Abstract
Calcium electroporation is a potential novel anticancer treatment, where high concentrations of calcium are introduced into the cell cytosol by electroporation. This is a method where short, high-voltage pulses induce a transient permeabilization of the cell membrane and thereby allow influx and efflux of ions and molecules. Electroporation is used in combination with chemotherapeutic drugs (electrochemotherapy) as a standard treatment for cutaneous metastases, and electroporation using a higher electric field and number of pulses (irreversible electroporation) is increasingly being used as an anticancer treatment. In this review, calcium electroporation is described with emphasis on the investigations of differences in the effect on normal and malignant cells and tissues in vitro and in vivo. Calcium electroporation has been shown to induce cell death in vitro and tumor necrosis in vivo with a difference in sensitivity between different tumor types. Normal cells treated in vitro are significantly less affected than cancer cells, and a similar trend is shown in vivo where muscle and skin tissue surrounding a treated tumor as well as muscle and skin directly treated with calcium electroporation were less affected than tumors. The mechanism behind this difference in sensitivity is not fully understood but might be affected by differences in electric impedance, membrane repair, and expression of plasma membrane calcium ATPases in normal and malignant cells. The research on calcium electroporation shows a potential novel anticancer treatment with significant effect on cancer cells and tissues while normal cells and tissues are clearly less affected.
Keywords: calcium electroporation, in vitro, in vivo, normal, malignant
Introduction
Electroporation is a method where short, high-voltage pulses induce a transient permeabilization of the cell membrane, allowing passage of ions and molecules into and out of the cell.1 Permeabilization is induced when the applied electric field leads to an increase in the transmembrane potential that surpasses the threshold for permeabilization, leading to the formation of aqueous pores.2 This permeabilization is reversible (reversible electroporation), and the method is used in combination with chemotherapeutic drugs (electrochemotherapy [ECT]) for treatment of cutaneous and subcutaneous metastases3–13 and is in clinical trial for treatment of tumors in internal organs.14,15 Electroporation has also been proposed to be used as a monotherapy (ie, without combining with chemotherapeutic or other drugs) for destruction of the cell membrane and thereby induce cell death.16 This is named irreversible electroporation (IRE) and is performed by using a far higher electric field and higher number of pulses than that used for reversible electroporation. Recently, a potential novel anticancer treatment was proposed by combining reversible electroporation and calcium (calcium electroporation [CaEP]).17 Results from the first clinical trial using CaEP for treatment of cutaneous metastases were recently published and showed a clear effect of CaEP comparable to the effect of ECT.18 The mechanism behind CaEP is still being elucidated, but interestingly differences in sensitivity between normal and malignant cells have been observed.
When testing new anticancer treatments, the effect on tumor cells as well as normal cells is crucial to investigate. Electrochemotherapy, IRE, and CaEP are local treatments, thus the normal tissue mostly affected is surrounding the treated tumor. In this review, we briefly describe the effects of ECT and IRE on normal cells and tissues followed by a description of the literature on differences in effects between malignant and normal cells and tissues after treatment with CaEP.
Electrochemotherapy
The first clinical trial with ECT was performed in 1990 to 199119 and has since been tested in several clinical trials for treatment of cutaneous and subcutaneous metastases3,4,7–13 as well as for tumors in internal organs.14,15 Electrochemotherapy is now in clinical use in more than 140 centers in Europe. The most commonly used chemotherapeutic drugs for ECT are bleomycin and cisplatin, and their cytotoxicity has been shown to increase 300 to 5000-fold and 2 to 13-fold, respectively, when combined with electroporation.1 It has been shown that ECT using bleomycin affects normal cells less than cancer cells in vitro in cells in suspension20 and in spheroids, a 3D tumor-like structure,21 which might be due to the effect of bleomycin on fast dividing cells compared to more quiescent cells, such as fibroblasts. In patients, the surrounding normal tissue is also less affected than the treated tumor, when the treatment also included normal tissue around the tumor.22 In a recent study, it was shown that the functionality of larger normal vessels was preserved while the tumor tissue was necrotic after ECT of liver metastases.23 Furthermore, tumor blood vessels have also been shown to be more affected than normal blood vessels.24 A factor contributing to the difference in sensitivity between normal and cancer cells is likely the increased effect of chemotherapeutic drugs on fast dividing cells.25 Another reason might be how the electric field influences specific cells and tissues. This could be differences in the electrical impedance of the tissues, where normal liver tissue has been shown to have higher impedance and thereby lower conductivity than cancerous liver tissue.26 Difference in membrane repair in vitro has also been shown between normal and cancer cells where normal cells repair faster than cancer cells which indicates that normal cells are permeabilized for a shorter period than cancer cells after electroporation.27 These differences in the effect of electroporation between normal and cancer cells might influence the effect of ECT, CaEP, and IRE as described subsequently and summarized in Table 1.
Table 1.
Contributing Factors for Difference in Sensitivity Between Normal and Malignant Tissues.a
| ECT | IRE | CaEP | Reference | |
|---|---|---|---|---|
| Electrical impedance | + | + | + | 26 |
| Membrane repair | + | ? | + | 27 |
| Vascular effect | + | + | ? | 24,32,40 |
| Drug cytotoxicity | + | NA | + | 25,52 |
| Drug removal | ? | NA | + | 52 |
aReasons that could influence the observed differences in sensitivity of ECT, IRE, and CaEP between normal and malignant cells/tissues. + indicates that the treatment is likely affected and ? indicates that this has not been investigated for the specific treatment.
Abbreviation: NA, not applicable.
Irreversible Electroporation
Electroporation without the addition of a chemotherapeutic drug is investigated as an anticancer treatment.16 In IRE, a higher number of pulses as well as a higher electric field are applied to the cells causing a permanent permeabilization of the cell membrane and thereby cell death. Irreversible electroporation has been shown to effectively inhibit proliferation in different cancer cells in vitro, both cells in suspension and 3D cultured cells.28–30 In vivo, IRE also induces tumor cell death which has been shown on tumors implanted on mice.31,32 Recently, high-frequency IRE has also been shown to be effective for hepatic ablation in a porcine model.33,34 The first clinical trial using IRE was performed on tumors in the kidney,35 and several studies have followed describing treatment of tumors in internal organs such as liver, lung, and pancreas as a treatment that seems safe and feasible.36–38 Irreversible electroporation has been shown to be used for tissue destruction, and normal tissue seems to also be affected.37,39 In a study on mice using a dorsal skinfold chamber, reduced blood perfusion was shown after IRE32; however, in a clinical trial where patients with hepatic cancer were treated with IRE, the venous structure adjacent to the treated area remained largely unaffected by the treatment.40 In a recent study, it was shown that nerves adjacent to tumors on rabbits treated with IRE were severely damaged, but their function and structure returned to normal in a few weeks.41 But in a canine sarcoma tumor treated with IRE, the tumor tissue was clearly affected while the adjacent nerves and muscle tissue were spared.42 In the studies where differences between normal and malignant cells have been observed, it might be due to differences in the electrical impedance of the different tissues and/or differences in membrane repair as described earlier (Table 1).
CaEP: Difference in Effect of Normal and Malignant Cells and Tissues
Calcium electroporation was described as a potential novel anticancer treatment in 2012.17 The treatment was shown to effectively induce cell death in vitro and tumor necrosis in vivo. Further studies have shown a similar effect of CaEP and ECT using bleomycin in vitro.43,44 The first clinical trial was recently conducted, where cutaneous metastases were randomized to treatment with CaEP or ECT using bleomycin, showing a comparable effect between the 2 treatments.18 The mechanism behind CaEP is likely complex, but it has been shown to be associated with adenosine triphosphate (ATP) depletion in vitro and in vivo,17,45–47 which might be due to direct loss of ATP through the permeabilized membrane,48 increased consumption of ATP by the Ca2+-ATPases to transport calcium out of the cells,49 and reduced production of ATP due to disruption in the mitochondrial membrane potential by the high intracellular calcium concentrations.50 The high calcium concentration might also cause generation of reactive oxygen species (ROS) and activation of lipases and proteases.51
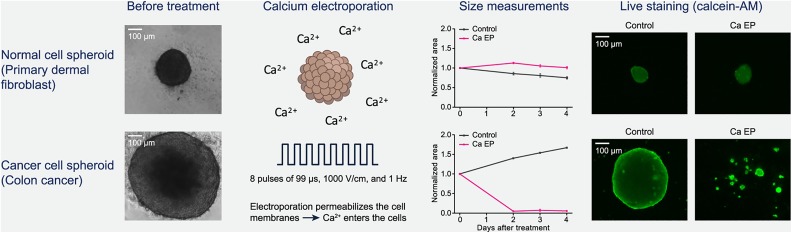
Several cancer cells have been tested in vitro, showing a clear dose–response but with a difference in sensitivity to CaEP.17,43,44,47 This difference has been confirmed in vivo, where 4 different tumor types (bladder, breast, colon, and small-cell lung cancer), injected or transplanted subcutaneously on immune-incompetent mice, have been treated with CaEP.17,52 The difference in sensitivity could be caused by the difference in electroporation effects (described above) as well as differences in the cellular response to increased calcium concentrations, but further research is needed, in vitro and in vivo as well as in the clinical setting. The effect of CaEP has also been investigated on normal cells and tissues. Interestingly, in 2 recent studies, normal rat and mouse skeletal muscle cells treated as cells in suspension were notably less affected by CaEP than the tested murine fibrosarcoma and human rhabdomyosarcoma cells, respectively.44,53 But in primary normal human dermal fibroblasts, no difference in viability was observed between the normal cells and 3 cancer cell lines when treated as cells in suspension.45 As described earlier, fibroblasts are more quiescent cells than cancer cells, and this could affect their sensitivity to treatment, but this is not seen in vitro on cells in suspension. However, when treated as cells in spheroids, a clear difference was observed. Normal fibroblasts were grown in spheroids, a 3D in vitro structure, and treated with similar conditions as in vivo. The size of the spheroids (1 normal and 3 malignant) was determined at days 2, 3, and 4 after treatment as a measure of treatment effect. All the malignant spheroids decreased in size after treatment with CaEP, similar to spheroids treated with ECT using bleomycin, while the normal cell spheroids treated with CaEP or ECT did not decrease in size compared to untreated spheroids (Figure 1). This showed a clear difference in effect between normal and malignant cells when treated as spheroids which are an in vitro model closer to the in vivo setting than cells in suspension. Interestingly, the ATP level decreased significantly in all the spheroids, normal and malignant, showing that the normal cells are able to survive this loss of ATP.46
Figure 1.
The effect of calcium electroporation (CaEP) on normal and cancer cell spheroids. In a spheroid study, colon cancer cell spheroids and normal dermal fibroblast spheroids were treated with CaEP. No decrease in spheroid size or viability was seen in normal cell spheroids, while a significant decrease in size and viability was observed in the colon cancer spheroids. The spheroids were treated 5 days after seeding the cells and due to differences in doubling time the size of the spheroids differs at treatment time. Adapted from Frandsen, Gibot et al, 2015.46
The effect of CaEP in vivo on normal tissue surrounding a treated tumor was investigated.52 Human breast cancer tumors on mice were treated with CaEP using different concentrations of calcium, and 2 days after treatment, tumors as well as the muscle below and the skin above the treated tumors were removed, and the effect on the different tissues was estimated by histological analyses. In the tumors, the fraction of necrosis increased significantly after treatment with CaEP, independent of the calcium concentration used. In the surrounding normal tissue, the effect was less pronounced. In 80% of the muscle samples located below the treated tumors, no necrosis was observed. In the skin tissue located above the treated tumor, inflammation, extravasation of erythrocytes, and edema were observed but to the same degree as in skin samples located above untreated tumors, indicating that the presence of the tumor caused the changes and not the CaEP treatment. In the same study, normal muscle and skin tissues were also directly treated with CaEP. In these muscle samples, only 40% of the samples showed no necrosis; however, when looking at the fraction of necrosis in the muscle samples, only 10% of the muscle tissue was necrotic; thus, most of the muscle samples showed some necrosis but it was only in a small part of each muscle sample. This fraction was much lower than that in the tumors treated with CaEP. In the skin tissue directly treated, no edema was seen, and a limited degree of inflammatory markers were present in the treated samples however to the same degree as was observed in control samples. Overall, this study shows a clear difference between normal and malignant tissues treated with CaEP.52
In the clinical trial comparing CaEP and ECT using bleomycin, the effect on the surrounding normal tissue was not directly investigated, but no damage on normal structures was reported.18 Investigations on the effect on normal tissue in patients may be added to future studies.
As described earlier, the mechanism behind CaEP is not yet fully understood. Thus, the reason for the observed differences between normal and malignant cells and tissues treated with CaEP is not completely known. However, a few differences between normal and malignant cells in relation to CaEP sensitivity have been shown. The different effects of electroporation on normal and malignant cells, described earlier, will likely also influence the overall effect of CaEP and therefore be part of the reason for the observed difference in sensitivity (Table 1). In a recent in vivo study,52 the intracellular calcium content was measured before and after treatment with CaEP. The calcium content was measured in tumor as well as the muscle below and the skin above the treated tumor. In the tumor tissue, intracellular calcium content increased significantly and was still significantly higher 4 hours after treatment than in untreated tumor tissue. Interestingly, intracellular calcium content was significantly higher in the skin tissue above tumors treated with CaEP 15 minutes and 60 minutes after treatment than in untreated skin, but the calcium content decreased 4 hours after treatment to a level comparable with the calcium content in untreated skin tissue. In the muscle tissue, no significant changes in intracellular calcium content were observed. These results indicate that the skin tissue is able to reestablish the intracellular calcium content unlike the tumor tissue. In the same study,52 the expression of the plasma membrane calcium ATPase (PMCA) in vitro was measured, showing a significantly lower expression of total PMCA in the tested cancer cell lines compared to normal dermal fibroblasts. In a recent study, it was shown that CaEP caused a decreased expression of PMCA as well as the sodium calcium exchanger (NCX1) in malignant muscle cells but not in normal muscle cells.53 In this study, it was also shown that CaEP affects the cytoskeleton structure more in malignant cells than in normal cells.53 Thus, normal cells might have a higher capability of pumping calcium out the cell as well as keeping normal cell structure compared to cancer cells and thereby reestablish intracellular calcium concentration and increase survival.
Conclusion and Perspectives
Since the first article on CaEP as a potential novel anticancer treatment was published, several papers have followed showing a clear anticancer effect in vitro, in vivo, and in the clinical setting. Furthermore, normal and malignant cells and tissues show different sensitivity to CaEP with normal cells being less affected than cancer cells. The mechanism behind this difference in sensitivity is not yet fully understood but might be influenced by differences in the response to the electroporation procedure between cell types, and differences in cytoskeleton structure after treatment as well as differences in the cellular capacity to handle increased intracellular calcium concentrations, such as differences in the PMCA and NCX1 expression. Further investigations are needed to understand the mechanism behind CaEP and thereby the differences in sensitivity between normal and malignant cells and tissues.
Abbreviations
- CaEP
calcium electroporation
- ECT
electrochemotherapy
- IRE
irreversible electroporation
- PMCA
plasma membrane calcium ATPase
- NCX1
sodium calcium exchanger
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A patent has been submitted—PCT/DK2012/050496. Therapeutic applications of calcium electroporation to effectively induce tumor necrosis (co-inventors: S.K. Frandsen and J. Gehl).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447. [DOI] [PubMed] [Google Scholar]
- 2. Levine ZA, Vernier PT. Calcium and phosphatidylserine inhibit lipid electropore formation and reduce pore lifetime. J Membr Biol. 2012;245(10):599–610. [DOI] [PubMed] [Google Scholar]
- 3. Guidance N. Electrochemotherapy for metastases in the skin from tumours of non-skin origin (IPG446). 2013; http://www.nice.org.uk/guidance/IPG446. Accessed July 05, 2018.
- 4. Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99(6):821–830. [DOI] [PubMed] [Google Scholar]
- 5. Campana LG, Marconato R, Valpione S, et al. Basal cell carcinoma: 10-year experience with electrochemotherapy. J Transl Med. 2017;15(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plaschke CC, Bertino G, McCaul JA, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: results from the treatment of mucosal cancers. Eur J Cancer. 2017;87:172–181. [DOI] [PubMed] [Google Scholar]
- 7. Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19(1):192–198. [DOI] [PubMed] [Google Scholar]
- 8. Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer. 1998;83(1):148–157. [DOI] [PubMed] [Google Scholar]
- 9. Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50(5):621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matthiessen LW, Johannesen HH, Hendel HW, Moss T, Kamby C, Gehl J. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51(6):713–721. [DOI] [PubMed] [Google Scholar]
- 11. Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. AnnSurgOncol. 2008;15(8):2215–2222. [DOI] [PubMed] [Google Scholar]
- 12. Sersa G, Cufer T, Paulin SM, Cemazar M, Snoj M. Electrochemotherapy of chest wall breast cancer recurrence. Cancer Treat Rev. 2012;38(5):379–386. [DOI] [PubMed] [Google Scholar]
- 13. Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32(28):3144–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edhemovic I, Gadzijev EM, Brecelj E, et al. Electrochemotherapy: a new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat. 2011;10(5):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miklavcic D, Sersa G, Brecelj E, et al. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput. 2012;50(12):1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. [DOI] [PubMed] [Google Scholar]
- 17. Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. [DOI] [PubMed] [Google Scholar]
- 18. Falk H, Matthiessen LW, Wooler G, Gehl J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–319. [DOI] [PubMed] [Google Scholar]
- 19. Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J, Jr, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer. 1993;72(12):3694–3700. [DOI] [PubMed] [Google Scholar]
- 20. Landstrom F, Ivarsson M, Vons AK, Magnuson A, Vonb M, Moller C. Electrochemotherapy - evidence for cell-type selectivity in vitro. Anticancer Res. 2015;35(11):5813–5820. [PubMed] [Google Scholar]
- 21. Gibot L, Madi M, Vézinet R, Rols MP. Mixed Spheroids as a Relevant 3D Biological Tool to Understand Therapeutic Window of Electrochemotherapy In: Jarm T, Kramar P, eds. 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies: Portorož, Slovenia, September 6 –10, 2015. Singapore: Springer Singapore; 2016:200–203. [Google Scholar]
- 22. Gehl J. Investigational treatment of cancer using electrochemotherapy, electrochemoimmunotherapy and electro-gene transfer[in Danish]. Ugeskr Laeger. 2005;167(34):3156–3159. [PubMed] [Google Scholar]
- 23. Gasljevic G, Edhemovic I, Cemazar M, et al. Histopathological findings in colorectal liver metastases after electrochemotherapy. PLoS One. 2017;12(7):e0180709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markelc B, Sersa G, Cemazar M. Differential mechanisms associated with vascular disrupting action of electrochemotherapy: intravital microscopy on the level of single normal and tumor blood vessels. PLoS One. 2013;8(3):e59557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mir LM, Tounekti O, Orlowski S. Bleomycin: revival of an old drug. GenPharmacol. 1996;27(5):745–748. [DOI] [PubMed] [Google Scholar]
- 26. Laufer S, Ivorra A, Reuter VE, Rubinsky B, Solomon SB. Electrical impedance characterization of normal and cancerous human hepatic tissue. Physiol Meas. 2010;31(7):995–1009. [DOI] [PubMed] [Google Scholar]
- 27. Frandsen SK, McNeil AK, Novak I, McNeil PL, Gehl J. Difference in membrane repair capacity between cancer cell lines and a normal cell line. J Membr Biol. 2016;249(4):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arena CB, Szot CS, Garcia PA, Rylander MN, Davalos RV. A three-dimensional in vitro tumor platform for modeling therapeutic irreversible electroporation. Biophys J. 2012;103(9):2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurata K, Matsushita M, Yoshii T, Fukunaga T, Takamatsu H. Effect of irreversible electroporation on three-dimensional cell culture model. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:179–182. [DOI] [PubMed] [Google Scholar]
- 30. Qin Q, Xiong ZA, Liu Y, et al. Effects of irreversible electroporation on cervical cancer cell lines in vitro. Mol Med Rep. 2016;14(3):2187–2193. [DOI] [PubMed] [Google Scholar]
- 31. Al-Sakere B, Andre F, Bernat C, et al. Tumor ablation with irreversible electroporation. PLoS One. 2007;2(11):e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qin Z, Jiang J, Long G, Lindgren B, Bischof JC. Irreversible electroporation: an in vivo study with dorsal skin fold chamber. Ann Biomed Eng. 2013;41(3):619–629. [DOI] [PubMed] [Google Scholar]
- 33. Arena CB, Sano MB, Rossmeisl JH, Jr, et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng Online. 2011;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siddiqui IA, Kirks RC, Latouche EL, et al. High-frequency irreversible electroporation: safety and efficacy of next-generation irreversible electroporation adjacent to critical hepatic structures. Surg Innov. 2017;24(3):276–283. [DOI] [PubMed] [Google Scholar]
- 35. Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34(1):132–138. [DOI] [PubMed] [Google Scholar]
- 36. Ansari D, Kristoffersson S, Andersson R, Bergenfeldt M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: a systematic review of safety and efficacy. Scand J Gastroenterol. 2017;52(11):1165–1171. [DOI] [PubMed] [Google Scholar]
- 37. Jiang C, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng. 2015;62(1):4–20. [DOI] [PubMed] [Google Scholar]
- 38. Vroomen L, Petre EN, Cornelis FH, Solomon SB, Srimathveeravalli G. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences? Diagn Interv Imaging. 2017;98(9):609–617. [DOI] [PubMed] [Google Scholar]
- 39. Rombouts SJE, van Dijck WPM, Nijkamp MW, et al. Clinical and pathological outcomes after irreversible electroporation of the pancreas using two parallel plate electrodes: a porcine model. HPB (Oxford). 2017;19(12):1058–1065. [DOI] [PubMed] [Google Scholar]
- 40. Dollinger M, Muller-Wille R, Zeman F, et al. Irreversible electroporation of malignant hepatic tumors--alterations in venous structures at subacute follow-up and evolution at mid-term follow-up. PLoS One. 2015;10(8):e0135773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo X, Qin Z, Tao H, et al. The safety of irreversible electroporation on nerves adjacent to treated tumors. World Neurosurg. 2017;108:642–649. [DOI] [PubMed] [Google Scholar]
- 42. Neal RE, Rossmeisl JH, Jr, Garcia PA, Lanz OI, Henao-Guerrero N, Davalos RV. Successful treatment of a large soft tissue sarcoma with irreversible electroporation. J Clin Oncol. 2011;29(13):e372–e377. [DOI] [PubMed] [Google Scholar]
- 43. Frandsen SK, Gissel H, Hojman P, Eriksen J, Gehl J. Calcium electroporation in three cell lines: a comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim Biophys Acta. 2014;1840(3):1204–1208. [DOI] [PubMed] [Google Scholar]
- 44. Zielichowska A, Daczewska M, Saczko J, Michel O, Kulbacka J. Applications of calcium electroporation to effective apoptosis induction in fibrosarcoma cells and stimulation of normal muscle cells. Bioelectrochemistry. 2016;109:70–78. [DOI] [PubMed] [Google Scholar]
- 45. Frandsen SK, Gehl J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. PLoS One. 2017;12(7):e0181839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frandsen SK, Gibot L, Madi M, Gehl J, Rols MP. Calcium electroporation: evidence for differential effects in normal and malignant cell lines, evaluated in a 3D spheroid model. PLoS One. 2015;10(12):e0144028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hansen EL, Sozer EB, Romeo S, Frandsen SK, Vernier PT, Gehl J. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS One. 2015;10(4):e0122973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rols MP, Teissie J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys J. 1990;58(5):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. [DOI] [PubMed] [Google Scholar]
- 50. Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 2002;99(3):1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int J Cell Biol. 2010;2010: 546163-546176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frandsen SK, Krüger MB, Mangalanathan UM, et al. Normal and malignant cells exhibit differential responses to calcium electroporation. Cancer Res. 2017;77(16):4389–4401. [DOI] [PubMed] [Google Scholar]
- 53. Szewczyk A, Gehl J, Daczewska M, et al. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget. 2018;9(14): 11604–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]