Abstract
Aim:
Spinal ependymomas are among the most common intramedullary neoplasms in both adults and children. While surgical resection is the golden treatment standard, the role chemotherapy and radiotherapy have in patients with spinal ependymomas remains unclear. The aim of this study is to determine the predictors of functional outcome following spinal ependymoma resection to single out patients that may require adjuvant therapy.
Methods:
We conducted a retrospective study on patients that underwent spinal ependymoma resection in our institution in a 10-year period. Magnetic resonance imaging of the spine was used to set the diagnosis of an intradural/intramedullary neoplasm. All patients underwent either gross tumor resection or tumor mass reduction. Histological diagnosis and histopathological grading of spinal ependymoma were done for all collected samples. Patients’ general and neurological examination were performed early after the surgery (within the 1st week) and in a 6-month follow-up.
Results:
A total of 51 intradural and intramedullary ependymoma resection surgeries on 43 patients were performed. There were slightly more male patients (57%) and the average patient age was 41 years. About 76.5% of patients presented with a tumor affecting one vertebrae level, while 23.5% presented with tumors expanding over two or more spinal regions. Gross tumor resection was achieved in 80% of cases, while 25% of procedures were performed on a recurring ependymomas. Most of the tumors (57%) were classified as G2 histological grade, while 8% were anaplastic ependymomas. In 80% of cases, early postoperative patient status was either better or equivalent to the preoperative one, while in a 6-month follow-up, up to 60% of cases showed a significant improvement over the preoperative status. Different demographic and clinical parameters were not proven to be predictors of postsurgical patient outcome including age, gender, and initial neurological presentation. Interestingly, most tumor characteristics were also not associated with postoperative functional outcome (histological grade, number of vertebrae levels affected, whether it is a primary or recurrent tumor). Even the scope of surgical procedure did not affect the functional outcome. The spinal region affected by the tumor was proven to be a predictor of early postoperative outcome (ρ= 0.346, P = 0.033), with lumbar spine being associated with the best outcomes. As expected, the scope of the surgery and whether gross tumor resection or tumor mass reduction was performed were the only significant predictors of tumor recurrence (ρ= 0.391, P = 0.005).
Conclusions:
Spinal ependymoma resection is an efficient procedure that improves the patient outcomes. Spinal region affected by the tumor is likely to be the most important predictor of functional outcome, while the procedure scope seems to be the most important predictor of tumor recurrence.
Keywords: Functional outcome, gross total tumor resection, predictors, spinal ependymoma, tumor recurrence
INTRODUCTION
Spinal ependymomas are benign, slow-growing spinal cord tumors that develop from ependymal cells and the adjacent tissues.[1,2] They are the most common intramedullary spinal cord neoplasms in adults and the second most common in children.[1,2,3,4] Although being such a prominent epidemiologic issue, our existing knowledge concerning their treatment modalities and prognostic factors remains sparse. Due to the benign nature of spinal ependymomas, surgical resection is deemed to be the golden treatment standard.[5,6,7,8,9,10,11,12,13,14] However, the role chemotherapy and radiotherapy have in patients with spinal ependymomas remains unclear.[1,2,3,4,5,6,7,8,9,10,11,12,13,14] Thus, the importance lays in the proper recognition of outcome predictors that can be used to single out patients with worse functional outcome, higher risk of recurrence, and a lower overall survival that may require adjuvant therapy.
Aims
Our aim was to detect the best predictors of postoperative functional outcome as well as to determine the predictors of tumor recurrence in a retrospective cohort of patients with spinal ependymomas treated at our center.
METHODS
We performed a single-center retrospective analysis of patients treated for spinal ependymomas at our department (Department of Neurosurgery, UHC Zagreb), during a 10-year period (from 2006 to 2016). A total of 43 patients (average age 41, age span 16–76 years, 43% female) [Figure 1] diagnosed with intradural and intramedullary spinal neoplasm using magnetic resonance imaging, underwent a total of 51 surgical removal procedures. Surgical treatment included laminotomy or laminectomy, durotomy, midline myelotomy, dissection and debulking of tumor, and gross total tumor resection or tumor mass reduction. All procedures were performed using intraoperative neurophysiological monitoring. Histological diagnosis and histopathological grading of spinal ependymoma were done for all the collected samples. Follow-up evaluation was performed early after surgery and in a 3–6-month period. Each patient underwent extensive physical and neurological examination to adequately assess their early and late postoperative functional recovery. Statistical analysis included group comparison (Chi-square test), correlational analysis (Spearman coefficient), and univariate regression analysis. P < 0.05 was considered statistically significant and SPSS version 21 software (IBM Corporation, USA) was used.
Figure 1.
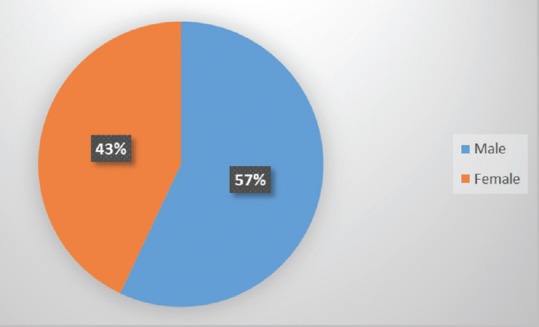
Patient gender distribution
All phases of this study were performed in accordance with the World Medical Association Declaration of Helsinki – Ethical principles for medical research involving human subjects.
RESULTS
When considering tumor characteristics, almost three quarters of all patients presented with a tumor affecting only one vertebral level (mostly lumbar), while the rest presented with a tumor expanding over two or more spinal regions (mostly lumbar and thoracic) [Figure 2]. In addition, two-thirds of the patients presented with a tumor affecting one or two vertebrae [Figure 3]. Pathohistology revealed most of the tumors to be G2 histological grade (57%) with a third being myxopapillary ependymomas, and 8% being anaplastic ependymomas [Figure 4].
Figure 2.
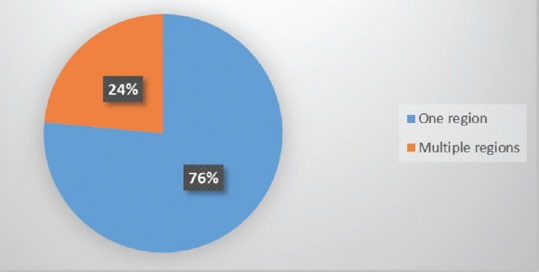
Patient distribution according to spinal regions affected by spinal ependymoma
Figure 3.
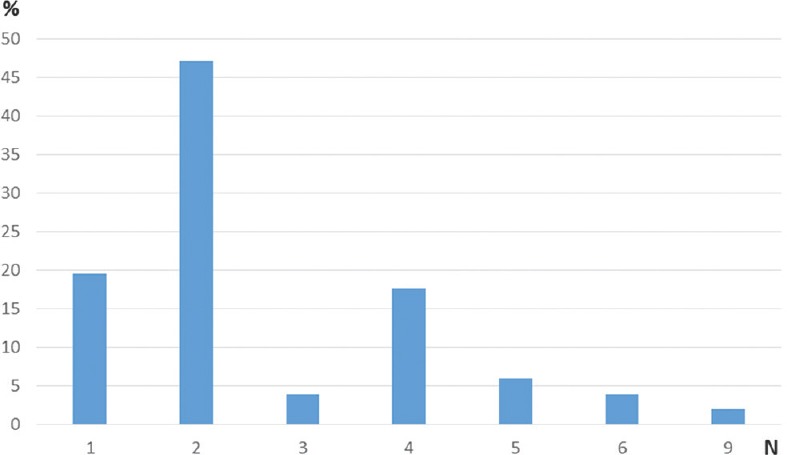
Patient distribution according to number of vertebrae affected by spinal ependymoma
Figure 4.
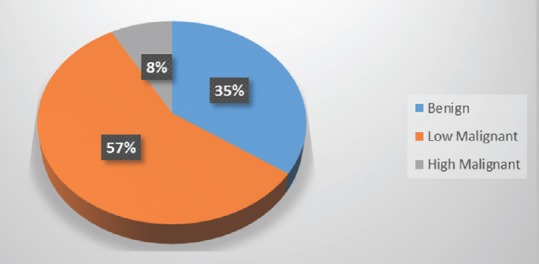
Patient distribution according pathohistological grade of spinal ependymoma tissue
As for surgical removal procedures, in most of the cases (over 80%), gross total tumor resection was achieved [Figure 5], with a quarter of procedures being performed on recurrent tumors.
Figure 5.
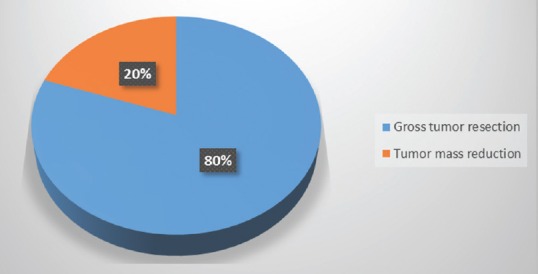
Distribution according to the achieved scope of surgical procedure
Early postoperative neurologic status was either better or equivalent to the preoperative one in over 80% of patients [Figure 6], with 60% of patients showing a significant improvement in a 6-month follow-up period [Figure 7].
Figure 6.
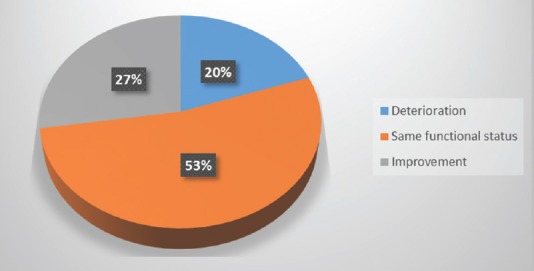
Early postoperative functional status
Figure 7.
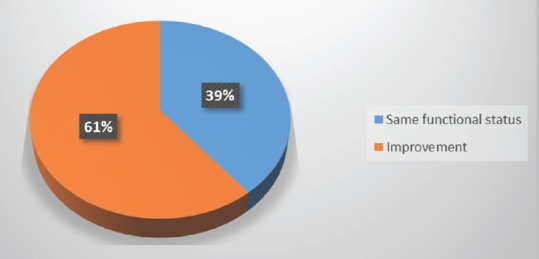
Functional status in a 3–6 months follow-up period
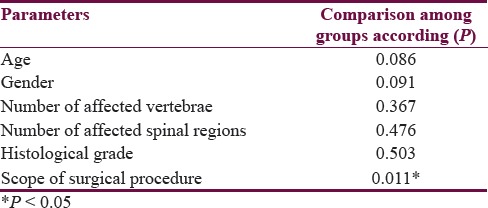
When dividing the patients into groups according to early and late postoperative outcomes, neither age nor gender was associated with a worse outcome [Table 1]. Interestingly, both the size of the tumor (measured as a number of affected vertebrae and spinal regions) and the histological grade were not found to be predictive of adverse outcome (worse neurological status) [Table 1]. This was also the case with the extent of the surgical procedure (gross tumor resection or tumor mass reduction) and whether it was a procedure on a recurrent tumor or not [Table 1]. These results were same in both early and late postoperative period [Table 1].
Table 1.
Comparison of different parameters among patient groups according to early and late functional outcomes
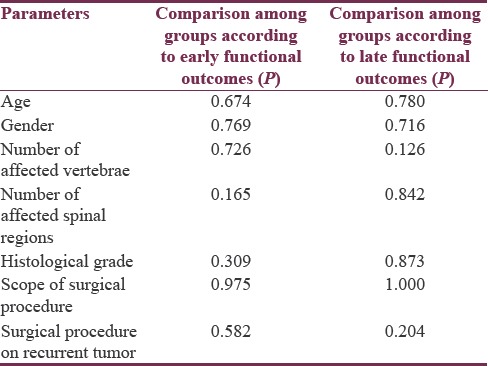
The only significant predictor of a good outcome was the spinal region affected with the tumor, with lumbar region being associated with best early outcomes (ρ= 0.346, P = 0.033). As it was expected, early outcomes correlated significantly with late outcomes (ρ= 0.273, P = 0.042).
Similarly to outcome analysis neither the tumor size nor the histological grade was found to correlate with tumor recurrence in our small cohort of patients [Table 2]. However, the extent of surgical procedure was significantly associated with tumor recurrence (ρ= 0.391, P = 0.005). In addition, a trend between younger age, male gender, and tumor recurrence was detected (ρ= −0.232, P = 0.100; ρ=0.237, P = 0.094, respectively).
Table 2.
Comparison of different parameters among patient groups according to spinal ependymoma recurrence
DISCUSSION
Although being a prominent neuroepidemiologic issue, especially among the younger population, spinal ependymoma incidence (and prevalence) remains relatively low (1–2 per one million people, per year).[1,2,3,4] This is in accordance with Croatian registries and our Center data. In addition, the average patient presentation age in our population is similar to the data presented by other researchers.[1,2,3,4,5,6,7,8,9,10,11,12,13,14] Nearly, equal gender distribution (shown by different registries) was detected in our population too.[1,2,3,4,5,6,7,8,9,10,11,12,13,14]
Histological analysis showed that most of the tumors in our patient cohort to be low malignant ependymomas (G2 grade). Only one-third of the patients presented with a benign (Grade I) ependymoma interestingly, all of them being myxopapillary tumors. The prevalence of high malignant ependymomas in our cohort was <1:12. The specific tumor distribution in our patient population can be due to the study inclusion bias (symptomatic patients undergoing surgical removal). However, the influence of unknown (genetic and environmental) features of Croatian population cannot be excluded from the study. A troublesome observation is that data on histological distribution of ependymomas in different populations remain sparse.
Most of our patients presented with a tumor expanding over two vertebrae levels; however, the majority being contained within a single spinal region (mostly lumbar). This is probably also due to the study inclusion criteria, which targeted symptomatic patients undergoing surgical removal.
Although the aim of this study was not to evaluate the survival of our patient cohort (mostly due to a short follow-up period), it is interesting to notice that 6 months survival was 100% in a population with over 60% of malignant ependymomas.
The completeness of tumor resection, localization, WHO grade, age, extent of the disease at presentation, and molecular characteristics were all already shown to be prognostic factors of survival in patients with spinal ependymomas.[1,2,3,4,5,6,7,8,9,10,11,12,13,14] However, data on postoperative functional status prognosis are sparse.[15] We found that age, WHO grade, and even the extent of the disease at the presentation do not correlate with postoperative functional outcome. The only important predictor of the functional outcome seems to be the tumor localization, with lumbar region being associated with best results. This could be due to the fact that complexity of surgical procedures depends more on the tumor localization than its size and histological type.
While the histological tumor characteristics have been shown to be reliable predictors of tumor recurrence, this was not detected in our patient cohort.[1,2,3,4,16] It remains unclear as to why this is so. As it was expected, the completeness of tumor resection was found to be the best predictor of tumor recurrence in our cohort.[5,6,7,8,9,10,11,12,13,14]
It is important to highlight the limitations of our study, among which the inclusion criteria manifest the most evident effects on the results. A relatively small cohort size and the fact that this is a single-center retrospective study have to be kept in mind when interpreting the results.
We would like to emphasize that these results were presented in the form of abstract at the 8th Congress of the Croatian Neurosurgical Society.
CONCLUSIONS
Surgical removal of spinal ependymomas remains the most effective treatment modality. Tumor localization is shown to have a crucial role in postoperative patient functional outcome. Therefore, it ought to be taken into account when considering all the spinal ependymoma treatment modalities available. Further research regarding the optimal treatment protocol is warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Utrecht: Netherlands Comprehensive Cancer Organisation (IKNL); 2014. Dutch Society for Neuro-Oncology. Ependymal Tumors in Adults – Evaluation and Treatment Protocol. [Google Scholar]
- 2.Reni M, Gatta G, Mazza E, Vecht C. Ependymoma. Crit Rev Oncol Hematol. 2007;63:81–9. doi: 10.1016/j.critrevonc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG, et al. Outcomes of malignant CNS ependymomas: An examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973-2005) J Surg Res. 2009;156:340–51. doi: 10.1016/j.jss.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amirian ES, Armstrong TS, Aldape KD, Gilbert MR, Scheurer ME. Predictors of survival among pediatric and adult ependymoma cases: A study using surveillance, epidemiology, and end results data from 1973 to 2007. Neuroepidemiology. 2012;39:116–24. doi: 10.1159/000339320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldron JN, Laperriere NJ, Jaakkimainen L, Simpson WJ, Payne D, Milosevic M, et al. Spinal cord ependymomas: A retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys. 1993;27:223–9. doi: 10.1016/0360-3016(93)90231-j. [DOI] [PubMed] [Google Scholar]
- 6.Lee TT, Gromelski EB, Green BA. Surgical treatment of spinal ependymoma and post-operative radiotherapy. Acta Neurochir (Wien) 1998;140:309–13. doi: 10.1007/s007010050103. [DOI] [PubMed] [Google Scholar]
- 7.Chang UK, Choe WJ, Chung SK, Chung CK, Kim HJ. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133–9. doi: 10.1023/a:1015789009058. [DOI] [PubMed] [Google Scholar]
- 8.Alkhani A, Blooshi M, Hassounah M. Outcome of surgery for intramedullary spinal ependymoma. Ann Saudi Med. 2008;28:109–13. doi: 10.5144/0256-4947.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaner T, Sasani M, Oktenoglu T, Solmaz B, Sarloglu AC, Ozer AF, et al. Clinical analysis of 21 cases of spinal cord ependymoma: Positive clinical results of gross total resection. J Korean Neurosurg Soc. 2010;47:102–6. doi: 10.3340/jkns.2010.47.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halvorsen CM, Kolstad F, Hald J, Johannesen TB, Krossnes BK, Langmoen IA, et al. Long-term outcome after resection of intraspinal ependymomas: Report of 86 consecutive cases. Neurosurgery. 2010;67:1622–31. doi: 10.1227/NEU.0b013e3181f96d41. [DOI] [PubMed] [Google Scholar]
- 11.Boström A, von Lehe M, Hartmann W, Pietsch T, Feuss M, Boström JP, et al. Surgery for spinal cord ependymomas: Outcome and prognostic factors. Neurosurgery. 2011;68:302–8. doi: 10.1227/NEU.0b013e3182004c1e. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Chung CK, Kim CH, Yoon SH, Hyun SJ, Kim KJ, et al. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: A retrospective multicenter study by the Korea Spinal Oncology Research Group. Neuro Oncol. 2013;15:921–9. doi: 10.1093/neuonc/not038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YH, Lin JW. Management and outcome of primary spinal ependymomas: A single center experience from Taiwan. Clin Neurol Neurosurg. 2013;115:2130–5. doi: 10.1016/j.clineuro.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Keil VC, Schmitt AJ, Martin SC, Cadoux-Hudson TA, Pereira EA. Optimising treatment strategies in spinal ependymoma based on 20 years of experience at a single centre. J Clin Neurosci. 2016;29:52–8. doi: 10.1016/j.jocn.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Gavin Quigley D, Farooqi N, Pigott TJ, Findlay GF, Pillay R, Buxton N, et al. Outcome predictors in the management of spinal cord ependymoma. Eur Spine J. 2007;16:399–404. doi: 10.1007/s00586-006-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervoni L, Celli P, Fortuna A, Cantore G. Recurrence of spinal ependymoma. Risk factors and long-term survival. Spine (Phila Pa 1976) 1994;19:2838–41. doi: 10.1097/00007632-199412150-00019. [DOI] [PubMed] [Google Scholar]


