given trial, an image appeared so that the image center was either 2° left of the screen center or 2° right of the screen center. The monkey’s head was stationary and centered in front of the screen. Each image was freely viewed for no more than 5 seconds, with up to 240 image presentations in a session. Between image presentations, the monkey received a fruit slurry reward for correctly performing several guided saccades. This arrangement allowed us to determine whether entorhinal neurons represent gaze position relative to the bounds of the image display.
Results yielded several discoveries. First, we discovered that neurons with stable representation of gaze position across trials did not all have the same reference frame. Half the neurons represented gaze position relative to the image display bounds, meaning that the neurons were sensitive to visual structure and reflected self-position relative to visible environmental features. In contrast, the other half of neurons did not shift their spatial representation along with the image display, meaning that they represented gaze position in a different spatial reference frame that was stationary throughout the experiment. These latter neurons might have been representing gaze position relative to the skull, screen, or even the room itself. Also, neurons with different reference frames were routinely recorded at the same time and in the same anatomical area, demonstrating that multiple spatial reference frames are represented simultaneously and intermixed within the entorhinal cortex.
Another major discovery in these data was that most of the recorded neurons represent gaze position. These neural representations of gaze position were reliable and specific enough so that we could use the neural activity to accurately decode where the monkey was looking.
Finally, results showed that the overwhelming majority of neurons representing gaze position do not show the grid-like spatial activity our laboratory originally observed.9 Instead, most neurons show irregular spatial representations without any obvious spatial periodicity.
Underappreciated Perspectives of the Hippocampal Memory System
Our results could be described in a way that corresponds to the customary research framework for HF spatial activity in rodents. For example, we could concentrate our discussion on the neurons that showed grid-like spatial activity and describe neurons with image-aligned activity as exhibiting the “allocentric” reference frame for which HF is known. However, our results and others in this brain region could be described with a different focus that emphasizes important, yet often-overlooked perspectives on what HF neural activity is, what causes it, and how it is used. Here, I describe our results from three underappreciated perspectives.
1. Visual input is a primary cause of spatial activity in the hippocampal memory system
Our finding that most entorhinal neurons represent gaze position emphasizes that visuospatial signals are prominent within the hippocampal memory system. This is also true in rodents, even though their vision seems rudimentary next to primates. For example, a hippocampal place cell fires selectively for a rodent’s position relative to visual environmental features and will show a drastically changed response if visual features of the environment are changed.6 This dependence on visual features means that a rodent place cell may be selectively firing for a particular visual input just like a hippocampal spatial view cell in a primate.10 Notably, in both primates and rodents, HF spatial selectivity that can persist in darkness is diminished and drifts.11-13 The profound potency of visual cues to HF neurons is also implicitly acknowledged by standard methodology which exploits it, because visual cue manipulation is the default manipulation in experiments studying HF spatial activity. Also, although other, nonvisual forms of selectivity have been discovered in rodent HF cells (eg, odor12,14), evidence demonstrates that visual cues are what which dominate HF response selectivity.15 Unlike nonvisual changes in the environment, visual change has been shown to affect the spatial activity of the entire network of simultaneously recorded cells.16 Therefore while there is no doubt that nonvisual information can influence HF activity, visual information so far appears to be the most powerful determinant of HF spatial representations.
However, many researchers who study the HF, or who study visual processing, do not regard the HF as a structure representing the visual world. This is because traditional visual areas are considered largely to be those with clear retinotopy (eg, neighboring neurons respond to neighboring parts of the visual world). It is also because basic visual ability does not seem to be affected in patients who have HF damage. Another barrier to recognizing the HF as directly driven by visual cues arises from the fact that much research is conducted in rodents, where monitoring eye position is technically challenging.17,18 So even though visual information matters immensely to HF spatial responses in rodents, it is difficult to match visually sensitive HF cell responses to exact visual input. If eye position were monitored, perhaps the spatial responses of some rodent HF neurons would prove to largely be responses selective for a particular visual input, as suggested by our finding of monkey entorhinal neurons selective for particular gaze position relative to a visual structure.
In sum, our results highlight a common finding that is not commonly expressed explicitly: visual input strongly drives neural activity in the hippocampal memory system.
2. Irregular spatial representations, not just grid or border representations, exist
Although we initially designed our experiment to examine the spatial reference frame of entorhinal neurons exhibiting grid or border spatial activity, our results revealed that most (90%) of spatial activity is neither grid nor border activity. Our results forced us to recognize that most entorhinal neurons exhibit stable spatial activity that is less aesthetically appealing and appears to have an irregular spatial layout. Our initial lack of investigative focus on this type of irregular spatial activity is not surprising, given that existing literature almost entirely ignores such spatial cells. Investigations of entorhinal neurons with spatial representations usually report exclusively on what happens to grid or border representations after an experimental manipulation. Only recently have some researchers drawn attention to this issue, establishing that spatial activity other than grid or border activity is the prevalent type of spatial activity within the rodent entorhinal cortex in the layers which output to the hippocampus.19,20 In addition, irregular spatial representations provide useful information,21 as demonstrated by our finding that neural activity of the cell population can be used to decode where the monkey is looking. Accumulating evidence from ours and other laboratories demonstrates the existence and potential relevance of neurons exhibiting irregular spatial representations. Hopefully, this encourages future studies to include data from these ubiquitous, yet overlooked, neurons within the HF.
3. Memory has a behavioral function, so the HF interacts with sensory and motor systems
A basic question looming over our findings and over similar research is, “What does this spatial activity have to do with memory?” This question inevitably arises because this spatial activity is observed within brain areas important for memory. In humans, damage to the HF results in profound memory deficit.2 Yet, memory is not often mentioned when speculating how spatial activity in the HF guides behavior. Instead, spatial activity in the HF is frequently discussed as reflecting ongoing “navigation.” Also, even though HF spatial activity is considered to underlie ongoing navigation, it is also viewed as “high-level” activity detached from “low-level” sensory or motor spatial activity. However, our finding that a large portion of entorhinal neurons are sensitive to gaze position, and visual structure in an eye-centered reference frame, could be interpreted as showing that HF responses resemble motor and sensory responses. Importantly, this resemblance does not preclude HF from having a role in memory or navigation but rather elucidates how it could do both. Spatial activity in the same reference frame as sensory and motor neurons could affect those neurons to alter their spatial representations with spatial memory, consequently guiding immediate movement with memory.
A few hundred milliseconds after an image appears, eye movements begin to reflect memory of whether the image has been seen before, and this mnemonic, navigatory behavior depends on the integrity of the HF (see Meister and Buffalo22 for review). The neural mechanism by which recognition memory guides this or any other movement is unknown, but our findings suggest that entorhinal neurons could support memory-guided looking behavior by encoding gaze position relative to recognized environmental structure. For example, the image-aligned activity we observed may enable the monkey to make a large saccade to the other side of the display using visual structure memory that there is an image region to inspect at the locus of saccade completion. Because primate foveal vision only allows us to perceive visual detail within the small area where we are currently fixating23 (Figure 1), we need to move our gaze intelligently to the most important parts of the environment using visual memory. In other words, visual perception outside the center gaze is severely diminished, so our intelligent eye movements to relevant parts of the world are guided by memory of visual layout. Consistent with this idea, patients who have HF damage are particularly impaired at remembering spatial layout of a scene compared with other scene aspects, even immediately after viewing it.24 Our observation of entorhinal neurons coding gaze position relative to major visual structure may be a neural signature of visual memory—driven by current visual input—that underlies memory-guided looking behavior.
Figure 1.
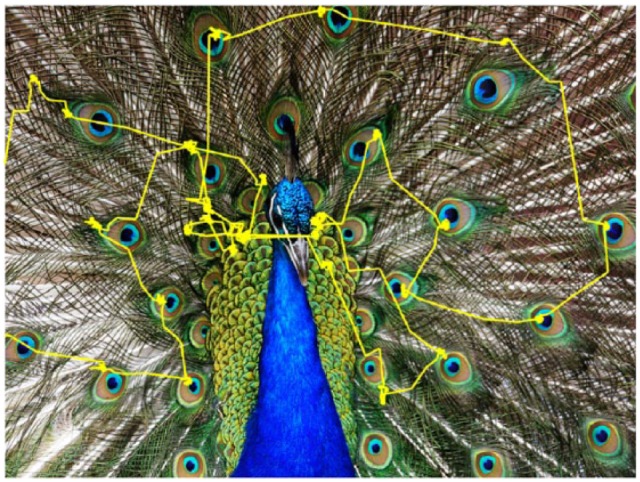
Viewing behavior consists of sequentially foveating different areas of a scene. A monkey’s eye position trace, shown in yellow, is superimposed on an image during free viewing. Poor peripheral vision requires the high-resolution fovea be directed to different locations to perceive visual detail across a scene.
Conclusions
Our findings in entorhinal cortex can be interpreted as promoting some underappreciated views of the hippocampal memory system, namely that it is flooded with visual inputs and usually signals spatial information in what appears to be irregular spatial form. Crucially, its activity drives motor output, which enables memory-guided behavior. Future studies aiming to expose the neural basis of memory could benefit from these perspectives by widening the scope of data to include irregular spatial cells, as well as a record of visual input that may drive cellular activity. Ultimately, probing the immediate, causal relationship between HF activity and motor output will yield needed insight as to how the hippocampal memory system normally functions.
Acknowledgments
The author thanks Elizabeth A Buffalo and Adam J Dede for comments on this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work is supported by funding from the National Institutes of Health.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MM wrote and developed the arguments presented in this Commentary.
ORCID iD: Miriam Meister  https://orcid.org/0000-0002-4479-0126
https://orcid.org/0000-0002-4479-0126
References
- 1. Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand. 1996;165:3–12. [DOI] [PubMed] [Google Scholar]
- 2. Annese J, Schenker-Ahmed NM, Bartsch H, et al. Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nat Commun. 2014;5:3122–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. [DOI] [PubMed] [Google Scholar]
- 4. Quirk GJ, Muller RU, Kubie JL, Ranck JB. The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J Neurosci. 1992;12:1945–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. [DOI] [PubMed] [Google Scholar]
- 6. O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. [DOI] [PubMed] [Google Scholar]
- 7. Taube JS, Muller RU, Ranck JB. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feigenbaum JD, Rolls ET. Allocentric and egocentric spatial information processing in the hippocampal formation of the behaving primate. Psychobiology. 1991;19:21–40. [Google Scholar]
- 9. Killian NJ, Jutras MJ, Buffalo EA. A map of visual space in the primate entorhinal cortex. Nature. 2012;5:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Araujo IE, Rolls ET, Stringer SM. A view model which accounts for the spatial fields of hippocampal primate spatial view cells and rat place cells. Hippocampus. 2001;11:699–706. [DOI] [PubMed] [Google Scholar]
- 11. Robertson RG, Rolls ET, Georges-Francois P. Spatial view cells in the primate hippocampus: effects of removal of view details. J Neurophysiol. 1998;79:1145–1156. [DOI] [PubMed] [Google Scholar]
- 12. Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Behav Neurosci. 1998;112:749–761. [DOI] [PubMed] [Google Scholar]
- 13. Zhang S, Schonfeld F, Wiskott L, Manahan-Vaughan D. Spatial representations of place cells in darkness are supported by path integration and border information. Front Behav Neurosci. 2014;8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acharya L, Aghajan ZM, Vuong C, Moore JJ, Mehta MR. Causal influence of visual cues on hippocampal directional selectivity. Cell. 2016;164:197–207. [DOI] [PubMed] [Google Scholar]
- 16. Fyhn M, Hafting T, Treves A, Moser M-B, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. [DOI] [PubMed] [Google Scholar]
- 17. Land MF. Animal vision: rats watch the sky. Curr Biol. 2013;23:R611–R613. [DOI] [PubMed] [Google Scholar]
- 18. Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JND. Rats maintain an overhead binocular field at the expense of constant fusion. Nature. 2013;498:65–69. [DOI] [PubMed] [Google Scholar]
- 19. Diehl GW, Hon OJ, Leutgeb S, Leutgeb JK. Grid and nongrid cells in medial entorhinal cortex represent spatial location and environmental features with complementary coding schemes. Neuron. 2017;94:83–92.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun C, Kitamura T, Yamamoto J, et al. Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc Natl Acad Sci U S A. 2015;112:9466–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hedrick KR, Zhang K. Megamap: flexible representation of a large space embedded with nonspatial information by a hippocampal attractor network. J Neurophysiol. 2016;116:868–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meister MLR, Buffalo EA. Getting directions from the hippocampus: the neural connection between looking and memory. Neurobiol Learn Mem. 2016;134:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Findlay JM, Gilchrist ID. Active Vision: The Psychology of Looking and Seeing. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 24. Urgolites ZJ, Hopkins RO, Squire LR. Medial temporal lobe and topographical memory. Proc Natl Acad Sci U S A. 2017;114:8626–8630. [DOI] [PMC free article] [PubMed] [Google Scholar]


