Abstract
Background
Oncolytic vesicular stomatitis virus (VSV) can be delivered intravenously to target primary and metastatic lesions, but the interaction between human peripheral blood leukocytes (PBLs) and VSV remains poorly understood. Our study aimed to assess the overall immunological consequences of ex vivo infection of PBLs with VSV.
Methods
Phenotypic analysis of lymphocyte subsets and apoptosis were evaluated with flow cytometry. Caspase 3/7 activity was detected by luminescence assay. Virus release was evaluated in a murine cell line (L929). Gene expression and cytokine/chemokine secretion were assessed by real-time PCR and multiplex assay, respectively.
Results
Ex vivo infection of PBLs with VSV elicited upregulated expression of RIG-I, MDA-5, tetherin, IFITM3, and MxA. VSV infection triggered rapid differentiation of blood monocytes into immature dendritic cells as well as their apoptosis, which depended on caspase 3/7 activation. Monocyte differentiation required infectious VSV, but loss of CD14+ cells was also associated with the presence of a cytokine/chemokine milieu produced in response to VSV infection.
Conclusions
Systemic delivery is a major goal in the field of oncolytic viruses. Our results shed further light on immune mechanisms in response to VSV infection and the underlying VSV-PBL interactions bringing hope for improved cancer immunotherapies, particularly those based on intravenous delivery of oncolytic VSV.
Keywords: Vesicular stomatitis virus, Oncolytic virus, Human peripheral blood leukocytes, Cytokines, Immature monocyte-derived dendritic cells
Introduction
Although significant progress in cancer treatment has been made in recent years, there are still certain types of cancer where a cure seems beyond our reach, and the side effects of conventional chemo- and radiotherapy remain undesirable. Consequently, there is an urgent need to find innovative strategies for cancer treatment. Oncolytic viruses are emerging as a promising alternative therapy that could be delivered intravenously, targeting both primary and metastatic lesions. Over the last decade, vesicular stomatitis virus (VSV), an oncolytic virus, has demonstrated oncolytic (cancer-killing) properties [[1] and references therein]. There are several advantages of using VSV as an anticancer therapy: VSV, a prototype of the nonsegmented negative-strand RNA viruses, is classified into the Rhabdoviridae family [2] and causes a relatively mild oral disease in livestock. Therefore, there is practically no preexisting immunity in humans, and the rare natural infections are generally asymptomatic [3]. VSV replicates within the cytoplasm of infected cells and does not undergo genetic recombination; moreover, it does not integrate any part of its genome into the host, and it is not known to have transformation potential [[1] and references therein]. Preferential tropism of VSV to tumor cells has been observed in the case of melanoma, glioblastoma, sarcoma, ovarian cancer, cervical cancer, breast cancer, and leukemia [1, 4]. VSV oncoselectivity is based largely on defective innate immune responses or tumor-related abnormalities in the regulation of mRNA translation or altered cellular signaling pathways. For instance, most cancers have a defective interferon (IFN) system, which makes them more susceptible to VSV infection [[1] and references therein]. It is worth mentioning that in addition to the direct oncolytic effect, genetically engineered VSV pseudotypes bearing other viral envelope proteins or immune response genes are ideal for vaccine development against various diseases or for stimulation of powerful tumor-specific immune responses [1, 5].
The basic principle of virotherapy is the use of live viruses. VSV has been shown to cause viral encephalitis in animal models. In addition, some scientists are skeptical regarding VSV safety and oncoselectivity; therefore, clinical safety following intravenous administration of VSV is given the highest priority, especially in individuals with immune systems compromised by disease or chemotherapy [6]. Despite the fact that neurotoxicity has been observed following intravascular VSV administration, little is known about the interaction of VSV with human peripheral blood leukocytes (PBLs) [[6], [7] and references therein]. Recently, a growing number of studies have demonstrated the capability of viral infection to trigger the differentiation of monocytes into dendritic cells (MDDCs), which are potent inducers of adaptive immune responses [8]. The mechanisms involved in DC differentiation from monocytes under physiological conditions or in response to viral infections are not fully understood. In our earlier studies, we found, in fact, that VSV can replicate in human PBLs with different efficiency, but the target leukocyte subpopulation remained elusive [9, 10, 11, 12].
In the present study, we investigated the interaction of VSV with human PBLs with a focus on the delineation of primary target cells for VSV infection. We have also examined immune responses to VSV via determining the expression profile of cytosolic RNA sensors, retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), IFN-stimulated genes (ISGs), as well as secretion profiles of cytokines and chemokines.
Taken together, these findings provide the link between VSV infection, cytokine release, monocyte survival, and their maturation. A better understanding of the immunoregulation and pathogenesis of VSV infection may be helpful in the design of future therapies through enhancing antitumoral immunity and improving safety and efficacy of VSV-based oncolytic therapies.
Materials and Methods
Subjects
Peripheral venous blood was obtained from 74 healthy adult volunteers and collected in tubes containing anticoagulant EDTA. This study has been reviewed, approved, and conducted in accordance with the guidelines of the Ethics Committee of the Wroclaw Medical University (No. KB-75/2016). Signed consent was obtained from all participants of the study. All plasma samples were tested for VSV-neutralizing activity and considered negative (data not shown).
Cells
L929 (ATCC CCL1), a murine fibroblast-like cell line, was maintained in complete RPMI 1640 medium (IIET, Wroclaw, Poland) with antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin), 2 mML-glutamine, and 10% fetal bovine serum (FBS) (all from Sigma-Aldrich, USA). PBLs were isolated according to a standard protocol from peripheral blood by gradient centrifugation in Gradisol G (Aqua-Med, Łódź, Poland) and maintained in the same medium as L929 cells. Whole blood cultures were prepared by suspension of freshly collected blood containing 1–2 × 106 cells after previous washing in complete medium. Monocyte-enriched blood cells from healthy donors' PBLs were negatively isolated using magnetic bead-based separation according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA) and suspended in complete medium. Purity was >55% as verified by flow cytometry.
Vesicular Stomatitis Virus
A wild-type Indiana VSV serotype was originally obtained from Dr. C. Buckler (National Institutes of Health, Bethesda, MD, USA). VSV was grown and titrated in L929 cells. The titer was expressed with reference to the TCID50 (tissue culture infectious dose) value, based on the cytopathic effect caused by this virus in approximately 50% of infected cells or by plaque assay.
In vitro VSV Infection
Isolated human PBLs, whole blood, or monocyte-enriched blood cells were cultured in complete medium. For in vitro infection, VSV was diluted in 2% FBS in RPMI medium. VSV was added at a multiplicity of infection (MOI) of 0.1, 1.0, or 10 to each well, containing 1–2 × 106 PBLs or 5 × 105 monocyte-enriched blood cells. After 40 min of incubation with the virus, cells were washed 5 times and cultured for 18 h in complete medium for FACS staining or the virus titer assay. Control infections using UV-inactivated virus were performed in parallel for all experiments. For VSV inactivation, viral stock was placed on ice and irradiated using a 254-nm UV Stratalinker (Stratagene, La Jolla, CA, USA) for 40 min. Sensitivity of PBLs to VSV infection was assessed according to a method described earlier; a VSV titer >4 log TCID50 was considered as a deficiency in innate immunity, and a titer of 0–1 log indicated complete resistance to VSV infection [11].
Blood Samples and Staining Protocol
Each blood sample (ca. 200 μL - based on the number of leukocytes calculated by hematology analyzer [Mythic18; Orphee Medical, Switzerland]) was stained with the specific antibodies (CD3-PE, CD14-APC [SouthernBiotech]; CD11c-Alexa Fluor 700, HLA-DR APC-Cy7, CD16-PE-CF594, CD56-PE-Cy7, CD20-PE-Cy7, CD123-PerCP-Cy5.5 [BD Bioscience]; anti-VSV-G-FITC [Abcam, UK], goat IgG isotype control [SouthernBiotech]). Erythrocytes were lysed with Cal-Lyse lysing solution (Invitrogen). The panel strategy and phenotypic analysis of lymphocyte populations were according to established and published methods [13]. Flow-cytometric analysis of VSV-infected cell populations was performed utilizing rabbit anti-VSV-G-FITC antibody (Abcam).
Data Acquisition and Sample Analysis
BD CompBeads stained with the same fluorochrome-labeled antibodies used in the experiment were used to calculate a compensation matrix. 50,000 events were acquired using a FACS- Fortessa™ cytometer (BD Biosciences, San Jose, CA, USA) within the combined lymphocyte-monocyte gate based on the FSC and SSC parameters. Finally, flow cytometry standard files were analyzed using DIVA software.
Percentages of Lymphocytes, Monocytes, and DC Subsets
The total white blood cell count was determined with an automated hematology analyzer (Mythic18). The percentages of PBL, monocyte, and DC populations were determined by flow cytometry from the whole blood sample. The panel strategy and phenotypic analysis of lymphocyte populations were according to established and published methods [13].
Activation of PBLs with TLR Ligands and Supernatants
Whole human blood cultures were cultured with TLR3 ligand-poly(I:C), (10 μg/mL) or TLR4 ligand-LPS (1 μg/mL) infected with VSV at the indicated MOI for 18 h. Cells were then analyzed by flow cytometry to assess the frequencies of various cell types. To assess the impact of cytokines and chemokines on monocyte differentiation, another batch of supernatants (pooled from 10 independent experiments) was prepared. Briefly, PBLs were infected with VSV for 18 h; supernatants were collected, screened for the presence of the infectious virus, and UV inactivated. Supernatants were then subdivided and pooled according to the level of VSV replication to: V0 and V3 groups, where V is defined as VSV infected. V0 represent supernatants obtained from cells resistant to VSV infection, while V3 are supernatants harvested from cells highly susceptible to VSV (TCID50 of VSV ≥3). M0 and M3 represent corresponding mock-infected supernatants (negative controls). Each PBL sample (obtained from 8 people) was then treated with 4 types of supernatants or culture medium (negative control) for 18 h and then analyzed by flow cytometry similarly to the VSV-infected samples. VSV-infected cells (MOI = 1) served as positive control (labeled as VSV). Differences in percentages of CD14+ cells in the PBL populations after treatment with supernatants were calculated in comparison to cells treated with culture medium only.
Analysis of Apoptosis in PBLs and CD14+ Cells
Staining of apoptotic cells was performed using the annexin V/PI protocol according to the manufacturer's instructions (BD Pharmingen, USA). Isolated PBLs were cultured with actinomycin (25 μg/mL) or were infected with VSV at MOI = 1 for 18 h. After blocking for 1 h with 2% FBS and 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), the cells were stained against VSV-G and CD14 and subsequently with annexin V-APC and vital dye (PI). Analysis of apoptotic cells in PBL and CD14+ populations was performed in a BD FACSCalibur™ flow cytometer.
Caspase 3/7 Activity Analysis
Caspase 3/7 intracellular activity was detected by the Caspase-Glo 3/7 Assay (Promega, USA) according to the manufacturer's instructions. PBLs isolated from freshly collected blood samples were infected with VSV at MOI = 1 for 18 h. Mock-infected samples served as a negative control and actinomycin-treated (3 µg/mL) cells served as a positive control of apoptosis. Luminescence was measured using a GloMax Luminometer (Promega).
Cytokine Profiling
The levels of interleukin (IL)-10, IFN-γ, and tumor necrosis factor (TNF)-α in supernatants from mock- and VSV-infected samples were detected using enzyme-linked immunosorbent assay (BD OptEIA™ human IL-10, IFN-γ, or TNF-α ELISA set, BD Biosciences). Concentrations of many other cytokines and chemokines in subsequent groups of patients were analyzed using the multiplex cytokine human magnetic 25-Plex panel (Invitrogen) detecting: eotaxin, GM-CSF, IFN-α, IFN-γ, IL-1β, IL-10, IL-12 (p40/p70), IL-13, IL-15, IL-17, IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IP-10, MCP-1, MIG, MIP-1α, MIP-1β, RANTES, and TNF-α on a Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA). The average of 3 measurements was recorded for each subject. Standard curves were drawn using a 5-PL curve and the data were analyzed using BioPlex Manager 6.0 software.
RNA Isolation and Real-Time PCR
PBLs (2 × 106 cells) were infected with VSV (MOI = 1) for 18 h, and real-time PCR was used to investigate mRNA expression. Total RNA (obtained from mock- or VSV-infected cells) was extracted with the RNeasy protect mini kit (Qiagen, Hilden, Germany), and reverse transcription was performed using the GoScript reverse transcription system (Promega, USA) according to the manufacturer's instructions. Real-time PCR was performed on a ViiA 7 real-time PCR system (ThermoFisher Scientific, Waltham, MA, USA) using the GoTaq qPCR MasterMix (Promega). The B2M (β2-microglobulin) gene was used to normalize Ct values. Results were analyzed using the Pfaffl mathematical model [14] and were shown as fold change compared to mock-infected samples. Primer sets used for these studies are listed in Table 1.
Table 1.
Primers used for real-time PCR
| Gene | Forward | Reverse | Reference |
|---|---|---|---|
| B2M | 5′-CTATCCAGCGTACTCCAAAG-3′ | 5′-GAAAGACCAGTCCTTGCTGA-3′ | 15 |
| Tetherin | 5′-CTGCAACCACACTGTGATG-3′ | 5′-ACGCGTCCTGAAGCTTATG-3′ | 16 |
| IFITM3 | 5′-ACTGTCCAAACCTTCTT CTCTC-3′ | 5′-AGCACAGCCACCTCGTGCTC-3′ | 17 |
| MxA | 5′-GCTACACACCGTGACGGATATGG-3′ | 5′-CGAGCTGGATTGGAAAGCCC-3′ | 18 |
| OAS2 | 5′-TCAGAAGAGAAGCCAACGTGA-3′ | 5′-CGGAGACAGCGAGGGTAAAT-3′ | 18 |
| MDA-5 | 5′-TCACGGACTTGCCCTCTCCA-3′ | 5′-GCAGCAATCCGGTTTCTGTCT-3′ | 18 |
| RIG-I | 5′-GTGCAAAGCCTTGGCATGT-3′ | 5′-TGGCTTGGGATGTGGTCTACTC-3′ | 18 |
| VSV_L | 5′-ATGATACAGTACAATTATTTTGGGACA-3′ | 5′-CAAAGACATGCCCGACACT-3′ | 19 |
Statistical Analyses
Statistical analyses were performed using the platform R-CRAN (version 3.3.2; www.r-project.org). Values are presented as the average value ± 95% confidence interval (CI) or as the median with interquartile range (IQR). Where it was needed, the data were log transformed or transformed using the method described by Box and Cox [20]. The optimal value of the transformation parameter λ, which represents the closest fit of the raw data set to a normal distribution, was estimated by the method of maximum likelihood. After transformation, the unpaired t test or exact permutation test was applied to compare 2 groups, and 1-way ANOVA was applied to compare >2 groups. The paired t test or Wilcoxon signed-rank test was used to compare 2 dependent groups. The Kendall rank correlation was used to investigate the association between 2 variables. All statements of significance were based on a probability value of * p < 0.05, unless stated otherwise.
Results
Detection of VSV in Infected PBLs
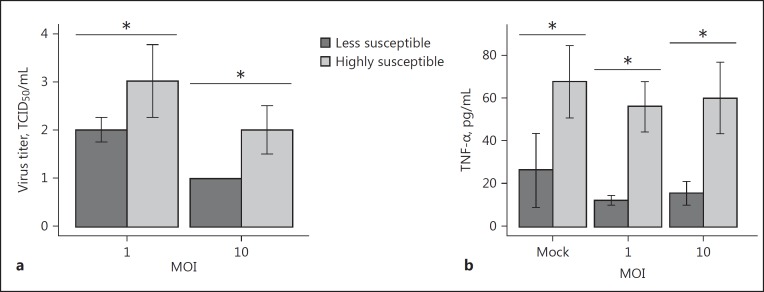
To determine the extent of VSV replication in PBLs obtained from healthy individuals, infectious VSV titers were assessed in the collected supernatants. We observed significant heterogeneity in virus titers between samples infected at the same MOI, which prompted us to separate examined samples into 2 groups: less susceptible and highly susceptible to VSV infection (Fig. 1a). We also divided our samples based on VSV titer at MOI = 10: the less susceptible group having VSV titer ≤1 log TCID50 and the highly susceptible group having virus titer >1 log TCID50. We hypothesized that virus replication in leukocytes sensitive to viral infection may be attributed to changes in the cytokine profile balance, i.e., TNF-α, IL-10, or IFN-γ production by VSV-infected leukocytes. Indeed, the level of TNF-α differed between groups with low and high virus titer in supernatants, with higher levels of TNF-α detected in the VSV-susceptible group (Fig. 1b). The observed difference was not dependent on the MOI used in the experiment; moreover, it was noticeable even in mock-treated samples (with a mean difference of 37.38 pg/mL between groups), which implies that spontaneous production of cytokines, or the activation of the antiviral state, have significant influence on the susceptibility of PBLs to VSV infection. There was no significant difference in IL-10 or IFN-γ production between groups (data not shown).
Fig. 1.
Susceptibility of PBLs to VSV infection (n = 26). a VSV-infected PBLs samples were further divided into two groups, accordingly to VSV titer in supernatants from PBLs infected at 18 hpi (less susceptible group, dark-grey bars, n = 7; highly susceptible group, light-grey bars, n = 19). Results are presented as the median percentage of infected cells with interquartile range. Mann-Whitney U test, * p < 0.05. b TNF-α production varied between these two groups. The error bars represent the average difference with a 95% confidence interval (t test, * p < 0.05).
VSV Infection Triggers RLR and ISG Expression in PBLs
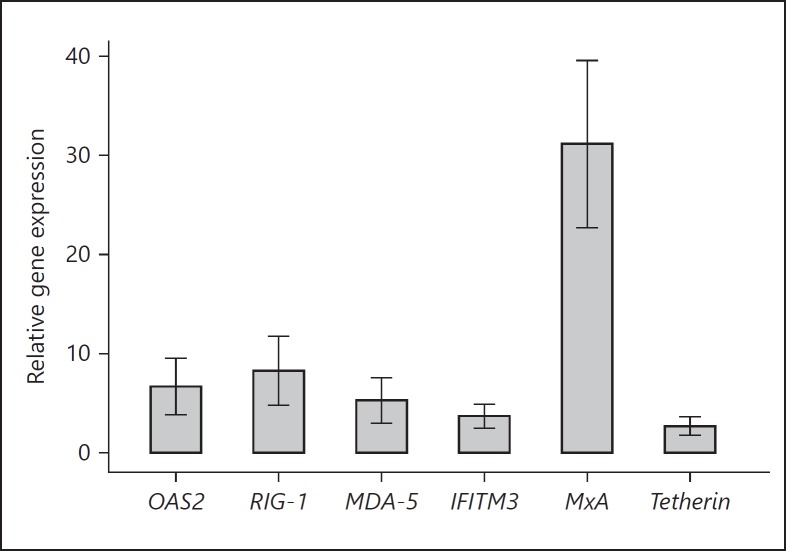
During infection, cytoplasmic viral RNA is sensed by RLRs ultimately driving IFN production. We, therefore, examined the expression of RIG-I and MDA-5 (melanoma differentiation-associated gene 5) mRNAs as well as IFN-stimulated genes - tetherin (encoding bone marrow stromal cell antigen 2), IFITM3 and OAS2 (encoding IFN-induced transmembrane protein 3 and 2′-5′-oligoadenylate synthetase 2, respectively), and MxA in VSV-infected PBLs. As shown in Figure 2, VSV infection led to a marked induction in RLR and ISG expression, respectively. Tetherin and IFITM3 genes were upregulated ≤5-fold relative to those of mock-infected cells. The expression levels of MDA-5, OAS2, and RIG-I were significantly increased with mean relative fold changes of 5.28, 6.66, and 8.27, respectively. Among all genes analyzed, MxA encoding myxovirus resistance protein 1, a key mediator of the IFN-induced antiviral response, was the most highly expressed gene with a mean of 31.15 relative fold change (95% CI 22.71–39.59).
Fig. 2.
Relative gene expression in PBLs after VSV infection (n = 11). The fold change of each gene transcript in the VSV-infected samples was normalized to the mock-infected samples. All samples were tested in triplicate. Whiskers represent 95% confidence interval of the mean. The results are statistically significant with p < 0.05 (exact permutation test).
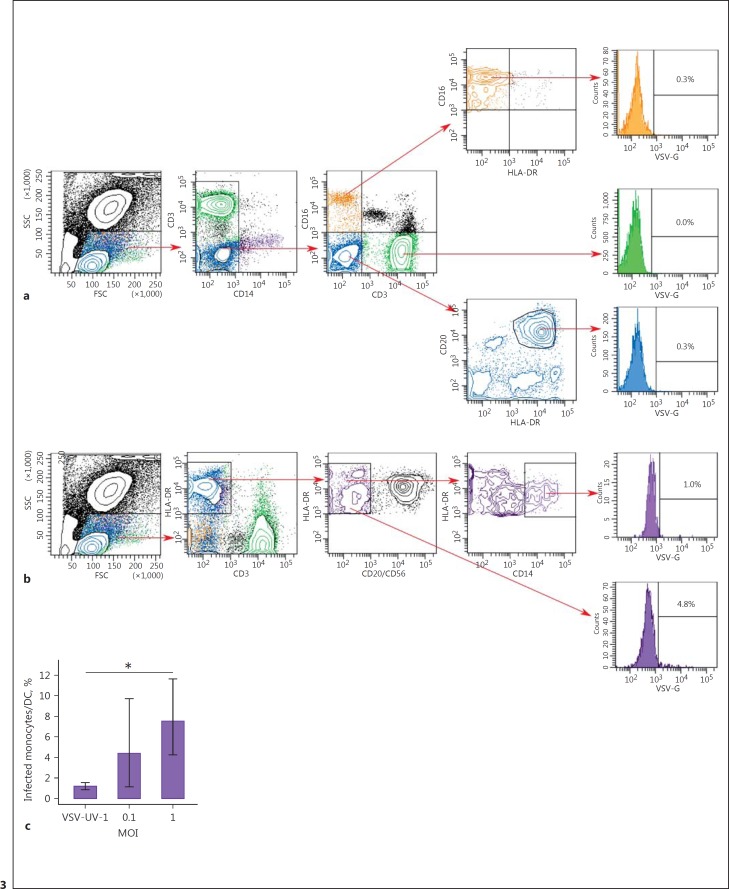
VSV Infects Monocytes and DCs in vitro
As shown in Figure 3, flow-cytometric analysis of the different PBL subsets of the representative donor revealed that no significant infection was observed in other subsets of PBLs (Fig. 3a) while VSV-G was detected within monocyte and DC populations (Fig. 3b). The number of CD14+ monocytes considerably decreased after VSV infection in all cases due to the apoptotic process or differentiation of peripheral blood monocytes into dendritic cells (MDDC). Thus, the final percentage of infected cells was calculated in the population expressing HLA-DR+/CD3-/CD20-/CD56-/CD14-, which might also include MDDCs. The number of VSV-G+ monocytes/DCs reached 5.48% (95% CI 0.34–10.61) at MOI = 0.1 and 7.00% (95% CI 3.30–10.70) at MOI = 1 (Fig. 3c). The difference in the percentage of infected monocytes/DCs between samples infected with VSV at MOI = 1 and mock- or VSV-UV-I-infected cells was statistically significant (p < 0.05).
Fig. 3.
Simultaneous detection of VSV-G in immune cell subsets. Flow cytometric analysis of T cells, B cells, and NK cells (a) as well as monocytes and monocytes/DC (b) 18 h after VSV infection at MOI = 1. A total of 50,000 cells were acquired. Data shown are from 1 representative donor. The percentage of VSV-G-positive (VSV-G+) cells are indicated. c Percentage of VSV-infected cells within HLA-DR+ population. The error bars represent the means of 4 donors ± 95% confidence interval (t test, * p < 0.05).
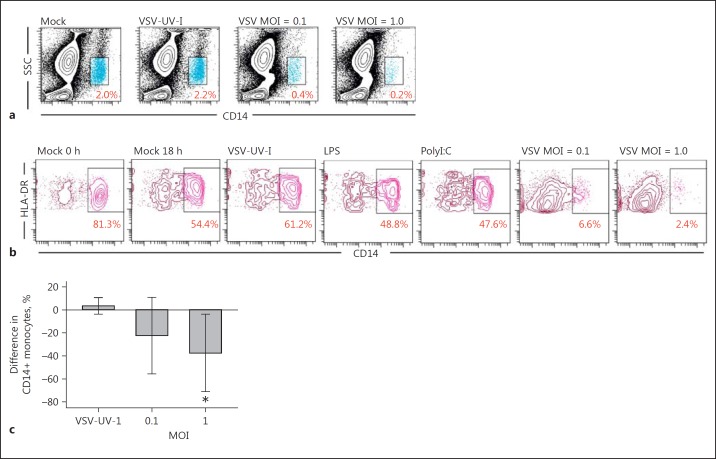
Loss of the CD14 Marker on Monocytes as an Effect of VSV Replication
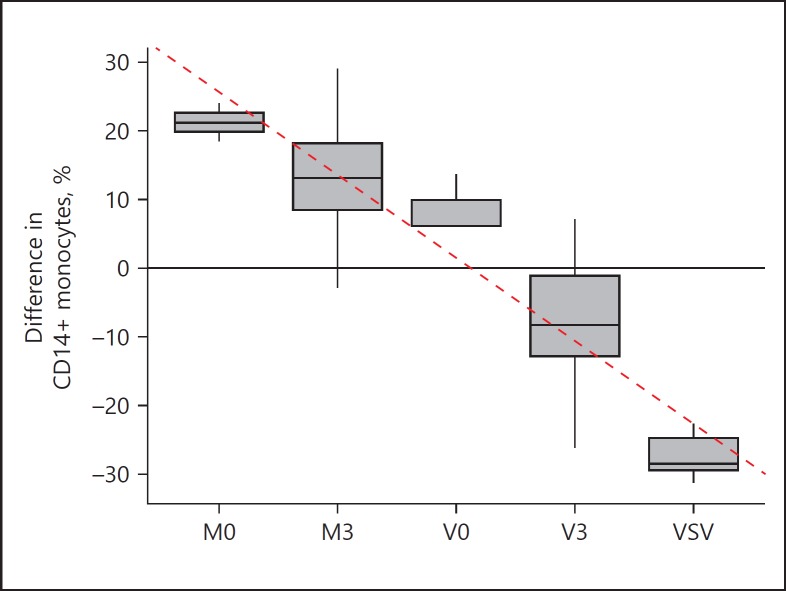
Experiments with UV-light-inactivated VSV (VSV-UV-I) or stimulation of the whole blood samples with stimulants poly(I:C) (TLR3 ligand) and LPS (TLR4 ligand) showed decreased expression of CD14 on VSV-infected leukocytes (the combined lymphocyte-monocyte gate) (Fig. 4a). Analysis of monocytes in HLA-DR+/CD3-/CD20-/CD56- populations (Fig. 4b) revealed that the number of CD14+ cells decreased to nearly zero. Infection with VSV-UV-I or stimulation with ligands of TLRs had only a slight effect on CD14 expression on monocytes. These results demonstrate that VSV replication was responsible for the observed loss of CD14. A statistically significant effect can be observed in VSV-infected samples at MOI = 1, where the percentage of CD14+ monocytes decreased by an average of 37.38% (95% CI 3.78–70.97) compared to mock-infected control samples (Fig. 4c). To eliminate the possibility that the observed loss of CD14+ cells is caused by soluble mediators secreted by activated cells, each PBL sample was treated with 4 different types of supernatants (UV inactivated) derived from VSV- or mock-infected PBLs (see Materials and Methods). As shown in Figure 5, expression of CD14 marker of the HLA-DR+/CD3-/CD20-/CD56- population (HLA-DR+ cells) increased in samples stimulated with supernatants from mock-infected cells (ANOVA, p < 0.05). This effect was more noticeable if supernatants were obtained from PBLs resistant to VSV infection (M0) with a mean of 21.21% (vs. nonstimulated mock-infected samples; 95% CI 14.40–28.02) and less pronounced if the source of supernatants were sensitive cells (M3) with a mean of 13.22% (vs. nonstimulated mock-infected samples; 95% CI 3.77–22.67). The changes in the percentage of CD14+ cells in HLA-DR+ population in samples stimulated with supernatants from VSV-infected cells compared to the mock-infected samples were not statistically significant. Evidence of a correlation was observed (Fig. 5; dashed line) between the percentage of CD14+ cells in the HLA-DR+ population and the type of supernatants used to stimulate PBLs (Kendall correlation, τ= −0.77, p < 0.05), which suggests differences (divergence) in supernatants obtained from PBLs with different susceptibility to VSV infection. Nevertheless, simultaneously performed infection with VSV resulted in a rapid loss of CD14+ cells, suggesting that the highest decline in monocyte population required virulent live VSV.
Fig. 4.
VSV infection caused a decrease in CD14 expression in monocytes. a, b Effect of VSV infection at indicated MOI (18 hpi) and stimulation with LPS or Poly(I:C) on PBL (a) and monocyte (b) populations. Numbers indicate the percentage of positive cells in the gate. Data shown are from 1 representative donor. c Comparison of the level of CD14+ surface expression on VSV-infected monocytes to uninfected control sample (mean difference) (n = 4). The error bars represent 95% confidence interval of the mean (t test, * p < 0.01).
Fig. 5.
Differences in the percentage of CD14+ surface expression on monocyte population (HLA-DR+ CD3- CD20- CD56-) stimulated with supernatants obtained from samples with different susceptibility to VSV infection (V0, M0, V3, M3), or VSV infected (VSV), compared to mock-infected, nonstimulated cells (n = 8). The box represents the interquartile range with the median in the middle as a bold line; whiskers represent the minimum and maximum in the data set. The correlation (tau = −0.77; p < 0.05) between the type of stimuli and change in expression of CD14 marker on HLA-DR+ CD3- CD20- CD56- cells is shown with the dashed line.
VSV Infection Triggers Rapid Differentiation of Human Blood Monocytes into Phenotypically Immature DCs
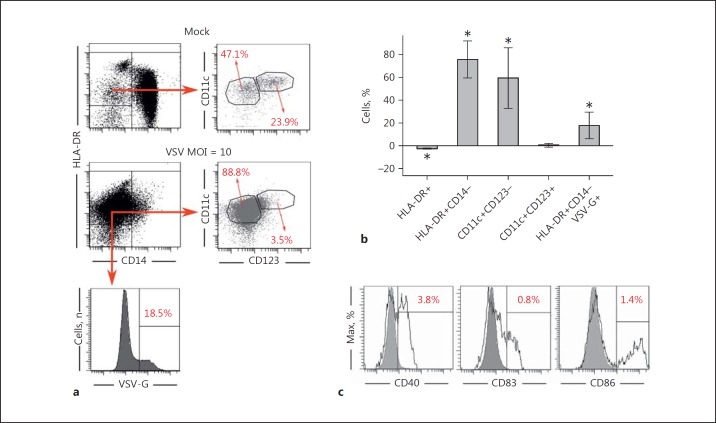
Several viruses are capable of regulating monocyte activation and maturation leading to either immune hyperactivation or suppression [8]. Therefore, we further characterized the phenotype of MDDCs induced by VSV infection with flow cytometry (Fig. 6a). The experiments with purified monocyte populations confirmed our previous observations that VSV infection triggered rapid loss of the CD14 marker in monocytes and thereby raised the percentage of HLA-DR+CD14- population (with a mean difference in the percentage of HLA-DR+CD14- cells in VSV-infected samples vs. mock-infected samples equal to 75.57%; 95% CI 59.27–91.88) (Fig. 6b). This time, however, we demonstrated that VSV infection induced HLA-DR+CD14- differentiation into CD11c+CD123- cells. The percentage of CD11c+CD123- cells among HLA-DR+ cells increased after infection with VSV on average by 59.47% (95% CI 32.83–86.11) in contrast to the CD11c+CD123+ population which was not significantly altered (mean 0.20%; 95% CI −1.26 to 1.66). VSV-infected cells comprised 17.68% of total DCs (HLA-DR+CD14- cells; 95% CI 6.14–29.22). Next, we investigated the expression of maturation markers on VSV-induced MDDCs. Our results have shown that VSV-induced MDDCs were characterized by very low expression of CD40, CD83, and CD86 markers (Fig. 6c), with mean expression levels of 2.10, 4.00, and 1.34%, respectively (data not shown). These data suggest that the population of VSV-induced MDDCs is mostly phenotypically immature.
Fig. 6.
VSV infection of human monocytes induces their differentiation into phenotypically immature dendritic cells. a Monocyte-enriched population isolated from fresh PBLs was mock-infected or VSV-infected with the MOI = 10. Flow cytometry analysis was performed to measure the expression of CD123 and CD11c markers. The percentage of VSV-G+ cells within total DC population (HLA-DR+CD14- cells) was assessed to prove the ongoing viral replication. The data from 1 representative sample are shown. b The results of phenotypic analysis of MDDCs after VSV infection (n = 4). Percentages of CD11c+ CD123- and CD11c+ CD123+ populations were calculated within HLA-DR+ population. Infection of VSV was measured in the total DC population (HLA-DR+CD14- VSV-G+). The average of 4 replicates (mean ± 95% confidence interval) is shown (t test, * p < 0.05). c Analysis of expression of CD40, CD83, and CD86 markers on the total DC population by flow cytometry (n = 4). Data from 1 representative sample are shown (solid line, mock-infected; filled histogram, VSV-infected).
VSV Infection Causes Apoptosis in CD14+ Cells
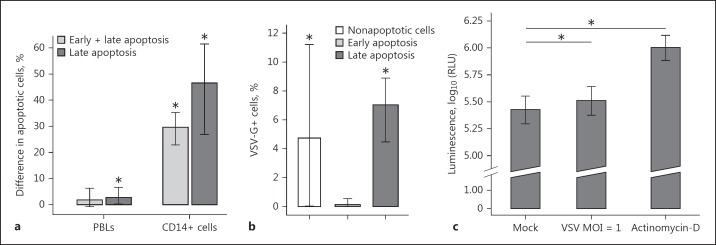
VSV infection was sufficient to induce monocyte differentiation into immature DC. However, sudden loss of monocyte counts also suggested that VSV infected monocytes were undergoing an apoptotic mode of death. Indeed, VSV induced apoptosis mainly in CD14+ cells (Fig. 7a). The difference in the percentage of cells in late apoptosis after VSV infection (compared to mock-infected cells) in the CD14+ population reached on average 46.55% (95% CI 26.93–61.50) and 2.76% (95% CI 0.18–6.66) within the combined lymphocyte-monocyte gate. These data suggest that monocytes were the main population of PBLs to undergo apoptosis after VSV infection. Surprisingly, CD14+ cells that were apoptotic remained mostly uninfected with an average percentage of VSV-G+ cells within late apoptotic CD14+ cells in VSV-infected samples of only 7.02% (95% CI 4.49–8.91; Fig. 7b). These findings suggest that mechanisms other than virus replication itself are involved in the apoptosis of CD14+ cells. It has been shown that VSV induces apoptosis in mouse fibroblasts [21]. Therefore, we measured caspase 3/7 activities to determine the pathway(s) by which VSV induces apoptosis in PBLs. We observed some evidence of an increase in caspase 3/7 activity in VSV-infected PBLs compared to mock-infected samples (Fig. 7c). Taken together, these results indicate that VSV infection markedly decreases monocyte survival by enhancing apoptosis.
Fig. 7.
VSV infection caused apoptosis in PBLs and CD14+ cells. Isolated PBLs were mock- or VSV-infected or incubated with actinomycin for 18 h. a Analysis was applied to PBLs within the combined lymphocyte-monocyte gate or to CD14+ cells only (n = 7). The bars with whiskers represent the average value with its 95% confidence interval obtained by a t test on data transformed using the Box-Cox method, * p < 0.05. b The percentage of VSV-infected (VSV-G+) cells within cells in different stages of apoptosis (n = 7). The average value with its 95% confidence interval is presented (t test on data transformed using the Box-Cox method, * p < 0.05). c Caspase-3/7 activity (n = 10). The average value with a 95% confidence interval is shown (one-sided exact permutation test for paired data, * p < 0.05).
PBLs from Donors Susceptible or Resistant to VSV Infection Differs in Spontaneous and Virus-Induced Cytokine Production
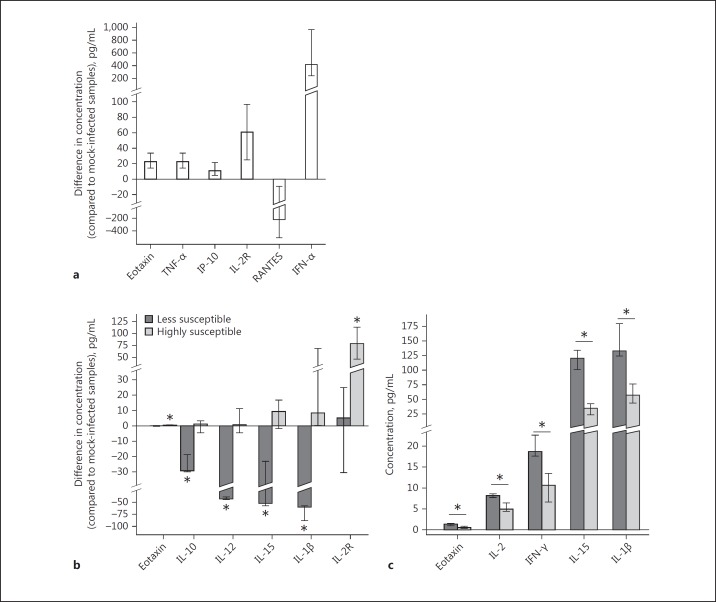
In light of the above observations, it become important to show the impact of VSV infection in leukocytes on cytokine and chemokine production. We noticed statistically significant changes in the concentration of 6 cytokines in samples treated with VSV compared to uninfected controls (Fig. 8a). Four of these (eotaxin, TNF-α, IL-2R, and IP-10) had slightly elevated levels which did not exceed 100 pg/mL. The level of IFN-α, one of the first transcribed antiviral agents after viral entry, was much higher in infected samples (median = 412.70 pg/mL, IQR = 720.90), as expected. On the other hand, the level of RANTES decreased after VSV infection by 218.06 pg/mL (IQR = 492.79). We also observed differences in cytokine production in VSV-infected samples between PBLs obtained from donors who are less susceptible and those who are highly susceptible to the virus (Fig. 8b). The levels of IL-1β, IL-10, IL-12, and IL-15 were significantly lower in the group of samples from donors less sensitive to the virus (medians = −59.70, −29.22, −43.22, and −51.42 pg/mL, respectively; p < 0.05), while no significant changes were observed in the group of samples from highly susceptible donors. On the contrary, the production of IL-2R was higher in the highly susceptible group (median = 78.68 pg/mL, IQR = 66.05; p < 0.05), while there was no significant alteration in the group of samples from resistant donors. Analysis of the spontaneous production of the cytokine panel was performed to investigate whether the observed differences resulted from the cytokine environment wherein the infection takes place rather than the consequence of viral replication. Statistically significant differences in cytokine concentrations between less sensitive and highly susceptible groups were found for IL-1β, IL-15, IFN-γ, eotaxin, and IL-2 (Fig. 8c), but the most pronounced effect was noted regarding IL-1β and IL-15 levels, where differences reached 94.54 and 81.42 pg/mL, respectively.
Fig. 8.
The production of cytokines by PBLs ex vivo in response to VSV infection from donors highly susceptible or less susceptible to VSV. a Differences in the concentration of cytokines (compared to mock-infected controls) after VSV infection (n = 13; Wilcoxon signed-rank test or Student's t test, p < 0.05). The bar represents mean/median (depending on whether a nonparametric or parametric test was used during the analysis) with the whiskers as interquartile range or 95% confidence interval for the mean. All presented results are statistically significant. b Differences in the concentration of cytokines after VSV infection between donors less susceptible (dark-grey bars, n = 3) or highly susceptible (light-grey bars, n = 10) to the virus. Results are presented as median with interquartile range (exact permutation test, * p < 0.05). c Spontaneous cytokine production in uninfected samples from donors less susceptible (dark-grey bars, n = 3) or highly susceptible (light-grey bars, n = 10) to VSV infection. Results are presented as median with interquartile range (exact permutation test, * p < 0.05).
Discussion
Systemic delivery of oncolytic viruses offers the opportunity to treat both primary and metastatic lesions, and, therefore, it is crucial to understand the impact of transient VSV viremia after intravenous administration. VSV is classified as a nonhemotropic virus, and, so far, conflicting results are reported in regard to the isolation of this virus from blood. Nevertheless, it has been shown that VSV can naturally infect and destroy the endothelium of the tumor vasculature [22, 23]. In our earlier studies, we showed that VSV is capable of infecting PBLs in vitro, but the interaction of peripheral blood cell subsets with VSV was unclear [12, 24, 25]. Therefore, in the present study, we first identified the immune consequences of VSV infection of human blood leukocytes, and next we sought to define blood subpopulations targeted by VSV.
In our previous studies, VSV infection of PBLs induced secretion of type I IFNs [10]. In the current study, however, we also established a potent upregulation of cytosolic RNA sensors (RIG-I and MDA-5) and induction of IFN-stimulated antiviral genes (MxA, OAS2, IFITM3, and tetherin). The MxA gene, which encodes a MX dynamin-like GTPase 1, was found to be the most significantly upregulated gene. Interestingly, MxA has been shown to play a role in the inhibition of VSV transcription [26]. More recent studies have shown that another gene besides MxA, OAS2, encoding a member of the 2–5A synthetase family, promotes widespread degradation of viral and cellular RNAs and is crucial for the resistance of cancer cells to VSV [27]. In agreement with the study of Weidner et al. [28], we found upregulated expression of other ISGs (tetherin and IFITM3) in response to VSV infection, but their expression in particular blood populations needs to be further elucidated. As we described above, several IFN-stimulated genes are critical for controlling viral infection; therefore, inhibiting IFN signaling through implementation of combination therapy might be beneficial to maximize the efficacy of VSV-based oncolytic virotherapies.
To pinpoint the target cell(s) of VSV infection in human leukocytes, we examined the effect of VSV infection on PBLs obtained from multiple healthy donors. Our results revealed that only monocytes and DCs are indeed permissive for VSV replication, which is in agreement with a study by Hou et al. [8]. Moreover, experiments with lymphocytes treated with supernatants from noninfected and VSV-infected PBLs clearly showed that only infectious VSV was responsible for the most pronounced decline in the monocyte population, although supernatants obtained from VSV-infected PBLs also reduced CD14 surface expression. Therefore, we hypothesized that loss of CD14 expression on monocytes might be related either to their differentiation into DCs or to apoptosis of VSV-infected monocytes. Monocytes and DCs have been considered the key elements in nonspecific and specific immune defenses against viral infection, but very little is known about the interactions of VSV with these cell types. In our study, MDDCs induced by VSV infection expressed high levels of MHC class II and integrin CD11c resembling myeloid DCs. Myeloid DCs have a strong capability to capture antigens, which enables them to stimulate T cells. However, VSV-induced MDDCs, unlike conventional DCs, were characterized by low expression of costimulatory molecules, despite the presence of TNF-α, IL-1β, and IFN-α - cytokines which are often present in DC maturation cocktails [29]. Therefore, we speculate that the activation signal provided by VSV infection is potent enough to initiate activation but not to induce full maturation of MDDCs.
A noteworthy finding was the high rate of apoptosis of monocytes after exposure to VSV. Even though apoptosis during VSV infection was observed more than 1 decade ago, the molecular mechanism by which VSV induces apoptosis, especially in lymphocytes, is poorly understood. Lai et al. [30] observed that VSV-based vaccine caused VSV viremia in parallel with transient loss of blood cells, which may be related to apoptosis. It has been shown that apoptosis induced by VSV involves the activation of multiple apoptotic pathways and varies among cell types [21]. In our study, only live VSV triggered such a substantial loss of monocytes, mainly via caspase 3/7 activation. However, it is also worth noting that the high percentage of apoptotic cells within the monocyte population was not accompanied by a high VSV infection rate suggesting bystander apoptosis. The same phenomenon was observed in the study by Sur et al. [31] in which the majority of cells in neural tissues did not stain simultaneously for both VSV-RNA and apoptosis. These results suggest that VSV causes apoptosis also through an indirect mechanism via a cytokine-dependent pathway induced by recruited inflammatory cells or reactive microglia.
Since our results demonstrated that either infection with live VSV or the treatment of monocytes with supernatants obtained from VSV-infected lymphocyte cultures triggered rapid loss of monocytes and their differentiation into MDDCs, we decided to look into the dynamics of cytokine and chemokine responses during the course of VSV replication more thoroughly. Two groups of samples distinguished on the basis of the susceptibility to VSV infection demonstrated a difference in the TNF-α levels produced. Higher production of TNF-α, however, did not have an impact on VSV titer, as leukocytes sensitive to VSV infection produced more TNF-α suggesting that TNF-α can exacerbate VSV infection. Indeed, the study by Publicover et al. [32] demonstrated that VSV pathogenesis in mice is linked to the induction of TNF-α in the organs as well as in plasma. Moreover, increased levels of serum TNF-α may systemically sensitize the patient to a cytokine shock-like response triggered by intravenous delivery of VSV [33]. Similarly to this study, apart from upregulated TNF-α production, we found elevated levels of IFN-α, IP-10 (IFN-γ inducible protein-10), and eotaxin in the supernatants of VSV-infected lymphocytes [30]. IP-10 induces apoptosis through activation of effector caspase-3, which might partially explain the loss of monocytes during VSV infection [34]. Another upregulated chemokine upon VSV infection of PBLs observed in our study was eotaxin (CCL11). It has been shown that VSV infection of the mouse central nervous system can cause acute encephalitis, and eotaxin was one of the chemokines expressed at the peak of disease [[35] and references therein]. Slight upregulation of IL-2R levels upon in vitro VSV infection indicates immune system activation. Elevated levels of soluble IL-2R in culture supernatants of VSV-infected leukocytes were especially noticeable in samples that were susceptible to VSV infection suggesting possible disruption in IL-2 signaling in this group, which might have affected immune responses. This finding is in agreement with the fact that spontaneous production of IL-2 was higher in the group with leukocytes less susceptible to VSV infection. Infection with VSV also caused a significant decrease in IL-15 and IL-1β levels in the supernatants of leukocytes less susceptible to VSV infection suggesting an important role of these cytokines in VSV infection. Indeed, Schluns et al. [36] demonstrated the importance of IL-15 for the generation and the subsequent maintenance of antiviral memory CD8 T cells in response to VSV infection in models of intravenous infection of mice and promotes the development of DC [36, 37]. IL-1β has been a well-described mediator of acute inflammatory responses in mice and humans, and the immunization of mice with recombinant VSV vaccine vectors induced local and systemic production of IL-1β [[38] and references therein, [39]].
In conclusion, this study shows that VSV, an oncolytic virus, offers a promising new platform that selectively targets and destroys cancer cells, but systemic delivery of oncolytic viruses should proceed with great caution. We demonstrated here that in vitro VSV infection of PBLs induced strong activation of innate mechanisms with upregulated expression of RLR and ISG genes, as well as elevated cytokine and chemokine production. Moreover, VSV infection caused apoptosis of monocytes and their rapid differentiation into immature DCs. We believe that understanding these processes can potentially lead to a more rational design of VSV-based oncolytic therapies and offer improved options regarding their feasibility for intravenous delivery.
Disclosure Statement
The authors declare that they have no competing interests.
Funding Sources
This work was supported by Wroclaw Center for Biotechnology, program: The Leading National Research Centre (KNOW) for years 2014–2018 and the “To Rescue Children with Cancer” Foundation (GR8/F/2015), Pediatric Oncology and Hematology Clinic of the Medical University of Wroclaw.
Author Contributions
B.U.O. developed the study concept, experimental design, and conceived and wrote the manuscript, T.T. performed the experiments and data analysis, wrote the Results, I.S. and M.K-K. performed the experiments, G.W., R.C., and E.P. revised the manuscript critically for important intellectual content. All authors were involved in reviewing the paper and had final approval of the submitted and published versions.
Acknowledgment
The authors thank Ms. Izabella Orzechowska, LSHTM, London, UK, for English language editing.
References
- 1.Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93:2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge P, Tsao J, Schein S, Green TJ, Luo M, Zhou ZH. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science. 2010;327:689–693. doi: 10.1126/science.1181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iowa State University College of Veterinary Medicine Vesicular Stomatitis, Sore Mouth of Cattle and Horses, Indiana Fever. 2016 www.cfsph.iastate.edu/IICAB. [Google Scholar]
- 4.Orzechowska BU, Jędryka M, Zwolińska K, Matkowski R. VSV based virotherapy in ovarian cancer: the past, the present and …future? J Cancer. 2017;8:2369–2383. doi: 10.7150/jca.19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomczyk T, Orzechowska B. Vesicular stomatitis virus (VSV) as a vaccine vector for immunization against viral infections (in Polish) Postepy Hig Med Dosw (Online) 2013;67:1345–1358. doi: 10.5604/17322693.1083016. [DOI] [PubMed] [Google Scholar]
- 6.Yasmeen A, Zhang L, Al Moustafa A-E. Does the vesicular stomatitis virus really have a selective oncolytic effect in human cancer? Int J Cancer. 2010;126:2509–2510. doi: 10.1002/ijc.24922. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan VS, Furr SR, Sterka DG, Nelson DA, Moerdyk-Schauwecker M, Marriott I, Grdzelishvili VZ. Vesicular stomatitis virus infects resident cells of the central nervous system and induces replication-dependent inflammatory responses. Virology. 2010;400:187–196. doi: 10.1016/j.virol.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Hou W, Gibbs JS, Lu X, Brooke CB, Roy D, Modlin RL, Bennink JR, Yewdell JW. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119:3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orzechowska B, Antoszków Z, Błach-Olszewska Z. Individual differentiation of innate antiviral immunity in humans; the role of endogenous interferons and tumor necrosis factor in the immunity of leukocytes. Arch Immunol Ther Exp (Warsz) 2003;51:51–60. [PubMed] [Google Scholar]
- 10.Orzechowska B, Antoszków Z, Siemieniec I, Lorenc M, Jatczak B, Błach-Olszewska Z. Cytokine production by human leukocytes with different expressions of natural antiviral immunity and the effect of antibodies against interferons and TNF-α. Arch Immunol Ther Exp (Warsz) 2007;55:111–117. doi: 10.1007/s00005-007-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orzechowska B, Chaber R, Wiśniewska A, Pajtasz-Piasecka E, Jatczak B, Siemieniec I, Gulanowski B, Chybicka A, Błach-Olszewska Z. Baicalin from the extract of Scutellaria baicalensis affects the innate immunity and apoptosis in leukocytes of children with acute lymphocytic leukemia. Int Immunopharmacol. 2014;23:558–567. doi: 10.1016/j.intimp.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Rybka K, Orzechowska B, Siemieniec I, Leszek J, Zaczynska E, Pajak J, Błach-Olszewska Z. Age related natural antiviral non-specific immunity of human leukocytes. Med Sci Monit. 2003;9:BR413–BR417. [PubMed] [Google Scholar]
- 13.Autissier P, Soulas C, Burdo TH, Williams KC. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A. 2010;77:410–419. doi: 10.1002/cyto.a.20859. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Yang Q, Bai J, Xuan Y, Wang Y. Identification of appropriate reference genes for human mesenchymal stem cell analysis by quantitative real-time PCR. Biotechnol Lett. 2015;37:67–73. doi: 10.1007/s10529-014-1652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Liu Z, Mi Z, Li X, Jia P, Zhou J, Yin X, You X, Yu L, Guo F, Ma J, Liang C, Cen S. High-throughput assay to identify inhibitors of Vpu-mediated down-regulation of cell surface BST-2. Antiviral Res. 2011;91:321–329. doi: 10.1016/j.antiviral.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Weston S, Czieso S, White IJ, Smith SE, Kellam P, Marsh M. A membrane topology model for human interferon inducible transmembrane protein 1. PLoS One. 2014;9:e104341. doi: 10.1371/journal.pone.0104341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology. 2013;436:221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camacho CPC, Escobar Mármol L, Borda CFE, Medina MJS, Gutiérrez AAF. Detection of seven viruses and Mycoplasma in fetal bovine serum by real time PCR. Rev Colomb Cienc Pecu. 2011;24:585–597. [Google Scholar]
- 20.Box GE, Cox DR. An analysis of transformations. J R Stat Soc Ser B Methodol. 1964:211–252. [Google Scholar]
- 21.Gaddy DF, Lyles DS. Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol. 2005;79:4170–4179. doi: 10.1128/JVI.79.7.4170-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breitbach CJ, De Silva NS, Falls TJ, Aladl U, Evgin L, Paterson J, Sun YY, Roy DG, Rintoul JL, Daneshmand M, Parato K, Stanford MM, Lichty BD, Fenster A, Kirn D, Atkins H, Bell JC. Targeting tumor vasculature with an oncolytic virus. Mol Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008;28:1882–1893. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piasecki E, Knysz B, Zwolińska K, Gąsiorowski J, Lorenc M, Zalewska M, Gladysz A, Siemieniec I, Pazgan-Simon M. Inhibition of vesicular stomatitis virus replication in the course of HIV infection in patients with different stages of immunodeficiency. Viral Immunol. 2010;23:567–576. doi: 10.1089/vim.2010.0054. [DOI] [PubMed] [Google Scholar]
- 25.Jatczak B, Leszek J, Siemieniec I, Sochocka M, Wiśniewska A, Tarkowski R, Bebenek M, Błach-Olszewska Z. Age- and disease-related innate immunity of human leukocytes ex vivo. Exp Gerontol. 2012;47:8–13. doi: 10.1016/j.exger.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Schwemmle M, Weining KC, Richter MF, Schumacher B, Staeheli P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 27.Hastie E, Cataldi M, Moerdyk-Schauwecker MJ, Felt SA, Steuerwald N, Grdzelishvili VZ. Novel biomarkers of resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus. Oncotarget. 2016;7:61601–61618. doi: 10.18632/oncotarget.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidner JM, Jiang D, Pan X-B, Chang J, Block TM, Guo J-T. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han TH, Jin P, Ren J, Slezak S, Marincola FM, Stroncek DF. Evaluation of three clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother. 2009;32:399–407. doi: 10.1097/CJI.0b013e31819e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai L, Davey R, Beck A, Xu Y, Suffredini AF, Palmore T, Kabbani S, Rogers S, Kobinger G, Alimonti J, Link CJ, Jr, Rubinson L, Ströher U, Wolcott M, Dorman W, Uyeki TM, Feldmann H, Lane HC, Mulligan MJ. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. JAMA. 2015;313:1249–1255. doi: 10.1001/jama.2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sur J-H, Allende R, Doster AR. Vesicular stomatitis virus infection and neuropathogenesis in the murine model are associated with apoptosis. Vet Pathol. 2003;40:512–520. doi: 10.1354/vp.40-5-512. [DOI] [PubMed] [Google Scholar]
- 32.Publicover J, Ramsburg E, Robek M, Rose JK. Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha. J Virol. 2006;80:7028–7036. doi: 10.1128/JVI.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rommelfanger DM, Compte M, Grau MC, Diaz RM, Ilett E, Alvarez-Vallina L, Thompson JM, Kottke TJ, Melcher A, Vile RG. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol Ther. 2013;21:348–357. doi: 10.1038/mt.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, Stiles JK. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ireland DDC, Reiss CS. Gene expression contributing to recruitment of circulating cells in response to vesicular stomatitis virus infection of the CNS. Viral Immunol. 2006;19:536–545. doi: 10.1089/vim.2006.19.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 37.Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34:66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 38.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 39.Athearn K, Sample CJ, Barefoot BE, Williams KL, Ramsburg EA. Acute reactogenicity after intramuscular immunization with recombinant vesicular stomatitis virus is linked to production of IL-1β. PLoS One. 2012;7:e46516. doi: 10.1371/journal.pone.0046516. [DOI] [PMC free article] [PubMed] [Google Scholar]