Abstract
Background
Radioimmunotherapy (RIT) is effective in treating relapsed/refractory follicular lymphoma (FL), with durable remissions in first-line consolidation. We hypothesized that RIT with ibritumomab tiuxetan (Zevalin®) would result in durable remissions by eliminating minimal residual disease after cytoreduction.
Methods
Patients with FL received 2 cycles of ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin) every 28 days, followed by Zevalin 4–6 weeks later if there was no disease progression and bone marrow biopsy showed < 25% involvement.
Results
Twenty-eight patients were treated, with a median age of 61 years, median of 3 prior therapies, 49% high-risk disease (Follicular Lymphoma International Prognostic Index, FLIPI), and 39% progressive disease. Three patients did not receive Zevalin due to residual bone marrow involvement. The main toxicities were cytopenias, with 11% febrile neutropenia. The overall response rate (ORR) was 72%, with 45% achieving complete response. With a median follow-up of 73 months, 1-year progression-free survival (PFS) was 38%, and median PFS was 10 months, but median overall survival (OS) was not reached.
Conclusion
The study did not reach its primary endpoint of a 1-year PFS of 67.3%. Reasons for this could include low accrual, high-risk disease, and inadequate debulking provided by 2 cycles of ESHAP. However, this protocol was associated with tolerable toxicity, high ORR, and high OS. Further studies would optimize debulking and focus on high-risk FL patients.
Keywords: Follicular lymphoma, Indolent non-Hodgkin lymphoma, Phase II trial, Relapsed/refractory lymphoma, CD20
Introduction
Follicular lymphoma (FL) is the second most common type of non-Hodgkin lymphoma (NHL) and the most common subtype of indolent NHL [1]. FL is incurable but has a variable clinical course. There is no one standard therapy, but most treatment choices utilize an anti-CD20 monoclonal antibody such as rituximab, often combined with chemotherapy [2, 3].
ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin) is a salvage regimen used in aggressive NHLs, but has good efficacy in relapsed low-grade lymphomas as well. One study showed a 75% overall response rate (ORR) and 36% complete response (CR) rate in patients with relapsed indolent lymphomas and substantially improved overall survival (OS) compared to DHAP [4]. In another study of 122 patients with relapsed, refractory NHL, ESHAP was associated with an 82% CR rate in indolent lymphoma [5]. Although ESHAP is very active in low-grade NHL, it is not often utilized by community oncologists as a salvage regimen. Therefore, most pretreated patients have not seen these agents and we hypothesized that their lymphomas should be responsive to ESHAP.
Ibritumomab tiuxetan (Zevalin®) is a murine anti-CD20 monoclonal antibody conjugated to yttrium-90. In a prospective study of 143 patients with relapsed or refractory NHL where a vast majority of patients had relapsed refractory FL, ibritumomab tiuxetan was associated with a significantly higher response rate compared to rituximab [6]. In a landmark trial, patients with FL who received ibritumomab tiuxetan had a notable increase in PFS [7]. About 20–25% of patients receiving ibritumomab tiuxetan have obtained durable remissions [8].
We postulated that radioimmunoconjugates may significantly improve the outcome for patients with FL, particularly when given for lower disease burden which could likely be achieved after treatment with 2 cycles of ESHAP. We therefore hypothesized that treatment with ibritumomab tiuxetan after 2 cycles of ESHAP would prolong time to progression (TPP), possibly changing the natural history of relapsed disease.
The only difference between ESHAP as originally published and as administered at our institution is the daily intravenous (IV) bolus dosing of cisplatin, instead of continuous infusion. The rationale for continuous infusion of cisplatin originally was to abrogate nephrotoxicity. However, it is well understood that limiting cisplatin doses to < 50 mg/m2/dose in combination with IV normal saline-based hydration is sufficient to minimize nephrotoxicity [9]. Cisplatin is amenable to administration via daily bolus infusion due to cell cycle-independent toxicity and an extracellular half-life of 16–53 h. Importantly, bolus administration results in high intracellular concentrations in lymphocytes [10].
Methods
Patients and Methods
We enrolled patients with histologically confirmed CD20-positive relapsed FL. Patients had bulky stage II, III, or IV disease by Ann Arbor classification. Bulky disease was defined as any tumor measuring 10 cm or greater or occupying more than one third of chest diameter. Patients were required to have at least 1 but less than 4 prior therapies. Patients were older than 18 years of age and required to have ECOG performance status 0–2, measurable disease, signed informed consent, normal serum creatinine, and bilirubin < 2.0 × upper limit of normal. Patients with platelet counts ≥150,000 were able to receive 0.4 mCi/kg of ibritumomab tiuxetan; patients with platelet counts 100,000–150,000 received a dose of 0.3 mCi/kg of ibritumomab tiuxetan.
At our institution, ESHAP has been adapted to an outpatient setting, consisting of etoposide 40 mg/m2/day IV bolus on days 1–4 (160 mg/m2 per cycle), cisplatin 25 mg/m2/day IV bolus on days 1–4 (100 mg/m2 per cycle), methylprednisolone 250 mg IV daily on days 1–4, and cytarabine 2,000 mg/m2 IV bolus given once on day 4. Patients were restaged after 2 cycles of outpatient ESHAP, given every 28 days as described. Patients with residual involvement of > 25% bone marrow after the 2 cycles of ESHAP were taken off treatment and followed up for TPP and survival. Patients who did not experience progression and had < 25% residual bone marrow involvement proceeded to receive ibritumomab tiuxetan 4–6 weeks after completion of the second cycle of ESHAP, with imaging for bio distribution as described originally [11]. Patients were subsequently re-evaluated for response assessment 12 weeks after completion of Zevalin. Thereafter, patients were followed up at 3-month intervals (short-term follow-up) for 2 years, and then every 6 months (long-term follow-up) for an additional 3 years. Once the patients completed long-term follow-up they were followed up on an annual basis (±30 days) for survival and disease status.
Statistical Analysis
The primary objective of this open-label single-arm phase II clinical trial was to evaluate the 1-year progression-free survival (PFS) of patients with relapsed follicular NHL treated with ESHAP chemotherapy for cytoreduction (2 cycles) followed by ibritumomab tiuxetan radioimmunotherapy (RIT), compared to what would be expected historically for patients treated with ibritumomab tiuxetan without prior cytoreduction. Secondary objectives included evaluation of median TTP, ORR, and CR rates.
A 1-year PFS of 67.3% was considered highly encouraging, compared to a historical rate of around 50% [4]. These values corresponded to approximate median TPP of 21 and 12 months, respectively, assuming exponential survival. Fifty-two evaluable patients would be required to test the null hypothesis that the true 1-year PFS is ≤50%, versus an alternative hypothesis that the true rate is ≥67.3% with this therapeutic approach. All patients who received at least one dose of chemotherapy were considered evaluable and included in the analysis. Sample size calculation was computed for 90% power and 10% type I error. Descriptive and summary statistics for demographic and clinical variables were obtained. The incidences of reported adverse events (AEs) were also tabulated. Kaplan-Meier survival analysis for PFS and OS was performed on TPP. All the analyses were performed using Stata [12].
Results
Patient Characteristics
Twenty-eight patients were enrolled in the study, with a median follow-up of 73.0 months (95% CI: 51.6–89.0). The study was closed per institutional mandate for slow accrual. One patient was ineligible due to re-review of pathology showing diffuse large B-cell lymphoma. The median age was 61 years and 36% of the patients were male. More than half the patients had B symptoms, 43% had bone marrow involvement, 39% had elevated β2-microglobulin, 44% had elevated lactate dehydrogenase, and none had bulky disease, although 25% of patients had tumors measuring 5 cm or more. The Follicular Lymphoma International Prognostic Index (FLIPI) showed 36% of patients had intermediate-risk and 46% had high-risk disease (49% in 27 eligible patients). The median number of prior therapies was 3 (range 1–4), with 43% of the patients receiving more than one prior regimen. One patient had prior autologous stem cell transplant. Eleven patients (39%) had progressive disease with the last chemotherapy. Table 1 describes additional baseline characteristics of the patients.
Table 1.
Patient characteristics (n = 28 patients)
| Baseline characteristics | |
| Median age (SD), years | 61 (11.9) |
| Male | 10 (36) |
| White | 27 (96) |
| Ethnicity (Hispanic) | 3 (11) |
| Clinical characteristics | |
| Karnofsky performance status | |
| 60 | 1 (4) |
| 70 | 4 (14) |
| 80 | 8 (29) |
| 90 | 7 (25) |
| 100 | 8 (29) |
| Baseline B symptoms | 15 (54) |
| Bone marrow involvement | 12 (43) |
| Prior transplant history | 1 (4) |
| Tumor bulk >5 cm | 7 (25) |
| Elevated β2-microglobulin | 11 (39) |
| FLIPI | |
| 0 | 1 (4) |
| 1 | 4 (14) |
| 2 | 10 (36) |
| 3 | 8 (29) |
| 4 | 5 (18) |
| FLIPI-2 | |
| 0 | 4 (14) |
| 1 | 9 (32) |
| 2 | 8 (29) |
| 3 | 5 (18) |
| 4 | 2 (7) |
| Prior chemotherapy regimens | |
| 0 | 0 (0) |
| 1 | 16 (57) |
| 2 | 7 (25) |
| 3 | 3 (11) |
| 4 | 2 (7) |
| Response to prior chemotherapy regimens | |
| CR | 9 (32) |
| PR | 4 (14) |
| SD | 1 (4) |
| PD | 11 (39) |
| UK | 3 (11) |
Values are n (%) unless otherwise indicated. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; UK, unknown.
Safety
AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs, v3.0, as detailed in Table 2 [13]. AEs were reported in most patients, and 25 patients had grade 3 or higher AE. The most common grade 3 AEs were cytopenias (not prolonged) and fatigue. Notably, the rate of neutropenia was 32% and the rate of febrile neutropenia was 11%, and neuropathy (sensory) was noted in 5 patients. There were 6 serious AEs reported on this study. One patient had a perforated duodenal ulcer, and 1 patient experienced nausea, vomiting, diarrhea, and functional decline. These 2 patients withdrew from the study and were not evaluable for response. Two patients developed myelodysplastic syndrome (MDS) after study completion, with no patients developing acute myelogenous leukemia. One patient had severe thrombocytopenia, leukopenia requiring admission, and transfusion support that qualified as a serious AE. Furthermore, 1 patient had a diagnosis of metachronous colon carcinoma diagnosed after completion of study.
Table 2.
Reported adverse events (n = 28 patients)
| Category | All grades, n (%) | Grade ≥3, n (%) |
|---|---|---|
| Any adverse event | 27 (96) | 25 (89) |
| Thrombocytopenia | 21 (75) | 17 (61) |
| Fatigue | 21 (75) | 5 (18) |
| Anemia | 21 (75) | 4 (14) |
| Leukopenia | 20 (71) | 11 (39) |
| Nausea | 19 (68) | 1 (4) |
| Pain | 16 (57) | 3 (11) |
| Vomiting | 12 (43) | 2 (7) |
| Hair loss/alopecia (scalp or body) | 10 (36) | |
| Neutropenia | 9 (32) | 2 (7) |
| Anorexia | 9 (32) | |
| Constipation | 7 (25) | |
| Dyspnea (shortness of breath) | 7 (25) | |
| Diarrhea | 6 (21) | 1 (4) |
| Dizziness | 6 (21) | |
| Dehydration | 5 (18) | 1 (4) |
| Neuropathy (sensory) | 5 (18) | |
| Weight loss | 5 (18) | |
| Cough | 4 (14) | |
| Tinnitus | 4 (14) | |
| Febrile neutropenia | 3 (11) | 3 (11) |
Efficacy
Twenty-two patients completed the protocol and were evaluable for response. In addition to the 2 patients who withdrew secondary to toxicities, 3 patients were not evaluable for response secondary to bone marrow showing residual involvement of > 25% that prohibited them from receiving ibritumomab tiuxetan. The ORR for the entire study was 77.3%, with 10 (45%) achieving CR, 32% having partial response (PR) and 14% with stable disease. There was a slight difference in response rates between patients that received one prior regimen (ORR 83%), and those that received more than one prior regimen (ORR 70%). Patients that received one prior regimen achieved 42% CR, while patients with more than one prior regimen achieved 50% CR. Patients with progressive disease with prior therapies had 50% CR and 25% PR, similar to the response in patients with non-progressive disease.
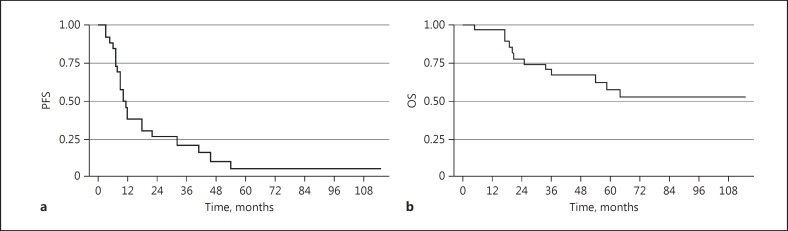
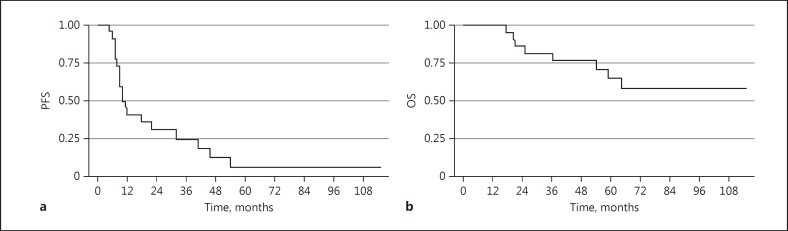
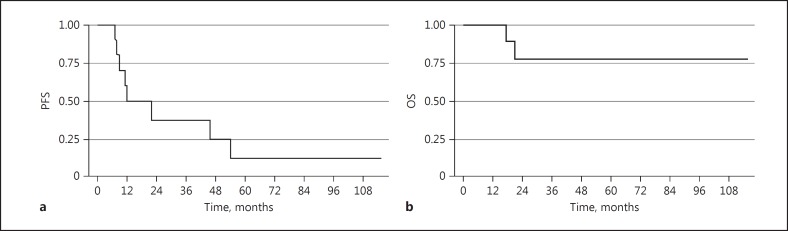
The median follow-up time was 73.0 months (95 % CI: 51.6–89.0). In an intent-to-treat analysis, the median PFS was 10.0 months (95% CI: 8.0–18.0) (Fig. 1a), and the median OS was not reached (95% CI: 34.0–not reached) (Fig. 1b). For the 22 evaluable patients, 1-year PFS was 38%, median TTP was 18.0 months (95% CI: 9.2–39.4), median PFS was 10.0 months (95% CI: 8.0–32.0) (Fig. 2a), and median OS was not reached (Fig. 2b) (95% CI: 54.0 months-not reached). Results were best for the patients in CR, with 1-year PFS of 50%, median PFS of 12 months (95% CI: 7.0–-54.0), and OS not reached (95% CI: 17 months–not reached) (Fig. 3a, b). Patients treated with one prior regimen had a 1-year PFS of 42%, with a median PFS of 9 months (95% CI: 7.0–32.0), while median OS was not reached. In patients treated with more than one prior regimen, 1-year PFS was 40%, with a median PFS of 11 months (95% CI: 6.0–46.0), and OS was also not reached.
Fig. 1.
Kaplan-Meier survival estimates of all consented patients (n = 28). a Median progression-free survival (PFS). b Median overall survival (OS).
Fig. 2.
Kaplan-Meier survival estimates of evaluable patients (n = 22). a Median progression-free survival (PFS). b Median overall survival (OS).
Fig. 3.
Kaplan-Meier survival estimates of evaluable patients who achieved complete response. a Median progression-free survival (PFS). b Median overall survival (OS).
Discussion
FL is a biologically heterogeneous disease with evolving therapeutic options in the relapsed refractory setting [14]. This study was open to accrual prior to the development of novel chemotherapeutic, immunotherapeutic, and targeted agents including bendamustine, idelalisib, venetoclax, and obinutuzumab. Bendamustine in particular adversely affected accrual in this study, leading to institutionally mandated closure.
ESHAP followed by ibritumomab tiuxetan had tolerable toxicity, with only 11% experiencing febrile neutropenia and no significant renal complications. Only 2 patients withdrew due to toxicity. Two patients did develop MDS, to which ibritumomab tiuxetan could have contributed, but that cannot be separated from the contribution of marrow toxicity from prior chemotherapy regimens [15], In fact, 1 patient subsequently underwent an allogeneic bone marrow transplant prior to the development of MDS. This is lower than estimates reported in the literature, where the risk of MDS/acute myelogenous leukemia has been estimated to be up to 14% [16]. Further, ESHAP lends itself to combinations nicely [17], and it was not associated with increased nephrotoxicity or neuropathy, thus proving itself as a feasible salvage regimen.
Several factors could have contributed to the trial not reaching its primary endpoint. The trial was closed before reaching its target due to low accrual. It enrolled a high-risk population, with 49% having high-risk disease (FLIPI), and 39% having progressive disease with the last prior therapy. Two cycles of ESHAP may have provided inadequate debulking, evidenced by 3 patients not being able to proceed with ibritumomab tiuxetan due to significant residual bone marrow involvement. Rituximab was not combined with ESHAP so as not to compete with ibritumomab tiuxetan for CD20 binding, and thus interfere with its efficacy [18]
However, the ORR rate of our study was similar to other studies of chemotherapy followed by RIT; for example, Illidge et al. [19] used 3 cycles of R-CHOP or R-CVP prior to ibritumomab tiuxetan in 50 patients. The ORR was 98%, CR was 30% (improving to 44% on follow-up), with a median PFS of 23.1 months and OS of 77.5% at 5 years. Pisani et al. [20] treated 9 patients with 4 cycles of FCR (fludarabine, cyclophosphamide, rituximab) followed by ibritumomab tiuxetan, with 7 patients achieving CR and 2 PR (converted to CR after ibritumomab tiuxetan). OS or PFS were both 67% at 7.5 years, with 3 patients dying while still in CR. While the majority of chemotherapy-RIT consolidation studies reporting excellent outcomes have been conducted in previously untreated patients [21], in our study there was notably no significant difference in the ORR, PFS, or OS of patients that had received one versus more than one prior regimen, and this protocol also effectively salvaged patients with progressive disease to prior therapies. In two reports, data from the four registration trials of Zevalin were analyzed retrospectively. Long-term responses (TTP ≥12 months) were noted in only 37% of 211 patients treated in these trials. The achievement of CR seemed to be the best predictor for achievement of long-term response [22, 23]. This is also noted in our data set, where responders in CR had the best TTP and PFS. Other predictors for durable remissions in a stepwise multivariate logistic regression analysis were tumor size (< 5 cm) and stage I or II disease at the time of RIT [22, 23]. In our study, at least half the patients had bone marrow involvement and one fourth had tumors > 5 cm, which may have adversely affected the response rates and PFS.
Another paradigm shift in the landscape of FL has been the recent realization of a high-risk group of patients that progress within 24 months after therapy, especially following an anthracycline-based therapy (R-CHOP) [24]. Our study enrolled and accrued prior to this discovery.
In summary, while we report a negative trial with respect to the primary endpoint of 1-year PFS with 2 cycles of ESHAP followed by Zevalin, this protocol was associated with low toxicity, good ORR, and good OS, regardless of the number of prior lines of therapy. Future directions of study would include conducting this trial in a high-risk patient population of FL such as that with event-free survival < 24 months, and optimizing debulking with ESHAP to improve subsequent clearance of lymphoma from the bone marrow space prior to treatment with Zevalin.
Statement of Ethics
All subjects who participated gave their informed consent to this study protocol which was approved by the institute's committee on human research.
Disclosure Statement
Dr. Anwer has received personal fees from Seattle Genetics and Incyte. Dr. Persky has received research funding from Merck and Spectrum Pharmaceuticals. Dr. Puvvada has received personal fees from Pharmacyclics, AbbVie, Gilead, and Seattle Genetics and research funding from Takeda, Spectrum, AbbVie, and Genentech Pharmaceuticals.
Funding Sources
Research funding for this study was received from Spectrum Pharmaceuticals. The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health (award No. P30 CA023074).
References
- 1.Press OW, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus 131iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol. 2013;31:314–320. doi: 10.1200/JCO.2012.42.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiddemann W, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Monge EJ, Cabanillas F, Long-term follow-up of platinum-based lymphoma salvage regimens The M.D. Anderson Cancer Center experience. Hematol Oncol Clin North Am. 1997;11:937–947. doi: 10.1016/s0889-8588(05)70471-8. [DOI] [PubMed] [Google Scholar]
- 5.Velasquez WS, et al. ESHAP – an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 7.Morschhauser F, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 8.Gordon LI, et al. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: long-term follow-up of a phase 1/2 study. Blood. 2004;103:4429–4431. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 9.Launay-Vacher V, et al. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61:903–909. doi: 10.1007/s00280-008-0711-0. [DOI] [PubMed] [Google Scholar]
- 10.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993;67:1171–1176. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zevalin® (ibritumomab tiuxetan) (package insert). 2001. www.zevalin.com/downloads/Zevalin_Package_Insert.pdf (accessed April 5, 2017)
- 12. StataCorp: Stata Statistical Software. Stata/SE. StataCorp LLC, College Station, USA.
- 13.Trotti A, et al. Amsterdam: Elsevier; 2003. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment; in: Seminars in Radiation Oncology. [DOI] [PubMed] [Google Scholar]
- 14.Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127:2055–2063. doi: 10.1182/blood-2016-06-721902. [DOI] [PubMed] [Google Scholar]
- 15.Czuczman MS, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25:4285–4292. doi: 10.1200/JCO.2006.09.2882. [DOI] [PubMed] [Google Scholar]
- 16.Reiss J, et al. Long-term follow up of rates of secondary malignancy and late relapse of two trials using radioimmunotherapy consolidation following induction chemotherapy for previously untreated indolent lymphoma. Leuk Lymphoma. 2015;56:2870–2875. doi: 10.3109/10428194.2015.1016929. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Sanz R, et al. Brentuximab vedotin plus ESHAP (BRESHAP) is a highly effective combination for inducing remission in refractory and relapsed Hodgkin lymphoma patients prior to autologous stem cell transplant: a trial of the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO) (abstract) Ann Meet Am Soc Hematol, San Diego. 2016 Dec;:3–6. [Google Scholar]
- 18.Gopal AK, et al. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112:830–835. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Illidge TM, et al. Short duration immunochemotherapy followed by radioimmunotherapy consolidation is effective and well tolerated in relapsed follicular lymphoma: 5-year results from a UK National Cancer Research Institute Lymphoma Group study. Br J Haematol. 2016;173:274–282. doi: 10.1111/bjh.13954. [DOI] [PubMed] [Google Scholar]
- 20.Pisani F, et al. Long term efficacy and safety of Fludarabine, Cyclophosphamide and Rituximab regimen followed by 90Y-ibritumomab tiuxetan consolidation for the treatment of relapsed grades 1 and 2 follicular lymphoma. Exp Hematol Oncol. 2015;4:17. doi: 10.1186/s40164-015-0012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morschhauser F, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-Line Indolent trial. J Clin Oncol. 2013;31:1977–1983. doi: 10.1200/JCO.2012.45.6400. [DOI] [PubMed] [Google Scholar]
- 22.Witzig TE, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90ibritumomab tiuxetan. Cancer. 2007;109:1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 23.Emmanouilides C, et al. Treatment with yttrium 90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47:629–636. doi: 10.1080/10428190500376076. [DOI] [PubMed] [Google Scholar]
- 24.Casulo C, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33:2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]