Abstract
Background:
Endonasal endoscopic approach for transsphenoidal excision of pituitary adenoma has undergone remarkable evolution in the last two decades. It is considered less invasive and less stressful, with results comparable to the previous “gold standard” technique of microscopic transsphenoidal excision of pituitary adenoma. The aim of this study was to compare the various perioperative anesthetic and surgical factors which differ in the two approaches (endoscopic vs. microscopic) for pituitary adenoma excision, during the period when surgeons increasingly started using endoscope at our center.
Materials and Methods:
Data of 307 patients from January 2011 to December 2013 were reviewed in this retrospective study. Various parameters were divided and compared on the basis of the type of approach for pituitary tumor resection vis-à-vis microscope-assisted sublabial transsphenoidal (MSLTS) resection or microscope-assisted transnasal transsphenoidal (MTNTS) resection or endoscope-assisted endonasal transsphenoidal (ETSS) resection.
Results:
Demographic variables (except age); tumor type, dimensions, and invasiveness; patients’ comorbidities; postoperative nausea/vomiting, electrolyte imbalance, respiratory, and cardiovascular problems were comparable among three groups. Duration of surgery and anesthesia were shortest for MTNTS group and longest for ETSS group (P < 0.001). Blood loss was higher in ETSS technique (median 300 mL) and least in MTNTS (median 100 mL), and the difference was significant across all three groups (P = 0.0003). Postoperative cerebrospinal fluid rhinorrhea was 17% in the MSLTS group compared to 6.5% in MTNTS and 7.9% in ETSS (P = 0.047).
Conclusion:
ETSS with the expected advantage of being less invasive offers a better chance for complete resection of adenoma. Neuroanesthesiologist must be prepared for longer surgical time and more blood loss as compared to previous microscopic approach, at least till the surgeons expertise in this newer technique.
Keywords: Endoscopic, microscopic, perioperative, transsphenoidal pituitary surgery
INTRODUCTION
Endoscopic approach for endonasal skull base surgery has undergone a remarkable evolution in the last two decades.[1] Endonasal endoscopic approach for transsphenoidal excision of pituitary adenoma is considered less invasive and less stressful to patients, with results comparable to the previous “gold standard” technique of microscopic transsphenoidal excision of pituitary adenoma.[1,2,3] Despite having its own disadvantages, like not being able to provide stereoscopic vision to surgeon and a learning curve, endoscopic technique, still, has the advantage of providing a panoramic view of the sella. Many centers are therefore gradually shifting over to the newer endoscopic approach for transsphenoidal excision of pituitary adenoma. Data about management of pituitary surgery are surplus in literature, but none of the studies have compared anesthetic problems and complications encountered in the newer endoscopic resection with the older microscopic technique for resection of pituitary tumors.
We planned this study to compare the various perioperative anesthetic and surgical factors which differ in the two approaches (endoscopic vs. microscopic) and influence patients’ outcome after transsphenoidal pituitary surgery.
MATERIALS AND METHODS
After obtaining Institute Ethics Committee approval, this retrospective study was conducted. Hospital records of all patients operated through transsphenoidal approach for pituitary adenoma excision from January 2011 to December 2013 were reviewed. Pituitary adenoma patients operated under neuronavigation in MRI suite were excluded. The microscopic technique was further divided into sublabial or transnasal route. Various parameters were recorded and compared on the basis of the type of approach for pituitary tumor resection, that is, microscope-assisted sublabial transsphenoidal (MSLTS) resection or microscope-assisted transnasal transsphenoidal (MTNTS) resection or endoscope-assisted endonasal transsphenoidal (ETSS) resection.
Data collection
Various preoperative parameters recorded were demographic profile, type, and dimensions of pituitary adenoma and baseline investigations. Intraoperative data included the type of anesthetic techniques, amount of blood loss, amount of fluids infused, hemodynamic changes, anesthesia and surgical duration, and extubation details. Postoperative details noted were anesthesia or surgical complications, computed tomography (CT)-head finding, number of days on ventilator, and duration of Intensive Care Unit (ICU) and hospital stay. Post-operative recovery was assessed using Glasgow outcome scale (GOS) at discharge.
Intraoperatively, the patient was considered to have a bradycardia if heart rate (HR) was <50/min, tachycardia if HR was >100/min, hypotension if systolic blood pressure (SBP) decreased to <90 mmHg, and hypertension if SBP increased to >160 mmHg. Maximum and minimum values of HR, SBP, diastolic blood pressure (DBP), and mean blood pressure (MAP) recorded intraoperatively were noted. Chief operating neurosurgery consultant was classified based on years of experience as “senior consultant” with more than 6 years of experience or “junior consultant” with less than 6 years of experience, after obtaining masters in postgraduate degree.
Statistical analysis
Statistical analysis was done using the software Stata 11.0 (College Station, TX, USA). Data were presented as number (%), mean ± standard deviation, or median (range), as appropriate. The three-group analysis for continuous variables which followed the normal distribution was done by ANOVA test, and for variables which did not follow normal distribution by Kruskal–Wallis test. Two-group comparison was done for continuous variables using the t-test for independent variables, and for variables which did not follow normal distribution by the Wilcoxon rank-sum test. Categorical variables were compared using the Chi-square test/Fisher exact test. P < 0.05 was considered statistically significant.
RESULTS
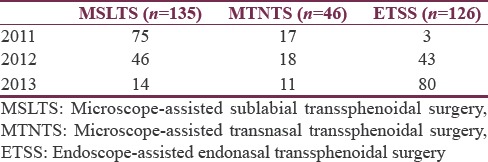
Record of 307 patients operated for pituitary adenoma excision through transsphenoidal route were retrieved and analyzed. Table 1 shows the increasing number of cases being done through ETSS technique over progressive years.
Table 1.
Year-wise distribution of number of cases
Anesthesia protocol
A similar management protocol was observed for all patients. Monitoring included electrocardiogram, SpO2, invasive blood pressure, EtCO2 and skin temperature in all patients. General anesthesia was induced with either propofol or thiopentone along with fentanyl and rocuronium and was maintained with oxygen, nitrous oxide, and isoflurane/sevoflurane/desflurane along with intermittent boluses of fentanyl and vecuronium. Few of the patients also received dexmedetomidine infusion intraoperatively. Mechanical ventilation was adjusted to EtCO2 of 35 ± 2 mmHg. Crystalloids ringer lactate and 0.9% normal saline and colloid 6% hydroxyethyl starch were infused to maintain intravascular volume status. At the end of surgery, trachea was either extubated or patient shifted with the endotracheal tube in situ for further management in the neurosurgical ICU.
Preoperative data
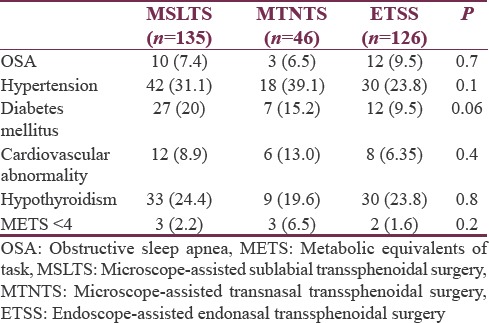
Demographic variables (sex, weight), American Society of Anesthesiology (ASA) grade, and blood groups were comparable among the three groups [Table 2]. However, age of patients in MTNTS group was higher than other groups (P < 0.05). Although record for the type and description of the tumor was available for all cases, exact dimensions of macroadenoma (pituitary adenoma >10 mm in dimension) were available for 70 (59.3%) patients in MSLTS group, 27 (67.5%) in MTNTS group, and 74 (65.4%) in ETSS group. Overall, 116 patients (37.8%) had a functioning pituitary adenoma. Hypothyroidism due to the pressure effect of adenoma on normal gland was present in 72 patients (23.4%). Comorbidities were comparable among all groups [Table 3].
Table 2.
Preoperative variables (n [%]/median [range] or mean±standard deviation)
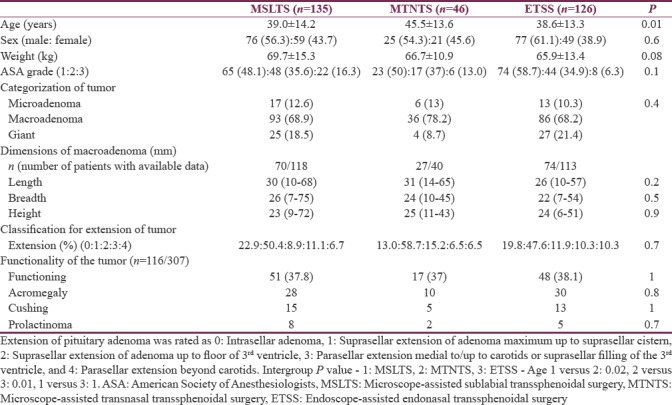
Table 3.
Associated comorbid conditions (n [%])
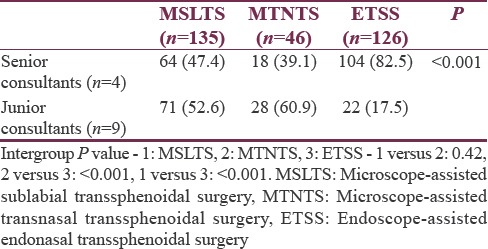
A total of thirteen neurosurgery consultants operated on the cases over 3 years [Table 4]. More number of ETSS was conducted by the “senior consultants” in neurosurgery team as compared to microscopic technique (P < 0.001).
Table 4.
Classification of neurosurgery consultants based on experience (n [%])
Intraoperative data
Higher percentage of patients in MSLTS and MTNTS groups (12.6% and 15.2%, respectively) received dexmedetomidine infusion compared to 4% in ETSS group (P = 0.016). Duration of surgery and anesthesia were shortest for MTNTS group and longest for ETSS group (P < 0.001) [Table 5].
Table 5.
Intraoperative parameters (n [%]/median [range] or mean±standard deviation)
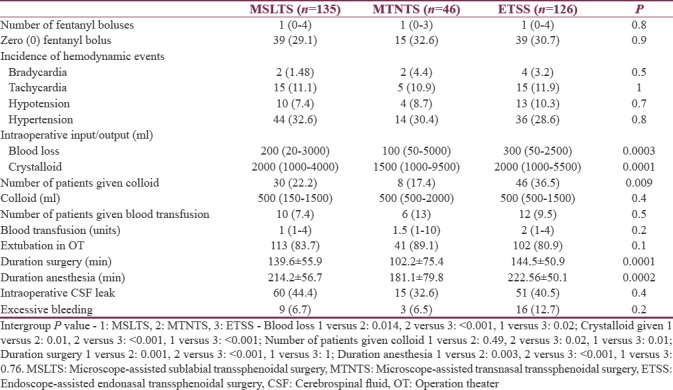
Analgesic requirement as judged by number of fentanyl boluses repeated intraoperatively, besides the induction dose, was similar across all three groups (P = 0.8). Likewise, number of patients who did not need any repeat fentanyl bolus was also similar across all three groups (P = 0.9).
Blood loss was highest in ETSS technique and least in MTNTS. In proportion to the blood loss, volume of crystalloids infused was least in MTNTS group and most in ETSS group. Number of patients who received colloid was also more in ETSS group.
Baseline pulse rate was higher in MTNTS group compared to MSLTS and ETSS [Figure 1]. Baseline SBP, DBP, and MAP were comparable across all three groups [Figures 2-4]. Minimum and maximum values of pulse, SBP, DBP, and MAP recorded intraoperatively were similar among groups [Figures 1-4]. Regarding hemodynamic instability, the number of patients with incidence of bradycardia, tachycardia, hypotension, and/or hypertension was comparable among the three groups [Table 5]. The incidence of intraoperative dura breach leading to cerebrospinal fluid (CSF) leak was also comparable among groups.
Figure 1.
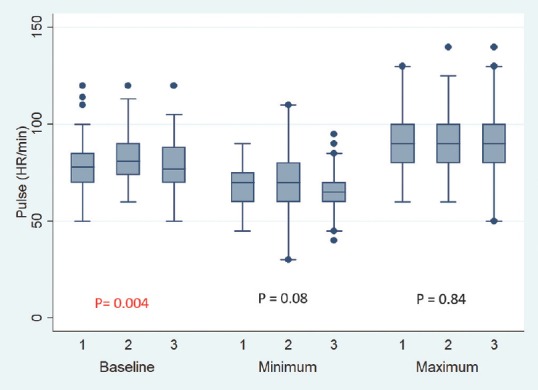
Baseline, minimum and maximum values of intraoperative heart rate. Values are in mean. Boxes indicate 25-75 percentile value. Whiskers indicate 2.5–97.5 percentile value. Median is represented as horizontal line in the bar. Dots represent outliers. P value in between the groups is mentioned. On X-axis 1 = Microscope-assisted sublabial transsphenoidal surgery, 2 = Microscope-assisted transnasal transsphenoidal surgery, and 3 = Endoscope-assisted endonasal transsphenoidal surgery
Figure 2.
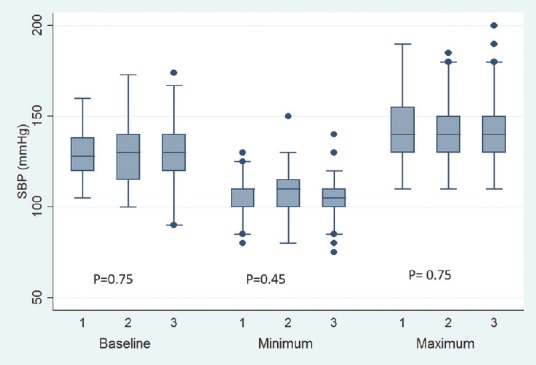
Baseline, minimum and maximum values of intraoperative systolic blood pressure. Values are in mean. Boxes indicate 25-75 percentile value. Whiskers indicate 2.5–97.5 percentile value. Median is represented as horizontal line in the bar. Dots represent outliers. P value in between the groups is mentioned. On X-axis 1 = Microscope-assisted sublabial transsphenoidal surgery, 2 = Microscope-assisted transnasal transsphenoidal surgery, and 3 = Endoscope-assisted endonasal transsphenoidal surgery
Figure 4.
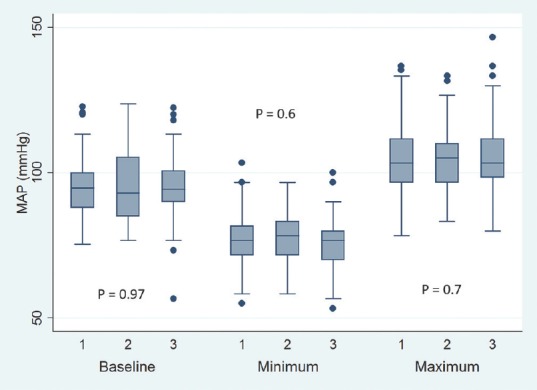
Baseline, minimum and maximum values of intraoperative mean blood pressure (MAP). Values are in mean. Boxes indicate 25-75 percentile value. Whiskers indicate 2.5–97.5 percentile value. Median is represented as horizontal line in the bar. Dots represent outliers. P value in between the groups is mentioned. On X-axis 1 = Microscope-assisted sublabial transsphenoidal surgery, 2 = Microscope-assisted transnasal transsphenoidal surgery, and 3 = Endoscope-assisted endonasal transsphenoidal surgery
Figure 3.
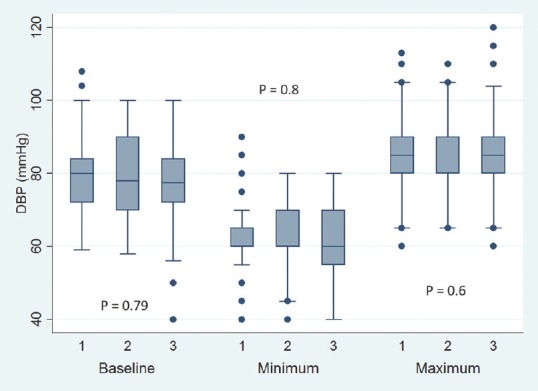
Baseline, minimum and maximum values of intraoperative diastolic blood pressure. Values are in mean. Boxes indicate 25-75 percentile value. Whiskers indicate 2.5–97.5 percentile value. Median is represented as horizontal line in the bar. Dots represent outliers. P value in between the groups is mentioned. On X-axis 1 = Microscope-assisted sublabial transsphenoidal surgery, 2 = Microscope-assisted transnasal transsphenoidal surgery, and 3 = Endoscope-assisted endonasal transsphenoidal surgery
Postoperative parameters
Postoperatively, nausea/vomiting, electrolyte imbalance, respiratory and cardiovascular problems, and CT brain imaging findings were comparable among all the three groups. The incidence of postoperative CSF rhinorrhea was 17% in the MSLTS group compared to 6.5% in MTNTS and 7.9% in ETSS. Similarly, number of patients who underwent postoperative lumbar puncture/drainage of CSF was significantly more in the MSLTS group [Table 6].
Table 6.
Postoperative parameters (n [%] or median [range])
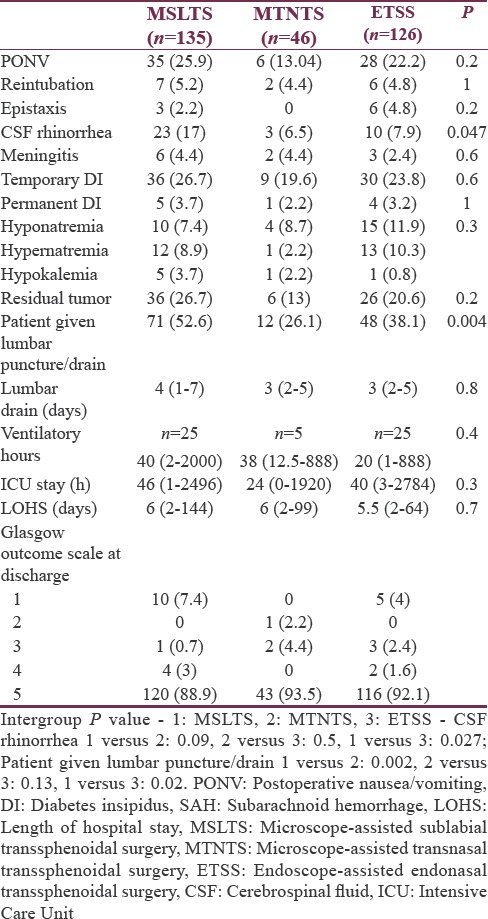
A total number of patients needing mechanical ventilation and length of ICU/hospital stay were comparable among all three groups. At discharge from hospital, 279 patients (90.9%) were with GOS 5 (good functional recovery), and the distribution was similar in all three groups. Overall mortality (GOS = 1) was 4.9% (15 patients), of which 10 patients were in MSLTS group and 5 patients in ETSS group. Rate of gross total excision as judged by incidence of residual tumor on postoperative CT scan was also comparable among all three groups.
Microscope-assisted TSS versus endoscope- assisted TSS–two-group analysis
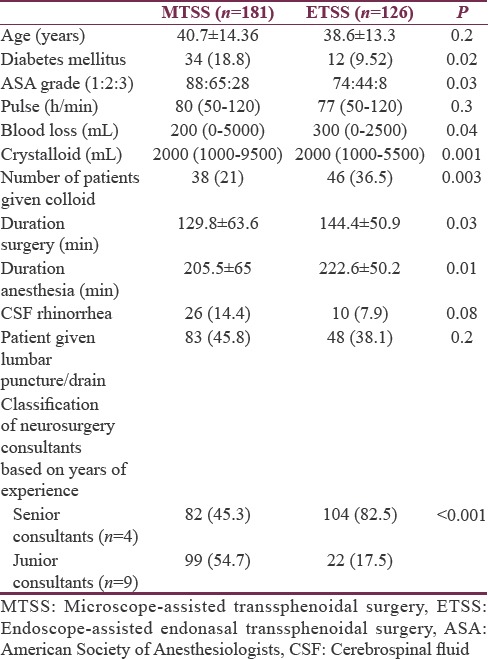
All the findings were reanalyzed by combining the MSLTS and MTNTS approaches into one group as microscope-assisted transsphenoidal surgery (MTSS) and compared with the ETSS. Statistically significant findings of the earlier three-group analysis when reanalyzed into two groups (MTSS and ETSS) are presented in Table 7. Differences observed earlier between three groups with respect to age, baseline pulse, and postoperative CSF rhinorrhea were not seen when two-group analyses was performed. The duration of surgery and anesthesia was more in ETSS group than MTSS, and the former technique was associated with more blood loss and fluid infusion, intraoperatively. This is despite the fact that ETSS was predominantly conducted by “senior consultants” of neurosurgery (82.5%).
Table 7.
Demographic profile and other perioperative parameters in microscope-assisted transsphenoidal surgery and endoscope-assisted endonasal transsphenoidal surgery (n [%]/median [range] or mean±standard deviation)
DISCUSSION
The newly introduced endoscopic technique for transsphenoidal pituitary adenoma excision (ETSS) requires the neuroanesthesiologist to be more watchful with the steps of the procedure. Although surgical duration of ETSS may be prolonged, completion may be sudden and surgeon may not pack the nasal cavity unlike the sublabial approach where tissue closure may give an opportunity to neuroanesthesiologist to titrate the maintenance agent. For this reason, drugs such as propofol and sevoflurane, which are short-acting agents, are preferred.[4] At our institute, balanced anesthesia with inhalational agents is preferred and shift from isoflurane to sevoflurane/desflurane seems the obvious need as the surgeons shifted to ETSS technique.
Intraoperative hemodynamic changes are expected to be more in the MSLTS and MTNTS groups due to sympathetic response at the insertion of a self-retaining speculum.[5] In contrast, ETSS is less invasive and expected to be relatively less painful, with cases having been performed even under local anesthesia and sedation.[6] However, in the present study, the incidence of hemodynamic changes were similar in all three approaches [Table 5 and Figures 1-4]. Number of fentanyl boluses given intraoperatively was also comparable across all three groups [Table 5]. These findings suggest that ETSS is as stressful as microscopic approach (i.e., MSLTS and MTNTS), if not more. One confounding factor is the fact that intraoperative use of dexmedetomidine was more frequent in the MSLTS and MTNTS groups compared to ETSS, which blunts the sympathetic responses. However, dexmedetomidine was used in ≤15% of cases, so it would not have influenced the overall results to a large extent.
ETSS have been shown to be of shorter duration in some studies;[7] in others, the duration has been similar to MTSS. In our study, ETSS took longer time to completion and mean surgical duration was about 20 min more as compared to the MTNTS group, which was of shortest duration. This could be explained by the fact that MTNTS was an already established surgical technique whereas endoscopic technique demands its own learning curve and duration. Hence, findings from our study show that the advantage of quicker resection of tumor by endoscopic technique was not present at the initial 2 years of introduction of this newer technique at our institute. In a study by O’Malley et al.,[8] authors found that the learning curve for a neurosurgeon for the newer endoscopic approach can be ≤17 procedures. Duration of operation, CSF leak, and other complications decrease as the surgeons gain more experience and become more familiar with the endoscopic technique.
The difference which stands out distinguishably is the higher blood loss in the ETSS group. The incidence of severe bleeding was almost double in ETSS group compared to MSLTS or MTNTS group (12.7% vs. 6.7/6.5%) although this finding was not statistically significant (P = 0.24). Higher blood loss translated into more amount of crystalloid infusion, more number of patients being infused with colloid, and higher urine output in the ETSS group. Ammirati et al.[9] in a recent meta-analysis involving 38 studies have shown that the reported number of vascular complications were higher and with no short-term advantage of endonasal endoscopic pituitary adenoma excision over the microscopic technique. Other authors have however demonstrated decreased blood loss in the endoscopic technique.[1,10] Higher blood loss in the ETSS group in our study may again reflect the significance of learning curve.
The incidence of CSF leak has been interpreted by different authors in varied context. Rotenberg et al.[11] have used placement of lumbar drain as means to gauge the incidence of CSF leak, others have used the term “CSF leak” for complication occurring anytime intra- or postoperatively.[9] We observed that despite the incidence of intraoperative CSF leak being similar across all three groups, the incidence of postoperative CSF rhinorrhea was almost double in the MSLTS group compared to ETSS (17% vs. 7.9%; P = 0.027). Placement of lumbar drain/puncture was also more in the MSLTS group (P = 0.004). Lower incidence of CSF leak in ETSS may be because of better visualization of tumor boundaries, thereby avoiding trauma to the duramater and confirmation of better seal of duramater, once membrane breaches. However, Strychowsky et al.[10] have observed increased incidence of “post-operative CSF leak” in the endoscopic approach, possibly implicated by efforts of the surgeons to extend their limits to achieve complete resection. On the contrary, Rotenberg et al.[11] have shown a decrease in the incidence of CSF leak in the ETSS group in their meta-analysis and stated that better success of reconstructive technique following dissection is the major determinant of lesser CSF leak.
Postoperatively, the incidence of diabetes insipidus (transient or permanent) and meningitis was comparable between the groups. The incidence of epistaxis, nausea/vomiting, respiratory, and cardiovascular complications [Table 6] were, also, comparable between the groups; which is in agreement with recent meta-analysis by Gao et al.[7] and Ammirati et al.[9] Neal et al.[12] in a three-group analysis as ours have shown a much higher incidence of diabetes insipidus in sublabial approach (33%) compared to the MTNTS (5%) and ETSS (7%). Several other studies have shown comparable or lesser incidence of DI in ETSS compared to MTSS.[13] Meticulous preservation of the neurovascular structures of the hypothalamus, infundibulum, and neurohypophysis helps to decrease the incidence of diabetes insipidus.[13]
Regarding the effectiveness of tumor debulking, the number of patients with residual pathology though less in ETSS than MSLTS was not statistically significant. A major limiting factor for complete pituitary tumor removal is the extent of cavernous sinus and internal carotid artery involvement.[14] Endoscopes with panoramic vision and ability to reach previously inaccessible areas have the advantage over tunneled vision provided by microscopes. Likewise, Gao et al.[7] in their meta-analysis of 15 studies found that the rate of gross tumor removal was higher in the endoscopic group than in the microscopic group.
A shorter length of hospital stay gives an indirect evidence of less complicated hospital course. A decreased hospital stay has been shown a clear advantage of endoscopic procedure in recent meta-analysis.[7,11] However, the hours of ICU stay and days of hospital stay were comparable among all groups in our study. However, it could be that discharge to home policy is different in our hospital compared to other institutes.
We compared the data as three-group analysis of MSLTS, MTNTS, and ETSS; and later as two-group analysis by combining both the microscope-assisted techniques (viz., MSLTS and MTNTS) as MTSS and comparing with ETSS. This helped us to better analyze the surgical and anesthesia technique. For example, while if only MTSS and ETSS were to be compared, no difference would have been found between two techniques; but a three-group analysis highlighted that the postoperative CSF rhinorrhea and number of patients requiring lumbar puncture/drain were highest in MSLTS group compared to other techniques. Similarly, when a two-group analysis showed lesser number of diabetic and better ASA grade patients in the ETSS group than MTSS group, which would have suggested selection bias of healthier patients to ETSS group, the three-group analysis, however, suggested that such distribution was only incidental and not clinically significant. Thus, if MTSS is not separated into the MSLTS and MTNTS subgroups and analysis is done under two broad categories (i.e., MTSS and ETSS), the results may conceal some of the relevant findings and highlight others; therefore, one must be careful while interpreting findings of few earlier studies.[1,15]
This study has few limitations. First, this being retrospective study carries all the drawbacks of such study plans. Second, a large number of consultants (13) have conducted the cases over 3-year period. It was not possible to remove the bias arising from hierarchy (experience) of neurosurgical consultants. Third, we have not used hormonal assay to measure the success of tumor debulking and have only used postoperative imaging to check for residual pathology. Fourth, the study duration is limited till the period of hospital stay of the patients. Long-term follow-up and outcome of the procedures cannot be derived from this data, and it only reflects short-term results. Finally, although the MTNTS may seem a better approach in several aspects, the smaller sample size fails us to draw a definitive judgment.
CONCLUSION
Endoscope-assisted technique is gradually replacing the microscope-assisted technique for transsphenoidal resection of pituitary adenomas. ETSS with the expected advantage of being less invasive offers a better chance for complete resection of adenoma. However, neuroanesthesiologist must be prepared for longer surgical time and more blood loss as compared to previous microscopic approach, at least until the surgeons have achieved expertise in this newer technique. While lesser incidence of postoperative CSF rhinorrhea is observed in endoscopic approach, incidence of rest of the complications seems similar to previous MTSS approach in our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: A systematic review and meta-analysis. Clin Otolaryngol. 2011;36:212–20. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 2.Lucas JW, Zada G. Endoscopic surgery for pituitary tumors. Neurosurg Clin N Am. 2012;23:555–69. doi: 10.1016/j.nec.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Hirsch NP. Pituitary disease and anaesthesia. Br J Anaesth. 2000;85:3–14. doi: 10.1093/bja/85.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Dunn LK, Nemergut EC. Anesthesia for transsphenoidal pituitary surgery. Curr Opin Anaesthesiol. 2013;26:549–54. doi: 10.1097/01.aco.0000432521.01339.ab. [DOI] [PubMed] [Google Scholar]
- 5.Ali Z, Prabhakar H, Bithal PK, Dash HH. Bispectral index-guided administration of anesthesia for transsphenoidal resection of pituitary tumors: A comparison of 3 anesthetic techniques. J Neurosurg Anesthesiol. 2009;21:10–5. doi: 10.1097/ANA.0b013e3181855732. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Zou J, Liu S, An H, Liang C. Anesthesia selection in hypophysectomy by endoscopic transnasal sphenoidal approach. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20:348–50. [PubMed] [Google Scholar]
- 7.Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C, et al. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: A meta-analysis. World J Surg Oncol. 2014;12:94. doi: 10.1186/1477-7819-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Malley BW, Jr, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: Single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25:E10. doi: 10.3171/FOC.2008.25.12.E10. [DOI] [PubMed] [Google Scholar]
- 9.Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:843–9. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strychowsky J, Nayan S, Reddy K, Farrokhyar F, Sommer D. Purely endoscopic transsphenoidal surgery versus traditional microsurgery for resection of pituitary adenomas: Systematic review. J Otolaryngol Head Neck Surg. 2011;40:175–85. [PubMed] [Google Scholar]
- 11.Rotenberg B, Tam S, Ryu WH, Duggal N. Microscopic versus endoscopic pituitary surgery: A systematic review. Laryngoscope. 2010;120:1292–7. doi: 10.1002/lary.20949. [DOI] [PubMed] [Google Scholar]
- 12.Neal JG, Patel SJ, Kulbersh JS, Osguthorpe JD, Schlosser RJ. Comparison of techniques for transsphenoidal pituitary surgery. Am J Rhinol. 2007;21:203–6. doi: 10.2500/ajr.2007.21.2981. [DOI] [PubMed] [Google Scholar]
- 13.Schreckinger M, Szerlip N, Mittal S. Diabetes insipidus following resection of pituitary tumors. Clin Neurol Neurosurg. 2013;115:121–6. doi: 10.1016/j.clineuro.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Gondim JA, Schops M, de Almeida JP, de Albuquerque LA, Gomes E, Ferraz T, et al. Endoscopic endonasal transsphenoidal surgery: Surgical results of 228 pituitary adenomas treated in a pituitary center. Pituitary. 2010;13:68–77. doi: 10.1007/s11102-009-0195-x. [DOI] [PubMed] [Google Scholar]
- 15.D’Haens J, Van Rompaey K, Stadnik T, Haentjens P, Poppe K, Velkeniers B, et al. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: A retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol. 2009;72:336–40. doi: 10.1016/j.surneu.2009.04.012. [DOI] [PubMed] [Google Scholar]


